Abstract
Background
Acute aortic dissection is a life-threatening condition that requires urgent surgical treatment. The frozen elephant trunk (FET) technique, including the Thoraflex hybrid prosthesis, has emerged as an effective strategy for treating complex aortic pathologies. With the widespread application of the FET technique, it continues to evolve, aiming to simplify procedures and reduce complications. These advancements provide improved outcomes and help save lives in patients with acute aortic dissection.
Methods
For this review, PubMed databases were utilized from inception to March 2023. A descriptive approach was employed to identify and present the evidence regarding the application of the FET technique in acute settings and its clinical implications on the postoperative course.
Results
In the reviewed studies, FET was a commonly used treatment approach for acute type A aortic dissection. A comprehensive analysis of 12 studies, comprising over 4056 FET procedures, revealed varying rates of early mortality (up to 21.1%), perioperative stroke (ranging from 2.7 to 18.0%), and spinal cord ischemia (ranging from 0 to 8.2%). During the follow-up period, which ranged from 6 to 108 months, the mortality rate was reported to be as high as 38%.
Conclusions
The surgical management of acute aortic dissection remains challenging, but FET has shown promising results. Experienced teams have achieved acceptable in-hospital mortality and stroke rates, along with a lower risk of spinal cord injury compared to conventional repair. Furthermore, the FET technique has demonstrated positive alterations in the structure of the distal aorta, potentially improving long-term survival and reducing the necessity for future procedures.
Keywords: Aortic, FET, Aortic dissection, TEVAR, Arch replacement
Introduction
Acute aortic dissection is a highly complex and life-threatening cardiovascular disorder, and it is the most prevalent condition affecting the aorta in its acute form. The classification of aortic dissection is based on its location within the anatomy and the time since the onset of symptoms [1].
Mortality rates for type A acute aortic dissection managed with medical treatments are approximately 20% at 24 h, 30% at 48 h, 40% at 1 week, and 50% at 1 month. The most common causes of death in these cases are aortic rupture, stroke, visceral ischemia, and cardiac tamponade. Therefore, the urgent surgical treatment of acute type A aortic dissection is considered the optimal therapeutic approach [2].
Given the principle of emergency surgery as the standard of care for preserving lives, is it justifiable to perform extensive repairs on the aortic arch and distal aorta? In this literature review, we focused on the reasoning behind and the outcomes associated with extensive repairs of the thoracic aorta.
Total arch replacement according to the frozen elephant trunk (FET) technique has proven itself a valid therapeutic strategy to treat complex surgical aortic arch and proximal descending aortic pathologies [3]. The first FET procedure at Bologna University was performed in 2007, with a focus on treating aneurysms located in the distal arch and proximal descending thoracic aorta. During that time, the only available device in Europe for this technique was the Jotec E-vita open. Over the years, we expanded our indications to include chronic aortic dissections. In 2013, with the introduction of the Thoraflex hybrid prosthesis, we further expanded our indications to also cover acute type A and B aortic dissections [4]. The Thoraflex prosthesis had a different stent shape and a slightly different length with a tetrafurcated graft, providing advantages for re-operations on the aortic arch where the use of a branched graft simplified procedures such as re-implantation of the supraaortic vessels and reperfusion after hypothermic circulatory arrest. Previously, when using the E-vita open prosthesis, we had to replace the tubular graft with an additional branched graft. We compared the two devices and found no differences in terms of in-hospital mortality. Furthermore, we believe that the stent length of 100 mm in the Thoraflex prosthesis is sufficient to stabilize the intimal flap and the downstream aorta, reducing notably the risk of spinal cord injury.
In recent years, the FET technique has gained widespread acceptance. Modifications have been made to the procedure to simplify it and reduce the duration of circulatory arrest, aiming to minimize the occurrence of significant complications such as spinal cord injury [5, 6].
Surgical indications for FET in acute aortic dissection
The FET technique is a valuable addition to total arch replacement procedures in patients who have distal aortic malperfusion with compression of the true lumen, complex primary and reentry tears involving the distal arch or proximal descending thoracic aorta, distal arch or descending thoracic aorta rupture, an aneurysmal arch and proximal descending thoracic aorta, or severely damaged aortic arch that makes safe distal aortic arch anastomosis challenging [7].
For patients experiencing malperfusion, the FET technique offers the potential to fully open the compressed true lumen and cover additional entry tears in the proximal descending thoracic aorta, which helps maintain pressurization of the false lumen. In cases without malperfusion, FET hybrid prostheses simplify surgery by eliminating the need for a complex and risky distal anastomosis performed deep in the proximal descending thoracic aorta, where there is a high risk of rupture or bleeding due to the fragile dissected aortic wall [7, 8]. By utilizing the distal stent graft segment of the FET prosthesis, a safer distal anastomosis can be performed at a more proximal level (such as proximal to the left subclavian or left carotid arteries), while still excluding the tear in the distal arch. In cases of acute dissection, extensive FET interventions aim to address both secondary entry tears in the proximal descending thoracic aorta (DTA) and the false lumen at that location [8, 9]. This approach is believed to reduce DTA dilatation, leading to improved long-term survival by reducing aortic-related deaths and the necessity for complex distal aortic reinterventions [9].
