Abstract
Germline pathogenic variants in DICER1 predispose individuals to develop a variety of benign and malignant tumors. Accurate variant curation and classification is essential for reliable diagnosis of DICER1-related tumor predisposition and identification of individuals who may benefit from surveillance. Since 2015, most labs have followed the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) sequence variant classification guidelines for DICER1 germline variant curation. However, these general guidelines lack gene-specific nuances and leave room for subjectivity. Consequently, a group of DICER1 experts joined ClinGen to form the DICER1 and miRNA-Processing Genes Variant Curation Expert Panel (VCEP), to create DICER1- specific ACMG/AMP guidelines for germline variant curation. The VCEP followed the FDA-approved ClinGen protocol for adapting and piloting these guidelines. A diverse set of 40 DICER1 variants were selected for piloting, including 14 known Pathogenic/Likely Pathogenic (P/LP) variants, 12 known Benign/Likely Benign (B/LB) variants, and 14 variants classified as variants of uncertain significance (VUS) or with conflicting interpretations in ClinVar. Clinically meaningful classifications (i.e., P, LP, LB, or B) were achieved for 82.5% (33/40) of the pilot variants, with 100% concordance among the known P/LP and known B/LB variants. Half of the VUS or conflicting variants were resolved with four variants classified as LB and three as LP. These results demonstrate that the DICER1-specific guidelines for germline variant curation effectively classify known pathogenic and benign variants while reducing the frequency of uncertain classifications. Individuals and labs curating DICER1 variants should consider adopting this classification framework to encourage consistency and improve objectivity.
Keywords: DICER1, variant curation, ClinGen, ClinVar, cancer predisposition, germline pathogenic variants, pediatric cancer
1. INTRODUCTION
The DICER1 gene (NM_177438.3), is located on chromosome 14q32.13, and contains 27 exons encoding 1,922 amino acids. Germline pathogenic variation in DICER1 is associated with increased risk for the development of tumors in childhood and adulthood (OMIM # 601200)(de Kock et al., 2019; Foulkes et al., 2014; Hill et al., 2009).The DICER1 protein is an endoribonuclease that converts a hairpin-shaped miRNA precursor (pre-miRNA) to a mature miRNA duplex by removing the terminal loop. The RNase IIIa and RNase IIIb domains of DICER1 form two catalytic cores (Zhang et al., 2004), cleaving at the 3’ and 5’ side of the terminal loop respectively, which are required to generate miRNAs derived from the 3p-arm (3p miRNAs) and 5p-arm (5p miRNAs) of the pre-miRNA accordingly.
DICER1-related tumor predisposition was first described in families with pleuropulmonary blastoma, a rare pediatric lung tumor (Hill et al., 2009). The phenotypic spectrum has since expanded to include a wide range of benign and malignant neoplasms in both children and adults such as Sertoli-Leydig cell tumors, cervical and ovarian embryonal rhabdomyosarcoma, Wilms tumor, nasal chondromesenchymal hamartoma, pituitary blastoma, pineoblastoma, thyroid lesions, and other rare sarcomas (de Kock et al., 2019; González et al., 2022). Surveillance recommendations aimed at early tumor detection exist for those with DICER1-related tumor predisposition due to germline variants in DICER1 (Bakhuizen et al., 2021; Schultz, Rednam, et al., 2017; Schultz et al., 2018).
Germline variant classification relies on the weighing of many pieces of evidence, such as functional data, population frequency, clinical phenotype, and family segregation data. In 2015, the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) issued a joint publication of standards and guidelines for classification of germline sequence variants (Richards et al., 2015) as a starting point to standardize variant classification procedures. The Clinical Genome Resource (ClinGen) (Rehm et al., 2015), a National Institutes of Health (NIH)-funded resource aimed at further refining and centralizing gene and variant curation processes, has since created a number of variant curation expert panels (VCEPs) (Rivera-Muñoz et al., 2018) that follow the Food and Drug Administration (FDA)-recognized guidance for Public Human Genetic Variant Databases and the ClinGen Expert Panel process to tailor and pilot gene-specific modifications of the ACMG/AMP variant curation guidelines (Fortuno et al., 2021; Lee et al., 2018; Mester et al., 2018; Wu et al., 2020).
The ClinGen DICER1 and miRNA-Processing Gene VCEP, hereafter referred to as the DICER1 VCEP, was formed with the goal of developing such tailored germline sequence variant curation guidelines for DICER1 and eventually other miRNA-processing genes associated with inherited syndromes (https://clinicalgenome.org/affiliation/50050/). Here we describe the process of our VCEP formation, evidence code specification for DICER1, and pilot curation.
2. METHODS
In 2019, a variety of DICER1 experts from across North America convened virtually to form the DICER1 VCEP (https://clinicalgenome.org/affiliation/50050/), following the ClinGen VCEP protocol (https://clinicalgenome.org/site/assets/files/3635/variant_curation_expert_panel_vcep_protocol_version_9-2_3.pdf). Membership included clinicians, basic scientists, laboratory geneticists, and variant scientists. Initially, 22 group members were divided into four subgroups (phenotype, penetrance, computational, and functional) to critically assess and modify a subset of the ACMG/AMP variant curation evidence codes for DICER1-specific germline variant curation. A preliminary set of specifications was defined in November 2020 using MANE transcript NM_177438.2 and MONDO:0017288.
The specifications were piloted on 40 DICER1 variants with submissions in ClinVar. These included 14 known pathogenic or likely pathogenic (P/LP) variants, 12 known benign or likely benign (B/LB) variants, and 14 variants with conflicting interpretations or classified as variants of uncertain significance (VUS). Classifications reflect ClinVar submissions as of November 2020 except for two of the P/LP variants which were updated more recently due to a known incongruence between one laboratory’s ClinVar submissions (VUS) and internal classifications (LP) at the time of the data pull. Pilot variants were intentionally selected such that missense, nonsense, frameshift, synonymous, and intronic variants were represented, giving the opportunity to test the performance of as many evidence codes as possible.
Each variant was double-curated by two of six biocurators to ensure evidence codes were being interpreted and applied uniformly. All biocurators had prior variant curation experience through other ClinGen VCEPs and/or employment at a commercial genetic testing laboratory offering clinical genetic testing for the DICER1 gene. In addition to published cases, relevant internal case-level data stripped of personal identifiable information was obtained by VCEP members working at testing laboratories, clinics, and the Pleuropulmonary Blastoma/DICER1 Registry (www.ppbregistry.org, NCT03382158) using an organized spreadsheet guide. Variants were curated within the ClinGen Variant Curation Interface (Preston et al., 2022). Final classifications were determined according to the original evidence code combinations (Richards et al., 2015) plus a handful of pre-determined combinations supported by a Bayesian framework (Tavtigian et al., 2018). In cases of conflicting benign and pathogenic evidence codes, a Bayesian points system was employed to reach a final classification (Tavtigian et al., 2020).
Evidence codes were further adapted as appropriate during the pilot, and the final specifications were approved by the ClinGen Sequence Variant Interpretation (SVI) Committee in May 2022.
Our ACMG/AMP specifications will be updated periodically, to find the most current information please visit https://clinicalgenome.org/affiliation/50050/ or https://cspec.genome.network/cspec/ui/svi/doc/GN024.
3. RESULTS
3.1. DICER1-specific variant curation criteria
The DICER1 VCEP specifications to the ACMG/AMP variant curation criteria are summarized in Table 1. Eight evidence codes (PM3, PM6, PP2, PP5, BP1, BP3, BP5, and BP6) were excluded due to redundancy, irrelevance with respect to DICER1, or published ClinGen guidance (Biesecker & Harrison, 2018). The remaining 20 criteria were kept with clarifications and/or gene-specific modifications to strength or scope.
Table 1.
Summary of DICER1-specific specifications of the ACMG/AMP variant curation guidelines.
