Abstract
The conformational landscapes of peptide/human leucocyte antigen (pHLA) protein complexes encompassing tumor neoantigens provide a rationale for target selection towards autologous T cell, vaccine, and antibody-based therapeutic modalities. Here, using complementary biophysical and computational methods, we characterize recurrent RAS55-64 Q61 neoepitopes presented by the common HLA-A*01:01 allotype. We integrate sparse NMR restraints with Rosetta docking to determine the solution structure of NRASQ61K/HLA-A*01:01, which enables modeling of other common RAS55-64 neoepitopes. Hydrogen/deuterium exchange mass spectrometry experiments alongside molecular dynamics simulations reveal differences in solvent accessibility and conformational plasticity across a panel of common Q61 neoepitopes that are relevant for recognition by immunoreceptors. Finally, we predict binding and provide structural models of NRASQ61K antigens spanning the entire HLA allelic landscape, together with in vitro validation for HLA-A*01:191, HLA-B*15:01, and HLA-C*08:02. Our work provides a basis to delineate the solution surface features and immunogenicity of clinically relevant neoepitope/HLA targets for cancer therapy.
Subject terms: Solution-state NMR, Oncogenes, Supramolecular assembly
The authors use an integrative structural biology approach to elucidate molecular features of the RAS Q61 family of public tumor antigens. This information can be used to develop targeted therapeutics to combat a range of cancers.
Introduction
The display of epitopic peptides by class I major histocompatibility complex (MHC-I) proteins to CD8+ cytotoxic T lymphocytes (T cells) forms the cornerstone of adaptive immunity, enabling surveillance of the endogenous proteome against invading pathogens and intracellular oncoproteins1. Properly conformed peptide/MHC-I complexes (pMHC-I) are assembled in the endoplasmic reticulum, where a multi-subunit peptide loading complex coordinates the association of transported peptides with heavy and light chain components1–3. While the light chain (human β2-microglobulin, hβ2m) is invariant across each pMHC-I, the heavy chain is encoded by the most polymorphic loci in the human genome, with thousands of identified allotypes categorized in the canonical human leukocyte antigen (HLA) A-, B-, and C- classes4. The HLA peptide binding groove comprises polymorphic residues distributed among six distinct pockets (termed A through F) that define the repertoire of epitopic peptides displayed by each HLA5. Peptide binding specificity is determined by anchoring interactions with HLA side chains forming the A-F-pockets, which impose chemical and steric restrictions on peptide docking6. Thus, the conformational landscape of pMHC-I structures is defined by both the peptide sequence and HLA groove polymorphisms, which has important ramifications for recognition by antibodies, T-cell receptors, and NK cell receptors7–11. Since the surface chemistry of pMHC-I proteins dictates recognition by T-cell receptors (TCRs) and therapeutic monoclonal antibodies12–14, structure determination of disease-associated complexes is essential for understanding immune responses, and the development of targeted therapies.
In recent years, there has been significant progress in the identification of immunogenic, mutated self-peptides (termed neoepitopes) presented on the surface of cancerous cells by HLA molecules15,16. Functional and structural characterization of neoepitope/HLA complexes enables design of targeted immunotherapies against cancer, such as engineered TCRs, antibodies, and cancer vaccines17–19. In particular, mutations in the RAS family of GTPase genes (NRAS, KRAS, and HRAS) are found in approximately 25% of patients with neuroblastoma and melanoma, where mutation at consensus residues 12, 13, and 61 are associated with driving oncogenic activity20,21. While structures of HLAs presenting RAS5−14 neoepitopes derived from mutations at residues 12 and 13 have been solved22,23, pMHC-I structures derived from RAS55-64 residue 61 neoepitopes have remained elusive. Using an integrated workflow to analyze tumor sequencing data from neuroblastoma patients, ProTECT24, we previously predicted that a public, decameric epitope derived from the recurrent NRAS55-64 mutation, Q61K (ILDTAGKEEY), could interact with the common HLA-A*01:01 molecule, in agreement with experimental validation of binding in vitro and in vivo by other groups25–27. More than 60% of NRAS positive tumors harbor Q61 mutants, making this epitope an attractive target for cancer therapy21,28. In addition, targeting of NRASQ61K by T cells has shown great promise in inhibiting tumor growth and angiogenesis in a peptide/HLA-restricted manner27,29,30. Notwithstanding, the structure of the NRASQ61K/HLA-A*01:01 complex has not yet been solved, limiting insights into how different RAS55-64 Q61 mutations could influence recognition by TCRs and antibody engineering approaches. While pMHC-I studies by X-ray crystallography have provided hundreds of structures in the Protein Data Bank9,31, many important pMHC-I complexes are not amenable to X-ray diffraction studies, despite exhaustive screening efforts32. Computational approaches have enabled modeling of pMHC-I structures using de novo or homology-based approaches33–35. However, both the precision and accuracy of these methods are limited by the large conformational space, which can be adopted by different peptide sequences within the HLA groove33.
To address these bottlenecks, we used complementary biophysical techniques to elucidate the assembly, structure, and conformational plasticity of peptide: HLA complexes encompassing different NRAS epitopes in a physiologically relevant, aqueous environment. Focusing on the NRASQ61K/HLA-A*01:01/hβ2m complex, we demonstrate that empty (peptide-free) HLA molecules undergo a conformational transition to sample a peptide-receptive conformation, which can spontaneously associate with NRAS peptides. We further implemented methyl-selective isotopic labeling for recording intermolecular NMR restraints, and combined our NMR data with Rosetta flexible docking simulations to determine the solution structure of the pMHC-I complex. We then utilized molecular dynamics (MD) simulations alongside hydrogen-deuterium exchange mass spectrometry (HDX-MS) to probe the solvation and conformational dynamics of sites within the MHC groove of NRASQ61K, as well as wild-type (Q61) and three additional, common neoepitopes (Q61H, Q61L, Q61R). Our data reveal that single amino acid changes at residue 61 of NRAS alter the peptide conformational landscape, where Q61K and Q61L display the greatest and least amount of mobility, respectively. Finally, we examined the HLA binding repertoire of NRAS neoepitopes using both in silico and in vitro approaches. These results underscore the public presentation of RAS55-64 Q61 neoepitopes beyond HLA-A*01:01, including the HLA-A*01:191, HLA-B*15:01, and HLA-C*08:02 allotypes. Taken together, our results highlight differences in conformational plasticity, solvation patterns, and surface accessibility across NRAS/HLA protein complexes, which can be exploited to design neoepitope-selective immunoreceptors and other therapeutic modalities.
Results
NRASQ61K binds to the common HLA-A*01:01 allotype with a moderate affinity
The RAS55-64 epitope sequence is strongly conserved across the RAS protein family (Supplementary Fig. 1). While we chose the NRAS55-64 Q61K peptide as a model system, Q61 mutations in KRAS55-64, NRAS55-64, and HRAS55-64 result in identical neoepitope sequences. Thus, NRAS55-64 Q61K is a representative for the entire family of RAS55-64 Q61K neoepitopes. Towards biophysical and structural characterization, we recombinantly expressed HLA-A*01:01 heavy chain and hβ2m light chain proteins in E. coli and performed in vitro refolding together with a 10-fold molar excess of the NRASQ61K peptide36. Purification by size exclusion chromatography (SEC) revealed properly folded, monomeric pMHC-I complexes (Supplementary Fig. 2a). Liquid chromatography–mass spectrometry (LC–MS) confirmed the presence of the bound NRASQ61K peptide with an observed mass of 1138 Da (Supplementary Fig. 2b). Differential scanning fluorimetry (DSF) revealed a moderately stable complex with melting temperature (Tm) of 53 °C (Supplementary Fig. 2c), consistent with reports of pMHC-I complexes exhibiting Tm’s ranging from 41 to 67 °C37,38,39. We next carried out fluorescence anisotropy competition experiments to estimate the affinity of NRASQ61K for the HLA-A*01:01/hβ2m complex40. Incubation of TAMRA-dye labeled NRASQ61K with NRASQ61K/HLA-A*01:01/hβ2m and varying concentrations of unlabeled competitor NRASQ61K yielded an IC50 of 260 nM (Supplementary Fig. 2d), consistent with a predicted IC50 of 218.5 nM by netMHCpan-4.141. We also attempted to solve the structure of the NRASQ61K/HLA-A*01:01/hβ2m complex by X-ray crystallography. However, following multiple crystallization screens, we were unable to find conditions that allowed crystal formation. Together, these results suggest that, while NRASQ61K can associate with the HLA-A*01:01 groove, the resulting complex is moderately stable leading to high levels of background exchange with the empty form, which likely prohibits the formation of an ordered crystal lattice for structural studies by X-ray diffraction.
Assembly of NRASQ61K with empty HLA-A*01:01 induces global structural changes
We sought to study the NRASQ61K/HLA-A*01:01/hβ2m assembly mechanism and structure using solution NMR, where structures of proteins up to 82 kDa have been determined de novo42. The size and complexity of our system (a non-symmetrical 45 kDa heterotrimer) necessitated the use of selective isotope labeling schemes (Supplementary Fig. 3a–e, Supplementary Table 1). Here, we applied complementary labeling schemes, inspired by the amino acid composition of the HLA-A*01:01 groove where methyl-bearing (Ala, Ile, Leu, Val) and aromatic (Phe, Tyr) residues lining the A- to F-pockets which anchor the peptide (Supplementary Fig. 3b). Our selective labeling allowed us to obtain unambiguous chemical shift assignments of resonances corresponding to both the HLA-A*01:01 heavy chain and NRASQ61K peptide residues, as well as to record intra- and intermolecular NOEs between HLA-A*01:01 and NRASQ61K protons. Chemical shift assignments of HLA-A*01:01 were established using TROSY-based 3D HNCA, 3D HN(CA)CB, and 3D HNCO triple-resonance together with 3D NOESY experiments, as shown for multiple other pMHC-I systems in previous studies by our group8,43,44–48,49. Free NRASQ61K peptide chemical shift assignments were performed using 2D 1H-1H NOESY and TOCSY experiments50. Bound peptide resonance assignments were achieved using transfers from the free state, followed by validation with peptide/HLA NOE cross-peaks obtained using samples with 15N/13C labeled NRASQ61K. Altogether, we achieved 100% stereospecific assignment of the 89 AILV methyl, 85% of the 21 FY aromatic as well as 93% of the amide groups of HLA-A*01:01 (Supplementary Fig. 3c–e), and complete assignment of peptide atoms in proximity to the groove.
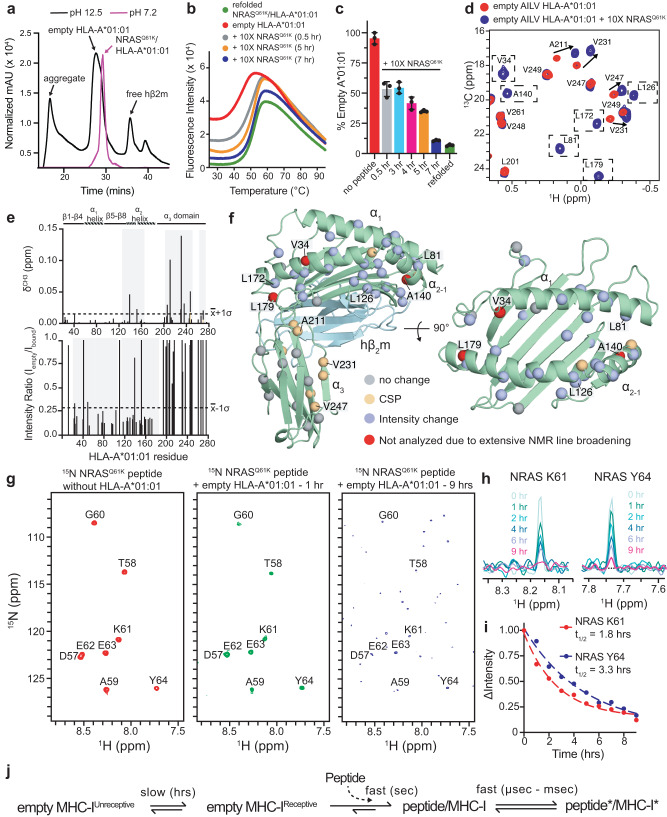
Direct peptide interactions with empty MHC-I molecules remain poorly understood but are thought to be a driving factor in antigen selection and pMHC-I cell surface stability. Thus, we sought to characterize how NRASQ61K could affect the structure and stability of empty MHC-I complex. In vitro studies of peptide interactions with empty MHC-I complexes have been previously reported in the literature, and are often employed as a first approximation pMHC-I interactions in vivo38,51–53. To characterize how NRASQ61K assembles with HLA-A*01:01, we first emptied HLA-A*01:01/hβ2m complexes of their in vitro refolded peptide by brief exposure to pH 12.5 followed by SEC purification (Fig. 1a), as previously described54–56. LC–MS confirmed that basic pH treatment of HLA-A*01:01/hβ2m resulted in monomeric, empty molecules, while control treatment with pH 7.2 revealed the NRASQ61K peptide was still bound in the complex (Supplementary Fig. 4a, b). Empty HLA-A*01:01/hβ2m complexes were receptive for binding to NRASQ61K as revealed by both DSF and solution NMR titrations (Fig. 1b–d). To determine the kinetics of peptide binding to the HLA, DSF experiments were performed where purified, empty HLA-A*01:01 was offered 10-fold molar excess of NRASQ61K peptide, and incubated for periods ranging from 0.5 to 7 h (Fig. 1b, c). The addition of NRASQ61K resulted in a reduction in the population of empty HLA-A*01:01 by approximately 50% within the first 0.5 h of incubation (Fig. 1b, c). Interestingly, complete reduction in the population of empty HLA-A*01:01 to levels observed in control experiments using refolded NRASQ61K/HLA-A*01:01 required incubation with NRASQ61K for up to 7 h (Fig. 1b, c). These results suggest the presence of a population of empty, peptide-receptive HLA-A*01:01 molecules, which can associate spontaneously with NRASQ61K, as well as a population of species undergoing a slow conformational change (several hours) towards the peptide-receptive state.
