Abstract
目的
探究肌肽对糖尿病肾脏病变的保护作用及机制。
方法
将70只大鼠随机分成对照组(CON组,n=14)和高糖高脂组(n=56)。CON组普通饲料喂养,高糖高脂组采用高糖高脂喂养,并结合腹腔注射链脲佐菌素建立损伤模型,将制备损伤模型成功的大鼠随机分为糖尿病组(DM组,n=12)及肌肽低(Car-L,n=13)、中(Car-M,n=13)、高剂量组(Car-H,n=13),分别按照0、100、300和900 mg/(kg·d)肌肽灌胃。测量大鼠体质量及血糖,实验结束时收集大鼠24 h尿液、血清及肾脏,分别测量尿量、尿蛋白含量、血肌酐(Scr)、肾脏质量及HE染色,TBA法检测肾脏组织丙二醛(MDA)含量、NBT法检测超氧化物歧化酶(SOD)活性、Western blot法检测肾脏组织中蛋白激酶B(AKT)、p-AKT、哺乳动物雷帕霉素靶蛋白(mTOR)、p-mTOR、微管相关轻链蛋白1轻链3(LC3)、选择性自噬接头蛋白(p62)的表达。
结果
与CON组相比,DM组大鼠出现毛色暗淡、毛发潮湿等现象;体质量下降,血糖、尿蛋白含量、24h尿量、Scr、肾脏质量均上升;肾小球肿胀变形,肾小管狭窄;SOD活性下降、MDA含量上升;肾脏组织中p-AKT/AKT和p-mTOR/mTOR比值降低,LC3Ⅱ/Ⅰ比值和p62蛋白表达增高(P < 0.01)。与DM组相比,肌肽各组的大鼠毛发恢复光泽、体质量回升,血糖、尿蛋白含量、24 h尿量、Scr、肾脏质量有所下降;肾小球肿胀变形、肾小管狭窄程度有所缓解;SOD活性上升、MDA含量下降(P < 0.01);肾脏组织中p-AKT/AKT和p-mTOR/mTOR比值升高、LC3 Ⅱ/Ⅰ比值降低、p62蛋白表达降低(P < 0.01)。
结论
肌肽对糖尿病大鼠肾脏病变具有保护作用,其保护作用与肌肽抑制氧化应激,激活AKT/mTOR通路,进而恢复自噬功能有关。
Keywords: 肌肽, 糖尿病肾病, 自噬, 蛋白激酶B, mTOR
Abstract
Objective
To explore the mechanisms mediating the protective effect of carnosine against nephropathy in rats with diabetes mellitus (DM).
Methods
Rat models of DM established by high-fat diet feeding and streptozotocin injection were randomized into DM group and 3 treatment groups with daily carnosine treatment at 100, 300, and 900 mg/kg. Body weight and blood glucose level changes of the rats were measured regularly. After the treatment, 24-h urine, serum samples and kidneys of the rats were collected to measure urine volume, urine protein content, blood creatinine, and kidney mass; renal pathology was observed using HE staining, and MDA content and SOD activity in the kidney tissues were detected. Western blotting was performed to detect the protein expressions of p-AKT, AKT, p-mTOR, mTOR, LC3 and p62 in the kidney tissues.
Results
Compared with normal control rats, the diabetic rats exhibited dull and wet hair and showed decreased body weight, increased blood glucose, urinary protein content, 24-h urine volume, blood creatinine, and kidney mass with obvious swelling and deformation of the glomeruli, narrowing of the renal tubules, decreased SOD activity and increased MDA content, lowered p-mTOR/mTOR and p-AKT/AKT ratios and increased LC3 Ⅱ/Ⅰ ratio and p62 protein expression in the kidney tissue. The diabetic rats receiving carnosine treatments had dry hair with normal luster and showed increased body weight and slightly decreased blood glucose, urinary protein content, 24-h urine volume, blood creatinine, and kidney mass. The treatment also improved renal pathology, increased SOD activity, decreased MDA content, increased p-mTOR/mTOR and p-AKT/AKT ratios and lowered LC3 Ⅱ/Ⅰ ratio and p62 protein expression in renal tissue of the diabetic rats.