Evolution of FET technique
Prior to 2014, our institution consistently performed the distal anastomosis beyond the left subclavian artery (LSA) in the arch zone 3. However, we subsequently made a practice change and shifted our target to arch zone 2, which lies between the left carotid artery and the LSA. This allowed for the reimplantation of the LSA and increased deployment of the stent graft. In arch zone 2, we increasingly utilized the Thoraflex hybrid prosthesis, particularly in cases of acute aortic dissections. This decision was based on the aforementioned reasons, as well as the fact that a significant intimal tear is often located near the LSA and can be effectively excluded. Additionally, the use of a shorter endograft length is an important factor in reducing the rate of spinal cord injury [10, 11].
Methods
Literature search criteria
The initial search performed using PubMed databases from inception to March 2023 yielded 409 articles, selecting publications in English, with no time restrictions. Further selection was performed by filtering literature using ‘frozen elephant trunk’ OR ‘acute aortic dissection’ OR ‘type A acute aortic dissection,’ ‘frozen elephant trunk in acute aortic dissection’ as either keywords or Medical Subject Headings (MeSH) terms. The initial search identified 409 studies, 351 of which were excluded, following screening of the titles and abstracts, because of not being relevant for the analysis. Case reports, editorial, expert opinion, and comment types of publication were excluded, and particular attention was paid in evaluating review articles, because of the potential doubling of results. Further selection according to the relevance of the topics treated, as well as the removal of duplicate data, led to twelve studies included in the literature review (Table 1).
Table 1.
Literature search and evidence
| Study | Year | No. pat | Age (mean, y) | Mortality (n/%) | Stroke (n/%) | SCI (n/%) |
|---|---|---|---|---|---|---|
| Roselli [23] | 2023 | 90 | - | 9/10% | 8/11% | 2/2.4% |
| Beckmann [21] | 2022 | 115 | 50.8 | 12/10.4% | - | - |
| Chivasso [24] | 2021 | 1295 | - | 7.8% | 3.5% | 1.7% |
| Yoshitake [18] | 2020 | 139 | 59.6 | 2/1.4 % | 7/5% | 1/0.7% |
| Ma [25] | 2020 | 518 | 46.2 | 39/7.5% | 15/2.9% | 13/2.5% |
| Chabry [26] | 2020 | 109 | 60 | 23/21.1% | 17/15.6% | 9/8.2% |
| Berger [8] | 2019 | 88 | 59 | 11% | 6/18% | 6% |
| Mariscalco [27] | 2019 | 66 | 62 | 8/12% | 11/17% | 0 |
| Matt [28] | 2016 | 37 | 60 | 0 | 3/8.1% | 0 |
| Lin [29] | 2015 | 881 | 45.4–66.8 | 8% | 3% | 4% |
| Katayama [19] | 2015 | 120 | 64.4 | 7/6% | 4/3% | 2/2% |
| Ma [20] | 2013 | 398 | 46.0 – 11.0 | 31/7.8% | 10/2.5% | 10/2.5% |
The main objective of this scoping review was to provide an overview of the existing literature on FET in acute settings, specifically focusing on the hospital mortality rate and complications. To achieve this, a descriptive approach was employed to identify and present these aspects. No protocol is available for this scoping review (Fig. 1).
Fig. 1.
Step-by-step graft size selection method
Data extraction and appraisal
All data were extracted from article text and tables. Two investigators independently reviewed each retrieved article (F.C., V.O.). The results were reviewed by two senior investigators (G.M., L.D.). All values are represented as numbers (percentages), mean ± standard deviation, or median. This retrospective study was approved by the local Institutional Review Board and did not require patient informed consent (IRB 2021/Disp/AOUBo).
Statistical analysis
Categorical variables were summarized as absolute and percentage frequencies, while continuous variables were summarized as mean and standard deviation (SD). Time-to-event (death and reinterventions) was estimated using Kaplan-Meier curves. Data for survival analyses were censored at death or at 10-year follow-up, whichever came first. Statistical analyses have been conducted using SPSS Statistics v26 (Statistical Package for Social Science, IBM, Armonk, NY, USA).
