Abstract
Background
Epidermal growth factor receptor (EGFR) exon 20 insertion (ex20ins) mutation is the third most common EGFR-mutant form, accounting for 10–12% of all EGFR mutations in non-small cell lung cancer (NSCLC). Chemotherapy was the first-line treatment for patients with EGFR ex20ins mutation in the era when EGFR ex20ins tyrosine kinase inhibitors (EGFR ex20ins-TKIs) were inaccessible. Although EGFR ex20ins-TKIs have since then demonstrated certain efficacy, the population benefit rate is not high due to the high cost of the drug and limited benefit to the population. Therefore, the choice of treatment modality when a patient does not have access to EGFR ex20ins-TKIs or are resistant to them remains an avenue worth exploring.
Case Description
In this report, we present two cases of patients with lung adenocarcinoma and EGFR ex20ins mutation. The two patients were middle-aged Asian women with no smoking history, and both had one or more metastatic lesions. Both achieved long-term clinical benefit (progression-free survival ≥12 months) after receiving combined treatment, suggesting that this is a promising treatment modality.
Conclusions
To the best of our knowledge, this is the first report supporting the combination of stereotactic body radiotherapy and apatinib and camrelizumab as an effective treatment strategy in patients with advanced EGFR ex20ins-positive NSCLC who have been previously treated with chemotherapy. The therapy described in this report might serve as a potential alternative approach for clinical oncologists.
Keywords: Epidermal growth factor receptor exon 20 insertion mutation (EGFR ex20ins mutation), stereotactic body radiotherapy (SBRT), camrelizumab, apatinib, case report
Highlight box.
Key findings
• We have identified for the first time a promising treatment modality as a higher-line treatment option for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) exon 20 insertion (ex20ins) mutation.
What is known and what is new?
• A clinical trial showed that camrelizumab in combination with apatinib was effective in NSCLC patients with EGFR ex20ins mutation [median progression-free survival (PFS): 8.3 (1.9–8.3) months].
• We combined this therapeutic strategy with stereotactic body radiotherapy (SBRT), and patients achieved long-term survival (PFS ≥12 months). We expect this treatment combination will bring clinical benefit for more patients.
What is the implication, and what should change now?
• We report for the first time that SBRT plus apatinib plus camrelizumab was an effective treatment strategy in NSCLC patients with EGFR ex20ins mutation, as both patients achieved long-term clinical benefit (PFS ≥12 months) after receiving this combined treatment, suggesting it is a promising treatment modality. Naturally, additional prospective clinical studies are needed to confirm these findings.
Introduction
Epidermal growth factor receptor (EGFR) is the most frequent mutant driver gene in non-small cell lung cancer (NSCLC) which accounts for 80–85% of all lung cancer cases (1). The successful use of EGFR tyrosine kinase inhibitors (EGFR-TKIs) has greatly improved the survival of patients with EGFR-positive NSCLC (2,3). The EGFR exon 20 insertion (EGFR ex20ins) mutation is the third most common mutant form after EGFR exon 19 deletion and L858R exon 21 mutation, accounting for 10–12% of all EGFR mutations in NSCLC (4,5).
EGFR ex20ins is a highly heterogeneous family of activating mutations, and due to its unique spatial site block, patients with EGFR ex20ins-mutated NSCLC are not sensitive to treatment with first- and second-generation EGFR-TKIs, thus limiting the efficacy of conventional EGFR-TKIs (6-9). In the era when EGFR ex20ins tyrosine kinase inhibitors (EGFR ex20ins-TKIs) were not available, treatment was dominated by chemotherapy. In recent years, a subset of patients with NSCLC and the EGFR ex20ins mutation have benefited from the inclusion of EGFR ex20ins inhibitors targeting the EGFR ex20ins mutation subtype with mobocertinib (TAK-788) and amivantamab (JNJ-61186372) in the National Comprehensive Cancer Network (NCCN) guidelines (10). However, due to its limited efficacy and high cost, it is not an option available for clinical treatment of a large proportion of patients. Therefore, the choice of treatment modality when a patient has no access to EGFR ex20ins-TKIs or are resistant to them remains an area of valuable research.
Here, we discuss a new treatment option of SBRT combined with immunotherapy and anti-angiogenesis therapy in cases for whom EGFR ex20ins-TKIs were unavailable. Two cases of pretreated advanced NSCLC with EGFR ex20ins mutation benefitted from this treatment modality for an extended period, supporting this regimen’s therapeutic value. We present this article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-542/rc).
Case presentation
Case 1
On November 22, 2017, a 53-year-old female with a first diagnosis of stage IIIA (cT1N2M0) NSCLC was treated with left superior lobectomy at a local hospital. Postoperative chemotherapy and radiotherapy were not administered, and disease-free survival was 12.7 months. Next-generation sequencing (NGS) analysis revealed presence of EGFR ex20ins mutation (p.P772-H773insTNP) and no co-mutation, the expression of programmed death-ligand 1 (PD-L1) was 10%, and the tumor mutation burden (TMB) was 6.4 mutations/Mb.
On December 13, 2018, the patient underwent positron emission tomography-computed tomography (PET-CT), which showed enlarged mediastinal zone 6 lymph nodes with increased metabolism. The patients thus underwent thoracoscopic mediastinal tumor resection in our hospital. Postoperative pathological testing revealed the patient had lung adenocarcinoma metastasis, and immunohistochemical staining results indicated the following: CK7+, TTF-1+, CK20−, CDX2−, and villin−.
