Abstract
Background
IgA nephropathy may recur in patients receiving kidney transplantation due to IgA nephropathy induced renal failure. The risk factors for recurrence are still at issue. The aim of this study was to conduct a systematic review and meta-analysis to assess risk factors and outcomes for IgA nephropathy recurrence.
Methods
We used PubMed, EMBASE, Cochrane Library, Web of Science, Scopus, CNKI, WanFang, VIP and CBM to search for relevant studies published in English and Chinese. Cohort or case-control studies reporting risk factors or outcomes for IgA nephropathy recurrence were included.
Results
Fifty-eight studies were included. Compare to no recurrence group, those with IgAN recurrence had younger age (mean difference [MD]=-4.27 years; risk ratio [RR]=0.96), younger donor age (MD=-2.19 years), shorter time from IgA nephropathy diagnosis to end stage renal disease (MD=-1.84 years; RR=0.94), shorter time on dialysis (MD=-3.14 months), lower human leukocyte-antigen (HLA) mismatches (MD=-0.11) and HLA-DR mismatches (MD=-0.13). HLA-B46 antigen (RR=0.39), anti-IL-2-R antibodies induction (RR=0.68), mycophenolate mofetil (RR=0.69), and pretransplant tonsillectomy (RR=0.43) were associated with less IgAN recurrence. Of note, male recipient gender (RR=1.17), related donor (RR=1.53), retransplantation (RR=1.43), hemodialysis (RR=1.68), no induction therapy (RR=1.73), mTOR inhibitor (RR=1.51), angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers (RR=1.63) were risk factors for IgAN recurrence. Recurrence increased the risk of graft loss (RR=2.19).
Conclusions
This study summarized the risk factors for recurrence of IgA nephropathy after kidney transplantation. Well-designed prospective studies are warranted for validation.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=377480, identifier CRD42022377480.
Keywords: IgA nephropathy, risk factors, kidney transplantation, recurrence, graft survival, systematic review
1. Introduction
Immunoglobulin A nephropathy (IgAN) is the most prevalent glomerular disease worldwide that can lead to end-stage renal disease (ESRD) with a 10-year renal survival rate ranging from 57% to 91% (1, 2). Kidney transplantation (KT) is the optimal treatment for patients with ESRD. However, there is a risk of IgAN recurrence in renal allografts with a recurrence rate of between 9% and 60%, depending on the time after KT and the IgAN recurrence increased the risk of graft failure (3).
The identification of risk factors for IgAN recurrence is crucial in pre-transplant evaluation, and many studies have been conducted to investigate this issue. However, the results are inconsistent, with some studies indicated that younger age, high human leukocyte-antigen (HLA) matching, and related donor are risk factors, while others found no significant association (4–9). The discrepancy is largely due to the fact that most studies were single-center or had small sample sizes, highlighting the need for further research.
To date, no studies have systematically evaluated the risk factors for IgAN recurrence after KT. Therefore, the present systematic review and meta-analysis aimed to identify risk factors for IgAN recurrence and quantify its impact on clinical outcomes.
2. Materials and methods
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) statement and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (10, 11). The protocol was registered in PROSPERO (CRD42022377480).
2.1. Search strategy
English or Chinese literature from the following databases were considered: PubMed, EMBASE, Cochrane Library, Scopus, Web of Science, CNKI, Wanfang, CBM, and VIP (from inception to October 3, 2022). We developed a search strategy for each database ( Supplementary Table 1 ).
2.2. Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) Patients: those who received a KT due to IgAN induced renal failure; (2) Exposure: various potential risk factors for IgAN recurrence, such as donor and recipient characteristics, primary disease characteristics, immunosuppressive therapy, biomarkers, etc.; (3) Outcome: recurrence of IgAN in kidney allografts; (4) Study design: prospective/retrospective cohort studies or case-control studies. Studies reporting the effect of recurrence on clinical outcomes were also included. The excluded criteria were as follows: (1) Excluded study types: reviews, systematic reviews, case reports, case series, animal studies, comments, conference abstracts, and letters without detailed data, (2) Difficult to differentiate the IgAN recurrence and de novo IgAN in allografts, (3) Studies published in languages other than English or Chinese.
2.3. Selection of studies and risk bias assessment
Two researchers (YL and YT) independently screened all relevant titles and abstracts of retrieved publications to identify eligible studies. The inclusion and exclusion criteria were then applied to the full text screening. A third reviewer (TS or TL) was consulted to resolve disputes and reach a consensus. The quality and methodological strength of the included studies were assessed by two researchers (YL and YT) using the Newcastle-Ottawa Scale (NOS) (12). Scores of 0-4, 5-6, and 7-9 correspond to poor, moderate, and high quality, respectively.
2.4. Data extraction and risk factors identification
Following items were extracted from selected studies: the name of the first author, year of publication, location, research design, demographic characteristics, inclusion and exclusion criteria, follow-up time, and effect of recurrence on outcomes. Number of events and total or associated effect sizes of all reported risk factors were extracted, and those risk factors assessed only by single publication were omitted. Zero counts in a two-by-two table were replaced by 0.5 according to continuity correction. For categorical variables, risk ratios (RRs) and 95% confidence intervals (CIs) were calculated independently by two researchers if reported as number of events and total. Considering that overlapping cohort studies may report different risk factors, the data for each risk factor was screened for overlapping cohort studies and only the studies with the largest sample size were included.
2.5. Statistical analysis
For categorical variables, the pooled RRs and 95% CIs were calculated using the Inverse variance method. For continuous variables, mean differences (MDs) or standardized mean differences (SMDs) and 95% CIs were used to pool the differences between the recurrence and non-recurrence groups. The mean and standard deviation were estimated based on data reported as median (interquartile or full range) (13, 14). Heterogeneity across studies was assessed using the I² statistic. If I² > 50%, the heterogeneity is considered to be significant, and the random effects model is used; otherwise, the fixed effect model is used. If the number of studies is greater than 10, Egger’s test is used to evaluate publication bias. All analyses were performed using R software (version 4.2.1).
3. Results
3.1. Study selection
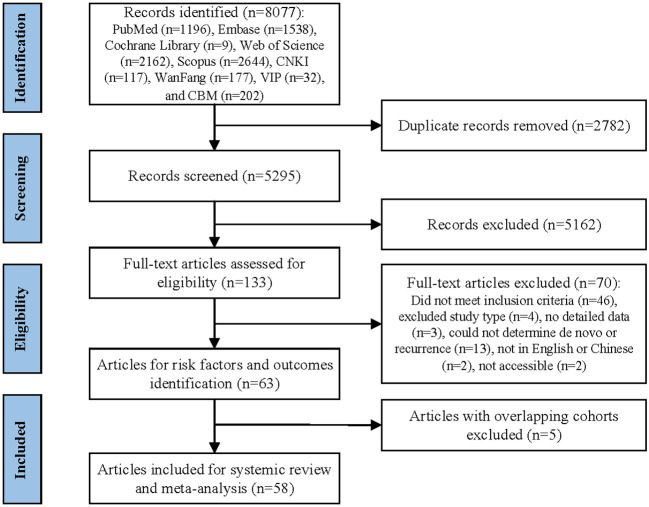
The study selection process is summarized in Figure 1 . The initial search retrieved 8077 records, and then 2787 duplicates were removed. After screening the titles and abstracts, 5162 records were excluded and 133 full text articles were assessed for eligibility. Out of these, 58 studies met the inclusion criteria and were included in the systematic review and meta-analysis (4–9, 15–66). The excluded studies and the reasons for their exclusion after full text screening are detailed in Supplementary Table 2 .
Figure 1.
Study selection flowchart.
3.2. Study characteristics
The characteristics of the included studies are summarized in Table 1 . The studies were published between 1984 and 2022, with 35 of them being published after 2010. The sample sizes ranged from 13 to 2501. The majority of the studies were retrospective cohort studies (56), while only two were case-control studies. The studies were conducted in various countries, with the majority being from Asia (23), Europe (22), and the USA (7).
Table 1.
