Introduction
In this recent issue of Journal of Thoracic Disease, Fong et al. describe practice patterns pertaining to the management of asymptomatic brain metastases secondary to epidermal growth factor receptor (EGFR)-mutant or anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC) (1). As the incidence of brain metastases reaches 50–60% in this population, how to most effectively treat intracranial disease in such patients remains an important clinical question (2,3).
Historically, the management of brain metastases has relied on local treatment modalities such as surgery or radiation due to the limited access of systemic therapies into the central nervous system (CNS) compartment (4,5). While surgical resection is primarily limited to solitary, large, symptomatic brain metastases, stereotactic radiosurgery (SRS) has classically been considered standard-of-care in patients with limited brain metastases due to its excellent local control and favorable toxicity profile compared to whole brain radiation therapy (WBRT) (6).
This treatment paradigm of upfront SRS for brain metastases is now evolving, however, due to the improved intracranial activity of newer-generation tyrosine kinase inhibitors (TKIs) targeting EGFR and ALK. Although effective, whether these systemic treatments can replace local brain-directed therapies is an area of ongoing debate. Fong et al.’s finding that medical oncologists, radiation oncologists, and neurosurgeons advocate different therapeutic approaches highlights the lack of consensus across specialties and represents a call for further research to establish the optimal treatment approach in the modern targeted therapy era (1).
International survey of clinical practice
In their study, “Recommended first-line management of asymptomatic brain metastases from EGFR mutant and ALK positive non-small cell lung cancer varies significantly according to specialty: an international survey of clinical practice”, Fong et al. administered an international survey to medical oncologists, clinical oncologists, radiation oncologists, and neurosurgeons to assess their recommended management of brain metastases in patients with EGFR-mutant or ALK-rearranged NSCLC. Clinical scenarios were subdivided according to the number (1–3, 4–9, and >9) and size (≤2 or >2 cm) of brain metastases and the type of driver mutation present (EGFR or ALK). Across all four cohorts of respondents for medical, clinical, radiation, and neurosurgical oncologists, most were attending staff (76–97%), part of a multidisciplinary oncology team (81–92%), and had practiced for ≥5 years (56–89%). Affiliation with an academic center (50–88%) and location of clinical practice (Asia vs. Europe vs. North America) varied. For both medical and clinical oncology respondents, over half (60–66%) reported seeing 5 or more new patients with primary lung cancers per month, and 38–40% reported seeing 3–10 patients with new brain metastases from lung cancer per month.
In clinical scenarios of small (≤2 cm), low burden [1–3] brain metastases for both EGFR- and ALK-positive NSCLC, medical and clinical oncologists were far more likely to recommend upfront systemic therapy alone compared to neurosurgeons and radiation oncologists. As the number of brain metastases increased (≥4), recommendations for upfront radiation therapy increased, regardless of physician specialty.
In clinical scenarios of EGFR- or ALK-positive NSCLC with large (>2 cm) but low burden [1–3] brain metastases, upfront surgery was more likely to be recommended by all cohorts, and neurosurgeons were numerically more likely to recommend resection over radiation therapy. As the number of brain metastases increased (≥4), recommendations for upfront radiation therapy again increased, but surgery continued to be more commonly recommended by neurosurgeons than by other specialists.
Not surprisingly, recommended therapies fell largely along specialty lines. Medical oncologists and clinical oncologists were more likely to recommend initial TKI monotherapy compared to radiation oncologists or neurosurgeons, especially for patients with fewer number of small intracranial lesions. Similarly, neurosurgeons were more likely to recommend their own treatment modality—surgical resection—for patients with larger brain metastases (>2 cm). Aside from the robust number of surveys included, a notable strength of this study is the variety of clinicians who were surveyed, including clinical oncologists, who prescribe both systemic anti-cancer therapies and radiation treatments. Across most scenarios, this group was more likely to recommend radiation therapy when compared to medical oncologists but less likely to recommend radiation therapy when compared to radiation oncologists. With physicians more likely to recommend their own treatment modalities, the clinical oncologist group provided a potentially less biased perspective given their experience treating with both systemic and local therapies.