After surgery, the patient underwent six cycles of PP chemotherapy (pemetrexed plus cisplatin) and radiotherapy (lung and mediastinal lymph node) at a local hospital. This was the patient’s first-line treatment regimen, and progression-free survival (PFS) 1 was 12.3 months.
On January 6, 2020, the patient was reexamined at a local hospital with CT, which indicated multiple nodules in both lungs and in the left pleura, with the possibility of metastasis. The carcinoembryonic antigen (CEA) concentration in the blood was 199.06 ng/mL, and progressive disease (PD) was considered. On January 13, 2020, the patient was treated with one cycle of TP chemotherapy (nab-paclitaxel plus carboplatin). Following this, the patient underwent five cycles of chemotherapy with TP regimen (nab-paclitaxel plus nedaplatin) at the local hospital. This was the patient’s second-line regimen, and PFS2 was approximately 1 month.
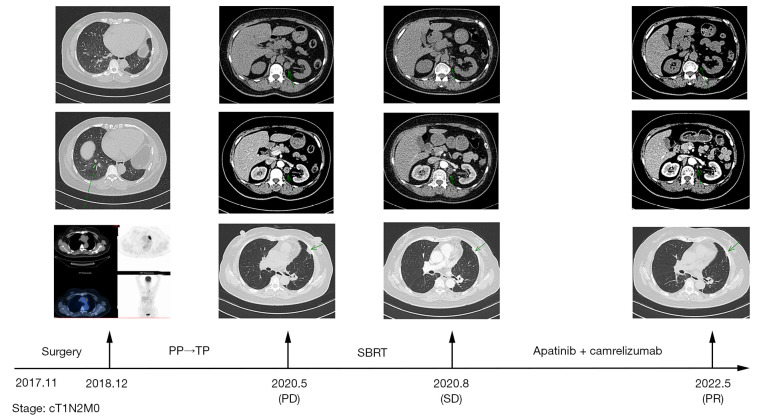
From February 2020 to May 2020, the patient’s reexamination suggested a progressive increase in the size of the anteromedial left renal node, and PD was again considered. The patient underwent radiotherapy with SBRT with a planning target volume (PTV) of 45 Gy/5 F in the left para-aortic node on July 6, 2020. The patient was administered 18 cycles of camrelizumab (200 mg) and apatinib (250 mg) per day (day 1 to day 5) from July 11, 2020, to October 17, 2021. Our combination treatment model was used as third-line treatment, during which no significant disease progression was observed in regular follow-up.
On May 19, 2022, the patient was reexamined at our hospital, and the evaluation was stable disease (SD). As of May 19, 2022, the patient is in a healthy state, and PFS has been sustained for more than 22.5 months after administration of SBRT combined with immunotherapy and anti-angiogenesis therapy (Figure 1). Thereafter, the patient continued to receive maintenance therapy with camrelizumab and apatinib locally.
Figure 1.
Timeline showing the treatment history and imaging dates of case 1. The arrows in the pictures point out the location of the tumor. PP, pemetrexed plus cisplatin; TP, paclitaxel plus carboplatin; PD, progressive disease; SBRT, stereotactic body radiotherapy; SD, stable disease; PR, partial response.
Case 2
On November 28, 2019, a 59-year-old female with no smoking history underwent CT examination at a local hospital for “right-sided chest pain”, which revealed an occupying lesion in the left upper lung. After percutaneous lung puncture biopsy pathology, the woman was diagnosed with invasive lung adenocarcinoma on December 3, 2019. Subsequently, the patient underwent PET-CT in our hospital, which showed an irregularly shaped mass in the anterior segment of the left upper lobe of the lung (2.1 cm × 1.5 cm) with increased metabolism, a soft tissue mass in the left anterior mediastinum (5.1 cm × 3.2 cm) with an abnormally increased metabolism and multiple nodules in both lungs and pleura with partially increased metabolism; these masses were considered to be malignant lesions. Immunohistochemical staining results were as follows: TTF-1+, C-MET+, and ALK−. The patient was definitively diagnosed with stage IV left lung adenocarcinoma (cT4N3M1), and NGS revealed the presence of the EGFR ex20ins mutation (p.Ala767_Val769dup) and no co-mutation, the TMB was 1.0 mutation/Mb. In addition, we did not observe the expression of PD-L1.
The patient underwent three cycles of PP chemotherapy regimen (pemetrexed + nedaplatin) plus bevacizumab (500 mg q3w) on December 7, 2019. However, this treatment was not significantly beneficial, and the patient developed atrial fibrillation and second degree gastrointestinal reaction. Patients and their families requested a change in treatment. Therefore, the chemotherapy regimen was changed, and the patient was treated with two cycles of pemetrexed monotherapy on March 5 and April 7, 2020. This was the first-line treatment regimen for this patient and yielded a PFS1 of 5.9 months.