Surgical and endovascular approach
Our surgical technique for FET implantation has previously been described [12]. It involves a complete sternotomy. The site for arterial cannulation is evaluated individually for each patient, according to the specific characteristics of the pathology. The most common sites are the right axillary artery, the brachiocephalic trunk, the right carotid artery, and (in fewer cases) the femoral artery. Crystalloid cardioplegia is administered (Custodiol; Koehler Chemie, Alsbach-Haenlein, Germany), either antegrade, through the aortic bulb or selectively in the coronary ostia, or—less frequently—retrogradely. Concomitant cardiac procedures, as well as the ones that involve the aortic root or ascending aorta, are routinely performed while the patient is cooled down to a 25°C nasopharyngeal temperature. Bilateral cerebral perfusion is achieved according to Kazui’s technique. The distal anastomosis site for the FET implantation is performed either in zone 2 or zone 3 (Fig. 1). Both the Thoraflex hybrid prosthesis (Vascutek, Inchinnan, UK) and the E-vita Open (Jotec Inc., Hechingen, Germany) prosthesis were used. Every elective case is routinely performed after implantation of cerebrospinal fluid (CSF) drainage the previous day to minimize the risk of spinal cord injury.
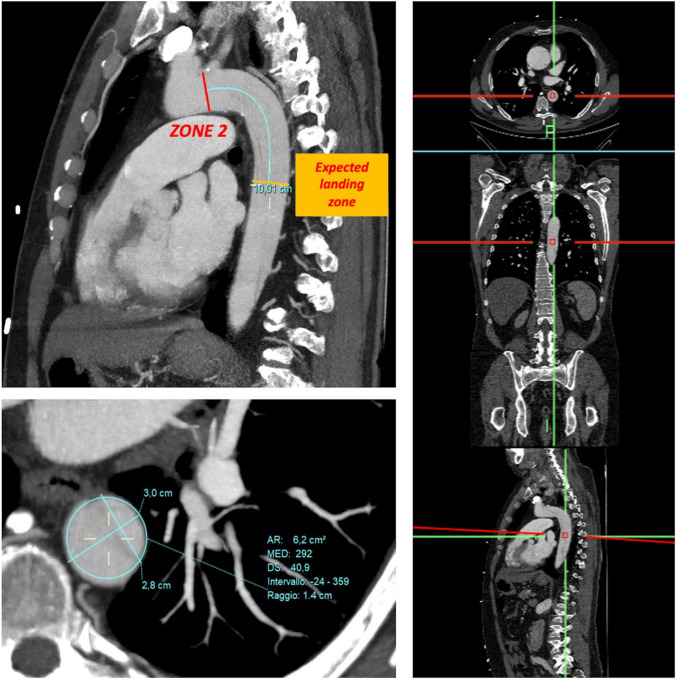
To properly select the size of the FET device, our protocol recommends using a curved MPR (multi-planar reconstruction) based on angio CT and electrocardiogram-gated computed tomography scans (EKG gated CT scan). This allows for precise evaluations and reduces the risk of incorrect measurements. First, the sagittal plane acquisition is performed and zone 2 is highlighted. Next, the length of the stent graft is chosen based on the required coverage of the descending thoracic aorta. Then, we follow the outer curve of the aorta (recently, the aortic central line) to determine the predicted location of the distal end of the prosthesis. At this level, a transverse plane is used, which is orthogonal (perpendicular) to the longitudinal axis of the aorta. On this plane, the diameters of the aorta are measured, as well as its circumference, using the approximation of a regular circle (Fig. 1).
When choosing the size of the device in treating degenerative aneurysms, we perform a slight oversizing that does not exceed 20%. No oversizing is performed for aortic dissections, either acute or chronic.
Regarding endovascular procedures, the femoral vessels are accessed surgically, and the femoral artery is reconstructed at the end of the endovascular procedure. We usually extend thoracic endovascular aortic repair (TEVAR) coverage of the thoracoabdominal aorta, with the distal end in the proximity of the celiac trunk offspring.
