Abstract
Thyroid storm is a life-threatening clinical condition that is usually triggered by untreated or interrupted treatment of Graves' disease, leading to the sudden onset of severe thyrotoxicosis, which requires an immediate diagnosis and treatment based on diagnostic criteria. Cases of thyroid storm caused by painless/painless subacute thyroiditis are very rare. We herein report an 85-year-old man with features of severe thyrotoxicosis caused by painless/painless subacute thyroiditis who had no uptake of 99mTcO4 and was negative for thyroid-stimulating hormone receptor antibodies. In thyroid storm patients in whom the findings are inconsistent with Graves' disease, careful follow-up and management are necessary, assuming the possibility of painless or painless subacute thyroiditis.
Keywords: thyroid storm, painless thyroiditis, thyrotoxicosis
Introduction
Thyroid storm is a life-threatening situation caused by high thyroid hormone levels and physical stress, leading to multiple organ failure. A high fever, circulatory failure, impaired consciousness, diarrhea, jaundice, and other symptoms are common in thyroid storm. The treatment includes antithyroid medication, inorganic iodine, and corticosteroids. Although most cases are caused by poorly controlled Graves' disease, other causes, such as thyroid-stimulating hormone (TSH)-producing tumors and destructive thyroiditis (due to immune checkpoint inhibitors, amiodarone, or lithium therapy), have also been reported (1,2).
Painless thyroiditis is usually characterized by mild to moderate thyrotoxicosis, moderate goiter, a low radioiodine uptake, and negativity for TSH receptor antibodies. Histologically, the thyroid gland shows lymphocytic infiltration, sometimes with germinal centers, focal Hürthle cell changes, and/or focal hyperplasia of the thyroid epithelium surrounding the areas of lymphocytic infiltration (3). Painless thyroiditis is an autoimmune-mediated inflammation of the thyroid gland, with release of thyroid hormones resulting in transient hyperthyroidism, lasting about two months before recovery, and usually does not require treatment. Subsequently, a period of hypothyroidism often ensues, before recovery of the thyroid function. The timing of the transition to permanent hypothyroidism can be early or late, ranging from many years to decades later (4).
Subacute thyroiditis tends to present with pain in the anterior neck followed by upper respiratory tract infection or sore throat and is postulated as being caused by viral infection. Inflammatory symptoms, such as nodular or diffuse enlargement of the goiter with spontaneous pain, tenderness and a fever, are also seen, as are symptoms of thyrotoxicosis, such as palpitations (5). Echography shows an indistinct hypoechoic area with reduced blood flow signals, in line with the induration, enlargement or pain, and in about 60% of cases, enlarged lymph nodes around the thyroid gland are reported (6) It is rare in children and the elderly (7), and most cases heal within a few months, although about 20% relapse during recovery (8,9), and 15% develop permanent hypothyroidism (9).
We herein report a rare case of a patient who developed thyroid storm secondary to painless destructive thyroiditis.
Case Report
An 85-year-old Japanese man (163.0 cm tall, weighing 50.7 kg, body mass index 19.1 kg/m2) with atrial fibrillation and Lewy body dementia presented to the emergency department of another hospital with a fever and palpitations (Day 0). The fever and palpitations did not improve despite the use of antipyretics, and the patient was admitted to the hospital six days later with congestive heart failure.
Laboratory tests after admission revealed thyrotoxicosis with TSH <0.007 μIU/mL [normal value (NV): 0.61-4.23] and free T4 >7.77 ng/dL (NV: 0.76-1.65). Fifteen milligrams of thiamazole was started on Day 10 but was discontinued because of TSH receptor antibody (TRAb) negativity. On the same day, he developed psychiatric symptoms, watery diarrhea, and a high fever of 40.6°C. Due to suspicion of thyroid storm, the patient was urgently transferred to our hospital and admitted to our department.