Fig. 1. Conformational changes and assembly kinetics for NRASQ61K association with empty HLA-A*01:01/hβ2m.
a SEC traces of HLA-A*01:01/hβ2m following short exposure to neutral (magenta) or basic (black) phosphate buffer. b DSF of 7 μM HLA-A*01:01/hβ2m following short exposure to pH 12.5 phosphate buffer. Red curve – no peptide added. 10× excess NRASQ61K peptide was added and incubated for 0.5 (gray), 5 (orange), and 7 (blue) hrs. Green curve – refolded NRASQ61K/HLA-A*01:01/hβ2m control. c Percent empty HLA-A*01:01 determined by DSF upon incubating empty HLA-A*01:01 without or with 10× excess NRASQ61K peptide for the indicated amount of time. Refolded complex is shown for reference. Data are mean ± SD for n = 3 technical replicates. d 2D 1H-13C methyl HMQC spectra of 50 μM AILV labeled HLA-A*01:01 refolded with natural abundance hβ2m following exposure to pH 12.5 phosphate buffer (red – no peptide; blue – 10× excess NRASQ61K peptide added) recorded at 25 °C at a 1H field of 800 MHz. Dotted boxes represent methyl resonances exhibiting line broadening in the empty state. e Chemical shift perturbations (CSP, δCH3, ppm) (top) and intensity ratios (Iempty/Ibound) (bottom) for AILV methyl probes of HLA-A*01:01 with 10× excess NRASQ61K (Ibound) relative empty HLA-A*01:01 (Iempty). Dotted lines represent the average plus one standard deviation for CSP analysis or minus one standard deviation for intensity ratio analysis. Gray boxes highlighted affected regions. The protein domains of HLA-A*01:01 are shown for reference. f Mapping of HLA-A*01:01 methyl residues with resonances exhibiting either CSP or intensity changes upon binding to NRASQ61K onto the X-ray structure of HLA-A*01:01 (PDB ID 6AT9, peptide atoms removed). g 2D 1H-15N HMQC spectra of 15N labeled NRASQ61K without (red) or with empty unlabeled HLA-A*01:01/hβ2m after 1 h (green) or 9 h (blue) of incubation recorded at a 1H field of 800 MHz at 25 °C. h 1D 1H spectral slices for the amide of K61 and Y64 of 15N labeled NRASQ61K throughout incubation in panel (g). i The change in NMR signal intensity (ΔIntensity) as a function of incubation time and fitted half-life (t1/2) values for K61 and Y64. j Schematic of the timescale for different conformational changes in the MHC-I complex.
We also performed NMR titrations to uncover structural differences in the empty versus peptide bound HLA-A*01:01 groove. Compared to the NRASQ61K bound state, 2D 1H-13C methyl HMQC spectra of AILV labeled empty HLA-A*01:01 exhibited both chemical shift perturbations (CSPs) and exchange-induced line broadening of NMR resonances corresponding to methyl groups spanning the entire HLA groove, including sites on the α1 helix, α2 helix, and pleated β-sheet (Fig. 1d–f, Supplementary Fig. 5a, b). These findings are in agreement with previous NMR studies of empty MHC-I molecules49,56,57. Intensity changes were primarily observed for NMR resonances corresponding to methyl groups located in the HLA-A*01:01 groove, suggesting the presence of dynamic motions on the intermediate (microseconds to milliseconds) timescale in the absence of bound peptide (Fig. 1e, f). The observation of CSPs at the interface between the HLA-A*01:01 α3 domain and hβ2m, distal to the peptide binding groove, revealed a rearrangement of the MHC-I domain structure (Fig. 1e, f). Together, these data support a global conformational change in the HLA-A*01:01/hβ2m complex, induced by peptide binding.
To follow peptide binding kinetics, we obtained U-[15N] labeled NRASQ61K and performed a series of incubations with empty HLA-A*01:01/hβ2m complex, prepared at natural isotopic abundance. We monitored peptide/MHC-I association as a function of time using 2D 1H-15N HMQC spectra. Upon binding to HLA-A*01:01/hβ2m, NMR resonances corresponding to the NRASQ61K amide groups exhibited increased line broadening, likely due to increased transverse relaxation rate of the peptide 15N and 1H atoms within the much larger (45 kDa) pMHC-I complex (Fig. 1g, h). The change in NRASQ61K amide NMR signal intensity was followed as a function of time and fit to a single-phase exponential decay model, which revealed half-lives of peptide association with empty HLA-A*01:01/hβ2m complex of up to 3.7 h (Fig. 1i, Supplementary Fig. 6). Taken together with previous studies58–60, our DSF and NMR results revealed that empty MHC-I molecules undergo a conformational change to a peptide-receptive state, which occurs on a slow (hours) timescale (Fig. 1j). Once a receptive state is formed, peptide association with the MHC-I proceeds via a fast (msec-sec timescale) process (Fig. 1j). Following pMHC-I complex formation, further conformational changes can be observed for both the bound peptide and the HLA groove on a fast (μsec - msec) timescale (Fig. 1j), in agreement with previous studies7,8,56,61,62.
The NRASQ61K/HLA-A*01:01 NMR structure reveals key features for targeted recognition
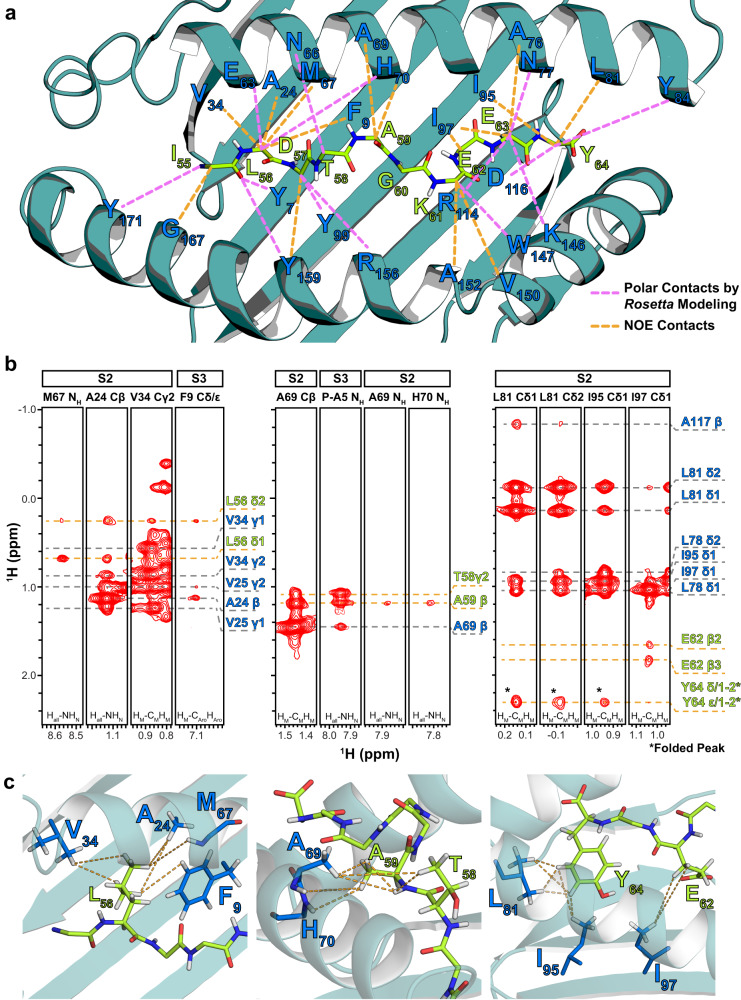
Towards determining the structure of the NRASQ61K/HLA-A*01:01 complex, we collected distance restraints in the form of intra- and intermolecular NOEs (Fig. 2a–c, Supplementary Fig. 3a, Supplementary Table 2, Supplementary Table 3). We recorded a suite of 3D SOFAST NOESY-HMQC experiments to obtain NOE cross-peaks at high signal-to-noise and spectral resolution63. These experiments allowed for simultaneous detection and assignment of intramolecular NOEs (between HLA-A*01:01 residues) and intermolecular NOEs (between HLA-A*01:01 and NRASQ61K residues) spanning the entire HLA groove (Fig. 2a–c). NOE cross-peaks recorded using 3D SOFAST NOESY-HMQC experiments were also observed in an analogous isotope-filtered/edited pulse sequences performed under independent conditions, albeit with lower sensitivity (Supplementary Fig. 7a–e)64.
Fig. 2. Intermolecular NOE contacts observed between the HLA-A*01:01 groove and NRASQ61K.
a Intermolecular NOEs observed between HLA-A*01:01 residues and NRASQ61K residues (dotted orange lines) in 3D SOFAST NOESY experiments are shown with HLA-A*01:01 groove (dark green) and peptide residues (green) labeled. Intermolecular polar contacts not observed experimentally, but observed in the final structure following high-resolution refinement using the Rosetta FlexPepDock ab-initio protocol are also shown (dotted purple lines). b Representative 1HM-1HM NOESY strips corresponding to intra- (gray dotted lines) and inter-molecular (orange dotted lines) NOE contacts, shown for regions spanning the HLA-A*01:01 groove (A/B pocket – left, B/C pocket – center, and E/F pocket – right). The top of the strip is labeled with the name of the sample used (S2 or S3) and the residue/atom name corresponding to the strip. The bottom of the strip is labeled with the NMR experiment where the NOE strip was obtained. Atoms corresponding to HLA-A*01:01 are labeled blue, while atoms corresponding to NRASQ61K are labeled green. NOE cross-peaks that are folded due to sweep width restrictions are indicated with an asterisk (*). c View of intermolecular NOE contacts (dotted lines) observed in panel B with HLA-A*01:01 (dark green) and peptide (light green) side chains shown as sticks. Our final refined structure of the complex (PDB ID 6MPP) was used to generate structure views.