Conclusion
Carnosine offers protection against nephropathy in rats with DM possibly by inhibiting oxidative stress, activating the AKT/mTOR pathway, and restoring autophagy in the kidneys.
Keywords: carnosine, diabetic kidney disease, autophagy, AKT, mTOR
糖尿病肾脏疾病(DKD)是糖尿病的常见并发症,其发病人数占全球糖尿病患者的20%~40%[1],已成为终末期肾病的第一病因[2, 3]。DKD患者在中国的住院率从2010年的3.58%上升到2017年的4.95%,上升趋势明显[4],同时DKD患者发生不良心血管病、感染和死亡的风险明显升高,给患者及家庭带来巨大经济和精神压力[5]。DKD发病机制十分复杂,现研究主要聚焦在氧化应激、炎症和自噬等方面[3, 6]。DKD治疗方法有限,主要通过控制血压和血糖,降低白蛋白尿来延缓DKD的进展,但效果并不理想[7-9]。因此广泛探索DKD发病机制,并在此基础上开展相关药物研究显得尤为迫切[8-10]。
肌肽是一种天然的、广泛存在于各种脏器的水溶性内源性二肽,具有抗氧化应激、抗炎、抑制蛋白质非酶糖基化等诸多生理功能[11-13]。本团队前期研究表明,肌肽对DKD大鼠具有保护作用,其机制可能与其抑制氧化应激和NF-κB信号通路异常激活有关[14]。但肌肽对DKD大鼠Akt/mTOR信号通路及自噬的影响却未见报道。本实验利用高糖高脂饲料喂养结合STZ腹腔注射制备糖尿病(DM)大鼠模型,通过观察大鼠的体质量和血糖的变化,比较血肌酐(Scr)、尿蛋白、24 h尿量及组织学变化,探讨肌肽对DKD的保护作用,同时进一步观察肾脏组织中AKT、mTOR、LC3、p62蛋白表达的趋势变化,探讨肌肽的作用机制,为DKD的治疗提供理论依据。
1. 材料和方法
1.1. 实验动物
70只健康雄性SD大鼠,体质量150~160 g,购自辽宁长生生物技术股份有限公司。实验动物合格证号:SCXK(辽)2020-0001。动物饲养室温度22±2℃,湿度(55±2)%。动物实验通过锦州医科大学伦理学审批(No.2022032101)。
1.2. 主要试剂和仪器
肌肽,纯度99.9%以上(苏州富士莱医药);高糖高脂饲料、普通饲料(北京博爱港生物技术);链脲佐菌素(Sigma);Akt、p-AKT、mTOR、p-mTOR、LC3、p62、β-actin抗体(武汉爱博泰克生物科技);HE染色试剂盒、SOD、MDA试剂盒、BCA试剂盒(北京索莱宝科技);肌酐和尿蛋白定量试剂盒(南京建成生物)。显影液、RIPA裂解液(沈阳万类生物);快速制胶试剂盒(苏州新赛美生物科技)。倒置显微镜(Olympus);石蜡切片机,德国莱卡;电泳仪、凝胶成像仪、转膜机(Bio-Rad)。MK3酶标分析仪(上海赛默飞世尔)。
1.3. 实验方法
1.3.1. 动物分组和大鼠DM模型建立
