Abstract
Background
Higher resting heart rate (HR) in patients with heart failure (HF) and sinus rhythm (SR) is associated with increased mortality. In patients hospitalized for HF, the aim herein, was to assess the use and dosage of guideline-recommended HR lowering medications, HR control at discharge and predictors of HR control.
Methods
In the present study, were Polish participants of the European Society of Cardiology HF Long-Term (ESC-HF-LT) Registry. Those selected were hospitalized for HF, with reduced ejection fraction (HFrEF) and SR at discharge (n = 236). The patients were divided in two groups (< 70 bpm and ≥ 70 bpm). Logistic regression was used to identify the predictors of HR ≥ 70 bpm.
Results
Of patients with HFrEF and SR, 59% had HR ≥ 70 bpm at hospital discharge. At discharge, 96% and only 0.5% of the patients with HFrEF and SR received beta-blocker and ivabradine, respectively. In the HF groups < 70 bpm and ≥ 70 bpm, only 11% and 4% of patients received beta-blocker target doses, respectively. There was no difference in the use of other guideline-recommended medications. Age, New York Heart Association class, HR on admission and lack of HR lowering medications were predictors of discharge HR ≥ 70 bpm.
Conclusions
Heart rate control after hospitalization for HFrEF is unsatisfactory, which may be attributed to suboptimal doses of beta-blockers, and negligence in use other HR lowering drugs (including ivabradine).
Keywords: acute heart failure, hospitalization, sinus rhythm, target heart rate, beta-blocker, ivabradine
Introduction
Large studies have shown that a higher resting heart rate (HR) in patients with heart failure (HF) is associated with an increased risk of major adverse cardiovascular events, including patient death [1–7]. Consequently, HR reduction has been shown to improve clinical outcomes in patients with chronic HF [8–13]. However, data regarding the targeted HR control are inconclusive for these patients and the new HF guidelines do not indicate the exact recommended resting HR value [14]. The lack of clear recommendations may be due to the heterogeneity of the HF group consisting of patients with atrial fibrillation/in sinus rhythm (SR), different values of left ventricular ejection fraction (LVEF), varying severity of symptoms and comorbidities, and therefore inconsistent effectiveness of HF medications. It can be hypothesized that the optimal target resting HR may differ in different HF subgroups. The available data refer mostly to patients with HF with reduced ejection fraction (HFrEF), suggesting a HR recommended value of ≤ 70 beats per minute (bpm) (based mainly on the findings from the SHIFT trial on ivabradine in SR) [15].
There is a lack of data on whether therapeutic goals in patients with HF are achieved in everyday clinical practice [16]. More valuable data can be obtained from registries that represent real-world patients and are of particular importance to the analysis of the HR distribution and its control in patients with HF.
The aim of this study was to assess HR control at discharge in real-life Polish patients with HFrEF and SR. The secondary goal was to analyze the association between HR control at discharge and clinical characteristics, use and dosage of guideline-recommended HR lowering medications. HR was also assessed at discharge in patients with HF with mildly reduced LVEF (HFmrEF), and HF with preserved LVEF (HFpEF).
Methods
Study design
The European Society of Cardiology (ESC) Heart Failure Pilot (ESC-HF-Pilot) survey and the ESC Heart Failure Long-Term (ESC-HF-LT) Registry were multicenter, prospective, observational surveys conducted in European countries, including a significant number of Polish centers. Patient recruitment in the ESC-HF-Pilot survey lasted from October 2009 to May 2010 and in the ESC-HF-LT Registry from April 2011 to April 2013. Both registries included all outpatients with HF and patients admitted for new-onset or worsening HF. More data on the design of the registries have been published previously [17, 18]. Briefly, records collected in both registries refer to clinical characteristics, laboratory parameters, HF management, and 1-year follow-up. Both HF registries collected the same type of clinical data. Adult patients with HF were enrolled, without specific exclusion criteria. The study protocol was approved by the local ethics committees. All participating patients gave written informed written consent for the study.
The current analysis included Polish patients hospitalized for HF included in the ESC-HF-LT Registry (the data from ESC-HF-Pilot survey was used for the comparison of HR control at discharge and concomitant pharmacotherapy over time). Only patients with a known heart rhythm and HR at discharge were included in the analysis. A case report form enabled the investigators to choose only one leading heart rhythm for each patient (SR, atrial fibrillation, paced rhythm, or other) based on a 12-lead electrocardiogram (ECG). The exclusion criteria were as follows: death during index hospitalization, rhythm other than SR or lack of ECG documentation on the leading heart rhythm, and lack of information on HR at discharge.