Summary characteristics of included studies.
| Study | Study design | Country or region | Study interval |
Sample size | Recurrence rate |
Female | NOS points |
|---|---|---|---|---|---|---|---|
| Ahn (2015) | Retrospective cohort | Korea | 1989.01-2005.12 | 56 | 30.36% | 46.43% | 6 |
| Allen (2017) | Retrospective cohort | Australia, New Zealand |
1985-2014 | 2501 | 9.00% | NA | 8 |
| Andresdottir (2001) | Retrospective cohort | Netherlands | 1969-1997 | 79 | 8.86% | NA | 6 |
| Avasare (2017) | Retrospective cohort | USA | 2001.01-2012.12 | 62 | 22.58% | 35.48% | 7 |
| Bachman (1986) | Retrospective cohort | USA | 1969-1984.05 | 13 | 46.15% | 7.69% | 5 |
| Bantis (2008) | Retrospective cohort | Germany | 1986-2004 | 103 | 15.53% | 20.39% | 6 |
| Berger (1984) | Retrospective cohort | France | NA | 32 | 53.13% | NA | 6 |
| Berthoux (2008) | Retrospective cohort | France | 1979.03-2005.12 | 116 | 28.45% | NA | 6 |
| Berthoux (2015) | Case-control | France | 2000-2012 | 60 | NA | 40.00% | 7 |
| Berthoux (2017) | Retrospective cohort | France | 1985.01-2007.12 | 96 | 35.42% | 17.71% | 7 |
| Bjørneklett (2011) | Retrospective cohort | Norway | 1988-2004 | 106 | NA | NA | 6 |
| Bumgardner (1998) | Retrospective cohort | USA | 1980.06-1994.12 | 61 | 29.51% | 34.43% | 8 |
| Chacko (2007) | Retrospective cohort | India | 1990.01-2004.05 | 20 | 25.00% | 25.00% | 5 |
| Chandrakantan (2005) | Retrospective cohort | USA | 1984.01-2003.08 | 156 | 12.82% | NA | 6 |
| Choy (2003) | Retrospective cohort | China | 1984.01-2001.12 | 75 | 18.67% | 37.33% | 8 |
| Coppo (2007) | Retrospective cohort | Italy | 1984-2002.11 | 61 | 49.18% | 32.79% | 7 |
| Courtney (2006) | Retrospective cohort | UK | 1977.08-2004.04 | 75 | 17.33% | 14.67% | 6 |
| Di Vico (2018) | Retrospective cohort | Italy | 1995.01-2012.12 | 51 | 54.90% | NA | 7 |
| Freese (1999) | Retrospective cohort | Sweden | 1985-1996.12 | 104 | 12.50% | 20.19% | 7 |
| Garnier (2018) | Retrospective cohort | France | 2003.01-2013.12 | 67 | 20.90% | 17.91% | 6 |
| Han (2009) | Retrospective cohort | Korea | NA | 221 | 19.91% | 42.08% | 7 |
| Jäger (2022) | Retrospective cohort | Switzerland | 2008.05-2016.12 | 161 | 26.71% | 17.39% | 8 |
| Jeong (2008) | Retrospective cohort | Korea | 1992-2003 | 77 | 57.14% | 27.27% | 7 |
| Ji (2016) | Retrospective cohort | China | 1996.01-2009.04 | 148 | 31.08% | 74.32% | 5 |
| Jiang (2018) | Retrospective cohort | Australia, New Zealand |
1985-2013 | 2393 | 9.65% | 24.15% | 6 |
| Jo (2019) | Retrospective cohort | Korea | 2011.1-2015.10 | 69 | 15.94% | 42.03% | 6 |
| Kamal Aziz (2012) | Retrospective cohort | France | 1982-2012 | 142 | 17.61% | NA | 5 |
| Kavanagh (2022) | Retrospective cohort | USA | 2005-2019 | 282 | 28.37% | 30.85% | 7 |
| Kawabe (2016) | Retrospective cohort | Japan | 1987-2015 | 21 | 28.57% | 61.90% | 6 |
| Kennard (2017) | Retrospective cohort | Australia, New Zealand |
1985-2013 | 2393 | NA | NA | 6 |
| Kessler (1996) | Retrospective cohort | France | 1985.01-1991.06 | 28 | 46.43% | 14.29% | 6 |
| Kim (2001) | Retrospective cohort | Korea | 1984.02-1998.10 | 43 | 44.19% | NA | 7 |
| Kim (2017) | Retrospective cohort | Korea | 1990.02-2016.02 | 95 | NA | NA | 6 |
| Lee (2019) | Retrospective cohort | Korea | 1995.02-2015.03 | 218 | NA | NA | 5 |
| Lionaki (2021) | Retrospective cohort | Greece | 2000-2018 | 96 | 23.96% | 29.17% | 7 |
| Maixnerova (2021) | Retrospective cohort | Czech | 1991-2017 | 313 | 14.06% | 19.17% | 8 |
| Martín‐Penagos (2019) | Retrospective cohort | Spain | 1993.01-2015.12 | 35 | 40.00% | 20.00% | 6 |
| McDonald (2006) | Retrospective cohort | Australia, New Zealand |
1987.10-2004.12 | 1386 | 7.94% | NA | 7 |
| Moriyama (2005) | Retrospective cohort | Japan | 1992-1999 | 49 | 26.53% | 34.69% | 6 |
| Moroni (2013) | Retrospective cohort | Italy | 1981-2010 | 190 | 22.11% | 21.58% | 7 |
| Nakamura (2021) | Retrospective cohort | Japan | NA | 15 | 46.67% | 33.33% | 5 |
| Namba (2004) | Retrospective cohort | Japan | 1980-2001 | 30 | 80.00% | 33.33% | 6 |
| Ng (2007) | Retrospective cohort | Singapore | 1984.11-2004.12 | 29 | 27.59% | NA | 6 |
| Nijim (2016) | Retrospective cohort | USA | 1993.04-2014.11 | 122 | 18.85% | 31.15% | 6 |
| Noguchi (2020) | Retrospective cohort | Japan | 2002.12-2018.12 | 135 | NA | 54.07% | 7 |
| Odum (1994) | Retrospective cohort | Australia | 1977-1992.09 | 51 | 33.33% | 6.25% | 5 |
| Okumi (2019) | Retrospective cohort | Japan | 1995.01-2015.03 | 299 | 26.76% | 44.15% | 7 |
| Ortiz (2012) | Retrospective cohort | Finland, Spain | 2001.01-2010.04 | 65 | 32.31% | 15.38% | 8 |
| Park (2021) | Retrospective cohort | Korea | 2009-2016 | 27 | 48.15% | 37.04% | 5 |
| Ponticelli (2001) | Retrospective cohort | Italy | 1973.07-1999.09 | 106 | 32.08% | 24.53% | 7 |
| Rodas (2020) | Retrospective cohort | Spain | 1992-2016 | 86 | 26.74% | 26.74% | 7 |
| Sato (2013) | Retrospective cohort | Japan | 1990-2005 | 184 | 38.04% | 44.02% | 6 |
| Sofue (2013) | Retrospective cohort | Japan | 2003.08-2011.02 | 35 | 34.29% | 28.57% | 6 |
| Temurhan (2017) | Case-control | Turkey | NA | 41 | NA | 26.83% | 6 |
| Uffing (2021) | Retrospective cohort | Europe, North and South America | 2005.01-2015.12 | 504 | 16.27% | 28.17% | 9 |
| Von Visger (2014) | Retrospective cohort | USA | 1989.06-2008.11 | 124 | 21.77% | 27.42% | 6 |
| Wang (2001) | Retrospective cohort | China | 1985.01-1998.12 | 48 | 29.17% | 54.17% | 6 |
| Wang (2021) | Retrospective cohort | China | 2008.01-2019.12 | 149 | 26.85% | 44.30% | 6 |
NOS, Newcastle–Ottawa scale; NA, not available.
3.3. Risk of bias
The risk of bias was assessed using the NOS and the results are presented in Table 1 . The overall quality of the included studies was medium, with 34 studies having a medium quality and 24 having a high quality.
3.4. Risk factors for IgAN recurrence
The results of meta-analysis examining risk factors for IgAN recurrence are presented in Table 2 .
Table 2.
Meta-analysis of risk factors and outcomes for IgAN recurrence.
