Abstract
This study explores the role of combining the controlling nutritional status (CONUT) score and the carcinoembryonic antigen (CEA) level on predicting tumor stage and prognosis in gastric cancer (GC) patients. A total of 682 GC patients were included in this retrospective study. CONUT scores and CEA levels were combined to establish a new scoring system: CONUT-CEA score. cutoff values for distinguishing patients between stage IV and non-stage IV were established by receiver operating characteristic curves. cutoff values for predicting prognosis were determined by maximum χ2 method. The CONUT and CEA cutoff values for discriminating stage IV patients from non-stage IV patients were 2.0 and 5.58 ng/mL, respectively. Logistic regression model demonstrated that high CONUT-CEA score was related to advanced tumor stage. Among non-stage IV patients, CONUT and CEA cutoff values of 2.0 and 9.50 ng/mL predicted overall survival (OS), respectively. The Cox proportional risk model revealed that high CONUT-CEA score was notable related to decreased OS (2 vs 0: hazard ratios (HR) = 2.358, 95% confidence intervals (CI) = 1.412–3.940, P = .001) and decreased disease-free survival (2 vs 0: HR = 1.980, 95% CI = 1.072–3.656, P = .003). The CONUT-CEA score may be a good biomarker for predicting tumor stage and prognosis in GC patients.
Keywords: carcinoembryonic antigen, controlling nutritional status, gastric cancer, prognosis, tumor stage
1. Introduction
Despite great progress in diagnosis and treatment, gastric cancer (GC) remains the fourth most common malignant tumor and the third leading cause of cancer death globally.[1] There are no symptoms or only nonspecific symptoms present at the time of early-stage diseases; consequently, GC is generally diagnosed in advanced stages.[2] Furthermore, the prognosis of patients with the same tumor stage may differ due to heterogeneity.[3] Accordingly, accurately evaluating the tumor progression and prognosis of GC patients is helpful for developing individualized treatment programmes to improve prognosis. Tumour progression is determined not only by tumor characteristics but also by the host’s nutritional status, systemic inflammation status and immune-compromised status.[4]
Controlling nutritional status (CONUT), a newly developed scoring instrument, consists of 3 blood indexes: serum albumin (ALB) concentration, total number of peripheral blood lymphocytes and total cholesterol concentration. CONUT was initially reported as a screening instrument to evaluate the nutritional status of patients.[5–8] The prognostic values of CONUT score for a variety of human tumors have been reported in recent years. It can be used to predict the prognosis of breast, bladder, lung cancer and GC.[7,9–16] However, there were relatively few studies on the impact of CONUT score on the prognosis of GC patients.
Carcinoembryonic antigen (CEA) is a glycoprotein expressed on the surface of tumor cells, which is one of the most commonly used biomarkers for in many cancers, including gastric cancer.[17] CEA levels are often used in the early detection of cancer, and some studies have shown that CEA levels are related to preoperative predictions and can reflect tumor characteristics.[18] CEA levels have been used as a prognostic indicator in GC.[19–21] However, because of its low sensitivity and high false-positive rate, the use of CEA as an independent prognostic marker has always been controversial.[22,23] Although CEA levels have limited value as an independent prognostic factor, some research has shown that the combinations of CEA levels and other indicators can increase their prognostic sensitivity.[24,25]
In this retrospective study, CONUT scores and CEA levels were combined to establish a new scoring system: CONUT-CEA score. This study analyzed the role of combinations of pretreatment CONUT score and CEA levels on tumor stage, and accessed the predictive value of the combined detection of the CONUT score and CEA levels on the prognosis of GC patients.
2. Methods
2.1. Patient population
This analysis included patients with GC who were diagnosed and treated at the First Affiliated Hospital of Fujian Medical University in Fujian, China between 2008 and 2011. This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University and obtained the informed consent of all participants.
The exclusion criteria were as follows: patients who had gastroduodenal diseases, hematologic diseases, autoimmune diseases, systemic inflammatory diseases, infections, thyroid disease, coronary artery disease, renal function failure, severe liver disease or other malignancies; patients who had incomplete clinical and pathologic data; patients who had previous surgery or radiotherapy and chemotherapy; and patients who had used corticosteroids, acetylsalicylic acid or statins within the previous 3 months.
Based on these criteria, we enrolled 682 patients, including 504 men and 178 women, the median age was 61.0 years (interquartile range (IQR) = 54.0–69.0 years, range: 18–87 years). Of the patients, 148 cases (21.7%) were Tumour-Node-Metastasis (TNM) stage I, 183 cases (26.8%) were TNM stage II, 227 cases (33.3%) were TNM stage III and 124 cases (18.2%) were TNM stage IV. Stage I, II, and III were defined as non-stage IV. Tumours were classified according to the seventh edition of the AJCC/TNM tumor staging system.[24] In the non-stage IV patients, 282 (50.5%) had proximal GC and 276 (49.5%) had distal GC; all the non-stage IV patients were subjected to radical operation. Seventy-four patients with stage IV GC were subjected to surgery, and the remaining 50 patients did not receive surgery and were diagnosed as stage IV by imaging examinations.
Stage IV GC was diagnosed as follows: metastasis of the liver, lung, bone, pancreas and other organs; peritoneal dissemination; and metastasis of distant lymph nodes.
2.2. Clinical assessment and laboratory data
Medical records and laboratory results were retrospectively reviewed. Age, sex, smoking status, alcohol intake, clinical characteristics, lymphocytes, ALB and total cholesterol level were collected from the patients’ medical records. All peripheral venous blood samples were collected in the morning after fasting for 1 night (at least 8 hours). The CONUT score, which reflects the nutritional status, is shown in Table 1, calculated by the ALB concentration, total blood cholesterol level and total peripheral lymphocyte count (Table 1).
Table 1.