| Original ACMG/AMP Evidence Codes | DICER1 Specifications | |
|---|---|---|
| Criteria | Criteria Description | |
| PVS1 | Null variant in a gene where loss of function is a known mechanism of disease. | Follow SVI-approved decision tree (Figure S1) with DICER1 specific modifications:
|
| PS1 | Same amino acid change as a previously established pathogenic variant regardless of nucleotide change. | Other variant must be interpreted as pathogenic by the DICER1 VCEP. Likely pathogenic changes do not apply. Same amino acid change: must confirm there is no difference in splicing. Non-canonical intronic splicing variants at same nucleotide: should have equal or worse splicing impact. Caveat: do not apply PM1 (full strength) or PM5 is PS1 is applied. |
| PS2 | De novo (proven or assumed) in a patient with disease and no family history. | Follow the point structure outlined in the manuscript and summarized in Table 3. PS2_Very Strong: ≥4 points PS2: ≥2 but less than 4 points PS2_Moderate: ≥1 but less than 2 points PS2_Supporting: ≥0.5 but less than 1 point |
| PS3 | Well-established in vitro or in vivo functional studies supportive of a damaging effect. | PS3: RNA assay demonstrates splicing impact that is out-of-frame OR in-frame with ≥ 193 residues affected OR disrupting the RNase IIIb domain. (Downgrade to PS3_Moderate if PVS1_Strong is also met) PS3_Moderate: RNA assay demonstrates splicing impact that is in-frame and disrupts < 193 residues, leaving the RNase IIIb domain intact. PS3_Supporting: In vitro cleavage assay with positive and negative controls demonstrates severely reduced capacity to produce 5p and/or 3p miRNA from pre-miRNA. Caveat: Do not apply PS3 at any strength if PVS1 is applied at full strength. |
| PS4 | The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls. | Follow the points structure outlined in the manuscript and summarized in Table 3. PS4: ≥4 points PS4_Moderate: 2–3.5 points PS4_Supporting: 1–1.5 points Caveats: Do not apply for variants that meet BA1 or BS1. Do not apply proband points for an individual who has another germline variant that could have reasonably contributed to the phenotype or whose tumor sequencing suggests sporadic tumorigenesis. |
| PM1 | Located in a mutational hot spot and/or critical and well-established functional domain. | PM1: Putative missense variants at residues affecting RNase IIb domain metal ion-binding (p.S1344, p.E1705, p.D1709, p.D1713, p.G1809, p.D1810, p.E1813). PM1_Supporting: Putative missense variants affecting other residues in the RNase IIIb domain (p.Y1682 – p.S1846). Caveat: The full strength rule cannot be applied with PS1 or PM5. |
| PM2 | Absent/rare from controls in an ethnically-matched cohort population sample. | This rule code is only applicable at a supporting level. PM2_Supporting: Allele frequency <0.000005 across gnomAD (non-cancer) with no more than one allele in any subpopulation and at least 20x coverage. |
| PM3 | For recessive disorders, detected in trans with a pathogenic variant. | N/A – DICER1 syndrome follows autosomal dominant inheritance |
| PM4 | Protein length changes due to in-frame deletions/insertions in a non-repeat region or stop-loss variants. | PM4: In-frame indels with a residue within the RNase IIIb domain (p.Y1682 – p.S1846). PM4_Supporting: In-frame indels outside of the RNase IIIb domain (p.Y1682 – p.S1846) and repeat regions (p.D606-p.D609; p.E1418-p.E1420; p.E1422-p.E1425). |
| PM5 | Missense change at an amino acid residue where a different missense change determined to be pathogenic has been seen before. | Other variant must be interpreted as pathogenic by the DICER1 VCEP. Likely pathogenic changes do not apply. The variant under assessment should have an equal or worse Grantham score. MaxEntScan and SpliceAI should demonstrate no splicing impact. Caveat: Do not apply with PS1 or with full-strength PM1. |
| PM6 | Assumed de novo, but without confirmation of paternity and maternity. | N/A – considered redundant after PS2 modifications |
| PP1 | Co-segregation with disease in multiple affected family members. | Phenotype-positive individuals should have high, moderate, or low-specificity phenotypes (see Table 2). PP1_Strong: ≥7 meioses across ≥2 families PP1_Moderate: 5 or 6 meioses across ≥1 family PP1: 3 or 4 meioses across ≥1 family Caveats: Do not apply for variants that meet BA1 or BS1. Segregation with a single low-specificity phenotype across multiple individuals does not fulfill PP1. |
| PP2 | Missense variant in a gene that has a low rate of benign missense variation and where missense variants are a common mechanism of disease. | N/A - While DICER1 does meet recommended cutoff for missense constraint z score of ≥3.09 established by the SVI (4.23 on gnomAD), the DICER1 VCEP recommends this rule not be used due to the presence of various missense variants throughout the gene that are clinically interpreted as benign (9) or likely benign (30) in ClinVar. |
| PP3 | Multiple lines of computational evidence support a deleterious effect on the gene or gene product. | Missense variants: REVEL score ≥0.75 OR concordance of MaxEntScan and SpliceAI for prediction of splice impact. Splicing variants: Concordance of MaxEntScan and SpliceAI for prediction of splice impact. Caveat: Do not apply in combination with PVS1. |
| PP4 | Patient’s phenotype or family history is highly specific for a disease with a single genetic etiology. | Tumor testing of a neoplasm within the DICER1 syndrome phenotypic spectrum in a proband with the germline variant under assessment reveals the following:Caveats: PP4 cannot be applied to germline curation of variants in the DICER1 hotspot codons (p.S1344, p.E1705, p.D1709, p.D1713, p.G1809, p.D1810, or p.E1813). PP4 cannotbe applied if tumor testing reveals any additional DICER1 non-hotspot variant(s). |
| PP5 | Reputable source recently reports variant as pathogenic but the evidence is not available to the laboratory to perform an independent evaluation. | N/A per published SVI guidance |
| BA1 | Allele frequency is above 5% in Exome Sequencing Project, 1000 Genomes, or ExAC. | Allele frequency >0.003 (0.3%) in gnomAD (non-cancer) subpopulations. Subpopulations must have >2,000 alleles tested and a minimum of 5 alleles present. |
| BS1 | Allele frequency is greater than expected for disorder. | Allele frequency >0.0003 (0.03%) in gnomAD (non-cancer) subpopulations. Subpopulations must have >2,000 alleles tested and a minimum of 5 alleles present. |
| BS2 | Observed in a healthy adult. | BS2: 40+ unrelated females from a single source have reached age 50 without a tumor diagnosis (ratio of BS2-eligible females to PS4-eligible probands must be ≥ 40:1) OR 2+ observations of homozygosity in healthy individuals OR 1+ observation(s) of homozygosity in a healthy individual with status confirmed by parental testing. BS2_Supporting: 10+ unrelated females from a single source have reached age 50 without a tumor diagnosis (ratio of BS2-eligible females to PS4-eligible probands must be ≥ 10:1) OR 2+ observations of homozygosity in individuals lacking clinical information. |
| BS3 | Well-established in vitro or in vivo functional studies shows no damaging effect on protein function. | BS3: For intronic or synonymous variants, ≥2 observations of no splicing impact via RNA assay. BS3_Supporting: In vitro cleavage assay with positive and negative controls demonstrates retained ability to produce 5p and 3p miRNA from pre-miRNA. |
| BS4 | Lack of segregation in affected members of a family. | Variant observed in at least one phenotype-positive (must be high- or moderate-specificity phenotype; see Table 2), genotype-negative 1st, 2nd, or 3rd degree relative(s) of the proband. This rule does not apply to phenotype-negative, genotype-positive family members. |
| BP1 | Missense variant in gene where primarily truncating variants cause disease. | N/A – truncating variants account for only a portion of disease-causing variants |
| BP2 | Observed in trans with a pathogenic variant for a fully penetrant dominant gene/disorder; or observed in cis with a pathogenic variant in any inheritance pattern. | Observed in trans with a pathogenic or likely pathogenic DICER1 variant (phase confirmed) in at least 1 individual OR observed in cis and/or phase unknown in at least 3 individuals, at least 2 of whom carry unique pathogenic/likely pathogenic DICER1 variants. This rule code can only be used to compare variants asserted as pathogenic by the ClinGen DICER1 VCEP. Homozygous cases are not relevant for BP2 and should instead contribute to BS2. |
| BP3 | In-frame deletions/insertions in a repetitive region without a known function. | N/A |
| BP4 | Multiple lines of computational evidence suggest no impact on gene or gene product. | Missense variants: REVEL score < 0.50 AND concordance of MaxEntScan and SpliceAI predicting no splice effects. Synonymous, intronic, and non-coding variants: Concordance of MaxEntScan and SpliceAI predicting no splice effects. |
| BP5 | Variant found in a case with an alternate molecular basis for disease. | N/A - Given the broad spectrum of DICER1-related neoplasms and the general lack of evidence of other high-penetrance germline variants that could account for such neoplasms (except perhaps for some low-specificity phenotypes), this rule should not be used at this time. |
| BP6 | Reputable source recently reports variant as benign but the evidence is not available to the laboratory to perform an independent evaluation. | N/A per published SVI guidance |
| BP7 | A synonymous (silent) variant for which splicing prediction algorithms predict no impact to the splice consensus sequence nor the creation of a new splice site AND the nucleotide is not highly conserved. | This rule applies to silent variants and intronic variants at or beyond +7 to −21 positions. For other intronic or non-coding variants, BP7 may be applied if the variant is the reference nucleotide in ≥1 primate and/or ≥4 mammalian species. Caveat: BP7 cannot be applied unless BP4 is also met. |
3.2. Population data (BA1, BS1, and PM2)
BA1 and BS1:
BA1 is stand-alone and BS1 is strong evidence for benign variation, based on the frequency of a variant in the general population. To determine frequency cutoffs, the VCEP first calculated a realistic maximum allele frequency for a pathogenic DICER1 variant using the Whiffin-Ware equation: maximum credible population allele frequency = disease prevalence x maximum allelic contribution / disease penetrance (Whiffin et al., 2017). Disease prevalence was set to 1 in 10,600 people (1 in 21,200 alleles) based on estimates from population databases (Kim et al., 2017). Maximum allelic contribution was set to 0.07 based on the proportion of the most common P/LP DICER1 variant from Invitae internal data. Disease penetrance was set to 0.1 (i.e., 10%) based on the lower end of published penetrance estimates for individuals aged 50–60 years (Stewart et al., 2019). The resulting frequency, 0.00003, was conservatively increased one order of magnitude for a BS1 cutoff of 0.0003 and another order of magnitude for a BA1 cutoff of 0.003. The VCEP chose to use non-cancer gnomAD subpopulations to minimize inclusion of cases. Generally, the most recent version of gnomAD with a non-cancer subpopulation should be used. However, earlier versions should be considered as relevant (e.g., superior sample size). Per published guidance, continental subpopulations must have greater than 2,000 alleles tested and a minimum of five alleles present (Ghosh et al., 2018).