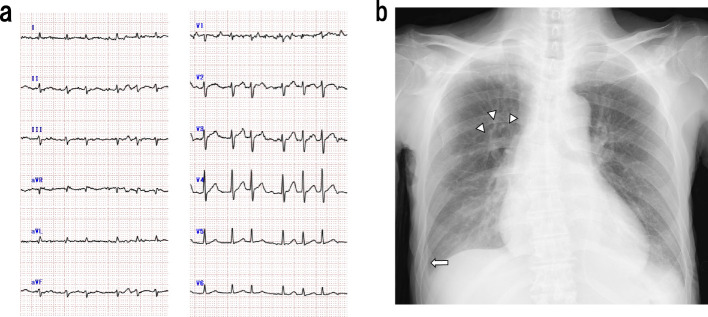
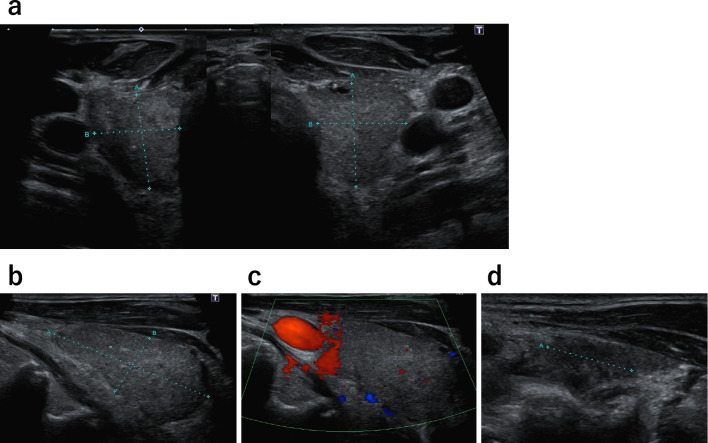
A physical examination on admission showed an impaired consciousness (Glasgow Coma Scale score: E3V2M5), fever of 38-40°C, blood pressure of 85/53 mmHg, peripheral oxygen saturation on pulse oximetry (SpO2) of 97.0% under 1 L/min oxygen via a nasal cannula, and tachycardia with arrhythmia (136 beats/min). There was no cervical swelling, and no vascular murmur was noted. Coarse crackles were present in both lung fields, and intestinal peristalsis was increased. An electrocardiogram showed tachycardic atrial fibrillation (132/min) with no ST-T changes (Fig. 1a). Chest X-ray demonstrated congestion in the lung fields bilaterally, along with pleural effusion and peribronchial cuffing, with a cardiothoracic ratio of 59.7% (Fig. 1b). An ultrasound examination of the thyroid gland showed no glandular enlargement (estimated thyroid volume approximately 17 g) and no hypervascularization. The thyroid parenchyma showed heterogeneous echogenicity, with a hypoechoic area 14 mm in size in the left lobe (Fig. 2).
Figure 1.
Electrocardiography and chest radiography findings. a) Electrocardiography showed tachycardic atrial fibrillation. b) Posteroanterior chest radiography. The arrowheads indicate peribronchial cuffing, and the arrow indicates pleural thickening-like findings.
Figure 2.
Thyroid ultrasound. a) The thyroid gland showed no enlargement and presented heterogeneous parenchyma. b) Right thyroid lobe in a sagittal view (41×21×19 mm). c) Thyroid doppler ultrasound showed no hypervascularization. d) Sagittal view of the left thyroid lobe (42×23×17 mm) showed the presence of a 14-mm hypoechoic area. The estimated thyroid volume was approximately 17 g.
The laboratory findings at the time of admission showed an increased leukocyte count [white blood cells (WBCs)] with leftward nuclear migration, elevated C-reactive protein (CRP) levels, mild anemia, and coagulation abnormalities [WBCs 21,820 /μL, neutrophils 201,000 /μL lymphocytes 1,100 /μL, CRP level 4.89 mg/dL, hemoglobin (Hb) level 10.1 g/dL, prothrombin time-international normalized ratio (PT-INR) 2.09, fibrinogen degradation product level 6.32 μg/mL, and D-dimer (D-D) level 1.72 μg/mL] (Table). In addition, elevated alkaline phosphatase (ALP), γ-glutamyltransferase (γ-GTP), direct bilirubin (D-Bil), adrenocorticotropic hormone (ACTH), cortisol (CORT), and brain natriuretic peptide (BNP) values (ALP 172 U/L, γ-GTP 131 U/L, D-Bil 0.58 mg/dL, ACTH 211 pg/mL, CORT 20.2 μg/dL and BNP 497.5 pg/dL) and a decrease in the estimated glomerular filtration rate (eGFR) and albumin (Alb) level (eGFR 40.7 mL/min/1.73 m2 and Alb 2.42 g/dL) were observed (Table).