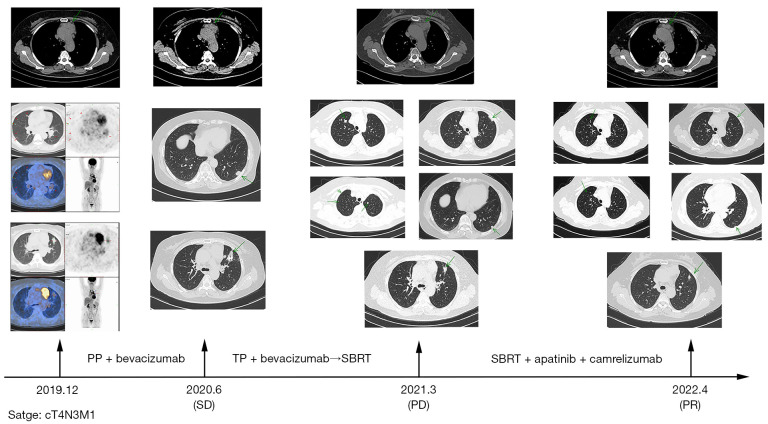
On June 3, 2020, the patient was reviewed in the outpatient clinic, which revealed that the intrapulmonary and mediastinal lymph node lesions were partially enlarged. The patient was treated with three cycles of TP chemotherapy plus bevacizumab (500 mg) on June 12, 2020. On August 25, 2020, the patient underwent CT-guided lung puncture with golden marker implantation and began SBRT with a PTV of 50 Gy/5 F for the left lung lesion on September 7, 2020. This was the second-line treatment regimen for this patient and yielded a PFS2 of 8.7 months.
On March 3, 2021, the patient underwent thoracic CT examination, revealing multiple small nodules in both lungs (the larger one is located in the upper lobe of the right lung), which were considered to be multiple metastases, and multiple enlarged lymph nodes in the anterior mediastinum. Given the progression of the patient’s disease, the patient underwent SBRT with a PTV of 24 Gy/3 F in the right lung lesion on March 12, 2021, which was followed by ten cycles of camrelizumab plus apatinib from March 16 to October 29. The yielded an evaluation of SD.
After an evaluation of SD on November 19, 2021, nine cycles of camrelizumab plus apatinib treatment were applied. On June 5, 2022, this patient was reexamined for tumor progression. Our treatment modality (SBRT plus apatinib plus camrelizumab) was applied as third-line treatment. The patient did well clinically, and the baseline respiratory status did not worsen until 14.8 months after SBRT (Figures 2,3). But we regret that the patient has not been able to control tumor progression with subsequent therapies and has reached OS in June 2023.
Figure 2.
Timeline showing the treatment history and imaging dates of case 2. The arrows in the pictures point out the location of the tumor. PP, pemetrexed plus cisplatin; SD, stable disease; TP, paclitaxel plus carboplatin; PD, progressive disease; SBRT, stereotactic body radiotherapy; PR, partial response.
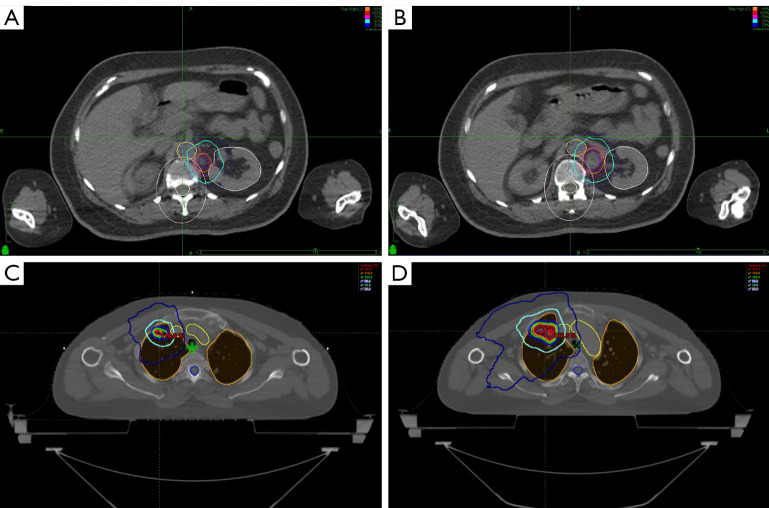
Figure 3.
SBRT plan. (A,B) SBRT plan used for case 1; (C,D) SBRT plan used for case 2. SBRT, stereotactic body radiotherapy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International Multidisciplinary Team (iMDT) discussion
Discussion among physicians from Union Hospital affiliated to Huazhong University of Science and Technology
EGFR mutations are the most common targetable genomic driver of NSCLC (11). Ninety percent of EGFR mutations are EGFR exon 19 deletions or exon 21 L858R mutations, both of which are known as common mutations (12). With the improvement of NGS and various authoritative guidelines emphasizing the need for rare EGFR mutation detection, uncommon EGFR mutations are being more fully recognized, and EGFR ex20ins are gaining more attention as the third most common EGFR mutation subtype (13-18). Patients with the EGFR ex20ins mutation have similar clinical features but have a poorer survival prognosis compared to those with the more common EGFR mutations (7,19).
We conducted intradisciplinary and multidisciplinary consultations of these two cases. Both patients were considered to have lung adenocarcinoma harboring EGFR ex20ins: one with initial stage IIIA progression to stage IV and the other with initial stage IV.