| Risk factors or outcomes | No. of studies | n | Heterogeneity | Effects model | ES | ES [95% CI] | Z | P | Egger’s test, P | |
|---|---|---|---|---|---|---|---|---|---|---|
| I² | P | |||||||||
| Recipient | ||||||||||
| Age at KT (year) | 28 | 3248 | 71.60% | < 0.0001 | R | MD | -4.27 [-5.76, -2.78] | -5.63 | < 0.0001 | 0.7522 |
| Age at KT (per year) | 9 | 3519 | 29.30% | 0.1848 | F | RR | 0.96 [0.95, 0.97] | -8.31 | < 0.0001 | – |
| Recipient sex (male) | 27 | 3795 | 6.40% | 0.3693 | F | RR | 1.17 [1.01, 1.35] | 2.07 | 0.0380 | 0.1139 |
| Recipient body mass index (kg/cm²) | 4 | 677 | 81.80% | 0.0009 | R | MD | -1.53 [-3.75, 0.69] | -1.35 | 0.1762 | – |
| Recipient body mass index (per 1 kg/cm²) | 2 | 722 | 0.00% | 1.0000 | F | RR | 0.96 [0.92, 1.02] | -1.68 | 0.0936 | – |
| Donor | ||||||||||
| Donor sex (male) | 7 | 983 | 0.00% | 0.5525 | F | RR | 0.90 [0.73, 1.11] | -1.00 | 0.3171 | – |
| Donor age at KT (year) | 15 | 1956 | 34.70% | 0.0910 | F | MD | -2.19 [-3.46, -0.93] | -3.40 | 0.0007 | 0.4067 |
| Donor age at KT (per year) | 3 | 389 | 0.00% | 0.4354 | F | RR | 0.99 [0.97, 1.01] | -0.79 | 0.4314 | – |
| Living donor | 26 | 4472 | 48.80% | 0.0029 | F | RR | 1.02 [0.90, 1.14] | 0.28 | 0.7781 | 0.0003 |
| Related donor | 21 | 2559 | 62.90% | < 0.0001 | R | RR | 1.53 [1.24, 1.88] | 4.02 | < 0.0001 | 0.0001 |
| Living related donor (vs. living unrelated donor) | 10 | 1221 | 21.40% | 0.2461 | F | RR | 1.69 [1.31, 2.18] | 3.99 | < 0.0001 | 0.1001 |
| Primary disease | ||||||||||
| Age at IgAN diagnosis (year) | 6 | 909 | 71.70% | 0.0034 | R | MD | -2.52 [-6.73, 1.69] | -1.17 | 0.2412 | – |
| Age at IgAN diagnosis (per year) | 2 | 417 | 86.00% | 0.0075 | R | RR | 0.99 [0.93, 1.05] | -0.43 | 0.6699 | – |
| Time from IgAN diagnosis to ESRD (year) | 13 | 1448 | 0.00% | 0.6548 | F | MD | -1.84 [-2.43, -1.25] | -6.14 | < 0.0001 | 0.8399 |
| Time from IgAN diagnosis to ESRD (per year) | 3 | 293 | 0.00% | 0.4097 | F | RR | 0.94 [0.91, 0.97] | -3.41 | 0.0007 | – |
| History of KT | ||||||||||
| History of KT | 12 | 4421 | 47.40% | 0.0342 | F | RR | 1.43 [1.24, 1.65] | 4.98 | < 0.0001 | 0.5062 |
| Dialysis history | ||||||||||
| Time on dialysis (month) | 19 | 2164 | 44.60% | 0.0193 | F | MD | -3.14 [-4.18, -2.09] | -5.88 | < 0.0001 | 0.5114 |
| Hemodialysis | 3 | 689 | 1.70% | 0.3615 | F | RR | 1.68 [1.04, 2.71] | 2.13 | 0.0331 | – |
| Pre-emptive transplant | 10 | 1569 | 52.70% | 0.0249 | R | RR | 1.12 [0.77, 1.62] | 0.60 | 0.5501 | 0.5854 |
| Comorbidities | – | |||||||||
| Hypertension | 7 | 600 | 44.80% | 0.0923 | F | RR | 1.09 [0.76, 1.57] | 0.49 | 0.6245 | – |
| Diabetes | 3 | 276 | 0.00% | 0.6065 | F | RR | 1.22 [0.73, 2.03] | 0.75 | 0.4547 | – |
| Histocompatibility | ||||||||||
| Panel reactive antibodies | 4 | 689 | 65.90% | 0.0320 | R | MD | 0.95 [-3.55, 5.45] | 0.41 | 0.6795 | – |
| Panel reactive antibodies >50% | 3 | 543 | 0.00% | 0.5899 | F | RR | 0.66 [0.34, 1.29] | -1.21 | 0.2259 | – |
| Pretransplant donor specific antibody | 5 | 1267 | 67.70% | 0.0148 | R | RR | 1.01 [0.53, 1.94] | 0.04 | 0.9650 | – |
| ABO-incompatibility | 4 | 505 | 0.00% | 0.8607 | F | RR | 0.89 [0.60, 1.31] | -0.60 | 0.5487 | – |
| HLA-A/B/DR mismatches | 19 | 2414 | 38.40% | 0.0455 | F | MD | -0.11 [-0.22, -0.00] | -2.00 | 0.0455 | 0.6727 |
| HLA-A/B mismatches | 2 | 251 | 0.00% | 0.7481 | F | MD | 0.09 [-0.05, 0.22] | 1.20 | 0.2294 | – |
| HLA-DR mismatches | 2 | 251 | 0.00% | 0.7494 | F | MD | -0.13 [-0.22, -0.05] | -3.00 | 0.0027 | – |
| HLA-A/B/DR full match | 4 | 905 | 31.3% | 0.2244 | F | RR | 1.88 [1.14, 3.11] | 2.47 | 0.0135 | – |
| HLA-A full match | 2 | 98 | 0.00% | 0.8560 | F | RR | 1.48 [0.70, 3.09] | 1.03 | 0.3021 | – |
| HLA-B full match | 4 | 180 | 36.70% | 0.1921 | F | RR | 1.12 [0.72, 1.76] | 0.51 | 0.6084 | – |
| HLA-DR full match | 4 | 229 | 85.80% | < 0.0001 | R | RR | 0.71 [0.15, 3.38] | -0.43 | 0.6693 | – |
| HLA identied related donor (vs. other) | 2 | 152 | 78.70% | 0.0301 | R | RR | 2.91 [0.7, 12.01] | 1.47 | 0.1406 | – |
| HLA identied related donor (vs. not identied related donor) | 3 | 92 | 18.50% | 0.2932 | F | RR | 1.14 [0.70, 1.88] | 0.53 | 0.5964 | – |
| HLA-A2 | 7 | 661 | 36.90% | 0.1471 | F | RR | 1.03 [0.78, 1.37] | 0.21 | 0.8300 | – |
| HLA-B35 | 8 | 1051 | 0.00% | 0.7456 | F | RR | 1.25 [0.95, 1.64] | 1.59 | 0.1116 | – |
| HLA-B46 | 3 | 290 | 0.00% | 0.5821 | F | RR | 0.39 [0.16, 0.95] | -2.06 | 0.0392 | – |
| HLA-DR3 | 2 | 466 | 0.00% | 0.9142 | F | RR | 1.14 [0.84, 1.55] | 0.86 | 0.3883 | – |
| HLA-DR4 | 4 | 397 | 82.80% | 0.0006 | R | RR | 2.91 [0.94, 8.97] | 1.86 | 0.0630 | – |
| Induction therapy | ||||||||||
| None | 9 | 1453 | 70.50% | 0.0007 | R | RR | 1.73 [1.16, 2.58] | 2.69 | 0.0071 | – |
| Anti-IL-2-R antibodies | 14 | 2102 | 79.80% | < 0.0001 | R | RR | 0.68 [0.47, 0.99] | -2.02 | 0.0429 | 0.8046 |
| Antithymocyte globulin | 13 | 1844 | 74.10% | < 0.0001 | R | RR | 0.97 [0.64, 1.47] | -0.15 | 0.8784 | 0.0189 |
| Anti-CD20 antibodies | 2 | 312 | 74.20% | 0.0488 | R | RR | 0.63 [0.22, 1.82] | -0.85 | 0.3951 | – |
| Maintenance therapy | ||||||||||
| Tacrolimus | 16 | 2146 | 52.20% | 0.0077 | R | RR | 0.90 [0.68, 1.19] | -0.72 | 0.4741 | 0.5163 |
| Cyclosporine | 15 | 1701 | 41.90% | 0.0445 | F | RR | 1.07 [0.90, 1.28] | 0.77 | 0.4414 | 0.2776 |
| MMF | 12 | 1846 | 24.90% | 0.1996 | F | RR | 0.69 [0.56, 0.86] | -3.28 | 0.0010 | 0.9129 |
| Azathioprine | 6 | 684 | 2.00% | 0.4036 | F | RR | 1.18 [0.85, 1.64] | 0.99 | 0.3239 | – |
| mTOR inhibitor | 7 | 602 | 0.00% | 0.5686 | F | RR | 1.51 [1.10, 2.06] | 2.57 | 0.0102 | – |
| Steroids | 10 | 3504 | 74.1% | < 0.0001 | R | RR | 0.88 [0.56, 1.38] | -0.55 | 0.5831 | 0.3347 |
| Graft rejection | ||||||||||
| Rejection | 8 | 1305 | 36.00% | 0.1414 | F | RR | 1.10 [0.83, 1.46] | 0.69 | 0.4932 | – |
| T cell-mediated rejection | 2 | 361 | 34.80% | 0.2155 | F | RR | 1.42 [0.96, 2.09] | 1.75 | 0.0800 | – |
| Antibody-mediated rejection | 3 | 374 | 0.00% | 0.9932 | F | RR | 0.85 [0.52, 1.38] | -0.67 | 0.5042 | – |
| Acute rejection | 13 | 2063 | 0.00% | 0.6052 | F | RR | 1.13 [0.96, 1.34] | 1.43 | 0.1532 | 0.9843 |
| Acute T cell-mediated rejection | 2 | 754 | 0.00% | 0.6059 | F | RR | 1.39 [0.99, 1.94] | 1.92 | 0.0547 | – |
| Acute antibody-mediated rejection | 2 | 754 | 81.70% | 0.0195 | R | RR | 1.25 [0.35, 4.47] | 0.35 | 0.7279 | – |
| Chronic rejection | 3 | 468 | 28.60% | 0.2465 | F | RR | 1.33 [0.91, 1.93] | 1.47 | 0.1403 | – |
| Other therapy | ||||||||||
| ACEI/ARB | 8 | 640 | 0.00% | 0.6797 | F | RR | 1.80 [1.42, 2.28] | 4.87 | < 0.0001 | – |
| Plasma exchange | 2 | 312 | 0.00% | 0.4185 | F | RR | 0.80 [0.54, 1.18] | -1.12 | 0.2639 | – |
| Tonsillectomy | 2 | 350 | 0.00% | 0.6420 | F | RR | 0.43 [0.23, 0.79] | -2.69 | 0.0072 | – |
| Steroids use before KT | 2 | 173 | 0.00% | 0.3936 | F | RR | 0.97 [0.58, 1.62] | -0.13 | 0.8995 | – |
| Immunosuppression use before KT | 3 | 279 | 62.00% | 0.0718 | R | RR | 1.27 [0.60, 2.71] | 0.62 | 0.5363 | – |
| Other | ||||||||||
| Cold ischemia time (hour) | 7 | 1356 | 12.90% | 0.3312 | F | MD | 0.10 [-0.76, 0.96] | 0.23 | 0.8188 | – |
| Delayed graft function | 5 | 443 | 18.60% | 0.2960 | F | RR | 0.70 [0.43, 1.12] | -1.48 | 0.1377 | – |
| Cytomegalovirus infection | 2 | 476 | 0.00% | 0.8245 | F | RR | 0.79 [0.45, 1.38] | -0.84 | 0.4023 | – |
| BK virus infection | 2 | 517 | 0.00% | 0.5896 | F | RR | 1.32 [0.67, 2.57] | 0.81 | 0.4205 | – |
| Outcomes | ||||||||||
| Graft loss | 30 | 5986 | 82.80% | < 0.0001 | R | RR | 2.19 [1.60, 3.01] | 4.88 | < 0.0001 | 0.0040 |
| Death | 4 | 703 | 0.00% | 0.5712 | F | RR | 0.77 [0.42, 1.41] | -0.85 | 0.3952 | – |
| Infection | 2 | 339 | 0.00% | 0.8135 | F | RR | 1.02 [0.66, 1.59] | 0.10 | 0.9231 | – |
IgAN, immunoglobulin A nephropathy; ES, effect size; CI, confidence interval; KT, kidney transplantation; R, random effects model; F, fixed effect model; MD, mean difference; RR, risk ratio; ESRD, end stage renal disease; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers.