Limitations of the analysis were well-described by the authors. First, the survey scenarios presented only asymptomatic lesions and did not include brain metastases involving the brainstem, lesions >2 cm involving eloquent locations of the brain, or leptomeningeal disease. Although treatment of symptomatic brain metastases or large lesions located in eloquent locations is considered straightforward in warranting local therapy, a recent retrospective study suggests benefit with TKI therapy alone in the treatment of even large burden CNS disease (7). A second limitation is that the type of radiation technique or TKI was not specified in each scenario. Survey responses may have been impacted if WBRT versus SRS were specified, or if osimertinib versus a less CNS-penetrant TKI such as erlotinib or gefitinib were offered. Due to the negative impact of WBRT on neurocognition, physicians may be adverse to recommending radiation if WBRT is the available modality, especially for scenarios in which there are numerous small lesions (8,9).
Current management of brain metastases
With respect to local therapy for brain metastases, initial randomized clinical trials showed that SRS preserves cognitive outcomes without a decrement in overall survival compared to WBRT, quickly making it the preferred treatment for patients with ≤3 metastases (8,9). Today, there is further evidence supporting similar cognitive and survival outcomes with SRS for increasing numbers of brain metastases, recently up to 15 (6,10). Although SRS minimizes neuro-toxicities compared to WBRT, patients still remain at risk for side-effects such as radionecrosis (11,12).
In the case of systemic therapy for EGFR-mutant and ALK-rearranged NSCLC, numerous studies have established the intracranial activity of next-generation TKIs (Table 1). The FLAURA trial reported an intracranial objective response rate (ORR) of 66% in EGFR-mutant NSCLC patients with any measurable or unmeasurable brain metastases and 91% in patients with one or more measurable lesions with osimertinib. At 12 months, the estimated probability of observing a CNS progression event was 8% with osimertinib compared to 24% with erlotinib or gefitinib (16). Of note, the FLAURA trial included patients with untreated brain metastases but those with initially symptomatic metastases were enrolled only if stable for at least 2 weeks after completion of definitive therapy and steroids. In addition, among those who had measurable or unmeasurable brain metastases at study entry, 25% received prior brain radiation, and intracranial outcomes were not analyzed separately for those receiving osimertinib alone versus osimertinib plus radiation (16).
Table 1. Intracranial efficacy of TKIs for EGFR- and ALK-positive NSCLC in recent select trials.
| Study characteristics | AURA extension and AURA2 (13) | AURA3 (14) | OCEAN (15) | FLAURA (16) | ALEX (17) | CROWN (18) | Brigatinib trial (19) |
|---|---|---|---|---|---|---|---|
| Patient eligibility | T790M-positive, progressed following prior EGFR-TKI | T790M-positive, progressed following prior EGFR-TKI | T790M-positive, progressed following prior EGFR-TKI | Untreated EGFR mutation-positive advanced NSCLC | Untreated, ALK-positive NSCLC | ALK-positive NSCLC | ALK-positive NSCLC |
| BM eligibility | Stable, asymptomatic CNS metastases | Stable, asymptomatic CNS metastases | Radiation naïve BM, excludes BM requiring RT or resection | Asymptomatic, stable BM, or symptomatic, or unstable BM if stable for ≥2 weeks after therapy and steroids |
Asymptomatic brain or leptomeningeal metastases, either treated or untreated | Asymptomatic CNS metastases, either treated or untreated, no prior radiation | Asymptomatic, untreated CNS metastases |
| Treatment | Osimertinib 80 mg daily |
Osimertinib 80 mg daily | Osimertinib 80 mg daily | Osimertinib 80 mg daily (vs. gefitinib 250 mg or erlotinib 150 mg daily) | Alectinib 600 mg twice daily (vs. crizotinib 250 mg twice daily) | Lorlatinib 100 mg daily (vs. crizotinib 250 mg twice daily) | Brigatinib 180 mg daily (vs. crizotinib 250 mg twice daily) |
| Key results | iORR: 54%; median CNS DOR: not reached | iORR: 70%; median CNS DOR: 8.9 months | BMRR: 70%; iORR: 40.5% | Patients with measurable CNS lesions iORR: 91%; median CNS DOR: 15.2 months | Patients with measurable CNS lesions iORR: 81% | iORR: 82%; intracranial CR: 71% | Patients with measurable CNS lesions iORR: 78% |
TKI, tyrosine kinase inhibitor; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer; BM, brain metastases; CNS, central nervous system; iORR, intracranial objective response rate; DOR, duration of response; RT, radiation therapy; BMRR, brain metastases response rate; CR, complete response.