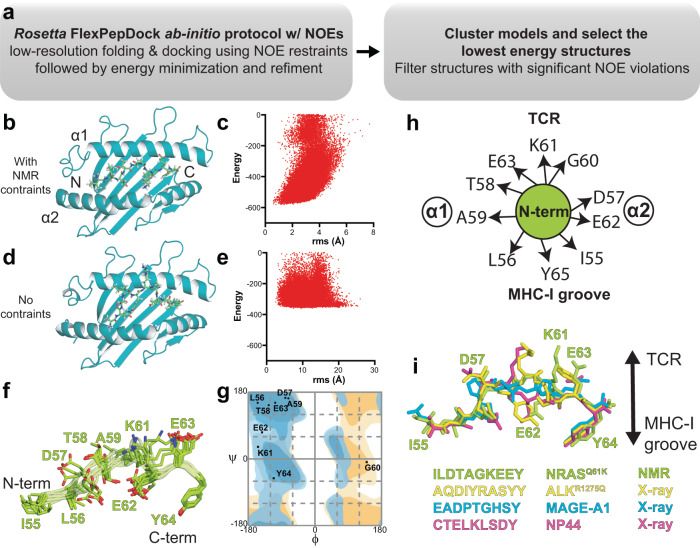
We then used both intra- and intermolecular NOE-derived constraints to perform structure calculations of the NRASQ61K/HLA-A*01:01/hβ2m complex with the FlexPepDock ab-initio protocol65 (Fig. 3a). FlexPepDock was chosen as previous benchmarks showed that even in the absence of experimental constraints, the protocol can sample near native pMHC-I conformations64,66. As a starting input template, we used a previously solved high-resolution structure of HLA-A*01:01 in complex with a different decameric peptide (PDB ID 6AT9) with the atoms corresponding to the original peptide removed26. Following refinement of the HLA-A*01:01 heavy chain using intramolecular NOEs, an atomic model of the free extended NRASQ61K peptide was placed near the HLA-A*01:01 groove in an orientation where P2 and P9 anchor positions of the peptide were oriented towards the A- and F-pockets of the MHC-I, respectively. This orientation is based on our observed peptide/HLA NOEs (Fig. 2a), which corroborates known peptide binding motifs associated with the HLA-A*01:01 groove, including canonical position 2 anchors (preferred ST, tolerated AILMV) and C-terminal anchors (preferred Y, tolerated F)67. A total of 50,000 models were generated from the initial starting structure, followed by fast low-resolution modeling and refinement of low-resolution models with and without intra- and intermolecular NOE-derived NMR constraints (Fig. 3b–e). We clustered the resulting structures, sorted them according to the total Rosetta energy score, and filtered out structures with NOE violations. Following the FlexPepDock ab-initio protocol combined with NMR restraints, we obtained a final structure where the NRASQ61K orientation within the HLA-A*01:01 groove agreed completely with the experimentally derived NOE constraints (Figs. 2a and 3b). The 10 lowest energy bound NRASQ61K conformations provided a well-defined ensemble (backbone RMSD of 0.61 Å and all-atom RMSD of 1.01 Å) (Fig. 3f). Notably, the overall Rosetta energy of the resulting decoys was much lower when NMR restraints were used (Fig. 3c, e), highlighting that experimental restraints were essential for guiding peptide docking towards the native structure. Together, these results support that a sparse set of NMR distance restraints, obtained using selective methyl/aromatic-labeled samples, are both necessary and sufficient for accurate modeling of the NRASQ61K/HLA-A*01:01/hβ2m complex solution structure.
Fig. 3. Molecular surface features of the NRASQ61K/HLA-A*01:01 groove for TCR recognition.
a Flow chart of the combined NMR and Rosetta FlexPepDock ab-initio protocol. b, d The Rosetta FlexPepDock ab-initio protocol was performed b with and d without NMR restraints. The HLA-A*01:01 groove is shown in dark green and NRASQ61K is shown in light green. The peptide cannot properly dock into the HLA-A*01:01 groove without NMR restraints due to geometric restrictions imposed by the MHC-I pockets. c, e Energy landscape plots showing energy (Rosetta energy units) versus backbone heavy atom root mean squared deviation (r.m.s., Å) to the lowest energy structure are shown for 50,000 models of FlexPepDock ab-initio protocol performed with c NOE-derived NMR constraints (Supplementary Table 2) and e without NMR constraints. f Overlay of the ten lowest energy NRASQ61K conformations in complex with HLA-A*01:01/β2m (not shown for clarity). g Ramachandran plot showing the φ/ψ angles of each residue of NRASQ61K peptide bound to HLA-A*01:01. The φ/ψ were computed on the peptide in the lowest energy structure HLA-A*01:01 bound NRASQ61K structure using the RAMPAGE server. Blue – general amino acid favored/allowed. Orange – glycine favored/allowed. h Cartoon schematic of the side chain orientation of the NRASQ61K (bound to HLA-A*01:01) displayed to TCRs as viewed from the N-terminal end relative to the MHC-I α1/α2 helices. i Comparison of the conformation and surface features of NRASQ61K (PDB ID 6MPP) displayed by HLA-A*01:01 to TCRs with peptides taken from X-ray structures of other known HLA-A*01:01-restricted epitopic peptides (ALKR1275Q - PDB ID 6AT9, MAGE-A1 - PDB ID 3BO8, NP44 - PDB ID 4NQV).
To characterize neoantigen features that are relevant for interactions with receptors, we examined the orientation of the NRASQ61K peptide relative to the HLA-A*01:01 groove and accessible molecular surface12. We focused on the representative lowest-energy structure, as there were no major differences among the NMR ensemble of HLA-A*01:01 bound NRASQ61K conformations (Fig. 3f). A Ramachandran plot of the HLA-A*01:01 bound NRASQ61K revealed backbone dihedral angles within the expected favored/allowed regions (Fig. 3g). NRASQ61K was oriented such that the side chains of G60, K61, and E63 formed an exposed molecular surface, whereas those for I55, L56 and Y64 were buried in the MHC-I pockets (Fig. 3h, i). Since the K61 side chain is solvent-exposed, the divergent electrostatic surface features of NRASQ61K relative to the wild-type NRASQ61 peptide can be exploited for targeted recognition using autologous or engineered immunoreceptors. Finally, we performed a superposition of the NRASQ61K peptide conformation with X-ray structures of other known immunodominant HLA-A*01:01 epitopes. The peptide backbone conformation of the bound NRASQ61K was compared with other peptide/HLA-A*01:01 complexes, such as ALKR1275Q (AQDIYRASYY – PDB ID 6AT9), MAGE-A1 (EADPTGHSY – PDB ID 3BO8), and NP44 (CTELKLSDY – PDB ID 4NQV). While the conformation of the peptide N- and C-termini was conserved and anchored through interactions within the A/B- and F-pockets of HLA-A*01:01, significant divergence was observed between residues 4 and 7 (Fig. 3i), revealing a unique surface chemistry and geometry for NRASQ61K presented to T-cell repertoires, relative to other known HLA-A*01:01 presented epitopes.
NRAS55-64 epitopes show differences in solvent accessibility and conformational plasticity
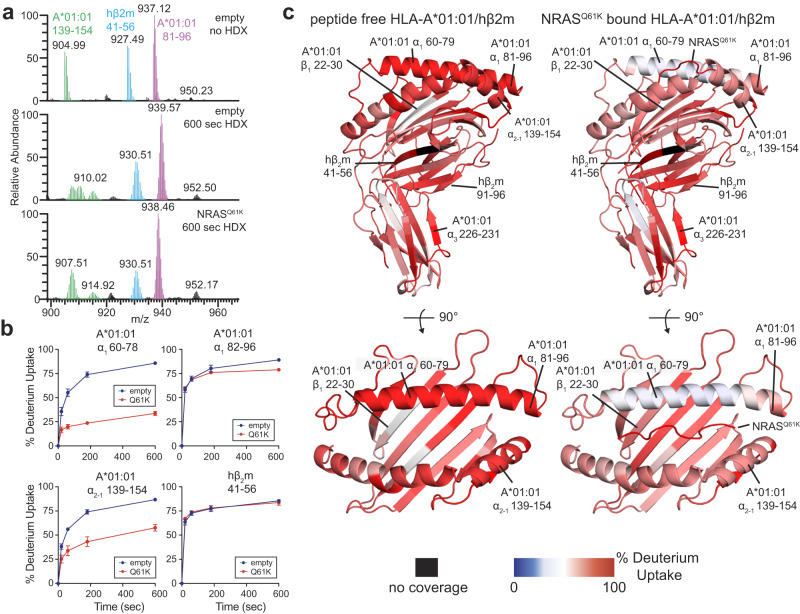
To compare the conformation and solvent accessibility of the MHC-I groove in empty versus NRASQ61K-bound HLA-A*01:01/hβ2m, we performed hydrogen/deuterium exchange (HDX) followed by proteolysis and mass spectrometry analysis of the resulting peptide fragments68,69. Compared to control experiments performed in the absence of deuterium, HDX performed on empty and NRASQ61K-bound HLA-A*01:01/hβ2m samples for 600 s resulted in different levels of deuterium uptake for peptide fragments spanning the entire HLA-A*01:01 and hβ2m molecules (Fig. 4a–c, Supplementary Table 5, Supplementary Data 1–3). Analysis of percent deuterium uptake as a function of HDX reaction time revealed saturation of most peptide fragments within the 600 s timescale and distinct profiles between empty and NRASQ61K bound HLA-A*01:01/hβ2m (Fig. 4b, Supplementary Data 2 and 3). Specifically, regions corresponding to α1 helix (residues 60–78 and 82–96) and α2-1 helix (residues 139–155) showed significant differences in HDX behavior between empty versus NRASQ61K bound states (Fig. 4b, c, Supplementary Figs. 8 and 9, Supplementary Data 3). In contrast, the HLA-A*01:01 α3 domain and hβ2m showed similar HDX rates in the absence or presence of peptide (Fig. 4b, c, Supplementary Fig. 9). Together, our HDX results demonstrate that the absence of NRASQ61K peptide destabilizes the HLA-A*01:01 groove, consistently with our DSF and NMR experiments (Fig. 1b, d).
Fig. 4. Global conformational plasticity of empty vs NRASQ61K bound HLA-A*01:01/hβ2m visualized by hydrogen/deuterium exchange.
a Mass spectrometric envelopes for peptide fragments comprising residues HLA-A*01:01 81–96 (magenta), HLA-A*01:01 139–154 (green), and hβ2m 41–56 (blue) for emptied HLA-A*01:01 with no HDX (i.e., all H sample) and 600 s HDX compared with NRASQ61K bound HLA-A*01:01 at 600 s HDX. The peptide fragment masses are noted. b Kinetic graphs of % deuterium uptake (back-exchange corrected) for different peptide fragments as a function of HDX time (0, 20, 60, 180, or 600 s) shown for emptied (blue) versus NRASQ61K bound (red) HLA-A*01:01/hβ2m. Data are mean % deuterium uptake ± SD from triplicate experiments. c Structure view of average % deuterium uptake at 600 s (back-exchange corrected) for empty and NRASQ61K bound HLA-A*01:01/hβ2m plotted onto PDB IDB 6MPP without or with atoms corresponding to NRASQ61K. Color ranges from deep blue (no deuterium uptake) to red (100% deuterium uptake). Black indicates regions where peptides were not obtained. Mean % deuterium uptakes at 600 s (back-exchange corrected) were resolved to individual peptide fragments and obtained from three independent samples.