Nutritional status assessment according to CONUT scoring system.
| Parameters | Undernutrition degree | |||
|---|---|---|---|---|
| Normal | Light | Moderate | Severe | |
| Serum albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocyte count (/mm3) | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| CONUT score = Serum albumin score + Total lymphocyte count score + Total cholesterol score | ||||
CONUT-CEA score was established by combining CONUT scores and CEA levels, including CONUT-CEA score-1 and CONUT-CEA score-2. cutoff values of CONUT scores and CEA levels for distinguishing between stage IV and non-stage IV patients were established, and the CONUT-CEA score-1 was calculated based on the above cutoff values. cutoff values of CONUT scores and CEA levels for predicting overall survival (OS) in non-stage IV patients were established, and the CONUT-CEA score-2 was calculated based on the above cutoff values.
2.3. Follow-up
For the non-stage IV patients, follow-up visits were conducted every 3 months for the first 2 years after surgery, every 6 months for the third to fifth years after surgery, and every 1 year thereafter. Postoperative follow-up included physical examination, laboratory examination, gastroscopy and chest/abdominopelvic computed tomography. The last follow-up was performed on September 1, 2019. The definition of OS was from the date of radical operation to death or the last follow-up. The definition of disease-free survival (DFS) was from the date of radical operation to the date of local tumor recurrence and/or metastasis or the date of the last follow-up. The main endpoint was OS, and the secondary endpoint was DFS.
2.4. Statistical analyses
Receiver operating characteristic (ROC) curves were drawn to establish cutoff values for distinguishing between stage IV and non-stage IV patients, and sensitivity and specificity were also tested. A binary logistical regression model was used to established parameters related to stage IV GC. The best CONUT and CEA cutoff values for predicting the prognosis of non-stage IV patients were determined by maximum χ2 method. This analysis uses the R maxstat software package (R Foundation for Statistical Computing, Vienna, Austria). The Kaplan–Meier method was used to determine the influence of each variable on OS and DFS, and log-rank tests were used to compare the survival curves. To determine the influence of clinical parameters on the prognosis of OS and DFS, a Cox proportional risk model was used for analysis, including univariate and multivariate analyses. Significant variables in the univariate analysis (P < .05) were entered into the multivariate regression models, and the data were represented as hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses were performed using SPSS software 19.0 (SPSS Inc., Chicago, IL) and R version 3.5.2 software (Institute for Statistics and Mathematics, Vienna, Austria). A P value < .05 was considered significant.
3. Results
3.1. Patient characteristics
Among the 682 patients, the CONUT score ranged from 0 to 11. The median CONUT value was 1 (IQR = 0–3). The CEA level ranged from 0.200 to 1000.0 ng/mL, the median CEA level was 2.41 ng/mL (IQR = 1.44–5.21 ng/mL). Of the non-stage IV patients, 282 (50.5%) had proximal GC and 276 (49.5%) had distal GC. After radical surgery in the non-stage IV cases, the median follow-up duration was 101 (range: 1–130) months; for patients who were alive at the end of this study, the median follow-up duration was 109 (range: 71–130) months. The baseline clinical characteristics of the patients are shown in Table 2.
Table 2.
Demographic and clinical characteristics of the study population.
| Characteristics | Value | Percentage | Median (IQR) |
|---|---|---|---|
| Number of patients | 682 | ||
| Age (yr) | |||
| ≥75 | 76 | 11.1% | 61 (54, 69) |
| <75 | 606 | 88.9% | |
| Sex (male) | |||
| Male | 504 | 73.9% | |
| Female | 178 | 26.1% | |
| Smoking | |||
| Yes | 168 | 24.6% | |
| No | 514 | 75.4% | |
| Alcohol intake | |||
| Yes | 64 | 9.38% | |
| No | 618 | 90.6% | |
| BMI (kg/m2) | |||
| ≥25 | 97 | 14.2% | 21.9 (19.8, 23.9) |
| <25 | 585 | 85.8% | |
| ALB (g/dL) | 3.78 (3.50, 4.08) | ||
| Lymphocyte (mm3) | 1710 (1340, 2050) | ||
| Cholesterol (mg/dL) | 170.9 (147.3201.1) | ||
| CONUT | 1 (0, 3) | ||
| CEA | 2.41 (1.44, 5.21) | ||
| Clinical TNM stage | |||
| I | 148 | 21.7% | |
| II | 183 | 26.8% | |
| III | 227 | 33.3% | |
| IV | 124 | 18.2% | |
| Tumor location (TNM stage I–III) | 558 | ||
| Proximal | 282 | 50.5% | |
| Distal | 276 | 49.5% | |
| T stage (TNM stage I–III) | |||
| T1 | 108 | 19.4% | |
| T2 | 72 | 12.9% | |
| T3 | 142 | 25.5% | |
| T4 | 236 | 42.3% | |
| N stage (TNM stage I–III) | |||
| N0 | 229 | 41.0% | |
| N1 | 98 | 17.6% | |
| N2 | 88 | 15.8% | |
| N3 | 143 | 25.6% | |
| Histological type (TNM stage I–III) | |||
| Differentiated | 209 | 37.5% | |
| Undifferentiated | 349 | 62.5% | |
| Postoperative complications (TNM stage I–III) | |||
| No | 463 | 83.0% | |
| Yes | 95 | 17.0% | |
| Neurovascular invasion (TNM stage I–III) | |||
| No | 292 | 52.3% | |
| Yes | 266 | 47.7% | |
| Adjuvant chemotherapy (TNM stage I–III) | |||
| Absent | 233 | 41.8% | |
| Present | 325 | 58.2% | |
ALB = albumin, BMI = body mass index, CEA = carcinoembryonic antigen, CONUT = controlling nutritional status, IQR = interquartile range, TNM = Tumour-Node-Metastasis.
The baseline clinical characteristics of the non-stage IV and stage IV cases are shown in Table 3. No significant differences were noted in sex, age, BMI, smoking status and alcohol intake (P > .05).
Table 3.