Table.
Laboratory Findings on Admission.
| Peripheral blood | WBC | 21,820 | /μL | (3,300-8,600) |
| NEUT (Neut) | 20.1 (92.0) | ×103/μL (%) | [1.50-7.50 (42.0-74.0)] | |
| LYMPH (Lymph) | 1.1 (5.0) | ×103/μL (%) | [1.00-4.00 (18.0-50.0)] | |
| RBC | 330 | ×104/μL | (435-555) | |
| Hb | 10.1 | g/dL | (13.7-16.8) | |
| MCV | 86.1 | fL | ||
| MCH | 30.6 | pg | ||
| MCHC | 35.6 | g/dL | (31.7-35.3) | |
| HCT | 28.4 | % | (40.7-50.1) | |
| PLT | 22.4 | ×104/μL | ||
| Coagulation profile | PT | 34 | % | (82.7-117.7) |
| PT-INR | 2.09 | (0.98-1.08) | ||
| APTT | 30.3 | s | ||
| Fib | 390 | mg/dL | ||
| FDP | 6.32 | μg/mL | (<5) | |
| D-dimer | 1.72 | μg/mL | (<1) | |
| Biochemistry | AST | 33 | U/L | |
| ALT | 15 | U/L | ||
| LDH | 221 | U/L | ||
| ALP | 172 | U/L | (38-113) | |
| γ-GTP | 131 | U/L | (13-64) | |
| T-Bil | 0.94 | mg/dL | ||
| D-Bil | 0.58 | mg/dL | (0.00-0.30) | |
| CK | 124 | U/L | ||
| TP | 5.39 | g/dL | (6.60-8.10) | |
| Alb | 2.42 | g/dL | (4.10-5.10) | |
| BUN | 29.7 | mg/dL | (8.0-20.0) | |
| CRE | 1.3 | mg/dL | (0.65-1.07) | |
| eGFR | 40.7 | mL/min/1.73 m2 | (60<) | |
| FPG | 104 | mg/dL | ||
| CRP | 4.89 | mg/dL | (0.00-0.14) | |
| Na | 138 | mmol/L | ||
| K | 3.9 | mmol/L | ||
| Cl | 102 | mmol/L | ||
| Urinalysis | Protein | (-) | ||
| WBC | (-) | |||
| Occult blood | (-) | |||
| Others | ACTH | 211 | pg/mL | (7.2-63.3) |
| CORT | 20.2 | μg/dL | (7.1-19.6) | |
| BNP | 497.5 | pg/mL | (0.0-18.4) | |
| SARS-CoV-2 PCR | (-) |
The reference ranges of data showing abnormal values are shown in brackets. WBC: white blood cell, NEUT: neutrophil (absolute value), Neut: neutrophil (percentage), LYMPH: lymphocyte (absolute value), Lymph: lymphocyte (percentage), RBC: red blood cell, Hb: hemoglobin, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, HCT: hematocrit, PLT: platelets, PT: prothrombin time, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time, Fib: fibrinogen, FDP: fibrin/fibrinogen degradation products, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyltransferase, ChE: cholinesterase, T-Bil: total bilirubin, D-Bil: direct bilirubin, CK: creatine kinase, TP: total protein, Alb: albumin, Na: sodium, K: potassium, Cl: chloride, BUN: blood urea nitrogen, CRE: creatinine, UA: uric acid, eGFR: estimated glomerular filtration rate, FPG: fasting blood glucose, CRP: C-reactive protein, ACTH: adrenocorticotropic hormone, CORT: cortisol, BNP: brain natriuretic peptide, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
The results of endocrinological tests were as follows: TSH: 0.028 μIU/mL; free T4: 5.27 ng/dL; and free T3: 14.32 pg/mL (NV: 2.48-4.14). Since the patient's laboratory test findings met the diagnostic criteria of thyrotoxicosis, and given that he had relevant symptoms, such as central nervous system effects, a fever, tachycardia, cardiovascular dysfunction, and watery diarrhea, thyroid storm was diagnosed as the probable cause of his condition (2,3).