3.4.1. Demographic characteristics of recipients and donors
The study found that recipients with recurrent IgAN were younger at KT than those without recurrence (MD = -4.27 years, 95% CI: -5.76 to -2.78, I² = 71.60%). The pooled RR showed that the risk of recurrence decreased by 4% for each increase in age of 1 year at KT (RR = 0.96, 95%CI: 0.95-0.97, I² = 29.30%). The donor age was also found to be younger in the recurrent group (MD = -2.19 years, 95% CI: -3.46 to -0.93, I² = 34.70%), but the pooled RR of 389 participants from 3 studies was not significant (RR = 0.99, 95%CI: 0.97 to 1.01, I² = 0.00%). Male recipients had a 17% increased risk of recurrence compared to female recipients (RR = 1.17, 95%CI: 1.01 to 1.35, I² = 6.40%). There were no significant differences between the recurrence and non-recurrence groups with respect to recipient body mass index or donor sex.
3.4.2. Donor type
There was no difference in the risk of recurrence among recipients with living donors compared to those with deceased donors (RR = 1.02, 95% CI: 0.90 to 1.14, I² = 48.80%). Recipients with related donors had a higher risk of recurrence compared to those with unrelated donors (RR = 1.53, 95% CI: 1.24 to 1.88, I² = 62.90%). Further analysis found that recipients with living related donors had a higher risk of recurrence compared with those with living unrelated donors (RR = 1.69, 95% CI: 1.31 to 2.18, I² = 21.40%).
3.4.3. Primary disease
The age at diagnosis of IgAN did not differ between the recurrence and non-recurrence groups. However, the time from IgAN diagnosis to ESRD was shorter in the recurrence group compared to the non-recurrence group, with an MD of -1.84 years (95% CI: -2.43 to -1.25, I² = 0.00%). The RR value for the time from diagnosis to ESRD showed each additional year was associated with a 6% reduction in recurrence (RR = 0.94, 95% CI: 0.91 to 0.97, I² = 0.00%). This suggests that patients with a faster progression of primary disease were more susceptible to recur.
3.4.4. Dialysis history
Recurrent group had short dialysis duration (MD = -3.14 months, 95% CI: -4.18 to -2.09, I² = 44.60%) and hemodialysis (RR = 1.68, 95% CI: 1.04 to 2.71, I² = 1.70%) were identified as risk factors for recurrence, while pre-emptive transplantation was not found to be a significant risk factor.
3.4.5. Histocompatibility features
In patients with recurrent IgAN, lower total HLA mismatches (MD = -0.11, 95% CI: -0.22 to -0.00, I² = 38.40%) and lower HLA-DR mismatches (MD = -0.13, 95% CI: -0.22 to -0.05, I² = 0.00%) were observed, but no significant difference was found in HLA-A and B. The pooled RR showed an increased risk of recurrence in recipients with HLA full match (RR = 1.88, 95% CI: 1.14 to 3.11, I² = 31.3%). However, compared with more than 1 mismatch, HLA-A, B, or DR full match were all found to have no effect on recurrence. For specific HLA antigens, HLA-B46 reduced the risk of recurrence (RR = 0.39, 95% CI: 0.16 to 0.95, I² = 0.00%), while HLA-A2, HLA-B35 HLA-DR3, and HLA-DR4 had no effect. ABO incompatibility, donor-specific antibodies, and panel reactive antibodies did not affect recurrence.
3.4.6. Immunosuppressive therapy
Patients without induction therapy had an increased risk of recurrence (RR = 1.73, 95% CI: 1.16 to 2.58, I² = 70.50%). The use of anti-IL-2-R antibodies was found to reduce the risk of recurrence by 32% (RR = 0.68, 95% CI: 0.47 to 0.99, I² = 79.80%), while antithymocyte globulin and anti-CD20 antibodies had no effect. For maintenance agents, mycophenolate mofetil (MMF) reduced the risk of recurrence (RR = 0.69, 95% CI: 0.56 to 0.86, I² = 24.90%) and use of mTOR inhibitor is associated with a higher risk (RR = 1.51, 95% CI: 1.10 to 2.06, I² = 0.00%), while steroids, tacrolimus, cyclosporine, and azathioprine had no significant effect. Pretransplant steroids or immunosuppressant exposure did not affect recurrence.
3.4.7. Other therapy
Recipients with recurrent IgAN were more likely to use angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) (RR = 1.63, 95%CI: 1.30 to 2.05, I² = 0.00%). Patients who had tonsillectomy before KT had a 57% lower risk of recurrence (RR = 0.43, 95% CI: 0.23 to 0.79, I² = 0.00%). Plasma exchange had no effect on recurrence.
3.4.8. Other factors
Recipients with KT history had a 43% increased risk of recurrence compared to those with first KT (RR = 1.43, 95% CI: 1.24 to 1.65, I² = 47.40%). Factors such as graft rejection, hypertension, diabetes, cold ischemia time, delayed graft function, cytomegalovirus or BK virus infection, did not have an impact on recurrence.
3.4.9. Serum biomarkers
Our findings indicated that serum immunoglobulin G autoantibodies (IgG), IgA, and galactose-deficient IgA1 (Gd-IgA1) levels for recurrence were not predictive of IgAN recurrence ( Table 3 ).
Table 3.
Meta-analysis of laboratory data for IgAN recurence.
| Laboratory parameters | No. of studies | n | Heterogeneity | Effects model | ES | ES [95% CI] | Z | P | |
|---|---|---|---|---|---|---|---|---|---|
| I² | P | ||||||||
| IgG (g/L) | |||||||||
| Baseline | 3 | 268 | 79.70% | 0.0073 | R | MD | 1.48 [-0.19, 3.16] | 1.74 | 0.0827 |
| IgA (mg/L) | |||||||||
| Baseline | 5 | 615 | 83.20% | < 0.0001 | R | MD | 0.48 [-0.10, 1.05] | 1.63 | 0.1033 |
| 6 month | 2 | 225 | 88.40% | 0.0033 | R | MD | 0.19 [-0.83, 1.20] | 0.36 | 0.7197 |
| 1 year | 3 | 561 | 81.30% | 0.0048 | R | MD | 0.32 [-0.28, 0.92] | 1.04 | 0.2997 |
| Gd-IgA1 | |||||||||
| Baseline | 5 | 400 | 2.70% | 0.3911 | F | SMD | 0.05 [-0.17, 0.27] | 0.44 | 0.6634 |
| Hematuria | |||||||||
| 1 year | 3 | 232 | 89.50% | 0.0021 | R | SMD | 1.29 [0.10, 2.47] | 2.13 | 0.0328 |
| 3 year | 3 | 232 | 97.90% | < 0.0001 | R | SMD | 1.94 [-0.65, 4.54] | 1.47 | 0.1425 |
| 5 year | 2 | 183 | 99.30% | < 0.0001 | R | SMD | 5.09 [-3.10, 13.28] | 1.22 | 0.2231 |
| Post KT * | 6 | 646 | 77.20% | 0.0005 | R | RR | 3.27 [1.63, 6.55] | 3.33 | 0.0009 |
| Proteinuria | |||||||||
| 6 month | 2 | 173 | 67.50% | 0.0795 | R | SMD | 0.33 [-0.31, 0.96] | 1.01 | 0.3128 |
| 1 year | 5 | 477 | 33.40% | 0.1989 | F | SMD | 0.37 [0.17, 0.58] | 3.52 | 0.0004 |
| 3 year | 2 | 197 | 0.00% | 0.9672 | F | SMD | 1.76 [1.41, 2.11] | 9.78 | < 0.0001 |
| 5 year * | 2 | 355 | 58.50% | 0.1208 | R | RR | 2.19 [1.12, 4.31] | 2.28 | 0.0227 |
| Time at biopsy | 4 | 182 | 0.00% | 0.6860 | F | SMD | 0.22 [-0.08, 0.53] | 1.46 | 0.1431 |
| Time at biopsy * | 2 | 334 | 74.90% | 0.0461 | R | RR | 3.91 [0.88, 17.25] | 1.80 | 0.0722 |
| Last follow up | 7 | 587 | 85.30% | < 0.0001 | R | SMD | 1.25 [0.55, 1.94] | 3.49 | 0.0005 |
| eGFR (ml/min/1.73m2) | |||||||||
| Baseline | 2 | 197 | 0.00% | 0.8518 | F | MD | -0.05 [-2.28, 2.18] | -0.05 | 0.9626 |
| 6 month | 5 | 708 | 29.70% | 0.2233 | F | MD | 0.05 [-2.23, 2.33] | 0.04 | 0.966 |
| 2 year | 2 | 307 | 95.10% | < 0.0001 | R | MD | -14.37 [-37.20, 8.46] | -1.23 | 0.2173 |
| 3 year | 3 | 608 | 98.90% | < 0.0001 | R | MD | -11.31 [-30.18, 7.57] | -1.17 | 0.2403 |
| 5 year | 3 | 608 | 99.30% | < 0.0001 | R | MD | 12.87 [-38.14, 12.40] | -1.00 | 0.3181 |
| Last follow up | 4 | 410 | 0.00% | 0.9340 | F | MD | -12.37 [-17.25, -7.49] | -4.97 | < 0.0001 |
| Serum creatinine (mg/dL) | |||||||||
| Baseline | 4 | 358 | 83.80% | 0.0003 | R | MD | -0.05 [-0.18, 0.08] | -0.76 | 0.4490 |
| 6 month | 2 | 173 | 92.30% | 0.0003 | R | MD | -0.23 [-0.78, 0.33] | -0.80 | 0.4240 |
| 1 year | 7 | 469 | 0.00% | 0.7372 | F | MD | -0.10 [-0.30, 0.10] | -1.00 | 0.3186 |
| 3 year | 3 | 253 | 89.30% | < 0.0001 | R | MD | 0.58 [-0.14, 1.30] | 1.57 | 0.1156 |
| 5 year | 4 | 307 | 99.80% | < 0.0001 | R | MD | 1.01 [-0.55, 2.57] | 1.27 | 0.2050 |
| Last follow up | 8 | 562 | 37.90% | 0.1269 | F | MD | 0.49 [0.35, 0.64] | 6.60 | < 0.0001 |
IgAN, immunoglobulin A nephropathy; ES, effect size; CI, confidence interval; R, random effects model; F, fixed effect model; MD, mean difference; SMD, standardized mean difference; RR, risk ratio; IgG, immunoglobulin G; IgA, immunoglobulin A; Gd-IgA1, galactose deficiency IgA1; KT, kidney transplantation; eGFR, estimated glomerular filtration rate.