PM2:
The PM2 criterion is intended to provide evidence of pathogenicity for variants that are absent from population databases or present only at low levels. The VCEP identified 19 P/LP or putative loss of function DICER1 variants in non-cancer gnomAD at low frequencies and expects that more will inevitably be present as databases grow. For this reason, the VCEP chose to establish a PM2 cutoff rather than to require absence. Based off the data from those 19 variants, the VCEP elected to apply PM2 for variants with frequency less than 0.000005 across non-cancer gnomAD with no more than one allele in any subpopulation and at least 20x coverage for that region of the gene in gnomAD. Such conditions would allow PM2 application for 15 of the 19 variants described previously. Per ClinGen SVI recommendations, PM2 should only be applied at a supporting level (https://www.clinicalgenome.org/site/assets/files/5182/pm2_-_svi_recommendation_-_approved_sept2020.pdf).
3.3. Computational and predictive data (PVS1, PS1, PM1, PM4, PM5, PP3, BP4, and BP7)
PVS1:
PVS1 provides very strong evidence of pathogenicity for null variants in a gene where loss of function is a known mechanism of disease. This code is particularly relevant to DICER1, as most germline causative alleles are loss of function (Brenneman et al., 2015; de Kock et al., 2019). The VCEP adopted previously published recommendations for PVS1 application (Abou Tayoun et al., 2018) but provided DICER1-specific details to simplify application such as the nonsense-mediated decay cutoff and which exons, if skipped, would result in in-frame deletions. Notably, the VCEP deviated from the typical recommendation by precluding PVS1 application for start codon variants, as the p.Met1 site is not highly conserved in DICER1, and there are three possible in-frame alternate methionine residues at p.Met11, p.Met17, and p.Met24. Furthermore, internal lab data showed that, in multiple individuals, p.Met1 variants are not associated with any DICER1 phenotype. A DICER1-specific PVS1 flowchart is provided in Supplementary Figure 1.
PP3 and BP4:
PP3 and BP4 are supporting level evidence codes based on computational predictors. The VCEP assessed the performance of several computational predictors, including metaSVM, CADD, BayesDel, and REVEL, on 15 known P/LP and 27 known B/LB DICER1 missense variants. The best separation was attained using REVEL, a computational meta-predictor whose score reflects 13 individual computational tools (Ioannidis et al., 2016). Attempts were made to trichotomize REVEL score cutoffs for PP3 and BP4 in a Bayesian fashion by calculating the odds of pathogenicity for a variant above or below a chosen threshold based on the test set of variants. Such a calculation could also be used to modify the strength of the evidence code if it could be shown, for example, that variants above a particular threshold had moderate or strong odds of pathogenicity (Tavtigian et al., 2018). Because few confidently curated missense variants in DICER1 currently exist, the VCEP was unable to establish cutoffs through a Bayesian approach (Pejaver et al., 2022) and instead selected ≥0.75 and <0.50 as the PP3 and BP4 cutoffs, respectively, based on general REVEL use guidelines (Ioannidis et al., 2016) and good visual separation of 15 pathogenic and 27 benign variants. PP3 and BP4 may also be applied to splicing and non-coding variants based on concordance of two splice predictors, MaxEntScan and SpliceAI. Until sufficient data are available to determine gene-specific splice predictor thresholds, standard MaxEntScan and SpliceAI thresholds should be used. PP3 sho0uld not be used in combination with PVS1.
BP7:
BP7 is intended for silent variants not predicted to impact splicing. BP4 must be applied as a prerequisite for BP7 consideration. For variants meeting BP4, any silent or intronic variant at +7 to −21 positions automatically qualifies for BP7. Non-coding variants outside the +7 to −21 intronic positions may have BP7 applied if the variant is the reference nucleotide in one or more primate and/or four or more mammalian species, indicating lack of conservation of the nucleotide.
PM1:
Variation in critical gene regions or hotspot codons is considered moderate evidence of pathogenicity under PM1. DICER1 has seven recognized hotspot codons: p.Ser1344, p.Glu1705, p.Asp1709, p.Asp1713, p.Gly1809, p.Asp1810, p.Glu1813 (Brenneman et al., 2015; de Kock et al., 2019; Pontén et al., 2022). Variation in these codons impairs activity of the DICER1 RNase IIIb domain while leaving the IIIa cleavage domain intact. While variants in these hotspot codons are more commonly somatic in origin, they have been observed in a mosaic state and thus are still relevant for germline curation considerations (Brenneman et al., 2015; de Kock et al., 2014). The VCEP decided it was appropriate to apply PM1 at a supporting level for missense variants affecting other residues within the RNase IIIb domain (p.Y1682 – p.S1846).
PM4:
Similarly, the VCEP decided that in-frame protein length changes, considered moderate evidence of pathogenicity under PM4, were more likely to be pathogenic if located in the RNase IIIb domain (Apellaniz-Ruiz et al., 2018; Apellaniz-Ruiz et al., 2019). For this reason, PM4 can be applied at full moderate strength to in-frame indels within the RNase IIIb domain (p.Y1682 – p.S1846) and at a supporting level to in-frame indels outside that domain. PM4 should not be applied to indels in repeat regions of DICER1 (p.D606-p.D609; p.E1418-p.E1420; p.E1422-p.E1425).
PS1 and PM5:
The PS1 and PM5 codes are intended for missense variants observed at an amino acid residue where the same (PS1) or a different (PM5) predicted amino acid change has been established as pathogenic. For both codes, the VCEP specified that the other variant must have reached a pathogenic classification (likely pathogenic does not suffice) by the DICER1 VCEP and that splice effects should be ruled out by RNA data or concordance of MaxEntScan and SpliceAI. For PM5, the missense variant under investigation should have an equal or worse (i.e., higher) Grantham score than the other pathogenic variant (Grantham, 1974). The VCEP further expanded the scope of PS1 by allowing it to apply to non-canonical intronic nucleotide substitutions where a pathogenic splice site variant has been observed before if MaxEntScan and SpliceAI both predict an equal or greater splice impact for the variant under investigation. Because PS1, PM5, and PM1 are similar evidence types, they should not be applied together. The strongest evidence code should be used for variants meeting two or more of these codes. PM1 at the supporting strength may be combined with PS1 or PM5.
3.4. Functional data (PS3, BS3)
PS3 and BS3:
In vivo and in vitro functional studies provide another critical piece of evidence for variant curation under PS3 and BS3. The VCEP identified various types of functional evidence applicable to DICER1 that can be applied at different strength levels. To apply PS3 at full strength, a patient-derived RNA assay must demonstrate an out-of-frame splicing impact or an in-frame splicing impact removing more than 10% (193 residues) of the protein or disrupting the RNase IIIb domain. If a variant also has PVS1_Strong applied, PS3 should be dropped to moderate application. PS3 can also be applied at a moderate level if RNA data demonstrates an in-frame splicing impact removing less than 10% of the protein and not affecting the RNase IIIb domain. Similarly, a patient-derived RNA assay demonstrating no splicing impact qualifies for BS3, though this should be observed in more than one patient to minimize the possibility of dropout. Another functional assay of utility for DICER1 variant classification is an in vitro cleavage assay which assesses the ability of a DICER1 protein to generate 3p and 5p miRNAs (Wu et al., 2018). Evidence of impaired or retained DICER1 cleavage function through such an assay may be used to apply PS3 or BS3, respectively, at a supporting level, provided that appropriate positive and negative controls were used. A higher strength level is not appropriate at this time as these assays are low-throughput and dependent on operator experience. PS3 cannot be applied at any strength if PVS1 is applied at full strength.
3.5. Clinical data
3.5.1. Phenotype (PS4, PP4)
PS4:
The VCEP critically evaluated known DICER1-associated phenotypes; the specificity of these phenotypes for an underlying pathogenic germline DICER1 variant was also considered. PS4 was initially intended to be an evidence code for variant-level case control studies, with a reduced-strength option for rare variants observed in multiple affected patients but lacking statistically significant case-control studies (Richards et al., 2015). The code has since evolved into a sophisticated proband-counting code with variable strength applications where affected, unrelated probands are allotted 0, 0.5, or 1 point each based on the specificity of their phenotypes, and point total determines PS4 strength application (Fortuno et al., 2021; Mester et al., 2018). The VCEP kept this framework in mind when considering the DICER1 phenotypic spectrum.
A high-specificity phenotype deserving a full proband point should reflect a greater than 80% likelihood of an underlying pathogenic germline variant in the gene of interest; a moderate-specificity phenotype deserving a half proband point should reflect a 60–80% likelihood of an underlying causative germline variant (Mester et al., 2018). Of nearly 30 DICER1-associated phenotypes gathered from the literature (de Kock et al., 2019; González et al., 2022; Guillerman et al., 2019) and panel members, few had published data on the frequency of underlying germline DICER1 variants in unselected patient cohorts. Studies of pleuropulmonary blastoma (Brenneman et al., 2015) and pituitary blastoma (de Kock et al., 2014) suggest greater than 80% specificity for an underlying pathogenic germline DICER1 variant, while cystic nephroma (Doros et al., 2014) and Sertoli-Leydig cell tumors and gynandroblastoma (Schultz, Harris, et al., 2017) appear to fall in the 60–80% range. More recently, studies of primary intra-cranial sarcomas (Diaz Coronado et al., 2022; Koelsche et al., 2018) and multinodular goiter in young adults (Altaraihi et al., 2021) suggest less than 60% specificity for germline DICER1 variants.