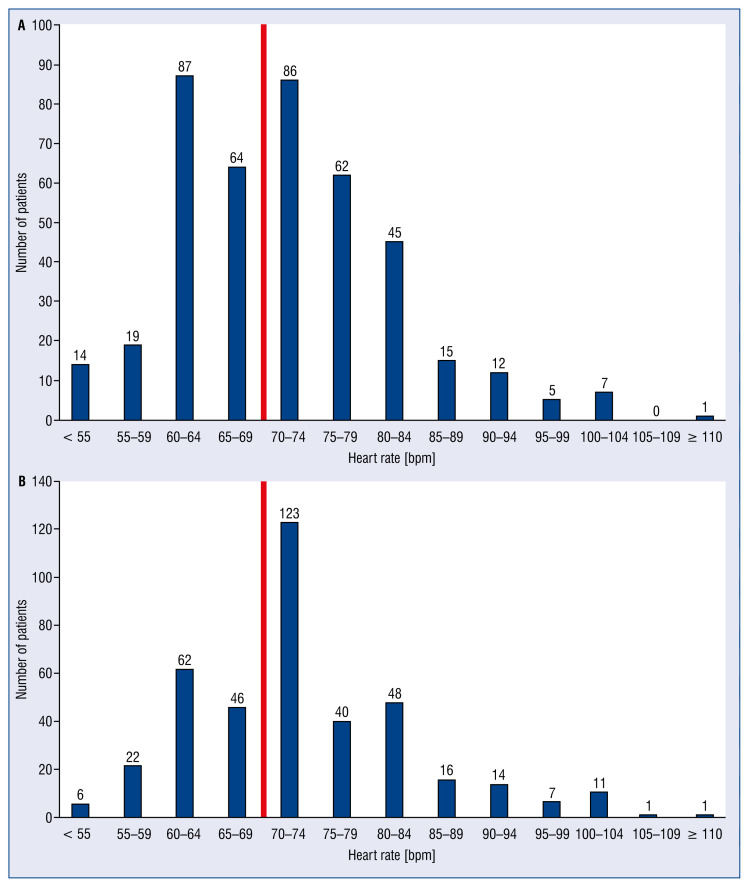
Bar graphs were plotted to present HR distribution at discharge in HF patients in SR in both registries.
The patients were further divided into three groups based on LVEF: HFrEF (LVEF of ≤ 40%), HFmrEF (LVEF of 41–49%), and HFpEF (LVEF of ≥ 50%) [14]. As the current ESC recommendations on HR control mainly account for patients with HFrEF in SR, we focused on this group in further analyzes. Patients with HFrEF in SR included in the ESC-HF-LT Registry were divided into two groups with an HR at discharge of < 70 bpm or ≥ 70 bpm and were compared in terms of baseline characteristics. The use and dosage of guideline-recommended HR lowering medications in these groups were also assessed. In order to analyze the predictors of poor HR control (HR ≥ 70 bpm) at discharge in the HFrEF group, common risk factors for increased HR in HF patients in SR were collected from the literature.
Statistical analysis
The Fisher exact test for the comparison of categorical variables and the Mann–Whitney test for continuous and ordinal variables were used, respectively. The results were presented as median and quartiles for continuous variables and as frequencies and percentages for ordinal variables. Logistic regression analysis was used for the analysis of the predictors of poor HR control (≥ 70 bpm). P value below 0.05 was considered significant for all tests. All tests were two-tailed. Statistical analyses were performed using SPSS software, version 22 (IBM SPSS Statistics 22, USA, New York).
Results
Baseline characteristics and HR distribution
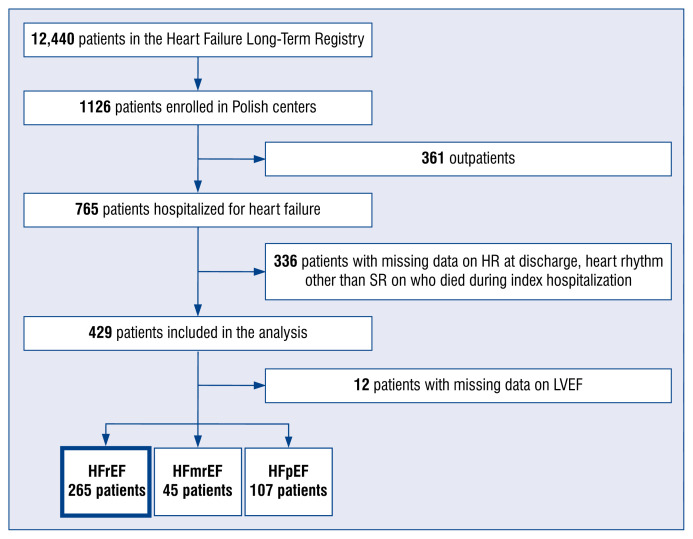
Figure 1 shows the flow chart of patient selection for the present study. The final analysis included 429 hospitalized Polish patients from the ESC-HF-LT Registry in SR and with a known HR at discharge. The median patient age was 76 years; men constituted 67.9% of the population. By comparison, the ESC-HF-Pilot survey included 399 hospitalized patients in SR (the median age was 73 years; men constituted 64.4%). In both registries, the majority of the patients had resting HR ≥ 70 bpm at hospital discharge (65.7% and 56.6%; p = 0.01 in the ESC-HF-Pilot and ESC-HF-LT registries, respectively). The same was also observed in a subgroup of patients in SR with HFrEF (66.3% and 56.8%; p = 0.048, respectively) (Table 1). Based on these results, it seems that the HR management only slightly improved over time in patients participating in the ESC-HF registries.
Figure 1.
