Abstract
PURPOSE
In CheckMate 649, first-line nivolumab plus chemotherapy prolonged overall survival versus chemotherapy in patients with advanced/metastatic non–human epidermal growth factor receptor 2 (HER2)-positive gastric/gastroesophageal junction cancer (GC/GEJC) or esophageal adenocarcinoma (EAC). We present exploratory patient-reported outcomes (PROs).
METHODS
In patients (N = 1,581) concurrently randomly assigned 1:1 to nivolumab plus chemotherapy or chemotherapy and in those with tumor PD-L1 expression at a combined positive score (CPS) of ≥5, health-related quality of life (HRQoL) was assessed using the EQ-5D and Functional Assessment of Cancer Therapy-Gastric (FACT-Ga), which included the FACT-General (FACT-G) and Gastric Cancer subscale (GaCS). The FACT-G GP5 item assessed treatment-related symptom burden. Longitudinal changes in HRQoL were assessed using mixed models for repeated measures in the PRO analysis population (randomly assigned patients with baseline and ≥1 postbaseline assessments). Time to symptom or definitive deterioration analyses were also conducted.
RESULTS
In the PRO analysis population (n = 1,360), PRO questionnaire completion rates were mostly >80% during treatment. Patient-reported symptom burden was not increased with nivolumab plus chemotherapy versus chemotherapy. Mean improved changes from baseline were greater with nivolumab plus chemotherapy versus chemotherapy for FACT-Ga total, GaCS, and EQ-5D visual analog scale in patients with a CPS of ≥5; results were similar for the overall PRO analysis population. In CPS ≥5 and all randomly assigned populations, nivolumab plus chemotherapy reduced the risk of symptom deterioration versus chemotherapy, on the basis of FACT-Ga total score and GaCS; time to definitive deterioration was longer, and the risk of definitive deterioration in HRQoL was reduced with nivolumab plus chemotherapy across EQ-5D and most FACT-Ga measures (hazard ratio [95% CI] <1).
CONCLUSION
Compared with chemotherapy alone, first-line nivolumab plus chemotherapy showed stable or better on-treatment HRQoL in patients with advanced/metastatic non–HER2-positive GC/GEJC/EAC and also showed decreased risk of definitive HRQoL deterioration.
1L NIVO + chemo maintains HRQoL with lower deterioration risk in gastric/esophageal adenocarcinoma
INTRODUCTION
Gastric/gastroesophageal junction cancer (GC/GEJC) and esophageal adenocarcinoma (EAC) are among the leading causes of global cancer-related mortality.1 Strong similarities in molecular profiles between GC/GEJC and EAC suggest that these cancers could be considered a single disease entity.2,3 Chemotherapy, the standard first-line treatment for patients with unresectable advanced or metastatic human epidermal growth factor receptor 2 (HER2)–negative GC/GEJC, has been associated with poor prognosis (median overall survival [OS] is <1 year) and patient-reported symptom burden, with some regimens resulting in significant treatment-related toxicity.4-7 Similar clinical outcomes with systemic chemotherapy have been reported for EAC.8-10 First-line targeted therapies for HER2-negative GC/GEJC have not shown significant improvements in efficacy or safety compared with chemotherapy.11-14 Considering the limited life expectancy of patients with advanced disease, it is important to assess the health-related quality of life (HRQoL) to weigh the benefits and risks of treatment from the patient's perspective.15 This is especially relevant for advanced gastroesophageal cancers16 as deterioration of HRQoL correlated with deterioration in Eastern Cooperative Oncology Group (ECOG) performance status (PS) and with disease progression in the second-line setting as shown by the RAINBOW/REGARDS studies.17 In the first-line setting, chemotherapy showed stable or improved HRQoL versus best supportive care in patients with advanced esophagogastric cancer.18 Recently, immunotherapy with or without chemotherapy has shown significant survival benefit versus chemotherapy alone in multiple tumor types, with no detrimental effects on HRQoL.19,20 In patients with PD-L1–positive advanced or metastatic GC/GEJC, first-line pembrolizumab, a PD-1 inhibitor, was noninferior to chemotherapy for OS and HRQoL was similar between treatment arms.21,22
CONTEXT
Key Objective
Gastric/gastroesophageal junction cancer (GC/GEJC) or esophageal adenocarcinoma (EAC) is associated with poor prognosis and high mortality. The phase III CheckMate 649 study showed clinical benefit with first-line nivolumab plus chemotherapy versus chemotherapy alone in patients with advanced GC/GEJC or EAC. This exploratory analysis evaluated the health-related quality of life (HRQoL) in patients treated with nivolumab plus chemotherapy versus chemotherapy alone using patient-reported outcomes (PROs).
Knowledge Generated
Patients treated with nivolumab plus chemotherapy versus chemotherapy alone maintained their HRQoL with a reduced risk of definitive deterioration in disease-related and overall health status and without increased treatment-related symptom burden. PRO results complement the previously reported clinically meaningful efficacy benefit with nivolumab plus chemotherapy and support its use as a first-line treatment for advanced or metastatic GE/GEJC or EAC.
Relevance (A.H. Ko)
-
These PRO data can be helpful when counseling patients with advanced or metastatic GE/GEJC or EAC, providing reassurance that the benefits of adding nivolumab to chemotherapy extend not only to improved survival, but also to preservation of their quality of life and prolonged symptom control.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
Nivolumab, a fully human anti–PD-1 antibody that restores antitumor T-cell function,23,24 has shown significant survival benefit and acceptable safety, while maintaining or improving HRQoL versus standard of care in the first- or second-line setting for multiple tumor types.25-31
CheckMate 649 (ClinicalTrials.gov identifier: NCT02872116), a phase III, randomized open-label study, evaluated first-line nivolumab-based therapies in patients with advanced or metastatic GC/GEJC or EAC.32 The study demonstrated superior OS along with a progression-free survival benefit and an acceptable safety profile with nivolumab plus chemotherapy versus chemotherapy in patients whose tumors expressed PD-L1 at a combined positive score (CPS) of ≥5 (primary population) or a CPS of ≥1 and in all randomly assigned patients.32,33 On the basis of these results, first-line nivolumab in combination with fluoropyrimidine- and platinum-based chemotherapies is approved in multiple countries for patients with advanced or metastatic GC/GEJC or EAC regardless of tumor PD-L1 expression34,35 and in the European Union for those with a CPS of ≥5.36 Initial analyses suggested that HRQoL was maintained with nivolumab plus chemotherapy.32,33 Here, we present detailed results of the exploratory HRQoL analyses from CheckMate 649, using data from the July-2021 database lock.
METHODS
Study Design and Treatment
The study design for CheckMate 649 was described previously.32 Adult patients with previously untreated advanced or metastatic non–HER2-positive (defined as HER2-negative or unknown HER2 status) GC/GEJC or EAC were initially randomly assigned 1:1:1 to receive nivolumab (360 mg once every 3 weeks or 240 mg once every 2 weeks) plus investigator's choice of chemotherapy (capecitabine and oxaliplatin [XELOX] or fluorouracil, leucovorin, and oxaliplatin [FOLFOX]), nivolumab plus ipilimumab, or chemotherapy alone after the nivolumab plus chemotherapy arm was added; 1:1 random assignment to nivolumab plus chemotherapy or chemotherapy continued after enrollment in the nivolumab plus ipilimumab arm was subsequently closed.32 Results from the nivolumab plus ipilimumab arm were not included in this analysis. Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, or ≤2 years for nivolumab.
This study was conducted according to Good Clinical Practice guidelines developed by the International Council for Harmonisation and the Declaration of Helsinki principles. The study Protocol (online only) was approved by an institutional review board or independent ethics committee at each center. All patients provided written informed consent.
