Abstract
Objective
A T-SPOT.TB can yield indeterminate results under two test observation conditions: a high response to the nil in negative control wells (high nil-control) or a low response to the mitogen in positive control wells (low mitogen-control). The most strongly influential factors for these indeterminate results, however, have yet to be identified.
Methods
From June 1, 2015, to June 30, 2021, we conducted a 1:1 matched case-control, retrospective study.
Patients
Patients who underwent a T-SPOT.TB test at Chiba University Hospital.
Results
The study included 5,956 participants. Indeterminate results were found in 63 participants (1.1%), including high nil-control in 37 and low mitogen-control in 26. Human T-cell leukemia virus type 1 (HTLV-1) positivity was the only influencing factor associated with high nil-control (adjusted odds ratio=98.5, 95% confidence interval: 6.59-1,480).
Conclusion
Regarding the indeterminate results, all HTLV-1 positive participants had a high nil response and no low mitogen response. It was suspected that abnormally produced interferon γ caused a nonspecific reaction to the negative control well, resulting in a high nil response. Low mitogen-control, conversely, did not appear to have any statistically significant influential factors.
Keywords: indeterminate, HTLV-1, high nil-control, low mitogen-control
Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis that primarily affects the lungs. Every year, 10 million people become ill with TB. Although TB is a preventable and curable disease, 1.5 million people die from it each year, making it the world's leading infectious killer (1). TB is one of the leading causes of death, particularly in low- and middle-income countries, and it ranks among the top 10 killer diseases (2).
However, not everyone infected with M. tuberculosis becomes ill. As a result, there are two TB-related conditions: latent TB infection (LTBI) and TB disease (3). LTBI is defined as a persistent immune response to M. tuberculosis antigen stimulation in the absence of clinically manifest active TB. There is no gold-standard test for LTBI, but it can be detected using a tuberculin skin test (TST) or an interferon-γ release assay (IGRA) (4).
For the diagnosis of M. tuberculosis infection, two IGRAs based on the in vitro detection of interferon (INF)-γ release by T cells in response to antigens specific to M. tuberculosis are available: T-SPOT.TB (Oxford Immunotec, Oxfordshire, UK) and QuantiFERON™-TB Gold (Cellectis, Carnegie, Australia). These tests are more specific than a TST for diagnosing TB infection. However, unlike a TST, IGRAs can yield indeterminate results (5).
For the T-SPOT.TB in particular, indeterminate results are reported with a frequency of 0-5.4% in the general population and 3.5-33% in immunocompromised patients (6). The cluster of differentiation 4 (CD4) counts, the age, lymphocyte counts, and underlying diseases have been reported as factors influencing T-SPOT.TB indeterminate results (1-12). However, not all studies experience indeterminate results (7,9,13,14). Indeed, indeterminate results can be caused by two T-SPOT.TB results: a high response to the nil in negative control wells (high nil-control) or a low response to the mitogen in positive control wells (low mitogen-control) (15).
Active TB is a major factor influencing low mitogen-control values, meaning that TB itself makes the diagnosis difficult. Furthermore, infectious diseases that affect the immune system, such as human immunodeficiency virus (HIV) and human T-cell leukemia virus type 1 (HTLV-1), are factors that influence indeterminate results. With an increase in the number of patients receiving immunosuppressive drugs, the presence of a history of TB is becoming increasingly important, often influencing the treatment course.
However, details concerning the factors most strongly influential for indeterminate results have not yet been clarified. We herein report our experience and analysis results.
Materials and Methods
Study design and participants
We conducted a 1:1 matched case-control study on patients who had received a T-SPOT.TB, comparing those with determinate and indeterminate results. From June 1, 2015, to June 30, 2021, Chiba University Hospital performed TB tests. T-SPOT.TB testing was mostly done on patients who were suspected of having TB or who were planned to be started on immunosuppressive drugs. TB was classified as active, old, latent TB infection, and not infected based on a medical interview, chest imaging (X-ray and computed tomography), and sputum culture.
Patients with indeterminate results who had clinical or laboratory data in the Electronic Health Record were deemed eligible as case patients. Indeterminate results included a high nil response (defined as a negative control spot count >10) and a low mitogen response (defined as a positive control spot count <20). If a patient was tested multiple times, only the most recent indeterminate result was used.