Flow chart of patient enrollment in the current analysis; LVEF — left ventricular ejection fraction; HFrEF — heart failure with reduced LVEF; HFmrEF — heart failure with mildly reduced LVEF; HFpEF — heart failure with preserved LVEF; HR — heart rate; SR — sinus rhythm.
Table 1.
Comparison of heart rate (HR) control at discharge and concomitant pharmacotherapy between patients with heart rate with reduced ejection fraction (HFrEF) in sinus rhythm (SR) participating in the ESC-HF-Pilot and ESC-HF-LT registries (patient recruitment 2009–2010 and 2011–2013, respectively).
| Total (n = 464) | HFrEF-Pilot Registry (n = 199; 42.9%) | HFrEF-LT Registry (n = 265; 57.1%) | P | |
|---|---|---|---|---|
| HR at admission [bpm] | 78 (70–90) | 80 (70–100) | 80 (70–95) | 0.05 |
| HR at discharge [bpm] | 70 (64–78) | 70 (65–80) | 70 (63–76) | 0.08 |
| Change in HR [bpm]* | 8 (0–20) | 10 (0–25) | 10 (0–20) | 0.20 |
| Patients with HR < 70 bpm at hospital discharge | 169 (38.9%) | 67 (33.7%) | 102 (43.2%) | 0.048 |
| Pharmacotherapy at hospital discharge | ||||
| Beta-blocker | 412/434 (94.9%) | 185/198 (93.4%) | 227 (96.2%) | 0.27 |
| Dose of beta-blocker | 5.0 (2.5–5.0); 401 | 5.0 (5.0–5.0); 185 | 5.0 (2.5–5.0); 216 | < 0.001 |
| Ivabradine | 2/434 (0.5%) | 0/198 (0%) | 2 (0.8%) | – |
| Digoxin | 79/434 (18.2%) | 40/198 (20.2%) | 39 (16.5%) | 0.38 |
| Amiodaron | 56/434 (12.9%) | 20/198 (10.1%) | 31 (13.0%) | 0.12 |
| Other antiarrhythmics | 36/434 (8.3%) | 28/198 (14.1%) | 8 (3.4%) | < 0.001 |
| Diuretics | 379/434 (87.3%) | 167/198 (84.3%) | 212 (89.8%) | 0.11 |
| Aldosterone antagonist | 333/434 (76.7%) | 149/198 (75.3%) | 184 (78.0%) | 0.57 |
| ACEI | 349/434 (80.4%) | 157/198 (79.3%) | 192 (81.4%) | 0.63 |
| ARB | 41/434 (9.4%) | 18/198 (9.1%) | 23 (9.7%) | 0.87 |
| CCB | 44/434 (10.1%) | 25/198 (12.6%) | 19 (8.1%) | 0.15 |
| Statins | 331/434 (76.3%) | 157/198 (79.3%) | 174 (73.7%) | 0.21 |
| Anticoagulants | 117/433 (27.0%) | 51/197 (25.9%) | 66 (28.0%) | 0.67 |
| Antiplatelets | 341/434 (78.6%) | 161/198 (81.3%) | 180 (76.3%) | 0.24 |
From admission to discharge. Bolded text indicates p-values < 0.05. Numbers in italics indicate available cases for the analyzed continuous variable.
ACEI — angiotensin-converting-enzyme inhibitor; ARB — angiotensin receptor blocker; bpm — beats per minute; CCB — calcium channel blocker; ESC-HF-LT — European Society of Cardiology Heart Failure Long-Term Registry; ESC-HF-Pilot — European Society of Cardiology Heart Failure Pilot Registry
Heart rate distribution at discharge among patients in SR participating in the ESC-HF-LT Registry and the ESC-HF-Pilot survey are presented in Figures 2A and 2B, respectively.
Figure 2.
Distribution of heart rate at discharge in heart failure patients in sinus rhythm participating in the European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT) (A) and European Society of Cardiology Heart Failure Pilot Registry (ESC-HF Pilot survey) (B). Red line separates the graph into two heart rate groups: < 70 bmp and ≥ 70 bpm.
HFrEF patients (n = 265) in SR included in the ESC-HF-LT Registry were divided into two groups with an HR at discharge of < 70 or ≥ 70 bpm (112 patients, 41.5% and 153 patients, 58.5%, respectively). There were no relevant differences between the groups in most baseline clinical characteristics (Table 2). Median HR on admission was 73 (60–80) bpm and 85 (75–100) bpm (p < 0.001), respectively. While on discharge, the median HR was 60.0 (60–65) and 76.0 (70–80) bpm (p < 0.001), respectively. There was no significant change in HR from admission to discharge between both groups. Similar analyzes for patients with HFmrEF and HFpEF are presented in Supplementary Tables S1 and S2, respectively.
Table 2.
