Abstract
Purpose of review
Historical evidence suggests a shared underlying etiology for migraine and gastrointestinal (GI) disorders that involves the gut-brain axis. Here we provide narrative review of recent literature on the gut-brain connection and migraine to emphasize the importance of tailoring treatment plans for patients with episodic migraine who experience GI comorbidities and symptoms.
Recent findings
Recent population-based studies report the prevalence of migraine and GI disorders as comorbidities as well as overlapping symptomology. American Headache Society (AHS) guidelines have integrated GI symptoms as part of migraine diagnostic criteria and recommend nonoral therapies for patients with GI symptoms or conditions. Nasal delivery is a recommended nonoral alternative; however, it is important to understand potential adverse events that may cause or worsen GI symptoms in some patients due to the site of drug deposition within the nasal cavity with some nasal therapies. Lastly, clinical perspectives emphasize the importance of identifying GI symptoms and comorbidities in patients with episodic migraine to best individualize migraine management.
Summary
Support for an association between the gut-brain axis and migraine continues to prevail in recent literature; however, the relationship remains complex and not well elucidated. The presence of GI comorbidities and symptoms must be carefully considered when making treatment decisions for patients with episodic migraine.
Keywords: Gut-brain, Migraine, Episodic migraine
Introduction
The bidirectional communication between the central nervous system and gastrointestinal (GI) system is referred to as the gut-brain axis and has been implicated in the pathogenesis of several neurological conditions [1••, 2••, 3]. As such, the Rome Foundation adopted the term disorder of gut-brain interaction (DGBI), defined as a group of disorders classified by GI symptoms related to any combination of motility disturbances, visceral hypersensitivity, altered mucosal and immune function, gut microbiota, and central nervous system processing. DGBIs were previously referred to as functional GI disorders. Historically, these disorders have been characterized by their symptoms and were without known structural abnormalities. Examples of DGBIs are functional dyspepsia, cyclic vomiting syndrome (CVS), irritable bowel syndrome (IBS), functional constipation, and functional diarrhea [4]. Migraine is a debilitating headache disorder with a high prevalence and burden, and like DGBIs, migraine is more prevalent in women than men [5–9]. According to the Global Burden of Disease Study in 2019, migraine is the second among the world’s causes of disability and the top cause for young women aged 15–49 years [8]. The International Classification of Headache Disorders version 3 (ICHD-3) defines migraine as a recurrent headache disorder with moderate or severe headache attacks lasting 4 to 72 h that are accompanied by nausea and photophobia and/or phonophobia. Individuals can further experience chronic migraine (CM), defined as ≥ 15 headaches per month, or episodic migraine (EM), defined as ≤ 14 headaches per month [5, 10]. In addition to nausea, individuals with migraine routinely experience other GI symptoms, including vomiting, diarrhea, and constipation and GI comorbidities such as celiac disease, gastroesophageal reflux disease (GERD), IBS, Helicobacter pylori infection, gastroparesis, functional dyspepsia, and CVS [11–19]. Further, several therapies are indicated to treat both migraine and accompanying symptoms and GI disorders, which include metoclopramide, tricyclic antidepressants (TCAs), antiepileptic drugs, antiemetics, and noninvasive vagal nerve stimulation [20, 21•, 22–26]. These associations suggest a relationship between migraine and the GI system, implicating pathophysiology of the gut-brain axis. Recent studies demonstrate a highly complex interplay between multiple physiological systems to produce the broad range of symptomology observed with migraine and DGBI or GI disorders. Several comprehensive review papers have been published on the topic of the gut-brain axis and its relationship to migraine [1••, 27••, 28–31]. Here, we provide a narrative review of current evidence for an association between migraine and GI comorbidities and symptoms, with an emphasis on episodic migraine; explore the effects of EM treatments on those symptoms based on recent evidence from the literature; and make treatment recommendations to optimize outcomes in patients with EM who experience GI comorbidities and symptoms.
Evidence Linking Migraine and GI Disorders
Historically, a shared pathophysiology between migraine and GI disorders has been based on the observed overlap of their symptom profiles and prevalence studies demonstrating a comorbid relationship. For example, individuals with migraine can experience nausea, vomiting, and delayed gastric emptying, which also constitute part of the symptomology of many well-known GI disorders, such as gastroparesis, functional dyspepsia, and CVS [4, 5, 12, 32]. Autonomic dysfunction has been described in migraine and GI disorders and may account for overlapping symptoms [33–39]. According to a prospective, cross-sectional study of 605 participants with migraine, visceral autonomic symptoms are common in individuals with migraine in all phases of the migraine cycle. Results showed that 41% of participants reported that 100% of their migraine attacks were accompanied by autonomic symptoms. Visceral symptoms present before, during, and after a migraine attack included stomach fullness, bloating, nausea, vomiting, eructing, constipation, diarrhea, frequent defecation, and frequent urination, and occurred more frequently during a migraine attack. Participants with migraine and ≥ 1 visceral symptom during their migraine attack reported a longer duration for their migraine compared with participants without visceral symptoms (24.4 ± 29.6 h vs 16.8 ± 19.8 h; p = 0.008). Participants with visceral symptoms also experienced migraine attacks of greater severity based on the numeric rating scale-11 compared with participants without visceral symptoms both before (8 ± 1.7 vs 7.6 ± 1.8; p = 0.02) and during (8.01 ± 1.7 vs 7.09 ± 1.9; p = 0.001) a migraine attack [37]. A prospective longitudinal study in 43 participants from a single tertiary academic center who were diagnosed with episodic or CM used the Composite Autonomic Symptom Scale (COMPASS-31) questionnaire to determine the presence of autonomic symptoms, which sums the scores of 6 domains: orthostatic intolerance (range 0–40), vasomotor (range 0–5), secretomotor (range 0–15), GI (range 0–25), bladder (range 0–10), and pupillomotor (range 0–5). A total score of 0 indicates no presence of autonomic symptoms and a score of 100 represents severe autonomic symptoms. Autonomic symptoms were reported in this cohort of patients with migraine and no change in the COMPASS-31 score before and after treatment over 12 months was observed (30.3 vs 30.3, respectively; p = 0.885), suggesting there is no correlation between treatment response and severity of autonomic symptoms [38].