Patient-Reported Outcome Assessments and End Points
Patient-reported outcomes (PROs) were prespecified exploratory end points and collected using the three-level EQ-5D, which assessed the impact of treatment on the general health status of patients, and the Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) questionnaire, which assessed the impact of treatment on cancer-related quality of life. The EQ-5D comprised a utility index (EQ-5D UI) on the basis of the UK value set and a EQ-5D visual analog scale (EQ VAS).37,38 The FACT-Ga included the 27-item FACT-General (FACT-G) questionnaire (consisting of the physical well-being, social well-being [SWB], emotional well-being, and functional well-being subscales) and selected components, including a 19-item Gastric Cancer Subscale (GaCS).39-41 Prespecified meaningful change thresholds (MCTs) were defined for each PRO measure (Data Supplement, Table S1 [online only]) on the basis of published ranges for minimally important differences for the respective scales.38,39,41,42 Higher scores indicated better HRQoL for all PRO measures. The single FACT-G item GP5 (“I am bothered by side effects of treatment”) was evaluated separately to assess the patient's experience with side effects. Scores indicating treatment bother ranged from 0 (not at all) to 4 (very much).43
All PRO questionnaires were completed by patients before dosing at baseline and every 6 weeks (±3 days) thereafter before treatment, regardless of the treatment schedule. To decrease patient burden, a reduced set of questionnaires were completed by patients during the follow-up period. Only the GaCS or the abbreviated 7-item version of FACT-G, FACT-G7,44 was administered along with the EQ-5D during follow-up visits 1 (30 days [±7] after last dose) and 2 (84 days [±7] after follow-up visit 1) and every 3 months thereafter at survival follow-up visits.
Statistical Analysis
The statistical analysis for the PRO end points was descriptive and did not include hypothesis testing.
PRO questionnaire completion rates corresponded to the proportion of questionnaires received out of the expected number (ie, the number of patients still on treatment or follow-up at any given timepoint). Responses to the FACT-G GP5 item were analyzed descriptively by treatment arm at each assessment.
Mean changes from baseline in PRO scale scores were estimated from a mixed model for repeated measures (MMRM) using restricted maximum likelihood with within-patient correlations modeled by an R-side unstructured covariance structure in patients with a CPS of ≥5 and all randomly assigned patients with an evaluable PRO assessment at baseline (day 1, assessment before administration of treatment on the day of first dose) and ≥1 evaluable postbaseline PRO assessment (CPS ≥5 and overall PRO analysis populations, respectively). The change in score from baseline at each postbaseline assessment was modeled as a linear function of treatment arm, study assessment, baseline score, trial stratification factors (tumor PD-L1 expression level [≥1%, <1%, or indeterminate], geographical region [United States, Asia, or rest of the world], ECOG PS [0 or 1], planned chemotherapy regimen [XELOX or FOLFOX]), interaction terms between treatment the arm and study assessment, interaction terms between baseline score and study assessment, and any potential confounders. For the GaCS, further analyses of missing data patterns were performed to investigate missing at random assumptions. Assessments with ≥10 patients per treatment arm were included for most PRO scales/subscales; for the EQ-5D UI and FACT-G7, assessments with ≥20 patients per treatment arm in the overall PRO analysis population were included to achieve model convergence. Data from both on-treatment and follow-up PRO assessments were included if sample size requirements were met. Model-adjusted least-squares (LS) mean change in score from baseline, associated 95% CIs, and descriptive P values were reported at each postbaseline assessment.
Time to deterioration analyses were performed in the CPS ≥5 and all randomly assigned populations and included all PRO assessments conducted before or on the date of treatment discontinuation. Time to symptom deterioration (TTSD) was defined as the time from random assignment until the first decline in PRO score from baseline, which met or exceeded the MCT. Time to definitive deterioration (TTDD) was defined as the time from random assignment until the first decline in PRO score from baseline, which met or exceeded the MCT, when all subsequent assessments also met or exceeded the MCT. TTSD and TTDD were compared between treatment arms using stratified log-rank tests and plotted using the Kaplan-Meier (KM) product-limit method; descriptive P values were reported. The hazard ratio (HR) and associated two-sided 95% CIs were calculated using a stratified Cox model with the treatment group as the only covariate.
Missing PRO assessment data were not imputed. All analyses were conducted using SAS version 9.4 or higher (SAS Institute; Cary, NC).
Additional details on treatment, PRO assessments, and statistical analyses are included in the Data Supplement.
RESULTS
Patients
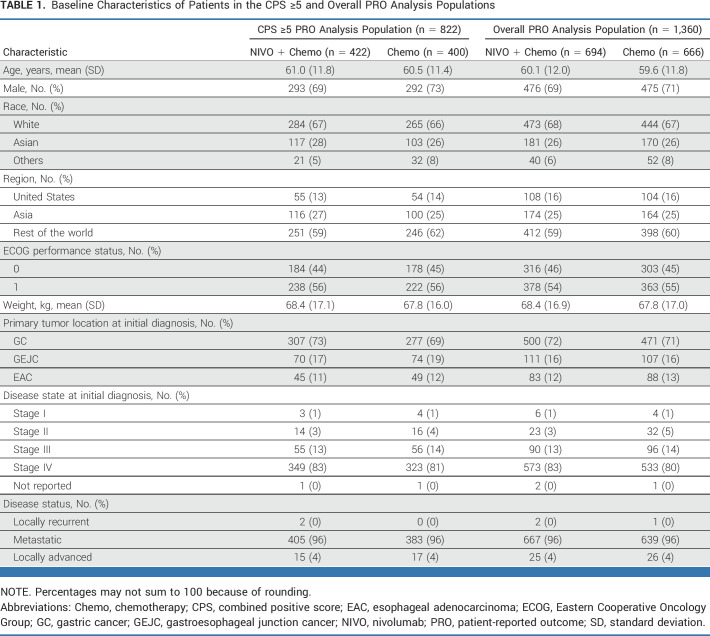
Among all randomly assigned patients (N = 1,581), 60% (nivolumab plus chemotherapy; n = 473 of 789) and 61% (chemotherapy; n = 482 of 792) had a PD-L1 CPS of ≥5; the proportion of patients with a CPS of ≥5 was similar in the overall PRO analysis population (n = 1,360; 61% [422 of 694] and 60% [400 of 666], respectively; Data Supplement, Fig S1). Baseline characteristics were generally balanced between treatment arms in the CPS ≥5 PRO analysis population, between the CPS ≥5 and overall PRO analysis populations, and between the overall PRO analysis and all randomly assigned populations (Table 1).32 The mean patient age in the CPS ≥5 PRO analysis population was 61.0 (nivolumab plus chemotherapy) and 60.5 (chemotherapy) years. Most patients were male (71%) and White (67%), had an ECOG PS of 1 (56%), and had GC as the primary tumor location at initial diagnosis (71%).
TABLE 1.
Baseline Characteristics of Patients in the CPS ≥5 and Overall PRO Analysis Populations
PRO Questionnaire Completion Rates
In the CPS ≥5 and overall PRO analysis populations, >95% of patients had an evaluable baseline assessment. In the CPS ≥5 and overall PRO analysis populations, EQ-5D and FACT-Ga questionnaire completion rates were >80% on most on-treatment assessments with ≥10 patients (until week 133) in both treatment arms; EQ-5D completion rates during follow-up were slightly lower (Data Supplement, Table S2).