Baseline characteristics, clinical course of index hospitalization and pharmacotherapy in hospitalized heart rate with reduced ejection fraction (HFrEF) patients in sinus rhythm (SR) stratified by heart rate at discharge < 70 and ≥ 70 bpm (from the Polish cohort of the ESC-HF-LT Registry).
| HFrEF, SR < 70/min (n = 112; 41.5%) | HFrEF, SR ≥ 70/min (n = 153; 58.5%) | P | |
|---|---|---|---|
| Demographics | |||
| Age [years] | 66.5 (57.6–75.3) | 63.9 (57.2–72.3 | 0.09 |
| Male | 90 (80.4%) | 118 (77.1%) | 0.55 |
| Body mass index [kg/m2] | 27.7 (24.6–29.5) | 27.8 (24.7–31.0) | 0.58 |
| Medical history | |||
| Previous HF hospitalization | 63 (56.2%) | 79 (51.6%) | 0.53 |
| Ischemic etiology | 82 (73.2%) | 105 (68.6%) | 0.50 |
| Valve disease | 6 (5.4%) | 4 (2.6%) | 0.33 |
| Dilated cardiomyopathy | 17 (15.2%) | 32 (20.9%) | 0.27 |
| Hypertension | 82/111 (73.2%) | 102/152 (66.7%) | 0.28 |
| Atrial fibrillation | 25 (22.3%) | 34 (22.2%) | 1.00 |
| Coronary artery disease | 80 (71.4%) | 101 (66.0%) | 0.42 |
| Prior PCI or CABG | 55 (49.1%) | 72 (47.1%) | 0.80 |
| Peripheral artery disease | 16 (14.3%) | 22/152 (14.5%) | 1.00 |
| Diabetes | 43 (38.4%) | 50 (32.7%) | 0.36 |
| Chronic kidney disease | 25 (22.3%) | 46 (30.1%) | 0.20 |
| COPD | 14 (12.5%) | 22 (14.4%) | 0.71 |
| Stroke | 10 (8.9%) | 15 (9.8%) | 0.83 |
| Current smoking | 82 (73.2%) | 105 (68.6%) | 0.49 |
| Alcohol1 | 79/108 (73.1%) | 108/146 (74%) | 0.88 |
| Clinical status at admission | |||
| NYHA class: | 0.16 | ||
| I | 0 (0%) | 0 (0%) | – |
| II | 32 (28.6%) | 35 (22.9%) | – |
| III | 52 (46.4%) | 66 (43.1%) | – |
| IV | 26 (23.2%) | 52 (34.0%) | – |
| Heart rate [bpm] | 73.0 (60.0–80.0) | 85.0 (75.0–100.0) | < 0.001 |
| SBP [mmHg] | 130.0 (110.0–140.0) | 120.0 (110.0–140.0) | 0.04 |
| DBP [mmHg] | 80.0 (70.0–86.2) | 80.0 (70.0–84.0) | 0.88 |
| Laboratory findings at admission | |||
| Hemoglobin [g/dL] | 13.4 (12.6–14.6); 111 | 13.2 (11.3–14.4); 152 | 0.11 |
| Serum creatinine [mg/dL] | 1.1 (0.9–1.3); 111 | 1.1 (0.9–1.4); 152 | 0.45 |
| Serum sodium [mmol/L] | 139.0 (137.0–141.0); 111 | 139.0 (136.9–141.0); 152 | 0.76 |
| Serum potassium [mmol/L] | 4.4 (4.1–4.7); 111 | 4.5 (4.1–4.9); 152 | 0.37 |
| BNP [pg/mL] | 901.5 (199.8–1449.0); 14 | 766.5 (319.4–1058.2); 16 | 0.67 |
| NT-proBNP [pg/mL] | 4263.5 (1505.0–8783.8); 48 | 4819.5 (2565.8–10108.5); 74 | 0.27 |
| Clinical status at discharge | |||
| NYHA class: | 0.22 | ||
| I | 2 (1.8%) | 9 (5.9%) | – |
| II | 61 (54.5%) | 90 (58.8%) | – |
| III | 46 (41.1%) | 52 (34.0%) | – |
| IV | 3 (2.7%) | 2 (1.3%) | – |
| Heart rate [bmp] | 60.0 (60.0–65.0) | 76.0 (70.0–80.0) | < 0.001 |
| Change in heart rate [bmp]2 | −10.0 (−21.2–0.0) | −7.0 (−20.0–1.0) | 0.15 |
| SBP [mmHg] | 120.0 (105.0–130.0) | 118.0 (105.0–126.0) | 0.66 |
| DBP [mmHg] | 70.0 (64.5–79.5); 111 | 70.0 (65.0–80.0); 153 | 0.59 |
| Laboratory findings at discharge | |||
| Hemoglobin [g/dL] | 13.2 (12.0–14.7); 62 | 12.4 (10.4–13.4); 88 | 0.002 |
| Serum creatinine [mg/dL] | 1.1 (0.9–1.3); 81 | 1.1 (0.9–1.4); 106 | 0.88 |
| Serum sodium [mmol/L] | 138.9 (137.0–141.4); 82 | 139.0 (137.0–141.1); 114 | 0.65 |
| Serum potassium [mmol/L] | 4.4 (4.1–4.7); 85 | 4.4 (4.1–4.7); 115 | 0.34 |
| Pharmacotherapy at hospital discharge | |||
| Beta-blocker | 108 (96.4%) | 147 (96.1%) | 1.00 |
| Dose of beta-blocker3 | 5.0 (2.5–5.0); 99 | 5.0 (2.5–5.0); 143 | 0.96 |
| Digoxin | 16 (14.3%) | 24 (15.7%) | 0.86 |
| Ivabradine | 0 (0.0%) | 2 (1.3%) | 0.51 |
| Amiodaron | 24 (21.4%) | 15 (9.8%) | 0.01 |
| Other antiarrhythmics | 3 (2.7%) | 6 (3.9%) | 0.73 |
| Diuretics | 95 (84.8%) | 135 (88.2%) | 0.46 |
| ACEI | 91 (81.2%) | 130 (85.0%) | 0.50 |
| Dose of ACEI4 | 5.0 (2.5–5.0); 89 | 5.0 (2.5–5.6); 128 | 0.22 |
| ARB | 12 (10.7%) | 11 (7.2%) | 0.37 |
| Aldosterone antagonist | 86 (76.8%) | 113 (73.9%) | 0.66 |
