Abstract
Activated brown fat (aBAT) is known to affect the evaluation of 18F-FDG PET scans, especially in young patients. The aim of this study was to determine factors influencing the occurrence of aBAT, and to investigate the effectiveness of the two preventive measures, warming and beta-blocker (propranolol) administration. Five-hundred-twenty-eight 18F-FDG-PET scans of 241 EuroNet-PHL-C2 trial patients from 41 nuclear medicine departments in Germany and Czech Republic were screened for aBAT. The occurrence of aBAT was analyzed with patient characteristics (age, sex, body mass index, predisposition to aBAT), weather data at the day of 18F-FDG PET scanning as well as the preventive measures taken. Potentially important factors from univariate analyses were included into a logistic regression model. Warming as a preventive measure was used in 243 18F-FDG-PET scans, propranolol was administered in 36, warming and propranolol were combined in 84, and no preventive measures were taken in 165 scans. Whereas age, sex and body mass index had no clear impact, there was an individual predisposition to aBAT. Logistic regression model revealed that the frequency of aBAT mainly depends on the outside temperature (p = 0.005) and can be effectively reduced by warming (p = 0.004), the administration of unselective beta-blocker or the combination of both. Warming is a simple, cheap and non-invasive method to reduce the frequency of aBAT. However, the effect of warming decreases with increasing outside temperatures. Administration of propranolol seems to be equally effective and provides advantages whenever the positive effect of warming is compromised. The combination of both preventive measures could have an additive effect.
Subject terms: Cancer imaging, Paediatric cancer
Introduction
Activated brown adipose tissue (aBAT) on 18F-FDG-PET scans is a well-known problem in children and young adults, which may markedly hamper appropriate scan evaluation1,2. This type of fat is rich in mitochondria and has the ability to produce heat, in particular needed by babies and toddlers, to prevent their body from hypothermia. It is frequently located in the neck and the axillae, but also around mediastinal blood vessels, paravertebral ganglia, and organs such as liver, spleen or kidneys3,4. Various potential factors influencing the occurence of aBAT are described in literature5–7. They can be grouped into patient-related factors and environmental factors5–7.
In young lymphoma patients 18F-FDG-PET scans are acquired at staging to determine lymphoma spread, and at restaging to evaluate response to chemotherapy8. At restaging metabolic activity of lymphoma residues is determined to decide on further treatment, in particular, whether radiotherapy is necessary or not9,10. However, residual lymphoma lesions embedded in aBAT are extremely difficult, if not impossible, to evaluate8. If an 18F-FDG-PET scan is not evaluable, it has to be either repeated, meaning additional radiation burden to the patient or radiotherapy cannot be omitted8. However, radiotherapy is highly problematic in the treatment setting of children and young adults with Hodgkin lymphoma because it markedly increases the risk of treatment related late effects such as secondary neoplasms or cardio-vascular diseases9–14.
In the second prospective trial of the European Network for Pediatric Hodgkin Lymphoma (EuroNet-PHL-C2; EudraCT: 2012-004053-88; ClinicalTrials.gov Identifier: NCT02797717), 18F-FDG-PET was compulsory at staging and following two courses of vincristine, etoposide, prednisone, and doxorubicin (OEPA) chemotherapy. In all patients with a negative interim 18F-FDG-PET scan (Deauville scores ≤ 3) irradiation was omitted. Patients with intermediate (Ann Arbor stages IE, IIA plus risk factors, IIB, IIE, IIIA) or advanced stages (Ann Arbor stages IIBE, IIIB, IIBE, all IV) who remained 18F-FDG-PET positive (Deauville scores > 3) received another 18F-FDG-PET scan at the end of their chemotherapy15. Final decision on irradiation fields and irradiation doses was based on randomization (standard arm versus experimental arm) as well as on the results of the two (interim- and end-of-treatment) 18F-FDG-PET scans. Thus, evaluability of the 18F-FDG-PET images was of utmost importance for precise treatment planning. To prevent aBAT, the imaging protocol of the EuroNet-PHL-C2 trial recommended two measures: Warming the patient and, unless there were contraindications or the patient was younger than 10 years, the administration of an unselective betablocker (propranolol; 1 mg/kg, up to 40 mg in total)15.
The aim of this study was to investigate retrospectively different factors, which may have an influence on the occurrence of aBAT on 18F-FDG-PET scans and to determine the effectiveness of the two preventive measures (warming, propranolol).
Methods
Data sources and data acquisition
Every EuroNet-PHL-C2 trial patient received 18F-FDG-PET (as PET/CT or PET/MRI) scans at initial staging and following two cycles of OEPA chemotherapy (early response assessment). Intermediate and advanced stage patients with partial metabolic response (Deauville > 3) after two cycles of OEPA had another 18F-FDG-PET scan at the end of their consolidation chemotherapy (late response assessment). The study protocol gave only recommendations on how to perform the 18F-FDG-PET scans15, but did not define mandatory criteria. Thus, imaging data closely reflect the clinical reality of numerous PET centres.