Descriptive Analyses of Treatment-Related Symptom Burden
In the CPS ≥5 and overall PRO analysis populations, most patients in both treatment arms reported that they were not at all or a little bothered by treatment-related side effects, as assessed by the FACT-G GP5 item. The proportion of patients reporting not at all bothered by treatment-related side effects increased over time in the nivolumab plus chemotherapy arm and was higher than that in the chemotherapy arm at most postbaseline timepoints (Data Supplement, Fig S2). In the CPS ≥5 PRO analysis population, the proportion of patients reporting not at all or a little bothered by treatment side effects at postbaseline assessments ranged from 63% (not at all, n = 91 of 336; a little, n = 120 of 336) at week 13 to 94% (n = 12 of 16; n = 3 of 16) at week 115 for nivolumab plus chemotherapy and 62% (n = 92 of 347; n = 124 of 347) at week 7 to 84% (n = 11 of 25; n = 10 of 25) at week 79 for chemotherapy; in the overall PRO analysis population, the proportions ranged from 62% (n = 137 of 538; n = 196 of 538) at week 13 to 100% (n = 11 of 11; n = 0 of 11) at week 133 and 60% (n = 60 of 282; n = 109 of 282) at week 25 to 86% (n = 18 of 44; n = 20 of 44) at week 79, respectively.
Analyses of Changes in PROs Over Time
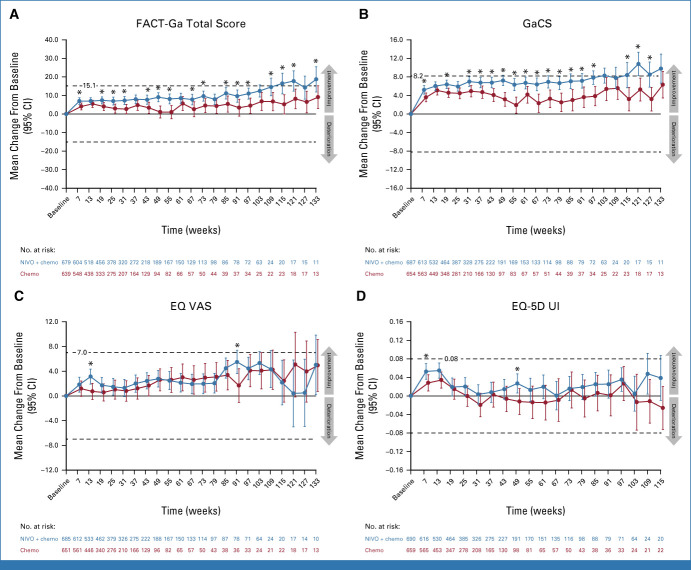
In the CPS ≥5 PRO analysis population, FACT-Ga total, GaCS, and EQ VAS scores generally improved from baseline at most on-treatment assessments for both treatment arms. LS mean changes from baseline favored nivolumab plus chemotherapy over chemotherapy for all three measures (Figs 1A-1C). EQ-5D UI scores showed a trend toward improvement from baseline over time with nivolumab plus chemotherapy but showed minimal changes from baseline with chemotherapy (Fig 1D). Similar results were observed for the overall PRO analysis population (Figs 2A-2D). LS mean changes in FACT-G total, including the abbreviated FACT-G7, from baseline were generally similar across both treatment arms in both the CPS ≥5 and overall PRO analysis populations (Data Supplement, Figs S3A-S3D).
FIG 1.
Least-squares mean change (95% CI) from baseline in (A) FACT-Ga total score, (B) GaCS, (C) EQ VAS, and (D) EQ-5D UI scores in the CPS ≥5 PRO analysis population. Dashed lines indicate MCTs. Circles indicate point estimates, and vertical bars indicate 95% CIs. *P < .05 was not formally tested. Only timepoints with ≥10 patients per treatment arm were included. Chemo, chemotherapy; CPS, combined positive score; EQ-5D UI, EQ-5D utility index; EQ VAS, EQ-5D visual analog scale; FACT-Ga, Functional Assessment of Cancer Therapy-Gastric; GaCS, Gastric Cancer Subscale; MCT, meaningful change threshold; NIVO, nivolumab; PRO, patient-reported outcome.
FIG 2.
Least-squares mean change (95% CI) from baseline in (A) FACT-Ga total score, (B) GaCS, (C) EQ VAS, and (D) EQ-5D UI scores in the overall PRO analysis population. Dashed lines indicate MCTs. Circles indicate point estimates, and vertical bars indicate 95% CIs. *P < .05 was not formally tested. Only timepoints with ≥10 patients per treatment arm were included for FACT-Ga total score, GaCS, and EQ VAS; timepoints with ≥20 patients per treatment arm were included for EQ-5D UI. Chemo, chemotherapy; CPS, combined positive score; EQ-5D UI, EQ-5D utility index; EQ VAS, EQ-5D visual analog scale; FACT-Ga, Functional Assessment of Cancer Therapy-Gastric; GaCS, Gastric Cancer Subscale; MCT, meaningful change threshold; NIVO, nivolumab; PRO, patient-reported outcomes.
Time to Deterioration Analyses
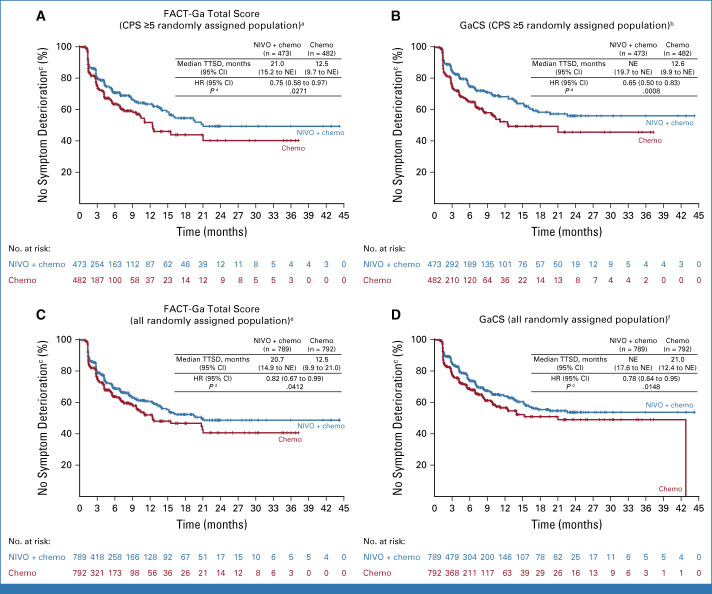
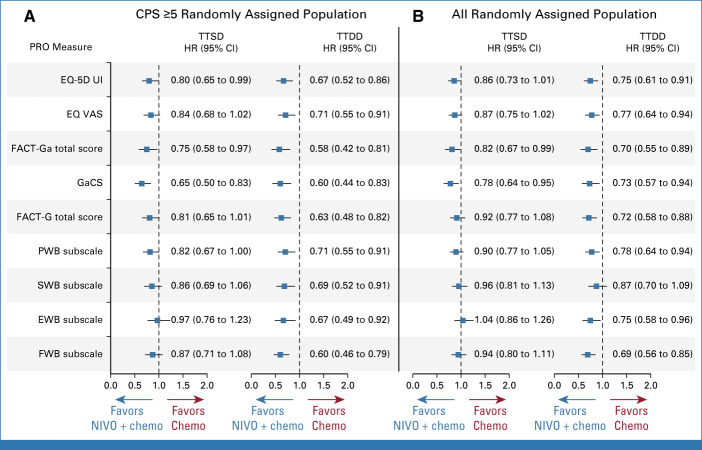
In the CPS ≥5 randomly assigned population, nivolumab plus chemotherapy delayed TTSD and reduced the risk of symptom deterioration versus chemotherapy during treatment, on the basis of the FACT-Ga total score (HR, 0.75 [95% CI, 0.58 to 0.97]), GaCS (HR, 0.65 [95% CI, 0.50 to 0.83]), and EQ-5D UI (HR, 0.80 [95% CI, 0.65 to 0.99]; Figs 3A, 3B and 4A; Data Supplement, Fig S4). TTSD also favored nivolumab plus chemotherapy over chemotherapy on the basis of EQ VAS, FACT-G total, and the four FACT-G subscales (HR <1 for all measures); however, these results were not statistically significant (Fig 4A; Data Supplement, Figs S4B and S4C). Results were generally consistent between the CPS ≥5 and all randomly assigned populations (Figs 3 and 4; Data Supplement, Fig S4).