The treatment landscape for patients with metastatic ALK-rearranged NSCLC has similarly evolved due to the advent of CNS-penetrant targeted therapies (Table 1). The ALEX trial enrolled patients with treated and untreated asymptomatic brain metastases and found an intracranial response rate of 81% and 59% in patients with measurable and measurable or unmeasurable CNS lesions treated with alectinib, respectively (17). A further detailed analysis of reported 12-month cumulative incidence rates of CNS progression with alectinib of 16% and 4.6% in patients with and without baseline CNS metastases, respectively. Intracranial progression-free survival (PFS) was longer with alectinib when compared with crizotinib regardless of prior radiation, although it was still numerically longer in those patients receiving alectinib after prior intracranial radiation versus no radiation [not reached (NR) vs. 14.0 months] (20). In the CROWN trial, lorlatinib, a third-generation ALK inhibitor, was also found to have an impressive intracranial response rate of 82% in patients with measurable brain metastases, with 71% having an intracranial complete response (18). Similarly, a phase III trial of brigatinib showed a promising intracranial response rate of 78% in patients with measurable CNS lesions (19). Notably, all of these studies included only patients with asymptomatic brain metastases, and there remains a paucity of data surrounding patients with symptomatic brain lesions.
In addition, no randomized trials to-date evaluating upfront TKIs alone versus in combination with local therapies have been reported, although retrospective analyses are available. A multi-institutional analysis by Magnuson et al., for example, found that upfront SRS or WBRT in addition to TKIs was associated with significantly improved survival in patients with EGFR-mutant NSCLC (21). Although this large retrospective study suggested the ongoing importance of radiation in the treatment of intracranial disease, most of these patients (98%) received erlotinib, which has limited intracranial activity compared to osimertinib. In contrast, another retrospective multi-institutional analysis by Thomas et al. found no significant differences in time to intracranial progression or time to treatment failure between patients receiving next-generation, CNS-penetrant TKIs alone (osimertinib, rociletinib, alectinib, lorlatinib, brigatinib, or ensartinib) versus with upfront radiation, suggesting that intracranial radiation may be deferred in carefully selected patients. However, the authors reported that a higher percentage of patients in the radiation cohort had symptomatic intracranial disease and were more likely to have received steroids prior to treatment (22). Despite this selection bias with unfavorable factors more common in the radiation cohort, outcomes were similar, suggesting that upfront radiation may have provided additional intracranial control in patients with more aggressive disease. Although a smaller phase II study evaluating the efficacy of osimertinib against radiation-naïve brain metastases included a minority of patients (20%) with limited symptomatic brain metastases, patients with symptomatic brain lesions that required radiation or surgical resection were excluded (15).
A call for consensus
Careful patient selection for local brain-directed therapies versus TKI monotherapy is critical to balancing oncologic outcomes with patient quality of life and treatment-related toxicities. Although the use of SRS decreases neurological symptoms and treatment-related morbidity compared to WBRT, radionecrosis can cause significant morbidity, and its incidence varies with total dose of radiation, total treated brain volume, and volume of brain receiving a specified dose (11,12,23).
