Introduction
Approximately 20% of the U.S. population has a sexually transmitted infection (STI) at any given time. Incidence rates of trichomoniasis, chlamydia, syphilis, and genital herpes have continually increased each year between 2010-2019.1 Trichomoniasis, caused by the parasitic pathogen Trichomonas vaginalis, is the most common nonviral STI.2 Unlike other common STIs such as chlamydia and gonorrhea, T. vaginalis is not a nationally reportable disease in the U.S.3, thus epidemiological data related to T. vaginalis are from population and clinic-based studies. According to recent estimates, over 1 million people in the U.S. are infected with T. vaginalis each year.4 Many individuals infected with T. vaginalis remain asymptomatic, facilitating its transmission.5 Additionally, almost 75% of male sexual partners of infected women can also be infected, demonstrating a high transmission rate.6 T. vaginalis is associated with multiple adverse sexual and reproductive health outcomes in both women and men including increased rates of adverse birth outcomes (low birth weight, preterm birth, pre-labor rupture of membranes), as well as increased risk of acquisition of HIV and other STIs, pelvic inflammatory disease (PID), infertility, and cervical cancer.7–15 The most common clinical presentation among those with symptoms is vaginitis in women and urethritis in men. Although T. vaginalis is a common STI and can have detrimental effects, there are currently no routine screening recommendations for any population except women living with HIV.16 Additionally, traditional diagnostic methods have low sensitivity, although multiple recent advances have been made in this area including the advent of highly sensitive and specific nucleic acid amplification tests (NAATs), including several that can be done on-demand or as point-of-care (POC) tests.17–24 This review will provide an update on the epidemiology, pathogenesis, and clinical significance of trichomoniasis, as well as discuss current approaches to diagnosis, and treatment of this common STI.
Epidemiology
Based on data from the World Health Organization (WHO), the most recent prevalence estimates of trichomoniasis in women and men were 5.3% and 0.6%, respectively, with an estimated incidence of 156 million cases worldwide.2 Since trichomoniasis is not a nationally reportable STI in the U.S., the most accurate national prevalence data in women and men ages 18-59 years comes from the 2013-2014 cycle of the National Health and Nutrition Examination survey (NHANES), published in 2018.25 T. vaginalis prevalence in this NHANES cohort was 1.8% among women and 0.5% among men, all of whom were screened using the Hologic Aptima® T. vaginalis NAAT test on urine specimens.25 In the NHANES cohort, T. vaginalis infection was associated with older age, lower educational level, lower socioeconomic status, and having multiple sexual partners.25 Regarding older age and T. vaginalis infection, a recent systemic review of the literature also supports this association. Lindrose et al investigated the prevalence and incidence of trichomoniasis among U.S. adults ≥45 years. They found that the prevalence of T. vaginalis in this age group ranged from 0.2% to 21.4% among participants in the 20 articles included in their review, with several studies finding increased risk among older versus younger age groups.26 The highest prevalence of T. vaginalis was seen among individuals seeking diagnostic testing for STIs. These data highlight the need for sexual health education and consideration of screening for T. vaginalis among older adults.
T. vaginalis also disproportionately affects Black individuals. The prevalence of T. vaginalis among Black women and men in the U.S. NHANES study was 6.8% compared to 0.4% among other racial and ethnic groups.25 A recent review of T. vaginalis in African Americans found compelling evidence that structural racism has generated and maintained the significant racial disparity regarding this STI among the Black community, arguing that current efforts to reduce its prevalence have failed globally, especially in the U.S. This is compounded by a failure of strategies to control this infection including a lack of public awareness, non-compliance with prophylactic use of protective barrier methods, inconsistent STI testing, lack of a prophylactic T. vaginalis vaccine, and lack of mandatory national and global surveillance programs.27 The authors concluded that critical strategies must be incorporated to reduce the negative burden of T. vaginalis infection on the Black community, including cultural competence training among healthcare providers, social workers, and sexual health educators, as well as access to high-quality clinical services that serve minority communities, particularly focusing on Black women. Overall, multiple demographic and socioeconomic factors such as age, race, educational level, and income play a significant role in the epidemiology of trichomoniasis in the U.S. and globally.27
Since it has traditionally been viewed as a benign infection in men, few studies have explored the epidemiology of trichomoniasis among this population.28 As already noted, the prevalence of trichomoniasis is notably lower among men compared to women. In theory, however, prevalence should be similar between these two groups given that trichomoniasis is a highly transmissible STI. One explanation for this discrepancy is that spontaneous resolution of T. vaginalis infection may occur in some men, although the parasite and host factors influencing this phenomenon are not well understood.29, 30 The exact frequency at which this occurs is also not clear, but several small studies have reported spontaneous resolution to occur in 36%-69% of cases of T. vaginalis infection in men.29–31
Similar to women, a significant racial disparity regarding T. vaginalis infection also exists among men, with the U.S. NHANES data finding Black men to be 7 times more likely to be infected with T. vaginalis that white men.32 This study also found higher rates of infection among men who smoked, had HSV-2 infection, and reported high numbers of lifetime sexual partners.32
To date, the majority of T. vaginalis research has focused on cisgender women. There is a need for further epidemiologic studies exploring the impact of trichomoniasis on gender diverse populations. A recent systematic review summarized HIV and STI prevalence among transgender individuals and found that there were no studies reporting the prevalence of trichomoniasis in this population.33 Given the varied sexual identities and practices as well as diverse genital anatomy that is represented in this population, this is a clear gap in the literature which should be investigated further.