| Dose of aldosterone antagonist5 | 25.0 (25.0–25.0); 85 | 25.0 (25.0–50.0); 113 | 0.50 |
| CCB | 10 (8.9%) | 13 (8.5%) | 1.00 |
| Statins | 89 (79.5%) | 109 (71.2%) | 0.15 |
| Anticoagulants | 33 (29.5%) | 33 (29.5%) | 0.66 |
| Antiplatelets | 87 (77.7%) | 116 (75.8%) | 0.77 |
Bolded text indicates p-values < 0.05. Numbers in italics indicate available cases for the analyzed continuous variable.
Former or sometimes;
Change was calculated as the heart rate on admission minus the heart rate at discharge;
Total daily doses of commonly used beta-blockers converted to the corresponding dose of bisoprolol;
Total daily doses of commonly used ACE-Is converted to the corresponding dose of ramipril;
Total daily doses of commonly used aldosterone antagonists converted to the corresponding dose of eplerenone.
ACEI — angiotensin-converting-enzyme inhibitor; ARB — angiotensin receptor blocker; BNP — B-type natriuretic peptide; bpm — beats per minute; CABG — coronary artery bypass grafting; CCB — calcium channel blocker; COPD — chronic obstructive pulmonary disease; DBP — diastolic blood pressure; ESC-HF-LT — European Society of Cardiology Heart Failure Long-Term Registry; HF — heart failure; NT-proBNP — N-terminal-pro-B-type natriuretic peptide; NYHA — New York Heart Association; PCI — percutaneous coronary intervention; SBP — systolic blood pressure
Almost all HFrEF patients in SR in the ESC-HF-LT Registry received beta-blockers (approx. 96%) irrespective of the resting HR group. However, patients with lower HR (< 70 bpm) were treated with amiodarone more frequently (21.4%) compared to patients with HR ≥ 70 bpm (9.8%; p = 0.01). The doses of beta-blockers in both groups were suboptimal (10.9% and 4.4% patients received 100% of the beta-blocker target dose, respectively) (Table 3). Ivabradine was used in a marginal percentage (0.01%) of patients in both groups. There was no difference in terms of the use of other guideline-recommended medications (Table 2).
Table 3.
Use of guideline-recommended heart rate lowering drugs among hospitalized heart rate with reduced ejection fraction patients in sinus rhythm (SR) stratified by heart rate at discharge < 70 and ≥ 70 bpm (from the Polish cohort of the ESC-HF-LT Registry). Use of ivabradine was not presented, as only 2 patients were administered this drug.
| Beta-blockers, target dose [mg/d] | Patients, n (%) | Dose [mg/d], mean (SD) | Target dose, % | |
|---|---|---|---|---|
|
| ||||
| ≥ 50% of target dose | 100% of target dose | |||
| SR < 70 bpm | ||||
| Any beta-blocker | 108/112 (96.4%) | – | 41.1%* | 10.9%* |
| Bisoprolol, 10 mg/d | 29/108 (26.9%) | 3.88 (2.66) | 37.9% | 6.9% |
| Carvedilol, 50 mg/d | 58/108 (53.7%) | 19.1 (12.5) | 37.9% | 10.3% |
| Metoprolol succinate, 200 mg/d | 13/108 (12%) | 78.8 (60.2) | 38.5% | 15.4% |
| Nebivolol, 10 mg/d | 8/108 (7.4%) | 3.75 (1.34) | 50% | 0% |
| Other | 0/108 (0%) | – | – | – |
| SR ≥ 70 bpm | ||||
| Any beta-blocker | 147/153 (96.1%) | – | 46.1%* | 4.4%* |
| Bisoprolol, 10 mg/d | 43/147 (29.3%) | 3.95 (2.08) | 53.5% | 4.7% |
| Carvedilol, 50 mg/d | 71/147 (48.3%) | 17.5 (11.2) | 35.2% | 5.6% |
| Metoprolol succinate, 200 mg/d | 16/147 (10.9%) | 71.9 (37.5) | 31.3% | 0% |
| Nebivolol, 10 mg/d | 14/147 (9.5%) | 4.55 (2.28) | 64.2% | 7.1% |
| Other | 3/147 (2%) | – | – | – |
Patients on other beta-blockers (not recommended in the HFrEF) were not included in the analysis;
bpm — beats per minute; ESC-HF-LT — European Society of Cardiology Heart Failure Long-Term Registry; SD — standard deviation
Predictors of poor HR control
Age, New York Heart Association (NYHA) class, HR on admission and lack of HR lowering medications were the predictors of poor HR control (≥ 70 bpm) at discharge in patients with HFrEF in SR (Table 4).