Angio-CT evaluation according to the Bologna protocol
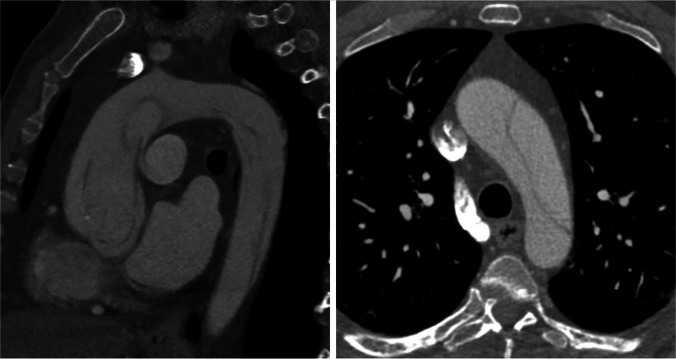
CT scans are widely recognized as the most effective imaging technique for identifying and locating aortic pathologies. They offer high sensitivity and specificity. Our standard approach follows the recommendations outlined in the latest consensus document on acute aortic dissection [13]. It involves contrast-enhanced, electrocardiogram-gated computed tomography scans (EKG-gated CT scans), which minimize motion artifacts throughout the entire thoracic aorta [14, 15] (Fig. 2). Despite improvements in the surgical outcome of type A acute aortic dissections (TAAD), the postoperative mortality rate remains high. The frozen elephant trunk technique has shown advantages such as clotting the false lumen, reducing the occurrence of patent false lumen, preventing secondary and re-entry tears, improving blood flow, and decreasing late aneurysm formation. However, there is ongoing debate regarding its technical complexity and the longer time needed for cerebral perfusion and hypothermic circulatory arrest. These factors may increase the risks of mortality and neurological complications.
Fig. 2.
Preoperative CT scan image of an acute type A aortic dissection with involvement of the aortic arch
Our protocol includes acquiring delayed images, particularly after stent graft repair, to identify any endoleaks. For patients being considered for FET (fenestrated endovascular repair) for aortic dissection, double oblique reconstruction allows for more accurate measurement of aortic diameters along the entire length of the stent graft. This enables a precise determination of the level at which the distal landing zone is located, a critical factor in avoiding stent graft oversizing.
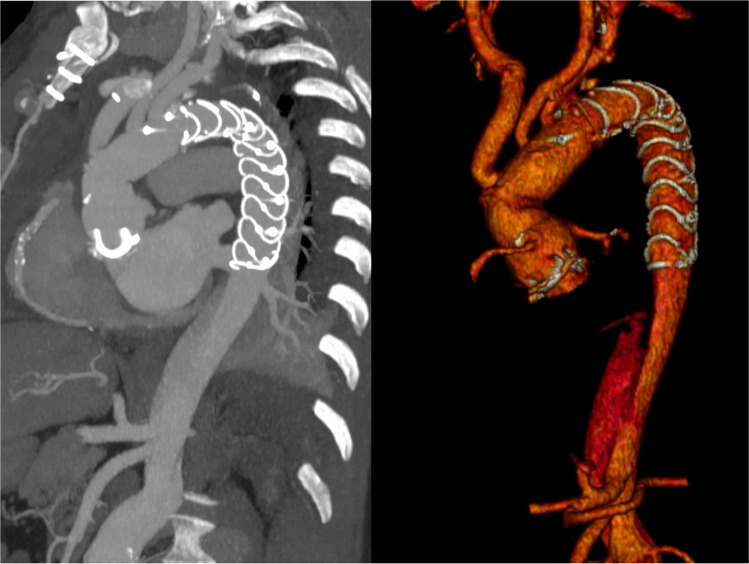
Postoperative angio-CT scans are performed before the patient is discharged from the hospital. Based on the findings of the initial CT scan, additional planned CT scans are scheduled at intervals of 3 months, 6 to 12 months, and then annually (Fig. 3).
Fig. 3.
Postoperative CT scan image after aortic arch replacement with the frozen elephant trunk technique showing complete thrombosis of the false lumen
Results
Literature review
In the reviewed studies, total arch replacement (TAR) with stented elephant trunk was utilized as a treatment approach for acute type A aortic dissection. Table 1 presents a summary of retrospective single- and multiple-center experiences, as well as meta-analyses, providing data on FET treatment for acute type A aortic dissection. Through the literature review, 12 studies were identified and analyzed, encompassing a total 4056 FET procedures. The findings indicated that the early mortality rate ranged up to 21.1%. The perioperative incidence of stroke ranged from 2.7 to 18.0%, while the incidence of spinal cord ischemia ranged from 0 to 8.2%.
During the follow-up period, which varied between 6 and 108 months across the reviewed studies, the mortality rate was reported to reach as high as 38%. The reported mean follow-up time ranged from 6 to 108 months, with a mortality rate from 2.8 up to 38%. This information reflects the long-term outcomes and highlights the importance of ongoing monitoring and care for patients who undergo the FET technique for the treatment of acute type A aortic dissection. Due to the lack of data across the studies examined, it was impossible to retrieve specific information relative to reoperation during the follow-up period.