Pathogenesis
Humans are the only known hosts for T. vaginalis. The parasite is predominately spread through sexual contact from an infected person to an uninfected person. T. vaginalis has a trophozoite stage where it is actively grows and feeds in preparation for replication.34 It subsequently undergoes replication through longitudinal binary fission in the lower genital tract of women (vaginal, urethra, and endocervix) and the urethra and prostate of men. The average incubation period is between 5 and 28 days, however, infection can persist over longer periods of time.35 T. vaginalis does not exist in a cyst form and does not survive well in the environment, although it has been identified outside the human body in warm and wet locations (i.e., moist towels) for >3 hours.36
T. vaginalis infects squamous epithelial cells of the human genital tract through membrane surface glycolipids and glycoproteins which attach to surface proteins on host genital epithelial cells.37, 38 This attachment, in combination with the release of proteases that contribute to cytoadherence, mucus membrane degradation, and cytotoxicity, elicits a host immune response.39 This host immune response includes increased levels of cytokines including interleukin (IL)-1b, IL-6, IL-8, IL-17, IL-22, IL-23, regulated and normal T cell expressed and secreted protein (RANTES), C–C motif chemokine ligand 2 (CCL2), interferon (IFN)β, macrophage inflammatory protein (MIP)-3α, and tumor necrosis factor (TNF).37, 38, 40 The same proteases used for cytoadherence and break down of mucous membranes can also be used to aid in the evasion of host immune defenses.39
T. vaginalis virus (TVV, a double-stranded RNA [dsRNA] virus) and Mycoplasma hominis (bacterial pathogen) have also been known to infect T. vaginalis.41 There have been studies that have found associations between the presence of TVV in T. vaginalis and pro-/anti-inflammatory responses in the human host, as well as increased susceptibility of T. vaginalis to metronidazole (MTZ), one of the 5-nitroimidazoles commonly used to treat this infection.40 The presence of specific TVV subspecies (TVV1 and TVV2) has also been associated with severity of symptoms in one study.42 In contrast, in a study of 355 U.S. T. vaginalis isolates from women participating in a clinical trial, of which 40% were positive for TVV, there were no associations between TVV positivity and genital symptoms, repeat infections, or MTZ resistance, suggesting that TVV may be commensal to T. vaginalis.43 In contrast, the presence of M. hominis in T. vaginalis has been associated with increased MTZ resistance and decreased levels of genes involved in resistance mechanisms.44
Methods of transmission
Penile-vaginal sexual contact is the primary method in which T. vaginalis is transmitted, with partner studies in the 1950s and 1960s originally demonstrating this phenomenon.36, 45 Given its propensity to infect vaginal mucosal tissue, digital sexual activity involving the vagina, including mutual masturbation between women, can also transmit trichomoniasis.46 Nonsexual transmission is uncommon, but several modes have been described. One study in Zambia among adolescent girls ages 13 to 16 found that the overall prevalence of trichomoniasis among 397 self-reported virgins was 24.7%. In multivariate analyses in this study, borderline significant associations were found between trichomoniasis and the use of pit latrines or bushes instead of toilets as well as suboptimal bathing conditions including inconsistent soap use and shared bathing water.47 Another proposed mechanism of nonsexual transmission is through use of infected fomites such as shared sex toys and wet washcloths, the latter of which is supported by a study demonstrating transmission of trichomoniasis among one female-female sexual partnership using shared washcloths following receptive oral sex.48 Iatrogenic transmission of trichomoniasis has also been described, though this is very rare and typically occurs in low resource settings where adequate hand hygiene among practitioners is not always possible. For example, one case from the Gambia involved a traditional healer transmitting trichomoniasis to a female patient via an ungloved digital vaginal exam.49
Clinical Significance
Women
Despite its reputation as a nuisance infection, T. vaginalis can have a devastating impact on the sexual and reproductive health of women.50 T. vaginalis has been associated with multiple adverse health outcomes in women including adverse birth outcomes (discussed in the section on trichomoniasis and pregnancy)8, increased risk of HIV and other STI acquisition11–13, PID14, infertility15, and cervical cancer.9 PID can manifest in a variety of ways, including endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis. In recent years, there are mounting data implicating T. vaginalis as a potential pathogenic organism in PID,14, 51 especially among women co-infected with viral pathogens such as HIV and HSV-2.10, 52 If left untreated, PID can result in infertility, higher risk for ectopic pregnancy, and chronic pelvic pain.53, 54
A recent systematic review by Zhang et al summarized findings in support of a correlation between T. vaginalis infection and infertility.15 Proposed mechanisms for T. vaginalis induced infertility in women include host immune system activation leading to uterine and fallopian tube epithelial cell damage and inflammation,55, 56 as well as direct damage to oocytes and blockage of ovulation.55, 57
Women with T. vaginalis infection are also at an increased risk of cervical cancer, as was demonstrated by a 2018 meta-analysis including 7,715 cases and 67,598 controls from 17 studies finding a significant association between these two entities.9 The underlying pathophysiological explanation for this relationship is not well understood. This meta-analysis and other studies have proposed that this is due in part to increased co-infection rates with high-risk HPV types and T. vaginalis, as well as an increased risk of HIV/STI acquisition.9, 58–62
Men
Similar findings of increased risk of HIV/STI acquisition and infertility have been noted in T. vaginalis-infected men.11, 15, 63, 64 The proposed mechanism for infertility in men is impairment of sperm motility via attachment to sperm glycoproteins, which can induce phagocytosis56 and/or interfere with horizontal sperm movement63, as well as direct damage and destruction of sperm cells.55, 64 T. vaginalis infection has also been associated with prostate cancer, but the studies reporting this relationship are not conclusive.65–70 Some studies have proposed that T. vaginalis infection can induce prostate epithelial cell proliferation66 and chronic inflammation71, and one study found that one-third of benign prostate hyperplasia (BPH) patients had a positive polymerase chain reaction (PCR) test for T. vaginalis.67 Additionally, Sutcliffe et al observed that serologic evidence of previous T. vaginalis infection was associated with an increased risk of prostate cancer.71 However, others have observed no relationship between T. vaginalis and prostate cancer.68, 69 A more recent study by Yang et al explored the association between trichomoniasis and BPH, prostate cancer, and bladder cancer, and observed that trichomoniasis was associated with both BPH and prostate cancer.70 In a meta-analysis of 6 studies, it was shown that the risk of prostate cancer was 1.17 fold higher in men with previous T. vaginalis exposure however this was not statistically significant.68 Lack of adequate studies and/or heterogeneity concerns may have biased the authors’ deductions about the impact of T. vaginalis on the outcome in this meta-analysis. Thus, whether T. vaginalis infection is a cause of prostate cancer remains controversial and additional research on this topic is needed.
Extra-genital sequelae have also been rarely observed in men with trichomoniasis, such as rectal trichomoniasis and pharyngitis, but this is extremely uncommon and routine screening of these locations is not recommended.72, 73
Trichomoniasis and Pregnancy
Since there is an immense burden of trichomoniasis in women of childbearing potential2, the consequences of this STI in pregnant women should be taken into consideration. Unfortunately, this STI has been understudied in pregnant women, thus, epidemiologic data on incidence and prevalence estimates are limited. Recent WHO prevalence estimates on trichomoniasis do not report on the subset of pregnant women captured in the studies included in their systematic review.2 Prevalence estimates of the burden of T. vaginalis infection among pregnant women in the U.S. are also limited since T. vaginalis is not a reportable STI and data on pregnant women are not presented in the recent NHANES study.74 Thus, available data are taken mainly from observational studies. One systematic review from 2016, which included only low- to middle-income countries, estimated a wide T. vaginalis prevalence range from 3.9% in Latin America to 24.6% in Southern Africa among pregnant women, after adjusting for age, test, and healthcare setting.75 Among another study of 1,821 U.S. pregnant women living with HIV, T. vaginalis prevalence was estimated to be 14.5%, however 30.3% of the women in this cohort were not tested for T. vaginalis.76 Similarly, a 20% prevalence of T. vaginalis was noted in one South African cohort of pregnant women with HIV.77 The high prevalence of T. vaginalis in both of these cohorts of pregnant women living with HIV underscores the importance of screening guideline adherence in this population of women.
Untreated trichomoniasis in pregnancy is not without consequence. A 2021 systematic review and meta-analysis, which included >80,000 pregnant women and 19 studies worldwide, demonstrated significant associations between T. vaginalis and preterm delivery, pre-labor rupture of membranes, and delivery of low birth weight infants.8 One potential pathophysiological mechanism of preterm delivery and pre-labor rupture of membranes in women with T. vaginalis relates to the post-infection maternal innate immune inflammatory response prompting these outcomes via early cervical ripening and dilation.78 The mechanisms of low birth weight in the setting of T. vaginalis may be related to the infection inducing intrauterine inflammation that can impede placental blood flow.79 Direct causal links between infection with T. vaginalis and these adverse birth outcomes are difficult to ascertain though, as some studies included in this systematic review and meta-analysis did not include data on co-infection with BV and other STIs. Thus, it is unknown if co-infection confounds the effect of T. vaginalis on perinatal morbidity or if BV and/or other STIs could also be part of the causal pathway between trichomoniasis and adverse birth outcomes. Additional studies are needed to further clarify this important relationship.