FIG 3.
TTSD on the basis of FACT-Ga total score and GaCS in the (A and B) CPS ≥5 and (C and D) all randomly assigned populations. aOne hundred twenty-five (26.4%) patients in the NIVO + chemo arm and 122 (25.3%) patients in the chemo arm showed symptom deterioration; b116 (24.5%) patients in the NIVO + chemo arm and 127 (26.3%) patients in the chemo arm showed symptom deterioration; cMCTs were 15.1 points for FACT-Ga total score and 8.2 points for GaCS; dP < .05 was not formally tested; e213 (27.0%) patients in the NIVO + chemo arm and 200 (25.2%) patients in the chemo arm showed symptom deterioration; f199 (25.2%) patients in the NIVO + chemo arm and 195 (24.6%) patients in the chemo arm showed symptom deterioration. Chemo, chemotherapy; CPS, combined positive score; FACT-Ga, Functional Assessment of Cancer Therapy-Gastric; GaCS, Gastric Cancer Subscale; HR, hazard ratio; MCT, meaningful change threshold; NE, nonestimable; NIVO, nivolumab; PRO, patient-reported outcomes; TTSD, time to symptom deterioration.
FIG 4.
TTSD and TTDD in the (A) CPS ≥5 and (B) all randomly assigned populations for all PRO measures. HR reports NIVO + chemo versus chemo. Chemo, chemotherapy; CPS, combined positive score; EQ-5D UI, EQ-5D utility index; EQ VAS, EQ-5D visual analog score; EWB, emotional well-being subscale; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-Ga, Functional Assessment of Cancer Therapy-Gastric; FWB, functional well-being subscale; GaCS, Gastric Cancer Subscale; HR, hazard ratio; NIVO, nivolumab; PRO, patient-reported outcome; PWB, physical well-being subscale; SWB, social well-being subscale; TTSD, time to symptom deterioration; TTDD, time to definitive deterioration.
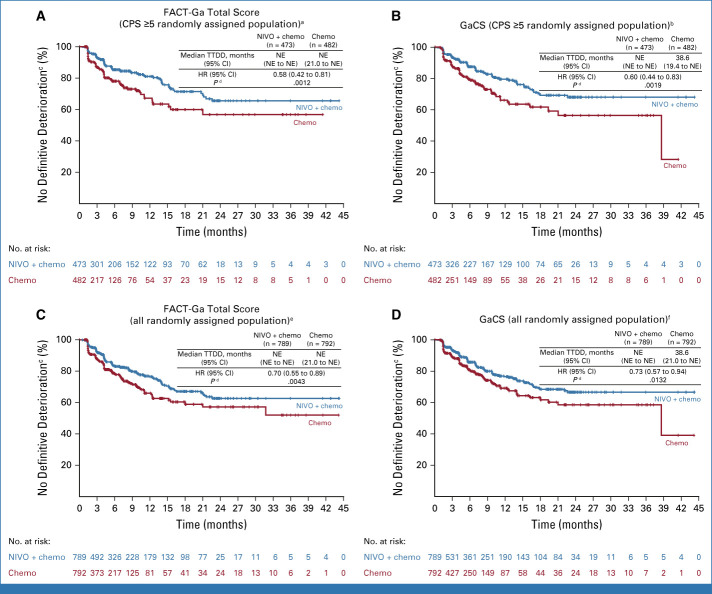
Nivolumab plus chemotherapy showed a statistically significant and clinically meaningful delay in TTDD and reduced the risk of definitive deterioration in HRQoL versus chemotherapy during treatment in the CPS ≥5 randomly assigned population across all PRO measures (HR [95% CI] <1; Figs 4A, 5A and 5B; Data Supplement, Figs S5A-S5C). Similar results were observed in all randomly assigned patients for all components of the EQ-5D and FACT-Ga measures except for the SWB subscale (Figs 4B, 5C and 5D; Data Supplement, Figs S5D-S5F).
FIG 5.
Time to definitive deterioration on the basis of FACT-Ga total score and GaCS in the (A and B) CPS ≥5 and (C and D) all randomly assigned populations. aSeventy-two (15.2%) patients in the NIVO + chemo arm and 79 (16.4%) patients in the chemo arm showed definitive deterioration; b75 (15.9%) patients in the NIVO + chemo arm and 85 (17.6%) patients in the chemo arm showed definitive deterioration; cMCTs were 15.1 points for FACT-Ga total score and 8.2 points for GaCS; dP <.05 was not formally tested; e131 (16.6%) patients in the NIVO + chemo arm and 132 (16.7%) patients in the chemo arm showed definitive deterioration; f129 (16.3%) patients in the NIVO + chemo arm and 131 (16.5%) patients in the chemo arm showed definitive deterioration. Chemo, chemotherapy; CPS, combined positive score; FACT-Ga, Functional Assessment of Cancer Therapy-Gastric; GaCS, Gastric Cancer Subscale; HR, hazard ratio; MCT, meaningful change threshold; NE, nonestimable; NIVO, nivolumab; TTDD, time to definitive deterioration.
DISCUSSION
In CheckMate 649, nivolumab plus chemotherapy demonstrated superior OS versus chemotherapy in first-line advanced or metastatic non–HER2-positive GC/GEJC or EAC.32 Here, we report that relative to patients treated with chemotherapy alone, patients treated with nivolumab plus chemotherapy also experienced clinically meaningful HRQoL benefits, as assessed by both gastric cancer–specific and overall health status PRO instruments using prespecified MCTs.38,39,41,42 These findings are consistent with initial results from the study.32,33 Previous studies on chemotherapy-based combination regimens, mainly in elderly patients, have shown early declines in HRQoL during the initial follow-up period in the combination therapy arm versus the control arm.16,45,46 However, the addition of immunotherapy to chemotherapy has shown stable or improved HRQoL versus chemotherapy alone in the first-line setting in other advanced solid tumor types.20,47-49 In this study, it is notable that the addition of nivolumab to chemotherapy did not negatively affect HRQoL or increase treatment-related symptom burden, despite a difference in safety profiles in the nivolumab plus chemotherapy versus chemotherapy arms (38% v 25% of patients discontinued treatment because of treatment-related adverse events).33
Longitudinal MMRM analyses showed a trend toward improved HRQoL with nivolumab plus chemotherapy versus chemotherapy although conclusions may be limited by smaller sample sizes in the chemotherapy arm at later time points. These results complement the clinical findings of the CheckMate 649 2-year update,33 in which nivolumab plus chemotherapy continued to improve OS versus chemotherapy in both the CPS ≥5 (HR, 0.70; 95% CI, 0.61 to 0.81) and all randomly assigned populations (HR, 0.79; 95% CI, 0.71 to 0.88). The longer median durations of response (CPS ≥5: 9.7 v 7.0 months; all randomly assigned: 8.5 v 6.9 months) and higher proportions of patients with ongoing response (CPS ≥5: 13% v 6%; all randomly assigned: 11% v 4%) in the nivolumab plus chemotherapy versus chemotherapy arms may also correlate with the improvement in disease-related health status over time observed in patients treated with nivolumab plus chemotherapy.33 Further analyses are needed to explore the correlation between PROs and clinical outcomes in this study.