Results in Bologna
Our center has conducted a total of 382 FET procedures for the treatment of acute and chronic aortic syndromes, as well as for chronic degenerative aneurysms, between January 2007 and May 2023. We have successfully used both Thoraflex and Evita Open grafts for a wide range of patients, either for degenerative aneurysm or aortic dissections. The main surgical indication was a chronic/residual aortic dissection, with 202 (52.9%), followed by 103 (26.9%), degenerative aneurysms. Sixty-six patients (54 males and 12 females, 81.8 and 18.2%, mean age 59.0 ± 11.0 years) underwent TAR with FET technique for acute aortic syndromes, either type A or type B (48 and 18, respectively). The demographic and preoperative characteristics of our cohort of patients are described in Table 2. Forty-four patients (66.7%) have been implanted with the Thoraflex device, while 22 (33.3%) have been treated with the E-Vita Open graft. Mean cardiopulmonary bypass (CPB) time (251.1 ± 77.2), cross clamp (153.1 ± 53.3), and antegrade selective cerebral perfusion (ASCP) (105.1 ± 48.9) times are portrayed in Table 3.
Table 2.
Patients’ characteristics
| Total patients | N = 66 |
|---|---|
| Age (mean ± SD) | 59.0 ± 11.0 |
| Male (n, %) | 54 (84.4) |
| Chronic obstructive pulmonary disease (COPD) (n, %) | 3 (4.7) |
| Diabetes mellitus (DM_ (n, %) | 3 (4.7) |
| Smoking (n, %) | 29 (45.3) |
| Hypertension (n, %) | 47 (73.4) |
| Marfan (n, %) | 3 (4.7) |
| REDO (n, %) | 2 (3.1) |
| Malperfusion syd (n, %) | 20 (31.3) |
| Aberrant subclavian artery (n, %) | 5 (7.8) |
| Type A/B (n, %) | 46 (71.9) – 18 (28.1) |
Table 3.
Intraoperative characteristics
| Total patients | N = 66 | |
|---|---|---|
| Cannulation sites | Femoral artery | 19 (29.7) |
| Innominate artery | 17 (26.6) | |
| Ascending Ao | 12 (18.8) | |
| Axillary artery | 11 (17.2) | |
| Carotid artery | 5 (7.8) | |
| Thoraflex | 44 (66.7) | |
| Evita | 22 (33.3) | |
| Cardiopulmonary bypass (CPB) time (min; mean ± SD) | 251.1 ± 77.2 | |
| Cross clamp (Xclamp) time (min; mean ± SD) | 153.1 ± 53.3 | |
| Antegrade selective cerebral perfusion (ASCP) time (min; mean ± SD) | 105.1 ± 48.9 | |
| Visceral ischemia (min; mean ± SD) | 49.7 ± 17.5 | |
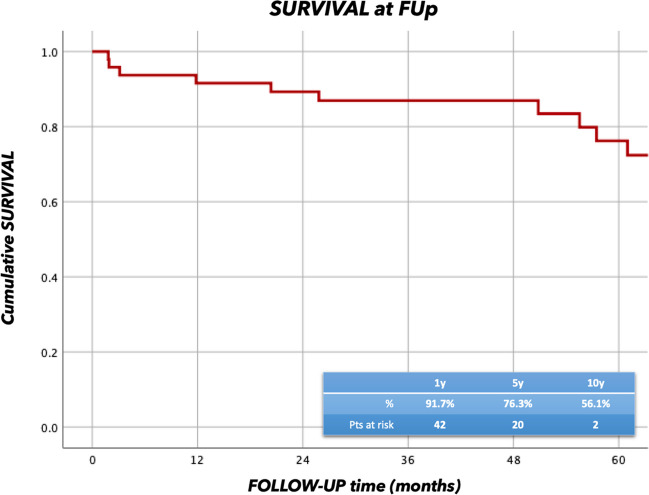
The overall follow-up time was 41.8 ± 42.4 months, and it was 100% complete. Seventeen procedures (25.8%) involved aortic root (15 Bentall and 2 David operations). Survival rates at 1 and 5 years were 91.7% and 76.3% respectively (Fig. 4).
Fig. 4.
Survival analysis at follow-up (FUp) for patients that received FET for acute aortic dissection
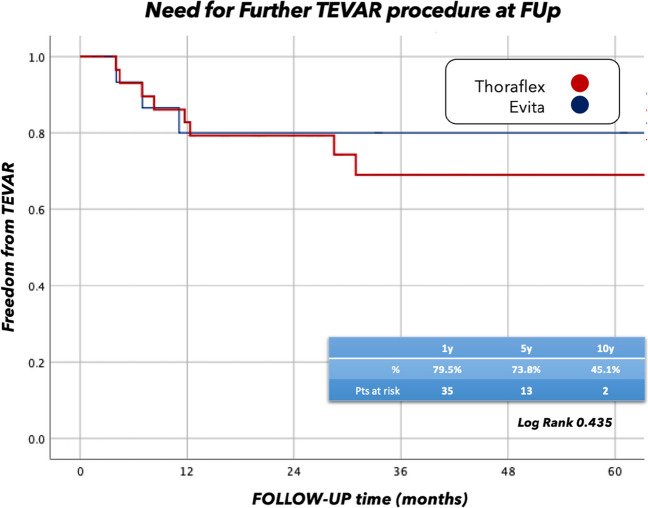
A further endovascular procedure (TEVAR) was necessary for eleven patients (22.0%), whereas three patients underwent other cardiac interventions during the follow-up time, not related to the aortic procedure. Freedom from TEVAR was not different statistically in patients undergoing E-vita open implantation compared to Thoraflex hybrid where it was 79.3% vs. 80.1% at 1 year, and 69.2% vs. 72.0% at 5 years, respectively (Log-rank = 0.435) (Fig. 5).