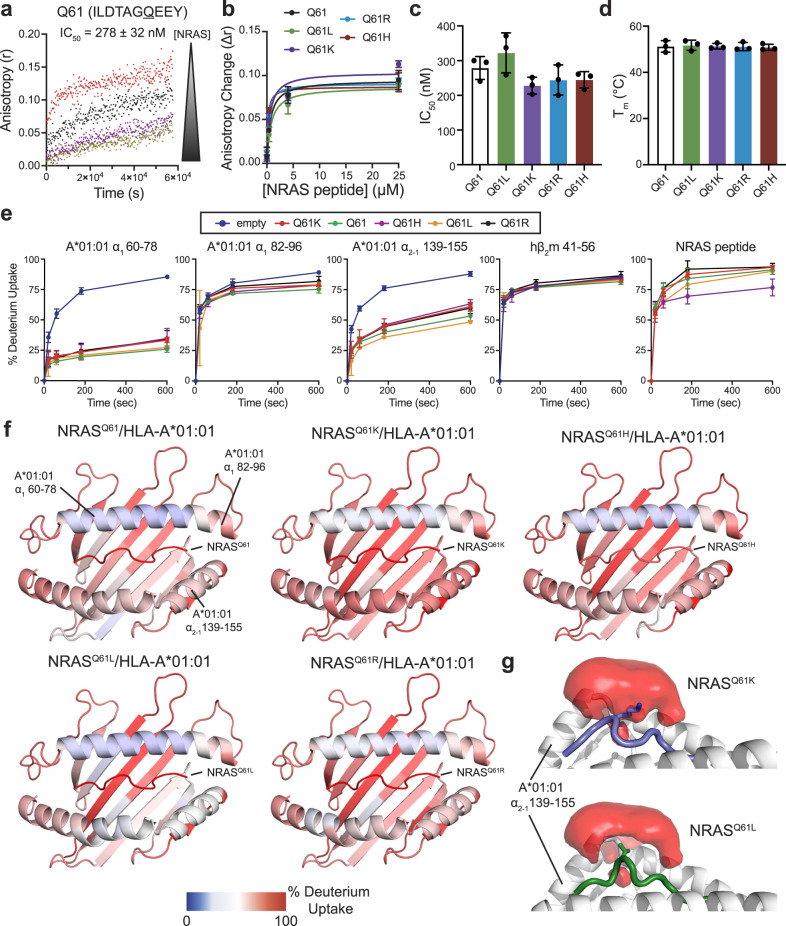
The Q61K mutation represents only a subset of cancer-associated RAS55-64 epitopes. Thus, we sought to compare the assembly of HLA-A*01:01 with alternative NRAS55-64 neoepitopes commonly found in tumors, such as Q61R, Q61L, and Q61H, relative to wild-type Q61 and Q61K20,70. Incubation of TAMRA-dye labeled NRASQ61K with unlabeled NRASQ61K/HLA-A*01:01/hβ2m complex and varying concentrations of unlabeled competitor (wild-type or residue 61 mutant NRAS55-64 peptide) revealed IC50 values between 225 and 329 nM, demonstrating mutation at residue 61 does not drastically alter the binding affinity of NRAS55-64 epitope with HLA-A*01:01 (Fig. 5a–c, Supplementary Fig. 10a–e), as supported by our NMR structure where the side chain for residue 61 does not form any interactions with groove residues (Fig. 3f–i). In agreement, DSF experiments revealed that complexes formed between HLA-A*01:01/hβ2m and wild-type or mutant NRAS55-64 peptides exhibited similar thermal stabilities in the 51 to 53 °C range (Fig. 5d, Supplementary Fig. 10f). Together, these data provide experimental evidence that HLA-A*01:01 can present a panel of naturally occurring Q61 neoepitopes of RAS55-64 with similar binding affinities.
Fig. 5. Affinity, stability, and conformational plasticity of cancer specific NRAS55-64 neoepitopes within the HLA-A*01:01 groove.
a Fluorescence anisotropy (r) of 25 nM TAMRA-NRASQ61K in the presence of 4 μM unlabeled NRASQ61K/HLA-A*01:01/hβ2m and varying concentrations of the specific unlabeled competitor NRAS Q61 peptide. [NRAS] shown in μM units are 0.0 (red), 0.25 (black), 4.0 (purple), 25 (yellow). The data were analyzed by global fitting using Dynafit 4 (http://www.biokin.com/dynafit). Estimated IC50 value for NRAS Q61 binding to HLA-A*01:01/hβ2m is noted. Data are mean ± SD for n = 3 independent experiments. b Comparison of anisotropy change (Δr) for different NRAS55-64 Q61 mutants as a function of competitor concentration extracted from competition fluorescence anisotropy experiments. Data are mean ± SD for n = 3 independent experiments. c Summary of fluorescence anisotropy measured IC50 values for different NRAS55-64 Q61 mutants competing for binding to NRASQ61K/HLA-A*01:01/hβ2m. Data are mean ± SD for n = 3 independent experiments. d Summary of DSF measured thermal stability values (Tm, °C) for different NRAS55-64 Q61 mutants in complex with HLA-A*01:01/hβ2m. Data are mean ± SD for n = 3 technical replicates. e Kinetic graphs of % deuterium uptake (back-exchange corrected) for different peptide fragments as a function of HDX time (0, 20, 60, 180, or 600 s) shown for emptied (blue) versus HLA-A*01:01/hβ2m bound to different NRAS Q61 mutant peptides. Data are mean % deuterium uptake ± SD from biological triplicates. f Structure view of average % deuterium uptakes for peptide fragments of HLA-A*01:01/hβ2m bound to different NRAS Q61 mutant peptides at 600 s (back-exchange corrected) plotted onto PDB IDB 6MPP. Color ranges from deep blue (no deuterium uptake) to red (100% deuterium uptake). Mean % deuterium uptakes at 600 s (back-exchange corrected) were resolved to individual peptide fragments and obtained from biological triplicates. g Average water occupancy maps across 3 × 1 μs MD simulations for NRASQ61K/HLA-A*01:01 and NRASQ61L/HLA-A*01:01 complexes generated using VolMap tool in VMD and visualized in PyMOL v2.5.2 with isosurface contour level 0.2.
We next examined whether Q61 mutations influence the conformational plasticity of the peptide/HLA-A*01:01/hβ2m complexes using our established HDX assay (Fig. 5, Supplementary Figs. 11 and 12). Regions corresponding to the HLA-A*01:01 C-terminal end of the α1 helix (residues 82–96) and hβ2m (residues 41–56) exhibited similar HDX rates for HLA-A*01:01 complexes prepared with different NRAS55-64 neoepitopes (Fig. 5e, Supplementary Fig. 8). In contrast, we observed differences in deuterium uptake for HLA-A*01:01 bound to different NRAS55-64 neoepitopes for specific regions of the HLA groove, including the α1 helix (residues 60–78) and the α2-1 helix (residues 139–155). In particular, the α2-1 helix of NRASQ61L/HLA-A*01:01 exhibited significantly reduced deuterium uptake (more protection) compared to NRASQ61H and NRASQ61R bound samples, while NRASQ61K/HLA-A*01:01 exhibited highest deuterium uptake among the peptide-loaded samples (Fig. 5e, f, Supplementary Fig. 13). Peptide fragments corresponding to the NRAS peptide showed similar deuterium uptake levels of approx. 90%, with the exception of NRASQ61H, which showed increased protection with % deuterium uptake levels of approx. 75% (Fig. 5e, f, Supplementary Fig. 8). To obtain a mechanistic basis for our observed differences in deuterium uptake for NRASQ61K versus Q61L in HDX experiments, we performed 3 independent 1 μs all-atom molecular dynamics (MD) simulations in explicit solvent for HLA-A*01:01 bound to NRASQ61K or Q61L peptides, and compared solvent occupancies near the HLA-A*01:01 α2-1 helix. Visualization of water occupancy maps revealed that, in contrast to Q61K, the hydrophobic side chain of Q61L was stably packed against the α2-1 helix, with reduced exposure to solvent molecules (Fig. 5g), in agreement with increased HDX protection levels of the α2-1 helix (Fig. 5f). Thus, our HDX data and MD-derived water occupancy maps reveal different solvent accessibility profiles for different NRAS Q61 variants, which underscore changes in both the average conformation and dynamics of captured NRAS55-64 neoepitopes within the HLA-A*01:01 groove.
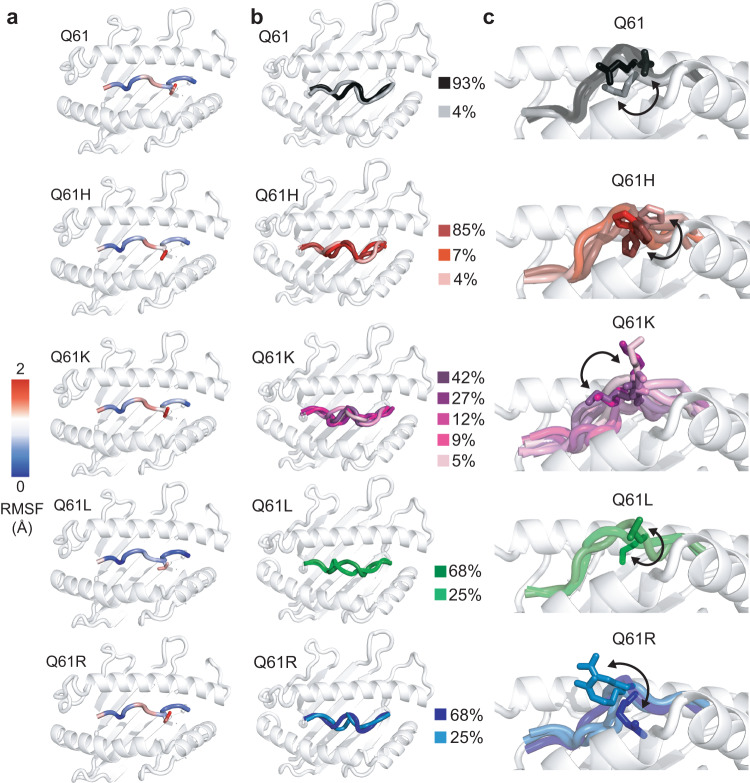
Finally, to examine how Q61 mutations influence the conformational landscape of NRAS55-64 peptide within the HLA-A*01:01 groove, which dictates recognition by TCRs or designed antibodies7,71, we complemented our MD simulations of HLA-A*01:01 bound to NRASQ61K or NRASQ61L with 3 × 1 μs MD trajectories of HLA-A*01:01 bound to either NRASQ61, NRASQ61H, or NRASQ61R (9 μs in total), all modeled by threading to our NMR structure of NRASQ61K/HLA-A*01:01. The major conformation of NRASQ61K obtained in MD simulations exhibited deviations from the NMR/docking-derived model, as would be expected for a dynamic peptide/protein complex (Supplementary Table 4). We computed average all heavy atom root mean square fluctuation (RMSF) values, which revealed differences in local backbone and side chain fluctuations for each peptide (Fig. 6a). In particular, the peptide backbone and side chain of Q61L were significantly more conformationally restricted than other mutants, while Q61K displayed the greatest mobility. We then characterized specific backbone and side chain motions contributing to the global conformational ensemble of each peptide. First, we computed order parameters (S2) for backbone φ (phi) and ψ (psi) dihedral angles, which quantify the degree of conformational restriction relative to an internal coordinate system. S2 values range from 0 (conformationally unrestricted) to 1 (rigid) (Supplementary Fig. 14). These analyses demonstrated that the backbone of Q61L is significantly more rigid relative to the other peptides, with a single trajectory showing increased mobility for the backbone of G60. Likewise, χ1 side chain dihedral angles determined across MD trajectories showed variations in side chain dynamics between different NRAS55-64 peptides (Supplementary Fig. 15). We performed single-linkage clustering, which allowed us to extract representative backbone conformations for each peptide (Fig. 6b). For each backbone cluster, we also performed a second round of clustering using all heavy atoms, generating backbone-specific side chain clusters (Fig. 6c). In agreement, we observed a reduction of conformational space sampled by the Q61L peptide, which can be described by only two major clusters of side chain conformations relative to a larger number of clusters for the other peptides (3, 3, 4, and 5 for Q61, Q61R, Q61H, and Q61K, respectively) (Fig. 6c). Taken together, these results reveal distinct conformational ensembles sampled by NRAS55-64 neoepitopes, which would differentially prime each peptide for specific interactions with TCRs. Specifically, our HDX experiments and MD simulations show that the Q61L neoepitope side chain is less solvent-exposed due to stable packing against the α2-1 helix, whereas the Q61K and Q61R side chains are oriented toward the TCR interface. While this could limit accessibility to TCRs, we speculate that the observed conformational rigidity of the Q61L peptide in the pMHC state could also reduce the entropy loss upon pMHC-I/TCR complex formation, leading to a more favorable binding free energy.
Fig. 6. MD simulations of NRAS55-64 neoepitope/HLA-A*01:01 complexes highlight differences in peptide backbone and side chain conformational landscapes.
a Root mean square fluctuation (RMSF, Å) of Cα atoms (for the peptide backbone, shown as colored cartoon) and heavy atoms (for residue 61, shown as sticks) averaged across 3 × 1 μs MD simulations. b Peptide backbone clusters generated using a backbone root mean squared deviation (RMSD, Å) cut-off of 0.5 Å. The percentage of frames corresponding to each cluster relative to the total number of frames across the three replicate trajectories (3000) is noted. c Peptide side chain clusters generated from the backbone clusters in (b) using a heavy atom RMSD cut-off of 1 Å. Arrows denote movement of the side chain for residue 61. In a–c the HLA-A*01:01 groove is shown in light gray cartoon for reference.