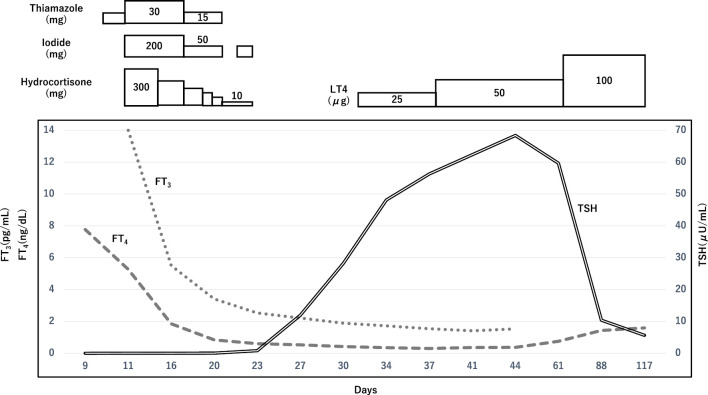
The patient was subsequently admitted to the intensive care unit for intensive monitoring. Oxygen was administered via a nasal cannula at 4-5 L/min on day 1, with maintenance of SpO2 at 94-96%; after day 2, similar SpO2 levels were maintained by an oxygen flow of 1-2 L/min via nasal cannula. He was cooled and managed with a continuous infusion of landiolol (3 γ), 5 mg propranolol, hydrocortisone (100 mg ×8 h), 30 mg thiamazole, and 200 mg iodide administered orally via a gastric tube. With this treatment, his level of consciousness improved to E4V4M5 the next day, and his thyroid hormone levels improved relatively quickly to free T4 of 1.85 ng/dL and free T3 of 5.5 pg/mL on the 6th day from the start of treatment. Since the patient's heart rate remained under control from the second day of starting treatment, the dose of propranolol was increased to 7.5 mg, and landiolol was reduced to 2 γ following the advice of the cardiologist. Landiolol was further reduced to 1 γ on the 4th day and discontinued on the 5th day. Subsequently, propranolol was reduced to 5 mg on the 13th day, and to 2.5 mg on the 23rd day.
Since the patient's thyroglobulin levels were elevated [537 ng/mL (NV: ≤33.7)] with the various thyroid antibody titers remaining within normal limits [anti-thyroglobulin antibody: 11.1 IU/mL (NV: <28.0); anti-thyroid peroxidase antibody: 4.7 IU/mL (NV: <16.0); TRAb (3rd): 0.7 IU/L (NV: <2.0), and thyroid-stimulating antibody (TSAb): 104% (NV: ≤120)], the possibility of transient thyrotoxicosis caused by thyroid follicular cell destruction was considered. In addition, although the patient received daily doses of rivaroxaban 10 mg, azosemide 30 mg, donepezil 5 mg, levodopa 200 mg/benserazide hydrochloride 50 mg, ramelteon 8 mg, lemborexant 5 mg, and 1.5 mg of clonazepam prior to the onset of thyroid storm, induction of thyroiditis by these drugs was ruled out.