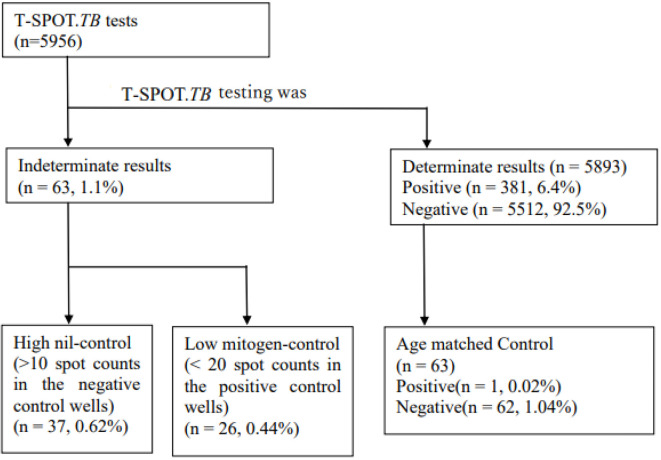
We referred to previous reports where withholding of T-SPOT.TB. indetermination results were influenced by age, we selected the same number of age-matched patients who had been tested for T-SPOT.TB. The matched control for each case was selected using the following criteria: 1) determinate results (positive or negative); 2) available clinical and laboratory data in the Electronic Medical Record System (Figure).
Figure.
Flowchart of T-SPOT. TB patients with indeterminate results (high nil-control and low mitogen-control). TB testing was performed on 5,956 patients. In this group, 63 patients had undetermined outcomes. There were 37 high nil-control patients and 26 low mitogen-control patients.
This study was approved by the Ethics Committee of Chiba University Hospital (reference number was M10119).
T-SPOT.TB assays
T-SPOT.TB assays (Oxford Immunotec) were performed by the SRL Clinical Laboratory Company (Tokyo, Japan), according to the manufacturer's instructions. We added T-cell Xtend to the samples after blood collection and conducted measurements within 24 hours.
T-SPOT.TB test is intended to detect effector T cells that respond to antigens specific to M. tuberculosis. A whole-blood sample is separated into peripheral blood mononuclear cells (PBMCs), which are incubated with the antigens to stimulate any sensitized T cells that may be present. Specific antibodies on the membrane capture the secreted cytokine, which is then cleaved by the bound enzyme to form an insoluble precipitate at the site of the reaction.
The test involves four panels: a positive control, a negative control, panel A (TB-specific antigen ESAT-6), and panel B. (TB-specific antigen CFP-10). The number of spots in (1) panel A-minus negative control or (2) panel B-minus negative control determines the outcome. Three patterns were considered decisive outcomes: if (1) or (2) or both had six or more spots, the results were positive. If both (1) and (2) had five spots or fewer, the result was negative.
However, the assays were deemed indeterminate if the negative control spot count was >10 (high nil-control) or the positive control spot count was <20 (low mitogen-control) (15).
Demographic, clinical, and laboratory data collection
Demographic data, underlying conditions, and medications within four weeks before performing T-SPOT.TB assays were obtained from electronic health records. The dosage of various kinds of glucocorticoids was standardized into the equivalent dose of prednisone.
Statistical analyses
Statistical analyses were performed using EZR, R version 4.1.2. (16). Continuous variables with normal distributions were expressed as the mean and standard deviation in the Kolmogorov-Smirnov test, whereas data without normal distributions were described as the median and interquartile range. Proportions were used to represent categorical variables. The indeterminate results participants were divided into high nil-control and low mitogen-control groups and compared to the determinate results group. For normal and non-normal data, the paired sample t-test and Mann-Whitney U test were used to compare continuous variables between case patients and control subjects. Fisher's exact test was used to compare categorical data. p<0.05 was regarded as statistically significant. The explanatory variables were chosen based on previous research and analysis data. Variables with p<0.10 were added to a conditional multivariate logistic model to assess the independent impact of each influencing factor on high-nil and low-mitogen responses.
Results
Participants' characteristics
A total of 5,956 participants received the T-SPOT.TB test. Of these, 381 participants (6.4%) exhibited positive results, 5,512 participants (92.5%) negative results, and 63 participants (1.1%) indeterminate results (high nil-control in 37 and low mitogen-control in 26). These 63 participants with available medical records were included in the matched case-control analysis.
The underlying disease included malignant solid tumors, hematological malignancies, rheumatic diseases, inflammatory bowel diseases, TB (including clinically active TB, a history of TB, and LTBI), HIV positivity, and HTLV-1 positivity (Table 1).