Table 4.
Multivariable analysis of predictors of poor heart rate control (≥ 70 bpm) at discharge in hospitalized heart rate with reduced ejection fraction patients in sinus rhythm (SR) (from the Polish cohort of the ESC-HF-LT Registry).
| Covariates | OR | 95% CI | P |
|---|---|---|---|
| Age | 0.96 | 0.93–0.99 | 0.02 |
| Male | 0.62 | 0.29–1.29 | 0.20 |
| Coronary artery disease | 1.54 | 0.77–3.12 | 0.22 |
| Chronic obstructive pulmonary disease | 1.00 | 0.42–2.43 | 0.99 |
| Chronic kidney disease | 2.06 | 0.93–4.63 | 0.08 |
| SBP at discharge | 1.00 | 0.98–1.01 | 0.65 |
| NYHA class III/IV (NYHA I/II as reference) at discharge | 0.39 | 0.20–0.75 | 0.01 |
| Heart rate at admission | 1.05 | 1.03–1.07 | < 0.001 |
| Hemoglobin concentration at admission | 0.89 | 0.75–1.04 | 0.16 |
| Left ventricular ejection fraction | 1.00 | 0.96–1.04 | 0.90 |
| Beta-blocker dosage at discharge | 1.05 | 0.96–1.17 | 0.28 |
| Drugs lowering heart rate* | 0.43 | 0.21–0.86 | 0.02 |
Bolded text indicates p-values < 0.05.
Other than beta-blockers;
bpm — beats per minute; CI — confidence interval; ESC-HF-LT — European Society of Cardiology Heart Failure Long-Term Registry; NYHA — New York Heart Association; OR — odds ratio; SBP — systolic blood pressure
Discussion
The results of this analysis provided important epidemiological data on HR control and associated clinical characteristics in real-life patients with HF. The study showed that HR over 70 bpm was present in the majority of HF patients in SR and that the patients were treated sub-optimally with HR lowering drugs. What is more, the study revealed the predictors of poor HR control.
Despite strict guidelines on the treatment of HFrEF, the readmission rate within 6 months of hospitalization for HF is as high as 50% [14, 19]. It is known that increased resting HR in patients with HF is associated with higher mortality [4, 20], particularly when above 110 bpm and with concomitant atrial fibrillation [21, 22]. HR can also contribute to tachyarrhythmic cardiomyopathy and HF decompensation. In the previous analysis from the ESC-HF registries, among all tested major electrocardiography (ECG) abnormalities in patients with HF (regardless of the type of HF), only tachycardia (> 100 bpm) remained an independent predictor of all-cause death in multivariable analysis [23].
In patients hospitalized for HF decompensation doses of guideline-recommended medications should be initially uptitrated before discharge and/or in the early post-discharge period. HR control should be assessed during every follow-up appointment to further adjust the medications [14]. In patients with HF, HR reduction is achieved mostly by beta-blocker therapy through their negative chronotropic effect and direct antagonization of toxic effects of catecholamines. Ivabradine acts by selectively and specifically inhibiting the channel responsible for the cardiac pacemaker current (If), and thus it regulates the HR in patients in SR. Other guideline-recommended medications commonly used in chronic HF also have the ability to reduce the HR by reducing sympathetic and renine–angiotensine system activity [24]. The international QUALIFY survey (QUAlity of adherence to guideline recommendations for Life-saving treatment in heart failure) has demonstrated that the adherence to guidelines is associated with an improved prognosis in patients with HF [25]. In the Polish population of the QUALIFY registry nearly 97.0% of the patients received beta-blockers, with only 17.7% of the patients reaching the target dose [25]. The general adherence score (calculated based on the use of five main guideline-recommended medications) in the study population was good in 72.2% of the patients [25]. In another analysis performed among the Spanish population included in the same registry (ESC-HF-LT Registry) showed that only 13% of patients with low ejection fraction received a beta-blocker in the recommended dosage [26]. In the current study, beta-blockers were administered in the majority of the patients (approx. 96%). However, only 10.9% of the patients with HR < 70 bpm and 4.4% ≥ 70 bpm reached the target dose of the beta-blocker. Ivabradine was administered only to 0.5% of patients with LVEF ≤ 35%. To compare, in the QUALIFY registry, ivabradine was prescribed to 13.9% of patients, but the target dose was attained only in 13.8% of patients [25]. It should be highlighted that ivabradine reduces the risk of cardiovascular mortality and HF hospitalization and should be considered in symptomatic patients with reduced ejection fraction (≤ 35%) in SR and a resting HR ≥ 70 bpm despite optimal medical therapy with other HF recommended-medications (or in patients who are unable to tolerate or have contraindications to beta-blockers) [14, 27].