All imaging data (18F-FDG-PET/CT or –PET/MR, CT, MR, bone scans) performed within the EuroNet-PHL-C2 trial underwent reference reading. They were stored on a central server for re-evaluation and research purposes16.
For the current study, 528 consecutive 18F-FDG-PET/CT or –PET/MR scans of 241 pediatric patients were reviewed. The scans were performed between November 3, 2015 and August 30, 2017 at 41 different nuclear medicine departments in Germany and Czech Republic. All 241 patients had one 18F-FDG-PET scan for staging. Upon availability their early (n = 232) and late response assessment (n = 55) scans were considered as well.
The scans were checked for aBAT by two experienced technologists (both > 20 years of working experience). Beforehand, both underwent an intensive training conducted by a nuclear medicine physician who served as the reference reader for the EuroNet-PHL-C2 trial. Subject of the training was a set of 30 18F-FDG-PET/CT or –PET/MR scans in which aBAT was present in varying degrees, and at all typical and less typical sites.
In addition to reviewing the 528 18F-FDG-PET scans for the presence of aBAT, patient age, sex, height and weight were retrieved from the EuroNet-PHL-C2 trial database. Body mass index (BMI) was calculated on the basis of patient’s weight and height. However, since BMI is age dependent, we calculated the so-called BMI_sds using age and gender specific percentiles data from German children and adolescents17. The BMI_sds is Z-score based on a standard normal distribution.
Mean outside temperature in the respective town for the period of the 18F-FDG-PET scan was taken from public weather sources.
All 41 nuclear medicine departments were asked for their general policy to prevent aBAT and to provide detailed information on preventive measures for every 18F-FDG-PET scan included into the analysis.
Ethics declarations
Written informed consent was obtained from all patients and/or their legal guardians before inclusion into the EuroNet-PHL-C2 trial. The trial was performed in accordance with good clinical practice and the Declaration of Helsinki. The Ethics Committee of the Medical Faculty of the University of Leipzig which is registered as Institutional Review Board (IBF) at the Office of Human Research Protections (OHRP) approved the evaluation presented here (498/17-ek).
Statistics
This is a retrospectively performed observational study. The preventive measures analyzed were not randomized or prescribed, but followed mainly site-specific policies. Therefore, our strategy of analyses was to first look at single factors (gender, age, BMI_sds, outside temperature, predisposition to aBAT) which may have an impact on aBAT using univariate statistical methods for all scans together (Chi2-test for cross-tables and logistic regression for the influence of a metric on a binary factor). A logistic regression model then analyzed potentially important factors, with patient as random effect to account for patient disposition. Data were first collected in SPSS Statistics 23. All analyses were carried out using R, version 4.3 (R Core Team, Vienna, Austria).
Results
General results
aBAT was detected on 94 of the 528 18F-FDG-PET scans (17.8%). In 165 of 528 18F-FDG-PET scans (31.3%) no preventive measures against aBAT were taken, whereas at least one preventive measure was taken in 363 18F-FDG-PET scans (68.7%). Further details are listed in Table 1. However, warming was not documented in every individual case, in particular not, when warming was a general policy at the respective nuclear medicine department. For further analysis we, thus, assumed that the policy of warming was strictly followed. This appeared justified since there was no significant difference in aBAT rates between “Warming—general policy*” (153 × no aBAT, 27 × aBAT) and “Warming—confirmed**” (55 × no aBAT, 8 × aBAT) (p = 0.811). Considering this assumption, 18F-FDG-PET scans were performed after warming the patient in 243 (46.0%), after administration of only propranolol in 36 (6.8%), and after combining warming and propranolol in 84 scans (15.9%).
Table 1.
Overview of performed or not performed measures to prevent aBAT.
| Preventive measure | Frequency | % |
|---|---|---|
| None | 165 | 31.3 |
| Warming (general policy*) | 180 | 34.1 |
| Warming (confirmed**) | 63 | 11.9 |
| Propranolol only (confirmed) | 36 | 6.8 |
| Propranolol (confirmed) and Warming (general policy) | 54 | 10.2 |
| Propranolol (confirmed) and Warming (confirmed) | 30 | 5.7 |
| Σ | 528 | 100.0 |
General policy (*) means that young patients who undergo 18F-FDG-PET scans are regularly warmed before radiotracer injection. Confirmed (**) means that warming and/or administration of propranolol prior to radiotracer injection was documented in the patient file.
Univariate analysis of factors with possible influence on aBAT
Gender
Table 2 shows the frequency of aBAT depending on gender. No aBAT was evident in 211 18F-FDG-PET scans from male (83.4%) and 223 18F-FDG-PET scans from female (81.1%). aBAT was detectable in 42 18F-FDG-PET scans from male (16.6%) and 52 18F-FDG-PET scans from female (18.9%). These numbers suggest that gender did not influence the frequency of aBAT (p = 0.563).
Table 2.