NRASQ61K binding across the HLA allelic landscape displays distinct features to TCRs
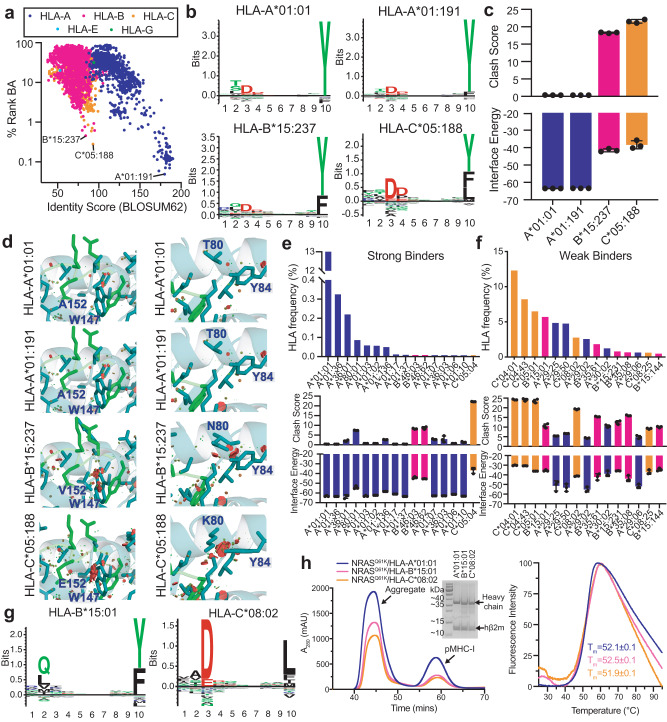
The HLA haplotypes of different patients influence their eligibility for pHLA-targeted therapies because only a subset of HLA allotypes can display neoepitope targets of interest16. To expand the set of known alleles that can display NRASQ61K, we first predicted binding against a total of 10,386 HLA-A-, B-, C-, E-, and G- alleles with netMHCpan-4.141. The top predicted binders for each classical class Ia were HLA-A*01:191, HLA-B*05:237, and HLA-C*05:188, which exhibited distinct antigen groove chemistries relative to HLA-A*01:01, based on a sequence alignment of groove residues (Fig. 7a, x-axis). Despite divergent groove amino acid compositions, the computationally derived peptide binding motifs of HLA-A*01:191, HLA-B*05:237, and HLA-C*05:188 resembled HLA-A*01:01, where Asp and Tyr are favored at residues 3 and 10 of the peptide, respectively (Fig. 7b). To examine how groove chemistry contributes to peptide stabilization from a structural perspective, we generated RosettaMHC models26,34 of NRASQ61K bound to different HLA alleles using our NRASQ61K/HLA-A*01:01 structure as a template, and compared structural and energetic features derived from each model. This approach allowed us to evaluate whether the NRASQ61K peptide can adopt a similar overall backbone conformation to that seen in our NRASQ61K/HLA-A*01:01 structure. NRASQ61K/HLA-A*01:191 models exhibited identical peptide/HLA interface energies, low clash scores, and formed a similar network of peptide/HLA contacts relative to the NRASQ61K/HLA-A*01:01 structure (Fig. 7c, d). In contrast, NRASQ61K/HLA-B*05:237 and NRASQ61K/HLA-C*05:188 models showed higher peptide/HLA interface energies and larger clash scores due to steric clashes introduced by amino acid polymorphisms in the MHC-I groove (Fig. 7c, d). Together, our in silico modeling suggests that NRASQ61K forms a similar structure in complexes formed with HLA-A*01:01 and HLA-A*01:191, but likely adopts a different conformation when bound to HLA-B*05:237 and HLA-C*05:18. In vitro refolding of HLA-A*01:191 with β2m in the presence of NRASQ61K peptide generates a good yield of NRASQ61K/HLA-A*01:191/β2m protein complexes, with an enhanced thermal stability, Tm, of 58.5 °C, relative to the HLA-A*01:01 complex (Supplementary Fig. 16). The experimental validation further confirmed our computational prediction that NRASQ61K is also a high-affinity peptide binder to HLA-A*01:191. Therefore, any peptide-focusing immunoreceptor of NRASQ61K in the context of HLA-A*01:01 should exhibit some level of cross-reactivity with NRASQ61K/HLA-A*01:191, while re-engineering of receptors is likely to be required for targeting the same neoepitope displayed by either HLA-B*05:237, or HLA-C*05:188.
Fig. 7. In silico and in vitro evaluation of the HLA binding repertoire of NRASQ61K.
a In silico prediction of NRASQ61K (ILDTAGKEEY) binding to 10,386 different HLA molecules with netMHCpan-4.1. The % rank binding affinity (BA) from netMHCpan-4.1 is compared to the BLOSUM62 identity score for all HLA alleles relative to HLA-A*01:01, calculated for HLA groove residues. The top predicted NRASQ61K binders for each HLA-A-, B-, and C- class are noted. b Decamer peptide binding motifs (generated by Seq2Logo) for the top predicted NRASQ61K binders for each HLA-A-, B-, and C- class. c Interface energy (REU) obtained from RosettaMHC models of NRASQ61K with the top predicted binders for each HLA-A-, B-, and C- classes and their MolProbity clash score. Our solved NMR structure (PDB ID 6MPP) was used as the template for modeling. Data are mean ± SD for n = 3 independent experiments. d Zoom view of RosettaMHC models of NRASQ61K with the top predicted binders for each HLA-A-, B-, and C- class. HLA residues within 3.5 Å of the peptide are shown. Van der Waals overlaps or steric clashes are represented with red/green discs. e and f Top: HLA frequency (%, obtained from the Allele Frequency Net Database for all populations) for the top predicted e strong and f weak NRASQ61K binders. Bottom: Interface energy (REU) obtained RosettaMHC models of NRASQ61K with the most frequently represented strong and weak binders in the global population and their MolProbity clash scores. PDB ID 6MPP was used as the template for modeling. Data are mean ± SD for n = 3 independent experiments. g Decamer peptide binding motifs (generated by Seq2Logo) for HLA-B*15:01 and HLA-C*08:02, which are predicted as weak binders of NRASQ61K. h Left: SEC traces of in vitro refoldings of NRASQ61K with HLA-A*01:01/hβ2m, HLA-B*15:01/hβ2m, and HLA-C*08:02/hβ2m. The SDS-PAGE gel of the purified pMHC-I complexes is also shown. Right: DSF experiments performed on the purified pMHC-I complexes with fitted thermal stability (Tm, °C) shown. Data are mean ± SD for n = 3 technical replicates.
To further identify and characterize HLA allotypes that can be targeted using NRASQ61-focused therapeutics, we sorted the 1,248 predicted NRASQ61K binders according to their observed global HLA frequency obtained from the HLA Allele Frequency Net Database72. HLA-A*01:01 was the only strong binder of NRASQ61K that is represented in >10% across different ethnic groups, with a global HLA frequency of approx. 13.2% (Fig. 7e). However, several allotypes represented across all populations, such as HLA-C*04:01 (12.3%), HLA-B*15:01 (5.7%), HLA-C*08:02 (2.8%), were predicted as weak binders by netMHCPan (Fig. 7f). Analysis of peptide/HLA interface energies and clash scores of structural models34,35 revealed similar trends in the predicted conformation of NRASQ61K across different HLA complexes (Fig. 7e, f). To validate our in silico predictions, we performed in vitro refolding of NRASQ61K with HLA-B*15:01 and HLA-C*08:02, which exhibited decamer peptide binding motifs that are consistent with the NRASQ61K sequence (Fig. 7g). In agreement, NRASQ61K assembled with both HLA-B*15:01 and HLA-C*08:02, as shown in SEC traces, albeit at lower efficiencies relative to HLA-A*01:01 (Fig. 7h, left). Purified HLA-B*15:01 and HLA-C*08:02 proteins with NRASQ61K exhibited moderate thermal stabilities in the 51 to 52 °C range, similar to the HLA-A*01:01 complex (Fig. 7h, right). Together, these results highlight the potential of NRASQ61K as a public neoepitope target for cancer immunotherapy. Our analysis further reveals sets of common HLA allotypes, which can present NRASQ61K in a similar overall conformation, suggesting that they can be targeted using a single therapeutic modality to maximize patient cohort coverage.
Discussion
Identification of RAS neoepitopes displayed on the surface of tumors and recognized by T cells or engineered therapeutics in an HLA-restricted manner has opened up new avenues for targeted cancer immunotherapy21,27,70,73–79. Efficient engineering of chimeric antigen receptors (CARs), TCRs, and directed antibodies with enhanced affinity and specificity requires detailed characterization of neoepitope/MHC-I complexes, together with receptor sequences (i.e. from TCR repertoire analysis80) and evaluation of cytotoxic tumor killing18,23. While landmark studies defining TCR repertoires and peptide/HLA mediated tumor killing have highlighted the potential of targeting NRASQ61K as a robust avenue for cancer therapy25,27,29, studies on the assembly, conformational plasticity, and structure of NRASQ61K/HLA complexes are currently lacking. Here, we provide mechanistic insights into how the decameric NRASQ61K peptide (ILDTAGKEEY), as well as related RAS55-64 derived neoepitopes, assemble with and stabilize the HLA-A*01:01/hβ2m complex. Furthermore, using NRASQ61K/HLA-A*01:01 as a model of a moderate affinity peptide that is refractory to X-ray crystallography, we outline an integrative pipeline for detailed characterization of the solution structure, conformational plasticity and HLA binding repertoire of clinically relevant neoepitope/HLA targets with desired features for cancer immunotherapy.
Our DSF, NMR, and HDX data support that NRASQ61K and hβ2m act in concert to stabilize and shape the conformation of the HLA-A*01:01 groove (Figs. 1a–f, 4b, c), in agreement with previous reports for analogous pMHC-I systems60,61. We find that association between NRASQ61K and empty HLA-A*01:01 requires several hours to reach saturation according to our NMR spectra, suggesting that HLA molecules undergo a slow transition to a peptide-receptive conformation (Fig. 1b, c, g–i). Alternative NRAS Q61 neoepitopes serving as clinical targets, such as NRAS Q61L, Q61R, and Q61H21,70, also assembled with HLA-A*01:01 with similar affinity, kinetics, and thermal stabilities, relative to NRASQ61K (Fig. 5a–d). Notably, HDX and MD simulations provide evidence that the precise amino acid at position 61 impacts the conformational ensembles sampled by both the NRAS55-64 peptide and the HLA-A*01:01 groove (Figs. 5e–g, 6). Our results further reveal that NRASQ61K can associate with common HLA alleles other than HLA-A*01:01, such as HLA-B*15:01 and HLA-C*08:02 (Fig. 7), thereby expanding the cohort of patients for which NRASQ61K-targeted immunotherapies can be applied. Like NRASQ61K/HLA-A*01:01, NRASQ61K/HLA-B*15:01 and NRASQ61K/HLA-C*08:02 complexes failed to form crystals for X-ray diffraction studies. Thus, future structure determination of these complexes using our integrative NMR-based approach provides an attractive avenue to validate our computational modeling results. One limitation of this study is that pMHC-I molecules were prepared lacking membrane association and glycosylation that are known to have important implications for peptide loading in vivo. Specifically, MHC-I glycosylation at the conserved Asn86 position influences interactions with molecular chaperones and other quality control molecules of the APP pathway, which mediate peptide repertoire optimization (Tapasin, TAPBPR, UGGT1), and could therefore influence cancer antigen selection and presentation81–84.
Our integrated approach highlights the powerful combination of sparse NMR data and integrative computational modeling using Rosetta65,85–87. This methodology will be particularly useful for cases in which pMHC-I and pMHC-II complexes fail to crystallize, or where in silico structural modeling35 is prohibited due to lacking of a structural template35, such as dynamic pMHC-I complexes, or non-canonical complexes displaying longer peptides up to 15 amino acids32,88,89. Our method also has the potential to be adapted and applied for structural characterization of a range of other peptide/proteins systems, for example PDZ-peptide and SH3-peptide interactions that play a role in cellular signaling, and trafficking90. To evaluate the performance of state-of-the-art structure prediction methods on a similar system, we compared our structure to an AlphaFold-Multimer91 derived model of the NRASQ61K/HLA-A*01:01 complex (Supplementary Figs. 17 and 18). We observed a Cα RMSD of 2.82 Å between the experimental and predicted NRASQ61K structures. While AlphaFold-Multimer correctly placed the NRASQ61K P2 and P10 anchoring residues within the HLA-A*01:01 groove, the predicted model deviated significantly from our solved structure at residues 4–7, which includes the mutation site at position 61 (Supplementary Fig. 17). The incorrect placement of these key side chains in the predicted model hinders their use to guide therapeutics development, or vaccine design efforts, and underscores a need for experimental validation of computationally derived models, as also shown in our recent study of a pMHC-targeting chimeric antigen receptor92.