将70只大鼠适应性喂养1周后随机分为对照组(CON,n=14)和高糖高脂组(n=56)。CON组普通饲料喂养,高糖高脂组高糖高脂饲料喂养。高糖高脂喂养4周后禁食不禁水12 h,一次性注射30 mg/kg STZ,72 h后Onetouch血糖仪测血糖,共有51只大鼠血糖≥16.7 mmol/L,视为造模成功。将造模成功大鼠随机进一步分为DM组(n=12只)及肌肽低(Car-L,n=13)、中(Car-M,n=13)、高剂量组(Car-H,n=13),各组分别按照每日0 mg/kg、100 mg/kg、300 mg/kg和900 mg/kg肌肽灌胃,各组依然高糖高脂饲料喂养继续喂养12周直到取材。
1.3.2. 样本采集
饲养大鼠期间,7 d/次测定体质量,定期测量血糖值。实验结束前,收集24 h尿液。收集血清后,处死大鼠后取肾脏,一侧肾脏储存于-80℃冰箱中,另一侧肾脏擦干水分放入10%多聚甲醛中进行固定。
1.3.3. 血肌酐(Scr)、24 h尿蛋白含量测定
随机取8个样品,采用肌氨酸氧化酶法测定血肌酐。CBB法,测定尿蛋白。具体按试剂盒说明书操作。
1.3.4. HE染色检测大鼠肾脏组织病理形态变化
将浸泡在4%多聚甲醛中进行固定的肾组织取出,经过脱水、二甲苯透明后进行液体石蜡包埋,等待石蜡冷却至室温后进行修剪蜡块、制作切片,石蜡切片厚度约为4 μm。将准备进行染色的蜡块进行二甲苯常规脱蜡和梯度酒精水化后,进行染色。染色后的切片经梯度乙醇脱水,二甲苯透明,中性树胶封片。光学显微镜观察并拍照。
1.3.5. 试剂盒检测大鼠肾脏组织SOD、MDA
严格按照说明书操作,随机取冻存8个肾脏组织,破碎后取上清液,记录实验数据;将数据按照公式进行计算,检测大鼠肾脏组织SOD活性、MDA含量。
1.3.6. Western blot检测大鼠肾脏组织中T-AKT、p-AKT、T-mTOR、p-mTOR、p62、LC3蛋白的表达
取冻存肾脏组织,提取蛋白,测定浓度,经SDS-PAGE凝胶电泳、转膜、封闭后分别用T-AKT、p-AKT、T-mTOR、p-mTOR、p62、LC3、β-actin抗体孵育过夜,洗膜后加入二抗,ECL成像。Image J软件分析并计算相应的蛋白条带与β-actin的灰度比值。
1.4. 统计学处理
运用SPSS 20.0软件对数据进行处理,实验数据以均数±标准差表示,组间比较采用单因素方差分析,以P < 0.05为差异有统计学意义。
2. 结果
2.1. 肌肽对DM大鼠状态、血糖和体质量的影响
实验结果显示,与CON组相比,DM组大鼠的反应较为迟钝、不喜运动、弓背卷体、多饮多尿明显、毛色发黄光泽差。与DM组相比,Car各组大鼠毛发清洁程度有改善,精神状态恢复。与CON组相比,DM组注射STZ后血糖水平显著升高,体质量显著降低,该趋势一直维持到实验结束,差异有统计学意义(P < 0.01)。与DM组相比,Car各组血糖略有下降,体质量升高,但只有Car-H组体质量具有统计学意义(P < 0.05,图 1)。
图 1.
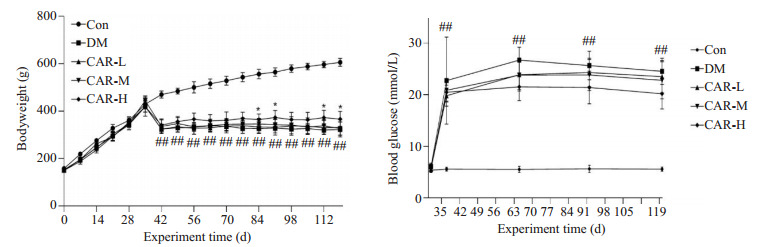
肌肽对DM大鼠血糖和体质量的影响
Effects of carnosine on blood glucose and body weight of the diabetic rats (Mean±SD, n=12-14). ##P < 0.01 vs CON group; *P < 0.05 vs DM group.