Clinical Presentation
Asymptomatic infection with T. vaginalis is common, more so among men than women. In men, T. vaginalis parasite burden is much lower compared to women.28 Among symptomatic men, the most common presentation is urethritis, which includes dysuria and/or clear or mucopurulent urethral discharge.28 As previously mentioned, spontaneous resolution of infection without treatment has been described in men29, 30, but the exact frequency at which this occurs is unknown. If left untreated, however, some men can experience symptoms of prostatitis or epididymitis.28, 80, 81 There have also been occasional case reports of rectal T. vaginalis infection leading to proctitis among men who have sex with men (MSM)82, however, this is exceedingly rare and routine screening of this location is not recommended.16
Women with trichomoniasis are more frequently symptomatic than men. One U.S. study found that women presenting with vaginal symptoms had higher rates of T. vaginalis infection (26%) compared to asymptomatic women who were screened for this infection (6.5%).83 Most commonly, symptomatic women present with symptoms of vaginitis, including copious yellow-green frothy vaginal discharge, vaginal odor, and vulvovaginal irritation. Symptomatic women with T. vaginalis may also note a wide range of additional symptoms including genital pruritis, dysuria, and dyspareunia. On physical exam, the characteristic discharge of trichomoniasis may be noted in the vaginal vault and, if the cervix is adequately visualized, may have a strawberry-like appearance (“colpitis macularis”); however, this is present in <5% of women.84 As mentioned earlier, PID has been associated with trichomoniasis, so while it is not the most common presentation, T. vaginalis diagnostic testing should be performed in women presenting with PID.51
Diagnosis
Table 1 details current diagnostic testing options available for T. vaginalis. Traditionally, trichomoniasis has been diagnosed using POC methods, the most common of which is wet mount microscopy of vaginal secretions. After preparation of a wet mount slide, motile trichomonads can be visualized (Figures 1A and 1B). The specificity of this finding is 100% although the sensitivity is low at 44-68%.85 This test should also be performed within 10 to 20 minutes of specimen collection to avoid the trichomonads losing viability, which increases the likelihood of a false negative test. Additionally, the requirement of a microscope and an experienced microscopist pose a challenge for diagnosing trichomoniasis in low resource settings with this method. In recent years, other POC tests that do not require microscopy have become available. The OSOM® rapid test (Sekisui Diagnostics, California) uses antibodies to detect protein antigens of T. vaginalis in vaginal secretion specimens. A positive test demonstrates the binding of antibodies to T. vaginalis antigens and results in a blue line on the test strip. However, the OSOM® rapid test should mainly be used in symptomatic women or contacts to T. vaginalis. This test yields results in <10 minutes and has a sensitivity of 82-95% and specificity 97-100%, compared to wet mount and culture.85
Table 1.
Diagnostic tests for Trichomonas vaginalis
| Test | Population | Specimen type | Sensitivity (Sens) & Specificity (Spec) | Time to results | Complexity, Limitations, Other Comments |
|---|---|---|---|---|---|
| Wet mount microscopy85 | Women | Women: Vaginal secretions | Sens: 44-68% Spec: 100% |
< 10 min (POC) | CLIA waived. Must be performed immediately after specimen collection Requires microscope & training |
| OSOM® rapid test85 | Symptomatic women | Women: Vaginal Secretions | Sens: 82-95% Spec: 97-100% |
< 10 min (POC) | CLIA waived. Detects antigen. No instrumentation needed. |
| InPouch® T. vaginalis culture system88 | Men and women | Men: urethral specimens, urine sediment, and semenα Women: vaginal specimens |
Men: Sens: 50-80% Spec: 100% Women: Sens: 75-96% Spec: 100% |
5-7 days | Requires incubation at 37°C, microscope, and training. CLIA moderately complex test |
| Hologic Aptima NAAT20 | Women | Women: Endocervical, vaginal, urine, and pap smear specimens (preserved in PreservCyt Solutions) | Women: Sens: 88-100% Spec 98-100% |
5.5 hours | CLIA high complexity test. Requires Panther, Viper, or Tigris instrumentation. |
| Becton Dickinson ProbeTec Qx NAAT21 | Women | Women: Endocervical vaginal, and urine specimens | Sens: 98-100% Spec: 98-100% |
< 8 hrs | CLIA high complexity. Requires Viper system. Off market. |
| Becton Dickinson (BD) Max CT/GC/TV2 NAAT23 | Men and women | Men: urine Women: vaginal specimens and urine |
Men: Sens: 81.1%-100% Spec: 98.7-100% Women Sens: 89.8-99.7% Spec: 98.1-99.5% |
3.5 hours | CLIA high complexity test. Requires BD Max system. |
| Cepheid GeneXpert NAAT18 | Men and women | Men: urine Women: endocervical and vaginal specimens, urine |
Men: Sens: 97.2-99.9% Spec: 97.2-99.9% Women: Sens: 99.5-100% Spec: 99.4-99.9% |
On demand results in 40-63 minutes | CLIA moderately complex |
| Solana® Trichomonas assay89 | Women | Women: Vaginal and urine specimens | Sens/Spec: >98% for vaginal samples, > 92% for urine samples | ~40 min | Not CLIA waived. Requires some instrumentation. |
| AmpliVue® Trichomonas assay19 | Women | Women: Vaginal specimens | Sens: 90.7% Spec: 98.9% |
~45 min | Not CLIA waived. Requires some instrumentation. |
| Roche cobas MG/TV NAAT22 | Men and women | Men: penile meatal specimens and urine Women: endocervical and vaginal specimens |
Men: Sens: 77.2-100% Spec: 97.2-99.9% Women: Sens: 96.4-100% Spec: 96.5-98.8% |
5 hours | CLIA high complexity test. For use on Cobas 6800/8800 systems. |
| Visby GC/CT/TV NAAT24 | Women | Women: Vaginal specimens | Sens: 99.2% Spec: 96.9% |
< 30 min (POC) | CLIA waived. Requires electrical outlet. |
| Abbott Alinity m STI assay90 | Women | Women: Endocervical, vaginal, pap smear, and urine specimens | < 2 hrs | CLIA high complexity. |
Abbreviations: CLIA=Clinical Laboratory Improvement Amendments; POC=point-of-care; STI=sexually transmitted infection; NAAT=nucleic acid amplification test; MG=Mycoplasma genitalium; GC=Neisseria gonorrhoeae, CT=Chlamydia trachomatis; TV=Trichomonas vaginalis
Inoculate the T. vaginalis culture with multiple male specimens, if possible, to increase likelihood of a positive result.
Figure 1.
A. Wet mount microscopy showing a motile trichomonad with a flagellum.
A. Wet mount microscopy showing two motile trichomonads.
For many years, prior to the advent of highly sensitive and specific NAATs, T. vaginalis culture was the gold standard for diagnosis. The InPouch® T. vaginalis culture system (BioMed Diagnostics, White City, OR) is the most commonly used culture method and has a sensitivity of 44-81% and specificity of 100%.85, 86 While this method has better sensitivity than wet mount and is highly specific, there are several challenges. First, the culture media should be inoculated with the genital specimen(s) within 1 hour of collection.85 T. vaginalis culture can be performed on specimens from both women (vaginal swabs) and men (urethral swabs, urine sediment, semen; multiple specimens are recommended in men to increase yield).85 Second, an inoculated InPouch® culture system must be immediately incubated at 37°C and specimens must be read multiple time over several days, making this a Clinical Laboratory Improvement Amendments (CLIA) moderately complex test.87, 88 Culture is advantageous, however, as drug susceptibility testing to 5-nitroimidazole medications (Figure 2) can be performed on positive specimens. This is especially useful in cases of persistent/resistant T. vaginalis infection.
Figure 2. 5-nitroimidazole Drug Susceptibility Testing.