*dichotomous variables.
3.5. Meta-analysis of hematuria and proteinuria in recurrent IgAN
The level of hematuria was found to be higher in patients with recurrence one year after transplantation, compared to those without recurrence (SMD = 1.29, 95% CI: 0.10 to 2.47, I² = 89.50%). Additionally, patients with recurrence were more likely to have hematuria, regardless of the time of occurrence (RR = 3.27, 95% CI: 1.63 to 6.55, I² = 77.20%). However, there was no significant difference in hematuria levels between the 3-year and 5-year follow-up periods. Urinary protein levels were also higher in patients with recurrence, and this difference was significant at follow-up periods (1 year, 3 years, 5 years, and the final follow-up).
3.6. Clinical outcomes of IgAN recurrence
A total of 27 studies were analyzed to determine the RR for graft loss in patients with IgAN recurrence. The results showed that the presence of IgAN recurrence was associated with poorer graft survival (RR = 2.19, 95% CI: 1.60 to 3.01, I² = 82.80%) ( Table 2 ). IgAN recurrence did not have impact on post-transplant death or infection rates. With respect to renal function, no significant difference was found during the other time periods, except for worse renal function in patients with recurrence at the final follow-up (estimated glomerular filtration rate [eGFR]: MD = -12.37 ml/min/1.73m2, 95% CI: -17.25 to -7.49, I² = 0.00%; serum creatinine: MD = 0.49 mg/dL, 95% CI: 0.35 to 0.64, I² = 37.90%) ( Table 3 ).
3.7. Publication bias
The majority of the P values of Egger’s test were not significant, indicating that there was no significant publication bias ( Table 2 ). However, it is important to note that publication bias may be present in the analysis of the pooled RRs for donor types (living donor: p = 0.0003; related donor: p = 0.0001), induction with antithymocyte globulin (p = 0.0189) and graft loss outcome (p = 0.0040).
4. Discussion
In the present systematic review and meta-analysis, our aim was to evaluate the risk factors associated with the recurrence of IgAN and provide a comprehensive summary of the outcomes based on relevant articles published until now. The immediate consequence of IgAN recurrence is an increased risk of graft loss, and similar to primary IgAN, treatment options for recurrent IgAN are limited. Thus, by studying the risk factors and employing appropriate risk stratification and preventive measures, it is possible to determine the likelihood of IgAN recurrence at an early stage and consequently reduce the recurrence rate. Although some previous studies have explored the risk factors for IgAN recurrence, their findings have been controversial due to variations in selection criteria, sample size, and study design. Therefore, in this meta-analysis, we aim to identify potential risk factors for IgAN recurrence, with key findings summarized in Table 4 .
Table 4.
Summary of risk factors for IgAN recurrence.
| Risk factors | Protective factors |
|---|---|
| Young recipient | HLA-B46 antigen |
| Young donor | Induction with anti-IL-2-R antibodies |
| Rapid progression from IgAN diagnosis to ESKD | MMF |
| Short time on dialysis | Pretransplant tonsillectomy |
| Low total HLA mismatches | |
| Low HLA-DR mismatches | |
| Male recipient | |
| Related donor | |
| Retransplantation | |
| Hemodialysis | |
| No induction therapy | |
| mTOR inhibitor | |
| ACEI/ARB |
IgAN, immunoglobulin A nephropathy; ESRD, end stage renal disease; HLA, human leukocyte antigen; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; MMF, mycophenolate mofetil.
IgAN is a systemic autoimmune disease which affects both the native and allograft kidney with a high recurrence (67). The “multi-hit” hypothesis is widely accepted as the pathogenesis of IgAN (67, 68). Although this hypothesis is yet to be proven, it has gained wide acceptance due to the available evidence (67). Therefore, we have attempted to establish a connection between our findings and the four stages of its pathogenesis.
The first step in the pathogenesis is an increase in circulating abnormal IgA (Gd-IgA1). The serum level of Gd-IgA1 was found to be elevated in patients with native IgAN (69). Additionally, patients who underwent KT also exhibited higher serum levels of Gd-IgA1 compared to healthy controls, both at diagnosis and at transplant (26). However, our pooled results indicate that serum Gd-IgA1 levels did not serve as a predictor for recurrence, but it is important to note that these measurements were taken at baseline. To the best of our knowledge, there have been no studies investigating the relationship between dynamic changes in serum Gd-IgA1 levels post-KT and the occurrence of recurrence. Further research in this area is imperative. The immune cells responsible for the production of Gd-IgA1 are present in the mucosa-associated lymphoid tissues, with the tonsil being a key component of these tissues (70, 71). Currently, the KDIGO clinical practice guidelines do not recommend tonsillectomy as a part of the treatment for native IgAN. However, studies have shown that tonsillectomy can lead to clinical remission and lower the incidence of ESRD in patients with native IgAN (72, 73). This finding has also been supported by a recent study with a large sample size (74). In the case of KT, our pooled results suggest that tonsillectomy may also prevent recurrence, though this conclusion is based on only 350 transplants from two studies (5, 34). Another aspect to consider is the associated risk of complications from tonsillectomy, which has been reported to range from 2.8% to 3.2% (74, 75). Therefore, it is necessary to conduct well-designed prospective studies to thoroughly evaluate the benefits and risks of tonsillectomy in the prevention and treatment of recurrent IgAN within the context of KT.
Anti-glycan immunoglobulin G autoantibodies (IgG) bind to abnormal IgA to form circulating immune complexes (76). However, no predictive effect of serum IgG on recurrence was detected in present study. Serum IgG antiglycan autoantibody level at transplant has been found to predict recurrence, but this was investigated in only one study, limiting further synthetic analysis (26). Genetic background, particularly major histocompatibility complex (MHC) sites, also play important roles in native IgAN disease (77–79). However, these antigens have not been systematically studied in the context of recurrent IgAN, and our results suggest that HLA-B46 is a protective factor. We found that patients with rapid progression of the primary disease were more likely to recur, possibly due to a stronger ongoing systemic autoimmune response after KT. Additionally, our study found that older recipients had a lower risk of recurrence, which may be due to a decrease in the production of autoantibodies by the immune system with age, consistent with what is observed in primary IgAN. Theoretically, regulation of the pathogenic immune pathway may alter the natural course of the disease. However, the role of immunosuppressive drugs in native IgAN remains controversial. Patients not receiving induction therapy had a 73% increased risk of recurrence, but this may be related to HLA matching, as patients with lower HLA mismatch were more likely not to receive induction therapy. The soluble IL-2 receptor α has been found to be associated with the progression of native IgAN (80). The anti-IL-2-R antibody targets the CD25 antigen (IL-2-R) on activated T lymphocytes, thereby blocking IL-2 binding (81). As a result, there is a cell cycle arrest in the G0 or G1 phase, which inhibits T cell proliferation. This suggests that the anti-IL-2-R antibody may inhibit the production of autoantibodies mediated by the above-mentioned pathway and therefore prevent the recurrence of IgAN after KT. Of the maintenance drugs, MMF was found to lower the risk of recurrence, possibly due to its ability to inhibit B and T lymphocyte proliferation and reduce the production of autoantibodies and Gd-IgA1 (82). Although mTOR inhibitors have also been associated with inhibition of T cell proliferation, their use was found to be associated with an increased risk of recurrence, possibly because mTOR inhibitors increase proteinuria and thus increase the chance of biopsy, leading to the detection of subclinical pathological recurrence findings (83, 84).