2.2. 肌肽对DM大鼠Scr、尿蛋白、24h尿量和肾脏质量的影响
与CON组相比,DM组的大鼠Scr、尿蛋白含量、24 h尿量和肾脏质量均有所升高(P < 0.01)。与DM组相比,CAR-M和Car-H组大鼠的尿蛋白含量、24 h尿量均显著降低(P < 0.05),肾脏质量与Scr下降有下降趋势,但差异无统计学意义(图 2)。
图 2.
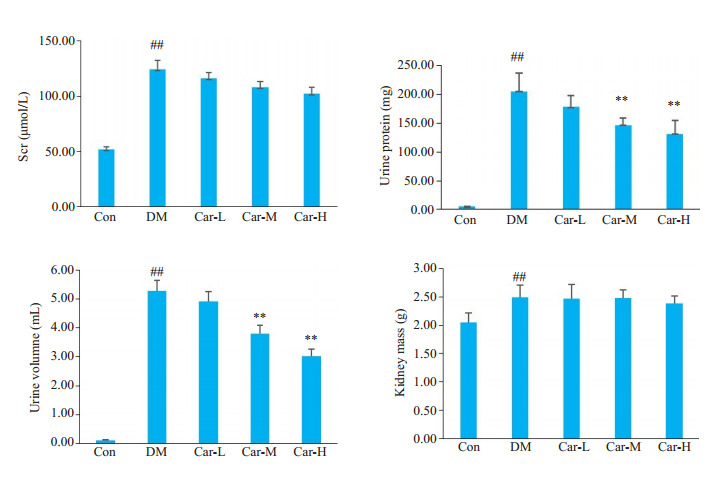
肌肽对DM大鼠的生理指标的影响
Effect of carnosine on renal indexes of DM rats (Mean±SD, n=8-14). ##P < 0.01 vs CON group; **P < 0.01 vs DM group.
2.3. 肌肽对DM大鼠肾脏组织形态的影响
CON组大鼠肾脏细胞均匀分布,肾小球形态圆润饱满,肾小管结构正常,基底膜系膜形态规则无增生。DM组大鼠肾脏组织细胞紊乱,肾小球肿胀变形,形状不规则,伴随基底膜增厚,系膜增生,肾小管出现狭窄现象。CAR组肾小球内系膜增生和基底膜增厚程度略有降低,肾小球形状逐渐圆润规整,空泡状肾小管上皮细胞有所恢复,尤其以高浓度组效果更佳(图 3)。
图 3.
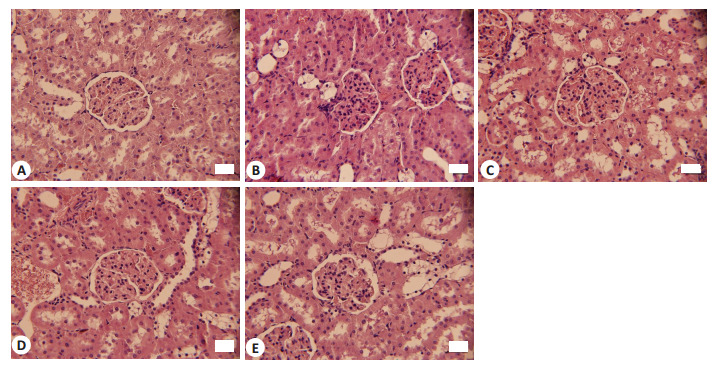
各组大鼠的肾脏HE染色结果
HE staining of renal tissue in each group (Original magnification: ×400). A: CON group. B: DM group. C: Car-L group. D: Car-M group. E: Car-H group.