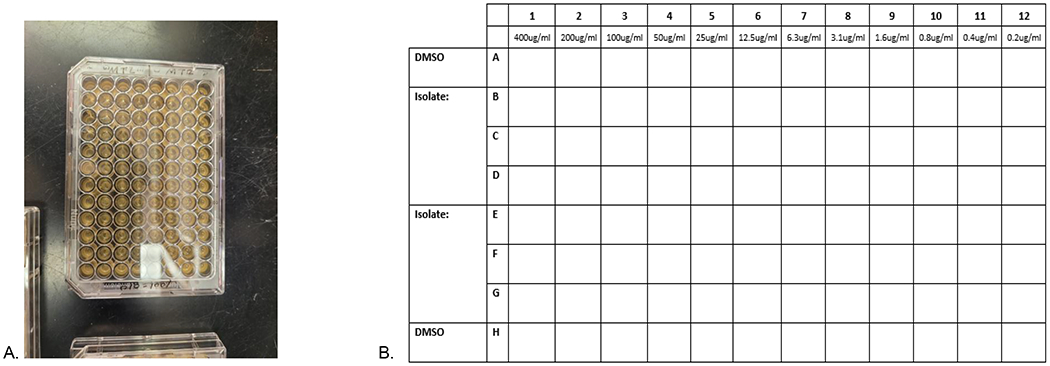
2A: 96-well plate used for 5-nitroimidazole drug susceptibility testing. 2B: Graphical representation of a portion of the 96 well plate for the 5-nitroimidazole drug susceptibility testing. Rows A and H are DMSO control lanes, while lanes B-G contain the 5-nitroimdazoles tested for specific T. vaginalis isolates tested in triplicates. 50 μl of media is initially added to each well. Next, 50 μl of drug/media is added to column 1 rows B-G, while 50 μl of DMSO/media are added to A and H and then serially diluted from left to right (column 1 [400 μg/ml] to column 12 [0.2 μg/ml]). 150 μl of the T. vaginalis sample is added to each well from the lowest concentration to the highest (column 12 [0.2 μg/ml] to column 1 [400 μg/ml]). The plates are then incubated for 48 to 52 hours before being read on an inverted microscope. MLC is determined when no motile TV is observed for all three wells with the same concentration.
A variety of molecular diagnostic tests have become available in the past decade for T. vaginalis diagnosis. The sensitivity of these molecular assays is far superior to wet mount microscopy and culture. Most recently, Visby has released an instrument-free molecular assay which can be used at the POC, and yields results in 25 minutes.24 This test can be performed on self-collected vaginal specimens and utilizes a one-time use handheld cartridge.
Two molecular amplified assays are also available for additional POC T. vaginalis diagnostics: the Solana® Trichomonas assay (Quidel, San Diego, CA)89 and AmpliVue® Trichomonas assay (Quidel, San Diego, CA).19 The Solana® Trichomonas assay qualitatively detects T. vaginalis with results on demand in <40 minutes. It is FDA-approved for use on female vaginal and urine specimens from asymptomatic and symptomatic women. This assay requires a testing instrument to run samples, therefore, an upfront investment is required, which could impact its cost-effectiveness in some settings. The AmpliVue® Trichomonas assay works in a similar way to the Solana® assay but can be performed using a small handheld cartridge instead of a testing platform. AmpliVue® results are available within 45 to 50 minutes. Both assays are highly sensitive and specific. Finally, the Cepheid GeneXpert T. vaginalis NAAT assay can provide on-demand results in 40-63 minutes and can be performed on a small device that can fit on a benchtop in a clinic.18
In addition to POC and on-demand molecular diagnostic tests for T. vaginalis, there are several additional NAAT test that require larger instruments for specimen analysis, thus, they are not able to be used at the POC. These include the Hologic Aptima T. vaginalis NAAT assay, the Becton Dickinson [BD] ProbeTec Qx T. vaginalis NAAT assay, the BD Max CT/GC/TV2 NAAT assay, the Roche cobas MG/TV NAAT assay, and the Abbott Alinity m STI assay (includes T. vaginalis NAAT testing).18, 20–23, 90
Treatment
5-nitroimidazole medications are the mainstay of treatment for trichomoniasis as they are the only class of antimicrobials with demonstrated in vitro anti-trichomonacidal activity.91 This drug class includes medications such as MTZ, tinidazole (TDZ), and secnidazole (SEC). Per the 2021 Centers for Disease Control and Prevention (CDC) STI treatment guidelines, the recommended treatment of uncomplicated urogenital trichomoniasis for both men and women is MTZ, however, for the first time, dosing and duration of treatment differ between genders.16 For women, a 7-day course of oral MTZ 500mg twice daily is now the recommended treatment regimen as clinical trial data suggest that the single-dose oral 2-g MTZ treatment is suboptimal. This was shown in a multicenter randomized controlled trial (RCT) among women in the U.S. without HIV infection which directly compared the multidose oral MTZ regimen to the single dose oral MTZ regimen. Those who received the multidose oral MTZ regimen were significantly less likely to retest positive for T. vaginalis at the one month test-of-cure (TOC) visit (11%) than women who received the single dose therapy (19%); p<0.0001.92 The multi-dose oral MTZ regimen was also shown to be more highly efficacious among women living with HIV in an earlier RCT;93, 94 treatment of trichomoniasis among women with HIV has also been shown to reduce vaginal HIV-1 shedding.7
For men with T. vaginalis, the recommended treatment remains the single-dose oral 2-g MTZ. This is due to lack of clinical trial data directly comparing oral single-dose MTZ and oral multi-dose MTZ regimens in men.
A single oral dose of 2-g TDZ is an alternative regimen for the treatment of uncomplicated trichomoniasis in women and men in the 2021 CDC STI Treatment Guidelines.16 Since the publication of those guidelines, new data have been published regarding a single dose of oral 2-g SEC being an additional option for treating trichomoniasis in women. SEC is a second generation 5-nitroimidazole with a longer half-life (17-19 hours) than MTZ (7-8 hours) and TDZ (11-12 hours).95 SEC is available in a granular formulation that must be administered to patients with a serving of either unsweetened apple sauce, pudding, or yogurt. A 2021 RCT including 147 women with trichomoniasis at 10 clinical sites across the U.S. demonstrated a microbiologic cure rate of 92.2% among women at the 6-12 day test-of-cure (TOC) receiving the single oral 2-g dose of SEC when compared to women receiving placebo (1.5%); p<0.001.96 SEC has since been FDA approved for T. vaginalis treatment in adolescent and adult women and men ages ≥ 12 years. While not in the current CDC or ACOG guidelines, these data support SEC as an alternative treatment for trichomoniasis in both women (and men).96
Current recommendations for the treatment of trichomoniasis in pregnant women are the same as those for non-pregnant women (i.e., multi-dose oral MTZ 500 mg twice daily for seven days is the recommended treatment).16 The mainstay of T. vaginalis treatment is MTZ, which crosses the placenta. Despite this fact, there are ample data to suggest that this medication poses minimal risk to the fetus in all trimesters of pregnancy and has no known teratogenic effects.97, 98 The 2021 CDC STI Treatment Guidelines recommend testing and treatment of all symptomatic pregnant women for T. vaginalis, in addition to counseling on partner treatment and condom use.16 Data are limited regarding the use of TDZ in pregnancy, although animal data have demonstrated potential risks to a fetus.99 Therefore, TDZ should be avoided for the treatment of T. vaginalis in pregnant women.16, 99 Limited data are available on the use of SEC in pregnant women however there is no evidence of adverse developmental outcomes in animal studies.95
For men, there have been no rigorous clinical trials directly comparing the efficacy of the multidose and single dose oral MTZ regimens. Thus, the single-dose oral 2-g MTZ remains the recommended treatment regimen for T. vaginalis in men. There are data, however, to suggest that this single dose regimen may be inadequate in men. One study found that treatment with the single dose 2-g MTZ regimen in men resulted in only 77.1% microbiological efficacy.100 Similar to the recommendations for women, a single-dose of oral 2-g TDZ is recommended as an alternative regimen.16 TDZ has also been inadequately studied in men, but does have better absorption in the prostate and other male genital tissues compared to MTZ.101, 102 The few studies that have been conducted in men have demonstrated that TDZ may have similar efficacy to MTZ in the treatment of T. vaginalis, but these were small studies and more data are needed.103, 104 Similarly, there are few studies investigating the efficacy of SEC for the treatment of T. vaginalis in men, though these studies suggest that SEC may also be a good alternative to oral MTZ.105-107 An RCT is needed to determine the optimal treatment regimen for men with trichomoniasis.