Previous studies have focused on HLAs presenting clinically relevant RAS5-14 neoepitopes, which contain driver mutations at positions 12 and 13 that are most commonly represented in HRAS and KRAS21,70. For example, the structures of KRAS5-14 G12V bound to HLA-A*02:0122 and KRAS5-14 G12D bound to HLA-C*08:0293 have provided valuable information towards development of engineered T cells and antibodies targeting pMHC-I surface markers. Our study addresses the RAS55-64 epitopic peptide, which encompasses Q61 mutants most commonly represented in HRAS and NRAS21,70. The overall structure of the NRASQ61K/HLA-A*01:01/hβ2m complex reveals a different surface chemistry for NRASQ61K where G60, K61 and E63 side chains are primed to engage cognate TCRs (Fig. 3h, i). HDX experiments and MD simulations highlight differences in the conformational landscape of residue 61 for Q61, Q61K, and Q61R (Figs. 5 and 6). These findings could provide a basis for modeling previously identified TCRs, such as N17.3.2 and N17.5,shown to specifically recognize NRASQ61K/HLA-A*01:01 complexes27. Our results also point to a strategy for designing neoantigen-selective receptors that avoid interactions with conformational ensembles found in the wild-type protein, while optimizing favorable interactions with the neoepitope pMHC. Specifically, we find that Q61K and Q61R have basic side chains oriented toward the TCR/scFv interface, which are conformationally distinct from wild-type Q61 as well as mutants Q61H and Q6L. These results have further implications for recognition of the pMHC-I surfaces by TCRs and therapeutic antibodies7,71. The information gleaned from our structure and MD simulations can be leveraged for modeling TCR/pMHC-I and antibody/pMHC-I complexes, or to engineer synthetic receptors with enhanced affinity and specificity. Taken together, our results provide a view of pMHC-I complexes as dynamic ensembles, providing a toehold for understanding antigen immunogenicity and cross-reactivity with TCRs.
Methods
Protein expression and purification
DNA encoding the luminal domain of the class I MHC-I heavy chain HLA-A*01:01, HLA-B*15:01, HLA-C*08:02, and the light chain human β2m (hβ2m) was graciously provided by the NIH Tetramer Core Facility. For the HLA-A*01:01 plasmid, a stop codon was introduced by PCR before the BirA tag due to known interference with NMR and X-ray crystallography experiments. BirAStop Forward Primer: 5′-ctg tct tcc cag ccc tga tcc ctg cat cat att - 3′; BirAStop Reverse Primer: 5′-aat atg atg cag gga tca ggg ctg gga aga cag - 3′. DNA sequences corresponding to NRASQ61K peptide (ILDTAGKEEY) were prepared as fusion constructs with an N-terminal a His6 tag, immunoglobulin-binding domain of streptococcal protein G (GB1) solubility tag, and enterokinase cleavage site (DDDDK) subcloned in pET22b vector49. Plasmids were transformed into Escherichia coli BL21(DE3) (Novagen) and then expressed in LB-broth (natural isotopic abundance samples) or 1× M9 minimal media (for isotopically labeled samples) at 37 °C. MHC-I heavy and light chain molecules were expressed separately as inclusion bodies, solubilized in 6 M guanidine-HCl/0.1 mM dithiothreitol (DTT), mixed together with antigenic peptide for refolding and then purified. NRASQ61K peptide was prepared from the His6-GB1-NRASQ61K fusion construct by purification with Ni-NTA Agarose affinity chromatography followed by cleavage of NRASQ61K from His6-GB1 with enterokinase followed by a final round of purification using a Aeris PEPTIDE XB-C18 column (3.6 μm, 150 × 4.6 mm, Phenomenex), with cleavage and purity validated by mass spectrometry. For in vitro refolding, 10 mg of peptide and 200 mg mixtures of heavy chain: light chain at a 1:3 molar ratio were slowly diluted over 24 h into refolding buffer (0.4 M Arginine-HCl, 2 mM EDTA, 4.9 mM reduced L-glutathione, 0.57 mM oxidized L-glutathione, 100 mM Tris pH 8.0) at 4 °C while stirring. Refolding proceeded for four days at 4 °C without stirring. Purification of pMHC-I complexes was performed by size exclusion chromatography (SEC) with a HiLoad 16/600 Superdex 75 pg column at 1 mL/min with running buffer (150 mM NaCl, 25 mM Tris pH 8). For HLA-A*01:01 complexes used in crystallization trails a second round of purification occurred via anion exchange chromatography with a mono Q 5/50 GL column at 1 mL/min using a 40 min 0–100% gradient of buffer A (50 mM NaCl, 25 mM Tris pH 8) and buffer B (1 M NaCl, 25 mM Tris pH 8). Proteins were extensively dialyzed into 150 mM NaCl, 25 mM Tris pH 8 prior to crystallography screens.
Generation of empty HLA-A*01:01/hβ2m complexes
HLA-A*01:01/hβ2m complexes were emptied of bound NRASQ61K peptide by brief exposure to pH 12.5 followed by SEC as previously described54. Briefly, 60 μL of 400 μM NRASQ61K/HLA-A*01:01/hβ2m (purified following in vitro refolding) was incubated for 30 min at 4°C. 10 μL of 0.2 M sodium phosphate pH 12.5 was then added to the sample at 4°C and incubated for 10 min. Samples were then spun at 13,000 r.p.m. for 10 min at 4°C. Samples were immediately loaded onto a Superdex 200 Increase 10/300 GL column at 0.5 mL/min with running buffer (50 mM NaCl, 20 mM sodium phosphate pH 7.2) for purification at 25 °C.
Liquid chromatography–mass spectrometry (LC–MS)
Validation of emptied and NRASQ61K bound HLA-A*01:01 samples (Supplementary Fig. 4) was achieved by LC–MS. LC–MS was carried out with passage through a Higgins PROTO300 C4 column (5 μm, 100 mm × 2.1 mm) followed by electron ion spray (ESI) mass spectroscopy performed on a Thermo Finnigan LC–MS/MS (LTQ). Analysis and deconvolution of LC–MS data were performed using MagTran.
Sample preparation for NMR
For all NMR samples, protein expression was achieved by induction with 1 mM isopropyl-D-thiogalactoside (IPTG) at an OD600 of 0.6 followed by cell growth at 37 °C for 4 h at 200 r.p.m. Purified isotopically labeled HLA-A*01:01 inclusion bodies from 1× M9 preparations in D2O were refolded with NRASQ61K and hβ2m with isotopic labeling schemes as noted below. Following refolding and purification pMHC-I samples were exhaustively buffer exchanged into NMR buffer (50 mM NaCl, 20 mM sodium phosphate pH 7.2, 0.01% (v/v) NaN3 and 1U Roche protease inhibitor cocktail in 90% H2O/10% D2O). Isotope labeling schemes for samples 01 through sample 04 are provided in Supplementary Table 1 and below:
Sample S1 In sample S1, HLA-A*01:01 is Ile 13CH3 for δ1 only; Leu 13CH3/12C2H3; Val 13CH3/12C2H3 labeled in an otherwise 2H, 13C, 15N background (herein referred to as ILV* labeled) where both NRASQ61K and hβ2m are at natural isotopic abundance. Val and Leu side chains exhibit linearized 13C spin systems that allow efficient magnetization transfer for larger proteins in solution94. Sample S1 is used for 3D experiments that permit both backbone assignment (3D HNCA, 3D HN(CA)CB, 3D HNCO) and methyl assignment (3D HMCM[CG]CBCA)94. The U-[15N, 13C, 2H] (Ile 13CH3 for δ1 only; Leu 13CH3/12C2H3; Val 13CH3/12C2H3) ILV* labeled sample was prepared in 1× M9 minimal media in D2O supplemented with 1 g/L 15NH4Cl and 3 g/L 13C, 2H glucose (Sigma #552151). Methyl labeling was achieved for Ile and Leu/Val, respectively, with addition of 70 mg/L 2-ketobutyric acid-13C4,3,3-2H2 (Sigma #607541) and 120 mg/L 2-keto-3-(methyl-d3)-butyric acid-1,2,3,4-13C4, 3-2H (Sigma #637858) added 1 h prior to induction. NRASQ61K and hβ2m were natural abundance. Sample S1 is also referred to as ILV* labeled HLA-A*01:01.
Sample S2 In sample S2, Ala, Ile, Leu, and Val methyl groups of HLA-A*01:01 are labeled in an otherwise 2H, 12C, 15N background (herein referred to as AILV labeled) where both NRASQ61K and hβ2m are at natural isotopic abundance. Sample S2 enables measurement and assignment of NOE cross-peaks with high sensitivity and resolution through the collection of methyl-methyl, amide-methyl, and amide-amide 3D SOFAST NOESY experiments, in addition to conventional filtered/edited NOESY methods63,64. The U-[2H, 12C, 15N] Ala 13Cβ; Ile 13Cδ1; Leu 13Cδ1/13Cδ2; Val 13Cγ1/13Cγ2 labeled sample was prepared in 1× M9 minimal media in D2O supplemented with 1 g/L 15NH4Cl, 3 g/L 12C, 2H glucose (Sigma #552003) and 0.2 g/L 2H,15N ISOGRO (Sigma #608300). To achieve methyl labeling for Ile, Leu/Val, and Ala methyls, respectively, 70 mg/L 2-ketobutyric acid-4-13C,3,3-2H2 (Sigma #589276) and 120 mg/L 2-keto-(3-methyl-13C)-butryic-4-13C,3-2H acid (Sigma #589063) were added 1 h prior to induction, while 100 mg/L L-alanine-3-13C,2-2H (Sigma #740055) was added 30 min prior to induction. NRASQ61K and hβ2m were natural abundance. Sample S2 is also referred to as AILV labeled HLA-A*01:01.
Sample S3 In sample S3, HLA-A*01:01 is labeled with AILV methyls in addition to Phe and Tyr aromatic side chains in an otherwise 2H, 12C, 15N background (herein referred to as FYAILV labeled)63 where hβ2m is U-[2H] and NRASQ61K peptide is U-[15N] labeled. Sample S3 allows for unambiguous assignment of intermolecular NOEs between NRASQ61K and HLA-A*01:01 without interference of signals arising from HLA contacts with hβ2m. The U-[2H, 12C, 15N] Ala 13Cβ; Ile 13Cδ1; Leu 13Cδ1/13Cδ2; Val 13Cγ1/13Cγ2; Phe 13C, 15N; Tyr 13C, 15N labeled sample was prepared in 1× M9 minimal media in D2O supplemented with 1 g/L 15NH4Cl, 3 g/L 12C, 2H glucose (Sigma #552003) and 0.2 g/L 2H,15N ISOGRO (Sigma #608300). To achieve methyl labeling for Ile, Leu/Val, and Ala methyls, respectively, 70 mg/L 2-ketobutyric acid-4-13C,3,3-2H2 (Sigma #589276) and 120 mg/L 2-keto-(3-methyl-13C)-butryic-4-13C,3-2H acid (Sigma #589063) were added 1 h prior to induction, while 100 mg/L L-alanine-3-13C,2-2H (Sigma #740055) was added 30 minutes prior to induction. To achieve aromatic labeling for Phe and Tyr, 50 mg/L U-[13C,15N]-Phe (Sigma #608017) and 50 mg/L U-[13C,15N]-Tyr (Sigma #607991) were added 1 h prior to induction. NRASQ61K was U-[15N] while hβ2m was U-[2H, 14N, 12C]. Sample S3 is also referred to as AILVFY labeled HLA-A*01:01 with 15N NRASQ61K.