Time to deterioration analyses also demonstrated favorable HRQoL outcomes with nivolumab plus chemotherapy over chemotherapy in both the CPS ≥5 and all randomly assigned populations. Patients treated with nivolumab plus chemotherapy showed reduced risk of disease-related symptom deterioration versus chemotherapy during treatment, as assessed by the FACT-Ga total score and GaCS. The reduced risk of symptom deterioration with nivolumab plus chemotherapy appeared to be more pronounced in patients with a CPS of ≥5 versus all randomly assigned patients, given the earlier separation of the KM curves for FACT-Ga total score in the CPS ≥5 population. Notably, in both populations, nivolumab plus chemotherapy prolonged TTDD and reduced the risk of definitive deterioration in HRQoL versus chemotherapy during treatment across most components of the EQ-5D and FACT-Ga. The delay in definitive deterioration of overall health status with nivolumab plus chemotherapy versus chemotherapy, as assessed by the EQ-5D UI, was greater in the CPS ≥5 versus all randomly assigned populations (10.7 v 7.6 months), which may correlate with the enriched survival benefit in patients with a CPS of ≥5 in CheckMate 649 analyses.32,33 However, it should be noted that sample sizes in the chemotherapy arm were smaller than those in the nivolumab plus chemotherapy arm at later timepoints in both the CPS ≥5 and all randomly assigned populations, reflecting a possible source of bias in the TTDD analyses.
The PRO results in this study further support the use of nivolumab plus chemotherapy as standard first-line treatment for advanced or metastatic non–HER2-positive GC/GEJC or EAC. Furthermore, a recent analysis showed that patients treated with nivolumab plus chemotherapy in CheckMate 649 experienced significantly longer quality-adjusted survival time spent without symptoms of disease progression or toxicity versus those treated with chemotherapy alone.50 Although cross-trial comparisons should be made with caution owing to differences in study design and PRO instruments, the PRO findings reported here are consistent with previous PRO studies that showed clinical benefit with first-line nivolumab and other PD-1 inhibitors with/without chemotherapy, with stable or improved HRQoL in patients, across several solid cancers, including advanced GC/GEJC.22,51-54 As patients with advanced or metastatic GC/GEJC or EAC are generally elderly and have other prognostic factors that limit the long-term use of chemotherapy, immunotherapy-based regimens with a favorable benefit-risk profile offer first-line treatment options without the burden of added toxicity.7,55,56 In addition, to extend the antitumor benefit beyond first-line treatment, maintenance immunotherapy is being increasingly considered for several tumor types, including GC/GEJC, to ensure optimal continuum of care for patients.57,58 Further research into long-term maintenance immunotherapy that helps maintain or improve HRQoL in patients with advanced or metastatic GC/GEJC or EAC is warranted.
Strengths of this study include the use of both general health-related and disease-specific PRO instruments, high PRO questionnaire completion rates at baseline and during treatment, and same timing of PRO evaluation between treatment groups. This study was limited by an open-label trial design, which might have potentially influenced patient responses to questionnaires.59 However, recent reports comparing PROs between study arms across multiple cancer trials found no differences in patient-reported symptoms despite significant disparities in treatment-related toxic effects.59,60 Other data also showed that patients were not reluctant to report symptomatic adverse event outcomes, and bias may be less pronounced for symptoms that are proximal outcomes to the physiology of the disease and treatment than other more distal outcomes.61 This study also used prespecified thresholds for meaningful change (Data Supplement, Table S1), which, in some cases, may be higher than what might be regarded as minimally important. Lower estimates of 4-7 points for the FACT-G total score, 3-5 points for the GaCS, and 7-10 points for FACT-Ga could be supported on the basis of a previous report62 and literature in other primary cancer types.63,64 Finally, sensitivity analyses were not performed in this study as data are not available at this time. Nonetheless, results from this PRO analysis indicated that nivolumab plus chemotherapy delayed definitive deterioration of HRQoL versus chemotherapy alone and, notably, demonstrated that the addition of immunotherapy to chemotherapy did not worsen HRQoL in patients with advanced or metastatic GC/GEJC or EAC.
In conclusion, nivolumab plus chemotherapy showed stable or better on-treatment HRQoL as it did not increase treatment-related symptom burden and decreased the risk of definitive HRQoL deterioration during treatment versus chemotherapy alone. These PRO results, in combination with the previously demonstrated clinically meaningful efficacy benefit and manageable safety profile,32,33 further support the use of nivolumab plus chemotherapy as a tolerable and efficacious first-line treatment for patients with advanced or metastatic non–HER2-positive GC/GEJC or EAC.
ACKNOWLEDGMENT
The authors thank the patients and their families for making the study possible, as well as the investigators and clinical study teams at Bristol Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Co Ltd (Osaka, Japan). Dako (an Agilent Technologies company, Santa Clara, CA) participated in the collaborative development of the PD-L1 IHC 28-8 pharmDx assay. Professional medical writing assistance was provided by Melissa Mehalick, PhD, of RTI Health Solutions at the time of study and Vidya Rajagopalan, PhD, of Evidence Scientific Solutions Inc, funded by Bristol Myers Squibb.