Table 1.
Characteristics of Indeterminate Results (High Nil-control, Low Mitogen-control) Compared with Determinate Results.
| Determinate results (n=63) | High nil (n=37) | p value* | Low mitogen (n=26) | p value** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 58.9±20.6 | 57.9±20.0 | 0.96 | 60.3±21.8 | 0.81 | |||||
| Male, n (%) | 31 (49.2) | 16 (43.2) | 0.68 | 12 (46.2) | 0.82 | |||||
| Underlying diseases | ||||||||||
| DM, n (%) | 6 (9.5) | 3 (8.1) | 1 | 8 (30.8) | <0.05 | |||||
| Malignant solid tumor, n (%) | 16 (25.4) | 8 (21.6) | 0.62 | 3 (11.5) | 0.17 | |||||
| Hematological malignancy, n (%) | 0 (0) | 0 (0) | ND | 1 (3.8) | 0.29 | |||||
| Rheumatic diseases, n (%) | 14 (22.2) | 11 (29.7) | 0.48 | 7 (26.9) | 0.78 | |||||
| Inflammatory bowel disease, n (%) | 6 (9.5) | 3 (8.1) | 1 | 2 (7.7) | 1 | |||||
| TB, n (%) | 2 (3.1) | 3 (8.1) | 0.36 | 5 (19.2) | <0.05 | |||||
| HIV positive, n (%) | 1 (1.9) | 0 (0) | 1 | 0 (0) | 1 | |||||
| HTLV-1 positive | 1 (1.6) | 13 (35.1) | <0.01 | 0 (0) | 1 | |||||
| Recent medication (within 4 weeks) | ||||||||||
| Corticosteroids, n (%) | 9 (14.3) | 7 (18.9) | 0.58 | 7 (26.9) | 0.22 | |||||
| Immunosuppressive agents, n (%) | 10 (15.9) | 8 (21.6) | 0.59 | 4 (15.4) | 1 | |||||
| Laboratory findings | ||||||||||
| Lymphocyte/μL | 1,403 [715-2,043] | 1,695 [1,176-2,162] | 0.70 | 1,014 [357-1,192] | <0.01 | |||||
| ALB, g/L | 38.1 [33.0-43.0] | 37.4 [35.0-43.0] | 0.84 | 29.2 [24.0-42.0] | <0.01 | |||||
| CRP, mg/L | 21.0 [0.9-18.4] | 19.8 [1.0-12.0] | 0.72 | 82.9 [6.1-126.5] | <0.01 | |||||
* High nil-control versus determinate results, ** Low mitogen-control versus determinate results
DM: diabetes mellitus, TB: active, old, and latent tuberculosis, HIV: human immunodeficiency virus, HTLV-1: human T-cell leukemia virus type 1, ALB: serum albumin, CRP: C-reactive protein, ND: not detected.
Continuous variables are expressed as means or median [IQR]. Categorical information is n (percent).
Glucocorticoids were administered to approximately 20% of patients, and immunosuppressive medications such as methotrexate, mycophenolate, calcineurin inhibitors, azathioprine, tumor necrosis factor inhibitors, and 5-aminosalicylic acid, were administered to approximately 17% of patients. There were no biological agents administered to patients.
High nil-control
We divided the indeterminate results into two groups as described above and analyzed the influencing factors (Table 1).
The only factor found to influence high nil-control was HTLV-1 positivity (p<0.01). Previously reported factors influencing high nil-control included male gender, Behçet's syndrome, use of corticosteroids, hypoalbuminemia, and HTLV-1 positivity (6,17). Age, gender, corticosteroid use, hypoalbuminemia, and HTLV-1 positivity were chosen as explanatory variables. There were no participants with Behçet's syndrome. The only factor found to influence high nil-control in the conditional multivariate logistic model was HTLV-1 positivity [adjusted odds ratio (OR)=98.5, 95% confidence interval (CI): 6.58-1,480] (Table 2).
Table 2.