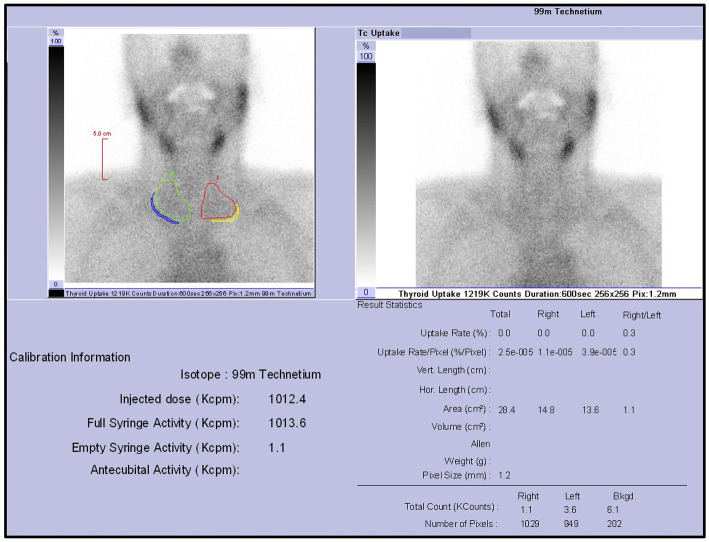
Since there were several criteria that were inconsistent with Graves' disease, such as rapid improvement of the thyroid hormone levels and TRAb or TSAb negativity, scintigraphy was performed to identify the cause of the thyrotoxicosis. Scintigraphy of the thyroid gland showed a radioactive 99mTcO4 uptake of 0% (TSH value 0.813 μIU/mL following a 50-mg dose of inorganic iodine administration on day 12), confirming the diagnosis of thyrotoxicosis due to destructive thyroiditis (Fig. 3). The patient's thyroid hormone levels normalized after 10 days without recurrence after tapering of hydrocortisone, thiamazole, and iodide. However, because of a further reduction in the thyroid hormone levels and a TSH value of 11.9 μIU/mL on Day 26, levothyroxine replacement therapy was started. The patient's level of consciousness did not improve beyond the Glasgow Coma Scale (GCS) score of E4V4M5 on day 2 of admission, probably due in part to Lewy body dementia, and he had difficulty with oral intake. However, his body weight increased by about 2 kg. On day 54, the patient was transferred back to the referring hospital to continue levothyroxine supplementation (Fig. 4).
Figure 3.
Thyroid static imaging with 99mTcO4 showed a decreased uptake in the thyroid gland (0%).
Figure 4.
The patient's clinical course. Treatment for thyroid storm that was presumed to be caused by Grave's disease was commenced after the patient's admission to the hospital. Subsequently, the patient’s thyroid hormone levels improved relatively quickly and normalized without recurrence after tapering of hydrocortisone, thiamazole, and iodide. Thereafter, the further reduction of hormone levels continued, and levothyroxine replacement therapy was started on Day 29.
Discussion
We suspected that physiological stress resulting from infectious disease had increased the ACTH levels via stimulation by inflammatory cytokines, such as Interleukin-6, leading to increased cortisol secretion, so we ruled out concomitant adrenal insufficiency.
Our patient had thyrotoxicosis with central nervous system symptoms, a fever over 38°C, tachycardia over 130/min, heart failure symptoms, and watery diarrhea, which led us to definitively diagnose him with thyroid storm (10). An evaluation using the Burch-Wartofsky point scale (11) also revealed a total score of 75 points, indicating a strong possibility of thyroid storm. However, the low total GCS score of 10 points in this patient only improved to 13 points despite a rapid recovery of his other symptoms, suggesting the difficulty in making judgments regarding central nervous system symptoms in cases complicated by dementia.
In the present case, although thyroid storm was definitively diagnosed by two different sets of criteria, the clinical picture was confounded by the absence of multiorgan failure (12) and limited improvement in neurological symptoms with treatment. Thyroid storm is a very rare condition with an annual incidence of just two cases per million people in Japan (10). It consists of severe hyperthyroidism with physiologic decompensation of one or more organ systems and carries a relatively high mortality rate (about 10%) (10,13,14). Graves' disease is the most common causative disease (about 98%), in which withdrawal of anti-thyroid therapy might trigger thyroid storm (10). In contrast, since destructive thyroiditis typically causes only mild to moderate thyrotoxicosis, it only rarely causes thyroid storm. In a nationwide survey conducted in Japan in 2012, only 5 cases of destructive thyroiditis were reported among 356 with thyroid storm (10), although there have been reports of subacute thyroiditis (15-19) and drug (immune checkpoint inhibitors, amiodarone, or lithium)-induced destructive thyroiditis leading to thyroid storm (1,2).