Department of Oncology
The standard first-line treatment regimen for patients with stage IV EGFR ex20ins lung adenocarcinoma is platinum-based chemotherapy, as recommended by NCCN guidelines. Three retrospective studies explored the efficacy of chemotherapy in patients NSCLC and EGFR ex20ins and showed that chemotherapy significantly prolonged PFS, with a median PFS of 5.5, 7.6, and 7.1 months each being reported (20-22). Despite the fact that chemotherapy represents a good option for the early treatment of patients with NSCLC harboring EGFR ex20ins, there is still a need for extended treatment maintenance, and clinical needs remain greatly unmet.
With the appearance of two EGFR ex20ins inhibitors (mobocertinib and amivantamab), new hope has been given to patients with EGFR ex20ins NSCLC. Clinical study results indicated amivantamab to be effective in 40% of patients with lung cancer who have failed platinum chemotherapy, with a median PFS of 8.3 months and a median overall survival of 22.8 months (23,24). Mobocertinib received accelerated United States Food and Drug Administration (FDA) approval on September 15, 2021, for the treatment of locally advanced or metastatic adult NSCLC for patients with the EGFR ex20ins mutation (25,26). Studies on mobocertinib reported that is resulted in significant tumor shrinkage or disappearance in 28% patients with lung cancer who failed platinum chemotherapy, yielding a disease control rate of 78%, a mean PFS of 7.3 months, and a mean overall survival of 20.2 months. Mobocertinib is the only oral TKI targeting the EGFR ex20ins mutation (27,28). Based on this, these two drugs have been included into the NCCN guidelines as second-line treatment options for patients who have failed chemotherapy. In addition, the clinical outcomes of targeted drugs including furmonertinib, osimertinib, afatinib, sunvozertinib, and poziotinib have also been reported (24,27,29-37) (Table 1).
Table 1. Overview of the efficacy of EGFR inhibitors in EGFR ex20ins-mutant NSCLC.
| Drug name | Year | Number of patients | ORR (%) | mPFS (months) | mOS (months) | Reference |
|---|---|---|---|---|---|---|
| Mobocertinib (TAK-788) | 2021 | 114 | 28 | 7.3 | 24 | (27) |
| Amivantamab (JNJ-372) | 2021 | 81 | 40 | 8.3 | 22.8 | (24) |
| CLN-081 (100 mg bid) | 2022 | 39 | 41 | 12 | NR | (29) |
| Sunvozertinib (DZD9008) | 2022 | 52 | 40.4 | NR | NR | (30) |
| Poziotinib | 2020 | 115 | 14.8 | 4.2 | NR | (31) |
| 2022 | 50 | 32 | 5.5 | NR | (32) | |
| Osimertinib | 2022 | 25 | 28 | 6.8 | NR | (33) |
| 2021 | 62 | 6.5 | 2.3 | NR | (35) | |
| Furmonertinib | 2022 | 15 | 53.5 | NR | NR | (34) |
| Afatinib | 2020 | 70 | 24.3 | NR | NR | (36) |
| 1st/2nd generation EGFR-TKIs | 2021 | 6 | 0 | 2 | 17 | (37) |
EGFR, epidermal growth factor receptor; ex20ins, exon 20 insertion; NSCLC, non-small cell lung cancer; ORR, overall response rate; mPFS, median progression-free survival; mOS, median overall survival; NR, not reported; TKI, tyrosine kinase inhibitor.
However, due to the high cost of EGFR ex20ins-TKIs and the fact that they are not accessible to all patients, additional treatment options need to be developed to benefit a broader population of patients with EGFR ex20ins. The two patients reported did not have access to EGFR ex20ins-TKIs and currently lack standard second-line treatment options. According to the results of Gao et al.’s study, camrelizumab in combination with apatinib in NSCLC patients with EGFR ex20ins mutation had an overall response rate (ORR) of 33.3% (0.8–90.6%), and the median PFS was 8.3 (1.9–8.3) months, suggesting that camrelizumab in combination with apatinib in EGFR ex20ins NSCLC may achieve good results (38).
Department of Radiotherapy
The use of immunotherapy provides patients more options, although there is no full consensus regarding the exact clinical benefit of immunotherapy in patients with advanced NSCLC and EGFR ex20ins mutation (Table 2); nonetheless, the combination of radiotherapy plus immunotherapy seems have some benefit. In recent years, it has been widely recognized that radiotherapy can be used not only as a local treatment, but also to stimulate a systemic immune response because of its “distant effect”, which provides a strong rationale for the combination of radiotherapy and immunotherapy (46). Radiotherapy promotes immunogenic effects and has the potential to convert irradiated tumors into in situ vaccines, thereby triggering innate and adaptive immune responses locally and systemically, and it also remodels the tumor microenvironment and exerts good immunomodulatory functions, thereby creating a therapeutically appropriate immune microenvironment that sensitizes chemotherapy and immunotherapy (47). SBRT in combination with immunotherapy has been reported to be considerably successful and is a treatment modality that is cost-effective and avoids the serious side effects of chemotherapy. In a phase II randomized controlled clinical trial named PEMBRO-RT, immunotherapy plus SBRT demonstrated good treatment efficacy, with a doubling of the ORR (18% vs. 36%) and a significantly increased median PFS (1.9 vs. 6.6 months) being observed (48). In the IMpower150 study, chemotherapy plus atezolizumab plus bevacizumab demonstrated the best efficacy in patients with EGFR lung cancer. This clearly demonstrates the therapeutic potential of anti-angiogenic therapy combined with immunotherapy in patients with EGFR mutations (49).