In the final stage of pathogenesis, mesangial deposition of circulating Gd-IgA1-antiglycan IgG immune complex in the renal allograft results in cell activation and glomerular injury, ultimately leads to recurrence. Transferrin receptor 1 (TfR1) was identified as an IgA1 receptor expressed on human mesangial cells and has affinity with the immune complex formed by Gd-IgA1 and IgG (85). Our observation that related donors and patients with low HLA mismatch are more likely to recur may indicate that allografts with similar genetic backgrounds may express more IgA1 receptors and therefore have higher affinity for host circulating immune complexes. Glomerular injury results in hematuria and proteinuria production, as our study found that patients had higher levels of urinary red blood cells and urinary protein before and after recurrence, which reminds us that more attention should be paid to urinalysis in the follow-up of these patients to determine the timing of biopsy. The role of ACEI/ARB in conservative therapy for native or recurrent IgAN is well established (1, 86). Our study found that recurrent patients were more likely to receive ACEI/ARB therapy, possibly because patients with recurrence were more prone to proteinuria during the course of the disease, leading to confounding bias. Furthermore, other medications, such as MMF and other immunosuppressants may conceal the protective effect of ACEI/ARB in KT (82).
Other risk factors were also identified in our study. Male recipients were more likely to recur, consistent with the higher prevalence observed in males in native IgAN (68). The donor age of recurrent recipients was also found to be younger. The duration of dialysis in recurrent patients was 3.14 months shorter than in non-recurrent patients. It is worth noting that the duration of dialysis in living related donor recipients is usually short, and we observed that the recurrence risk of these individuals is lower. However, preemptive transplant patients and patients receiving dialysis did not differ in recurrence risk. Since recurrence increases the risk of graft failure, and the same risk factors make it easier to recur after a second or subsequent transplant. Our study lacked analysis of immunopathological and histopathological risk factors. Previous studies have shown that complement deposition, such as C4d in the glomeruli, plays an important role in graft loss in recurrent IgAN (87). Given the low prevalence of procedural biopsy, it is difficult to analyze its predictive effect on the risk of recurrence.
This study has several limitations. All the studies included in this meta-analysis are retrospective studies, which may introduce inevitable biases. Furthermore, the overall quality of the studies is rated as medium. All the patients included in the analysis had a biopsy-confirmed diagnosis of IgAN, which could potentially result in selection bias and underestimate the recurrent incidence of IgAN. Additionally, some of the analysis is based on univariate data, which could be influenced by confounding factors. A number of the identified risk factors are based on limited studies and small sample sizes, so further research is required to verify these findings. Moreover, most of the results from Egger’s test showed p-values greater than 0.05, indicating no significant publication bias in the included literature. However, we were unable to analyze publication bias for the analysis with less than 10 included articles. This limitation arises from the fact that the power of Egger’s test greatly decreases when the number of studies is small. Additionally, we restricted the study to articles published in English and Chinese, potentially excluding studies in other relevant languages and introducing publication bias. As a result, it is advisable to exercise caution when interpreting the results of this meta-analysis.
In conclusion, this meta-analysis has identified several factors associated with IgAN recurrence after KT. As patients with recurrence had poor graft outcomes, our findings may improve pre-transplant evaluation of individuals with IgAN induced renal failure. By properly stratifying risk and implementing appropriate interventions, it might be of help to enhance long-term outcomes in this population.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
YL: data curation, formal analysis, investigation, methodology, software, visualization, writing – original draft. TY: writing – original draft, data curation, formal analysis, investigation. TL: funding acquisition, supervision, validation, writing – review & editing. TS: supervision, validation, writing – review & editing, conceptualization, funding acquisition, project administration, software.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of China (Grant number 81870513) and the Key Research funding for Sichuan province (Grant number 2021YFS0118).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1277017/full#supplementary-material
References
- 1. Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol (2017) 12(4):677–86. doi: 10.2215/CJN.07420716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis (2000) 36(2):227–37. doi: 10.1053/ajkd.2000.8966 [DOI] [PubMed] [Google Scholar]
- 3. Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol (2010) 5(12):2363–72. doi: 10.2215/CJN.06720810 [DOI] [PubMed] [Google Scholar]
- 4. Jäger C, Stampf S, Molyneux K, Barratt J, Golshayan D, Hadaya K, et al. Recurrence of IgA nephropathy after kidney transplantation: experience from the Swiss transplant cohort study. BMC Nephrol (2022) 23(1):178. doi: 10.1186/s12882-022-02802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okumi M, Okada D, Unagami K, Kakuta Y, Iizuka J, Takagi T, et al. Higher immunoglobulin A nephropathy recurrence in related-donor kidney transplants: The Japan Academic Consortium of Kidney Transplantation study. Int J Urol (2019) 26(9):903–9. doi: 10.1111/iju.14066 [DOI] [PubMed] [Google Scholar]
- 6. McDonald SP, Russ GR. Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation (2006) 82(6):759–62. doi: 10.1097/01.tp.0000230131.66971.45 [DOI] [PubMed] [Google Scholar]
- 7. Han SS, Huh W, Park SK, Ahn C, Han JS, Kim S, et al. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int (2010) 23(2):169–75. doi: 10.1111/j.1432-2277.2009.00966.x [DOI] [PubMed] [Google Scholar]
- 8. Kim Y, Yeo SM, Kang SS, Park WY, Jin K, Park SB, et al. Long-term clinical outcomes of first and second kidney transplantation in patients with biopsy-proven IgA nephropathy. Transplant Proc (2017) 49(5):992–6. doi: 10.1016/j.transproceed.2017.03.063 [DOI] [PubMed] [Google Scholar]
- 9. Noguchi H, Tsuchimoto A, Ueki K, Kaku K, Okabe Y, Nakamura M. Reduced recurrence of primary IgA nephropathy in kidney transplant recipients receiving everolimus with corticosteroid: A retrospective, single-center study of 135 transplant patients. Transplant Proc (2020) 52(10):3118–24. doi: 10.1016/j.transproceed.2020.05.022 [DOI] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj (2009) 339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res (2018) 27(6):1785–805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 15. Ji S, Ni X, Xie K, Li X, Wen J, Cheng D, et al. Recurrent IgA nephropathy after renal transplantation: not always a benign prognosis. Organ Transplant (2016) 7:94–9. doi: 10.3969 /j.issn.1674-7445. 2016. 02. 003 [Google Scholar]
- 16. Wang Y, Wang K, Li M, Qu Q. Risk factors for recurrence of IgA nephropathy after kidney transplantation. Prac J Organ Transplant (Electronic Version) (2021) 9:126–30. doi: 10.3969/j.issn.2095-5332.2021.02.009 [DOI] [Google Scholar]
- 17. Ahn S, Min SI, Min SK, Ha IS, Kang HG, Kim YS, et al. Different recurrence rates between pediatric and adult renal transplant for immunoglobulin A nephropathy: predictors of posttransplant recurrence. Exp Clin Transplant (2015) 13(3):227–32. doi: 10.6002/ect.2014.0291 [DOI] [PubMed] [Google Scholar]
- 18. Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int (2017) 92(2):461–9. doi: 10.1016/j.kint.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 19. Andresdottir MB, Hoitsma AJ, Assmann KJ, Wetzels JF. Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol (2001) 56(4):279–88. [PubMed] [Google Scholar]