Factors for High Nil-control in a Conditional Multivariate Logistic Model.
| aOR | 95% CI | p value | |
|---|---|---|---|
| Age >60 years | 0.44 | 0.11-1.74 | 0.24 |
| Male | 1.63 | 0.42-6.36 | 0.48 |
| Use of corticosteroids | 0.42 | 0.04-4.00 | 0.45 |
| ALB <35g/L | 1.27 | 0.29-5.63 | 0.75 |
| HTLV-1 positive | 98.5 | 6.58-1480 | <0.01 |
ALB: serum albumin, HTLV-1: human T-cell leukemia virus type 1, aOR: adjusted odds ratio, and CI: confidence interval
Low mitogen-control
In contrast to high nil-control, several factors were found to influence low mitogen-control, including diabetes mellitus (DM), TB, the lymphocyte count, serum albumin (ALB) level, and C-reactive protein (CRP) level (Table 1).
Previously reported factors influencing low mitogen-control were inactive TB, younger age, and HIV positivity (2,18). We selected gender, TB, lymphocyte count, ALB level, and CRP level as explanatory variables. There were no HIV-positive participants and a small number of participants under 5 years old (n=2); therefore, these two items were not analyzed.
In our conditional multivariate logistic model, no factor was found to be statistically significantly influential for low mitogen-control.
Discussion
In the present study, the frequency of indeterminate results was 1.1%. Indeterminate results have been reported with a frequency of 0-5.4% in the general population and 3.5-33% in immunocompromised patients for T-SPOT.TB (6). Our study showed a similar frequency to previous reports.
The factors influencing indeterminate results of T-SPOT.TB were reported to include the CD4 count, age, lymphocyte count, and underlying disease (1-12). However, some studies have reported in influence of these factors (7,9,13,14). The reason for these contradictory results was thought to be that the analysis was performed without distinguishing between high-nil-control and low-mitogen-control.
We compared the outcomes of cases of high nil-control and low mitogen-control. HTLV-1 positivity was the only influencing factor associated with high nil-control. In a conditional multivariate logistic model, there was no statistically significant factor associated with low mitogen-control, although several factors were listed as potential influencing factors in the univariate analysis, including DM, TB, a low lymphocyte count, hypoalbuminemia, and a high CRP level (See Tables 1-3). There was no consistent tendency toward high nil-control or low mitogen-control in patients with malignant solid tumors, rheumatoid arthritis, inflammatory bowel disease, and the use of corticosteroids and immunosuppressants. Due to the small number of subjects, HIV-positive and hematological malignancies were not statistically examined (Table 1). Our search revealed no reports of increased indeterminate results of T-SPOT.TB in diabetic participants, and conversely, there were reports that T-SPOT.TB was useful, even in diabetic participants (19). It has been reported that the production of IFN-γ is decreased in diabetic patients, and a negative correlation of production of IFN-γ with glycemic control was noted (20,21). Another report suggested increased IFN-γ production in diabetic patients, but this IFN-γ was dysfunctional because of glycation (22). A decrease in or dysfunction of IFN-γ may therefore have affected low mitogen-control.
Table 3.
Factors for Low Mitogen-control in a Conditional Multivariate Logistic Model.
| aOR | 95% CI | p value | |
|---|---|---|---|
| Male | 1.22 | 0.35-4.3 | 0.76 |
| DM | 4.28 | 0.74-24.8 | 0.11 |
| TB | 3.85 | 0.37-40.6 | 0.26 |
| Lymphocyte count <1,000/μL | 3.02 | 0.75-12.1 | 0.12 |
| ALB <35 g/L | 3.38 | 0.45-24.8 | 0.23 |
| CRP >40 mg/L | 1.68 | 0.40-18.0 | 0.31 |
DM: diabetes mellitus, TB: active and old tuberculosis, ALB: serum albumin, CRP:C-reactive protein, aOR: adjusted odds ratio, CI: confidence interval
TB itself can also influence low mitogen-control, so if T-SPOT.TB shows indeterminate results, we recommend that other tests be used for the diagnosis.
In our study, hypoalbuminemia, low lymphocyte counts, and low CRP levels were also influential factors for low mitogen-control. These laboratory results can be abnormal in cases of chronic inflammation and malnutrition. In chronic inflammation, induction of T helper lymphocyte 2 (Th2) may protect the organism from systemic “overshooting” with Th1 and pro-inflammatory cytokines, such as INF-γ (23). It has also been reported that malnutrition reduces INF-γ production (24). Unfortunately, no clear link has been found between these reports and a low-mitogen response.