Table 2. Overview of immunotherapy of EGFR ex20ins-mutant NSCLC.
| Study | Treatment | Year | Number of patients | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|
| Metro et al. (39) | ICB monotherapy | 2021 | 12 | 6.7 | 2 | 5.3 |
| Christopoulos et al. (40) | Chemotherapy + ICB | 2022 | 25 | 24 | 6.5 | NR |
| Yang et al. (41) | Chemotherapy + ICB | 2023 | 15 | 40 | 6.53 | NR |
| Trummer et al. (42) | Chemotherapy + atezolizumab + bevacizumab | 2022 | 9 | 88.9 | 13.6 | NR |
| Ou et al. (43) | 1L ICB monotherapy | 2021 | 11 | 9.1 | 3.1 | 11 |
| 1L ICB + platinum | 2021 | 16 | 18.8 | 4.5 | 11.3 | |
| ≥2L ICB monotherapy | 2021 | 32 | 3.1 | 2.3 | 8.1 | |
| Lau et al. (44) | Immunotherapy monotherapy | 2021 | 6 | 50 | 4.8 | NR |
| Gao et al. (38) | Camrelizumab + apatinib | 2022 | 3 | 33.3 | 8.3 | NR |
| Morita et al. (45) | ICBs | 2021 | 8 | 25 | 3.1 | NR |
EGFR, epidermal growth factor receptor; ex20ins, exon 20 insertion; NSCLC, non-small cell lung cancer; ORR, overall response rate; mPFS, median progression-free survival; mOS, median overall survival; ICB, immune checkpoint blockade; NR, not reported; 1L, first-line; 2L, second-line.
Previously, we have found that camrelizumab plus apatinib with or without SBRT was beneficial as a higher-line treatment for EGFR-mutant patients with NSCLC. For patients treated with SBRT, this regimen may have better efficacy. The results of this study were presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting (clinical trial information: ChiCTR1900028363) (50). Although this study targeted all EGFR-mutated lung cancers, due to the good treatment effect of camrelizumab plus apatinib in EGFR ex20ins NSCLC, we speculate that camrelizumab plus apatinib and SBRT will also be effective in treating EGFR ex20ins patients. Therefore, we treated these two patients with a treatment model of camrelizumab plus apatinib with SBRT and achieved an unexpected PFS result.
Case 1 had a PFS of 22.5 months (July 6, 2020, to May 21, 2022), and case 2 had a PFS of 14.8 months (April 12, 2021, to June 5, 2022). Both achieved long-term clinical benefit (PFS ≥12 months) after receiving combined treatment, suggesting this is a promising treatment modality. These two patients were middle-aged Asian women with no smoking history, and both had lung adenocarcinoma with one or more metastatic lesions. However, the PD-L1 expression status, TMB, and EGFR ex20ins type were not significantly similar in these two cases. We are not sure if it means that the treatment model of SBRT combined with immunotherapy and anti-angiogenic therapy does not depend on patients’ PD-L1 expression status, TMB, or EGFR ex20ins type and may benefit a wide range of patients. Of course, prospective clinical studies are needed to clarify this. In conclusion, we propose a new treatment paradigm for patients with advanced EGFR ex20ins NSCLC that yields an extended PFS.
Several further issues regarding the diagnosis and treatment of this patient were discussed
Which EGFR ex20ins NSCLC patients are the real beneficial population for immunotherapy?
Kenichi Suda: EGFR-mutated NSCLC has been recognized as one of the tumors that are resistant to cancer immunotherapies, which led to the exclusion of these patients in some trials using immunotherapeutic drugs. However, it is also true that some studies, that enrolled EGFR-mutated NSCLCs as well, have shown similar efficacy of immunotherapies even in EGFR mutation positive subgroup, e.g., adjuvant atezolizumab after pulmonary resection (51) (IMpower010 trial) or a combination therapy trial involving an anti-angiogenic drug, bevacizumab (49) (IMpower150 trial). Therefore, it is hypothesized that immunotherapy will work in EGFR-mutated NSCLCs in some circumstances, and one of important points of these two patients presented in this case report would be the addition of an anti-angiogenic drug to immunotherapy.
Mariacarmela Santarpia: The impact of mutation subtypes on response to immune checkpoint inhibitors (ICIs) is controversial. In retrospective analyses, uncommon EGFR mutations, including EGFR ex20ins, have been associated with significant better response and PFS compared to common EGFR mutations, suggesting that ICIs constitute an important therapeutic option for these patients (44,52,53). Among EGFR exon 20- and HER2-mutated patients, no difference in terms of clinical benefit was observed in tumors with in-frame insertions compared to missense mutations (44).
Samir Dalia: we do not know which mutation allows for beneficial immunotherapy versus an EGFR inhibitor.
What is the biomarker for anti-angiogenic therapy plus immunotherapy to achieve long survival?