Given the lack of large, unselected studies of these neoplasms, the VCEP elected to independently survey six clinical experts from the VCEP to categorize the phenotypes as high-specificity (much more likely than not to have a germline P/LP DICER1 variant), moderate-specificity (more likely than not to have a germline P/LP DICER1 variant), and low-specificity (less likely to have a germline P/LP DICER1 variant). Consensus was reached if 5 or more of the experts agreed on the categorization. VCEP members discussed cases of disagreement and conservatively downgraded specificity. Certain phenotypes were considered so non-specific (e.g., adult multinodular goiter, macrocephaly) that they were not deemed fit to qualify even for low-specificity. The final agreed upon designations are summarized in Table 2.
Table 2.
DICER1 syndrome phenotypes grouped by specificity. For use with the following evidence codes: PS4, PS2, PP1, PP4, BS4.
| Specificity | Phenotypes |
|---|---|
|
High-specificity
(much more likely than not to have germline P/LP DICER1) |
Pleuropulmonary blastoma (PPB) (Including Type 1r) Pituitary blastoma Anaplastic renal sarcoma Ciliary body medulloepithelioma Cystic nephroma (<18 yrs) Embryonal rhabdomyosarcoma (Ovarian) Embryonal rhabdomyosarcoma (Cervix) |
|
Moderate-specificity
(more likely than not to have germline P/LP DICER1) |
Differentiated thyroid cancer and/or Multinodular goiter (<18 years) Nasal chondromesenchymal hamartoma Ovarian Sertoli-Leydig cell tumors Ovarian sex-cord stromal tumor of mixed type (specifically, gynandroblastoma) |
|
Low-specificity
(less likely to have DICER1) |
Non-parasitic liver cysts (childhood) Wilms tumor Pineoblastoma Cerebral sarcoma Lung cysts (<18 yrs) |
| **For PP4 use ONLY** Additional neoplasms of very low or undetermined specificity | Thyroid neoplasms (any age) Sarcomas Juvenile hamartomatous polyps Primitive neuroectodermal/neuroepithelial neoplasms Infantile cerebellar embryonal tumors Fetal lung adenocarcinoma |
Using Table 3 as a guide, unrelated probands may be granted a full point on the basis of a high-specificity phenotype, two moderate-specificity phenotypes, a moderate-plus a low-specificity phenotype, or a moderate-specificity phenotype plus family history of a high- or moderate-specificity phenotype in a first- or second-degree relative. If the last combination is used and that family also contributes to PP1 meiosis counting, only a half point should be counted to avoid double-counting segregation. A proband with only one moderate-specificity phenotype should be given a half point. Anything less specific is not granted any points. Points summed across unrelated probands indicate the strength application of PS4: supporting (1 to <2 points), moderate (2 to <4 points), or strong (≥4 points). PS4 should not be applied when a variant also has population data meeting BA1 or BS1 since a common variant may be present in a proband by chance. Additionally, PS4 should not be applied to a proband with another germline variant that could have reasonably contributed to the observed phenotype or whose tumor sequencing suggests sporadic tumorigenesis.
Table 3.
Points per proband that can be applied toward PS2 and/or PS4 application based on proband phenotype and confirmed or assumed de novo status. Modified from “SVI Recommendation for De Novo Criteria (PS2 & PM6)” – Version 1.0
| Points per Proband | ||||
|---|---|---|---|---|
| PS2 | PS4 | Proband Phenotype (see Table 2) | ||
| Phenotypic Consistency | Confirmed | Assumed | ||
| Phenotype highly specific for gene | 2 | 1 | 1 |
|
| Phenotype consistent with gene but not highly specific | 1 | 0.5 | 0.5 | IV. 1 Moderate |
| Phenotype consistent with gene but not highly specific and high genetic heterogeneity ‡ | 0.5 | 0.25 | 0 | V. ≥1 Low |
If PP1 is applied and the proband’s family contributed to the PP1 meiosis count, use IV (1 Moderate) instead of III.B to avoid double counting family history.
Maximum allowable value of 1 may contribute to overall PS2 score to avoid counting multiple probands with only low-specificity phenotypes.
PP4:
Considering PS4 proband counting, many VCEPs have discarded PP4, a code focused on patient phenotype and family history, as redundant. However, it has been recognized that PP4 may be utilized as a tumor phenotype code when appropriate (Walsh et al., 2018). With few exceptions, both benign and malignant DICER1-driven neoplasms follow a distinct modified two-hit hypothesis: one loss of function variant plus one variant selectively impairing the RNase IIIb domain function (Brenneman et al., 2015; Chen et al., 2018; Foulkes et al., 2014; Garcia et al., 2022). In DICER1-related tumor predisposition, the germline variant is typically loss of function, and the somatic second hit generally occurs in one of a handful of hotspot codons. Because this pattern is a hallmark of DICER1-driven neoplasms, the VCEP determined that evidence from somatic tumor sequencing of any DICER1-associated neoplasm, regardless of specificity, should lead to PP4 application if three conditions are met. First, the variant under investigation should not itself be in a DICER1 hotspot codon. Second, in addition to retention of the germline variant in the tumor, somatic sequencing should reveal a previously reported somatic second hit (de Kock et al., 2019; Gadd et al., 2017; Wu et al., 2013) as summarized in Supplementary Table 1. Finally, no additional non-hotspot DICER1 variants or loss of heterozygosity should be revealed, as such a finding could reflect sporadic tumorigenesis. A flowchart simplifying PP4 application is shown in Figure 1. A single observation of such evidence is sufficient for PP4 application. Multiple observations cannot increase the code strength, as this would be considered proband counting. The VCEP will consider whether PP4 should be strengthened in future versions once a sufficient number of variants have been curated to allow for formal odds of pathogenicity calculations.
Figure 1.
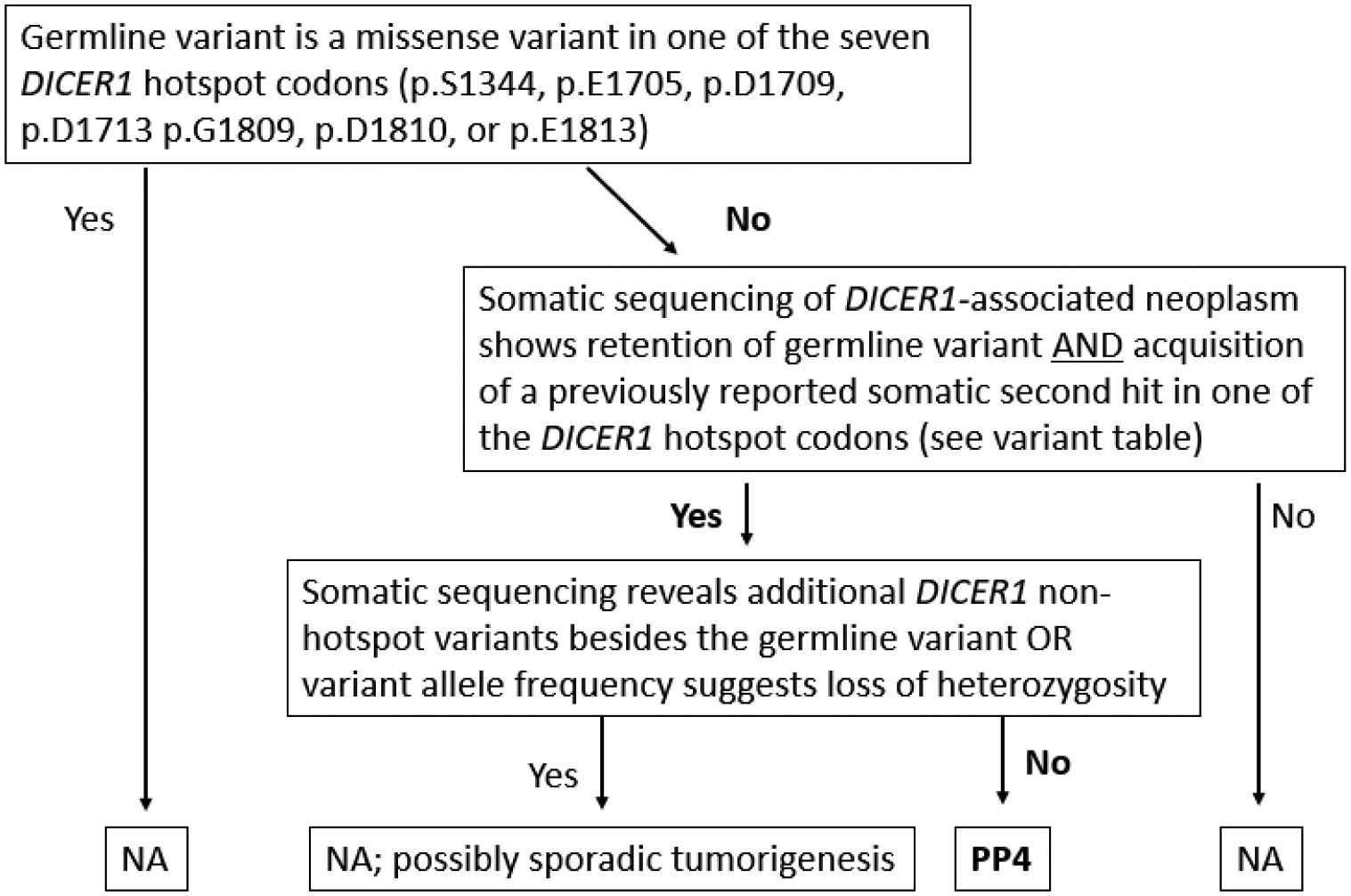
Flowchart for DICER1-specific PP4 code application.