NMR spectroscopy
For NMR chemical shift assignments of HLA-A*01:01, we utilized a multipronged approach previously applied to the β-chain-labeled B4.2.3 TCR43. Specifically, we acquired a suite of TROSY-based triple-resonance measurements (3D HNCA, 3D HN(CA)CB, 3D HNCO) and a suite of 3D SOFAST NOESY measurements (Supplementary Table 1) recorded at 800 MHz at 25 °C63. 3D HNCA, 3D HN(CA)CB, 3D HNCO experiments had acquisition times of 30 ms (15N), 20 ms (13CO) and 10/5 ms (13Cα /13Cβ) in the indirect dimension. Backbone amide assignments were validated by acquiring amide-amide NOES using 3D HN-NHN and 3D N-NHN SOFAST NOESY experiments. AILV methyl resonances were assigned using a 3D HMCM[CG]CBCA methyl out-and-back experiment94 recorded on 0.5 mM pMHC-I samples at 800 MHz at 25 °C. AILV methyl assignments were validated and stereo-specifically disambiguated by acquiring methyl-methyl NOEs with 3D HM-CMHM and 3D CM-CMHM NOESY experiments. Backbone amide and AILV methyl assignments were cross-validated by acquiring methyl-to-amide NOEs using 3D HN-CMHM and CM-NHN experiments. Phe and Tyr aromatic side chains were assigned using CAro-CMHM, CAro-NHN, HM-CAroHAro, and HNHAro-NHN 3D NOESY experiments63. Free state resonance assignments of NRASQ61K were obtained from 2D 1H-1H NOESY and 1H-1H TOCSY spectra of 1 mM NRASQ61K (natural isotopic abundance). Assignments of the bound state were obtained with HNHAro-CMHM and HNHAro-NHN 3D NOESY experiments using Sample S3. Intramolecular (HLA-A*01:01 to HLA-A*01:01) and intermolecular (HLA-A*01:01 to NRASQ61K) nuclear Overhauser effects (NOEs) were using experiments summarized in Supplementary Table 1 and Supplementary Table 2.
For NMR experiments to determine conformational changes in the HLA groove in the empty and peptide-loaded states, 2D 1H-13C SOFAST HMQC spectra of 50 μM emptied AILV labeled HLA-A*01:01/natural abundance hβ2m in the absence or presence of 50 μM NRASQ61K peptide were recorded at a 1H field of 800 MHz at 25 °C. In all samples 150 μM of excess natural isotopic abundance hβ2m added for stabilization of the complex. Chemical shift deviations (CSP, ppm) were determined using the equation: ΔδCH3 = [1/2(ΔδH2 + ΔδC2/4)]1/2 for AILV methyls. The change in peak intensity was determined by calculating the ratio of Iempty/Ipeptide bound from peak intensities extracted from NMRFAM-SPARKY. V34, L179, and A140 methyl groups were not used for either CSP or intensity analysis due to complete line broadening in the empty state (signal-to-noise <10).
For assembly kinetics experiments, 2D 1H-13C SOFAST HMQC experiments were recorded for 100 μM 15N labeled NRASQ61K in the free state. Next, 2D 1H-13C SOFAST HMQC spectra were acquired in 1 h time increments following the addition of 300 μM emptied (natural abundance) HLA-A*01:01/hβ2m at a 1H field of 800 MHz at 25 °C. At each time point, the changes in amide signal of free NRASQ61K resonance relative to the sample without HLA-A*01:01 were fit with a single-phase exponential decay in GraphPad Prism to determine the half-life (t1/2).
Relaxation delays (d1) for non-SOFAST experiments were set to 1.2 to 1.5 s, and 0.2 s in SOFAST experiments. Data were processed using nmrPipe95 and analyzed using NMRFAM-SPARKY96.
Rosetta FlexPepDock ab-initio protocol
Create the initial structure of the complex
Initial starting structure of the unbound HLA-A*01:01/hβ2m was obtained from the atomic coordinates of a previously solved HLA-A*01:01/hβ2m presenting a decameric peptide with the atomic coordinates corresponding to the peptide removed (PDB ID 6AT9). The initial NRASQ61K backbone conformation of the peptide was assumed to be extended and was created using the BuildPeptide Rosetta utility.
BuildPeptide.default.linuxgccrelease–database ~/rosetta/Rosetta/main/database -in:file:fasta nrasq61k.fasta -out:file:o nrasq61k_extended.pdb
The initial NRASQ61K peptide was then positioned manually near (~2.5 Å) the HLA-A*01:01 groove with an orientation of N to C of ILDTAGKEEY based on the known P2 and P8 anchor positions for the HLA-A*01:01 groove using PyMOL. The orientation of the peptide was derived from our NOE measurements. Finally, the PDB file was created in agreement with the specifications in the original FlexPepDock protocol (the peptide coordinates should be the last model)65.
Initial files and structures
The unbound HLA-A*01:01/hβ2m and HLA-A*01:01/hβ2m + NRASQ61K structures were “relaxed” and “prepacked”, respectively. The lowest energy initial structures were selected for restraint guided docking.
Command line for “Relax”:
/path/to/rosetta/binaries/relax.linuxgccrelease -relax:constrain_relax_to_start_coords -relax:coord_constrain_sidechains -relax:ramp_constraints false -s your_structure.pdb
Command lines for “Prepack”:
/path/to/rosetta/binaries/FlexPepDocking.linuxgccrelease -s "best_start_relaxed.pdb" -ex1 -ex2aro -use_input_sc -unboundrot "best_native_relaxed.pdb" -flexpep_prepack -nstruct 1
FlexPepdock protocol
Production runsqsub -pe orte 300 production.jobContent of production.job file### JOB TEMPLATE FOR BAKER CLUSTER## Job name#$ -N FlexPepDock_log_ab_init## User current working directory#$ -cwd## Don’t combine input and output#$ -j y#echo ""echo $PATHecho ""echo "Executing on: $HOSTNAME"echo "Number of hosts operating on: $NHOSTS"echo "Number of queued slots in use for parallel job: $NSLOTS"# where $NSLOTS is as submitted to pe command to the OpenGrid SGE schedulerecho ""echo "Running on $NSLOTS cpus …"##Current Rosetta binariesEXE = /path/to/rosetta/binaries/FlexPepDocking.mpi.linuxgccreleaseCMDLINE = "@flags/flags_A01_Nras_ab"echo $EXEecho $CMDLINEmpiexec -n $NSLOTS $EXE $CMDLINEexitFlag file “flags_A01_Nras_ab”:#io flags:-s pdbs/start.pdb-native pdbs/native.pdb-out:file:silent_struct_type binary-out:file:silent decoys.abinit.silent-scorefile score.flexpepdock.abinit.sc-out:prefix ab_#-out:suffix _preopt#number of structures to produce-nstruct 50000#flexpepdock flags:-lowres_abinitio-pep_refine#packing flags-ex1-ex2aro-use_input_sc-unboundrot pdbs/unbound.pdb#extra side chain rotamers-ex1aro-ex2-ex3-ex4#fragments for peptide-frag3 frags/frags.3mers.offset-frag5 frags/frags.5mers.offset-frag9 frags/frags.9mers.offset#NOE constraints-constraints:cst_file constraints/contacts_A01.cst-constraints:cst_weight 10-constraints:cst_fa_file constraints/contacts_A01.cst-constraints:cst_fa_weight 10#expert flags#start the protocol with the peptide in extended conformation (neglect original peptide conformation; extend from the anchor residue)-extend_peptide
Clustering top rank representatives
/path/to/rosetta/binaries/cluster.linuxgccrelease -in:file:silent decoys.abinit.silent -in:file:silent_struct_type binary -cluster:radius 2.3 -in::file::fullatom -in:file:tags tags.of.the.top.500.structures -silent_read_through_errors -cluster:limit_cluster_size 10 -cluster:limit_clusters 5
Fluorescence anisotropy
Fluorescence anisotropy (r) experiments were performed using a NRASQ61K peptide labeled with TAMRA-dye (ILDTAGKTAMRAEEY, herein called TAMRA-NRASQ61K) (Biopeptek Inc, Malvern, USA). TAMRA association with K61 was modeled to not disrupt interaction with the HLA-A*01:01 groove because K61 is solvent-exposed. For competition experiments, a mixture of purified 4 μM NRASQ61K/HLA-A*01:01/hβ2m complex and 25 nM TAMRA-NRASQ61K was treated with graded concentrations of competitor NRAS peptide (either NRASQ61, NRASQ61K, NRASQ61L, NRASQ61R, or NRASQ61H) with concentrations of 0, 0.25, 4 or 25 μM. NRAS peptides were prepared by chemical synthesis (GenScript, Piscataway, USA). Each experiment was performed at 25 °C in a volume of 100 μL and loaded onto a black 96-well polystyrene assay plate (Costar #3915). Data was recorded on a SpectraMax iD5 plate reader with an excitation filter of λex = 531 nm and an emission filter of λem = 595 nm with an PMT gain set to high, an integration time of 1200 msec and read height 0.5 mm. Each experiment was performed in triplicate (n = 3) and is representative of at least two independent experiments. Experimental values were subtracted from background r values obtained from incubation of TAMRA-NRASQ61K alone. All samples were prepared in matched buffer (50 mM NaCl, 20 mM sodium phosphate pH 7.2, 0.05% (v/v) Tween-20). IC50 values were obtain by fitting to a simple single-site (one phase association) model with DynaFit (http://www.biokin.com/dynafit).
Differential scanning fluorimetry (DSF)
DSF experiments were performed in triplicate (n = 3) on an Applied Biosystems 7900HT Fast Real-Time PCR System with excitation and emission wavelengths set to 470 nm and 569 nm with proteins in buffer of 50 mM NaCl, 20 mM sodium phosphate pH 7.2. Experiments were conducted in MicroAmp Fast 384-well plates with 20 μL total volume containing final concentrations of 7 μM protein and 10 × SYPRO orange dye (Thermo Fisher). Temperature was incrementally increased at a scan rate of 1 °C/min between 25 °C and 95 °C. Data analysis and fitting with a Boltzman sigmoidal curve were performed in GraphPad Prism v9. For experiments with emptied HLA-A*01:01, data were recorded for 7 μM emptied HLA-A*01:01/hβ2m as well as 7 μM emptied HLA-A*01:01/hβ2m incubated for different amounts of time with 70 μM NRASQ61K peptide. The % of empty HLA-A*01:01 was calculated by determining the ratio of the fluorescence intensity of each time point relative to the maximum fluorescence intensity for empty HLA-A*01:01 (no NRASQ61K peptide added) at 25 °C.
Molecular dynamics simulations
All-atom molecular dynamics (MD) simulations in explicit solvent were carried out as previously described in GROMACS version 2020.4 using an AMBER99SB-ILDN protein forcefield and TIP3P water model49. LINCS and SETTLE constraint algorithms were used to constrain peptide/protein and water molecules, respectively. An integration time step of 2 fs was used with coordinates output every 10 ps. Short range interactions were treated with a Verlet cut-off scheme with 10 Å electrostatic and van der Walls cutoffs and long-range electrostatics were treated with the PME method with a grid spacing of 1.2 Å and cubic interpolation. Periodic cubic boundaries were used. Simulations were carried out in cubic simulation boxes, and periodic boundary conditions were used in all three spatial dimensions. The thermodynamic ensemble was NPT with temperature kept constant at 300 K by a velocity rescaling thermostat with a stochastic term97 with 0.1 ps time constant and pressure kept constant at 1 bar pressure using an isotropic Berendsen barostat with 0.5 ps time constant and 4.5 × 10−5 bar−1 isothermal compressibility. The NRASQ61K/HLA-A*01:01/hβ2m structure (PDB ID 6MPP) served as the primary input, with the first state used in modeling. For modeling of other NRAS55-64 Q61 mutations, Maestro (Schrödinger) was used to generate mutations in the peptide sequence by replacing side chains without any change to the backbone (energy minimization was performed as stated below during the MD simulation). Each pMHC-I system was solvated to overall neutral charge and contained Na+ and Cl− ions to yield physiological concentration of 0.15 M. Following 500 steps of steepest-descent energy minimization, initial velocities were generated at 65 K with linear heating up to 300 K over 2 ns. Three independent trajectories for each pMHC-I complex were acquired for 1 μs using the Children’s Hospital Of Philadelphia Respublica computational cluster. Analysis was performed using GROMACS and the Visual Molecular Dynamics (VMD) package98. Visualizations were produced with PyMOL v2.5.2 and gnuplot v5.2.8.