2.4. 肌肽对DM大鼠肾脏组织SOD和MDA的影响
与CON组相比,DM组的大鼠的肾脏组织SOD活性下降,MDA含量上升(P < 0.05)。与DM组相比,Car各组大鼠肾脏组织中的SOD含量上升,MDA含量下降(P < 0.05),并且呈现剂量依赖性(图 4)。
图 4.
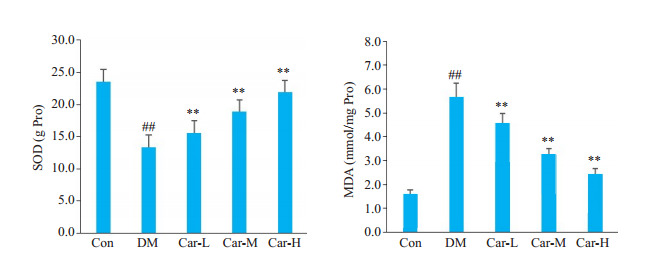
肌肽对DM大鼠肾脏中氧化应激的影响
Effect of carnosine on SOD activity and MDA content in the kidney of the rats with DM. ##P < 0.01 vs CON group; **P < 0.01 vs DM group.
2.5. 肌肽对DM大鼠肾脏组织AKT、mTOR、P-AKT、p-mTOR、LC3 Ⅰ、LC3 Ⅱ、p62的影响
与CON组相比较,DM组的大鼠肾脏组织中AKT和mTOR蛋白总量无明显差异,但p-AKT/AKT、p-mTOR/mTOR的比值明显降低(P < 0.05),p62蛋白表达及LC3-Ⅱ/LC3-Ⅰ比值明显升高(P < 0.01)。与DM组进行比较,Car各组p-AKT/AKT与p-mTOR/mTOR比值均有所上升,p62蛋白表达及LC3-Ⅱ/LC3-Ⅰ比值均下降,尤其以Car-M和Car-H组更明显(P < 0.01,图 5)。
图 5.
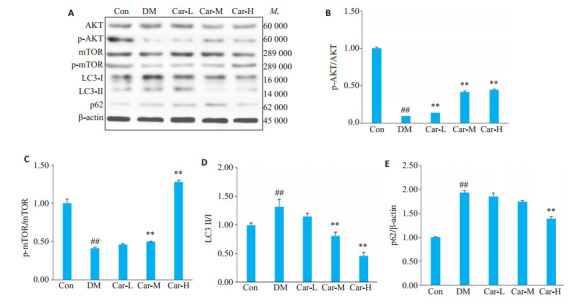
Western blot检测AKT、mTOR、P-AKT、p-mTOR、LC3、p62蛋白的表达水平
Renal expression of AKT, mTOR, P-AKT, LC3, and p62 proteins in the rats detected by Western blotting. A: Representative image of AKT, mTOR, P- AKT, LC3, p62 proteins detected by Western blots. B: The ratio of p- AKT/AKT protein expression. C: The ratio of p-mTOR/mTOR protein expression. D: The ratio of LC3 Ⅱ/Ⅰ protein expression. E: Relative expressions of P62 protein. ##P < 0.01 vs CON group; **P < 0.01 vs DM group.