- 20. Avasare RS, Rosenstiel PE, Zaky ZS, Tsapepas DS, Appel GB, Markowitz GS, et al. Predicting post-transplant recurrence of IgA nephropathy: the importance of crescents. Am J Nephrol (2017) 45(2):99–106. doi: 10.1159/000453081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bachman U, Biava C, Amend W. The clinical course of IgA-nephropathy and Henoch-Schonlein purpura following renal transplantation. Transplantation (1986) 42(5):511–5. doi: 10.1097/00007890-198611000-00014 [DOI] [PubMed] [Google Scholar]
- 22. Bantis C, Heering PJ, Aker S, Schwandt C, Grabensee B, Ivens K. Influence of interleukin-10 gene G-1082A polymorphism on recurrent IgA nephropathy. J Nephrol (2008) 21(6):941–6. [PubMed] [Google Scholar]
- 23. Berger J, Noël LH, Nabarra B. Recurrence of mesangial IgA nephropathy after renal transplantation. Contrib Nephrol (1984) 40:195–7. doi: 10.1159/000409749 [DOI] [PubMed] [Google Scholar]
- 24. Berthelot L, Robert T, Vuiblet V, Tabary T, Braconnier A, Dramé M, et al. Recurrent IgA nephropathy is predicted by altered glycosylated IgA, autoantibodies and soluble CD89 complexes. Kidney Int (2015) 88(4):815–22. doi: 10.1038/ki.2015.158 [DOI] [PubMed] [Google Scholar]
- 25. Berthoux F, El Deeb S, Mariat C, Diconne E, Laurent B, Thibaudin L. Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation (2008) 85(10):1505–7. doi: 10.1097/TP.0b013e3181705ad4 [DOI] [PubMed] [Google Scholar]
- 26. Berthoux F, Suzuki H, Mohey H, Maillard N, Mariat C, Novak J, et al. Prognostic value of serum biomarkers of autoimmunity for recurrence of IgA nephropathy after kidney transplantation. J Am Soc Nephrol (2017) 28(6):1943–50. doi: 10.1681/ASN.2016060670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bjørneklett R, Vikse BE, Smerud HK, Bostad L, Leivestad T, Hartmann A, et al. Pre-transplant course and risk of kidney transplant failure in IgA nephropathy patients. Clin Transplant (2011) 25(3):E356–365. doi: 10.1111/j.1399-0012.2011.01424.x [DOI] [PubMed] [Google Scholar]
- 28. Bumgardner GL, Amend WC, Ascher NL, Vincenti FG. Single-center long-term results of renal transplantation for IgA nephropathy. Transplantation (1998) 65(8):1053–60. doi: 10.1097/00007890-199804270-00008 [DOI] [PubMed] [Google Scholar]
- 29. Chacko B, George JT, Neelakantan N, Korula A, Chakko JK. Outcomes of renal transplantation in patients with immunoglobulin A nephropathy in India. J Postgrad Med (2007) 53(2):92–5. doi: 10.4103/0022-3859.32207 [DOI] [PubMed] [Google Scholar]
- 30. Chandrakantan A, Ratanapanichkich P, Said M, Barker CV, Julian BA. Recurrent IgA nephropathy after renal transplantation despite immunosuppressive regimens with mycophenolate mofetil. Nephrol Dial Transplant (2005) 20(6):1214–21. doi: 10.1093/ndt/gfh773 [DOI] [PubMed] [Google Scholar]
- 31. Choy BY, Chan TM, Lo SK, Lo WK, Lai KN. Renal transplantation in patients with primary immunoglobulin A nephropathy. Nephrol Dial Transplant (2003) 18(11):2399–404. doi: 10.1093/ndt/gfg373 [DOI] [PubMed] [Google Scholar]
- 32. Coppo R, Amore A, Chiesa M, Lombardo F, Cirina P, Andrulli S, et al. Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin Transplant (2007) 21(6):728–37. doi: 10.1111/j.1399-0012.2007.00730.x [DOI] [PubMed] [Google Scholar]
- 33. Courtney AE, McNamee PT, Nelson WE, Maxwell AP. Does angiotensin blockade influence graft outcome in renal transplant recipients with IgA nephropathy? Nephrol Dial Transplant (2006) 21(12):3550–4. doi: 10.1093/ndt/gfl506 [DOI] [PubMed] [Google Scholar]
- 34. Di Vico MC, Messina M, Fop F, Barreca A, Segoloni GP, Biancone L. Recurrent IgA nephropathy after renal transplantation and steroid withdrawal. Clin Transplant (2018) 32(4):e13207. doi: 10.1111/ctr.13207 [DOI] [PubMed] [Google Scholar]
- 35. Freese P, Svalander C, Nordén G, Nyberg G. Clinical risk factors for recurrence of IgA nephropathy. Clin Transplant (1999) 13(4):313–7. doi: 10.1034/j.1399-0012.1999.130406.x [DOI] [PubMed] [Google Scholar]
- 36. Garnier AS, Duveau A, Demiselle J, Croué A, Subra JF, Sayegh J, et al. Early post-transplant serum IgA level is associated with IgA nephropathy recurrence after kidney transplantation. PloS One (2018) 13(4):e0196101. doi: 10.1371/journal.pone.0196101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeong HJ, Park SK, Cho YM, Kim MS, Kim YS, Choi J, et al. Progression of renal allograft histology after renal transplantation in recurrent and nonrecurrent immunoglobulin A nephropathy. Hum Pathol (2008) 39(10):1511–8. doi: 10.1016/j.humpath.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 38. Jiang SH, Kennard AL, Walters GD. Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol (2018) 19(1):344. doi: 10.1186/s12882-018-1135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jo HA, Han SS, Lee S, Kim JY, Yang SH, Lee H, et al. The association of tumor necrosis factor superfamily 13 with recurrence of immunoglobulin A nephropathy in living related kidney transplantation. BMC Nephrol (2019) 20(1):33. doi: 10.1186/s12882-019-1222-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamal Aziz A, Mousson C, Berthoux F, Ducloux D, Chalopin JM. Renal transplantation outcome in selected recipients with IgA nephropathy as native disease: a bicentric study. Ann Transplant (2012) 17(3):45–51. doi: 10.12659/aot.883457 [DOI] [PubMed] [Google Scholar]
- 41. Kavanagh CR, Zanoni F, Leal R, Jain NG, Stack MN, Vasilescu ER, et al. Clinical predictors and prognosis of recurrent IgA nephropathy in the kidney allograft. Glomerular Dis (2022) 2(1):42–53. doi: 10.1159/000519834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kawabe M, Yamamoto I, Komatsuzaki Y, Yamakawa T, Katsumata H, Katsuma A, et al. Recurrence and graft loss after renal transplantation in adults with IgA vasculitis. Clin Exp Nephrol (2017) 21(4):714–20. doi: 10.1007/s10157-016-1336-y [DOI] [PubMed] [Google Scholar]
- 43. Kennard AL, Jiang SH, Walters GD. Increased glomerulonephritis recurrence after living related donation. BMC Nephrol (2017) 18(1):25. doi: 10.1186/s12882-016-0435-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kessler M, Hiesse C, Hestin D, Mayeux D, Boubenider K, Charpentier B. Recurrence of immunoglobulin A nephropathy after renal transplantation in the cyclosporine era. Am J Kidney Dis (1996) 28(1):99–104. doi: 10.1016/S0272-6386(96)90137-7 [DOI] [PubMed] [Google Scholar]
- 45. Kim YS, Moon JI, Jeong HJ, Kim MS, Kim SI, Choi KH, et al. Live donor renal allograft in end-stage renal failure patients from immunoglobulin A nephropathy. Transplantation (2001) 71(2):233–8. doi: 10.1097/00007890-200101270-00011 [DOI] [PubMed] [Google Scholar]
- 46. Lee KW, Kim KS, Lee JS, Yoo H, Kim K, Park JB, et al. Impact of induction immunosuppression on the recurrence of primary IgA nephropathy. Transplant Proc (2019) 51(5):1491–5. doi: 10.1016/j.transproceed.2019.01.115 [DOI] [PubMed] [Google Scholar]
- 47. Lionaki S, Makropoulos I, Panagiotellis K, Vlachopanos G, Gavalas I, Marinaki S, et al. Kidney transplantation outcomes in patients with IgA nephropathy and other glomerular and non-glomerular primary diseases in the new era of immunosuppression. PloS One (2021) 16(8):e0253337. doi: 10.1371/journal.pone.0253337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maixnerova D, Hruba P, Neprasova M, Bednarova K, Slatinska J, Suchanek M, et al. Outcome of 313 Czech patients with IgA nephropathy after renal transplantation. J Am Soc Nephrol (2021) 32:846. doi: 10.3389/fimmu.2021.726215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martín-Penagos L, Benito-Hernández A, San Segundo D, Sango C, Azueta A, Gómez-Román J, et al. A proliferation-inducing ligand increase precedes IgA nephropathy recurrence in kidney transplant recipients. Clin Transplant (2019) 33(4):e13502. doi: 10.1111/ctr.13502 [DOI] [PubMed] [Google Scholar]
- 50. Moriyama T, Nitta K, Suzuki K, Honda K, Horita S, Uchida K, et al. Latent IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transplant (2005) 19 Suppl 14:41–8. doi: 10.1111/j.1399-0012.2005.00403.x [DOI] [PubMed] [Google Scholar]
- 51. Moroni G, Longhi S, Quaglini S, Gallelli B, Banfi G, Montagnino G, et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant (2013) 28(5):1305–14. doi: 10.1093/ndt/gfs472 [DOI] [PubMed] [Google Scholar]
- 52. Nakamura T, Shirouzu T, Harada S, Sugimoto R, Nobori S, Yoshikawa M, et al. The abundance of antigalactose-deficient IgA1 autoantibodies results in glomerular deposition and IgA nephropathy recurrence after renal transplantation. Transplantation (2021) 105(12):e407–8. doi: 10.1097/TP.0000000000003879 [DOI] [PubMed] [Google Scholar]