3.5.2. Segregation data (BS4, PP1)
PP1 and BS4:
Variant segregation and lack of segregation with disease fall under PP1 and BS4, respectively. For counting PP1 meiosis, the DICER1 VCEP adopted the same cutoffs used by other VCEPs (Fortuno et al., 2021; Lee et al., 2018; Mester et al., 2018) and informed by prior work (Jarvik & Browning, 2016; Thompson et al., 2003). Namely, PP1 may be applied at supporting strength when 3 or 4 meioses are observed across one or more families, moderate strength when 5 or 6 meioses are observed across one or more families, and strong strength when seven or more meioses are observed across two or more families. Meioses are counted between phenotype-positive individuals with high-, moderate-, or low-specificity phenotypes as outlined in Table 2. PP1 was relaxed to include low-specificity phenotypes during the pilot, which improved its performance for pathogenic variants without resulting in excessive segregation counts. However, variant segregation with a single low-specificity phenotype (e.g., Wilms tumor) across multiple individuals is not sufficient for PP1 application. PP1 should not be applied when a variant also has population data meeting BA1 or BS1 since a common variant may appear to segregate with disease by chance. BS4 may be applied if a proband has a phenotype-positive (must be high- or moderate-specificity), genotype-negative first-, second-, or third-degree relative. Genotype-positive and phenotype-negative individuals do not count toward BS4 but may be considered for BS2 (see 3.5.4).
3.5.3. De novo data (PS2)
PS2:
The DICER1 VCEP followed SVI recommendations for de novo criteria (https://clinicalgenome.org/working-groups/sequence-variant-interpretation/). Under the recommended framework, probands with de novo germline variants contribute 0, 0.25, 0.5, 1, or 2 points toward a de novo score based on the phenotype of the proband and whether parental relationships were confirmed (e.g., trio exome, maternity/paternity testing) or unconfirmed. Under this framework, a curator may apply either of the two de novo evidence codes originally proposed in the ACMG/AMP guidelines (Richards et al., 2015). The DICER1 VCEP elected to adopt PS2 as the sole de novo evidence code and to exclude PM6 as redundant, instead using PS2 at lower evidence strength when maternity/paternity were unconfirmed. The proposed points combinations are summarized in Table 3, and phenotypes are organized in Table 2. Points summed across unrelated probands indicate the strength application of PS2: supporting (0.5 to 1 point), moderate (1 to <2 points), strong (2 to <4 points) or very strong (≥4 points).
3.5.4. Allelic data (BS2, BP2)
BS2:
Because pathogenic DICER1 variants have incomplete penetrance, the DICER1 VCEP initially excluded BS2, which is considered benign evidence for a variant observed in a healthy adult. However, it became apparent during the pilot that a modified version of BS2 would be needed for multiple known benign variants to comfortably reach a benign classification. Based on a conservative neoplasm penetrance estimate of 10% in individuals aged 50–60 years with germline DICER1 variants (Stewart et al., 2019) and higher penetrance in females than males, the VCEP determined that an observation of 10 or more unrelated females, who have reached 50 years of age without a tumor diagnosis, should qualify for BS2_Supporting, provided that the ratio of BS2-eligible females to PS4-eligible probands is equal to or greater than 10:1. Similarly, since a strong evidence code can be thought of as equivalent to four supporting level codes (Tavtigian et al., 2018), an observation of 40 or more unrelated females, who have reached 50 years of age without a tumor diagnosis should qualify for BS2 at full strength, provided that the ratio of BS2-eligible females to PS4-eligible probands is equal to or greater than 40:1. In both cases, all females should come from a single source (e.g., from a single laboratory, database, clinical cohort, or publication) to eliminate the possibility of double counting. Additionally, since homozygous loss of function variants in DICER1 are thought to be embryonic lethal (Bernstein et al., 2003; Teijeiro et al., 2018), homozygous observations can also qualify for BS2 application. The DICER1 VCEP allows BS2 to be applied at full strength if homozygosity is observed in two or more healthy individuals or one healthy individual if homozygosity is confirmed by parental testing. BS2_Supporting may be applied if two or more observations of homozygosity are made in individuals lacking clinical information.
BP2:
In cases where an additional P/LP germline DICER1 variant is found in a proband, BP2 may be applied if the P/LP variant is confirmed in trans with the variant under investigation. If the P/LP variant is in cis or in an unknown phase, three such observations are required for BP2 application, and the probands must not all carry the same P/LP variant. Similar to PS1 and PM5, the co-occurring P/LP variant must be classified by the DICER1 VCEP.
3.6. Evidence Code Combinations
Initially, the VCEP followed the originally recommended evidence code combinations (Richards et al., 2015) and stated that a single supporting evidence code should not be considered conflicting evidence if a clinically meaningful classification would otherwise be reached. However, the original combinations were not flexible enough to account for some of the combinations in round 1 of the pilot (e.g. 6 supporting pathogenic codes), and limitations with regards to resolving complex conflicting evidence code combinations are apparent. For those reasons, the VCEP pivoted to a flexible, modified Bayesian points approach for all evidence code combinations (Tavtigian et al., 2020) for the final pilot curations. In this approach, supporting, moderate, strong, and very strong evidence codes are weighted at one, two, four, and eight points, respectively, with pathogenic evidence weighted positively and benign evidence weighted negatively. A sum of the points results in the final classification as outlined in Table 4.
Table 4.
Points system for classifying DICER1 germline variants. Supporting, moderate, strong, and very strong codes receive 1, 2, 4, and 8 points, respectively, with pathogenic evidence codes in the positive direction, and benign evidence codes in the negative direction. Adapted Tavtigian et al. 2020 (PMID: 32720330)
| Category | Point ranges |
|---|---|
| Pathogenic | ≥ 10 |
| Likely Pathogenic | 6 to 9 |
| Uncertain | 0 to 5 |
| Uncertain with caveat† | −1 |
| Likely Benign | −2 to −6 |
| Benign | ≤ −7 |
A final point value of −1 may be overridden to Likely Benign only in cases where PM2_Supporting is applied AND no other pathogenic evidence codes are applied (e.g. BP4, BP7, PM2_Supporting).
3.7. Pilot
The VCEP tested the proposed evidence code specifications on 40 DICER1 variants as described in the Methods. Pilot results, including evidence codes applied, are summarized in Table 5. To improve performance, the VCEP modified PP1, BS2, and the method for evidence code combinations as described above between round 1 and round 2 of the pilot. The changes implemented between the initial and final round of pilot classifications led to stronger variant classifications (i.e., more pathogenic or more benign) in nine variants (22.5%), including five variants which shifted from VUS to LB or LP.
Table 5.
Classification of 40 germline DICER1 variants during the pilot phase of the DICER1-specific ACMG/AMP variant curation guidelines. Round 1 and Round 2 criteria reflect criteria from the preliminary and finalized guidelines, respectively.