Emerging evidence suggests an association between altered gut microbiota and migraine pathophysiology. A cross-sectional, case control study that evaluated gut microbiota in 42 participants with EM demonstrated altered gut microbiota when compared with 43 healthy controls. Moreover, the relative abundance of specific microbial genera was shown to be associated with migraine frequency and severity [40]. Altered gut microbiota have also been noted in individuals with fibromyalgia and IBS [41, 42], suggesting an association between gut microbiota, pain, and proinflammatory mediators. In support of this notion, a recent preclinical study demonstrated that microbiota dysbiosis enhanced migraine-like pain via upregulation of the proinflammatory cytokine tumor necrosis factor alpha (TNFα) in intraspinal trigeminal nucleus caudalis (Sp5C) [43]. A randomized, double-blind, placebo-controlled trial of 50 chronic and 50 episodic patients with migraine in Iran evaluated migraine treatment effect of supplementation with a 14-strain probiotic mixture over 8 and 10 weeks, respectively. In patients with EM who received probiotics compared with those who received placebo, the mean frequency of migraine attacks per month significantly decreased (-2.64 vs 0.06; respectively, p < 0.001) and the mean migraine severity as assessed by the visual analog scale decreased (-2.14 vs 0.11, respectively; p < 0.001). In patients with CM who received probiotics compared with those who received placebo, the mean frequency of migraine attacks significantly decreased (-9.67 vs -0.22; respectively, p ≤ 0.001) and the mean migraine severity decreased (-2.69 vs -0.22, respectively; p ≤ 0.001). The number of abortive drugs used weekly for EM and daily for CM also decreased compared with baseline (EM: -0.72; p < 0.001; CM: -1.02; p < 0.001) and with no significant change in the placebo group [44]. However, a recent meta-analysis of published randomized clinical trials found insufficient evidence that probiotic supplementation significantly affects migraine frequency or severity, indicating that probiotics alone may be insufficient to restore microbiota alterations associated with migraine pathogenesis and that further research is warranted in this field [45].
Several recent population-based studies have reported the prevalence of migraine and GI disorders as comorbidities; however, most of these studies do not disclose if migraine is chronic or episodic. For example, in the large Migraine in America Symptoms and Treatment (MAST) study (n = 6045), 75.7% of participants with migraine reported experiencing nausea, and 28.1% described nausea as their most bothersome symptom [46]. In a separate analysis of the MAST cohort (n = 15,133), participants with migraine were more than 3 times likely to experience gastric ulcers and GI bleeding than healthy controls (OR: 3.11; 95% CI: 2.8–3.5) [47]. In a study of 60 patients with functional dyspepsia, of whom 38 had postprandial distress syndrome and 22 had epigastric pain syndrome, 68% (41/60) were reported to have migraine without aura. Of those with postprandial distress syndrome and epigastric pain syndrome, 76% (29/38) and 54% (12/22) had migraine without aura, respectively. In patients with postprandial distress syndrome who had migraine, onset was related to meal ingestion and associated with dyspeptic symptoms for 89%, and there was a statistically significant correlation of migraine severity and postprandial modification of the gastric discomfort threshold (r = -0.73; p < 0.001) [13]. A cross-sectional Iranian study of overweight and obese individuals with migraine evaluated their prevalence of GI disorders, which was 18.8% for dyspepsia, 11% for constipation, 7.2% for heartburn, 6.1% for fatty liver, 4.4% for IBS, and 1.7% for cholelithiasis [48]. A retrospective analysis in the United States showed a high prevalence of headaches (48%) in participants with IBS (n = 1645) [49]. A cross-sectional, observational study of 341 Iranian participants undergoing gastric endoscopy demonstrated that 43.7% met diagnostic criteria for migraine, with a statistically significant higher incidence of women (67.8%) compared with men (32.2%; p = 0.003). Among the participants with GERD, H pylori infection, duodenal ulcer, and gastric ulcer, 78, 138, 37, and 10 participants had migraine, respectively, which was statistically significant (p ≤ 0.001) compared with participants who did not have migraine for all GI disorders with the exception of gastric ulcer [50]. The American Migraine Prevalence and Prevention Study of 11,603 eligible respondents with migraine showed that 7.4%, 10.2%, and 10.2% of patients with low-frequency (n = 7860; 0–3 monthly headache days), medium-frequency (n = 2051; 4–7 monthly headache days), and high-frequency (n = 898; 8–14 monthly headache days) EM, respectively, experienced ulcers of the stomach or intestines, which was statistically significant for high- vs medium-frequency EM (p = 0.026) [51]. A Japanese cross-sectional study of individuals with EM (n = 271) reported comorbidities of heartburn (38.4%), chronic constipation (27.3%), frequent diarrhea (23.6%), IBS (16.6%), GERD (15.9%), ulcers (10.3%), and ulcerative colitis (1.5%) [52]. Lastly, a multicenter, randomized, single-blind, phase 4 study in 65 patients with EM without significant GI symptoms evaluated colonic transit time of a single dose of a calcitonin gene-related peptide (CGRP) monoclonal antibody that targets the ligand (galcanezumab; Emgality®, Eli Lilly, Indianapolis, IN, USA) compared with a CGRP monoclonal antibody that targets the receptor (erenumab; Aimovig®, Amgen Inc, Thousand Oaks, CA, USA). Baseline demographic data revealed that gastric emptying time was severely delayed in 12.5% and 21.2%, small bowel transit time was delayed in 28.1% and 18.2%, colonic transit time was delayed in 12.5% and 12.1%, small and large bowel transit time was delayed in 12.5% and 12.1%, whole gut transit time was delayed in 15.6% and 15.2%, the Bristol Stool Form Scale was consistent with constipation in 9.4% and 9.1%, and the Bristol Stool Form Scale was consistent with diarrhea in 3.1% and 9.1% of patients in the erenumab and galcanezumab groups, respectively. This growing body of literature highlights the need for careful examination of migraine and any related GI symptoms or comorbidities, which in turn will serve to optimize EM treatment regimens and improve patient outcomes.
The Impact of GI Symptoms and Disorders on EM Treatment
EM can be managed with acute therapies to reduce the pain, associated symptoms, and disability or preventive therapies if a patient meets the appropriate diagnostic requirements [21•, 53, 54]. According to the American Headache Society (AHS) consensus, the main goals of acute therapies for migraine are rapid and consistent freedom from pain and associated symptoms without recurrence, restored ability to function, minimal need for repeat dosing or rescue medications, optimal self-care, reduced subsequent use of resources, minimal or no adverse events (AEs), and cost considerations [21•]. For migraine preventive therapies, the main goals are to reduce attack frequency, severity, duration, and disability; improve responsiveness to and avoid escalation in use of acute treatment; improve function; reduce disability; reduce reliance on poorly tolerated, ineffective, or unwanted acute treatments; reduce overall cost associated with migraine treatment; enable patients to manage their disease to enhance a sense of control; improve health-related quality of life; and reduce headache-related distress and psychological symptoms [21•]. Approved acute therapies can be divided into migraine-specific agents, such as triptans, ergotamine derivatives (eg, dihydroergotamine [DHE] mesylate), gepants, and ditans, and migraine-nonspecific agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and combination nonopioid analgesics. Approved preventive therapies for EM include the migraine-specific agents of CGRP monoclonal antibodies and migraine-nonspecific agents of beta-blockers, anticonvulsants, and antidepressants. Dual therapies for migraine can be used as both acute and preventive treatments, which include frovatriptan (for menstrual-related migraine), neuromodulation, behavioral therapy, and gepants (such as rimegepant) [21•]. Based on the consensus from several international guidelines as well as results from clinical trials, the aforementioned acute and preventive therapies are safe and efficacious for the management of migraine [21•, 53, 54], and “a nonoral formulation should be used in patients whose attacks are associated with severe nausea or vomiting, who do not respond well to traditional oral treatments, patients who experience significant nausea or vomiting early during attacks, or who have trouble swallowing orally administered medications” [7, 21•]. Most acute therapies are orally administered, with some available as parenteral or nasal, while preventive therapies are either oral or parenteral [21•, 55]. A strong understanding of how route of administration coupled with mechanism of action of migraine therapies affects treatment outcomes is critical for drug selection and meeting individualized needs.