- 53. Namba Y, Oka K, Moriyama T, Ichimaru N, Kyo M, Kokado Y, et al. Risk factors for graft loss in patients with recurrent IGA nephropathy after renal transplantation. Transplant Proc (2004) 36(5):1314–6. doi: 10.1016/j.transproceed.2004.05.044 [DOI] [PubMed] [Google Scholar]
- 54. Ng YS, Vathsala A, Chew ST, Chiang GS, Woo KT. Long term outcome of renal allografts in patients with immunoglobulin A nephropathy. Med J Malaysia (2007) 62(2):109–13. [PubMed] [Google Scholar]
- 55. Nijim S, Vujjini V, Alasfar S, Luo X, Orandi B, Delp C, et al. Recurrent IgA nephropathy after kidney transplantation. Transplant Proc (2016) 48(8):2689–94. doi: 10.1016/j.transproceed.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 56. Odum J, Peh CA, Clarkson AR, Bannister KM, Seymour AE, Gillis D, et al. Recurrent mesangial IgA nephritis following renal transplantation. Nephrol Dial Transplant (1994) 9(3):309–12. [PubMed] [Google Scholar]
- 57. Ortiz F, Gelpi R, Koskinen P, Manonelles A, Räisänen-Sokolowski A, Carrera M, et al. IgA nephropathy recurs early in the graft when assessed by protocol biopsy. Nephrol Dial Transplant (2012) 27(6):2553–8. doi: 10.1093/ndt/gfr664 [DOI] [PubMed] [Google Scholar]
- 58. Park WY, Kim Y, Paek JH, Jin K, Han S. Clinical significance of serum galactose-deficient immunoglobulin A1 for detection of recurrent immunoglobulin A nephropathy in kidney transplant recipients. Kidney Res Clin Pract (2021) 40(2):317–24. doi: 10.23876/j.krcp.20.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ponticelli C, Traversi L, Feliciani A, Cesana BM, Banfi G, Tarantino A. Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int (2001) 60(5):1948–54. doi: 10.1046/j.1523-1755.2001.00006.x [DOI] [PubMed] [Google Scholar]
- 60. Rodas LM, Ruiz-Ortiz E, Garcia-Herrera A, Pereira A, Blasco M, Ventura-Aguiar P, et al. IgA nephropathy recurrence after kidney transplantation: role of recipient age and human leukocyte antigen-B mismatch. Am J Nephrol (2020) 51(5):357–65. doi: 10.1159/000506853 [DOI] [PubMed] [Google Scholar]
- 61. Sato K, Ishida H, Uchida K, Nitta K, Tanabe K. Risk factors for recurrence of immunoglobulin a nephropathy after renal transplantation: single center study. Ther Apher Dial (2013) 17(2):213–20. doi: 10.1111/j.1744-9987.2012.01139.x [DOI] [PubMed] [Google Scholar]
- 62. Sofue T, Inui M, Hara T, Moritoki M, Nishioka S, Nishijima Y, et al. Latent IgA deposition from donor kidneys does not affect transplant prognosis, irrespective of mesangial expansion. Clin Transplant (2013) 27 Suppl 26:14–21. doi: 10.1111/ctr.12158 [DOI] [PubMed] [Google Scholar]
- 63. Temurhan S, Akgul SU, Caliskan Y, Artan AS, Kekik C, Yazici H, et al. A novel biomarker for post-transplant recurrent IgA nephropathy. Transplant Proc (2017) 49(3):541–5. doi: 10.1016/j.transproceed.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 64. Uffing A, Perez-Saez MJ, Jouve T, Bugnazet M, Malvezzi P, Muhsin SA, et al. Recurrence of IgA nephropathy after kidney transplantation in adults. Clin J Am Soc Nephrol (2021) 16(8):1247–55. doi: 10.2215/CJN.00910121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Von Visger JR, Gunay Y, Andreoni KA, Bhatt UY, Nori US, Pesavento TE, et al. The risk of recurrent IgA nephropathy in a steroid-free protocol and other modifying immunosuppression. Clin Transplant (2014) 28(8):845–54. doi: 10.1111/ctr.12389 [DOI] [PubMed] [Google Scholar]
- 66. Wang AY, Lai FM, Yu AW, Lam PK, Chow KM, Choi PC, et al. Recurrent IgA nephropathy in renal transplant allografts. Am J Kidney Dis (2001) 38(3):588–96. doi: 10.1053/ajkd.2001.26885 [DOI] [PubMed] [Google Scholar]
- 67. Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol (2011) 22(10):1795–803. doi: 10.1681/ASN.2011050464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, et al. IgA nephropathy. Nat Rev Dis Primers (2016) 2:16001. doi: 10.1038/nrdp.2016.1 [DOI] [PubMed] [Google Scholar]
- 69. Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int (2007) 71(11):1148–54. doi: 10.1038/sj.ki.5002185 [DOI] [PubMed] [Google Scholar]
- 70. Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, Horikoshi S, et al. Experimental evidence of cell dissemination playing a role in pathogenesis of IgA nephropathy in multiple lymphoid organs. Nephrol Dial Transplant (2013) 28(2):320–6. doi: 10.1093/ndt/gfs467 [DOI] [PubMed] [Google Scholar]
- 71. Muto M, Manfroi B, Suzuki H, Joh K, Nagai M, Wakai S, et al. Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in igA nephropathy. J Am Soc Nephrol (2017) 28(4):1227–38. doi: 10.1681/ASN.2016050496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu LL, Wang LN, Jiang Y, Yao L, Dong LP, Li ZL, et al. Tonsillectomy for IgA nephropathy: a meta-analysis. Am J Kidney Dis (2015) 65(1):80–7. doi: 10.1053/j.ajkd.2014.06.036 [DOI] [PubMed] [Google Scholar]
- 73. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int (2021) 100(4):753–79. doi: 10.1016/j.kint.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 74. Hirano K, Matsuzaki K, Yasuda T, Nishikawa M, Yasuda Y, Koike K, et al. Association between tonsillectomy and outcomes in patients with immunoglobulin A nephropathy. JAMA Netw Open (2019) 2(5):e194772. doi: 10.1001/jamanetworkopen.2019.4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen MM, Roman SA, Sosa JA, Judson BL. Safety of adult tonsillectomy: a population-level analysis of 5968 patients. JAMA Otolaryngol Head Neck Surg (2014) 140(3):197–202. doi: 10.1001/jamaoto.2013.6215 [DOI] [PubMed] [Google Scholar]
- 76. Rizk DV, Saha MK, Hall S, Novak L, Brown R, Huang ZQ, et al. Glomerular immunodeposits of patients with IgA nephropathy are enriched for igG autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol (2019) 30(10):2017–26. doi: 10.1681/ASN.2018111156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Feehally J, Farrall M, Boland A, Gale DP, Gut I, Heath S, et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol (2010) 21(10):1791–7. doi: 10.1681/ASN.2010010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet (2014) 46(11):1187–96. doi: 10.1038/ng.3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Doxiadis II, De Lange P, De Vries E, Persijn GG, Claas FH. Protective and susceptible HLA polymorphisms in IgA nephropathy patients with end-stage renal failure. Tissue Antigens (2001) 57(4):344–7. doi: 10.1034/j.1399-0039.2001.057004344.x [DOI] [PubMed] [Google Scholar]
- 80. Lundberg S, Lundahl J, Gunnarsson I, Sundelin B, Jacobson SH. Soluble interleukin-2 receptor alfa predicts renal outcome in IgA nephropathy. Nephrol Dial Transplant (2012) 27(5):1916–23. doi: 10.1093/ndt/gfr554 [DOI] [PubMed] [Google Scholar]
- 81. Cibrik DM, Kaplan B, Meier-Kriesche HU. Role of anti-interleukin-2 receptor antibodies in kidney transplantation. BioDrugs (2001) 15(10):655–66. doi: 10.2165/00063030-200115100-00003 [DOI] [PubMed] [Google Scholar]
- 82. Sievers TM, Rossi SJ, Ghobrial RM, Arriola E, Nishimura P, Kawano M, et al. Mycophenolate mofetil. Pharmacotherapy (1997) 17(6):1178–97. doi: 10.1002/j.1875-9114.1997.tb03082.x [DOI] [PubMed] [Google Scholar]
- 83. Sennesael JJ, Bosmans JL, Bogers JP, Verbeelen D, Verpooten GA. Conversion from cyclosporine to sirolimus in stable renal transplant recipients. Transplantation (2005) 80(11):1578–85. doi: 10.1097/01.tp.0000184623.35773.6a [DOI] [PubMed] [Google Scholar]
- 84. Stephany BR, Augustine JJ, Krishnamurthi V, Goldfarb DA, Flechner SM, Braun WE, et al. Differences in proteinuria and graft function in de novo sirolimus-based vs. calcineurin inhibitor-based immunosuppression in live donor kidney transplantation. Transplantation (2006) 82(3):368–74. doi: 10.1097/01.tp.0000228921.43200.f7 [DOI] [PubMed] [Google Scholar]
- 85. Nihei Y, Suzuki H, Suzuki Y. Current understanding of IgA antibodies in the pathogenesis of IgA nephropathy. Front Immunol (2023) 14:1165394. doi: 10.3389/fimmu.2023.1165394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Park S, Baek CH, Go H, Kim YH, Min SI, Ha J, et al. Possible beneficial association between renin-angiotensin-aldosterone-system blockade usage and graft prognosis in allograft IgA nephropathy: a retrospective cohort study. BMC Nephrol (2019) 20(1):354. doi: 10.1186/s12882-019-1537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Eder M, Kozakowski N, Omic H, Aigner C, Kläger J, Perschl B, et al. Glomerular C4d in Post-Transplant IgA Nephropathy is associated with decreased allograft survival. J Nephrol (2021) 34(3):839–49. doi: 10.1007/s40620-020-00914-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.