Fig. 5.
Freedom from endovascular reoperation at FUp Thoraflex vs. Evita
Discussion
In the last decade, several studies have been published demonstrating good early outcomes of the FET technique in the treatment of acute aortic dissection when performed by experience surgeons at high-volume centers compared to large registry results of conventional repair; however, the optimal surgical strategy remains controversial. Potential advantages of extended repair with complete replacement of the aortic arch over replacement of the proximal arch include a lower incidence of aortic-related complications in the downstream aorta and, consequently, a reduced need for future reoperations. However, to reap the benefits of extended repair, the patient must survive surgery. Given the high surgical mortality associated with acute aortic dissection, a more conservative, tear-oriented approach to aortic resection is generally preferred [16].
A meta-analysis by Yan et al. [17] comparing proximal arch repair and extended repair with total arch and elephant trunk implantation in patients with acute type A dissection showed that, compared with extended repair, proximal arch surgery was associated with lower early mortality but higher rates of postoperative aortic events, including reoperation of the distal aorta and significant dilatation of the false lumen. Similarly, a study by Yoshitake et al. [18] showed how replacement of the entire arch in acute aortic dissection improved long-term mortality compared with ascending or hemiarch replacement. The concern with FET is the risk of spinal cord injury (SCI). Katayama et al. [19] reported that the location of the distal portion of the stent graft is an independent risk factor of SCI after FET; in order to reduce the risk of SCI, we modified our technique using the Thoraflex hybrid prosthesis in arch zone 2 more frequently, especially in cases of acute aortic dissections. Even with improvements in the surgical outcome of AAAD in the past 20 years, there is still a high mortality rate after surgery. The frozen elephant trunk technique has been shown to have benefits such as clotting the false lumen, reducing the occurrence of patent false lumen, preventing secondary tears in the arch and re-entry tears in the proximal descending aorta, improving blood flow in the distal aorta, and decreasing the formation of late aneurysms. However, there is ongoing debate about its technical complexity and the longer time required for cerebral perfusion and hypothermic circulatory arrest. These factors may increase the risks of mortality and neurological complications [20].
In addition, a major concern is to perform extensive surgery especially when the aortic dissection also involves the aortic root. In this regard, Beckmann et al. [21] reported data that would show that complete aortic root replacement in patients in whom there is an indication to also perform FET for aortic dissection actually does not increase the perioperative risk of mortality and stroke. Highlighting a significant difference in the treatment indication for aortic dissections is imperative. Thorough evaluation of the preoperative AngioCT scan is essential, particularly when contemplating the use of the FET technique. Specifically, if visceral vessels arise from the false lumen without proximal re-entries, FET is not recommended, due to the high risk of visceral ischemia. In such cases, a conventional elephant trunk procedure with a wide fenestration of the intimal flap is preferable.
The selection of the most suitable stent graft size is of paramount importance. In Bologna, the choice of the device is influenced by the experience with both Thoraflex and Evita grafts. Thoraflex is favored due to its ease of deployment and circular stent design, whereas Evita is used when a longer coverage in the descending thoracic aorta is required.
The decision on graft size is based on the evaluation of Angio CT and EKG-gated images. The diameter of the distal landing zone is determined by measuring the MPR CT images, beginning from zone 2 as per the Ishimaru aortic map. Once the appropriate stent length is selected, the perimeter/diameter of the true lumen at the estimated site of the distal end of the stent is measured.
In acute cases, no oversizing is performed, while for chronic aortic dissection, the oversizing does not exceed 20%.
Future perspectives
The future surgical perspectives of the frozen elephant trunk (FET) procedure in acute aortic dissections are promising. As technology continues to progress and surgical techniques refine, we anticipate a range of potential advancements and developments in the management of patients who undergo FET for acute aortic dissections.
With the wide spread of minimally invasive approaches, hybrid procedures, as well as the improvement of the graft design, with evolution aiming to provide enhanced sealing, durability, and compatibility with each individual aortic anatomy, are a likely future direction. Moreover, thanks to the technological advance in the field of radiology and imaging (i.e., the improvement in 3D tridimensional reconstruction, as well as virtual reality), it will be easier to plan the procedures for each individual patient, offering a customized and tailored treatment, minimizing the occurrence of complications.