On the other hand, multiple variables including intracranial lesion size and location may necessitate the use of upfront radiation therapy to prevent future symptoms. A retrospective study providing lesion-level analyses in patients with EGFR-mutant NSCLC on osimertinib found a 12-month cumulative incidence of local recurrence in the brain of 0% in patients in patients with an initial complete response to osimertinib versus 4% and 11% in patients with partial response or stable disease, respectively (P=0.02) (24). Variables including uncontrolled primary tumor, increasing number of previous systemic therapies, and higher ECOG score were associated with CNS local recurrence, highlighting the importance of both patient and tumor factors in determining optimal intracranial management (24). Notably, number of intracranial lesions was not associated with local failure, which runs contrary to the results presented by Fong et al. in which more respondents recommended radiotherapy as the number of lesions increased. Clinicians may also favor SRS in the treatment of oligometastatic disease, where randomized trials have demonstrated improved survival with consolidative radiation therapy in combination with upfront systemic therapy (25,26).
Overall, given the evolving landscape of therapeutic treatment options and increasing complexity in treating nuanced cases of EGFR-mutant and ALK-rearranged NSCLC with brain metastases, management of these cases on an individual level warrants multidisciplinary input, as emphasized by Fong et al. On a broader level, while recent systemic therapy developments have drastically improved outcomes for patients with metastatic NSCLC, there are limited guidelines available outside of those from ASCO-SNO-ASTRO to aid physicians in the optimal sequencing of local therapies with these newer targeted agents (27). In addition, while we await results from ongoing randomized studies including the OUTRUN (NCT03497767) and LUOSICNS trials (NCT03796103), there is currently a lack of randomized published evidence to support the superiority of TKI therapy alone versus TKI plus radiation (28,29). These factors contribute to variability in treatment recommendations between and within specialties, and we therefore echo Fong et al. in calling for further research and guidelines that provide clinicians with a framework for managing brain metastases in patients with EGFR- or ALK-positive NSCLC.
Our institutional experience
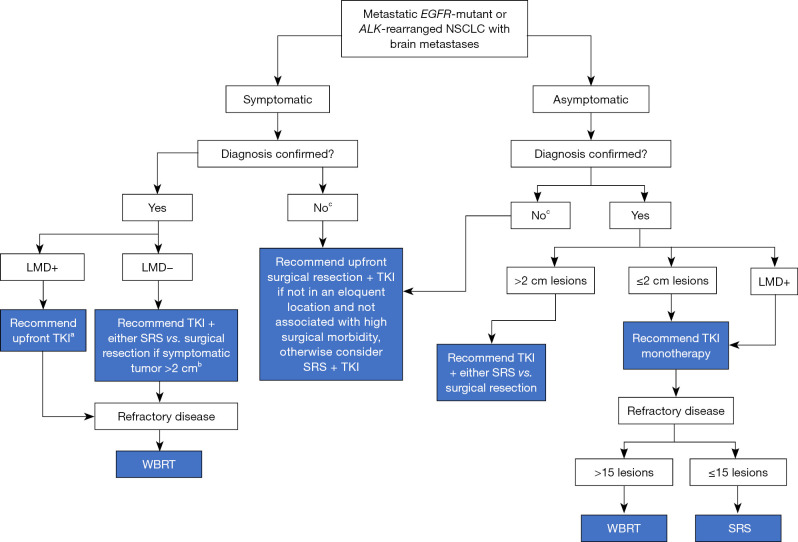
To provide additional perspective, we summarize here our institutional approach that is driven by the existing data, collective clinical experience, and collaboration between medical oncologists, radiation oncologists, and neurosurgeons (Figure 1). Clinical decision-making takes into account both multidisciplinary assessments and patient preferences.
Figure 1.
A flow diagram depicting our institutional approach to the management of brain metastases from EGFR-mutant or ALK-rearranged NSCLC. a, warrants informed discussion with the patient. Although WBRT classically used for symptomatic LMD, given the intracranial activity of newer-generation EGFR and ALK TKIs, we often allow the opportunity for response on TKI alone to avoid upfront toxicity of WBRT, depending on the degree of symptoms. b, in the case of multiple brain metastases, some of which are symptomatic and others of which are not, a hybrid approach is reasonable in which local therapy (radiation or surgery) is utilized for symptomatic lesions with monitoring of the remaining lesions for response on TKI. c, if diagnosis is unknown and other sites are not available for tissue sampling, may consider craniotomy for both diagnostic and therapeutic purposes. EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer; LMD, leptomeningeal disease; TKI, tyrosine kinase inhibitor; WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery.