Comparison of the clinical indexes between stage IV and non-stage IV groups.
| Total N = 682 (%) | Stage IV group N = 124 (%) | Non-stage IV group N = 558 (%) | χ2/F | P | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 178 (26.1) | 28 (22.6) | 150 (26.9) | 0.973 | .324 |
| Male | 504 (73.9) | 96 (77.4) | 408 (73.1) | ||
| Age | |||||
| <75 | 606 (88.9) | 107 (86.3) | 499 (89.4) | 1.008 | .315 |
| ≥75 | 76 (11.1) | 17 (13.7) | 59 (10.6) | ||
| Smoking | |||||
| No | 514 (75.4) | 90 (72.6) | 424 (76.0) | 0.634 | .426 |
| Yes | 168 (24.6) | 34 (27.4) | 134 (24.0) | ||
| Alcohol | |||||
| No | 613 (89.9) | 105 (88.2) | 508 (90.2) | 0.430 | .512 |
| Yes | 69 (10.1) | 14 (11.8) | 55 (9.8) | ||
| BMI (kg/m2) | |||||
| <25 | 585 (85.8) | 107 (86.3) | 478 (85.7) | 0.033 | .856 |
| ≥25 | 97 (14.2) | 17 (13.7) | 80 (14.3) | ||
| CONUT | 2.56 ± 2.29 | 1.86 ± 1.92 | 6.584 | .002 | |
| CEA | 45.70 ± 140.4 | 9.073 ± 52.63 | 63.245 | .005 | |
BMI = body mass index, CEA = carcinoembryonic antigen, CONUT = controlling nutritional status.
3.2. Relationship between pretreatment tumor stage and CONUT/CEA
The median (IQR) CONUT score of the stage IV patients was 2 (1–4), and that of the non-stage IV patients was 1 (0–3). The CONUT score of stage IV patients was significantly higher than that of non-stage IV patients (P = .002) (Table 3). The median (IQR) CEA of the stage IV patients was 4.30 (1.77–11.93) ng/mL, and that of the non-stage IV patients was 2.28 (1.41–4.43) ng/mL. The CEA of the stage IV patients was significantly higher than that of the non-stage IV patients (P = .005) (Table 3).
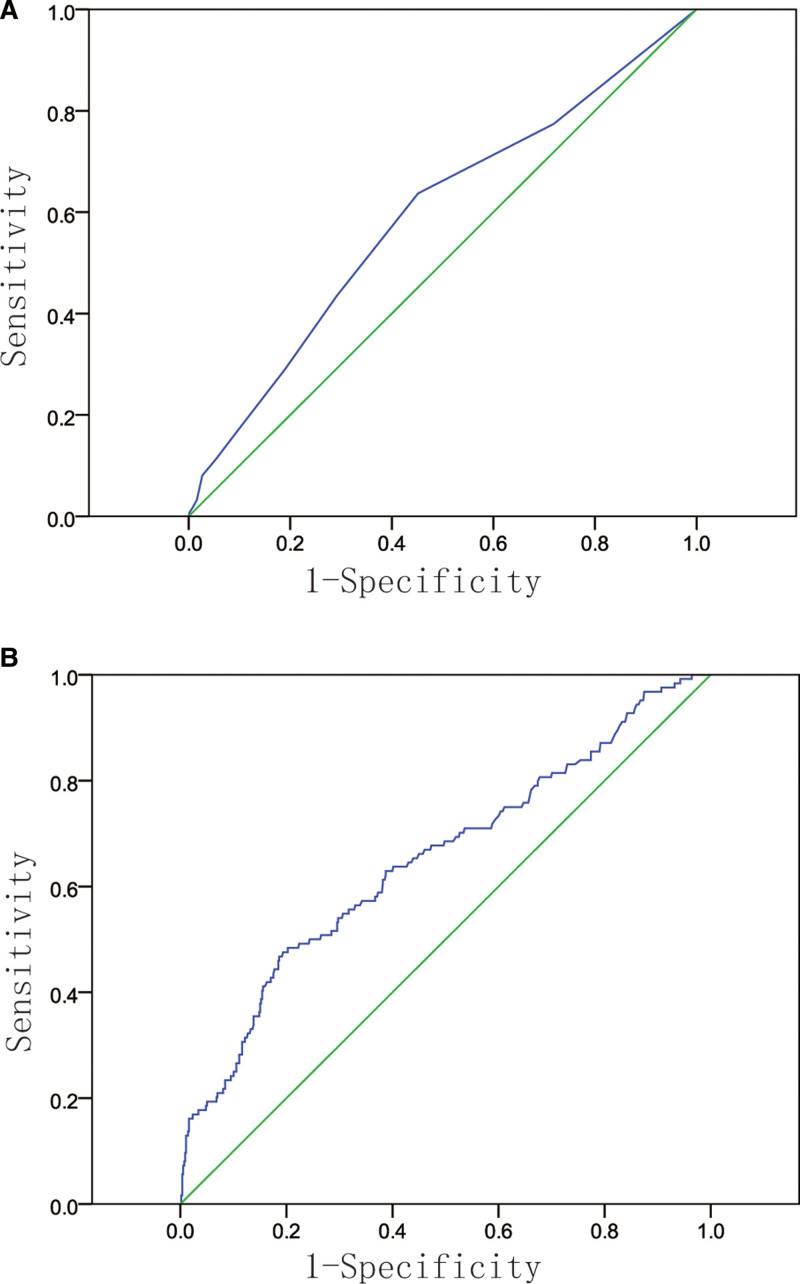
According to the ROC curves, the area under the cure was 0.592 (95% CI: 0.535–0.649) for discriminating stage IV patients from non-stage IV patients based on the CONUT score, and the area under the cure was 0.651 (95% CI: 0.580–0.695) for discriminating stage IV patients from non-stage IV patients based on the CEA level (Fig. 1A and B). According to the ROC analysis, the cutoff value of CONUT was 2.0, the sensitivity and specificity were 0.436 and 0.708, respectively. The cutoff value of CEA was 5.58 ng/mL, the sensitivity and specificity were 0.460 and 0.815, respectively.
Figure 1.
Receiver operating characteristic curves of CONUT score (A) and CEA (B) to discriminate stage IV group and non-stage IV group. CEA = carcinoembryonic antigen, CONUT = controlling nutritional status.