Observational studies have demonstrated that in every third patient with chronic HF, despite the use of the recommended treatment, the control of resting HR was insufficient [28]. In the QUALIFY registry less than 40% of the patients reached the target HR < 70 bpm [25]. In the present study, 66.3% and 56.8% of patients (ESC-HF-Pilot and ESC-HF-LT; p = 0.048) had HR over 70 bpm. There was a difference in terms of adequate HR control between the ESC-HF-Pilot and ESC-HF-LT groups in favor of the latter. Unfortunately, still more than half of the patients were not optimally treated (HR was ≥ 70 bpm). This was despite the fact that the percentage of the patients receiving HF guideline-recommended drugs was higher in the ESC-HF-LT registry in comparison with the ESC-HF-Pilot group [29].
On the other hand, it may be emphasized that the therapeutic benefit correlates with the degree of HR reduction, and not with the dose of the beta-blocker, and the optimal HR should be the goal of HF therapy. A meta-analysis by McAlister et al. [30] showed that a 5-bpm reduction in HR reduces mortality by 18%. While, every 1 bpm increase in HR the increases the risk by 3% [2]. In the current study the group with HR < 70 bpm patients had a more than twice the rate of amiodarone use than the group with HR > 70 bpm, which could result in better HR control.
In the present study, in the multivariable analysis it was observed that younger age, lower NYHA class at discharge, higher HR on admission and no use of HR lowering medications were independent predictors of poor HR control (HR ≥ 70 bpm). A study assessing, i.e., the extent of HR reduction achieved in clinical practice, showed that patients with increased HRs were younger, more often male, with a higher NYHA class and lower LVEF [31].
The suboptimal use of chronotropic negative therapy is observed especially in patients with coexisting lung diseases [32]. It should be noted that the use of beta-blockers in patients with chronic obstructive pulmonary disease is not only safe but it also reduces their all-cause and in-hospital mortality [32, 33]. Therefore, apart from typical HF medications, adequate HR control and treatment of comorbidities should constitute a cornerstone element of HF therapy.
Limitations of the study
The inclusion of real-life patients followed-up by cardiologists is an important advantage of the ESC-HF registries; however, there are limitations that have to be acknowledged. First, it is the partial incompleteness of the data and its observational character. Second, the registries did not primarily focus on heart rhythm analysis. Thirdly, taking into account the fact that the mean HR is slightly higher in females than in males of the same age, it should be noted that females were underrepresented in the current study. Fourthly, in the analysis there was a marked difference is hemoglobin levels between the HF groups with HR < 70 and ≥ 70 bpm [34]. Anemia is a known risk factor that can cause a hyperdynamic circulatory state and affect resting HR and could have altered the HR control [35].
Conclusions
The results of this study suggest that HR control in real-life patients with HF only slightly improved over time (between the ESC-HF-Pilot and ESC-HF-LT groups), and it remains unsatisfactory. This might be due to the suboptimal use and dosage of HF guideline-recommended medications, particularly of beta-blockers and ivabradine.
Supplementary Information
Footnotes
Conflict of interest: None declared
References
- 1.Greene S, Vaduganathan M, Wilcox J, et al. The prognostic significance of heart rate in patients hospitalized for heart failure with reduced ejection fraction in sinus rhythm. JACC: Heart Failure. 2013;1(6):488–496. doi: 10.1016/j.jchf.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Böhm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376(9744):886–894. doi: 10.1016/s0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 3.DeVore AD, Schulte PJ, Mentz RJ, et al. Relation of elevated heart rate in patients with heart failure with reduced ejection fraction to one-year outcomes and costs. Am J Cardiol. 2016;117(6):946–951. doi: 10.1016/j.amjcard.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laskey WK, Alomari I, Cox M, et al. Heart rate at hospital discharge in patients with heart failure is associated with mortality and rehospitalization. J Am Heart Assoc. 2015;4(4) doi: 10.1161/JAHA.114.001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vollmert T, Hellmich M, Gassanov N, et al. Heart rate at discharge in patients with acute decompensated heart failure is a predictor of mortality. Eur J Med Res. 2020;25(1):47. doi: 10.1186/s40001-020-00448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cierpka-Kmieć K, Hering D. Tachycardia: The hidden cardiovascular risk factor in uncomplicated arterial hypertension. Cardiol J. 2020;27(6):857–867. doi: 10.5603/CJ.a2019.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yetkin E, Ozturk S, Cuglan B, et al. Golden ratio in congestive heart failure: A promising proportion for prognosis and decompensation. Cardiol J. 2020;27(6):904–905. doi: 10.5603/CJ.2020.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess PL, Sheng S, Matsouaka R, et al. Strict versus lenient versus poor rate control among patients with atrial fibrillation and heart failure (from the Get With The Guidelines — Heart Failure Program) Am J Cardiol. 2020;125(6):894–900. doi: 10.1016/j.amjcard.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Sartipy U, Savarese G, Dahlström U, et al. Association of heart rate with mortality in sinus rhythm and atrial fibrillation in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21(4):471–479. doi: 10.1002/ejhf.1389. [DOI] [PubMed] [Google Scholar]