Conclusions
The surgical treatment of acute aortic dissection continues to pose significant difficulties, even for skilled surgeons. However, the FET technique has shown promising early outcomes.
Despite improvements in the surgical outcome of acute aortic dissection, the postoperative mortality rate remains high. The frozen elephant trunk technique has shown advantages such as clotting the false lumen, reducing the occurrence of patent false lumen, preventing secondary and re-entry tears, improving blood flow, and decreasing late aneurysm formation. However, there is ongoing debate regarding its technical complexity and the longer time needed for cerebral perfusion and hypothermic circulatory arrest. These factors may increase the risks of mortality and neurological complications.
When performed by experienced teams, the FET technique demonstrates acceptable rates of in-hospital mortality and stroke when compared to conventional repair [16, 21]. Additionally, the FET technique promotes positive changes in the structure of the distal aorta, which is likely to enhance long-term survival and reduce the need for further procedures in the future [22].
Author contribution
(I) Conception and design: All authors.
(II) Administrative support: All authors.
(III) Provision of study materials or patients: All authors.
(IV) Collection and assembly of data: All authors.
(V) Data analysis and interpretation: All authors.
(VI) Manuscript writing: All authors.
(VII) Final approval of manuscript: All authors.
Funding
None.
Data Availability
Data availability is available by the corresponding author as part of an Istitutional Database.
Declarations
Ethics approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
All the authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-234/coif). The series “Frozen Elephant Trunk” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sievers H-H, Rylski B, Czerny M, Baier ALM, Kreibich M, Siepe M, et al. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact Cardiovasc Thorac Surg. 2020;30:451–7. doi: 10.1093/icvts/ivz281. [DOI] [PubMed] [Google Scholar]
- 2.Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015;66:350–8. [DOI] [PubMed]
- 3.Tsagakis K, Pacini D, Grabenwöger M, Borger MA, Goebel N, Hemmer W, et al. Results of frozen elephant trunk from the international E-vita Open registry. Ann Cardiothorac Surg. 2020;9:178–88. doi: 10.21037/acs-2020-fet-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leone A, Murana G, Coppola G, Berardi M, Botta L, Bartolomeo RD, et al. Frozen elephant trunk—the Bologna experience. Ann Cardiothorac Surg. 2020;9:22022–222. doi: 10.21037/acs.2020.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Marco L, Mariani C, Murana G, Amodio C, Campanini F, Berardi M, et al. Why is frozen elephant trunk better than classical elephant trunk? Indian J Thorac Cardiovasc Surg. 2022;38:70–8. doi: 10.1007/s12055-021-01302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacini D, Murana G, Di Marco L. Frozen elephant trunk technique: ready to get back to the future? J Thorac Cardiovasc Surg. 2018;156:e79–80. [DOI] [PubMed]
- 7.Di Marco L, Leone A, Murana G, Castelli A, Alfonsi J, Di Bartolomeo R, et al. Acute type A aortic dissection: Rationale and outcomes of extensive repair of the arch and distal aorta. Int J Cardiol. 2018;267:145–9. doi: 10.1016/j.ijcard.2018.05.111. [DOI] [PubMed] [Google Scholar]
- 8.Berger T, Weiss G, Voetsch A, Arnold Z, Kreibich M, Rylski B, et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection†. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2019;56:572–8. doi: 10.1093/ejcts/ezz037. [DOI] [PubMed] [Google Scholar]
- 9.Kreibich M, Berger T, Rylski B, Chen Z, Beyersdorf F, Siepe M, et al. Aortic reinterventions after the frozen elephant trunk procedure. J Thorac Cardiovasc Surg. 2020;159:392–399.e1. doi: 10.1016/j.jtcvs.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 10.Hori D, Kusadokoro S, Adachi K, Kimura N, Yuri K, Matsumoto H, et al. Risk factors for spinal cord injury in patients undergoing frozen elephant trunk technique for acute aortic dissection. Gen Thorac Cardiovasc Surg. 2020;68:328–34. doi: 10.1007/s11748-019-01196-2. [DOI] [PubMed] [Google Scholar]
- 11.Khachatryan Z, Haunschild J, von Aspern K, Borger MA, Etz CD. Ischemic spinal cord injury-experimental evidence and evolution of protective measures. Ann Thorac Surg. 2022;113:1692–702. doi: 10.1016/j.athoracsur.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Di Bartolomeo R, Murana G, Di Marco L, Alfonsi J, Gliozzi G, Amodio C, et al. Is the frozen elephant trunk frozen? Gen Thorac Cardiovasc Surg. 2019;67:111–7. doi: 10.1007/s11748-018-0911-4. [DOI] [PubMed] [Google Scholar]