According to the CONUT and CEA cutoff values, cases were split into the following groups: high (≥2; n = 217) and low CONUT status (<2; n = 465) or high (≥5.58 ng/mL; n = 160) and low CEA status (<5.58 ng/mL; n = 522).
3.3. Relationship between tumor stage and CONUT-CEA score
The CONUT-CEA score-1 ranged from 0 to 2 as follows: score of 0, neither high CONUT (≥2) nor high CEA (≥5.58 ng/mL); score of 1, either high CONUT or high CEA; score of 2, both high CONUT and high CEA. A CONUT-CEA score-1 of 0, 1, and 2 was noted in 367 (53.8%), 253 (37.1%), and 62 (9.09%) cases. The CONUT-CEA score-1 of the stage IV group was significantly higher than that of the non-stage IV group (P = .000) (Table 4). The univariate and multivariate logistic regression model displayed that high CONUT-CEA score-1 was correlated with stage IV (P < .001) (Table 5).
Table 4.
Relationship between tumor stage and the CONUT-CEA score.
| CONUT-CEA score-1 (%) | P | |||
|---|---|---|---|---|
| 0 (n = 367) | 1 (n = 253) | 2 (n = 62) | ||
| Stage IV group (n = 124) | 40 (32.3) | 57 (46.0) | 27 (21.8) | .000 |
| Non-stage IV group (n = 558) | 327 (58.6) | 196 (35.1) | 35 (6.27) | |
Table 5.
Risk factors related to diagnose as stage IV: univariate and multivariate logistic regression analysis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex (female vs male) | 0.793 | 0.500–1.258 | .325 | 0.809 | 0.486–1.346 | .414 |
| Age (≥75 vs < 75) | 1.344 | 0.753–2.396 | .317 | 1.004 | 0.539–1.870 | .991 |
| Smoking (yes vs no) | 1.195 | 0.770–1.856 | .426 | 1.063 | 0.632–1.790 | .817 |
| Alcohol (yes vs no) | 1.293 | 0.690–2.422 | .422 | 1.324 | 0.652–2.688 | .437 |
| BMI (≥25 vs < 25) | 0.949 | 0.540–1.668 | .856 | 1.368 | 0.753–2.486 | .303 |
| CONUT-CEA score-1 | ||||||
| 1 vs 0 | 2.377 | 1.529–3.696 | .000 | 2.451 | 1.559–3.855 | .000 |
| 2 vs 0 | 6.306 | 3.462–11.489 | .000 | 6.810 | 3.649–12.710 | .000 |
3.4. The optimal cutoff values for forecasting the prognosis of non-stage IV cases
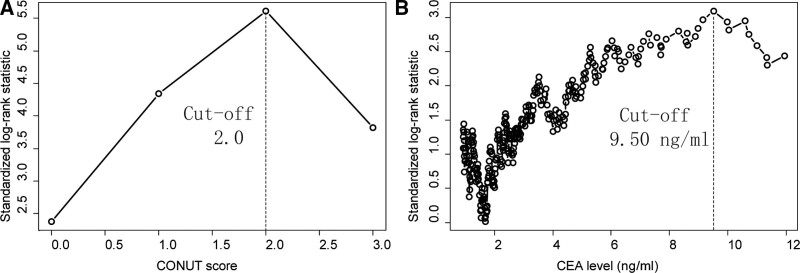
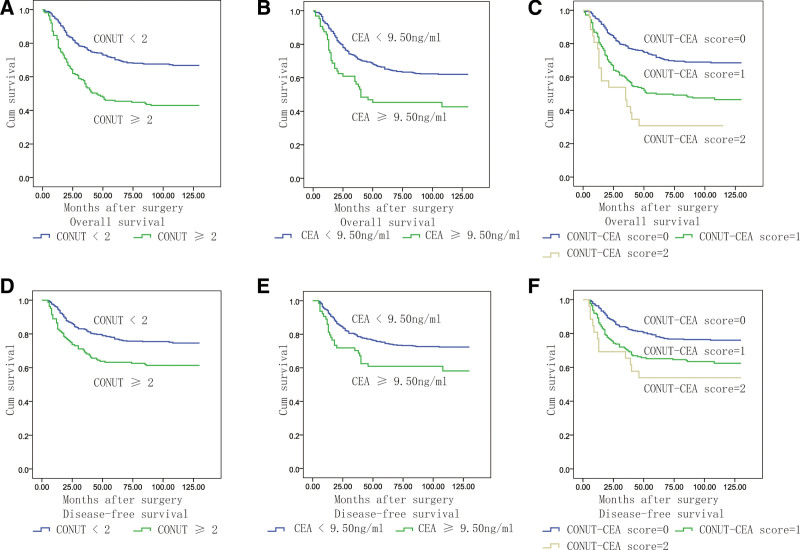
Cutoff values of CONUT and CEA to predict prognosis were determined by maximum χ2 method. The maximal χ2 method could define a subgroup with the greatest prognosis difference. The optimal CONUT and CEA cutoff values were 2.0 and 9.50 ng/mL, respectively, for predicting OS of non-stage IV cases (Fig. 2). The CONUT-CEA score-2 ranged from 0 to 2 as follows: score of 2, high CONUT (≥2) and high CEA (≥9.50 ng/mL); score of 1, either high CONUT or high CEA; score of 0, neither high CONUT nor high CEA. A CONUT-CEA score-2 of 0, 1, and 2 was noted in 357 (64.0%), 175 (31.4%), and 26 (4.66%) patients, respectively. CONUT, CEA and CONUT-CEA score-2 were significantly associated with the prognosis of OS and DFS based on the Kaplan–Meier method and log-rank tests (P < .05, Fig. 3).
Figure 2.
Cutoff value for CONUT (A) and CEA (B). The maximum difference in overall survival was achieved when the CONUT score was 2.0 and CEA level was 9.50 ng/mL. CEA = carcinoembryonic antigen, CONUT = controlling nutritional status.
Figure 3.