- 10.Swedberg K, Komajda M, Böhm M, et al. SHIFT Investigators. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59(22):1938–1945. doi: 10.1016/j.jacc.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Coats A, Fowler MB. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 12.Fowler MB, Vera-Llonch M, Oster G. Influence of carvedilol on hospitalizations in heart failure: incidence, resource utilization and costs. J Am Coll Cardiol. 2001;37(6):1692–1699. doi: 10.1016/s0735-1097(01)01190-1. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 15.Borer JS, Böhm M, Ford I, et al. Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study. Eur Heart J. 2012;33(22):2813–2820. doi: 10.1093/eurheartj/ehs259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramgobin D, Vo M, Golamari R, et al. Congestive heart failure clinics and telemedicine: The key to reducing hospital readmissions in the United States. Cardiol J. 2021 doi: 10.5603/CJ.a2021.0073. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2010;12(10):1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 18.Crespo-Leiro MG, Anker SD, Maggioni AP, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18(6):613–625. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 19.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 20.Kapłon-Cieślicka A, Balsam P, Ozierański K, et al. Resting heart rate at hospital admission and its relation to hospital outcome in patients with heart failure. Cardiol J. 2014;21(4):425–433. doi: 10.5603/CJ.a2013.0147. [DOI] [PubMed] [Google Scholar]
- 21.Ozierański K, Kapłon-Cieślicka A, Balsam P, et al. Effect of beta-blockers on 1-year survival and hospitalizations in patients with heart failure and atrial fibrillation: results from ESC-HF Pilot and ESC-HF Long-Term Registry. Pol Arch Intern Med. 2018;128(11):649–657. doi: 10.20452/pamw.4346. [DOI] [PubMed] [Google Scholar]
- 22.Simpson J, Castagno D, Doughty RN, et al. Is heart rate a risk marker in patients with chronic heart failure and concomitant atrial fibrillation? Results from the MAGGIC meta-analysis. Eur J Heart Fail. 2015;17(11):1182–1191. doi: 10.1002/ejhf.346. [DOI] [PubMed] [Google Scholar]
- 23.Tymińska A, Ozierański K, Balsam P, et al. The prevalence and association of major ECG abnormalities with clinical characteristics and the outcomes of real-life heart failure patients - Heart Failure Registries of the European Society of Cardiology. Kardiol Pol. 2021;79(9):980–987. doi: 10.33963/KP.a2021.0053. [DOI] [PubMed] [Google Scholar]
- 24.Hori M, Okamoto H. Heart rate as a target of treatment of chronic heart failure. J Cardiol. 2012;60(2):86–90. doi: 10.1016/j.jjcc.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Opolski G, Ozierański K, Lelonek M, et al. Adherence to the guidelines on the management of systolic heart failure in ambulatory care in Poland. Data from the international QUALIFY survey. Pol Arch Intern Med. 2017;127(10):657–665. doi: 10.20452/pamw.4083. [DOI] [PubMed] [Google Scholar]
- 26.Crespo-Leiro M, Segovia-Cubero J, González-Costello J, et al. Adherence to the ESC Heart Failure Treatment Guidelines in Spain: ESC Heart Failure Long-term Registry. Rev Esp Cardiol (Engl Ed) 2015;68(9):785–793. doi: 10.1016/j.rec.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376(9744):875–885. doi: 10.1016/s0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 28.Moran D, Buckley A, Daly K, et al. Heart rate awareness in patients with chronic stable heart failure. A multi-center observational study. Int J Cardiol. 2014;177(2):380–384. doi: 10.1016/j.ijcard.2014.08.119. [DOI] [PubMed] [Google Scholar]
- 29.Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Differences in clinical characteristics and one-year outcomes of hospitalized heart failure patients in succeeding European Society of Cardiology-Heart Failure Registries – Pilot and Long-Term. Pol Arch Intern Med. 2019;129(2):106–116. doi: 10.20452/pamw.4418. [DOI] [PubMed] [Google Scholar]
- 30.McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150(11):784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 31.Franke J, Wolter JS, Meme L, et al. Optimization of pharmacotherapy in chronic heart failure: is heart rate adequately addressed? Clin Res Cardiol. 2013;102(1):23–31. doi: 10.1007/s00392-012-0489-2. [DOI] [PubMed] [Google Scholar]
- 32.Villalobos RE, Tan IR, Divinagracia RM. Safety and benefits of beta-blockers in chronic obstructive pulmonary disease: a review of current evidence and meta-analysis. Eur Resp J. 2018;52(suppl 62):PA742. [Google Scholar]
- 33.Yang YL, Xiang ZJ, Yang JH, et al. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2020;41(46):4415–4422. doi: 10.1093/eurheartj/ehaa793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhavathi K, Selvi KT, Poornima KN, et al. Role of biological sex in normal cardiac function and in its disease outcome — a review. J Clin Diagn Res. 2014;8(8):BE01–BE04. doi: 10.7860/JCDR/2014/9635.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80–98. doi: 10.1161/CIRCULATIONAHA.118.030099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.