Kenichi Suda: Identifying predictive biomarkers, beyond PD-L1 and TMB, is difficult especially when ICIs are given as combination treatment. TMB is considered to be a biomarker for treatment with ICIs. Clinical studies have indicated that high TMB is associated with a survival benefit after treatment with ICIs in single cancer types or pan-cancer types (54,55). Some studies have suggested a distinct immune microenvironment of NSCLCs with uncommon EGFR mutations (such as G719X and S768I), however, these studies also suggested that NSCLCs with EGFR ex20ins may have similar immune microenvironment compared with tumors harboring common EGFR mutations (56,57). Therefore, it seems that the usefulness of this treatment may not be limited to NSCLCs with EGFR ex20ins mutation.
Mariacarmela Santarpia: Anti-tumor immunity is regulated by a complex crosstalk between tumor vasculature and immune cells (58). Preclinical studies have demonstrated that simultaneous targeting of angiogenesis and immune checkpoints can normalize aberrant vascular-immune crosstalk and potentiate cancer immunotherapy. In clinical studies, the combination of anti-angiogenic drugs and ICIs has been demonstrated to be an effective strategy and is currently approved for a variety of tumor types, including lung cancer (59). Several potential tissue- and serum-based biomarkers predictive of response to anti-angiogenic plus immunotherapy have been described. However, due to the complex nature of the interaction between tumor angiogenesis and immune response, it is difficult to identify a single, viable biomarker that could be useful to select patients that can respond to this treatment combination.
Samir Dalia: There is no biomarker that predicts improvement with anti-angiogenesis medications and immunotherapy. We just know that higher PD-L1 status predicts better outcome to immunotherapy.
What kind of radiotherapy fractionation pattern and radiotherapy site is the best treatment pattern for combined immunotherapy plus anti-angiogenic therapy?
Mariacarmela Santarpia: Combining immunotherapy with radiotherapy has a strong biological rationale. The use of radiotherapy can result in the release of antigens from tumors and can enhance antitumor T cell response by several mechanisms. Moreover, this combination can potentially enhance the abscopal effect, thereby leading to regression of nonirradiated lesions (60,61).
Different studies reported that the immune-modulating effect of hypofractionated radiotherapy was more pronounced compared with single-dose radiotherapy. SBRT may activate noninflamed NSCLC tumors toward an inflamed tumor microenvironment, rendering them receptive to ICIs, with acceptable toxicity (62). In the phase II randomized PEMBRO-RT trial, immunotherapy plus SBRT was associated with higher response rate and increased median PFS (48). However, more clinical data is needed to define the effects of radiotherapy dose, fractionation, and treatment site on the antitumor immune response.
Samir Dalia: No data yet on which type of radiotherapy fractionation is better with immunotherapy or anti-angiogenesis therapy.
Conclusions
To the best of our knowledge, this is the first case report of a combination of SBRT with apatinib and camrelizumab that proved to be an effective treatment strategy in EGFR ex20ins-positive patients with advanced NSCLC who were previously treated with chemotherapy. This regimen might serve as an alternative approach for clinical oncologists.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 32271267), National Natural Science Foundation of China (No. 82373228), CSCO Cancer Research Fund (Nos. Y-zai2022/ms-0190 and Y-2019Genecast-039), Chinese Thoracic Oncology Group (CTONG) (No. CTONG-YC20220118), and 2022Wu Jieping Medical Foundation (No. 320.6750.2022-22-64).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-542/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-542/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-542/coif). K.S. has received research grants from AstraZeneca and Boehringer Ingelheim, and has received honoraria from AstraZeneca, Chugai, and Taiho, outside the submitted work. M.S. is an invited speaker at scientific meetings for Novartis, BMS. The other authors have no conflicts of interests to declare.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. 10.1016/S0140-6736(21)00312-3 [DOI] [PubMed] [Google Scholar]
- 3.Singal G, Miller PG, Agarwala V, et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 2019;321:1391-9. 10.1001/jama.2019.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passaro A, Mok T, Peters S, et al. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J Thorac Oncol 2021;16:764-73. 10.1016/j.jtho.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. 10.1016/S1470-2045(11)70129-2 [DOI] [PubMed] [Google Scholar]