Kaplan–Meier analysis of the effects of each variable on OS and DFS. A: The OS curves of patients with different CONUT score. B: The OS curves of patients with different CEA score. C: The OS curves of patients with different CONUT-CEA score. D: The DFS curves of patients with different CONUT score. E: The DFS curves of patients with different CEA score. F: The DFS curves of patients with different CONUT-CEA score. CEA = carcinoembryonic antigen, CONUT = controlling nutritional status, DFS = disease-free status, OS = overall survival.
3.5. Univariate and multivariate analyses of OS
As shown in Table 6, CONUT ≥ 2, CEA ≥ 9.5 ng/mL, high CONUT-CEA score-2, age ≥ 75 years, T stage (T2 vs T1, T3 vs T1, T4 vs T1), N stage (N1 vs N0, N2 vs N0, N3 vs N0), TNM stage (II vs I, III vs I), histological type (undifferentiated vs differentiated) and neurovascular invasion (yes vs no) were established as significant prognostic factors of OS in the univariate analysis (all P < .05).
Table 6.
Univariate and multivariate analyses for OS and DFS among patients with gastric cancer.
| Overall survival | Disease-free survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| CONUT (≥2 vs <2) | 2.185 | 1.674–2.853 | .000 | – | – | – | 1.756 | 1.280–2.408 | .000 | – | – | – |
| CEA (≥9.5 vs <9.5) | 1.819 | 1.273–2.599 | .001 | – | – | – | 1.681 | 1.105–2.557 | .015 | – | – | – |
| CONUT-CEA score-2 (1 vs 0) | 2.083 | 1.582–2.744 | .000 | 1.647 | 1.241–2.188 | .001 | 1.757 | 1.272–2.427 | .001 | 1.452 | 1.046–2.016 | .026 |
| CONUT-CEA score-2 (2 vs 0) | 3.339 | 2.028–5.499 | .000 | 2.358 | 1.412–3.940 | .001 | 2.475 | 1.352–4.531 | .003 | 1.980 | 1.072–3.656 | .029 |
| Age (≥75 vs <75) | 2.423 | 1.713–3.426 | .000 | 2.400 | 1.672–3.444 | .000 | 1.511 | 0.964–2.369 | .072 | – | – | – |
| Sex (female vs male) | 1.323 | 0.997–1.756 | .052 | – | – | – | 1.375 | 0.990–1.909 | .057 | – | – | – |
| Smoking | 0.783 | 0.566–1.083 | .139 | – | – | – | 0.727 | 0.491–1.075 | .110 | – | – | – |
| Alcohol | 0.964 | 0.602–1.542 | .878 | – | – | – | 0.888 | 0.504–1.566 | .683 | – | – | – |
| BMI (≥25 vs <25) | 0.730 | 0.485–1.098 | .131 | – | – | – | 0.811 | 0.508–1.295 | .381 | – | – | – |
| Tumor location (distal vs proximal) | 0.778 | 0.597–1.014 | .063 | – | – | – | 0.846 | 0.621–1.153 | .289 | – | – | – |
| T stage (T2 vs T1) | 2.450 | 1.18–5.086 | .016 | – | – | – | 2.132 | 0.898–5.060 | .086 | – | – | – |
| T stage (T3 vs T1) | 5.262 | 2.844–9.736 | .000 | – | – | – | 4.615 | 2.262–9.418 | .000 | – | – | – |
| T stage (T4 vs T1) | 6.660 | 3.682–12.045 | .000 | – | – | – | 5.926 | 2.990–11.747 | .000 | – | – | – |
| N stage (N1 vs N0) | 2.810 | 1.822–4.335 | .000 | – | – | – | 2.878 | 1.660–4.988 | .000 | – | – | – |
| N stage (N2 vs N0) | 4.028 | 2.646–6.133 | .000 | – | – | – | 5.724 | 3.465–9.457 | .000 | – | – | – |
| N stage (N3 vs N0) | 5.850 | 4.031–8.490 | .000 | – | – | – | 6.221 | 3.907–9.907 | .000 | – | – | – |
| TNM stage (II vs I) | 3.889 | 2.254–6.710 | .000 | 2.721 | 1.543–4.799 | .001 | 3.813 | 1.916–7.588 | .000 | 2.820 | 1.378–5.772 | .005 |
| TNM stage (III vs I) | 8.697 | 5.179–14.606 | .000 | 5.848 | 3.352–10.205 | .000 | 9.566 | 5.003–18.290 | .000 | 6.634 | 3.300–13.335 | .000 |
| Histological type (undifferentiated vs differentiated) | 1.523 | 1.145–2.026 | .004 | 1.104 | 0.813–1.498 | .526 | 1.637 | 1.164–2.304 | .005 | 1.063 | 0.742–1.524 | .737 |
| Complications (yes vs no) | 0.677 | 0.217–2.115 | .502 | – | – | – | 1.099 | 0.735–1.642 | .646 | – | – | – |
| Neurovascular invasion (yes vs no) | 2.619 | 1.988–3.451 | .000 | 1.563 | 1.158–2.110 | .004 | 2.735 | 1.969–3.799 | .000 | 1.475 | 1.033–2.104 | .032 |
| Adjuvant chemotherapy (present vs absent) | 1.279 | 0.974–1.680 | .076 | – | – | – | 1.118 | 0.816–1.532 | .487 | – | – | – |
ALB = albumin, BMI = body mass index, CEA = carcinoembryonic antigen, CONUT = controlling nutritional status, DFS = disease-free survival, OS = overall survival, TNM = Tumour-Node-Metastasis.
In the Cox multivariate analysis, high CONUT-CEA score-2 (1 vs 0: HR = 1.647, 95% CI = 1.241–2.188, P = .001; 2 vs 0: HR = 2.358, 95% CI = 1.412–3.940, P = .001), age ≥ 75 years (HR = 2.400, 95% CI = 1.672–3.444, P = .000), elevated TNM stage (II vs I: HR = 2.721, 95% CI = 1.543–4.799, P = .001; III vs I: HR = 5.848, 95% CI = 3.352–10.205, P = .000), and neurovascular invasion (HR = 1.563, 95% CI = 1.158–2.110, P = .004) were notable related to decreased OS (Table 6).