While oral medications are convenient and easy to administer, their absorption and efficacy may be compromised in patients with GI symptoms or comorbidities. As early as the 1990s, studies have demonstrated that gastric emptying in patients with migraine is delayed during and outside the migraine attack [12, 56–59]. Complementing these results are early studies reporting slower absorption of orally administered drugs, including some triptans, during a migraine attack, which was shown to be increased when combined with agents that improve GI motility [60–66]. Further, nausea and vomiting are highly disabling symptoms accompanying migraine that may interfere with the efficacy or administration of oral therapies [46, 67–69]. A prospective cohort study using data from the American Migraine Prevalence and Prevention Study reported that 43.7% of participants with EM (n = 3182) experienced persistent frequent nausea and 3.4% progressed to CM within 2 years, indicating the importance of considering nausea during drug selection for managing EM in patients [70]. Although triptans are generally recommended as first-line acute therapies for moderate to severe migraine attacks, a recent retrospective claims analysis in the United States of 10,509 new triptan users reported that 30% were potential triptan-insufficient responders, with 68% of potential triptan-insufficient responders using opioids in the 24-month postindex period. This highlights the need for better, individualized acute treatment options that are nonoral [71]. An absence of baseline vomiting and nausea has been found to be a predictor of headache relief at 2 h with oral sumatriptan 100 mg using data from the Sumatriptan Naratriptan Aggregate Patient database, and in some studies, triptan use was reported to be the cause of nausea [72–75]. A post hoc analysis of the randomized, double-blind COMPASS study that compared AVP-825 (Onzetra® Xsail®; breath-powered intranasal delivery of powdered sumatriptan 22 mg) with oral sumatriptan tablets (100 mg) characterized the longitudinal trajectories of nausea across multiple migraine attacks using 3 models. The first model (n = 259) measured overall nausea (ie, longitudinal change in nausea from predose to 120 min for the whole sample, independent of baseline nausea). Compared to oral sumatriptan, overall nausea declined more rapidly during the first hour, and there were reduced odds of nausea from 30 to 120 min following AVP-825 treatment. The second model (n = 232) measured treatment-emergent nausea from 10 to 120 min postdose in migraine attacks without nausea at baseline. Compared to AVP-825, the risk of treatment-emergent nausea increased at a significantly faster rate over 45 min postdose, with significantly greater odds of treatment-emergent nausea at 45, 60, and 90 min postdose with oral sumatriptan. The third model (n = 167) measured nausea relief from 10 to 120 min postdose in eligible migraine attacks with nausea at baseline, and it showed reduced odds of nausea with AVP-825 treatment compared with oral sumatriptan but no differences in the rate of change in nausea over time between the 2 treatments [76]. A separate post hoc analysis of the COMPASS trial of 259 patients further revealed that AVP-825 significantly lowered mean pain intensity and mean disability from 10 to 90 min postdose (effect sizes: -0.09 to -0.29; p < 0.0001 to 0.01) and was associated with greater within-person consistency in migraine pain intensity and migraine-related disability across multiple migraine attacks, from 45 to 120 min postdose compared with oral sumatriptan [77]. Results from these studies suggest that nasal administration may provide more rapid and consistent drug absorption and provide better relief from nausea compared with oral administration.
Overall, nasal drug delivery can provide rapid onset of relief, bypasses first-pass metabolism, and improves drug bioavailability in a noninvasive and convenient manner [78–85], which is particularly important for patients with GI symptoms who have cycled through many oral agents without treatment success. However, the site of drug deposition within the nasal cavity needs to be considered because it can influence drug pharmacokinetics [79, 81, 86, 87]. Traditional nasal sprays generally deliver drug to the lower nasal space, where epithelium is not well suited for optimal drug absorption and where there is an increased likelihood of drug clearance due to nasal drip, swallowing, or mucociliary clearance, potentially resulting in variable absorption and suboptimal efficacy as well as AEs of dysgeusia [79, 83, 86–93]. According to the Food and Drug Administration Adverse Event Reporting System (FAERS) database, dysgeusia is a common adverse event of nasal sprays, which is most likely a result of the taste of the drug rather than the underlying mechanism of action of the therapies [94, 95]. Several factors may contribute to the incidence of dysgeusia associated with nasal sprays for migraine treatment, such as type of medication used, dosage, and individual characteristics. Traditional nasal sprays that use a nasal spray pump or an atomizer for delivery of the drug have shown to deposit drug in the lower nasal space [79, 83, 86, 93, 96], and dysgeusia may be caused by postnasal dripping of residual spray into the oral cavity and contact with the posterior tongue and taste buds, which activates the taste receptor cells [95, 97]. Regardless of the mechanism, dysgeusia is a concern for patients with migraine who experience GI symptoms because it can exacerbate existing nausea and vomiting and, in some cases, be the cause of it [95]. Dysgeusia rates for approved nasally administered acute therapies for migraine range from 1.1% to 25%, with the lowest value referring to DHE mesylate delivered to the upper nasal space by Precision Olfactory Delivery (POD®) or INP104 (TRUDHESA®, Impel Pharmaceuticals, Seattle, WA, USA) [84, 98–104]. INP104 is approved as an acute treatment for migraine with or without aura [103]. POD is a handheld and manually actuated device that gently delivers a narrow, focused plume of DHE mesylate through the nasal valve, reaching the hard-to-reach upper nasal space, where drug is less likely to drip out of the nose or be swallowed and is more likely to be absorbed within the richly vascularized olfactory epithelium [96]. The site of drug deposition and the POD technology are likely explanations for the low rates of dysgeusia (1.1%) in the phase 3 study of INP104 in patients with migraine [84]. Parenteral administration of migraine drugs is another alternative to oral agents and can provide rapid onset of relief; however, it may not be ideal for all patients. For example, intravenous DHE mesylate can produce strong adverse events of nausea and vomiting due to high maximum plasma concentrations and must be delivered in a clinical setting with an antiemetic, making it inconvenient for routine administration [83, 87, 105–107]. Further, parenteral administration is not ideal for patients who are needle-phobic.