HTLV-1 positivity was the only factor significantly influencing high nil-control in a multivariate analysis. On restricting the assessment to HTLV-1-positive cases with indeterminate results, all cases involved high nil-control, with none showing a low-mitogen response. Evidence has been shown that HTLV-1 infects Treg cells and produces IFN-γ, thus promoting inflammation. (25-29). HTLV-1-infected individuals have increased susceptibility to M. tuberculosis infection (30), but their response to T-SPOT.TB and its impact on the disease status is unknown. Our search found no reports investigating the relationship between HTLV-1 positivity and the QuantiFERON™-TB Gold. HTLV-1 is found globally, with some clusters of high endemicity found. The main regions where HTLV-1 is highly endemic are the Southwestern part of Japan, sub-Saharan Africa and South America, the Caribbean, and foci in the Middle East and Australia-Melanesia (31). These regions may benefit from the usefulness of T-SPOT.TB and a TB diagnosis.
Of the 5,956 participants, 40 were HTLV-1-positive, and 38 had determinate results (3 were positive and 35 were negative), while 2 had indeterminate results (data not presented). T-SPOT.TB is useful, but when it cannot be utilized for a diagnosis, other testing methods, such as QuantiFERON™-TB Gold, chest imaging, and culture, should be applied instead.
Several limitations associated with the present study warrant mention. First, because this was a retrospective study, some important laboratory examinations were not performed on all subjects, reducing the statistical power of identifying some potential risk factors, such as HIV. Second, there was a risk of selection bias because the T-SPOT.TB was not performed on all patients, such as those for whom immunosuppressive drug use was planned. Third, because of the small number of samples analyzed, there was a possibility of no significant difference, so more case accumulation is desirable. Fourth, previous research has shown that indeterminate results are more common in HIV patients than in others. We were unable to assess the impact of HIV positivity on the frequency of indeterminate results because only one HIV-positive patient was included. Fifth, of the 40 HTLV-1-positive participants, 38 had determinate results (3 were positive and 35 were negative), while 2 had indeterminate results (data not presented). Due to the small number of patients, we did not analyze the difference between determinate and indeterminate results of HTLV-1-positive participants.
In conclusion, indeterminate results were detected in 63 (1.1%) participants, including high nil-control in 37 and low mitogen-control in 26. The only influencing factor for high nil-control was HTLV-1 positivity. As a cause of a high-nil response, it was suspected that abnormally produced INF-γ may have caused a nonspecific reaction to the negative control well. Furthermore, in a univariate analysis, the possible influential factors for low mitogen-control included DM, TB, a low lymphocyte count, hypoalbuminemia, and a high CRP level. In these situations, it was suspected that a decrease or dysfunction of IFN-γ may have led to low mitogen-control. The association between TB and a low-mitogen response was unclear. It was suggested that the fact that TB itself influenced low mitogen-control makes the diagnosis more difficult.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This research was supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP22fk0108127.
Acknowledgement
We thank all staff of Chiba University Hospital for supporting sample collection.
References
- 1.World Health Organization. Tuberculosis [Internet]. [cited 2022 Mar 1]. Available from: https://www.who.int/health-topics/tuberculosis#tab=tab_1
- 2.World Health Organization. The top 10 causes of death [Internet]. [cited 2022 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 3.Centers for Disease Control and Prevention. Latent TB infection and TB disease [Internet]. [cited 2022 Mar 1]. Available from: https://www.cdc.gov/tb/topic/basics/tbinfectiondisease.htm
- 4.World Health Organization. Latent tuberculosis infection [Internet]. [cited 2022 Mar 1]. Available from: https://www.who.int/tb/publications/2018/executivesummary_consolidated_guidelines_ltbi.pdf?ua=1
- 5. Mazurek GH, Jereb J, Vernon A, Centers for Disease Control and Prevention, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infections - United States, 2010. MMWR 59: 1-25, 2010. [PubMed] [Google Scholar]