Frequency of aBAT in relation to gender.
| Gender | No aBAT | % | aBAT | % | Σ |
|---|---|---|---|---|---|
| Male | n = 211 | 83.4 | n = 42 | 16.6 | 253 |
| Female | n = 223 | 81.1 | n = 52 | 18.9 | 275 |
| Σ | n = 434 | 82.2 | n = 94 | 17.8 | 528 |
Age
Patients included in our analysis were between 2 and 18 years old. They were divided into two groups setting a cut-off at 11 years. The intention of this cut-off was to distinguish pre-pubertal from pubertal or post-pubertal patients.
The numbers in Table 3 indicate that aBAT was more frequent in patients > 11 years (19.8% versus 10.5%, p = 0.031). This result, however, did not remain significant on multivariate analysis (data not shown).
Table 3.
Frequency of aBAT in relation to age.
| < 11 years | % | ≥ 11 years | % | Σ | |
|---|---|---|---|---|---|
| No aBAT | n = 102 | 89.5 | n = 332 | 80.2 | 434 |
| aBAT | n = 12 | 10.5 | n = 82 | 19.8 | 94 |
| Σ | n = 114 | n = 414 | 528 |
BMI_sds
BMI_sds was only calculable for the patients of 506 of the 528 18F-FDG-PET scans as either weight or height were not available. Figure 1 shows the proportion of aBAT (y-axis) as a function of BMI_sds (x-axis). Red circles represent PET scans with aBAT, green circles PET scans without aBAT. The dashed line stands for the baseline rate of aBAT, which is at 17.8%. The light blue logistic regression curve shows a slight decrease in the rate of aBAT from lower BMI_sds (approx. 24%) to higher BMI_sds (approx. 16%). However, this trend was not significant (p = 0.136).
Figure 1.
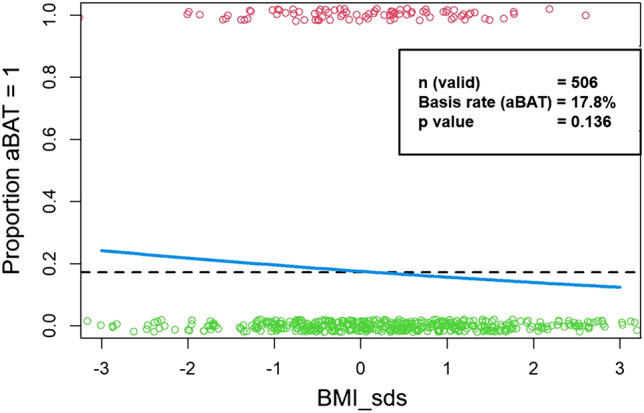
Influence of BMI_sds on the frequency of aBAT (blue curve). Green circles = no aBAT, red circles = aBAT. Dashed line = basis rate of aBAT.
Outside temperature
Figure 2 shows the proportion of aBAT (y-axis) as a function of average outside temperature stated in degrees Celcius (x-axis). Red circles represent PET scans with aBAT, green circles PET scans without aBAT. The dashed dark line stands for the baseline rate of aBAT, which is at 17.8%. The light blue logistic regression curve indicates that outside temperature has a clear influence on the occurrence of aBAT. Accordingly, aBAT significantly increased with lower and decreased with higher outside temperatures (p = 0.0001). However, aBAT frequency never reached 0 or 100%, suggesting that the outside temperature was an important, but not the only factor.
Figure 2.
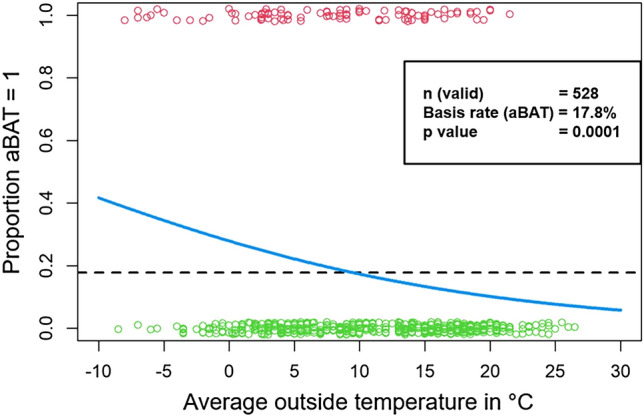
Influence of the average outdoor temperature on the frequency of aBAT (blue curve). Green circles = no aBAT, red circles = aBAT. Dashed line = basis rate of aBAT.
Influence of outside temperature on preventive measures
We first explored whether the outside temperature had an influence on the choice of the preventive measure: Fig. 3a displays the proportion of patients who received warming (y-axis) as a function of average outside temperature in degrees Celcius (x-axis). Red circles represent PET scans with aBAT, green circles PET scans without aBAT. The dashed dark line stands for the baseline rate of performed warming as preventive measure, which was at 61.7% (in 327 of 528 PET scans). The course of the light blue logistic regression curve remains flat, indicating that the average outside temperature had no influence on whether the patient was warmed or not (p = 0.80).
Figure 3.