- 13.Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS) Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2019;55:133–62. doi: 10.1093/ejcts/ezy313. [DOI] [PubMed] [Google Scholar]
- 14.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 15.Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW, Bolen MA, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334–482. doi: 10.1161/CIR.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luc JGY, Preventza O. Optimal extent of repair for acute type I aortic dissection-frozen elephant trunk? How long and why? Aorta Stamford Conn. 2022;10:169–74. doi: 10.1055/s-0042-1756664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Y, Xu L, Zhang H, Xu Z-Y, Ding X-Y, Wang S-W, et al. Proximal aortic repair versus extensive aortic repair in the treatment of acute type A aortic dissection: a meta-analysis. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2016;49:1392–401. doi: 10.1093/ejcts/ezv351. [DOI] [PubMed] [Google Scholar]
- 18.Yoshitake A, Tochii M, Tokunaga C, Hayashi J, Takazawa A, Yamashita K, et al. Early and long-term results of total arch replacement with the frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2020;58:707–13. doi: 10.1093/ejcts/ezaa099. [DOI] [PubMed] [Google Scholar]
- 19.Katayama K, Uchida N, Katayama A, Takahashi S, Takasaki T, Kurosaki T, et al. Multiple factors predict the risk of spinal cord injury after the frozen elephant trunk technique for extended thoracic aortic disease. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2015;47:616–20. doi: 10.1093/ejcts/ezu243. [DOI] [PubMed] [Google Scholar]
- 20.Ma W-G, Zheng J, Dong S-B, Lu W, Sun K, Qi R-D, et al. Sun’s procedure of total arch replacement using a tetrafurcated graft with stented elephant trunk implantation: analysis of early outcome in 398 patients with acute type A aortic dissection. Ann Cardiothorac Surg. 2013;2:621–8. doi: 10.3978/j.issn.2225-319X.2013.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckmann E, Martens A, Kaufeld T, Natanov R, Krueger H, Rudolph L, et al. Frozen elephant trunk in acute aortic type a dissection: risk analysis of concomitant root replacement. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2022;62:ezac051. [DOI] [PubMed]
- 22.Mehanna M, Elhamami M, Abolkasem A, Ramadan B, Almaghraby A, Mascaro J. Aortic remodelling and false lumen changes after the frozen elephant trunk technique using the thoraflex hybrid stented graft for aortic dissection. Egypt Heart J EHJ Off Bull Egypt Soc Cardiol. 2021;73:74. doi: 10.1186/s43044-021-00198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roselli EE, Kramer B, Germano E, Toth A, Vargo PR, Bakaeen F, et al. The modified frozen elephant trunk may outperform limited and extended-classic repair in acute type I dissection. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2023;63:ezad122. doi: 10.1093/ejcts/ezad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chivasso P, Mastrogiovanni G, Miele M, Bruno VD, Rosciano A, Montella AP, et al. Frozen elephant trunk technique in acute type A aortic dissection: is it for all? Medicina (Mex). 2021;57:894. doi: 10.3390/medicina57090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W-G, Chen Y, Zhang W, Li Q, Li J-R, Zheng J, et al. Extended repair for acute type A aortic dissection: long-term outcomes of the frozen elephant trunk technique beyond 10 years. J Cardiovasc Surg (Torino). 2020;61:292–300. doi: 10.23736/S0021-9509.20.11293-X. [DOI] [PubMed] [Google Scholar]
- 26.Chabry Y, Porterie J, Gautier C-H, Nader J, Chaufour X, Alsac JM, et al. The frozen elephant trunk technique in an emergency: THORAFLEX French National Registry offers new insights. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2020;ezaa325. [DOI] [PubMed]
- 27.Mariscalco G, Bilal H, Catarino P, Hadjinikolaou L, Kuduvalli M, Field M, et al. Reflection from UK Aortic Group: frozen elephant trunk technique as optimal solution in type A acute aortic dissection. Semin Thorac Cardiovasc Surg. 2019;31:686–90. doi: 10.1053/j.semtcvs.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Matt P, Banerjee P, Grapow M, Rueter F, Schurr U, Siegemund M, et al. Modified frozen elephant trunk for acute type A aortic dissection: a comparative study with standard repair technique. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2017;51:754–60. doi: 10.1093/ejcts/ezw412. [DOI] [PubMed] [Google Scholar]
- 29.Lin H-H, Liao S-F, Wu C-F, Li P-C, Li M-L. Outcome of frozen elephant trunk technique for acute type A aortic dissection: as systematic review and meta-analysis. Medicine (Baltimore). 2015;94:e694. doi: 10.1097/MD.0000000000000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is available by the corresponding author as part of an Istitutional Database.