The CGRP class of acute and preventive therapies, which act via antagonism of the CGRP pathway by targeting the CGRP receptor or its canonical receptor, or the CGRP molecule itself, have proven clinical efficacy and safety [21•, 108]. A recent narrative review provides evidence for the role of CGRP in migraine and GI disorders [109••]. Based on the FAERS database, GI-related AEs such as abdominal discomfort and pain, diarrhea, nausea, constipation, and vomiting have been reported with CGRP monoclonal antibody use. Constipation rates were higher for CGRP receptor antagonists compared with antagonists that target the CGRP ligand [109••], which may be due to differences in mechanisms of action [110]. Results from the phase 4 study in 65 patients with EM without significant GI symptoms that evaluated colonic transit time of a single dose of a CGRP monoclonal antibody that targets the ligand (galcanezumab) compared with a CGRP monoclonal antibody that targets the receptor (erenumab) demonstrated numerically reduced colonic transit times for galcanezumab and numerically increased colonic transit times for erenumab at 2 weeks post-treatment but were not statistically significant. Participants treated with erenumab experienced both a reduction in the number of spontaneous bowel movements and a hardening of stool at 2 and 4 weeks following treatment, whereas participants treated with galcanezumab showed no change in these measures. Notably, participants receiving both treatments reported significant increases in the constipation domain of the GI symptom rating scale [110]. This result is consistent with another study of 30 healthy participants in Denmark, in which 93% treated with infusions of CGRP experienced GI symptoms, including rumbling, nausea, diarrhea, and urge to defecate. Further, symptoms of GI hyperactivity were noted at the time of peak CGRP plasma concentration [111]. Taken together, these studies emphasize that additional care with respect to GI adverse events—especially constipation—should be taken into consideration for drug selection in patients with migraine and GI symptoms.
Migraine and Gut Disorders: a Clinical Perspective
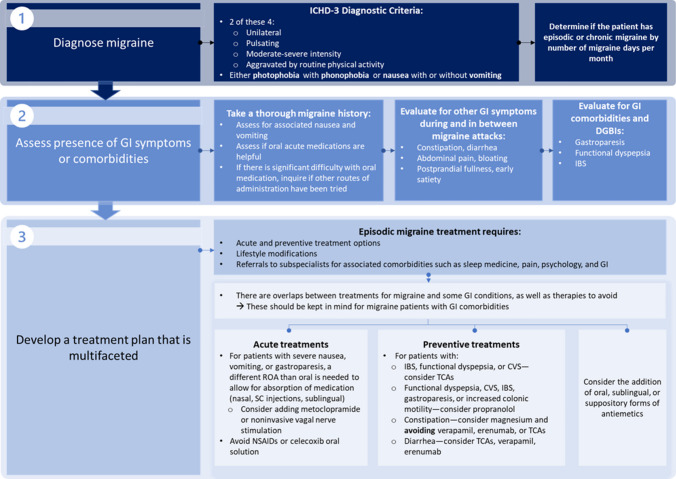
A growing body of literature highlights the importance of detecting GI comorbidities and symptoms in patients with migraine when considering available treatment options; however, established guidelines in the headache community are lacking. Migraine requires a multifaceted treatment approach because of its complex pathophysiology and broad array of potential comorbidities. An optimal treatment plan for EM requires acute and preventive therapeutic options, lifestyle modifications, and referrals to subspecialists for associated comorbidities, such as sleep medicine, pain, psychiatry, and GI. Referrals to GI specialists should be especially considered when patients are significantly bothered by GI symptoms and their migraine medications are not effective. To determine the presence of GI comorbidities or symptoms, it is recommended that clinicians take a thorough migraine history and inquire whether oral, acute medications are helpful. If the patient mentions difficulties with oral medications, question whether other routes of migraine drug administration have been attempted. Clinicians should also inquire about the presence of GI symptoms during and between migraine attacks, including constipation, diarrhea, abdominal pain, bloating, postprandial fullness, and early satiety. The choice of acute and preventive treatment options for EM should heavily consider the presence of and type of GI symptoms or comorbidities. For example, acute treatments for patients with severe nausea or vomiting or gastroparesis requires a different route of administration than oral to allow for absorption of medication. Metoclopramide can also be used for gastroparesis or to accelerate gastric emptying. Similarly, TCAs should be considered as a preventive migraine treatment for a patient with CVS or functional dyspepsia; however, if a patient experiences frequent constipation, then TCAs are not recommended (Fig. 1). According to the AHS consensus, nonoral alternatives include subcutaneous and intranasal sumatriptan, intranasal and intramuscular ketorolac, subcutaneous and intranasal DHE mesylate, and neuromodulatory devices. Some acute therapies for migraine may cause GI-related adverse events, and the benefit-risk profiles of these agents should be weighed carefully in patients with EM and GI symptoms or comorbidities. NSAIDs are a recommended acute treatment for migraine, but they can cause serious GI adverse events. Celecoxib oral solution should be avoided in patients with a history of peptic ulcer disease or GI bleeding because of an increased risk of spontaneous bleeding, ulceration, and perforation of the stomach or intestines. Further, alternatives to erenumab are recommended in patients with constipation [21•].
Fig. 1.
Proposed Step-by-step Algorithm for Diagnosing GI Symptoms or Comorbidities and Developing a Treatment Plan for Patients With Episodic Migraine [5, 21•]. Note: This figure was created based on clinical perspective of the authors as well as published guidelines from the American Headache Society Consensus Statement. CVS, cyclic vomiting syndrome; DGBI, disorder of gutbrain interaction; GI, gastrointestinal; IBS, irritable bowel syndrome; ICHD-3, International Classification of Headache Disorders version 3; NSAID, nonsteroidal anti-inflammatory drug; ROA, route of administration; SC, subcutaneous; TCA, tricyclic antidepressant
Conclusions
The association between the gut-brain axis and migraine has recently garnered considerable interest in the headache community and attention to GI comorbidities, and symptoms may be particularly important in managing patients with EM who do not experience relief from oral therapies or who cycle through many migraine therapies with suboptimal efficacy or adverse events. Other considerations include route of drug administration and formulation, which can affect absorption, and therefore the efficacy and tolerability of migraine therapies. Using a multifaceted treatment approach, understanding which migraine therapies to consider or avoid in patients with GI symptoms or comorbidities, and knowing when to refer to GI specialists can help patients with EM to achieve their individualized needs and optimize their migraine management.