Markus Moehler
Honoraria: Amgen, Roche/Genentech, Merck Serono, MSD Oncology, Bristol Myers Squibb, AstraZeneca/MedImmune, Servier, Pierre Fabre, Sanofi
Consulting or Advisory Role: Bayer, MSD, Merck Serono, Amgen, Taiho Pharmaceutical, Pfizer, Roche, Lilly, Servier, BeiGene, BMS, AstraZeneca
Research Funding: Amgen (Inst), Leap Therapeutics (Inst), Merck Serono (Inst), AstraZeneca (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Amgen, Merck Serono, Roche, Bayer, ASCO, German Cancer Society, MSD, ESMO, BeiGene
Hong Xiao
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Steven I. Blum
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb, GlaxoSmithKline
Elena Elimova
Employment: Merck
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Zymeworks (Inst), Adaptimmune (Inst), BeiGene (Inst), Astellas Pharma, Viracta Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Zymeworks (Inst), AstraZeneca Canada (Inst), Bold Therapeutics (Inst)
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Ipsen, Celcuity, Immunogen, Fulcrum Therapeutics
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Clovis Oncology (Inst)
Kohei Shitara
Honoraria: Bristol Myers Squibb, Takeda, Janssen
Consulting or Advisory Role: Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Astellas Pharma, Guardant Health, Janssen
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst)
Jaffer A. Ajani
Honoraria: Lilly, Bristol Myers Squibb, Merck, Aduro Biotech, DAVA Pharmaceuticals, AstraZeneca, Acrotech Biopharma, Zymeworks, Astellas Pharma, Amgen, Oncotherics, Daiichi Sankyo, Novartis, Servier, Gilead Sciences, BeiGene, Fresenius Kabi, Boehringer Ingelheim, GRAIL
Consulting or Advisory Role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol Myers Squibb, Novartis, Astellas Pharma, Gilead Sciences, Amgen, Servier, Geneos, Arcus Biosciences
Research Funding: Novartis, Bristol Myers Squibb, Taiho Pharmaceutical, Roche/Genentech, Amgen, Lilly/ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda, ProLynx, Zymeworks, Daiichi Sankyo, Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I have research funding from: Genentech, Roche, BMS, Taiho, MedImmune, Merck, Amgen, Lilly
Yelena Y. Janjigian
Stock and Other Ownership Interests: Rgenix
Consulting or Advisory Role: Pfizer, Merck, Bristol Myers Squibb, Merck Serono, Daiichi Sankyo, Rgenix, Bayer, Imugene, AstraZeneca, Lilly, Zymeworks, Basilea Pharmaceutical, Michael J. Hennessy Associates, Paradigm, Seagen, AmerisourceBergen, Arcus Biosciences, Geneos, GlaxoSmithKline, Imedex, Lynx Health, Peerview, Silverback Therapeutics, Mersana, Research to Practice, AskGene Pharma, Phanes Therapeutics
Research Funding: Bayer (Inst), Rgenix (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Lilly (Inst), NCI (Inst), Department of Defense (Inst), Cycle for Survival (Inst), Fred's Team (Inst), Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb Japan
Other Relationship: Clinical Care Options, Axis Medical Education, Research to Practice
Marcelo Garrido
Consulting or Advisory Role: MSD, AstraZeneca, Roche
Speakers' Bureau: Bristol Myers Squibb, Bayer, Merck
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst)
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: Roche
Lin Shen
Consulting or Advisory Role: MSD, Bristol Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbor BioMed, Merck, Boehringer Ingelheim, Sanofi
Research Funding: Nanjing Yaojieankang Biotechnology (Inst), Baiji Shenzhou (Beijing) Biotechnology (Inst), Beijing Xiantong Biomedical Technology (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst), Jacobio (Inst), CANbridge Pharmaceuticals (Inst)
Kensei Yamaguchi
Consulting or Advisory Role: Bristol Myers Squibb Japan, Daiichi Sankyo
Speakers' Bureau: Chugai Pharma, Bristol Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Daiichi Sankyo, Merck
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Gilead Sciences (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Eisai (Inst), MSD Oncology (Inst), Sanofi (Inst), Bristol Myers Squibb (Inst)
Michael Schenker
Research Funding: Bristol Myers Squibb, Roche, Amgen, MSD, Pfizer/EMD Serono, Lilly, Astellas Pharma, AstraZeneca, GlaxoSmithKline, Regeneron, Novartis, AbbVie, Gilead Sciences, Sanofi/Regeneron, Mylan, Bioven, Clovis Oncology, Tesaro, BeiGene, Five Prime Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb
Ruben Kowalyszyn
Consulting or Advisory Role: BMS Argentina, MSD Oncology, Astellas Pharma, Merck Serono, Takeda
Speakers' Bureau: BMS Argentina, Novartis
Research Funding: BMS, MSD Oncology, Novartis, Roche, Astellas Pharma, Lilly, Gemabiotech, Nektar, Poliphor, AstraZeneca, Pfizer
Travel, Accommodations, Expenses: Gador, Pfizer/EMD Serono
Arinilda Campos Bragagnoli
Speakers' Bureau: Bristol Myers Squibb/Medarex, AstraZeneca, Merck
Ricardo Bruges
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Pfizer, Bristol Myers Squibb/Medarex, MSD Oncology, Pfizer
Travel, Accommodations, Expenses: Pfizer, Tecnofarma, MSD Oncology, Tecnofarma, Pfizer
Roberto Pazo-Cid
Consulting or Advisory Role: Roche, Bristol Myers Squibb/Celgene, Eisai Europe, Astellas Pharma, AstraZeneca Spain, Servier, Ipsen
Speakers' Bureau: BMS GmbH & Co KG, Servier, AstraZeneca Spain, Astellas Pharma
Travel, Accommodations, Expenses: Lilly, BMS GmbH & Co KG
Shannon Hunter
Employment: Daiichi Sankyo
Research Funding: BMS (Inst)
Eric Davenport
Research Funding: RTI Internation-Health Solutions
Jinyi Wang
Research Funding: Multiple pharmaceutical companies, identities confidential (Inst)
Kaoru Kondo
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Mingshun Li
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Lucjan Wyrwicz
Honoraria: BeiGene, BMS, MSD
Consulting or Advisory Role: GlaxoSmithKline, Servier
Speakers' Bureau: BMS
Travel, Accommodations, Expenses: Servier
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at ASCO Annual Meeting, virtual, June 4-8, 2021; the Korean Society for Medical Oncology Annual Meeting, virtual, September 2-3, 2021; the ASCO Quality Care Symposium, Boston, MA, September 24-25, 2021; and the Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie Annual Meeting, Berlin, Germany, October 1-2, 2021.
SUPPORT
Supported by Bristol Myers Squibb.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data are available upon reasonable request. BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
AUTHOR CONTRIBUTIONS
Conception and design: Markus Moehler, Hong Xiao, Steven I. Blum, David Cella, Kohei Shitara, Jaffer A. Ajani, Yelena Y. Janjigian, Lin Shen, Vincenzo Montesarchio, Roberto Pazo-Cid, Kaoru Kondo, Mingshun Li
Financial support: Kensei Yamaguchi
Administrative support: Lin Shen
Provision of study materials or patients: Markus Moehler, Yelena Y. Janjigian, Marcelo Garrido, Lin Shen, Tianshu Liu, Michael Schenker, Ruben Kowalyszyn, Arinilda Campos Bragagnoli, Ricardo Bruges, Vincenzo Montesarchio, Roberto Pazo-Cid, Lucjan Wyrwicz
Collection and assembly of data: Markus Moehler, Hong Xiao, Elena Elimova, Kohei Shitara, Yelena Y. Janjigian, Marcelo Garrido, Lin Shen, Kensei Yamaguchi, Tianshu Liu, Michael Schenker, Ruben Kowalyszyn, Arinilda Campos Bragagnoli, Ricardo Bruges, Vincenzo Montesarchio, Roberto Pazo-Cid, Mingshun Li, Lucjan Wyrwicz
Data analysis and interpretation: Markus Moehler, Hong Xiao, Steven I. Blum, Elena Elimova, David Cella, Kohei Shitara, Jaffer A. Ajani, Yelena Y. Janjigian, Marcelo Garrido, Lin Shen, Michael Schenker, Ruben Kowalyszyn, Arinilda Campos Bragagnoli, Ricardo Bruges, Vincenzo Montesarchio, Roberto Pazo-Cid, Shannon Hunter, Eric Davenport, Jinyi Wang, Kaoru Kondo, Mingshun Li, Lucjan Wyrwicz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Health-Related Quality of Life With Nivolumab Plus Chemotherapy Versus Chemotherapy in Patients With Advanced Gastric/Gastroesophageal Junction Cancer or Esophageal Adenocarcinoma From CheckMate 649
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Markus Moehler
Honoraria: Amgen, Roche/Genentech, Merck Serono, MSD Oncology, Bristol Myers Squibb, AstraZeneca/MedImmune, Servier, Pierre Fabre, Sanofi
Consulting or Advisory Role: Bayer, MSD, Merck Serono, Amgen, Taiho Pharmaceutical, Pfizer, Roche, Lilly, Servier, BeiGene, BMS, AstraZeneca
Research Funding: Amgen (Inst), Leap Therapeutics (Inst), Merck Serono (Inst), AstraZeneca (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Amgen, Merck Serono, Roche, Bayer, ASCO, German Cancer Society, MSD, ESMO, BeiGene
Hong Xiao
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Steven I. Blum
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb, GlaxoSmithKline
Elena Elimova
Employment: Merck