- 6.Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. 10.1038/s41591-018-0007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett H, Emich H, Carroll C, et al. Epidemiological and clinical burden of EGFR Exon 20 insertion in advanced non-small cell lung cancer: A systematic literature review. PLoS One 2021;16:e0247620. 10.1371/journal.pone.0247620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J Thorac Oncol 2018;13:1560-8. 10.1016/j.jtho.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byeon S, Kim Y, Lim SW, et al. Clinical Outcomes of EGFR Exon 20 Insertion Mutations in Advanced Non-small Cell Lung Cancer in Korea. Cancer Res Treat 2019;51:623-31. 10.4143/crt.2018.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier T, Prasad V. Amivantamab and Mobocertinib in Exon 20 insertions EGFR Mutant Lung Cancer, Challenge To The Current Guidelines. Transl Oncol 2022;23:101475. 10.1016/j.tranon.2022.101475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramadhan HH, Taaban DF, Hassan JK. The Frequency of Epidermal Growth Factor Receptor (EGFR) mutations in Iraqi patients with Non-Small Cell Lung Cancer (NSCLC). Asian Pac J Cancer Prev 2021;22:591-6. 10.31557/APJCP.2021.22.2.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: A comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol 2021;160:103300. 10.1016/j.critrevonc.2021.103300 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Liu Z, Wan Z, et al. Clinicopathologic and molecular characteristics of Chinese lung adenocarcinoma patients with EGFR exon 20 insertion mutations. Ann Transl Med 2022;10:220. 10.21037/atm-22-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdrizet K, Stockley TL, Law JH, et al. Integrating comprehensive genomic sequencing of non-small cell lung cancer into a public healthcare system. Cancer Treat Res Commun 2022;31:100534. 10.1016/j.ctarc.2022.100534 [DOI] [PubMed] [Google Scholar]
- 15.Passiglia F, Malapelle U, Normanno N, et al. Optimizing diagnosis and treatment of EGFR exon 20 insertions mutant NSCLC. Cancer Treat Rev 2022;109:102438. 10.1016/j.ctrv.2022.102438 [DOI] [PubMed] [Google Scholar]
- 16.Ou SI, Hong JL, Christopoulos P, et al. Distribution and Detectability of EGFR Exon 20 Insertion Variants in NSCLC. J Thorac Oncol 2023;18:744-54. 10.1016/j.jtho.2023.01.086 [DOI] [PubMed] [Google Scholar]
- 17.Murakami S, Yokose T, Shinada K, et al. Comparison of next-generation sequencing and cobas EGFR mutation test v2 in detecting EGFR mutations. Thorac Cancer 2022;13:3217-24. 10.1111/1759-7714.14685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batra U, Nathany S, Sharma M, et al. Next generation sequencing for detection of EGFR alterations in NSCLC: is more better? J Clin Pathol 2022;75:164-7. 10.1136/jclinpath-2020-207212 [DOI] [PubMed] [Google Scholar]
- 19.Bazhenova L, Minchom A, Viteri S, et al. Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer 2021;162:154-61. 10.1016/j.lungcan.2021.10.020 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Li J, Zhou Y, et al. Tumor genomics and response to chemotherapy in advanced non-small cell lung cancer with exon 20 insertion epidermal growth factor receptor mutations. Ann Transl Med 2020;8:1297. 10.21037/atm-20-6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu CW, Wang WX, Wang D, et al. Pemetrexed-based chemotherapy for non-small-cell lung cancer patients with EGFR exon 20 insertion mutation: a multicenter study. Transl Lung Cancer Res 2020;9:1853-61. 10.21037/tlcr-20-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah MP, Aredo JV, Padda SK, et al. EGFR exon 20 Insertion NSCLC and Response to Platinum-Based Chemotherapy. Clin Lung Cancer 2022;23:e148-53. 10.1016/j.cllc.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah V, McNatty A, Simpson L, et al. Amivantamab-Vmjw: A Novel Treatment for Patients with NSCLC Harboring EGFR Exon 20 Insertion Mutation after Progression on Platinum-Based Chemotherapy. Biomedicines 2023;11:950. 10.3390/biomedicines11030950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol 2021;39:3391-402. 10.1200/JCO.21.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duke ES, Stapleford L, Drezner N, et al. FDA Approval Summary: Mobocertinib for Metastatic Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations. Clin Cancer Res 2023;29:508-12. 10.1158/1078-0432.CCR-22-2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chon K, Larkins E, Chatterjee S, et al. FDA Approval Summary: Amivantamab for the Treatment of Patients with Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations. Clin Cancer Res 2023;29:3262-6. 10.1158/1078-0432.CCR-22-3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Ramalingam SS, Kim TM, et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol 2021;7:e214761. 10.1001/jamaoncol.2021.4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riely GJ, Neal JW, Camidge DR, et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial. Cancer Discov 2021;11:1688-99. 10.1158/2159-8290.CD-20-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu HA, Tan DSW, Smit EF, et al. Phase (Ph) 1/2a study of CLN-081 in patients (pts) with NSCLC with EGFR exon 20 insertion mutations (Ins20). J Clin Oncol 2022;40:9007. [Google Scholar]
- 30.Janne PA, Wang M, Camidge DR, et al. Antitumor activity of sunvozertinib in NSCLC patients with EGFR Exon20 insertion mutations after platinum and anti-PD(L)1 treatment failures. J Clin Oncol 2022;40:9015. [Google Scholar]
- 31.Le X, Goldman JW, Clarke JM, et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol 2020;38:9514. [Google Scholar]
- 32.Elamin YY, Robichaux JP, Carter BW, et al. Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity. Cancer Cell 2022;40:754-767.e6. 10.1016/j.ccell.2022.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwierenga F, van Veggel B, Hendriks LEL, et al. High dose osimertinib in patients with advanced stage EGFR exon 20 mutation-positive NSCLC: Results from the phase 2 multicenter POSITION20 trial. Lung Cancer 2022;170:133-40. 10.1016/j.lungcan.2022.06.012 [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Dong H, Li P, et al. Short-term efficacy of furmonertinib in treatment of NSCLC patients with EGFR exon20 insertion. J Clin Oncol 2022;40:e21063. [Google Scholar]
- 35.Yang GJ, Li J, Xu HY, et al. Osimertinib for Chinese advanced non-small cell lung cancer patients harboring diverse EGFR exon 20 insertion mutations. Lung Cancer 2021;152:39-48. 10.1016/j.lungcan.2020.11.027 [DOI] [PubMed] [Google Scholar]