For patients with a limited number of symptomatic brain metastases, upfront local brain-directed therapy is often employed, either with surgical resection or radiation therapy. Larger solitary lesions (>2 cm) and need for definitive tissue diagnosis are factors that tend to favor surgical resection over radiation therapy. On the other hand, lesions that involve eloquent brain regions or are challenging to resect without a high risk of morbidity favor radiation. Given the toxicity associated with WBRT and the intracranial activity of CNS-penetrant EGFR and ALK targeted therapies, we favor SRS in almost all cases and usually reserve WBRT for parenchymal or leptomeningeal disease that is refractory to TKI therapy and/or SRS. In the case of patients who have numerous parenchymal metastases with some causing symptoms, we often utilize a hybrid approach with SRS to symptomatic or high-risk lesions and observation of the remaining asymptomatic lesions on TKI therapy. If treatment of all sites of disease is preferred because of symptoms or concern for lack of disease control with systemic therapy alone, there is data supporting SRS over WBRT for the treatment of up to 15 lesions (30).
For patients with asymptomatic disease, the number and size of the lesions is important to guide decision-making. Larger lesions may be more likely to experience local failure or cause future symptoms, and thus, we are more likely to treat these lesions with upfront radiation therapy or surgical resection. This is consistent with the results from the Fong et al. who noted that a higher proportion of respondents from all specialties recommended upfront surgical intervention for tumors >2 cm in size. For patients with multiple lesions that are small, asymptomatic, and not at high risk for local failure, we often observe on TKI therapy and defer radiation until there is evidence of TKI failure. Interestingly, the results from this article showed that when the number of brain metastases increased to ≥4, the proportion of clinicians recommending radiation therapy increased. However, as mentioned above, radiation techniques were not specified (SRS vs. WBRT) in each scenario (1). With increasing number of lesions, we may be more inclined to recommend TKI monotherapy due to our institutional preference to avoid WBRT.
Conclusions
Although it is challenging to evaluate the nuances on a case-by-case basis, Fong et al. were able to provide four different clinical scenarios that replicated a variety of real-world scenarios in the treatment of brain metastases from EGFR-mutant and ALK-rearranged NSCLC. Despite these scenarios not representing all possible patient presentations, the authors were able to show consistently that recommendations for the management of asymptomatic brain metastases vary significantly according to oncology specialties. Their work highlights the importance of both multidisciplinary guidelines and further research to optimize and standardize treatment approaches for brain metastases in patients with EGFR-mutant or ALK-rearranged NSCLC. Understanding institutional practices that are guided by the available literature can be another useful tool in the meantime to bridge gaps between specialties and the oncology community at-large.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1483/coif). E.L.P. received an honorarium from Varian Clinical School for her role of speaker/GI Course Instructor; E.L.P. received an honorarium from Vysioneer for her role of Advisory Board Member; and E.L.P. received an honorarium from GT Medical Technologies for her role of Advisory Board Member. The other authors have no conflicts of interest to declare.