- 6. Sun X, Wan S, Zhang L, Zhang Y, Liu X. Prevalence and influencing factors of the high nil-control spot count in T-SPOT.TB: a matched case-control study. Clin Chim Acta 487: 96-100, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Kobashi Y, Sugiu T, Shimizu H, et al. Clinical evaluation of the T-SPOT.TB test for patients with indeterminate results on the QuantiFERON TB-2G test. Intern Med 48: 137-142, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Lee YM, Kim SM, Park SJ, et al. Indeterminate T-SPOT.TB test results in patients with suspected extrapulmonary tuberculosis in routine clinical practice. Infect Chemother 45: 44-50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Sun J, Zhang R, et al. T-SPOT.TB in the diagnosis of active tuberculosis among HIV-infected patients with advanced immunodeficiency. AIDS Res Hum Retroviruses 27: 289-294, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Santin M, Munoz L, Rigau D. Interferon-γ release assays for the diagnosi of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One 7: e32482, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciappini E, Bonsignori F, Accetta G, et al. Interferon-γ release assays for the diagnosis of Mycobacterium tuberculosis infect in children: a literature review. Int J Immunopathol Pharmacol 25: 335-343, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Lagrange PH, Herrmann JL. Diagnosing latent tuberculosis infection in the HIV era. Open Respir Med J 2: 52-59, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meier NR, Volken T, Geiger M, Heininger U, Tebruegge M, Ritz N. Risk factors for indeterminate interferon-gamma release assay for the diagnosis of tuberculosis in children - a systematic review and meta-analysis. Front Pediatr 7: 208, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elzi L, Steffen I, Furrer H, et al. Improved sensitivity of an interferon-gamma release assay (T-SPOT.TB™) in combination with tuberculin skin test for the diagnosis of latent tuberculosis in the presence of HIV co-infection. BMC Infect Dis 11: 319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxford Immunotec. Package insert foe in vitro diagnostic use [Internet]. [cited 2022 Mar 1]. Available from: http://www.oxforddiagnosticlaboratories.eu/wp-content/media/PI-TB-IVD-UK-v3.pdf
- 16. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Umekita K, Hashiba Y, Iwao K, et al. Human T-cell leukemia virus type 1 may invalidate T-SPOT.TB assay results in rheumatoid arthritis patients: a retrospective case-control observational study. PLoS ONE 15: e0233159, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandalakas AM, Highsmith HY, Harris NM, Pawlicka A, Kirchner HL. T-SPOT.TB performance in routine pediatric practice in a low TB burden setting. Pedatr Infect Dis J 37: 292-297, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Walsh MC, Camerlin AJ, Miles R, et al. The sensitivity of interferon-γ release assays is not compromised in tuberculosis patients with diabetes. Int J Tuberc Lung Dis 15: 179-184, i-iii, 2001. [PMC free article] [PubMed] [Google Scholar]
- 20. Stalenhoef JE, Alisjahbana B, Nelwan EJ, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbil Infect Dis 27: 97-103, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Tsukaguchi K, Okamura H, Ikuno M, et al. The relation between diabetes mellitus and IFN-gamma, IL-12 and IL-10 productions by CD4+ alpha beta T cells and monocytes in patients with pulmonary tuberculosis. Kekkaku 72: 617-622 (in Japanese). [PubMed] [Google Scholar]
- 22. Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis 47: 634-641, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calcagni E, Elenkov I. Stress system activity, innate and T helper cutokines, and susceptibity to immune-related diseases. Ann Y Acad Sci 1069: 62-76, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez L, Gonzalez C, Flores L, Zamudio LJ, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol 12: 502-507, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamano Y, Araya N, Sato T, et al. Abnormally high levels of virus-infected IFN-γ+CCR4+CD4+CD25+Tcells in a retrovirus-associated neuroinflammatory disorder. PLos ONE 4: e6517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Futsch N, Prates G, Mahieux R, Casseb J, Dutartre H. Cytokine networks dysregulation during HTLV-1 infection and associated diseases. Viruses 10: 691, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welles SL, Tachibana N, Okayama A, et al. Decreased reactivity to PPD among HTLV-1 carriers in relation to virus and hematologic status. Int J Cancer 56: 337-340, 1994. [DOI] [PubMed] [Google Scholar]
- 28. Tachibana N, Okayama A, Ishizaki J, et al. Suppression pf tuberculin skin reaction in healthy HTLV-1 carriers from Japan. Int J Cancer 42: 829-831, 1988. [DOI] [PubMed] [Google Scholar]
- 29. Araya N, Sato T, Ando H, et al. HTLV-1 induces a Th1-like state in CD4+CCR4+T cells. J Clin Invest 124: 3431-3442, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Souaz A, Carcalho N, Neves Y, et al. Association of tuberculosis status with nerologic diseases and immune response in HTLV-1 infection. AIDS Res Hum Retroviruses 33: 1126-1133, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3: 388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]