- 36.Yang JC, Schuler M, Popat S, et al. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J Thorac Oncol 2020;15:803-15. 10.1016/j.jtho.2019.12.126 [DOI] [PubMed] [Google Scholar]
- 37.Chelabi S, Mignard X, Leroy K, et al. EGFR Exon 20 Insertion in Metastatic Non-Small-Cell Lung Cancer: Survival and Clinical Efficacy of EGFR Tyrosine-Kinase Inhibitor and Chemotherapy. Cancers (Basel) 2021;13:5132. 10.3390/cancers13205132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao G, Ni J, Wang Y, et al. Efficacy and safety of camrelizumab plus apatinib in previously treated patients with advanced non-small cell lung cancer harboring EGFR or ALK genetic aberration. Transl Lung Cancer Res 2022;11:964-74. 10.21037/tlcr-22-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metro G, Baglivo S, Bellezza G, et al. Sensitivity to Immune Checkpoint Blockade in Advanced Non-Small Cell Lung Cancer Patients with EGFR Exon 20 Insertion Mutations. Genes (Basel) 2021;12:679. 10.3390/genes12050679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christopoulos P, Kluck K, Kirchner M, et al. The impact of TP53 co-mutations and immunologic microenvironment on outcome of lung cancer with EGFR exon 20 insertions. Eur J Cancer 2022;170:106-18. 10.1016/j.ejca.2022.04.020 [DOI] [PubMed] [Google Scholar]
- 41.Yang G, Yang Y, Liu R, et al. First-line immunotherapy or angiogenesis inhibitor combined with chemotherapy for advanced non-small cell lung cancer with EGFR exon 20 insertions: Real-world evidence from China. Cancer Med 2023;12:335-44. 10.1002/cam4.4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trummer A, Bethge A, Dickgreber N, et al. NSCLC with uncommon EGFR mutations treated with atezolizumab plus bevacizumab and chemotherapy. Lung Cancer 2022;174:141-5. 10.1016/j.lungcan.2022.11.006 [DOI] [PubMed] [Google Scholar]
- 43.Ou SHI, Lin HM, Hong JL, et al. Real-world response and outcomes in NSCLC patients with EGFR exon 20 insertion mutations. J Clin Oncol 2021;39:9098. 10.1016/j.jtocrr.2023.100558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau SCM, Fares AF, Le LW, et al. Subtypes of EGFR- and HER2-Mutant Metastatic NSCLC Influence Response to Immune Checkpoint Inhibitors. Clin Lung Cancer 2021;22:253-9. 10.1016/j.cllc.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 45.Morita C, Yoshida T, Shirasawa M, et al. Clinical characteristics of advanced non-small cell lung cancer patients with EGFR exon 20 insertions. Sci Rep 2021;11:18762. 10.1038/s41598-021-98275-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Liu X, Chen D, et al. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther 2022;7:258. 10.1038/s41392-022-01102-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory Effects of Stereotactic Body Radiation Therapy: Preclinical Insights and Clinical Opportunities. Int J Radiat Oncol Biol Phys 2021;110:35-52. 10.1016/j.ijrobp.2019.02.046 [DOI] [PubMed] [Google Scholar]
- 48.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. 10.1001/jamaoncol.2019.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 50.Meng R, Wu L, Zhang K, et al. Efficacy of camrelizumab (SHR-1210) plus apatinib with or without stereotactic body radiotherapy (SBRT) as higher-line therapy for advanced EGFR-mutant non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:e21128. [Google Scholar]
- 51.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 52.Yamada T, Hirai S, Katayama Y, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med 2019;8:1521-9. 10.1002/cam4.2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pretelli G, Spagnolo CC, Ciappina G, et al. Overview on Therapeutic Options in Uncommon EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): New Lights for an Unmet Medical Need. Int J Mol Sci 2023;24:8878. 10.3390/ijms24108878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee KW, Van Cutsem E, Bang YJ, et al. Association of Tumor Mutational Burden with Efficacy of Pembrolizumab±Chemotherapy as First-Line Therapy for Gastric Cancer in the Phase III KEYNOTE-062 Study. Clin Cancer Res 2022;28:3489-98. 10.1158/1078-0432.CCR-22-0121 [DOI] [PubMed] [Google Scholar]
- 55.Cristescu R, Aurora-Garg D, Albright A, et al. Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors. J Immunother Cancer 2022;10:e003091. 10.1136/jitc-2021-003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma T, Jiao J, Huo R, et al. PD-L1 expression, tumor mutational burden, and immune cell infiltration in non-small cell lung cancer patients with epithelial growth factor receptor mutations. Front Oncol 2022;12:922899. 10.3389/fonc.2022.922899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lei Y, Wang K, Liu Y, et al. Various Subtypes of EGFR Mutations in Patients With NSCLC Define Genetic, Immunologic Diversity and Possess Different Prognostic Biomarkers. Front Immunol 2022;13:811601. 10.3389/fimmu.2022.811601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Hyeon DY, Hwang D. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med 2020;52:1428-42. 10.1038/s12276-020-0420-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 60.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51. 10.1186/s40425-016-0156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voronova V, Vislobokova A, Mutig K, et al. Combination of immune checkpoint inhibitors with radiation therapy in cancer: A hammer breaking the wall of resistance. Front Oncol 2022;12:1035884. 10.3389/fonc.2022.1035884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018;36:1611-8. 10.1200/JCO.2017.76.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as