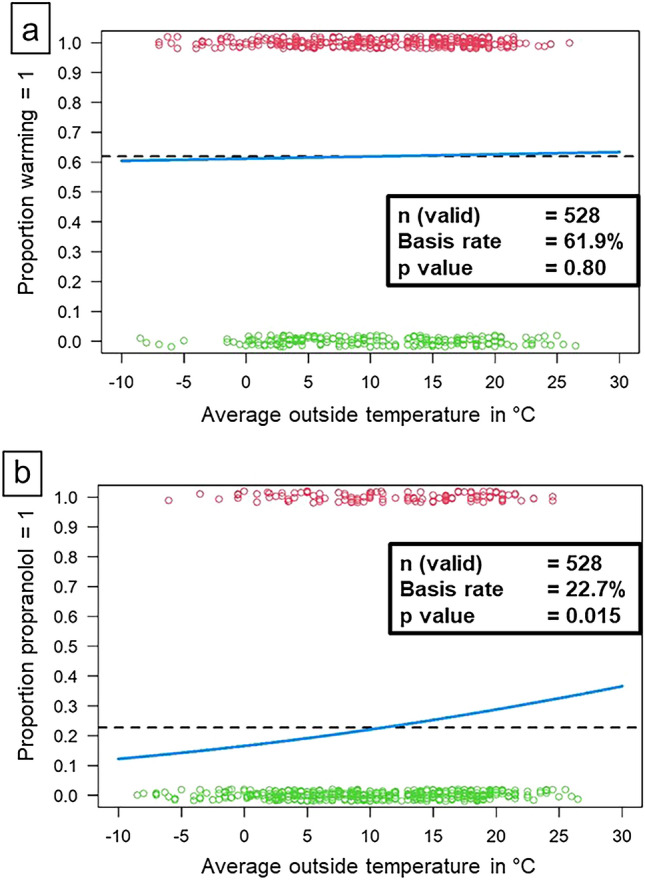
Interrelation between the outside temperature and the execution of preventive measures: (a) warming the patient (blue line = proportion of patients warmed), (b) administration of propranolol (blue line = proportion of patients in whom propranolol was given). Green circles = no aBAT, red circles = aBAT.
As a next step, we conducted the same analysis for propranolol: Fig. 3b shows the proportion of patients who received propranolol (y-axis) as a function of average outside temperature in degrees Celcius (x-axis). Red circles represent PET scans with aBAT, green circles PET scans without aBAT. The dashed dark line stands for the baseline rate of propranolol administration as preventive measure, which was at 22.7% (in 120 pf 528 PET scans). The course of the light blue logistic regression curve increases with increasing outside temperatures, meaning that the use of propranolol increased with higher temperatures. This trend turned out to be significant (p = 0.015).
Patient predisposition
We looked at pairs of 18F-FDG-PET scans of the same patient at staging and response assessment to see whether there is evidence for a patient predisposition for aBAT: 14 of 35 patients (40%) with aBAT and 28 of 196 (14.3%) without aBAT at staging presented with aBAT at response assessment (p < 0.001). This result suggests a patient predisposition.
Model analysis on the effect of preventive measures
To synthesize our findings, we fitted a logistic regression model (with patient as random effect to account for multiple images per patient) with all factors found to be relevant in the preceding analyses. From the model, we calculate the predicted probability of aBAT as a function of average outside temperature (in °C) and the preventive measures (warming and/or propranolol or none). The corresponding results are shown in Table 4 and in Fig. 4. Figure 4 displays the probability of aBAT predicted by the model as a function of the average outside temperature (in degrees Celsius) depending on the applied preventive measure (black graph = no preventive measure, n = 165; red graph = warming alone, n = 243; green graph = administering of propranolol only, n = 36; blue = combination of warming and administering of propranolol, n = 84). Table 4 and Fig. 4 demonstrate that both measures were effective to reduce aBAT. While warming patients significantly reduced the occurrence of aBAT (p = 0.004), the administration of propranolol was of borderline significance (p = 0.08) possibly due to the comparatively small number of patients in this group (Table 1). If both preventive measures were combined, there tended to be an additive effect (Fig. 4). Nevertheless, the effect of every preventive measure or its combination was significantly dependent on the outdoor temperature, i.e. the effectiveness increased with lower, but decreased with higher temperatures (Fig. 4).
Table 4.
Results of the aBAT prediction logistic regression model including average outside temperature and the two preventive interventions (warming, propranolol, and the combination of both).
| Estimate | Standard error | Probability (> │Z│) | |
|---|---|---|---|
| (Intercept) | − 0.526 | 0.281 | 0.062 |
| Average outside temperature | − 0.054 | 0.019 | 0.005 |
| Warming (true) | − 0.87 | 0.299 | 0.004 |
| Propranolol (true) | − 0.678 | 0.387 | 0.080 |
Figure 4.
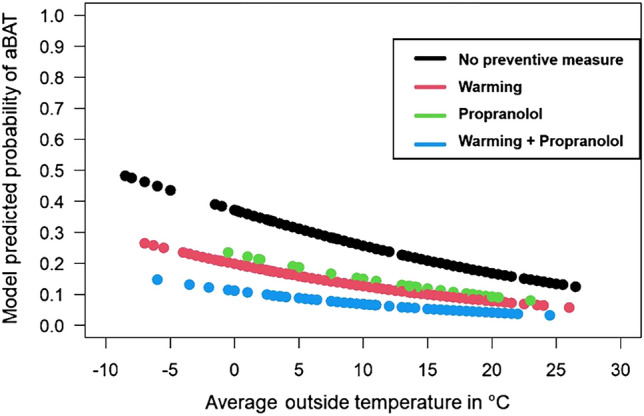
Probability of aBAT as a function of the average outdoor temperature and use of preventive measures.