Persistent infection with trichomoniasis is not uncommon, especially given the proclivity of the parasite to reinfect a person if their partner was not treated. Thus, when treating patients for trichomoniasis, counseling on partner treatment and consistent condom use at the completion of therapy is essential. Once reinfection has been excluded in the setting of treatment failure, consideration should be given to antimicrobial resistance against 5-nitroimidazoles. Collection of a T. vaginalis culture and sending the specimen for 5-nitroimidazole resistance testing can be a helpful in managing these difficult cases (Figure 2). Resistance testing is currently available through the CDC and can be requested at the following website: https://www.cdc.gov/laboratory/specimen-submission/detail.html?CDCTestCode=CDC-1023916 as well as some local laboratories in the US, including the University of Alabama at Birmingham. While awaiting susceptibility testing results, clinicians can consider longer courses of treatment with higher doses than standard therapies with MTZ or TDZ.16 While the optimal regimen for treating resistant or persistent trichomoniasis infection has not be established, regimens such as MTZ or TDZ 2-g orally daily for 7 days have been used successfully in some cases. In the case that the 7 day MTZ or TDZ 2-g oral regimen fails, combining longer courses of high dose oral TDZ 2-3 g daily in divided doses with intravaginal medications has also had successful results.108, 109 Two such regimens include (1) oral TDZ 1-g three times daily plus vaginal paromomycin (4 g of 6.25% vaginal cream nightly, both for 14 days or (2) oral TDZ 2-3 g daily in divided doses plus vaginal TDZ 500 mg twice daily for 14 days.110, 111 It is important to note that both vaginal paromomycin and vaginal TDZ must be formulated at a compounding pharmacy. Topical use of vaginal paromomycin cream can result in painful vulvar ulcers that are self-limited and resolve once treatment is discontinued. Use of lubricating jelly to the vulva before use has been successful in preventing the development of these ulcers in some women.112 Clinical data are limited regarding the efficacy in SEC as a treatment option for persistent or resistant trichomoniasis, but several studies suggest that SEC has better in vitro trichomonacidal activity compared to MTZ.91, 113 Thus, further clinical studies including SEC should be performed in this subset of patients.
Another difficult treatment scenario is trichomoniasis in the setting of 5-nitroimidazole hypersensitivity. While MTZ hypersensitivity prevalence is reported to be approximately 0.15%114, patients often report a variety of intolerances (i.e., nausea, vomiting) as “allergies” to providers. Therefore, obtaining a detailed allergy history is an essential first step in managing these patients in order to identify whether or not their prior history is truly consistent with a type 1, IgE-mediated hypersensitivity reaction.115 If a true hypersensitivity reaction is deemed to be present, consultation with an allergist is recommended so that desensitization can be considered.115 There are limited treatment options outside of the 5-nitroimidazole class for the treatment of trichomoniasis. For T. vaginalis-infected patients with serious allergies who are not candidates for desensitization, some successful treatment regimens containing drugs outside of the 5-nitroimidazole drug class have been reported. These include vaginal boric acid 600mg twice daily for at least 60 days116-118 and vaginal paromomycin 4-g of 6.25% vaginal cream nightly for 14 days.111, 112, 119, 120 Like intravaginal paromomycin and TDZ, vaginal preparations of boric acid must be compounded.
After treatment, women who are sexually active should be retested for T. vaginalis between 3 weeks and 3 months due to a high reinfection rate. There are currently insufficient data to support retesting men after treatment.58, 121 If re-testing by 3 months is not possible, women should be re-tested whenever they next seek medical care <12 months after treatment.16
Partner Management
Given high rates of asymptomatic infection as well as recurrence among men and women122, 123, appropriate management of sexual partners is essential in preventing transmission beyond the infected individual. It is currently recommended that all sexual partners of a person infected with T. vaginalis (both within the past 60 days and the last sexual partner, even if this was greater than 60 days) be treated presumptively and that the infected individual avoid any further sexual activities until treatment has been completed and they are asymptomatic.16 While not permitted in all states, expedited partner therapy (EPT) could be an important tool in partner management for T. vaginalis. There have been multiple studies investigating EPT for trichomoniasis. One RCT demonstrated that partner treatment with single dose oral 2-g TDZ resulted in a >4 fold reduction in repeat infections among T. vaginalis-infected index women 124. Two other studies using single dose 2-gram oral MTZ for male partners of T. vaginalis-infected women found either no effect of EPT 125 or a borderline effect 126. While it is possible that the two studies using oral MTZ were either underpowered or did not use a correct control arm, it is also possible that oral TDZ (or SEC) could be a better treatment for men. Thus, further studies in this area are needed.
Conclusion
Trichomoniasis has long been a neglected STI given the erroneous perception that infection is inconsequential. However, in recent years, mounting data suggest that this STI has the potential to yield significant adverse sexual and reproductive health outcomes for both women and men. Recent advances in diagnosis and treatment of T. vaginalis are promising in quelling the high rates of infection, but more public health attention is needed on this neglected STI to make a significant impact.
Key Points.
Trichomonas vaginalis is a prevalent, yet understudied, sexually transmitted infection that is associated with significant adverse sexual and reproductive health outcomes for both men and women.
T. vaginalis is most prevalent among women and Black individuals, making it clear that societal-level health disparities impact the epidemiology of this STI.
Options for T. vaginalis diagnostics have grown in the last decade, with multiple point-of-care and highly sensitive and specific nucleic acid amplification tests now available.
Treatment options for trichomoniasis are generally limited to 5-nitroimidazoles, including metronidazole, tinidazole, and secnidazole
Further studies are needed to determine the optimal treatment regimen for this STI among men.
Synopsis:
Trichomoniasis is the most common nonviral sexually transmitted infection worldwide. It has been associated with a variety of adverse sexual and reproductive health outcomes for both men and women. In this review, we discuss updates in its epidemiology, pathophysiology, clinical significance, diagnosis, and treatment.
Funding:
This work was funded in part by the UAB Centers for Clinical and Translational Sciences TL1 grant (5TL1TR003106-04/Ruth L. Kirschstein National Research Service Award to Author SAO) as well as the UAB Department of Medicine (2022 Frommeyer Fellowship in Investigative Medicine to Author OTVG).
Disclosure statement:
OTVG receives grant funding from the National Institutes of Health, Gilead Sciences, Inc, and Abbott Molecular and has also served on scientific advisory boards for Scynexis; Christina A. Muzny, MD, MSPH has received research grant support from NIH/NIAID, Lupin Pharmaceuticals, Abbott Molecular, and Gilead Sciences, Inc.; is a consultant for BioNTech, Scynexis, and Cepheid, and has received honoraria from Visby, Elsevier, Abbott Molecular, Cepheid, Roche Diagnostics, and Lupin Pharmaceuticals. All other authors have no pertinent disclosures.