3. 讨论
DKD是目前糖尿病最为常见也是最为严重的一种慢性微血管并发症,30%~40%的糖尿病患者会发展成DKD。采用高糖高脂饲养同时配合STZ腹腔注射建立了DM大鼠模型,实验结果表明,与CON组相比较,DM组的大鼠体质量明显的减轻(P < 0.05)、血糖、Scr、尿蛋白及24 h尿量升高明显(P < 0.05),肾小球出现基底膜增生、系膜增厚和肾小球肿胀变形等,说明DKD模型制备成功,与文献报道一致[15]。与DM组相比,肌肽各组可见以上各指标不同程度的减轻,其中肌肽高剂量组的效果最为明显,说明肌肽对DM肾脏病变具有保护作用。
血糖升高可产生过量自由基,同时清除能力下降,其导致的氧化应激失衡在DKD中起重要作用[16, 17]。SOD的主要功能是清除体内产生的氧自由基。MDA是脂质发生过氧化反应最终产物,其含量可以反映机体过氧化的程度,是常用的过氧化损伤的指标。实验结果显示,与CON组相比,DM组的大鼠血清中SOD活性下降,MDA含量上升,说明DM组大鼠氧化应激损伤明显。灌胃肌肽后,肌肽提高DM组大鼠SOD活性及降低MDA含量,因此推断肌肽可通过提升大鼠机体的抗氧化能力,从而对DM肾脏病变产生保护作用。
氧化应激与AKT/mTOR途径密切相关[18, 19],而AKT/mTOR途径又在DKD中发挥重要作用[20, 21]。AKT的苏氨酸和丝氨酸残基发生磷酸化后,直接或间接通过结节性硬化复合体激活mTOR蛋白,调节下游效应分子S6K1和4E-BP1的表达,从而调节细胞的增殖、分化、凋亡和自噬等生理活动[18, 22]。在肾脏中,mTOR活性的生理水平是支持肾细胞生长和分化、维持肾细胞完整性和正常肾单位功能,包括电解质、水和葡萄糖的运输所必需的,其活性失调会导致肾脏疾病[21]。结果显示,与CON组相比,DM组p-AKT/AKT和p-mTOR/mTOR比值降低,与文献结果一致[23]。同时也应该注意到不同种属、模型制备方法和时间不同的DM模型,p-AKT/AKT和p-mTOR/mTOR比值的变化趋势并不一致[24, 25],具体原因仍需进一步研究。灌胃肌肽后p-AKT/AKT和p-mTOR/mTOR比值升高,而以高浓度肌肽效果更明显,推测肌肽可通过激活AKT/mTOR途径发挥其保护作用。
Akt/mTOR信号通路对自噬过程具有重要调节作用[26, 27],可以抑制自噬体的合成[28, 29]。自噬是一种高度保守的溶酶体降解途径,对于维持细胞的稳态发挥重要作用[30],其调节失控在DKD的进展中起着重要作用[6, 31, 32]。微管相关轻链蛋白3(LC3)是一种可溶性蛋白质,通过对其C末端切割产生LC3-Ⅰ,进一步与磷脂酰乙醇胺结合产生LC3-Ⅱ。LC3-Ⅱ反映细胞自噬体相对数量,可作为自噬活性和DKD进展的标志物[33]。P62是自噬受体蛋白,与可作为自噬降解指标,与自噬活性呈负相关。本实验结果显示,与CON组相比,DM组p62蛋白表达及LC3-Ⅱ/Ⅰ比值明显均明显升高(P < 0.05),而肌肽灌胃后,随着肌肽浓度增加,p62蛋白表达及LC3-Ⅱ/Ⅰ比值逐渐降低,以上结果说明DM大鼠肾脏自噬过程受到干扰,而肌肽可以调节DM大鼠自噬恢复正常。
综上所述,肌肽可通过激活AKT/mTOR信号通路,改善自噬发挥对DM肾脏病变的保护作用。
Biography
任毅,硕士,E-mail: 564322481@qq.com
Funding Statement
辽宁省教育厅面上项目(2021LJKZ0823)
Contributor Information
任 毅 (Yi REN), Email: 564322481@qq.com.
杨 菁 (Jing YANG), Email: yangjing@jzmu.edu.cn.