| DICER1 Variant | ClinVar ID | ClinVar Classifications† | Round 1 Criteria Applied‡ | Initial Classification | Round 2 Criteria Added‡ | Points | Final Classification§ |
|---|---|---|---|---|---|---|---|
| c.4748T>G (p.L1583R) | 4468 | P/LP | PM2_P, PP3, PS3_P, PS4_P, PP1 | VUS | PP1 → PP1_M | 6 | LP |
| c.5125G>A (p.D1709N) | 690480 | P | PM2_P, PP3, PM1, PS3_P, PS4_M, PS2_VS | P | 15 | P | |
| c.5441C>T (p.S1814L) | 412119 | P/LP | PM2_P, PP3, PM1_P, PS3_P, PS4, PP4, PP1_S | P | 13 | P | |
| c.5465A>T (p.D1822V) | 254350 | P/LP | PM2_P, PP3, PM1_P, PS3_P, PS4_M | LP | 6 | LP | |
| c.5138A>T (p.D1713V) | 690454 | P/LP | PM2_P, PP3, PM1, PS3_P, PS2_P | LP | 6 | LP | |
| c.5104C>T (p.Q1702*) | 254344 | P | PM2_P, PVS1, PS4_P | P | 10 | P | |
| c.1408G>T (p.E470*) | 254287 | P | PM2_P, PVS1, PS4_P, PP4 | P | 11 | P | |
| c.3019C>T (p.Q1007*) | 429113 | P | PM2_P, PVS1, PS4_P, PP4 | P | 11 | P | |
| c.1880_1883del (p.I627fs) | 254298 | P | PM2_P, PVS1, PS4_P, PP1 | P | 11 | P | |
| c.878_881del (p.R293fs) | 254355 | P | PM2_P, PVS1, PS4_P, PP4 | P | 11 | P | |
| c.2650+1G>T | 254310 | P | PM2_P, PVS1, PS4_M | P | 11 | P | |
| c.1907+1G>A | 429148 | P/LP | PM2_P, PVS1, PS4_P, PM6 | P | 12 | P | |
| c.2988–1G>T | 429116 | P | PM2_P, PVS1, PS4_M, PS2 | P | 15 | P | |
| c.5479del (p.1827fs) | 477261 | P/LP | PVS1, PM2_P, PS4_P | P | 10 | P | |
| c.2614G>A (p.A872T) | 133967 | B/LB | BS1, BP4, BS3_P | LB | BS2 | −10 | B |
| c.4910C>T (p.S1637L) | 242127 | B/LB | BS1, BP4 | LB | BS2 | −9 | B |
| c.3828T>A (p.D1276E) | 794388 | LB | BS1, BP4 | LB | −5 | LB | |
| c.3428T>C (p.L1143P) | 220594 | B/LB | BS1, BP4 | LB | BS2 | −9 | B |
| c.1825G>T (p.D609Y) | 133965 | B/LB | BA1 (BS1, BP4) | B | BS2 | NA | B |
| c.884C>G (p.S295C) | 242151 | B/LB | BA1 (BP4) | B | BS2 | NA | B |
| c.59C>T (p.A20V) | 133964 | B/LB | BS1, BP4 | LB | BS2 | −9 | B |
| c.5052C>G (p.L1684=) | 242128 | LB | BS1, BP4, BP7 | LB | −6 | LB | |
| c.2808T>C (p.Y936=) | 751425 | LB | BP4, BP7 | LB | −4 | LB | |
| c.4647C>T (p.H1549=) | 417113 | B/LB | BP4, BP7 | LB | BS2 | −6 | LB |
| c.5521C>T (p.L1841=) | 477262 | LB | PM2_P, BP4, BP7 | LB | −1 | LB | |
| c.3269+14G>A | 315107 | B/LB | BP4, BP7 | LB | BS2 | −6 | LB |
| c.5096–12G>A | 580203 | VUS | PS3, PS4_M, PP4, PM2_P | LP | 8 | LP | |
| c.1722_1724del (p.E574del) | 566588 | VUS | PM2_P, PM4_P | VUS | 2 | VUS | |
| c.2651–4T>G | 543697 | Conflicting: LB(1); VUS(1) | BP4, BP7 | LB | −2 | LB | |
| c.238G>T (p.E80*) | 649946 | Conflicting: P(1); VUS(1) | PVS1, PM2_P | LP | 9 | LP | |
| c.5276A>G (p.K1759R) | 133974 | Conflicting: LB(1); VUS(1) | BP4, PM1_P | VUS | BS2 | −4 | LB |
| c.5107C>T (p.R1703C) | 242130 | VUS | PP3, PM1_P, PM2_P | VUS | 3 | VUS | |
| c.4178_4180dup (p.N1393dup) | 242100 | VUS | PM4_P | VUS | BS2_P | 0 | VUS |
| c.*5G>A | 315099 | Conflicting: LB(1); VUS(2) | BP4 | VUS | BS2_P | −2 | LB |
| c.5563C>T (p.R1855*) | 947388 | VUS | PVS1_M, PM2_P | VUS | 3 | VUS | |
| c.4024C>T (p.R1342C) | 412120 | VUS | PM2_P | VUS | 1 | VUS | |
| c.1907+3A>T | 820285 | Conflicting: LB(1); VUS(1) | PM2_P; BS3 | VUS | Points | −3 | LB |
| c.2T>C (p.M1T) | 242076 | VUS | PM4_P | VUS | 1 | VUS | |
| c.5422A>G (p.M1808V) | 477260 | VUS | PM1_P | VUS | 1 | VUS | |
| c.2642T>C (p.L881P) | 690445 | VUS | PP3, PP4, PM2_P, PS3_P, PS4_P | VUS | PP1, Points | 6 | LP |
ClinVar classifications were pulled in November 2020 with the exception of p.D1713V and p.Leu1827fs due to a known incongruence between one laboratory’s ClinVar submissions (VUS) and internal classifications (LP) at the time of the data pull.
Modified strength levels are denoted with an underscore followed by a P, M, S, or VS, denoting supporting, moderate, strong, or very strong strength.
Classifications that changed between Round 1 and Round 2 are in bold text.
Final VCEP classifications were clinically meaningful for 82.5% (33/40) of the pilot variants. Concordance for known P/LP and known B/LB pilot variants was 100% (14 of 14 P/LP and 12 of 12 B/LB). Pilot variants with conflicting or uncertain classifications in ClinVar reached 50% (7/14) resolution, with four variants reaching LB and three reaching LP.
4. DISCUSSION
Under the ClinGen framework, the DICER1 VCEP developed and piloted DICER1-specific sequence variant curation guidelines. These guidelines performed very well on a set of pilot variants, with more than 80% of pilot variants receiving a clinically meaningful classification. Furthermore, the pilot demonstrated that the guidelines could be interpreted and applied consistently by curators and that internal data sharing can be effectively integrated into the curation process. The pilot variants and evidence summaries have been submitted to ClinVar as three-star submissions (Landrum et al., 2018). Additional curation details for those variants are also available on the ClinGen Evidence Repository (https://erepo.clinicalgenome.org/evrepo/).
Past challenges in curating DICER1 missense variants have been recognized and even cited as a reason to exclude DICER1 from the ACMG Secondary Findings list (Miller et al., 2021). The success of our guidelines in clarifying DICER1 variant classification not only implies fewer patients will be faced with VUS results in the future but also reduces this barrier for future reconsideration of DICER1 for the ACMG Secondary Findings list.
The VCEP will continue to meet regularly to further variant curation progress and submit classifications for public use. Variants will be prioritized by ClinVar classification (conflicting interpretations or VUS by multiple submitters) and by request. ClinVar currently contains ~5,000 DICER1 variant entries, including ~150 with conflicting interpretations and ~860 VUS by multiple submitters. Variant interpretations will be submitted to ClinVar within 30 days of VCEP approval. The VCEP will re-curate variants classified as LP or VUS every two years to assess whether additional evidence is available. Medically significant discrepancies (i.e. P/LP vs. VUS/LB/B) between a VCEP submission and a more recent ClinVar submitter will be reviewed and updated as appropriate within six months of the discrepant submission. Other discrepancies (i.e. VUS vs. LB/B) will be reviewed within two years.
Due to the characteristic signature of DICER1 somatic mutations, the DICER1 VCEP chose to use somatic tumor testing as supporting evidence (PP4) (Walsh et al., 2018). The DICER1 VCEP is the first VCEP within the ClinGen Hereditary Cancer Clinical Domain to use somatic tumor testing to inform PP4 application, providing a model for other VCEPs.
As more is learned and published on the DICER1 gene and the phenotypic consequences of its pathogenic variation, the VCEP will re-evaluate the proposed guidelines and consider updates for future versions of the guidelines. For example, the phenotypic spectrum of the disorder may expand, or the specificity of certain phenotypes may need to be adjusted. Additionally, as more DICER1 variants are curated, the VCEP can revisit odds of pathogenicity calculations for various evidence codes such as PP4 tumor phenotype evidence or PP3 and BP4 in silico predictor cutoffs and modify the strength of the evidence codes as appropriate. Any modifications to evidence specifications will be submitted to the SVI for approval and made publicly available on the ClinGen website (https://clinicalgenome.org/affiliation/50050/) as a resource for others curating DICER1 variants.
CONCLUSIONS
The DICER1-specific sequence variant curation guidelines developed by the ClinGen DICER1 VCEP show promising results on a pilot set of 40 variants, with 80% reaching clinically meaningful classifications.
Consistent utilization of these guidelines may reduce the number of variants of uncertain significance returned to patients undergoing DICER1 sequencing. Future refinement of these guidelines over time is expected to further improve the clinical utility of variant classification.
Supplementary Material
Supplementary Table 1. DICER1 hotspot variants previously reported as somatic second hits.
Supplementary Figure 1. Flowchart for DICER1-specific PVS1 code application. Modified from Tayoun et al. 2018 (PMID 30192042)
ACKNOWLEDGEMENTS
The ClinGen DICER1 and miRNA-Processing Gene Variant Curation Expert Panel thanks Steven Harrison, Leslie Biesecker, Sharon Plon, and the ClinGen Sequence Variant Interpretation Group for guidance and feedback on the development of our guidelines. We also thank past member D. Ashley Hill for her contributions and the patients whose data made curation possible.
FUNDING STATEMENT
ClinGen is primarily funded by the National Human Genome Research Institute (NHGRI) with co-funding from the National Cancer Institute (NCI), through the following grants: Baylor/Stanford – U24HG009649, Broad/Geisinger – U24HG006834, and UNC/Kaiser – U24HG009650. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work of DRS, JNH, CH, MNF, and JK was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. WDF is funded by the Canadian Institutes of Health Research grant FDN-148390.
KAS receives funding from NIH NCI R01 CA244940 and the Pine Tree Apple Classic Fund.
CONFLICTS OF INTEREST
The following authors work for laboratories that offer fee-for-service testing of DICER1: MJA, ECC, HCC, SBC, SH, JM, JLM, NNY. The following authors have made substantial contributions to the DICER1 gene: disease literature: DRS, XB-D, KSC, WDF, SG, KAS, MKW. NSA-H is an employee and equity holder of 23andMe; serves as a scientific advisory board member for Allelica; received personal fees from Genentech, Allelica, and 23andMe; received research funding from Akcea; and was previously employed by Regeneron Pharmaceuticals.
DATA AVAILABILITY
The variant classifications made during this effort have been published in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and the curated evidence collected has been made publicly available through the ClinGen Evidence Repository (https://erepo.clinicalgenome.org/evrepo/). Some detailed internal patient-level data is not publicly available for ethical and privacy reasons.