References
- 1.Du M, Yan W, Jing W, et al. Increasing incidence rates of sexually transmitted infections from 2010 to 2019: an analysis of temporal trends by geographical regions and age groups from the 2019 Global Burden of Disease Study. BMC Infect Dis. Jun 26 2022;22(1):574. doi: 10.1186/s12879-022-07544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. Aug 1 2019;97(8):548–562p. doi: 10.2471/blt.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoots BE, Peterman TA, Torrone EA, Weinstock H, Meites E, Bolan GA. A Trich-y question: should Trichomonas vaginalis infection be reportable? Sex Transm Dis. Feb 2013;40(2):113–6. doi: 10.1097/OLQ.0b013e31827c08c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sexually Transmitted Diseases. 2021;48(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin Infect Dis. Nov 15 2007;45(10):1319–26. doi: 10.1086/522532 [DOI] [PubMed] [Google Scholar]
- 6.Seña AC, Miller WC, Hobbs MM, et al. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin Infect Dis. Jan 1 2007;44(1):13–22. doi: 10.1086/511144 [DOI] [PubMed] [Google Scholar]
- 7.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. Jan 2009;36(1):11–6. doi: 10.1097/OLQ.0b013e318186decf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gerwen OT, Craig-Kuhn MC, Jones AT, et al. Trichomoniasis and adverse birth outcomes: a systematic review and meta-analysis. Bjog. Nov 2021;128(12):1907–1915. doi: 10.1111/1471-0528.16774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Zhao W, Wang H, Wang Y, Li J, Wu X. Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. Sep 2018;228:166–173. doi: 10.1016/j.ejogrb.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 10.Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. Dec 2006;33(12):747–52. doi: 10.1097/01.olq.0000218869.52753.c7 [DOI] [PubMed] [Google Scholar]
- 11.Masha SC, Cools P, Sanders EJ, Vaneechoutte M, Crucitti T. Trichomonas vaginalis and HIV infection acquisition: a systematic review and meta-analysis. Sex Transm Infect. Feb 2019;95(1):36–42. doi: 10.1136/sextrans-2018-053713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker EK, Malekinejad M, Merai R, et al. Risk of Human Immunodeficiency Virus Acquisition Among High-Risk Heterosexuals With Nonviral Sexually Transmitted Infections: A Systematic Review and Meta-Analysis. Sex Transm Dis. Jun 1 2022;49(6):383–397. doi: 10.1097/olq.0000000000001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. Aug 2012;50(8):2601–8. doi: 10.1128/jcm.00748-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiringa AE, Ness RB, Darville T, Beigi RH, Haggerty CL. Trichomonas vaginalis, endometritis and sequelae among women with clinically suspected pelvic inflammatory disease. Sexually Transmitted Infections. 2020;96(6):436–438. doi: 10.1136/sextrans-2019-054079 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Li Y, Lu H, et al. A systematic review of the correlation between Trichomonas vaginalis infection and infertility. Acta Trop. Dec 2022;236:106693. doi: 10.1016/j.actatropica.2022.106693 [DOI] [PubMed] [Google Scholar]
- 16.Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. Jul 23 2021;70(4):1–187. doi: 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaydos CA, Manabe YC, Melendez JH. A Narrative Review of Where We Are With Point-Of-Care STI Testing in the United States. Sex Transm Dis. May 14 2021;doi: 10.1097/olq.0000000000001457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwebke JR, Gaydos CA, Davis T, et al. Clinical Evaluation of the Cepheid Xpert TV Assay for Detection of Trichomonas vaginalis with Prospectively Collected Specimens from Men and Women. J Clin Microbiol. Feb 2018;56(2)doi: 10.1128/jcm.01091-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaydos CA, Hobbs M, Marrazzo J, et al. Rapid Diagnosis of Trichomonas vaginalis by Testing Vaginal Swabs in an Isothermal Helicase-Dependent AmpliVue Assay. Sex Transm Dis. Jun 2016;43(6):369–73. doi: 10.1097/olq.0000000000000447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol. Dec 2011;49(12):4106–11. doi: 10.1128/jcm.01291-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper system. J Clin Microbiol. Mar 2014;52(3):885–9. doi: 10.1128/jcm.02966-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Der Pol B A profile of the cobas® TV/ MG test for the detection of Trichomonas vaginalis and Mycoplasma genitalium. Expert Review of Molecular Diagnostics. 2020/April/02 2020;20(4):381–386. doi: 10.1080/14737159.2020.1714440 [DOI] [PubMed] [Google Scholar]
- 23.Van Der Pol B, Torres-Chavolla E, Kodsi S, et al. Clinical Performance of the BD CTGCTV2 Assay for the BD MAX System for Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis Infections. Sex Transm Dis. Feb 1 2021;48(2):134–140. doi: 10.1097/OLQ.0000000000001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris SR, Bristow CC, Wierzbicki MR, et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. May 2021;21(5):668–676. doi: 10.1016/s1473-3099(20)30734-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR. Prevalence and Correlates of Trichomonas vaginalis Infection Among Men and Women in the United States. Clin Infect Dis. Jul 2 2018;67(2):211–217. doi: 10.1093/cid/ciy079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindrose AR, Htet KZ, O’Connell S, Marsh J, Kissinger PJ. Burden of trichomoniasis among older adults in the United States: a systematic review. Sexual health. Jun 2022;19(3):151–156. doi: 10.1071/sh22009 [DOI] [PubMed] [Google Scholar]
- 27.Bassey GB, Clarke AIL, Elhelu OK, Lee CM. Trichomoniasis, a new look at a common but neglected STI in African descendance population in the United States and the Black Diaspora. A review of its incidence, research prioritization, and the resulting health disparities. J Natl Med Assoc. Feb 2022;114(1):78–89. doi: 10.1016/j.jnma.2021.12.007 [DOI] [PubMed] [Google Scholar]
- 28.Van Gerwen OT, Camino AF, Sharma J, Kissinger PJ, Muzny CA. Epidemiology, Natural History, Diagnosis, and Treatment of Trichomonas vaginalis in Men. Clin Infect Dis. Sep 15 2021;73(6):1119–1124. doi: 10.1093/cid/ciab514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. Jan 15 2011;52(2):163–70. doi: 10.1093/cid/ciq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weston TE, Nicol CS. NATURAL HISTORY OF TRICHOMONAL INFECTION IN MALES. Br J Vener Dis. Dec 1963;39(4):251–7. doi: 10.1136/sti.39.4.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger JN, Verdon M, Siegel N, Holmes KK. Natural history of urogenital trichomoniasis in men. J Urol. Jun 1993;149(6):1455–8. doi: 10.1016/s0022-5347(17)36414-5 [DOI] [PubMed] [Google Scholar]
- 32.Daugherty M, Glynn K, Byler T. Prevalence of Trichomonas vaginalis Infection Among US Males, 2013-2016. Clinical Infectious Diseases. 2018;68(3):460–465. doi: 10.1093/cid/ciy499 [DOI] [PubMed] [Google Scholar]
- 33.Van Gerwen OT, Jani A, Long DM, Austin EL, Musgrove K, Muzny CA. Prevalence of Sexually Transmitted Infections and Human Immunodeficiency Virus in Transgender Persons: A Systematic Review. Transgend Health. Jun 1 2020;5(2):90–103. doi: 10.1089/trgh.2019.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira-Neves A, Ribeiro KC, Benchimol M. Pseudocysts in trichomonads--new insights. Protist. Oct 2003;154(3-4):313–29. doi: 10.1078/143446103322454095 [DOI] [PubMed] [Google Scholar]
- 35.Tulchinsky THVE. Communicable Diseases- Sexually Transmitted Infections. The New Public Health. 3rd ed. 2014:149–236. [Google Scholar]