Acknowledgements
Writing and editorial assistance was provided by IMPRINT Science, New York, NY, USA, and supported by Impel Pharmaceuticals Inc. The authors are fully responsible for the content, editorial decisions, and opinions expressed in the article.
Funding
This article is funded by Impel Pharmaceuticals.
Compliance with Ethical Standards
Conflict of Interest
Linda Nguyen is a consultant for Ardelyx, Atmo Biosciences, Evoke Pharma, Gemelli Biotech, Impel Pharmaceuticals, Ironwood Pharmaceuticals, Neurogastrx, Pendulum, Phathom Pharmaceuticals, and Takeda Pharmaceuticals. She has received research support from Bold Health, Impel Pharmaceuticals, Kate Farms, and Vanda Pharmaceuticals. Nada Hindiyeh is a consultant for AbbVie and Eli Lilly and a speaker for AbbVie and Impel Pharmaceuticals. Sutapa Ray and Sheena K. Aurora are full-time employees of Impel Pharmaceuticals and are stockholders in Impel Pharmaceuticals. Robert E. Vann was a full-time employee of Impel Pharmaceuticals at the time of manuscript development.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Arzani M, Jahromi SR, Ghorbani Z, Vahabizad F, Martelletti P, Ghaemi A, et al. Gut-brain axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21:15. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.•• Morais LH, Schreiber HL 4th, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021:19:241–255. 10.1038/s41579-020-00460-0. Important review of migraine and GI disorders. [DOI] [PubMed]
- 3.Singh A, Dawson TM, Kulkarni S. Neurodegenerative disorders and gut-brain interactions. J Clin Invest. 2021:131. 10.1172/jci143775. [DOI] [PMC free article] [PubMed]
- 4.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. 3rd ed. Cephalalgia. 2018;38:1–211. 10.1177/0333102417738202. [DOI] [PubMed]
- 6.Agosti R. Migraine burden of disease: from the patient's experience to a socio-economic view. Headache. 2018;58(Suppl 1):17–32. doi: 10.1111/head.13301. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Sakai F, Miyake H, Sone T, Sato M, Tanabe S, et al. Disability, quality of life, productivity impairment and employer costs of migraine in the workplace. J Headache Pain. 2021;22:29. doi: 10.1186/s10194-021-01243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21:137. doi: 10.1186/s10194-020-01208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palsson OS, Sperber AD, Bangdiwala S, Whitehead WE. Prevalence and associated factors of disorders of gut-brain interaction in the United States: comparison of two nationwide Internet surveys. Neurogastroenterol Motil. 2023;35:e14564. 10.1111/nmo.14564. [DOI] [PubMed]
- 10.Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16:86–92. doi: 10.1007/s11916-011-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aamodt AH, Stovner LJ, Hagen K, Zwart JA. Comorbidity of headache and gastrointestinal complaints. the Head-HUNT study. Cephalalgia. 2008;28:144–151. 10.1111/j.1468-2982.2007.01486.x. [DOI] [PubMed]
- 12.Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57–63. doi: 10.1111/j.1526-4610.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 13.Di Stefano M, Pucci E, Miceli E, Pagani E, Brondino N, Nappi G, et al. Prevalence and pathophysiology of post-prandial migraine in patients with functional dyspepsia. Cephalalgia. 2019;39:1560–1568. doi: 10.1177/0333102419857596. [DOI] [PubMed] [Google Scholar]
- 14.Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic vomiting syndrome in 41 adults: the illness, the patients, and problems of management. BMC Med. 2005;3:20. doi: 10.1186/1741-7015-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgescu D, Reisz D, Gurban CV, Georgescu LA, Ionita I, Ancusa OE, et al. Migraine in young females with irritable bowel syndrome: still a challenge. Neuropsychiatr Dis Treat. 2018;14:21–28. doi: 10.2147/ndt.s144955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 17.Moisset X, Bommelaer G, Boube M, Ouchchane L, Goutte M, Dapoigny M, et al. Migraine prevalence in inflammatory bowel disease patients: a tertiary-care centre cross-sectional study. Eur J Pain. 2017;21:1550–1560. doi: 10.1002/ejp.1056. [DOI] [PubMed] [Google Scholar]
- 18.Serratrice J, Disdier P, de Roux C, Christides C, Weiller PJ. Migraine and coeliac disease. Headache. 1998;38:627–628. doi: 10.1046/j.1526-4610.1998.3808627.x. [DOI] [PubMed] [Google Scholar]
- 19.Su J, Zhou XY, Zhang GX. Association between Helicobacter pylori infection and migraine: a meta-analysis. World J Gastroenterol. 2014;20:14965–14972. doi: 10.3748/wjg.v20.i40.14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakhatreh M, Jehangir A, Malik Z, Parkman HP. Metoclopramide for the treatment of diabetic gastroparesis. Expert Rev Gastroenterol Hepatol. 2019;13:711–721. doi: 10.1080/17474124.2019.1645594. [DOI] [PubMed] [Google Scholar]
- 21.Ailani J, Burch RC, Robbins MS. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021–1039. doi: 10.1111/head.14153. [DOI] [PubMed] [Google Scholar]
- 22.Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome Foundation working team report. Gastroenterology. 2018;154:1140–1171.e1141. doi: 10.1053/j.gastro.2017.11.279. [DOI] [PubMed] [Google Scholar]
- 23.Paulon E, Nastou D, Jaboli F, Marin J, Liebler E, Epstein O. Proof of concept: short-term non-invasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis. Frontline Gastroenterol. 2017;8:325–330. doi: 10.1136/flgastro-2017-100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatesan T, Levinthal DJ, Tarbell SE, Jaradeh SS, Hasler WL, Issenman RM, et al. Guidelines on management of cyclic vomiting syndrome in adults by the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association. Neurogastroenterol Motil. 2019;31(Suppl 2):e13604. doi: 10.1111/nmo.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988–1013. doi: 10.1038/ajg.2017.154. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Kuo B, Nguyen L, Vaughn VM, Petrey J, Greer K, et al. ACG clinical guideline: gastroparesis. Am J Gastroenterol. 2022;117:1197–1220. 10.14309/ajg.0000000000001874. [DOI] [PMC free article] [PubMed]
- 27.•• Aurora SK, Shrewsbury SB, Ray S, Hindiyeh N, Nguyen L. A link between gastrointestinal disorders and migraine: insights into the gut-brain connection. Headache. 2021;61:576–589. 10.1111/head.14099. Important review of migraine and GI disorders. [DOI] [PMC free article] [PubMed]