Discussion
aBAT on 18F-FDG-PET scans is an issue, especially in young patients with Hodgkin lymphoma. At worst, aBAT impairs the readability of the scans to such an extent that it becomes impossible to determine the degree of metabolic response, which is essential to decide on further treatment. Therefore, the aim of this study was to find out whether preventive measures such as warming and/or propranolol are able to suppress aBAT, and whether patient related and environmental factors influence its frequency. Therefore, we analyzed a total of 528 18F-FDG-PET scans of 241 consecutive patients from the EuroNet-PHL-C2 trial.
Our results show that aBAT frequency did not differ between male and female patients, which is in line with several previously performed studies (one with 385 scans, the other with 2792 scans in pediatric cancer patients)2,18. However, there seems to be a difference to adults as a large study with more than 15,000 18F-FDG-PET scans from patients who were on average 61 (± 13) years old, showed that aBAT occurred more frequently in women6.
With regard to age, we observed that pediatric patients under the age of 11 had less aBAT compared to patients over the age of 11. This result was of borderline significance on univariate analysis and turned out to be of non-significance on multivariate analysis. Nevertheless, it corresponds well to the trend seen in the data published by Brady et al.: In this study, the frequency of aBAT in patients under the age of 12 ranged from 7 to 17%, whereas it ranged from 22 to 29% in patients in between 12 and 20 years. Gilsanz et al.19 came to a similar conclusion. They explained the difference as a matter of pre-pubertal and pubertal status since pubertal status is associated with hormone changes as well as physical adaptations, such as an increase in muscle mass. Growth and sex hormones, in particular, appear to have an influence on aBAT. However, hormonal constellations and interactions are complex which make it extremely difficult to precisely work out its effect on each individual and on the occurrence of aBAT20,21. Regarding body composition—determined by BMI_sds—we found no significant association between BMI_sds and the frequency of aBAT even though we observed a slight trend towards higher aBAT rates in patients with lower BMI_sds. The latter trend is backed by research results from an adult population published by Steinberg and colleagues. They were able to demonstrate that aBAT was more frequently in patients with a lower body mass index6. Drubach and colleagues came to a similar result in children and adolescents2.
We were able to confirm that the occurrence of aBAT is dependent on the outdoor temperature, whereby aBAT frequency increased with decreasing outdoor temperatures and decreased with increasing outdoor temperatures, respectively6,22,23. Thus, warming as a preventive measure suggests itself. In our cohort, warming significantly reduced the frequency of aBAT, although the preventive effect of warming diminishes with increasing outdoor temperatures. This result, however, conflicts with findings of a large study including 1290 patients and 2792 scans18. The 1290 patients were divided into three groups: The first group (323 patients, 630 scans) received no prevention against aBAT, the second group (345 patients, 705 scans) underwent “warming” by keeping the room temperature constantly at 24 degrees during the 18F-FDG uptake phase, and the third group received oral premedication with propranolol 60 min before the 18F-FDG injection. The frequency of aBAT on 18F-FDG-PET scans was 32.2% in group 1, 40.6% in group 2 and 15% in group 318. These data suggest that warming even increases the rate of aBAT. This is, however, contrary to the experiences of many nuclear medicine physicians, and to the recommendations of current guidelines for PET imaging in children and adolescents1,24. A closer look at the “method of warming” used in group 2 provides possible explanations for that discrepancy: three imaging clinics/departments from Cincinnati, Ann Arbor and Memphis participated in this study. Average daily highs are above 24° in Memphis from May to September, in Ann Arbor from June to August, and in Cincinnati from June to September25–27. During these periods, 24 degrees room temperature does not mean warming the patient, but rather cooling. Thus, it could well be that the temperature difference between outside and inside triggered aBAT. Another explanation for the discrepant result could be running air-conditioning systems. They create a constant, conditioned airflow inside the room and, thus, also on the skin of the people who are in the room. Thereby, warmth is conduced from the skin surface. With time, this also cools the temperature of the venous blood flowing back to the centre of the body. Cold venous blood, in turn, triggers aBAT4.
Another finding of our research was that propranolol as an unselective beta-blocker had the same effect as warming. However, this result was of borderline significance, probably because propranolol was less often used as single preventive measure in our dataset. Nevertheless, this result corresponds well with evidence in the literature. Thereafter propranolol is highly effective in preventing aBAT1,5,18,28–31.
Finally, there is the suggestion that combining warming and propranolol may further reduce the risk of aBAT. However, this remains a hypothesis, which requires further confirmation since our data lack the power to prove this additive effect.