REFERENCES
- Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, & Harrison SM (2018). Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat, 39(11), 1517–1524. 10.1002/humu.23626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaraihi M, Hansen TVO, Santoni-Rugiu E, Rossing M, Rasmussen Å K, Gerdes AM, & Wadt K (2021). Prevalence of Pathogenic Germline DICER1 Variants in Young Individuals Thyroidectomised Due to Goitre - A National Danish Cohort. Front Endocrinol (Lausanne), 12, 727970. 10.3389/fendo.2021.727970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apellaniz-Ruiz M, de Kock L, Sabbaghian N, Guaraldi F, Ghizzoni L, Beccuti G, & Foulkes WD (2018). Familial multinodular goiter and Sertoli-Leydig cell tumors associated with a large intragenic in-frame DICER1 deletion. Eur J Endocrinol, 178(2), K11–k19. 10.1530/eje-17-0904 [DOI] [PubMed] [Google Scholar]
- Apellaniz-Ruiz M, Segni M, Kettwig M, Glüer S, Pelletier D, Nguyen VH, Wagener R, López C, Muchantef K, Bouron-Dal Soglio D, Sabbaghian N, Wu MK, Zannella S, Fabian MR, Siebert R, Menke J, Priest JR, & Foulkes WD (2019). Mesenchymal Hamartoma of the Liver and DICER1 Syndrome. N Engl J Med, 380(19), 1834–1842. 10.1056/NEJMoa1812169 [DOI] [PubMed] [Google Scholar]
- Bakhuizen JJ, Hanson H, van der Tuin K, Lalloo F, Tischkowitz M, Wadt K, & Jongmans MCJ (2021). Surveillance recommendations for DICER1 pathogenic variant carriers: a report from the SIOPE Host Genome Working Group and CanGene-CanVar Clinical Guideline Working Group. Fam Cancer, 20(4), 337–348. 10.1007/s10689-021-00264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, & Hannon GJ (2003). Dicer is essential for mouse development. Nat Genet, 35(3), 215–217. 10.1038/ng1253 [DOI] [PubMed] [Google Scholar]
- Biesecker LG, & Harrison SM (2018). The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet Med, 20(12), 1687–1688. 10.1038/gim.2018.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman M, Field A, Yang J, Williams G, Doros L, Rossi C, Schultz KA, Rosenberg A, Ivanovich J, Turner J, Gordish-Dressman H, Stewart D, Yu W, Harris A, Schoettler P, Goodfellow P, Dehner L, Messinger Y, & Hill DA (2015). Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in pleuropulmonary blastoma / DICER1 syndrome: a unique variant of the two-hit tumor suppression model. F1000Res, 4, 214. 10.12688/f1000research.6746.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, Stuart SH, Stroup EK, Shukla AS, Wang J, Rajaram V, Vujanic GM, Slone T, Rakheja D, & Amatruda JF (2018). Distinct DICER1 Hotspot Mutations Identify Bilateral Tumors as Separate Events. JCO Precis Oncol, 2. 10.1200/po.17.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock L, Sabbaghian N, Plourde F, Srivastava A, Weber E, Bouron-Dal Soglio D, Hamel N, Choi JH, Park SH, Deal CL, Kelsey MM, Dishop MK, Esbenshade A, Kuttesch JF, Jacques TS, Perry A, Leichter H, Maeder P, Brundler MA, Warner J, Neal J, Zacharin M, Korbonits M, Cole T, Traunecker H, McLean TW, Rotondo F, Lepage P, Albrecht S, Horvath E, Kovacs K, Priest JR, & Foulkes WD (2014). Pituitary blastoma: a pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol, 128(1), 111–122. 10.1007/s00401-014-1285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock L, Wu MK, & Foulkes WD (2019). Ten years of DICER1 mutations: Provenance, distribution, and associated phenotypes. Hum Mutat, 40(11), 1939–1953. 10.1002/humu.23877 [DOI] [PubMed] [Google Scholar]
- Diaz Coronado RY, Mynarek M, Koelsche C, Mora Alferez P, Casavilca Zambrano S, Wachtel Aptowitzer A, Sahm F, von Deimling A, Schüller U, Spohn M, Sturm D, Pfister SM, Morales La Madrid A, Sernaque Quintana R, Sarria Bardales G, Negreiros Chinchihuara T, Ojeda Medina L, Garcia-Corrochano Medina P, Campos Sanchez DA, Ponce Farfan J, Rutkowski S, & Garcia Leon JL (2022). Primary central nervous system sarcoma with DICER1 mutation-treatment results of a novel molecular entity in pediatric Peruvian patients. Cancer, 128(4), 697–707. 10.1002/cncr.33977 [DOI] [PubMed] [Google Scholar]
- Doros LA, Rossi CT, Yang J, Field A, Williams GM, Messinger Y, Cajaiba MM, Perlman EJ, K AS, Cathro HP, Legallo RD, LaFortune KA, Chikwava KR, Faria P, Geller JI, Dome JS, Mullen EA, Gratias EJ, Dehner LP, & Hill DA (2014). DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol, 27(9), 1267–1280. 10.1038/modpathol.2013.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuno C, Lee K, Olivier M, Pesaran T, Mai PL, de Andrade KC, Attardi LD, Crowley S, Evans DG, Feng BJ, Foreman AKM, Frone MN, Huether R, James PA, McGoldrick K, Mester J, Seifert BA, Slavin TP, Witkowski L, Zhang L, Plon SE, Spurdle AB, & Savage SA (2021). Specifications of the ACMG/AMP variant interpretation guidelines for germline TP53 variants. Hum Mutat, 42(3), 223–236. 10.1002/humu.24152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes WD, Priest JR, & Duchaine TF (2014). DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer, 14(10), 662–672. 10.1038/nrc3802 [DOI] [PubMed] [Google Scholar]
- Gadd S, Huff V, Walz AL, Ooms A, Armstrong AE, Gerhard DS, Smith MA, Auvil JMG, Meerzaman D, Chen QR, Hsu CH, Yan C, Nguyen C, Hu Y, Hermida LC, Davidsen T, Gesuwan P, Ma Y, Zong Z, Mungall AJ, Moore RA, Marra MA, Dome JS, Mullighan CG, Ma J, Wheeler DA, Hampton OA, Ross N, Gastier-Foster JM, Arold ST, & Perlman EJ (2017). A Children’s Oncology Group and TARGET initiative exploring the genetic landscape of Wilms tumor. Nat Genet, 49(10), 1487–1494. 10.1038/ng.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A, Desrosiers L, Scollon S, Gruner S, Reuther J, Gandhi I, Patil N, Fuller MY, Dai H, Muzny D, Gibbs RA, Bercaw-Pratt JL, Rao SL, Rainusso N, Fisher KE, Lin FY, Plon SE, Parsons DW, & Roy A (2022). Distinct somatic DICER1 hotspot mutations in three metachronous ovarian Sertoli-Leydig cell tumors in a patient with DICER1 syndrome. Cancer Genet, 262–263, 53–56. 10.1016/j.cancergen.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Harrison SM, Rehm HL, Plon SE, & Biesecker LG (2018). Updated recommendation for the benign stand-alone ACMG/AMP criterion. Hum Mutat, 39(11), 1525–1530. 10.1002/humu.23642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González IA, Stewart DR, Schultz KAP, Field AP, Hill DA, & Dehner LP (2022). DICER1 tumor predisposition syndrome: an evolving story initiated with the pleuropulmonary blastoma. Mod Pathol, 35(1), 4–22. 10.1038/s41379-021-00905-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R (1974). Amino acid difference formula to help explain protein evolution. Science, 185(4154), 862–864. 10.1126/science.185.4154.862 [DOI] [PubMed] [Google Scholar]
- Guillerman RP, Foulkes WD, & Priest JR (2019). Imaging of DICER1 syndrome. Pediatr Radiol, 49(11), 1488–1505. 10.1007/s00247-019-04429-x [DOI] [PubMed] [Google Scholar]
- Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, Williams G, Bracamontes D, Messinger Y, & Goodfellow PJ (2009). DICER1 mutations in familial pleuropulmonary blastoma. Science, 325(5943), 965. 10.1126/science.1174334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, Musolf A, Li Q, Holzinger E, Karyadi D, Cannon-Albright LA, Teerlink CC, Stanford JL, Isaacs WB, Xu J, Cooney KA, Lange EM, Schleutker J, Carpten JD, Powell IJ, Cussenot O, Cancel-Tassin G, Giles GG, MacInnis RJ, Maier C, Hsieh CL, Wiklund F, Catalona WJ, Foulkes WD, Mandal D, Eeles RA, Kote-Jarai Z, Bustamante CD, Schaid DJ, Hastie T, Ostrander EA, Bailey-Wilson JE, Radivojac P, Thibodeau SN, Whittemore AS, & Sieh W (2016). REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am J Hum Genet, 99(4), 877–885. 10.1016/j.ajhg.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik GP, & Browning BL (2016). Consideration of Cosegregation in the Pathogenicity Classification of Genomic Variants. Am J Hum Genet, 98(6), 1077–1081. 10.1016/j.ajhg.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Field A, Schultz KAP, Hill DA, & Stewart DR (2017). The prevalence of DICER1 pathogenic variation in population databases. Int J Cancer, 141(10), 2030–2036. 10.1002/ijc.30907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsche C, Mynarek M, Schrimpf D, Bertero L, Serrano J, Sahm F, Reuss DE, Hou Y, Baumhoer D, Vokuhl C, Flucke U, Petersen I, Brück W, Rutkowski S, Zambrano SC, Garcia Leon JL, Diaz Coronado RY, Gessler M, Tirado OM, Mora J, Alonso J, Garcia Del Muro X, Esteller M, Sturm D, Ecker J, Milde T, Pfister SM, Korshunov A, Snuderl M, Mechtersheimer G, Schüller U, Jones DTW, & von Deimling A (2018). Primary intracranial spindle cell sarcoma with rhabdomyosarcoma-like features share a highly distinct methylation profile and DICER1 mutations. Acta Neuropathol, 136(2), 327–337. 