- 28.Cámara-Lemarroy CR, Rodriguez-Gutierrez R, Monreal-Robles R, Marfil-Rivera A. Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol. 2016;22:8149–8160. doi: 10.3748/wjg.v22.i36.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doulberis M, Saleh C, Beyenburg S. Is there an association between migraine and gastrointestinal disorders? J Clin Neurol. 2017;13:215–226. doi: 10.3988/jcn.2017.13.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hindiyeh N, Aurora SK. What the gut can teach us about migraine. Curr Pain Headache Rep. 2015;19:33. doi: 10.1007/s11916-015-0501-4. [DOI] [PubMed] [Google Scholar]
- 31.van Hemert S, Breedveld AC, Rovers JM, Vermeiden JP, Witteman BJ, Smits MG, et al. Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol. 2014;5:241. doi: 10.3389/fneur.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen LA, Snape WJ., Jr Clinical presentation and pathophysiology of gastroparesis. Gastroenterol Clin North Am. 2015;44:21–30. doi: 10.1016/j.gtc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen L, Wilson LA, Miriel L, Pasricha PJ, Kuo B, Hasler WL, et al. Autonomic function in gastroparesis and chronic unexplained nausea and vomiting: relationship with etiology, gastric emptying, and symptom severity. Neurogastroenterol Motil. 2020;32:e13810. 10.1111/nmo.13810. [DOI] [PMC free article] [PubMed]
- 34.Salvioli B, Pellegatta G, Malacarne M, Pace F, Malesci A, Pagani M, et al. Autonomic nervous system dysregulation in irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:423–430. doi: 10.1111/nmo.12512. [DOI] [PubMed] [Google Scholar]
- 35.Miglis MG. Migraine and autonomic dysfunction: which is the horse and which is the jockey? Curr Pain Headache Rep. 2018;22:19. doi: 10.1007/s11916-018-0671-y. [DOI] [PubMed] [Google Scholar]
- 36.Cortez MM, Millsap L, Brennan KC, Campbell CL. Craniofacial autonomic dysfunction in migraine: implications for treatment and prognosis. J Neuroophthalmol. 2020;40:67–73. doi: 10.1097/wno.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 37.Togha M, Martami F, Jafari E, Ariyanfar S, Hashemi SM. The prevalence and characteristics of visceral autonomic symptoms among migraineurs: a population-based study. Cephalalgia. 2022;42:500–509. doi: 10.1177/03331024211056849. [DOI] [PubMed] [Google Scholar]
- 38.Ray JC, Cheema S, Foster E, Gunasekera L, Mehta D, Corcoran SJ, et al. Autonomic symptoms in migraine: results of a prospective longitudinal study. Front Neurol. 2022;13:1036798. doi: 10.3389/fneur.2022.1036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland AM, Bon-Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci. 2021;78:4713–4733. doi: 10.1007/s00018-021-03812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong D, Lee H, Min HG, Kim K, Oh HS, Chu MK. Altered gut microbiota in individuals with episodic and chronic migraine. Sci Rep. 2023;13:626. doi: 10.1038/s41598-023-27586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minerbi A, Gonzalez E, Brereton NJB, Anjarkouchian A, Dewar K, Fitzcharles MA, et al. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160:2589–2602. doi: 10.1097/j.pain.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 42.Pimentel M, Lembo A. Microbiome and its role in irritable bowel syndrome. Dig Dis Sci. 2020;65:829–839. doi: 10.1007/s10620-020-06109-5. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Liu S, Shu H, Yanagisawa L, Tao F. Gut microbiota dysbiosis enhances migraine-like pain via TNFα upregulation. Mol Neurobiol. 2020;57:461–468. doi: 10.1007/s12035-019-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martami F, Togha M, Seifishahpar M, Ghorbani Z, Ansari H, Karimi T, et al. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: a randomized double-blind controlled trial. Cephalalgia. 2019;39:841–853. doi: 10.1177/0333102418820102. [DOI] [PubMed] [Google Scholar]
- 45.Parohan M, Djalali M, Sarraf P, Yaghoubi S, Seraj A, Foroushani AR, et al. Effect of probiotic supplementation on migraine prophylaxis: a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci. 2022;25:511–518. doi: 10.1080/1028415x.2020.1764292. [DOI] [PubMed] [Google Scholar]
- 46.Munjal S, Singh P, Reed ML, Fanning K, Schwedt TJ, Dodick DW, et al. Most bothersome symptom in persons with migraine: results from the Migraine in America Symptoms and Treatment (MAST) study. Headache. 2020;60:416–429. doi: 10.1111/head.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buse DC, Reed ML, Fanning KM, Bostic R, Dodick DW, Schwedt TJ, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2020;21:23. doi: 10.1186/s10194-020-1084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martami F, Ghorbani Z, Abolhasani M, Togha M, Meysamie A, Sharifi A, et al. Comorbidity of gastrointestinal disorders, migraine, and tension-type headache: a cross-sectional study in Iran. Neurol Sci. 2018;39:63–70. doi: 10.1007/s10072-017-3141-0. [DOI] [PubMed] [Google Scholar]
- 49.Grover M, Kolla BP, Pamarthy R, Mansukhani MP, Breen-Lyles M, He JP, et al. Psychological, physical, and sleep comorbidities and functional impairment in irritable bowel syndrome: results from a national survey of U.S. adults. PLoS One. 2021;16:e0245323. 10.1371/journal.pone.0245323. [DOI] [PMC free article] [PubMed]
- 50.Hormati A, Akbari N, Sharifipour E, Hejazi SA, Jafari F, Alemi F, et al. Migraine and gastric disorders: are they associated? J Res Med Sci. 2019;24:60. doi: 10.4103/jrms.JRMS_464_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buse DC, Reed ML, Fanning KM, Bostic RC, Lipton RB. Demographics, headache features, and comorbidity profiles in relation to headache frequency in people with migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2020. 10.1111/head.13966. [DOI] [PubMed]
- 52.Kikui S, Chen Y, Ikeda K, Hasebe M, Asao K, Takeshima T. Comorbidities in patients with migraine in Japan: a cross-sectional study using data from National Health and Wellness Survey. BMJ Open. 2022;12:e065787. doi: 10.1136/bmjopen-2022-065787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sacco S, Amin FM, Ashina M, Bendtsen L, Deligianni CI, Gil-Gouveia R, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention - 2022 update. J Headache Pain. 2022;23:67. doi: 10.1186/s10194-022-01431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanderPluym JH, Halker Singh RB, Urtecho M, Morrow AS, Nayfeh T, Torres Roldan VD, et al. Acute treatments for episodic migraine in adults: a systematic review and meta-analysis. JAMA. 2021;325:2357–2369. doi: 10.1001/jama.2021.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yalcin H, Okuyucu EE, Ucar E, Duman T, Yilmazer S. Changes in liquid emptying in migraine patients: diagnosed with liquid phase gastric emptying scintigraphy. Intern Med J. 2012;42:455–459. doi: 10.1111/j.1445-5994.2012.02741.x. [DOI] [PubMed] [Google Scholar]