Few limitations of our study should be considered: it was a retrospective observational study without randomization. The group in which only propranolol was used was too small to obtain final results on its effectiveness. Information from the centres on the use of air conditioning systems in uptake rooms would have been of interest, since all data came from nuclear medicine departments in Germany and Czech Republic. The use of air conditioning systems is expectedly less frequent in these two countries compared to warmer regions in the European Union (e.g. Spain, Italy) or the United States.
Conclusion
Warming is a simple, cheap and non-invasive method to reduce the risk of aBAT. However, the effect of warming decreases with increasing outdoor temperatures and might be completely neutralized, e.g. as soon as air conditioning systems are in operation during the tracer uptake phase. The administration of propranolol appears to be similarly effective to warming and may be considered the first choice in countries where air-conditioning is regularly used. Warming and propranolol seem to have additive effects particularly when outdoor temperatures are low.
Acknowledgements
We acknowledge the efforts made by all nuclear medicine physicians and technologists to provide necessary information and image data: Prof. Dr. Peter Bartenstein, Department of Nuclear Medicine, LMU Munich; Prof. Dr. Frank M. Bengel, Department of Nuclear Medicine, Medizinische Hochschule Hannover; Prof. Dr. Ambros J. Beer, Department of Nuclear Medicine, University Hospital Ulm; Prof. Dr. Winfried Brenner, Department of Nuclear Medicine, Charité Berlin; Prof. Dr. Jan Alexander Bucerius, Department of Nuclear Medicine, University Hospital Göttingen; Prof. Dr. Andreas Buck, Department of Nuclear Medicine, University Hospital Würzburg; Dr. Elke Conrad, Department of Nuclear Medicine, Helios-Klinikum Erfurt; Prof. Dr. Stefan Dresel, Department of Nuclear Medicine, Helios-Klinikum Berlin-Buch; Prof. Dr. Alexander Drzezga, Department of Nuclear Medicine, University Hospital Cologne; Prof. Dr. Markus Essler, Department of Nuclear Medicine, University Hospital Bonn; Prof. Dr. Samer Ezziddin, Department of Nuclear Medicine, University Hospital Homburg; Prof. Dr. Christiane Franzius, ZEMODI, Bremen; Prof. Dr. Martin Freesmeyer; Department of Nuclear Medicine, University Hospital Jena; Prof. Dr. C. la Fougère, Department of Nuclear Medicine, University Hospital Tübingen; Prof. Dr. Frederik L. Giesel, Department of Nuclear Medicine, University Hospital Düsseldorf; Prof. Dr. Frank Grünwald, Department of Nuclear Medicine, University Hospital Frankfurt/Main; Prof. Dr. Uwe Haberkorn, Department of Nuclear Medicine, University Hospital Heidelberg; Prof. Dr. Dr. Alexander Heinzel, Department of Nuclear Medicine, University Hospital Halle (Saale); Prof. Dr. Dirk Hellwig, Department of Nuclear Medicine, University Hospital Regensburg; Prof. Dr. Ken Herrmann, Department of Nuclear Medicine, University Hospital Essen; Prof. Dr. Jörg Kotzerke, Department of Nuclear Medicine, University Hospital Dresden; Prof. Dr. Bernd J. Krause, Department of Nuclear Medicine, University Hospital Rostock; Prof. Dr. Susanne Klutmann, Department of Nuclear Medicine, University Hospital Hamburg-Eppendorf; Prof. Dr. Michael Kreißl, Department of Nuclear Medicine, University Hospital Magdeburg; Prof. Dr. Torsten Kuwert, Department of Nuclear Medicine, University Hospital Erlangen; Prof. Dr. Constantin Lapa, Department of Nuclear Medicine, University Hospital Augsburg; PD Dr. Ulf Lützen, Department of Nuclear Medicine, University Hospital Schleswig-Holstein Kiel; Prof. Dr. Dr. Ph. T. Meyer, Department of Nuclear Medicine, University Hospital Freiburg; Prof. Dr. F. M. Mottaghy, Department of Nuclear Medicine, University Hospital Aachen; Dr. C. Neumann, Department of Nuclear Medicine, Evangelisches Klinikum Bethel; Prof. Dr. Michael J. Reinhardt, Department of Nuclear Medicine, Pius Hospital Oldenburg; PD Dr. Christoph Rischpler, Department of Nuclear Medicine, Klinikum Stuttgart; Prof. Dr. Juri Ruf, Department of Nuclear Medicine, Städtisches Klinikum Karlsruhe gGmbH; Prof. Dr. M. Schäfers, Department of Nuclear Medicine, University Hospital Münster; Prof. Dr. Mathias Schreckenberger, Department of Nuclear Medicine, University Hospital Mainz; Dr. Dagmar Steiner, Department of Nuclear Medicine, University Hospital Giessen; Prof. Dr. Wolfgang Weber, Department of Nuclear Medicine, Klinikum rechts der Isar an der Technischen Universität Munich; Prof. Dr. Philipp Wiggermann, Department of Radiology and Nuclear Medicine, Städtisches Klinikum Braunschweig; Prof. Dr. Klaus Zöphel, Department of Nuclear Medicine, Klinikum Chemnitz—all in Germany; and Prof. MUDr. Jiri Ferda, Radiologieplzen.eu, Plzen, Czech Republic. This study was supported by the Mitteldeutsche Kinderkrebsforschung—Stiftung für Forschung und Heilung, Leipzig, Germany.