- 36.Burch TA, Rees CW, Reardon LV. Epidemiological studies on human trichomoniasis. Am J Trop Med Hyg. May 1959;8(3):312–8. doi: 10.4269/ajtmh.1959.8.312 [DOI] [PubMed] [Google Scholar]
- 37.Mercer F, Johnson PJ. Trichomonas vaginalis: Pathogenesis, Symbiont Interactions, and Host Cell Immune Responses. Trends Parasitol. Aug 2018;34(8):683–693. doi: 10.1016/j.pt.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fichorova RN, Buck OR, Yamamoto HS, et al. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect. Sep 2013;89(6):460–6. doi: 10.1136/sextrans-2013-051052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menezes CB, Tasca T. Trichomoniasis immunity and the involvement of the purinergic signaling. Biomed J. Aug 2016;39(4):234–243. doi: 10.1016/j.bj.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fichorova RN, Lee Y, Yamamoto HS, et al. Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PLoS One. 2012;7(11):e48418. doi: 10.1371/journal.pone.0048418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graves KJ, Ghosh AP, Kissinger PJ, Muzny CA. Trichomonas vaginalis virus: a review of the literature. Int J STD AIDS. Jan 9 2019:956462418809767. doi: 10.1177/0956462418809767 [DOI] [PubMed] [Google Scholar]
- 42.Graves KJ, Ghosh AP, Kissinger PJ, Muzny CA. Trichomonas vaginalis virus: a review of the literature. Int J STD AIDS. Apr 2019;30(5):496–504. doi: 10.1177/0956462418809767 [DOI] [PubMed] [Google Scholar]
- 43.Graves KJ, Ghosh AP, Schmidt N, et al. Trichomonas vaginalis Virus Among Women With Trichomoniasis and Associations With Demographics, Clinical Outcomes, and Metronidazole Resistance. Clin Infect Dis. Nov 27 2019;69(12):2170–2176. doi: 10.1093/cid/ciz146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graves KJ, Novak J, Secor WE, Kissinger PJ, Schwebke JR, Muzny CA. A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis. Parasitology. 2020;147(13):1383–1391. doi: 10.1017/S0031182020001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt L, Jennison RF. Incidence of Trichomonas vaginalis in marital partners. Br J Vener Dis. Sep 1960;36(3):163–6. doi: 10.1136/sti.36.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kellock D, O’Mahony CP. Sexually acquired metronidazole-resistant trichomoniasis in a lesbian couple. Genitourin Med. Feb 1996;72(1):60–1. doi: 10.1136/sti.72.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crucitti T, Jespers V, Mulenga C, Khondowe S, Vandepitte J, Buvé A. Non-sexual transmission of Trichomonas vaginalis in adolescent girls attending school in Ndola, Zambia. PLoS One. Jan 31 2011;6(1):e16310. doi: 10.1371/journal.pone.0016310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muzny CA, Rivers CA, Mena LA, Schwebke JR. Genotypic characterization of Trichomonas vaginalis isolates among women who have sex with women in sexual partnerships. Sex Transm Dis. Jul 2012;39(7):556–8. doi: 10.1097/OLQ.0b013e31824f1c49 [DOI] [PubMed] [Google Scholar]
- 49.Peterson K, Drame D. Iatrogenic transmission of Trichomonas vaginalis by a traditional healer. Sex Transm Infect. Oct 2010;86(5):353–4. doi: 10.1136/sti.2010.043125 [DOI] [PubMed] [Google Scholar]
- 50.Van Gerwen OT, Muzny CA, Marrazzo JM. Sexually transmitted infections and female reproductive health. Nat Microbiol. Aug 2022;7(8):1116–1126. doi: 10.1038/s41564-022-01177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell CM, Anyalechi GE, Cohen CR, Haggerty CL, Manhart LE, Hillier SL. Etiology and Diagnosis of Pelvic Inflammatory Disease: Looking Beyond Gonorrhea and Chlamydia. J Infect Dis. Aug 16 2021;224(12 Suppl 2):S29–s35. doi: 10.1093/infdis/jiab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. Feb 15 2002;34(4):519–22. doi: 10.1086/338399 [DOI] [PubMed] [Google Scholar]
- 53.McKee DL, Hu Z, Stahlman S. Incidence and sequelae of acute pelvic inflammatory disease among active component females, U.S. Armed Forces, 1996-2016. Msmr. Oct 2018;25(10):2–8. [PubMed] [Google Scholar]
- 54.Haggerty CL, Peipert JF, Weitzen S, et al. Predictors of Chronic Pelvic Pain in an Urban Population of Women With Symptoms and Signs of Pelvic Inflammatory Disease. Sexually Transmitted Diseases. 2005;32(5) [DOI] [PubMed] [Google Scholar]
- 55.Kranjcić-Zec I, Dzamić A, Mitrović S, Arsić-Arsenijević V, Radonjić I. [The role of parasites and fungi in secondary infertility]. Med Pregl. Jan-Feb 2004;57(1-2):30–2. Uloga parazita i gljiva u nastanku sekundarnog steriliteta. doi: 10.2298/mpns0402030k [DOI] [PubMed] [Google Scholar]
- 56.Benchimol M, de Andrade Rosa I, da Silva Fontes R, Burla Dias AJ. Trichomonas adhere and phagocytose sperm cells: adhesion seems to be a prominent stage during interaction. Parasitol Res. Mar 2008;102(4):597–604. doi: 10.1007/s00436-007-0793-3 [DOI] [PubMed] [Google Scholar]
- 57.Casari E, Ferrario A, Morenghi E, Montanelli A. Gardnerella, Trichomonas vaginalis, Candida, Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in the genital discharge of symptomatic fertile and asymptomatic infertile women. New Microbiol. Jan 2010;33(1):69–76. [PubMed] [Google Scholar]
- 58.Cu-Uvin S, Ko H, Jamieson DJ, et al. Prevalence, incidence, and persistence or recurrence of trichomoniasis among human immunodeficiency virus (HIV)-positive women and among HIV-negative women at high risk for HIV infection. Clin Infect Dis. May 15 2002;34(10):1406–11. doi: 10.1086/340264 [DOI] [PubMed] [Google Scholar]
- 59.Yang M, Li L, Jiang C, et al. Co-infection with trichomonas vaginalis increases the risk of cervical intraepithelial neoplasia grade 2-3 among HPV16 positive female: a large population-based study. BMC Infect Dis. Sep 1 2020;20(1):642. doi: 10.1186/s12879-020-05349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazenby GB, Taylor PT, Badman BS, et al. An association between Trichomonas vaginalis and high-risk human papillomavirus in rural Tanzanian women undergoing cervical cancer screening. Clin Ther. Jan 1 2014;36(1):38–45. doi: 10.1016/j.clinthera.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 61.Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. Aug 1994;23(4):682–90. doi: 10.1093/ije/23.4.682 [DOI] [PubMed] [Google Scholar]
- 62.Roeters AM, Boon ME, van Haaften M, Vernooij F, Bontekoe TR, Heintz AP. Inflammatory events as detected in cervical smears and squamous intraepithelial lesions. Diagn Cytopathol. Feb 2010;38(2):85–93. doi: 10.1002/dc.21169 [DOI] [PubMed] [Google Scholar]
- 63.Jarecki-Black JC, Lushbaugh WB, Golosov L, Glassman AB. Trichomonas vaginalis: preliminary characterization of a sperm motility inhibiting factor. Ann Clin Lab Sci. Nov-Dec 1988;18(6):484–9. [PubMed] [Google Scholar]
- 64.Gopalkrishnan K, Hinduja IN, Kumar TC. Semen characteristics of asymptomatic males affected by Trichomonas vaginalis. J In Vitro Fert Embryo Transf. Jun 1990;7(3):165–7. doi: 10.1007/bf01135682 [DOI] [PubMed] [Google Scholar]
- 65.Sutcliffe S, Alderete JF, Till C, et al. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int J Cancer. May 1 2009;124(9):2082–7. doi: 10.1002/ijc.24144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SS, Kim JH, Han IH, Ahn MH, Ryu JS. Inflammatory Responses in a Benign Prostatic Hyperplasia Epithelial Cell Line (BPH-1) Infected with Trichomonas vaginalis. Korean J Parasitol. Apr 2016;54(2):123–32. doi: 10.3347/kjp.2016.54.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitteregger D, Aberle SW, Makristathis A, et al. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Med Microbiol Immunol. Feb 2012;201(1):113–6. doi: 10.1007/s00430-011-0205-2 [DOI] [PubMed] [Google Scholar]