3.6. Univariate and multivariate analyses of DFS
According to the univariate analysis (Table 6), the following factors have significant differences in the impact of DFS: CONUT ≥ 2, CEA ≥ 9.5 ng/mL, high CONUT-CEA score-2, age ≥ 75 years, T stage (T2 vs T1, T3 vs T1, T4 vs T1), N stage (N1 vs N0, N2 vs N0, N3 vs N0), TNM stage (II vs I, III vs I), histological type (undifferentiated vs differentiated) and neurovascular invasion (yes vs no) (all P < .05).
According to the Cox multivariate analysis, high CONUT-CEA score-2 (1 vs 0: HR = 1.452, 95% CI = 1.046–2.016, P = .001; 2 vs 0: HR = 1.980, 95%C I = 1.072–3.656, P = .003), elevated TNM stage (II vs I: HR = 2.820, 95% CI = 1.378–5.772, P = .000; III vs I: HR = 6.634, 95% CI = 3.300–13.335, P = .000), and neurovascular invasion (yes vs No: HR = 1.475, 95% CI = 1.033–2.104, P = .000) were notable related to decreased DFS (Table 6).
4. Discussion
In the present study, CONUT scores and CEA levels were combined to establish the CONUT-CEA score, which is a new scoring system used for predicting the preoperative stage and postoperative prognosis of GC patients. The present study demonstrated that the CONUT-CEA score plays an important role in discriminating patients with stage IV GC from those with non-stage IV GC, and high CONUT-CEA score was notable related to poor OS and DFS in non-stage IV GC patients. To our knowledge, this study is the first to determine the role of the CONUT-CEA score in the staging and prognostic evaluation of GC.
A CONUT cutoff value of 2 was used to preoperatively determine whether the patient was stage IV or non-stage IV according to the ROC analysis results. The present study also showed that non-stage IV patients with high CONUT levels (≥2) had worse OS and DFS. The CONUT cutoff value in the present study was the same as that reported by Ryo S et al[14] However, previous research only analyzed patients with TNM stages I–III and did not include stage IV patients.[13–16] The treatment and prognosis of stage IV and non-stage IV patients are different. Therefore, evaluating the preoperative clinical stage is key to choosing the appropriate treatment. Inflammation, nutrition and other indicators may be altered in stage IV patients. Hence, the CONUT score may be able to predict preoperative stage IV GC.
The CONUT score is calculated according to ALB concentration, total blood cholesterol level and total peripheral lymphocyte count. In addition to reflecting nutritional status, ALB can reflect systemic inflammation.[26] Pro-inflammatory cytokines such as interleukin-6 or tumor necrosis factor-α decrease ALB by regulating the synthesis of albumin in the liver.[27,28] The total number of lymphocytes reflects the host’s immune response to the tumor.[29] Low peripheral blood lymphocyte counts lead to insufficient immune responses of the host to cancer cells, leading to cancer progression.[30–32] The total cholesterol concentration is related to tumor progression and the prognosis of various cancers.[33,34] Tumour growth requires cholesterol consumption, leading to cholesterol lowering.[35] The CONUT score can reflect nutritional status, the systemic inflammation status and immune response. Nutritional damage and immunosuppression in cancer patients promote the chronic inflammatory response of cancer cells.[36–38] Additionally, systemic inflammation and malnutrition are the main reasons for the decrease in the helper T cell population, interleukin-2 and interleukin-3 levels and T blastogenic reaction, leading to the impairment of tumor immune function and the increase of tumor cell proliferation.[36,38] The increased production of vascular endothelial cell growth factors is related to chronic inflammation, malnutrition and immunosuppression in GC patients.[39]
CEA is a glycosylated protein with a glycosylated form of salivary fucosylation.[40] As a selectin ligand, CEA promotes the metastasis of cancer cells.[41,42] CEA levels are closely associated with tumor burden, so the degree of elevation of CEA levels may be correlated with the pathological stage and prognosis of cancer. Some researches have found that CEA levels are related to pathological stage and can predict prognosis and recurrence in GC, and some studies also reported that high CEA levels above the upper limit of normal indicate a poor outcome in GC; however, the cutoff values of CEA remain unclear. Han et al[43] found that the CEA levels of GC patients with extensive peritoneal seeding were significantly higher than those of other stages, but the ROC curve did not determine the cutoff values of CEA. Liu et al[44] found that the increase of serum CEA levels was related to the GC pathological stage and lymph infiltration, but they did not show the cutoff value of CEA. A study conducted by Park et al[45] found a high recurrence rate in CEA-positive GC patients. Takahashi et al[46] founded that patients with high CEA levels, particularly a CEA ratio more than twice the normal upper limit, experience more frequent cancer recurrence. Lee et al[47] also found that patients with CEA levels more than twice the normal limit had a poor prognosis. Xiao et al[48] reported that a cutoff value of 30.02 ng/mL could be applied to distinguish between patients with a poor prognosis and good prognosis.
The present study showed that a CEA cutoff value of 5.58 ng/mL could be used to determine whether the patient was stage IV or non-stage IV preoperatively according to the ROC analysis results. When assessing prognosis, the maximal χ2 method was used to determine the cutoff value of CEA and showed that non-stage IV patients with high CEA levels ≥ 9.5 ng/mL had worse OS and DFS after radical operation. Moreover, the CEA levels were nearly twice as high as normal limits, which was close to the results reported by Kim et al[49] and Lee et al.[47]
Tumour progression is known to be associated with tumor characteristics, host nutritional status, systemic inflammation status and immunocompromised status. Therefore, identifying parameters that can reflect both tumor characteristics and host state can provide a better prognostic value. Meanwhile, CEA levels were reported to be related to the invasion and metastasis of tumor cells,[39–41] and the CONUT score can reflect the nutritional, inflammation and immune response state. The multivariate logistic analysis of the present study showed that a high CONUT-CEA score was associated with advanced tumor stage. Therefore, we believe that the CONUT score combined with the CEA level can improve the preoperative diagnosis of GC stage. Moreover, the current study demonstrated that the CONUT-CEA score was a more effective candidate prognostic biomarker in patients undergoing surgical resection of GC than the CONUT score or CEA level alone displayed in Table 6.