Abbreviations
- aBAT
Activated brown adipose tissue
- BAT
Brown adipose tissue
- BMI
Body mass index
- EuroNet-PHL-C2
European Network for Pediatric Hodgkin Lymphoma Classical Hodgkin Lymphoma, 2nd generation
- 18F-FDG-PET
18F-Fluorodeoxyglucose positron emission tomography
- HL
Hodgkin lymphoma
- IBF
Institutional Review Board
- OEPA
Vincristine, etoposide, prednisone, doxorubicine
- OHRP
Office of Human Research Protections
Author contributions
L.K., C.P., D.H., D.S., and R.K. analyzed the data and wrote the manuscript. C.M.K., D.K., and M.C. provided patient data. C.P. and S.N. analyzed the 18F-FDG-PET images and collected all necessary data. O.S., R.K., C.M.K., and D.K. provided administrative support. All authors reviewed the manuscript and edited it accordingly. All authors have approved the submitted version of this manuscript. Furthermore, they have agreed both to be personally accountable for their own contributions and ensure that questions related to the accuracy or integrity of any part of the work, even ones in which they were not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data (tables) that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: C. Pötzsch, Lars Kurch, D. Hasenclever and R. Kluge.
References
- 1.Vali R, Alessio A, Balza R, Borgwardt L, Bar-Sever Z, Czachowski M, Jehanno N, Kurch L, Pandit-Taskar N, Parisi M, Piccardo A, Seghers V, Skulkin BL, Zuchetta P, Lim R. SNMMI Procedure Standard/EANM Practice Guideline on Pediatric 18F-FDG PET/CT for Oncology 1.0. JNM. 2021;62(1):99–110. doi: 10.2967/jnumed.120.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drubach, L.A., Palmer, E.L., Connolly, L.P., Baker, A., Zurakowski, D., Cypress, A.M. Pediatric brown adipose tissue: Detection, epidemiology, and differences from adults. J. Pediatr. 939–944 (2011). [DOI] [PubMed]
- 3.Merkel M, Schmid SM, Iwen KA. Physiologie und klinische Bedeutung von weißem, beigem und braunem Fettgewebe. Der Internist. 2019;60:115–121. doi: 10.1007/s00108-018-0540-0. [DOI] [PubMed] [Google Scholar]
- 4.Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue—Functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–1790. doi: 10.2337/db12-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao R, Yuan L, Zhang N, Li C, Yang J. Brown adipose tissue: Distribution and influencing factors on FDG PET/CT scan. J. Pediatr. Endocr. Met. 2012;25:233–237. doi: 10.1515/jpem-2012-0029. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg JD, Vogel W, Vegt E. Factors influencing brown fat activation in FDG PET/CT: A retrospective analysis of 15,000+ cases. BJR. 2017 doi: 10.1259/bjr.201700093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzi-Engbeaya C, Salem V, Atkar RS, Dhillo WS. Insight into brown adipose tissue physiology as revealed by imaging studies. Adipocyte. 2015 doi: 10.4161/21623945.2014.965609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluge R, Kurch L, Georgi T, Metzger M. Current role of FDG-PET in Pediatric Hodgkin’s Lymphoma. Semin. Nucl. Med. 2017;47(3):242–257. doi: 10.1053/j.semnuclmed.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Mauz-Körholz C, Landman-Parker J, Balwierz W, Ammann RA, Anderson RA, Attarbaschi A, Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M, Claviez A, Daw S, Dieckmann K, Fernádez-Teijeiro A, Fossa A, Gattenlöhner S, Georgi T, Hjalgrim LL, Hraskova A, Karlén J, Kluge R, Kurch L, Leblanc T, Mann G, Montravers F, Pears J, Pelz T, Rajic V, Ramsay AD, Stoevesandt D, Uyttebroeck A, Vordermark D, Körholz D, Hasenclever D, Wallace WH. Response-adapated omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): A titration study with an open-label-embedded, multinational, non-inferiority, randomized controlled trial. Lancet Oncol. 2022;23:125–137. doi: 10.1016/S1470-2045(21)00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauz-Körholz C, Landman-Parker J, Fernández-Teijeriro A, Attarbaschi A, Balwierz W, Bartelt JM, Beishuizen A, Boudjemaa S, Cepelova M, Ceppi F, Claviez A, Daw S, Dieckmann K, Fossa A, Gattenlöhner S, Georgi T, Hjalgrim LL, Hraskova A, Karlén J, Kurch L, Leblanc T, Mann G, Montravers F, Pears J, Pelz T, Rajic V, Ramsay AD, Stoevesandt D, Uyttebroeck A, Vordermark D, Körholz D, Hasenclever D, Wallace WH. Response-adapated omission of radiotherapy in children and adolescents with early-stage classical Hodgkin lymphoma and an adequate response to vincristine, etoposide, prednisone, and doxorubicin (EuroNet-PHL-C1): A titration study. Lancet Oncol. 2023;24:252–261. doi: 10.1016/S1470-2045(23)00019-0. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, DeLaat C, Fossati-Bellani F, Morgan E, Oberlin O, Reaman G, Ruymann FB, Tersak J, Meadows AT, on behalf of the Late Effects Study Group High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. JCO. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Dörffel W, Riepenhausen M, Lüders H, Brämswig J, Schellong J. Secondary malignancies following treatment for Hodgkin’s lymphoma in childhood and adolescence—A cohort study with more than 30 years follow-up. Deutsches Ärzteblatt Int. 2015;112:320–327. doi: 10.3238/arztebl.2015.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schellong G, Riepenhausen M, Bruch C, Kotthoff S, Vogt J, Bölling T, Dieckmann K, Pötter R, Heinecke A, Brämswig J, Dörffel W. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: Report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr. Blood Cancer. 2010;55:1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 14.Morris B, Partap S, Yeom K, Gibbs IC, Fisher PG, King AA. Cerebrovascular disease in childhood cancer survivors: A Children’s Oncology Group Report. Neurology. 2009;73:1906–1913. doi: 10.1212/WNL.0b013e3181c17ea8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.skion.nl/workspace/uploads/EuroNet-PHL-C2_trial_protocol_final2-0-2015-07-27.pdf (27.04.2023)
- 16.Kurch L, Mauz-Körholz C, Bertling S, Wallinder M, Kaminska M, Marwede D, Tchavdarova L, Georgi TW, Elsner A, Barthel A, Stoevesandt D, Hasenclever D, Sattler B, Sabri O, Körholz D, Kluge R. The EuroNet paediatric Hodgkin network—Modern imaging data management for real time central review in multicentre trials. Klin. Padiatr. 2013;225:357–361. doi: 10.1055/s-0033-1354416. [DOI] [PubMed] [Google Scholar]
- 17.Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß HC, Hesse V, von Hippel A, Jaeger U, Johnsen D, Korte W, Menner K, Müller G, Müller JM, Niemann-Pilatus A, Remer R, Schaefer F, Wittchen H-U, Zabransky S, Zellner K, Ziegler A, Hebebrand J. Prezentile für den Body-mass-Index für das Kinder- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift Kinderheilkunde. 2001;149:807–818. doi: 10.1007/s001120170107. [DOI] [Google Scholar]
- 18.Brady SL, Wong KK, Doubrovin M, Han Y, Li Y, Wu S, Hossain AKMM, Chism CB, Naik MH, Rossi M, Shulkin BL. Effect of propranolol on 18F-fluorodeoxyglucose uptake in brown adipose tissue in children and young adults with neoplastic diseases. Mol. Imaging Biol. 2021;23:260–269. doi: 10.1007/s11307-020-01547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TAL, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J. Pediatr. 2012;160:604–609. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J. Pediatr. 2011;158:722–726. doi: 10.1016/j.jpeds.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaikaew K, Grefhorst A, Visser JA. Sex differences in brown adipose tissue function: Sex hormones, glucocorticoids, and their crosstalk. Front. Endocrinol. 2021 doi: 10.3389/fendo.2021.652444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: Prevalence is related to ambient outdoor temperature—Evaluation with 18F-FDG PET/CT. JNM. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 23.Kim SH, Krynyckyi BR, Machac J, Kim CK. Temporal relation between temperature chance and FDG uptake in brown adipose tissue. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:984–989. doi: 10.1007/s00259-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 24.Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, Drubach LA. Constant ambient temperature of 24 °C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:602–606. doi: 10.1007/s00259-008-0983-y. [DOI] [PubMed] [Google Scholar]
- 25.https://www.usclimatedata.com/climate/cincinnati/ohio/united-states/usoh0188 (19.04.2023)
- 26.https://www.usclimatedata.com/climate/ann-arbor/michigan/united-states/usmi0028 (19.04.2023)
- 27.https://www.usclimatedata.com/climate/memphis/tennesse/united-states/ustn0325 (19.04.2023)
- 28.Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1018–1022. doi: 10.1007/s00259-006-0318-9. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A, Nair N, Baghel NS. A novel approach for reduction of brown fat uptake on FDG PET. Br. J. Radiol. 2009;82:626–631. doi: 10.1259/bjr/24661539. [DOI] [PubMed] [Google Scholar]
- 30.Parysow O, Mollerach AM, Jager V, Racioppi S, Roman JS, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin. Nucl. Med. 2007;32:351–357. doi: 10.1097/01.rlu.0000259570.69163.04. [DOI] [PubMed] [Google Scholar]
- 31.George A, Sinha P, Conrad G, Memon AA, Dressler EV, Wagner LM. Pilot study of propranolol premedication to reduce FDG uptake in brown adipose tissue on PET scans of adolescent and young adult oncology patients. Pediatr. Hematol. Oncol. 2017;34:149–156. doi: 10.1080/08880018.2017.1338806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data (tables) that support the findings of this study are available from the corresponding author upon reasonable request.