- 68.Najafi A, Chaechi Nosrati MR, Ghasemi E, et al. Is there association between Trichomonas vaginalis infection and prostate cancer risk?: A systematic review and meta-analysis. Microb Pathog. Dec 2019;137:103752. doi: 10.1016/j.micpath.2019.103752 [DOI] [PubMed] [Google Scholar]
- 69.Marous M, Huang WY, Rabkin CS, et al. Trichomonas vaginalis infection and risk of prostate cancer: associations by disease aggressiveness and race/ethnicity in the PLCO Trial. Cancer Causes Control. Aug 2017;28(8):889–898. doi: 10.1007/s10552-017-0919-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang HY, Su RY, Chung CH, et al. Association between trichomoniasis and prostate and bladder diseases: a population-based case-control study. Sci Rep. Sep 13 2022;12(1):15358. doi: 10.1038/s41598-022-19561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutcliffe S, Giovannucci E, Alderete JF, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. May 2006;15(5):939–45. doi: 10.1158/1055-9965.Epi-05-0781 [DOI] [PubMed] [Google Scholar]
- 72.Francis SC, Kent CK, Klausner JD, et al. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005-2006. Sex Transm Dis. Sep 2008;35(9):797–800. doi: 10.1097/OLQ.0b013e318177ec39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter-Wicker K, Utuama O, Omole F. Can trichomoniasis cause pharyngitis? A case report. SAGE Open Med Case Rep. 2016;4:2050313x16682132. doi: 10.1177/2050313x16682132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR. Prevalence and Correlates of Trichomonas vaginalis Infection Among Men and Women in the United States. Clinical Infectious Diseases. 2018;67(2):211–217. doi: 10.1093/cid/ciy079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joseph Davey DL, Shull HI, Billings JD, Wang D, Adachi K, Klausner JD. Prevalence of Curable Sexually Transmitted Infections in Pregnant Women in Low- and Middle-Income Countries From 2010 to 2015: A Systematic Review. Sexually Transmitted Diseases. 2016;43(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young MR, Broadwell C, Kacanek D, et al. Sexually Transmitted Infections in Pregnant People Living With Human Immunodeficiency Virus: Temporal Trends, Demographic Correlates, and Association With Preterm Birth. Clinical Infectious Diseases. 2022;doi: 10.1093/cid/ciac321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Price CM, Peters RPH, Steyn J, et al. Prevalence and Detection of Trichomonas vaginalis in HIV-Infected Pregnant Women. Sex Transm Dis. May 2018;45(5):332–336. doi: 10.1097/olq.0000000000000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol. Dec 2009;83(1-2):185–9. doi: 10.1016/j.jri.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a Cause of Perinatal Morbidity: A Systematic Review and Meta-Analysis. Sexually Transmitted Diseases. 2014;41(6):369–376. doi: 10.1097/olq.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 80.Papeš D, Pasini M, Jerončić A, et al. Detection of sexually transmitted pathogens in patients with chronic prostatitis/chronic pelvic pain: a prospective clinical study. Int J STD AIDS. May 2017;28(6):613–615. doi: 10.1177/0956462417691440 [DOI] [PubMed] [Google Scholar]
- 81.Tsang SH, Peisch SF, Rowan B, et al. Association between Trichomonas vaginalis and prostate cancer mortality. Int J Cancer. May 15 2019;144(10):2377–2380. doi: 10.1002/ijc.31885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffman CM, Fritz L, Radebe O, et al. Rectal Trichomonas vaginalis infection in South African men who have sex with men. Int J STD AIDS. Dec 2018;29(14):1444–1447. doi: 10.1177/0956462418788418 [DOI] [PubMed] [Google Scholar]
- 83.Meites E, Llata E, Braxton J, et al. Trichomonas vaginalis in selected U.S. sexually transmitted disease clinics: testing, screening, and prevalence. Sex Transm Dis. Nov 2013;40(11):865–9. doi: 10.1097/olq.0000000000000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wølner-Hanssen P, Krieger JN, Stevens CE, et al. Clinical manifestations of vaginal trichomoniasis. Jama. Jan 27 1989;261(4):571–6. doi: 10.1001/jama.1989.03420040109029 [DOI] [PubMed] [Google Scholar]
- 85.Hobbs MM, Seña AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect. Sep 2013;89(6):434–8. doi: 10.1136/sextrans-2013-051057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health. Mar 1998;22(3):205–8. doi: 10.1016/s1054-139x(97)00214-0 [DOI] [PubMed] [Google Scholar]
- 87.Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. Nov 2013;51(11):3875–6. doi: 10.1128/jcm.02006-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol. Feb 2009;200(2):188.e1–7. doi: 10.1016/j.ajog.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 89.Gaydos CA, Schwebke J, Dombrowski J, et al. Clinical performance of the Solana® Point-of-Care Trichomonas Assay from clinician-collected vaginal swabs and urine specimens from symptomatic and asymptomatic women. Expert Rev Mol Diagn. Mar 2017;17(3):303–306. doi: 10.1080/14737159.2017.1282823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrmann B, Malm K. Comparison between Abbott m2000 RealTime and Alinity m STI systems for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium. Eur J Clin Microbiol Infect Dis. Oct 2021;40(10):2217–2220. doi: 10.1007/s10096-020-04135-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mtshali A, Ngcapu S, Govender K, Sturm AW, Moodley P, Joubert BC. In Vitro Effect of 5-Nitroimidazole Drugs against Trichomonas vaginalis Clinical Isolates. Microbiol Spectr. Aug 31 2022;10(4):e0091222. doi: 10.1128/spectrum.00912-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis. Nov 2018;18(11):1251–1259. doi: 10.1016/s1473-3099(18)30423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Howe K, Kissinger PJ. Single-Dose Compared With Multidose Metronidazole for the Treatment of Trichomoniasis in Women: A Meta-Analysis. Sex Transm Dis. Jan 2017;44(1):29–34. doi: 10.1097/olq.0000000000000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kissinger P, Mena L, Levison J, et al. A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J Acquir Immune Defic Syndr. Dec 15 2010;55(5):565–71. doi: 10.1097/QAI.0b013e3181eda955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muzny CA, Van Gerwen OT, Legendre D. Secnidazole: a treatment for trichomoniasis in adolescents and adults. Expert Rev Anti Infect Ther. Aug 2022;20(8):1067–1076. doi: 10.1080/14787210.2022.2080656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muzny CA, Schwebke JR, Nyirjesy P, et al. Efficacy and Safety of Single Oral Dosing of Secnidazole for Trichomoniasis in Women: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled, Delayed-Treatment Study. Clin Infect Dis. Sep 15 2021;73(6):e1282–e1289. doi: 10.1093/cid/ciab242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mann JR, McDermott S, Zhou L, Barnes TL, Hardin J. Treatment of trichomoniasis in pregnancy and preterm birth: an observational study. J Womens Health (Larchmt). Apr 2009;18(4):493–7. doi: 10.1089/jwh.2008.0964 [DOI] [PubMed] [Google Scholar]
- 98.Sheehy O, Santos F, Ferreira E, Berard A. The use of metronidazole during pregnancy: a review of evidence. Curr Drug Saf. 2015;10(2):170–9. doi: 10.2174/157488631002150515124548 [DOI] [PubMed] [Google Scholar]
- 99.Package Insert for Tinidazole.
- 100.Khrianin AA, Reshetnikov OV. [Clinical and microbiological efficacy of metronidazole and ornidazole in the treatment of urogenital trichomoniasis in men]. Antibiot Khimioter. 2006;51(1):18–21. [PubMed] [Google Scholar]