There were some limitations in this study. First of all, this was a retrospective study at one center, so the possibility of selection bias cannot be completely controlled. Second, we cannot assess the influence of the postoperative CONUT score and CEA level on the prognosis of GC. Third, different nutritional supports after surgery were inevitable, which may confuse our results.
5. Conclusions
In summary, the CONUT score and CEA level are useful for the differential diagnosis of stage IV and non-stage IV GC, and the combination of these measurements is more helpful than each factor alone. Additionally, the CONUT score and CEA level are helpful for forecasting the long-term OS and DFS in GC patients after radical gastrectomy. Assessing preoperative status based on the CONUT-CEA score may help develop effective strategies for GC treatment. CONUT and CEA, as inexpensive and convenient markers, can play an important role in treatment decision-making and follow-up in GC.
Author contributions
Conceptualization: Peiwen Wu.
Data curation: Xiuqing Chen, Linjing Huang, Peiwen Wu.
Funding acquisition: Peiwen Wu.
Investigation: Xiuqing Chen, Chen Chen, Linjing Huang.
Methodology: Xiuqing Chen, Chen Chen.
Project administration: Linjing Huang.
Resources: Chen Chen, Peiwen Wu.
Validation: Linjing Huang.
Writing – original draft: Xiuqing Chen, Chen Chen.
Writing – review & editing: Xiuqing Chen, Peiwen Wu.
Abbreviations:
- ALB
- albumin
- CEA
- carcinoembryonic antigen
- CI
- confidence intervals
- CONUT
- controlling nutritional status
- DFS
- disease-free survival
- GC
- gastric cancer
- HR
- hazard ratios
- OS
- overall survival
- ROC
- receiver operating characteristic
- TNM
- Tumour-Node-Metastasis
XC and CC contributed equally to this work.
This research was funded by the Fujian Provincial Medical Innovation Project (no. 2020CXA035) and Fujian Medical University Sailing Fund Project (no. 2021QH1068).
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (approval no: MRCTA, ECFAH of FMU [2019] 200). Written informed consent for data collection and analysis was obtained from the respective patients.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Chen X, Chen C, Huang L, Wu P. Pretreatment controlling nutritional status (CONUT) score and carcinoembryonic antigen level provide tumor progression and prognostic information in gastric cancer: A retrospective study. Medicine 2023;102:49(e36535).
Contributor Information
Xiuqing Chen, Email: ciece3@qq.com.
Chen Chen, Email: ciece3@qq.com.
Linjing Huang, Email: 530556292@qq.com.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Alsina M, Arrazubi V, Diez M, et al. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. 2023;20:155–70. [DOI] [PubMed] [Google Scholar]
- [3].Ho SWT, Tan P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019;110:3405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Demirelli B, Babacan NA, Ercelep O, et al. Modified glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. 2020;73:1–9. [DOI] [PubMed] [Google Scholar]
- [5].Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- [6].Lee K, Ahn JM, Kang DY, et al. Nutritional status and risk of all-cause mortality in patients undergoing transcatheter aortic valve replacement assessment using the geriatric nutritional risk index and the controlling nutritional status score. Clin Res Cardiol. 2020;109:161–71. [DOI] [PubMed] [Google Scholar]
- [7].Liang RF, Li JH, Li M, et al. The prognostic role of controlling nutritional status scores in patients with solid tumors. Clin Chim Acta. 2017;474:155–8. [DOI] [PubMed] [Google Scholar]
- [8].Elghiaty A, Kim J, Jang WS, et al. Preoperative controlling nutritional status (CONUT) score as a novel immune-nutritional predictor of survival in non-metastatic clear cell renal cell carcinoma of </= 7 cm on preoperative imaging. J Cancer Res Clin Oncol. 2019;145:957–65. [DOI] [PubMed] [Google Scholar]
- [9].Shoji F, Haratake N, Akamine T, et al. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37:741–7. [DOI] [PubMed] [Google Scholar]
- [10].Huang J, Zhao L, Wang K, et al. Controlling nutritional status score evaluates prognosis in patients with non-muscle invasive bladder cancer. Cancer Control. 2021;28:10732748211021078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saito A, Amiya E, Hatano M, et al. Controlling nutritional status score as a predictive marker for patients with implantable left ventricular assist device. ASAIO J. 2020;66:166–72. [DOI] [PubMed] [Google Scholar]
- [12].Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204–12. [DOI] [PubMed] [Google Scholar]
- [13].Zheng ZF, Lu J, Xie JW, et al. Preoperative skeletal muscle index vs the controlling nutritional status score: which is a better objective predictor of long-term survival for gastric cancer patients after radical gastrectomy? Cancer Med. 2018;7:3537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ryo S, Kanda M, Ito S, et al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer: analysis of a multi-institutional data set. Ann Surg Oncol. 2019;26:456–64. [DOI] [PubMed] [Google Scholar]
- [15].Suzuki S, Kanaji S, Yamamoto M, et al. Controlling nutritional status (CONUT) score predicts outcomes of curative resection for gastric cancer in the elderly. World J Surg. 2019;43:1076–84. [DOI] [PubMed] [Google Scholar]
- [16].Liu X, Zhang D, Lin E, et al. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric Cancer. BMC Cancer. 2018;18:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kiio LK, Onyatta JO, Ndangili PM, et al. Ultrasensitive immunosensor for multiplex detection of cancer biomarkers carcinoembryonic antigen (CEA) and yamaguchi sarcoma viral oncogene homolog 1 (YES1) based on eco-friendly synthesized gold nanoparticles. Talanta. 2024;266:124934. [DOI] [PubMed] [Google Scholar]
- [18].Hasbahceci M, Malya FU, Kunduz E, et al. Use of serum and peritoneal CEA and CA19-9 in prediction of peritoneal dissemination and survival of gastric adenocarcinoma patients: are they prognostic factors? Ann R Coll Surg Engl. 2018;100:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Uda H, Kanda M, Tanaka C, et al. Perioperative serum carcinoembryonic antigen levels predict recurrence and survival of patients with pathological T2-4 gastric cancer treated with curative gastrectomy. Dig Surg. 2018;35:55–63. [DOI] [PubMed] [Google Scholar]