A literature search revealed only one previous report of painless thyroiditis causing thyroid storm (20), indicating that it is extremely rare. The reason why thyroid storm due to subacute thyroiditis is reported more frequently than that due to painless thyroiditis is because subacute thyroiditis produces a strong inflammatory response, which might lead to thyroid storm due to the production of inflammatory cytokines and their effect on thyroid hormone metabolism (13). Furthermore, it has been reported that subacute thyroiditis is significantly more prone to causing hypoalbuminemia than painless thyroiditis (21), and the state of physical wasting due to intense inflammation might be a contributing factor. It is interesting that the case reported by Harada et al. was confirmed at pathological autopsy to be advanced chronic thyroiditis, possibly atrophic thyroiditis (20), and that the laboratory findings and course of the disease were very similar to those of our case.
In the present case, we were unable to identify any obvious risk factors for the development of thyroid storm. Given the elevated WBC and neutrophil counts and CRP level at the time of admission (Table), and the fact that the risk factors for Graves' disease leading to thyroid storm include infection and stress, in addition to those related to treatment and examinations, we speculate that some infection might have been involved in this case. A thyroid storm might occur in an elderly person with physiological impairment following the induction of thyrotoxicosis by painless thyroiditis and when the patient is under some kind of stress or infection. The mortality rate has been reported to be significantly higher in older patients with thyroid crisis than in younger patients (14). It is speculated that the low frequency of typical symptoms and the coexistence of nonspecific signs and symptoms caused by other diseases and aging might delay the diagnosis and treatment in this patient group (22), so they require more careful attention than others.
In cases of thyroid storm due to destructive thyroiditis, antithyroid drugs and inorganic iodine are ineffective and should not be administered. However, in the present case, it took five days for the TSH receptor antibody status to be known. In medical institutions where a real-time definitive diagnosis is as difficult as in our hospital, we believe that the high mortality rate of thyroid storm and the infrequency of the causative disease should not deter the prompt initiation of treatment (including the administration of antithyroid drugs and inorganic iodine) as in thyroid storm due to Graves' disease. However, if Graves' disease is ruled out, antithyroid drugs and inorganic iodine, which are ineffective for thyrotoxicosis due to destructive thyroiditis, should be promptly and rapidly discontinued, since high doses of antithyroid drugs can cause adverse drug reactions, such as liver dysfunction and agranulocytosis, which require careful monitoring.
In the present case, the diagnosis of destructive thyroiditis was made with an emphasis on the radioactive 99mTcO4 uptake of 0%, but its diagnostic accuracy is limited by the fact that the result was obtained after a large dose of inorganic iodine had been administered.
Thyrotoxicosis varies in severity, and its course is usually not protracted and might not require treatment. However, about 10% of patients with painless thyroiditis have recurrent episodes, and several authors have reported troublesome and recurrent cases requiring total thyroidectomy or radioactive iodine therapy. In our case, however, since a hypoechoic area was identified in the left lobe of the thyroid gland on ultrasound (Fig. 2d) and his CRP level was elevated (4.89 mg/dL) (Table), the possibility of painless subacute thyroiditis, which Neupane et al. reported as a cause of an unknown fever (23), could not be excluded.
Recently, cases of painless subacute thyroiditis have been reported in relation to COVID-19 infection (24). However, there have been no reports of painless subacute thyroiditis leading to thyroid storm, and if painless subacute thyroiditis was indeed the cause of the destructive thyroiditis in our case, this would make it the first such report. A limitation of this report is that we were unable to confirm the histological findings in the thyroid gland.
Conclusion
We herein report a rare case of thyroid storm presumably due to painless thyroiditis or painless subacute thyroiditis. The triggers for this patient's thyroid storm were not clear. Thyroid storm is a highly fatal condition that should be diagnosed and treated promptly using diagnostic criteria. While Graves' disease is the most common cause of thyroid storms, in cases with contradictory findings, the possibility of destructive thyroiditis, such as painless thyroiditis or painless subacute thyroiditis, should also be considered.
Written, informed consent was obtained from the patient and his family for publication of this case report and all accompanying images.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors wish to thank the patient and his family for their permission to publish this manuscript.