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Zymeworks (Inst), Adaptimmune (Inst), BeiGene (Inst), Astellas Pharma, Viracta Therapeutics
Research Funding: Bristol Myers Squibb (Inst), Zymeworks (Inst), AstraZeneca Canada (Inst), Bold Therapeutics (Inst)
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, Pfizer, Astellas Pharma, Novartis, Bristol Myers Squibb, Ipsen, Celcuity, Immunogen, Fulcrum Therapeutics
Research Funding: Novartis (Inst), Ipsen (Inst), Pfizer (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Clovis Oncology (Inst)
Kohei Shitara
Honoraria: Bristol Myers Squibb, Takeda, Janssen
Consulting or Advisory Role: Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Astellas Pharma, Guardant Health, Janssen
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst)
Jaffer A. Ajani
Honoraria: Lilly, Bristol Myers Squibb, Merck, Aduro Biotech, DAVA Pharmaceuticals, AstraZeneca, Acrotech Biopharma, Zymeworks, Astellas Pharma, Amgen, Oncotherics, Daiichi Sankyo, Novartis, Servier, Gilead Sciences, BeiGene, Fresenius Kabi, Boehringer Ingelheim, GRAIL
Consulting or Advisory Role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol Myers Squibb, Novartis, Astellas Pharma, Gilead Sciences, Amgen, Servier, Geneos, Arcus Biosciences
Research Funding: Novartis, Bristol Myers Squibb, Taiho Pharmaceutical, Roche/Genentech, Amgen, Lilly/ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda, ProLynx, Zymeworks, Daiichi Sankyo, Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I have research funding from: Genentech, Roche, BMS, Taiho, MedImmune, Merck, Amgen, Lilly
Yelena Y. Janjigian
Stock and Other Ownership Interests: Rgenix
Consulting or Advisory Role: Pfizer, Merck, Bristol Myers Squibb, Merck Serono, Daiichi Sankyo, Rgenix, Bayer, Imugene, AstraZeneca, Lilly, Zymeworks, Basilea Pharmaceutical, Michael J. Hennessy Associates, Paradigm, Seagen, AmerisourceBergen, Arcus Biosciences, Geneos, GlaxoSmithKline, Imedex, Lynx Health, Peerview, Silverback Therapeutics, Mersana, Research to Practice, AskGene Pharma, Phanes Therapeutics
Research Funding: Bayer (Inst), Rgenix (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Lilly (Inst), NCI (Inst), Department of Defense (Inst), Cycle for Survival (Inst), Fred's Team (Inst), Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb Japan
Other Relationship: Clinical Care Options, Axis Medical Education, Research to Practice
Marcelo Garrido
Consulting or Advisory Role: MSD, AstraZeneca, Roche
Speakers' Bureau: Bristol Myers Squibb, Bayer, Merck
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst)
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: Roche
Lin Shen
Consulting or Advisory Role: MSD, Bristol Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbor BioMed, Merck, Boehringer Ingelheim, Sanofi
Research Funding: Nanjing Yaojieankang Biotechnology (Inst), Baiji Shenzhou (Beijing) Biotechnology (Inst), Beijing Xiantong Biomedical Technology (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst), Jacobio (Inst), CANbridge Pharmaceuticals (Inst)
Kensei Yamaguchi
Consulting or Advisory Role: Bristol Myers Squibb Japan, Daiichi Sankyo
Speakers' Bureau: Chugai Pharma, Bristol Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Daiichi Sankyo, Merck
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Gilead Sciences (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Eisai (Inst), MSD Oncology (Inst), Sanofi (Inst), Bristol Myers Squibb (Inst)
Michael Schenker
Research Funding: Bristol Myers Squibb, Roche, Amgen, MSD, Pfizer/EMD Serono, Lilly, Astellas Pharma, AstraZeneca, GlaxoSmithKline, Regeneron, Novartis, AbbVie, Gilead Sciences, Sanofi/Regeneron, Mylan, Bioven, Clovis Oncology, Tesaro, BeiGene, Five Prime Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb
Ruben Kowalyszyn
Consulting or Advisory Role: BMS Argentina, MSD Oncology, Astellas Pharma, Merck Serono, Takeda
Speakers' Bureau: BMS Argentina, Novartis
Research Funding: BMS, MSD Oncology, Novartis, Roche, Astellas Pharma, Lilly, Gemabiotech, Nektar, Poliphor, AstraZeneca, Pfizer
Travel, Accommodations, Expenses: Gador, Pfizer/EMD Serono
Arinilda Campos Bragagnoli
Speakers' Bureau: Bristol Myers Squibb/Medarex, AstraZeneca, Merck
Ricardo Bruges
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Pfizer, Bristol Myers Squibb/Medarex, MSD Oncology, Pfizer
Travel, Accommodations, Expenses: Pfizer, Tecnofarma, MSD Oncology, Tecnofarma, Pfizer
Roberto Pazo-Cid
Consulting or Advisory Role: Roche, Bristol Myers Squibb/Celgene, Eisai Europe, Astellas Pharma, AstraZeneca Spain, Servier, Ipsen
Speakers' Bureau: BMS GmbH & Co KG, Servier, AstraZeneca Spain, Astellas Pharma
Travel, Accommodations, Expenses: Lilly, BMS GmbH & Co KG
Shannon Hunter
Employment: Daiichi Sankyo
Research Funding: BMS (Inst)
Eric Davenport
Research Funding: RTI Internation-Health Solutions
Jinyi Wang
Research Funding: Multiple pharmaceutical companies, identities confidential (Inst)
Kaoru Kondo
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Mingshun Li
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Lucjan Wyrwicz
Honoraria: BeiGene, BMS, MSD
Consulting or Advisory Role: GlaxoSmithKline, Servier
Speakers' Bureau: BMS
Travel, Accommodations, Expenses: Servier
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency, et al. : Integrated genomic characterization of oesophageal carcinoma. Nature 541:169-175, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem ME, Puccini A, Xiu J, et al. : Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist 23:1319-1327, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network, Inc: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Gastric Cancer V.2.2022, 2022. https://NCCN.org
- 5.Smyth EC, Verheij M, Allum W, et al. : Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38-v49, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Wang XS, Hess LM, Lin F-Y, et al. : Patient-reported symptom burden and functioning in patients with advanced esophageal, gastroesophageal junction (GEJ), and gastric cancer undergoing chemotherapy. J Clin Oncol 39:183, 2021 [Google Scholar]
- 7.Wagner AD, Syn NL, Moehler M, et al. : Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 8:Cd004064, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankaran V, Xiao H, Bertwistle D, et al. : A comparison of real-world treatment patterns and clinical outcomes in patients receiving first-line therapy for unresectable advanced gastric or gastroesophageal junction cancer versus esophageal adenocarcinomas. Adv Ther 38:707-720, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pape M, Vissers PA, Bertwistle D, et al. : A nationwide population-based study comparing survival in unresectable advanced or synchronous metastatic esophageal and gastric adenocarcinoma. J Clin Oncol 38:308, 2020 [Google Scholar]
- 10.Chau I, Norman AR, Cunningham D, et al. : The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma—Individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol 20:885-891, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Catenacci D, Tebbutt NC, Davidenko I, et al. : Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18:1467-1482, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lordick F, Kang YK, Chung HC, et al. : Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol 14:490-499, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Shah MA, Bang Y-J, Lordick F, et al. : Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: The METGastric randomized clinical trial. JAMA Oncol 3:620-627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs CS, Shitara K, Di Bartolomeo M, et al. : Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 20:420-435, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Vickery CW, Blazeby JM, Conroy T, et al. : Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer 37:966-971, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Kripp M, Al-Batran SE, Rosowski J, et al. : Quality of life of older adult patients receiving docetaxel-based chemotherapy triplets for esophagogastric adenocarcinoma: A randomized study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Gastric Cancer 17:181-187, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Chau I, Fuchs CS, Ohtsu A, et al. : Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: Exploratory analysis of RAINBOW and REGARD phase III trials. Eur J Cancer 107:115-123, 2019 [DOI] [PubMed] [Google Scholar]