10.1007/s00401-018-1871-6 [DOI] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W, Karapetyan K, Katz K, Liu C, Maddipatla Z, Malheiro A, McDaniel K, Ovetsky M, Riley G, Zhou G, Holmes JB, Kattman BL, & Maglott DR (2018). ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res, 46(D1), D1062–d1067. 10.1093/nar/gkx1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Krempely K, Roberts ME, Anderson MJ, Carneiro F, Chao E, Dixon K, Figueiredo J, Ghosh R, Huntsman D, Kaurah P, Kesserwan C, Landrith T, Li S, Mensenkamp AR, Oliveira C, Pardo C, Pesaran T, Richardson M, Slavin TP, Spurdle AB, Trapp M, Witkowski L, Yi CS, Zhang L, Plon SE, Schrader KA, & Karam R (2018). Specifications of the ACMG/AMP variant curation guidelines for the analysis of germline CDH1 sequence variants. Hum Mutat, 39(11), 1553–1568. 10.1002/humu.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester JL, Ghosh R, Pesaran T, Huether R, Karam R, Hruska KS, Costa HA, Lachlan K, Ngeow J, Barnholtz-Sloan J, Sesock K, Hernandez F, Zhang L, Milko L, Plon SE, Hegde M, & Eng C (2018). Gene-specific criteria for PTEN variant curation: Recommendations from the ClinGen PTEN Expert Panel. Hum Mutat, 39(11), 1581–1592. 10.1002/humu.23636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Lee K, Chung WK, Gordon AS, Herman GE, Klein TE, Stewart DR, Amendola LM, Adelman K, Bale SJ, Gollob MH, Harrison SM, Hershberger RE, McKelvey K, Richards CS, Vlangos CN, Watson MS, & Martin CL (2021). ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med, 23(8), 1381–1390. 10.1038/s41436-021-01172-3 [DOI] [PubMed] [Google Scholar]
- Pejaver V, Byrne AB, Feng B-J, Pagel KA, Mooney SD, Karchin R, O’Donnell-Luria A, Harrison SM, Tavtigian SV, Greenblatt MS, Biesecker LG, Radivojac P, Brenner SE, & Group CSVIW (2022). Evidence-based calibration of computational tools for missense variant pathogenicity classification and ClinGen recommendations for clinical use of PP3/BP4 criteria. bioRxiv, 2022.2003.2017.484479. 10.1101/2022.03.17.484479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén E, Frisk S, Taylan F, Vaz R, Wessman S, de Kock L, Pal N, Foulkes WD, Lagerstedt-Robinson K, & Nordgren A (2022). A complex DICER1 syndrome phenotype associated with a germline pathogenic variant affecting the RNase IIIa domain of DICER1. J Med Genet, 59(2), 141–146. 10.1136/jmedgenet-2020-107385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CG, Wright MW, Madhavrao R, Harrison SM, Goldstein JL, Luo X, Wand H, Wulf B, Cheung G, Mandell ME, Tong H, Cheng S, Iacocca MA, Pineda AL, Popejoy AB, Dalton K, Zhen J, Dwight SS, Babb L, DiStefano M, O’Daniel JM, Lee K, Riggs ER, Zastrow DB, Mester JL, Ritter DI, Patel RY, Subramanian SL, Milosavljevic A, Berg JS, Rehm HL, Plon SE, Cherry JM, Bustamante CD, & Costa HA (2022). ClinGen Variant Curation Interface: a variant classification platform for the application of evidence criteria from ACMG/AMP guidelines. Genome Med, 14(1), 6. 10.1186/s13073-021-01004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, Landrum MJ, Ledbetter DH, Maglott DR, Martin CL, Nussbaum RL, Plon SE, Ramos EM, Sherry ST, & Watson MS (2015). ClinGen--the Clinical Genome Resource. N Engl J Med, 372(23), 2235–2242. 10.1056/NEJMsr1406261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, & Rehm HL (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17(5), 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Muñoz EA, Milko LV, Harrison SM, Azzariti DR, Kurtz CL, Lee K, Mester JL, Weaver MA, Currey E, Craigen W, Eng C, Funke B, Hegde M, Hershberger RE, Mao R, Steiner RD, Vincent LM, Martin CL, Plon SE, Ramos E, Rehm HL, Watson M, & Berg JS (2018). ClinGen Variant Curation Expert Panel experiences and standardized processes for disease and gene-level specification of the ACMG/AMP guidelines for sequence variant interpretation. Hum Mutat, 39(11), 1614–1622. 10.1002/humu.23645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KAP, Harris AK, Finch M, Dehner LP, Brown JB, Gershenson DM, Young RH, Field A, Yu W, Turner J, Cost NG, Schneider DT, Stewart DR, Frazier AL, Messinger Y, & Hill DA (2017). DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: Clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol Oncol, 147(3), 521–527. 10.1016/j.ygyno.2017.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KAP, Rednam SP, Kamihara J, Doros L, Achatz MI, Wasserman JD, Diller LR, Brugières L, Druker H, Schneider KA, McGee RB, & Foulkes WD (2017). PTEN, DICER1, FH, and Their Associated Tumor Susceptibility Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin Cancer Res, 23(12), e76–e82. 10.1158/1078-0432.Ccr-17-0629 [DOI] [PubMed] [Google Scholar]
- Schultz KAP, Williams GM, Kamihara J, Stewart DR, Harris AK, Bauer AJ, Turner J, Shah R, Schneider K, Schneider KW, Carr AG, Harney LA, Baldinger S, Frazier AL, Orbach D, Schneider DT, Malkin D, Dehner LP, Messinger YH, & Hill DA (2018). DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res, 24(10), 2251–2261. 10.1158/1078-0432.Ccr-17-3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Best AF, Williams GM, Harney LA, Carr AG, Harris AK, Kratz CP, Dehner LP, Messinger YH, Rosenberg PS, Hill DA, & Schultz KAP (2019). Neoplasm Risk Among Individuals With a Pathogenic Germline Variant in DICER1. J Clin Oncol, 37(8), 668–676. 10.1200/jco.2018.78.4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian SV, Greenblatt MS, Harrison SM, Nussbaum RL, Prabhu SA, Boucher KM, & Biesecker LG (2018). Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genet Med, 20(9), 1054–1060. 10.1038/gim.2017.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavtigian SV, Harrison SM, Boucher KM, & Biesecker LG (2020). Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Hum Mutat, 41(10), 1734–1737. 10.1002/humu.24088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijeiro V, Yang D, Majumdar S, González F, Rickert RW, Xu C, Koche R, Verma N, Lai EC, & Huangfu D (2018). DICER1 Is Essential for Self-Renewal of Human Embryonic Stem Cells. Stem Cell Reports, 11(3), 616–625. 10.1016/j.stemcr.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Easton DF, & Goldgar DE (2003). A full-likelihood method for the evaluation of causality of sequence variants from family data. Am J Hum Genet, 73(3), 652–655. 10.1086/378100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MF, Ritter DI, Kesserwan C, Sonkin D, Chakravarty D, Chao E, Ghosh R, Kemel Y, Wu G, Lee K, Kulkarni S, Hedges D, Mandelker D, Ceyhan-Birsoy O, Luo M, Drazer M, Zhang L, Offit K, & Plon SE (2018). Integrating somatic variant data and biomarkers for germline variant classification in cancer predisposition genes. Hum Mutat, 39(11), 1542–1552. 10.1002/humu.23640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiffin N, Minikel E, Walsh R, O’Donnell-Luria AH, Karczewski K, Ing AY, Barton PJR, Funke B, Cook SA, MacArthur D, & Ware JS (2017). Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med, 19(10), 1151–1158. 10.1038/gim.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Luo X, Feurstein S, Kesserwan C, Mohan S, Pineda-Alvarez DE, & Godley LA (2020). How I curate: applying American Society of Hematology-Clinical Genome Resource Myeloid Malignancy Variant Curation Expert Panel rules for RUNX1 variant curation for germline predisposition to myeloid malignancies. Haematologica, 105(4), 870–887. 10.3324/haematol.2018.214221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MK, Sabbaghian N, Xu B, Addidou-Kalucki S, Bernard C, Zou D, Reeve AE, Eccles MR, Cole C, Choong CS, Charles A, Tan TY, Iglesias DM, Goodyer PR, & Foulkes WD (2013). Biallelic DICER1 mutations occur in Wilms tumours. J Pathol, 230(2), 154–164. 10.1002/path.4196 [DOI] [PubMed] [Google Scholar]
- Wu MK, Vujanic GM, Fahiminiya S, Watanabe N, Thorner PS, O’Sullivan MJ, Fabian MR, & Foulkes WD (2018). Anaplastic sarcomas of the kidney are characterized by DICER1 mutations. Mod Pathol, 31(1), 169–178. 10.1038/modpathol.2017.100 [DOI] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Jaskiewicz L, Westhof E, & Filipowicz W (2004). Single processing center models for human Dicer and bacterial RNase III. Cell, 118(1), 57–68. 10.1016/j.cell.2004.06.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. DICER1 hotspot variants previously reported as somatic second hits.
Supplementary Figure 1. Flowchart for DICER1-specific PVS1 code application. Modified from Tayoun et al. 2018 (PMID 30192042)
Data Availability Statement
The variant classifications made during this effort have been published in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and the curated evidence collected has been made publicly available through the ClinGen Evidence Repository (https://erepo.clinicalgenome.org/evrepo/). Some detailed internal patient-level data is not publicly available for ethical and privacy reasons.