References
- 1. McMillen B, Dhillon MS, Yong-Yow S. A rare case of thyroid storm. BMJ Case Rep 2016: bcr2016214603, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raza MA, Jain A, Mumtaz M, et al. Thyroid storm in a patient on chronic amiodarone treatment. Cureus 14: e24164, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mizukami Y, Michigishi T, Hashimoto T, et al. Silent thyroiditis: a histologic and immunohistochemical study. Hum Pathol 19: 423-431, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Akamizu T, Amino N. Hashimoto's Thyroiditis. In: Endotext [Internet]. Feingold KR, Anawalt B, Blackman MR, et al., Eds. MDText.com, South Dartmouth, 2017 Jul 17 [cited 2023 Feb 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK285557/
- 5.Hennessey Jv. Subacute Thyroiditis. In: Endotext [Internet]. Feingold KR, Anawalt B, Blackman MR, et al., Eds. MDText.com, South Dartmouth, 2018 Jun 12 [cited 2023 Feb 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279084/
- 6. Frates MC, Marqusee E, Benson CB, et al. Subacute granulomatous (de Quervain) thyroiditis: grayscale and color Doppler sonographic characteristics. J Ultrasound Med 32: 505-511, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Nishihara E, Ohye H, Amino N, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med 47: 725-729, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Mizukoshi T, Noguchi S, Murakami T, et al. Evaluation of recurrence in 36 subacute thyroiditis patients managed with prednisolone. Intern Med 40: 292-295, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Fatourechi V, Aniszewski JP, Fatourechi GZ, et al. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab 88: 2100-2105, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Akamizu T, Satoh T, Isozaki O, et al.; Japan Thyroid Association. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 22: 661-679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 22: 263-277, 1993. [PubMed] [Google Scholar]
- 12. Angell TE, Lechner MG, Nguyen CT, et al. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab 100: 451-459, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Akamizu T. Thyroid storm: a Japanese perspective. Thyroid 28: 32-40, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ono Y, Ono S, Yasunaga H, et al. Factors associated with mortality of thyroid storm: analysis using a national inpatient database in Japan. Medicine (Baltimore) 95: e2848, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherman SI, Simonson L, Ladenson PW. Clinical and socioeconomic predispositions to complicated thyrotoxicosis: a predictable and preventable syndrome? Am J Med 101: 192-198, 1996. [DOI] [PubMed] [Google Scholar]
- 16. Swinburne JL, Kreisman SH. A rare case of subacute thyroiditis causing thyroid storm. Thyroid 17: 73-76, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Salih AM, Kakamad FH, Rawezh QS, et al. Subacute thyroiditis causing thyrotoxic crisis; a case report with literature review. Int J Surg Case Rep 33: 112-114, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaballa S, Hlaing KM, Bos N, et al. A rare case of subacute painful thyroiditis causing thyroid storm and a successful trial of propylthiouracil. Cureus 29: e9461, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman K, Walthall L. A case of thyroid storm caused by thyroiditis. J Investig Med High Impact Case Rep 10: 23247096221129468, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harada Y, Handa M, Koyama K, et al. Atypical case of thyroid storm revealed by autopsy. Int J Case Rep Short Rev 3: 49-52, 2017. [Google Scholar]
- 21. Yanai H, Hakoshima M, Katsuyama H. Differences in clinical and laboratory findings among Graves' disease, painless thyroiditis and subacute thyroiditis patients with hyperthyroidism. J Endocrinol Metab 9: 37-42, 2019. [Google Scholar]
- 22. Goichot B, Caron P, Landron F, et al. Clinical presentation of hyperthyroidism in a large representative sample of outpatients in France: relationships with age, aetiology and hormonal parameters. Clin Endocrinol (Oxf) 84: 445-451, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Neupane B, Karki S, Tirthani E, et al. A case of painless subacute thyroiditis presenting as fever of unknown origin. Cureus 14: e24949, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dolkar T, Jitidhar F, Patel MJ, et al. Painless subacute thyroiditis in a patient with acute COVID-19 infection: a transient event. Cureus 14: e26924, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]