References
- 1.Fong CH, Meti N, Kruser T, et al. Recommended first-line management of asymptomatic brain metastases from EGFR mutant and ALK positive non-small cell lung cancer varies significantly according to specialty: an international survey of clinical practice. J Thorac Dis 2023;15:4367-78. 10.21037/jtd-22-697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. 10.1097/JTO.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 3.Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015;16:e510-21. 10.1016/S1470-2045(15)00013-3 [DOI] [PubMed] [Google Scholar]
- 4.Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45 Suppl 2:S253-7. 10.1016/j.lungcan.2004.07.967 [DOI] [PubMed] [Google Scholar]
- 5.Walbert T, Gilbert MR. The role of chemotherapy in the treatment of patients with brain metastases from solid tumors. Int J Clin Oncol 2009;14:299-306. 10.1007/s10147-009-0916-1 [DOI] [PubMed] [Google Scholar]
- 6.Li J. Stereotactic Radiosurgery Versus Whole-brain Radiation Therapy For Patients With 4-15 Brain Metastases: A Phase III Randomized Controlled Trial ASTRO; 2020 [cited 2023 Sep 14]. Available online: https://www.astro.org/ASTRO/media/ASTRO/News%20and%20Publications/Press%20Kits/PDFs/2020/AM20_PressSlidesLi41.pdf
- 7.Langston J, Patil T, Ross Camidge D, et al. CNS Downstaging: An Emerging Treatment Paradigm for Extensive Brain Metastases in Oncogene-Addicted Lung Cancer. Lung Cancer 2023;178:103-7. 10.1016/j.lungcan.2023.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 11.Lehrer EJ, Peterson JL, Zaorsky NG, et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys 2019;103:618-30. 10.1016/j.ijrobp.2018.10.038 [DOI] [PubMed] [Google Scholar]
- 12.Minniti G, Scaringi C, Paolini S, et al. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int J Radiat Oncol Biol Phys 2016;95:1142-8. 10.1016/j.ijrobp.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 2018;29:687-93. 10.1093/annonc/mdx820 [DOI] [PubMed] [Google Scholar]
- 14.Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. 10.1200/JCO.2018.77.9363 [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi H, Wakuda K, Fukuda M, et al. A Phase II Study of Osimertinib for Radiotherapy-Naive Central Nervous System Metastasis From NSCLC: Results for the T790M Cohort of the OCEAN Study (LOGIK1603/WJOG9116L). J Thorac Oncol 2021;16:2121-32. 10.1016/j.jtho.2021.07.026 [DOI] [PubMed] [Google Scholar]
- 16.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018. [Epub ahead of print]. doi: . 10.1200/JCO.2018.78.3118 [DOI] [PubMed] [Google Scholar]
- 17.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. 10.1056/NEJMoa2027187 [DOI] [PubMed] [Google Scholar]
- 19.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- 20.Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. 10.1093/annonc/mdy405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. 10.1200/JCO.2016.69.7144 [DOI] [PubMed] [Google Scholar]
- 22.Thomas NJ, Myall NJ, Sun F, et al. Brain Metastases in EGFR- and ALK-Positive NSCLC: Outcomes of Central Nervous System-Penetrant Tyrosine Kinase Inhibitors Alone Versus in Combination With Radiation. J Thorac Oncol 2022;17:116-29. 10.1016/j.jtho.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 23.Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011;6:48. 10.1186/1748-717X-6-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui C, Qu V, Wang JY, et al. Local control of brain metastases with osimertinib alone in patients with EGFR-mutant non-small cell lung cancer. J Neurooncol 2022;160:233-40. 10.1007/s11060-022-04145-x [DOI] [PubMed] [Google Scholar]
- 25.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XS, Bai YF, Verma V, et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer. J Natl Cancer Inst 2023;115:742-8. 10.1093/jnci/djac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol 2023;40:492-516. 10.1200/JCO.21.02314 [DOI] [PubMed] [Google Scholar]
- 28.Trans Tasman Radiation Oncology Group. A Randomised Phase II Trial of Osimertinib With or Without Stereotactic Radiosurgery for EGFR Mutated Non-Small Cell Lung Cancer (NSCLC) With Brain Metastases clinicaltrials.gov; 2022 Nov [cited 2023 Sep 15]. Report No.: NCT03497767. Available online: https://clinicaltrials.gov/study/NCT03497767
- 29.British Columbia Cancer Agency. Open Label, Multicenter, Phase II Study of Patients With Treatment Naïve Metastatic Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer (NSCLC) With Brain Metastases Randomized to Stereotactic Radiosurgery (SRS) and Osimertinib or Osimertinib Alone clinicaltrials.gov; 2021 Feb [cited 2023 Sep 15]. Report No.: NCT03769103. Available online: https://clinicaltrials.gov/study/NCT03769103
- 30.Hughes RT, Masters AH, McTyre ER, et al. Initial SRS for Patients With 5 to 15 Brain Metastases: Results of a Multi-Institutional Experience. Int J Radiat Oncol Biol Phys 2019;104:1091-8. 10.1016/j.ijrobp.2019.03.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as