References
- 1.Pozo Garcia L, Thomas SS, Rajesh H, et al. Progress in the management of patients with diabetes and chronic kidney disease. Curr Opin Nephrol Hypertens. 2022;31(5):456–63. doi: 10.1097/MNH.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan CM, Nee R, Ceckowski KA, et al. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J. 2017;10(2):257–62. doi: 10.1093/ckj/sfw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuttle KR, Agarwal R, Alpers CE, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102(2):248–60. doi: 10.1016/j.kint.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Yang C, Wang HB, Zhao XJ, et al. CKD in China: evolving spectrum and public health implications. Am J Kidney Dis. 2020;76(2):258–64. doi: 10.1053/j.ajkd.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle KR, Brosius FC 3rd, Cavender MA, et al. SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the national kidney foundation. Diabetes. 2021;70(1):1–16. doi: 10.2337/dbi20-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki T, Mimura I, Tanaka T, et al. Treatment of diabetic kidney disease: current and future. Diabetes Metab J. 2021;45(1):11–26. doi: 10.4093/dmj.2020.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu WJ, Huang WF, Ye L, et al. The activity and role of autophagy in the pathogenesis of diabetic nephropathy. Eur Rev Med Pharmacol Sci. 2018;22(10):3182–9. doi: 10.26355/eurrev_201805_15079. [DOI] [PubMed] [Google Scholar]
- 8.A/L B Vasanth Rao VR, Tan SH, Candasamy M, et al. Diabetic nephropathy: an update on pathogenesis and drug development. Diabetes Metab Syndr. 2019;13(1):754–62. doi: 10.1016/j.dsx.2018.11.054. [DOI] [PubMed] [Google Scholar]
- 9.Kitada M, Ogura Y, Monno I, et al. Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr Diab Rep. 2017;17(7):53. doi: 10.1007/s11892-017-0879-y. [DOI] [PubMed] [Google Scholar]
- 10.李 佳佳, 黄 皓, 陶 立坚, et al. 糖尿病肾病主要发病机制的研究进展. 生命科学. 2023;35(3):396–404. doi: 10.13376/j.cbls/2023048. [DOI] [Google Scholar]
- 11.Creighton JV, de Souza Gonçalves L, Artioli GG, et al. Physiological roles of carnosine in myocardial function and health. Adv Nutr. 2022;13(5):1914–29. doi: 10.1093/advances/nmac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters V, Yard B, Schmitt CP. Carnosine and diabetic nephropathy. Curr Med Chem. 2020;27(11):1801–12. doi: 10.2174/0929867326666190326111851. [DOI] [PubMed] [Google Scholar]
- 13.Kiliś-Pstrusińska K. Carnosine and kidney diseases: what we currently know? Cur Med Chem. 2020;27(11):1764–81. doi: 10.2174/0929867326666190730130024. [DOI] [PubMed] [Google Scholar]
- 14.何 鑫, 杨 文强, 李 宗泽, et al. 肌肽对糖尿病肾病大鼠氧化应激及NF-κB信号通路的影响. 中国临床解剖学杂志. 2020;38(3):289–94. doi: 10.13418/j.issn.1001-165x.2020.03.010. [DOI] [Google Scholar]
- 15.Mohany M, Ahmed MM, Al-Rejaie SS. The role of NF-κB and bax/bcl-2/caspase-3 signaling pathways in the protective effects of sacubitril/valsartan (entresto) against HFD/STZ-induced diabetic kidney disease. Biomedicines. 2022;10(11):2863. doi: 10.3390/biomedicines10112863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao JB, Liu XT, Tang J, et al. The effect of allograft inflammatory factor-1 on inflammation, oxidative stress, and autophagy via miR-34a/ATG4B pathway in diabetic kidney disease. Oxidative Med Cell Longev. 2022;2022:1–22. doi: 10.1155/2022/1668000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei PZ, Szeto CC. Mitochondrial dysfunction in diabetic kidney disease. Clin Chim Acta. 2019;496:108–16. doi: 10.1016/j.cca.