- 57.Yu YH, Jo Y, Jung JY, Kim BK, Seok JW. Gastric emptying in migraine: a comparison with functional dyspepsia. J Neurogastroenterol Motil. 2012;18:412–418. doi: 10.5056/jnm.2012.18.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aurora S, Kori S, Barrodale P, Nelsen A, McDonald S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007;47:1443–1446. doi: 10.1111/j.1526-4610.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 59.Boyle R, Behan PO, Sutton JA. A correlation between severity of migraine and delayed gastric emptying measured by an epigastric impedance method. Br J Clin Pharmacol. 1990;30:405–409. doi: 10.1111/j.1365-2125.1990.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krymchantowski AV, Filho PF, Bigal ME. Rizatriptan vs. rizatriptan plus trimebutine for the acute treatment of migraine: a double-blind, randomized, cross-over, placebo-controlled study. Cephalalgia. 2006;26:871–4. 10.1111/j.1468-2982.2006.01136.x. [DOI] [PubMed]
- 61.Sakamoto Y, Sekino Y, Yamada E, Higurashi T, Ohkubo H, Sakai E, et al. Effect of sumatriptan on gastric emptying: a crossover study using the BreathID system. World J Gastroenterol. 2012;18:3415–3419. doi: 10.3748/wjg.v18.i26.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulman EA, Dermott KF. Sumatriptan plus metoclopramide in triptan-nonresponsive migraineurs. Headache. 2003;43:729–733. doi: 10.1046/j.1526-4610.2003.03130.x. [DOI] [PubMed] [Google Scholar]
- 63.Thomsen LL, Dixon R, Lassen LH, Giboens M, Langemark M, Bendtsen L, et al. 311C90 (zolmitriptan), a novel centrally and peripheral acting oral 5-hydroxytryptamine-1D agonist: a comparison of its absorption during a migraine attack and in a migraine-free period. Cephalalgia. 1996;16:270–275. doi: 10.1046/j.1468-2982.1996.1604270.x. [DOI] [PubMed] [Google Scholar]
- 64.Tokola RA, Neuvonen PJ. Effects of migraine attack and metoclopramide on the absorption of tolfenamic acid. Br J Clin Pharmacol. 1984;17:67–75. doi: 10.1111/j.1365-2125.1984.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tokola RA, Neuvonen PJ. Effect of migraine attacks on paracetamol absorption. Br J Clin Pharmacol. 1984;18:867–871. doi: 10.1111/j.1365-2125.1984.tb02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volans GN. Migraine and drug absorption. Clin Pharmacokinet. 1978;3:313–318. doi: 10.2165/00003088-197803040-00004. [DOI] [PubMed] [Google Scholar]
- 67.Newman LC. Why triptan treatment can fail: focus on gastrointestinal manifestations of migraine. Headache. 2013;53(Suppl 1):11–16. doi: 10.1111/head.12111. [DOI] [PubMed] [Google Scholar]
- 68.Rapoport AM, Freitag F, Pearlman SH. Innovative delivery systems for migraine: the clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs. 2010;24:929–940. doi: 10.2165/11317540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 69.Silberstein SD. Migraine symptoms: results of a survey of self-reported migraineurs. Headache. 1995;35:387–396. doi: 10.1111/j.1526-4610.1995.hed3507387.x. [DOI] [PubMed] [Google Scholar]
- 70.Reed ML, Fanning KM, Serrano D, Buse DC, Lipton RB. Persistent frequent nausea is associated with progression to chronic migraine: AMPP study results. Headache. 2015;55:76–87. doi: 10.1111/head.12450. [DOI] [PubMed] [Google Scholar]
- 71.Marcus SC, Shewale AR, Silberstein SD, Lipton RB, Young WB, Viswanathan HN, et al. Comparison of healthcare resource utilization and costs among patients with migraine with potentially adequate and insufficient triptan response. Cephalalgia. 2020;40:639–649. doi: 10.1177/0333102420915167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christoph-Diener H, Ferrari M, Mansbach H. Predicting the response to sumatriptan: the Sumatriptan Naratriptan Aggregate Patient Database. Neurology. 2004;63:520–524. doi: 10.1212/01.wnl.0000133207.70312.30. [DOI] [PubMed] [Google Scholar]
- 73.Sheftell FD, Dahlöf CG, Brandes JL, Agosti R, Jones MW, Barrett PS. Two replicate randomized, double-blind, placebo-controlled trials of the time to onset of pain relief in the acute treatment of migraine with a fast-disintegrating/rapid-release formulation of sumatriptan tablets. Clin Ther. 2005;27:407–417. doi: 10.1016/j.clinthera.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Lipton RB, Pascual J, Goadsby PJ, Massiou H, McCarroll KA, Vandormael K, et al. Effect of rizatriptan and other triptans on the nausea symptom of migraine: a post hoc analysis. Headache. 2001;41:754–763. doi: 10.1046/j.1526-4610.2001.01139.x. [DOI] [PubMed] [Google Scholar]
- 75.Winner P, Landy S, Richardson M, Ames M. Early intervention in migraine with sumatriptan tablets 50 mg versus 100 mg: a pooled analysis of data from six clinical trials. Clin Ther. 2005;27:1785–1794. doi: 10.1016/j.clinthera.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Lipton RB, McGinley JS, Shulman KJ, Silberstein SD, Wirth RJ, Buse DC. AVP-825 (sumatriptan nasal powder) reduces nausea compared to sumatriptan tablets: results of the COMPASS randomized clinical trial. Headache. 2018;58:229–242. doi: 10.1111/head.13199. [DOI] [PubMed] [Google Scholar]
- 77.McGinley JS, Buse DC, Shulman KJ, Wirth RJ, Hugentobler E, Lipton RB. Evaluating mean level and within-person consistency in migraine pain intensity and migraine-related disability for AVP-825 vs oral sumatriptan: results from the COMPASS study, a randomized trial. Headache. 2019;59:1002–1013. doi: 10.1111/head.13530. [DOI] [PubMed] [Google Scholar]
- 78.Charlesworth BR, Dowson AJ, Purdy A, Becker WJ, Boes-Hansen S, Färkkilä M. Speed of onset and efficacy of zolmitriptan nasal spray in the acute treatment of migraine: a randomised, double-blind, placebo-controlled, dose-ranging study versus zolmitriptan tablet. CNS Drugs. 2003;17:653–667. doi: 10.2165/00023210-200317090-00005. [DOI] [PubMed] [Google Scholar]
- 79.Djupesland PG, Messina JC, Mahmoud RA. Breath powered nasal delivery: a new route to rapid headache relief. Headache. 2013;53(Suppl 2):72–84. doi: 10.1111/head.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11:129. doi: 10.3390/pharmaceutics11030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obaidi M, Offman E, Messina J, Carothers J, Djupesland PG, Mahmoud RA. Improved pharmacokinetics of sumatriptan with Breath Powered™ nasal delivery of sumatriptan powder. Headache. 2013;53:1323–1333. doi: 10.1111/head.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shrewsbury S, Hocevar-Trnka J, Hoekman J. Drug delivery via the upper nasal space: a novel route for anesthesiologists, intensivists and emergency department physicians? J Clin Anesth Intensive Care. 2021;2:8–14. 10.46439/anesthesia.2.007.