- [20].Deng K, Yang L, Hu B, et al. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shimada H, Noie T, Ohashi M, et al. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33. [DOI] [PubMed] [Google Scholar]
- [22].Lai IR, Lee WJ, Huang MT, et al. Comparison of serum CA72-4, CEA, TPA, CA19-9 and CA125 levels in gastric cancer patients and correlation with recurrence. Hepatogastroenterology. 2002;49:1157–60. [PubMed] [Google Scholar]
- [23].Choi SR, Jang JS, Lee JH, et al. Role of serum tumor markers in monitoring for recurrence of gastric cancer following radical gastrectomy. Dig Dis Sci. 2006;51:2081–6. [DOI] [PubMed] [Google Scholar]
- [24].Hirahara N, Matsubara T, Kaji S, et al. Novel inflammation-combined prognostic index to predict survival outcomes in patients with gastric cancer. Oncotarget. 2023;14:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Di T, Lai YR, Luo QY, et al. A novel nomogram integrated with PDL1 and CEA to predict the prognosis of patients with gastric cancer. Clin Transl Oncol. 2023;25:2472–86. [DOI] [PubMed] [Google Scholar]
- [26].Sheinenzon A, Shehadeh M, Michelis R, et al. Serum albumin levels and inflammation. Int J Biol Macromol. 2021;184:857–62. [DOI] [PubMed] [Google Scholar]
- [27].Peters SJ, Vanhaecke T, Papeleu P, et al. Co-culture of primary rat hepatocytes with rat liver epithelial cells enhances interleukin-6-induced acute-phase protein response. Cell Tissue Res. 2010;340:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Milan Manani S, Virzì GM, Clementi A, et al. Pro-inflammatory cytokines: a possible relationship with dialytic adequacy and serum albumin in peritoneal dialysis patients. Clin Kidney J. 2016;9:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang YJ, Fletcher R, Yu J, et al. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018;5:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang Y, Ma C, Wang M, et al. Prognostic significance of immune cells in the tumor microenvironment and peripheral blood of gallbladder carcinoma patients. Clin Transl Oncol. 2017;19:477–88. [DOI] [PubMed] [Google Scholar]
- [31].Oh SY, Heo J, Noh OK, et al. Absolute lymphocyte count in preoperative chemoradiotherapy for rectal cancer: changes over time and prognostic significance. Technol Cancer Res Treat. 2018;17:1533033818780065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tustumi F, Takeda FR, Brandao A, et al. Lymphocyte count and platelet volume predicts postoperative complications in esophagectomy for cancer: a cohort study. Arq Gastroenterol. 2019;56:377–85. [DOI] [PubMed] [Google Scholar]
- [33].Garrido MM, Marta JC, Ribeiro RM, et al. Serum lipids and prostate cancer. J Clin Lab Anal. 2021;35:e23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goupille C, Ouldamer L, Pinault M, et al. Identification of a positive association between mammary adipose cholesterol content and indicators of breast cancer aggressiveness in a French population. J Nutr. 2021;151:1119–27. [DOI] [PubMed] [Google Scholar]
- [35].Shin HJ, Roh CK, Son SY, et al. Prognostic value of hypocholesterolemia in patients with gastric cancer. Asian J Surg. 2021;44:72–9. [DOI] [PubMed] [Google Scholar]
- [36].Braumuller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. [DOI] [PubMed] [Google Scholar]
- [37].Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. [DOI] [PubMed] [Google Scholar]
- [38].Blaser H, Dostert C, Mak TW, et al. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–61. [DOI] [PubMed] [Google Scholar]
- [39].Nakamura I, Shibata M, Gonda K, et al. Serum levels of vascular endothelial growth factor are increased and correlate with malnutrition, immunosuppression involving MDSCs and systemic inflammation in patients with cancer of the digestive system. Oncol Lett. 2013;5:1682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yarizadeh K, Behbahani M, Mohabatkar H, et al. Computational analysis and optimization of carcinoembryonic antigen aptamers and experimental evaluation. J Biotechnol. 2019;306:1–8. [DOI] [PubMed] [Google Scholar]
- [41].Ferreira IG, Carrascal M, Mineiro AG, et al. Carcinoembryonic antigen is a sialyl Lewis x/a carrier and an Eselectin ligand in nonsmall cell lung cancer. Int J Oncol. 2019;55:1033–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Heidemann F, Schildt A, Schmid K, et al. Selectins mediate small cell lung cancer systemic metastasis. PLoS One. 2014;9:e92327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Han ES, Lee HH, Lee JS, et al. At which stage of gastric cancer progression do levels of carcinoembryonic antigen and carbohydrate antigen 19-9 increase? application in advanced gastric cancer treatment. J Gastric Cancer. 2014;14:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu X, Cai H, Wang Y. Prognostic significance of tumour markers in Chinese patients with gastric cancer. ANZ J Surg. 2014;84:448–53. [DOI] [PubMed] [Google Scholar]
- [45].Park SH, Kim DY, Heo JS, et al. Postoperative chemoradiotherapy for gastric cancer. Ann Oncol. 2003;14:1373–7. [DOI] [PubMed] [Google Scholar]
- [46].Takahashi Y, Takeuchi T, Sakamoto J, et al.; Tumor Marker Committee. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003;6:142–5. [DOI] [PubMed] [Google Scholar]
- [47].Lee JC, Lee SY, Kim CY, et al. Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer. J Korean Surg Soc. 2013;85:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xiao J, Ye ZS, Wei SH, et al. Prognostic significance of pretreatment serum carcinoembryonic antigen levels in gastric cancer with pathological lymph node-negative: a large sample single-center retrospective study. World J Gastroenterol. 2017;23:8562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim DY, Kim HR, Shim JH, et al. Significance of serum and tissue carcinoembryonic antigen for the prognosis of gastric carcinoma patients. J Surg Oncol. 2000;74:185–92. [DOI] [PubMed] [Google Scholar]