- 18.van Kleef JJ, Ter Veer E, van den Boorn HG, et al. : Quality of life during palliative systemic therapy for esophagogastric cancer: Systematic review and meta-analysis. J Natl Cancer Inst 112:12-29, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Boutros A, Bruzzone M, Tanda ET, et al. : Health-related quality of life in cancer patients treated with immune checkpoint inhibitors in randomised controlled trials: A systematic review and meta-analysis. Eur J Cancer 159:154-166, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Mansoor W, Kulkarni AS, Kato K, et al. : Health-related quality of life (HRQoL) of pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase III KEYNOTE-590 study. J Clin Oncol 39:168, 2021 [Google Scholar]
- 21.Shitara K, Van Cutsem E, Bang YJ, et al. : Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6:1571-1580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Cutsem E, Valderrama A, Bang YJ, et al. : Quality of life with first-line pembrolizumab for PD-L1-positive advanced gastric/gastroesophageal junction adenocarcinoma: Results from the randomised phase III KEYNOTE-062 study. ESMO Open 6:100189, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Drake CG, Wollner I, et al. : Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28:3167-3175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long GV, Atkinson V, Ascierto PA, et al. : Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: Results from the phase III CheckMate 066 study. Ann Oncol 27:1940-1946, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington KJ, Ferris RL, Blumenschein G, Jr, et al. : Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 18:1104-1115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reck M, Taylor F, Penrod JR, et al. : Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: Results from the CheckMate 017 study. J Thorac Oncol 13:194-204, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Reck M, Brahmer J, Bennett B, et al. : Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer 102:23-30, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Cho BC, Takahashi M, et al. : Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:1506-1517, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Escudier B, George S, et al. : Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 126:4156-4167, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shitara K, Ajani JA, Moehler M, et al. : Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 603:942-948, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration : FDA approves nivolumab in combination with chemotherapy for metastatic gastric cancer and esophageal adenocarcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-combination-chemotherapy-metastatic-gastric-cancer-and-esophageal
- 35.Ono Pharmaceutical Co, Ltd : Opdivo® intravenous infusion approved for the treatment of gastric cancer, gastroesophageal junction cancer and esophageal adenocarcinoma in combination with chemotherapy in Taiwan. https://www.ono-pharma.com/sites/default/files/en/news/press/enews20211020.pdf
- 36.European Medicines Agency : Opdivo (nivolumab). https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf
- 37.EuroQol Group : EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 16:199-208, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Pickard AS, Neary MP, Cella D: Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 5:70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garland SN, Pelletier G, Lawe A, et al. : Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-Ga) quality-of-life instrument. Cancer 117:1302-1312, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Brucker PS, Yost K, Cashy J, et al. : General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof 28:192-211, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Cella D, Hahn EA, Dineen K: Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual Life Res 11:207-221, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Yost KJ, Sorensen MV, Hahn EA, et al. : Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the Functional Assessment of Cancer Therapy-Biologic Response Modifiers (FACT-BRM) instrument. Value Health 8:117-127, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Pearman TP, Beaumont JL, Mroczek D, et al. : Validity and usefulness of a single-item measure of patient-reported bother from side effects of cancer therapy. Cancer 124:991-997, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanez B, Pearman T, Lis CG, et al. : The FACT-G7: A rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol 24:1073-1078, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Al-Batran SE, Pauligk C, Homann N, et al. : The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: A randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer 49:835-842, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Morgans AK, Chen YH, Sweeney CJ, et al. : Quality of life during treatment with chemohormonal therapy: Analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol 36:1088-1095, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazieres J, Kowalski D, Luft A, et al. : Health-related quality of life with carboplatin-paclitaxel or nab-paclitaxel with or without pembrolizumab in patients with metastatic squamous non-small-cell lung cancer. J Clin Oncol 38:271-280, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Garassino MC, Gadgeel S, Esteban E, et al. : Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21:387-397, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Adams S, Diéras V, Barrios CH, et al. : Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann Oncol 31:582-589, 2020 [DOI] [PubMed] [Google Scholar]
- 50.Sugarman R, Botteman M, Rusibamayila N, et al. : A quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis of patients in CheckMate 649: Nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC). J Clin Oncol 40:273, 2022 [Google Scholar]
- 51.Tykodi SS, Schadendorf D, Cella D, et al. : Patient-reported outcomes with nivolumab in advanced solid cancers. Cancer Treat Rev 70:75-87, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Nishijima TF, Shachar SS, Muss HB, et al. : Patient-reported outcomes with PD-1/PD-L1 inhibitors for advanced cancer: A meta-analysis. Oncologist 24:e565-e573, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garon EB, Cho BC, Luft A: 5MO—Patient-reported outcomes (PROs) with 1L durvalumab (D), with or without tremelimumab (T), plus chemotherapy (CT) in metastatic (m) NSCLC: Results from POSEIDON. Ann Oncol 33:S27-S70, 2022 [Google Scholar]
- 54.Monk BJ, Tewari KS, Hasegawa CK: Patient-reported outcomes from the phase 3 randomized, double-blind, KEYNOTE-826 trial of pembrolizumab plus chemotherapy versus placebo plus chemotherapy for the first-line treatment of persistent, recurrent, or metastatic cervical cancer. Presented at The Society of Gynecologic Oncology (SGO) 2022 Annual Meeting on Women’s Cancer, Phoenix, AZ, March 18-21, 2022
- 55.Högner A, Moehler M: Immunotherapy in gastric cancer. Curr Oncol 29:1559-1574, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marqués-Lespier JM, González-Pons M, Cruz-Correa M: Current perspectives on gastric cancer. Gastroenterol Clin North Am 45:413-428, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moehler M, Dvorkin M, Boku N, et al. : Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: Results from JAVELIN Gastric 100. J Clin Oncol 39:966-977, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grivas P, Monk BJ, Petrylak D, et al. : Immune checkpoint inhibitors as switch or continuation maintenance therapy in solid tumors: Rationale and current state. Target Oncol 14:505-525, 2019 [DOI] [PubMed] [Google Scholar]
- 59.Atkinson TM, Wagner J-S, Basch E: Trustworthiness of patient-reported outcomes in unblinded cancer clinical trials. JAMA Oncol 3:738-739, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efficace F, Cella D, Aaronson NK, et al. : Impact of blinding on patient-reported outcome differences between treatment arms in cancer randomized controlled trials. J Natl Cancer Inst 114:471-474, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roydhouse JK, Fiero MH, Kluetz PG: Investigating potential bias in patient-reported outcomes in open-label cancer trials. JAMA Oncol 5:457-458, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Yost KJ, Eton DT: Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Eval Health Prof 28:172-191, 2005 [DOI] [PubMed] [Google Scholar]
- 63.King MT, Stockler MR, Cella DF, et al. : Meta-analysis provides evidence-based effect sizes for a cancer-specific quality-of-life questionnaire, the FACT-G. J Clin Epidemiol 63:270-281, 2010 [DOI] [PubMed] [Google Scholar]
- 64.King MT, Cella D, Osoba D, et al. : Meta-analysis provides evidence-based interpretation guidelines for the clinical significance of mean differences for the FACT-G, a cancer-specific quality of life questionnaire. Patient Relat Outcome Meas 1:119-126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.