- 83.Silberstein SD, Shrewsbury SB, Hoekman J. Dihydroergotamine (DHE) — then and now: a narrative review. Headache. 2020;60:40–57. doi: 10.1111/head.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith TR, Winner P, Aurora SK, Jeleva M, Hocevar-Trnka J, Shrewsbury SB. STOP 301: a phase 3, open-label study of safety, tolerability, and exploratory efficacy of INP104, Precision Olfactory Delivery (POD®) of dihydroergotamine mesylate, over 24/52 weeks in acute treatment of migraine attacks in adult patients. Headache. 2021;61:1214–1226. doi: 10.1111/head.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Musumeci T, Bonaccorso A, Puglisi G. Epilepsy disease and nose-to-brain delivery of polymeric nanoparticles: an overview. Pharmaceutics. 2019;11. 10.3390/pharmaceutics11030118. [DOI] [PMC free article] [PubMed]
- 86.Martin V, Hoekman J, Aurora SK, Shrewsbury SB. Nasal delivery of acute medications for migraine: the upper versus lower nasal space. J Clin Med. 2021;10. 10.3390/jcm10112468. [DOI] [PMC free article] [PubMed]
- 87.Shrewsbury SB, Jeleva M, Satterly KH, Lickliter J, Hoekman J. STOP 101: a phase 1, randomized, open-label, comparative bioavailability study of INP104, dihydroergotamine mesylate (DHE) administered intranasally by a I123 Precision Olfactory Delivery (POD®) device, in healthy adult subjects. Headache. 2019;59:394–409. doi: 10.1111/head.13476. [DOI] [PubMed] [Google Scholar]
- 88.Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10:116. doi: 10.3390/pharmaceutics10030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoekman J, Ray S, Aurora SK, Shrewsbury SB. The upper nasal space—a novel delivery route ideal for central nervous system drugs. US Neurol. 2020;16:25–31. doi: 10.17925/USN.2020.16.1.25. [DOI] [Google Scholar]
- 90.Ladel S, Schlossbauer P, Flamm J, Luksch H, Mizaikoff B, Schindowski K. Improved in vitro model for intranasal mucosal drug delivery: primary olfactory and respiratory epithelial cells compared with the permanent nasal cell line RPMI 2650. Pharmaceutics. 2019;11:367. doi: 10.3390/pharmaceutics11080367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57:1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 92.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3:42–62. doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahsanuddin S, Povolotskiy R, Tayyab R, Nasser W, Barinsky GL, Grube JG, et al. Adverse events associated with intranasal sprays: an analysis of the Food and Drug Administration database and literature review. Ann Otol Rhinol Laryngol. 2021;130:1292–1301. doi: 10.1177/00034894211007222. [DOI] [PubMed] [Google Scholar]
- 95.Bossaller NA, Shelton RC. Real-world approach to managing dysgeusia following the use of esketamine nasal spray: a case report. Ann Gen Psychiatry. 2020;19:13. doi: 10.1186/s12991-020-00262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooper W, Ray S, Aurora SK, Shrewsbury SB, Fuller C, Davies G, et al. Delivery of dihydroergotamine mesylate to the upper nasal space for the acute treatment of migraine: technology in action. J Aerosol Med Pulm Drug Deliv. 2022;35:321–332. doi: 10.1089/jamp.2022.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiffman SS. Influence of medications on taste and smell. World J Otorhinolaryngol Head Neck Surg. 2018;4:84–91. doi: 10.1016/j.wjorl.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.IMITREX® nasal spray. Package insert. GlaxoSmithKline, Inc; 2017.
- 99.ZOMIG® nasal spray. Package insert. Amneal Pharmaceuticals; 2019.
- 100.ONZETRA® Xsail®. Package insert. Currax™ Pharmaceuticals; 2019.
- 101.TOSYMRA™ nasal spray. Package insert. Upsher-Smith Laboratories; 2019.
- 102.MIGRANAL®. Package insert. Bausch Health Companies, Inc; 2019.
- 103.TRUDHESA®. Package insert. Impel NeuroPharma, Inc; 2021.
- 104.ZAVZPRETTM nasal spray. Package insert. Pfizer; 2023.
- 105.Shrewsbury SB, Cook RO, Taylor G, Edwards C, Ramadan NM. Safety and pharmacokinetics of dihydroergotamine mesylate administered via a novel (Tempo™) inhaler. Headache. 2008;48:355–367. doi: 10.1111/j.1526-4610.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 106.Silberstein SD, Kori SH. Dihydroergotamine: a review of formulation approaches for the acute treatment of migraine. CNS Drugs. 2013;27:385–394. doi: 10.1007/s40263-013-0061-2. [DOI] [PubMed] [Google Scholar]
- 107.Baron EP, Tepper SJ. Orally inhaled dihydroergotamine: reviving and improving a classic. Future Neurol. 2011;6:327–333. doi: 10.2217/fnl.11.24. [DOI] [Google Scholar]
- 108.Russo AF, Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev. 2023;103:1565–1644. doi: 10.1152/physrev.00059.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.•• Ailani J, Kaiser EA, Mathew PG, McAllister P, Russo AF, Vélez C, et al. Role of calcitonin gene-related peptide on the gastrointestinal symptoms of migraine-clinical considerations: a narrative review. Neurology. 2022;99:841–53. 10.1212/wnl.0000000000201332. Important review of CGRP treatment for migraine and GI symptoms. [DOI] [PMC free article] [PubMed]
- 110.Kudrow D, Nguyen L, Semler J, Stroud C, Samaan K, Hoban DB, et al. A phase IV clinical trial of gastrointestinal motility in adult patients with migraine before and after initiation of a calcitonin gene-related peptide ligand (galcanezumab) or receptor (erenumab) antagonist. Headache. 2022;62:1164–1176. doi: 10.1111/head.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Falkenberg K, Bjerg HR, Olesen J. Two-hour CGRP infusion causes gastrointestinal hyperactivity: possible relevance for CGRP antibody treatment. Headache. 2020;60:929–937. doi: 10.1111/head.13795. [DOI] [PubMed] [Google Scholar]