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Dai ST, Tao J, et al. Taurine suppresses ROS-dependent autophagy via activating Akt/mTOR signaling pathway in calcium oxalate crystals-induced renal tubular epithelial cell injury. Aging. 2020;12(17):17353–66. doi: 10.18632/aging.103730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Yuan HY, Li WQ, et al. Polygonatum sibiricum polysaccharides protect against MPP- induced neurotoxicity via the Akt/mTOR and Nrf2 pathways. Oxid Med Cell Longev. 2021;2021:8843899. doi: 10.1155/2021/8843899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong RX, Zhang X, Liu YD, et al. Rutin alleviates EndMT by restoring autophagy through inhibiting HDAC1 via PI3K/AKT/mTOR pathway in diabetic kidney disease. Phytomedicine. 2023;112:154700. doi: 10.1016/j.phymed.2023.154700. [DOI] [PubMed] [Google Scholar]
- 21.Huynh C, Ryu J, Lee J, et al. Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat Rev Nephrol. 2023;19(2):102–22. doi: 10.1038/s41581-022-00648-y. [DOI] [PubMed] [Google Scholar]
- 22.Kma L, Baruah TJ. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl Biochem. 2022;69(1):248–64. doi: 10.1002/bab.2104. [DOI] [PubMed] [Google Scholar]
- 23.魏 爽, 李 冀, 韩 东卫, et al. 基于网络药理学探讨黄芪-葛根药对对T2DM模型大鼠PI3K/Akt/FoxO1信号通路的调节作用. 中国实验方剂学杂志. 2022;28(5):157–68. [Google Scholar]
- 24.尹 建平, 庞 欣欣. 补肾降糖方对糖尿病肾病小鼠肾脏功能及肾小管上皮细胞自噬的影响. 中国老年学杂志. 2023;43(1):92–97. [Google Scholar]
- 25.Liao T, Zhao LB, Liu H, et al. Liraglutide protects from renal damage via Akt-mTOR pathway in rats with diabetic kidney disease. Eur Rev Med Pharmacol Sci. 2019;23(3 Suppl):117–25. doi: 10.26355/eurrev_201908_18638. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Jiang L, Liu XQ, et al. Paeoniflorin binds to VEGFR2 to restore autophagy and inhibit apoptosis for podocyte protection in diabetic kidney disease through PI3K-AKT signaling pathway. Phytomedicine. 2022;106:154400. doi: 10.1016/j.phymed.2022.154400. [DOI] [PubMed] [Google Scholar]
- 27.Tang G, Du Y, Guan HC, et al. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2- mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 2022;179(1):159–78. doi: 10.1111/bph.15693. [DOI] [PubMed] [Google Scholar]
- 28.Yang F, Qu QS, Zhao CY, et al. Paecilomyces cicadae-fermented Radix astragali activates podocyte autophagy by attenuating PI3K/AKT/mTOR pathways to protect against diabetic nephropathy in mice. Biomed Pharmacother. 2020;129:110479. doi: 10.1016/j.biopha.2020.110479. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Zhang HB. Regulation of autophagy by mTOR signaling pathway. Adv Exp Med Biol. 2019;1206:67–83. doi: 10.1007/978-981-15-0602-4_3. [DOI] [PubMed] [Google Scholar]
- 30.Yang DY, Livingston MJ, Liu ZW, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. 2018;75(4):669–88. doi: 10.1007/s00018-017-2639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang ZW, Sun YT, Xue JJ, et al. The critical role of dysregulated autophagy in the progression of diabetic kidney disease. Front Pharmacol. 2022;13:977410. doi: 10.3389/fphar.2022.977410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez CD, Carro Negueruela MP, Nicora Santamarina C, et al. Autophagy dysregulation in diabetic kidney disease: from pathophysiology to pharmacological interventions. Cells. 2021;10(9):2497. doi: 10.3390/cells10092497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki H, Osawa T, Fujioka Y, et al. Structural biology of the core autophagy machinery. Curr Opin Struct Biol. 2017;43:10–7. doi: 10.1016/j.sbi.2016.09.010. [DOI] [PubMed] [Google Scholar]