For analysis, periodic boundary conditions were removed and 1000 equally-spaced frames from each trajectory (3000 frames in total for three independent replicates) were extracted in using gmx trjconv. Phi/psi/chi angles and dihedral order parameters were calculated using gmx chi. Peptide per-atom RMSF was calculated for each individual replicate and averaged across replicates using gmx rmsf. Backbone clusters were generated using gmx cluster (single-linkage, RMSD cut-off) on backbone atoms (no oxygen) and an RMSD cut-off of 0.5 Å. Frames corresponding to each cluster were extracted using gmx extract-cluster. Side chain clusters were generated using gmx cluster on protein heavy atoms with an RMSD cut-off of 1.0 Å. Water volume maps were generated in VMD using the VolMap tool, selecting all water atoms within 5 Å of HLA-A*01:01 residues 150-155 and calculating average occupancy with a 1.0 Å resolution and a 1.0 atom size cut-off, and were visualized in PyMOL using isosurface with a contour level of 0.2.
Hydrogen-deuterium exchange (HDX)
All HDX-MS experiments were designed to follow guidelines recommended by Masson et al.68 30 μM stock pMHC-I proteins were dialyzed into HDX Buffer 1 (50 mM NaCl, 20 mM sodium phosphate pH 6.5 in H2O). Emptied MHC-I was prepared using the method outlined in the section “Generation of empty HLA-A*01:01/hβ2m complexes.” For reference experiments (i.e., all H or no HDX), 5 μL of 30 μM emptied MHC-I or in vitro refolded pMHC-I was added to 20 μL HDX Buffer 1 and mixed. Next, 25 μL of HDX Quench Buffer (50 mM NaCl, 1 M TCEP (tris(2-carboxyethyl)phosphine), 20 mM sodium phosphate pH 2.35 in H2O) was added and the sample was mixed. The 50 μL samples were immediately injected for LC–MS/MS, which combines pepsin digestion, LC separation using a C8 5 μm column (TARGA, Higgins Analytical, TP-M501-C085), and MS/MS measurement on a Q Exactive Orbitrap Mass Spectrometer (Thermo Fisher Scientific). Following LC–MS/MS experiments for all H samples, peptide fragments corresponding to HLA-A*01:01 (96% sequence coverage), hβ2m (95% sequence coverage), and various NRAS peptides (100% sequence coverage) were identified using Thermo Proteome Discoverer v2.4. For HDX reactions, 5 μL of 30 μM pMHC-I was added to HDX Buffer 2 (50 mM NaCl, 20 mM sodium phosphate pD 6.5 in D2O), mixed, and incubated for 20, 60, 180, or 600 sec, followed by the addition of 25 μL of HDX Quench Buffer. Dfrac (fraction D/H) in the final HDX reactions was 0.8. The 50 μL samples were immediately injected into the LC–MS and underwent pepsin digestion, LC separation using a C8 5 μm column, and MS measurement. HDX data points were corrected for back-exchange by performing an additional experiment (i.e., all D) where peptide-free or peptide-loaded samples were heated to 35 °C and 46 °C to induce partial unfolded (5–10% unfolded) for 15 min, respectively, followed by HDX data collection. HDX data points were corrected for back-exchange using Eq. (1)68:
| 1 |
where mt = peptide centroid mass at a given time point, m0% is the peptide centroid mass for the non-deuterated peptide obtained from the all H sample, and m100% is peptide centroid mass for the peptide maximally obtained from the all D sample. HDX reactions were performed at 25 °C followed by pepsin digestion and MS data collection at 0 °C. ExMS2 software was used to identify and analyze deuterated peptides99. Percent deuterium uptakes (back-exchange corrected) were resolved to individual peptide fragments and obtained in biological triplicates (three independent in vitro refolding for each protein). Mean % deuterium uptakes for peptide fragments at 600 s were calculated based on triplicate measurements and averaged to each amino acid based on the start and end position and the length of each peptide. The kinetic plots and the B factor for the structure plot were generated by python3 and PyMOL. The Brown-Forsythe and Welch version of one-way ANOVA tests was performed on data corresponding to three biological triplicates using GraphPad Prism to determine statistical significance.
HLA binding repertoire analysis
netMHCpan-4.141 was used to predict binding of NRASQ61K to a total of 10,386 HLA-A-, B, C, E, and G- alleles. A list of strong and weak binders was obtained together with their % rank binding affinity (BA) from the netMHCpan-4.1 output. BLOSUM62 identity scores100 relative to HLA-A*01:01 for all 10,386 alleles were generated from a Clustal Omega v1.2.4 alignment of residues representing a pseudo MHC groove (MHC_pseudo.dat file located in $netMHCpan-4.1/data). Decamer Kullback–Leibler sequence (peptide motif) logos were generated by Seq2Logo101. Code used for structural modeling35 is available on Zenodo35. pMHC-I models26,34 of the complex between NRASQ61K and all strong or weak binding HLA alleles were generated from the NRASQ61K/HLA-A*01:01 structure template (PDB ID 6MPP) with the following run.sh script, which runs RosettaMHC and then outputs the interface energy (REU) between the peptide and HLA groove: python3 main.py -nstruct 3 –idealize_relax -relax_after_threading -template_pdb 6MPP.pdb -mhcs mhc_list -peptides pep_list -mhc_chain A -peptide_chain D -pep_start_index 181 -interface_cutpoint 180 -out_file nras_HLA_interface_energies.csv Clash scores were determined with MolProbity. Visualization of clashes in the resulting structures was achieved in PyMOL v2.4.2 (Schrödinger, Inc.) using show_bumps.py (via Thomas Holder - https://pymolwiki.org/index.php/Show_bumps). HLA frequencies were obtained from the HLA Frequency Net Database72 using the Estimation of Global Allele Frequencies tool for A, B, and C loci with options set to “all” except for Sample Size > 5000.
AlphaFold-Multimer modeling
An Alphafold-Multimer model for NRAS55-64Q61K peptide bound to HLA-A*01:01 was generated using the Google Colab implementation of Alphafold 2.1.0 in multimer mode (https://colab.research.google.com/github/deepmind/alphafold/blob/main/notebooks/AlphaFold.ipynb). Amino acid sequences were taken from PDB structure 6MPP, with 3 glycine residues added to each end of the peptide to satisfy the 16 amino acid size constraint.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors thank Drs. A. Rao, S. Tripathi, and D. Haussler (University of California, Santa Cruz), M. Yarmarkovich, S. Gupta, and J. Maris (Children’s Hospital of Philadelphia). This research was supported by NIAID (5R01AI143997), NIDDK (3U01DK112217) and NIGMS (5R35GM125034) grants to N.G.S. The authors acknowledge the Johnson Research Foundation and Dr. L. Mayne (University of Pennsylvania) for assistance with HDX instrumentation. This work was delivered as part of the NexTGen team supported by the Cancer Grand Challenges partnership funded by Cancer Research UK (CGCATF-2021/100002) and the National Cancer Institute (CA278687-01) and the Mark Foundation.
Author contributions
N.G.S., A.C.M., S.E.G., and D.F-S. designed research. A.C.M., D.F-S., J.S.T., and M.C.Y. prepared recombinant protein samples. A.C.M. and D.F-S. performed NMR experiments, resonance assignments, and data analysis. D.F-S. performed Rosetta flexible docking simulations and solved the NRASQ61K/HLA-A*01:01 complex structure. A.C.M. and J.S.T. recorded DSF, fluorescence anisotropy experiments. A.C.M. performed in silico binding repertoire analysis, molecular modeling, and analyzed data. Y.S. performed HDX-MS experiments and carried out revisions of the manuscript. S.E.G. carried out MD simulations, assisted with HDX data analysis, and prepared the AlphaFold-Multimer model. A.C.M., Y.S., S.E.G., and N.G.S. wrote the paper with feedback from all authors. N.G.S. acquired funding and supervised the project.
Peer review
Peer review information
Nature Communications thanks Lars Schäfer, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The atomic coordinates for the NRASQ61K/HLA-A*01:01/hβ2m structure have been deposited in the Protein Data Bank with PDB ID 6MPP. Other crystal structures used in this study include 6AT9, 3BO8, and 4NQV. NMR chemical shift assignments of HLA-A*01:01 heavy chain have been deposited into the Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu) under accession number 27632 [doi:10.13018/BMR27632]. The HDX-MS data have been deposited to the ProteomeXchange Consortium via the PRIDE102 partner repository with the dataset identifier PXD044838. The protein sequences and sequence coverage maps for HDX-MS can be accessed from Figshare [10.6084/m9.figshare.24417373]. The kinetics plots for each peptide fragment of the HDX-MS biological triplicates can be accessed from Figshare for peptide-free [10.6084/m9.figshare.24415921], NRASQ61K[10.6084/m9.figshare.24415918], NRASQ61[10.6084/m9.figshare.24415942], NRASQ61H[10.6084/m9.figshare.24415939], NRASQ61L[10.6084/m9.figshare.24415945], and NRASQ61R[10.6084/m9.figshare.24415948] peptide-loaded HLA-A*01:01/hβ2m. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andrew C. McShan, David Flores-Solis.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43654-9.
References
- 1.Rock KL, Reits E, Neefjes J. Present yourself! by MHC class I and MHC class II molecules. Trends Immunol. 2016;37:724–737. doi: 10.1016/j.it.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu. Rev. Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margulies DH, et al. Chaperones and catalysts: how antigen presentation pathways cope with biological necessity. Front. Immunol. 2022;13:859782. doi: 10.3389/fimmu.2022.859782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serçinoğlu O, Ozbek P. Sequence-structure-function relationships in class I MHC: A local frustration perspective. PLoS ONE. 2020;15:e0232849. doi: 10.1371/journal.pone.0232849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson J, et al. Distinguishing functional polymorphism from random variation in the sequences of >10,000 HLA-A, -B and -C alleles. PLoS Genet. 2017;13:e1006862. doi: 10.1371/journal.pgen.1006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaitoua AJ, Kaur A, Raghavan M. Variations in MHC class I antigen presentation and immunopeptidome selection pathways. F1000Res. 2020;9:F1000 Faculty Rev–1177. doi: 10.12688/f1000research.26935.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayres CM, et al. Dynamically driven allostery in MHC proteins: peptide-dependent tuning of class I MHC global flexibility. Front. Immunol. 2019;10:966. doi: 10.3389/fimmu.2019.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McShan AC, et al. Molecular determinants of chaperone interactions on MHC-I for folding and antigen repertoire selection. Proc. Natl Acad. Sci. USA. 2019;116:25602–25613. doi: 10.1073/pnas.1915562116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieczorek M, et al. Major Histocompatibility Complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djaoud Z, Parham P. HLAs, TCRs, and KIRs, a triumvirate of human cell-mediated immunity. Annu. Rev. Biochem. 2020;89:717–739. doi: 10.1146/annurev-biochem-011520-102754. [DOI] [PubMed] [Google Scholar]
- 11.Papadaki GF, et al. Decoupling peptide binding from T cell receptor recognition with engineered chimeric MHC-I molecules. Front Immunol. 2023;14:1116906. doi: 10.3389/fimmu.2023.1116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossjohn J, et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015;33:169–200. doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- 13.Yarmarkovich M, et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature. 2021;599:477–484. doi: 10.1038/s41586-021-04061-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Høydahl LS, Frick R, Sandlie I, Løset GÅ. Targeting the MHC ligandome by use of TCR-like antibodies. Antibodies. 2019;8:32. doi: 10.3390/antib8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher TN, Hacohen N. Neoantigens encoded in the cancer genome. Curr. Opin. Immunol. 2016;41:98–103. doi: 10.1016/j.coi.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Saini SK, Rekers N, Hadrup SR. Novel tools to assist neoepitope targeting in personalized cancer immunotherapy. Ann. Oncol. 2017;28:xii3–xii10. doi: 10.1093/annonc/mdx544. [DOI] [PubMed] [Google Scholar]
- 17.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021;18:215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Kranz DM. Recent advances in T-cell engineering for use in immunotherapy. F1000Res. 2016;5:F1000 Faculty Rev–2344. doi: 10.12688/f1000research.9073.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell–based treatments for solid tumors. Nat. Med. 2019;25:1488–1499. doi: 10.1038/s41591-019-0596-y. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs GA, Der CJ, Rossman KL. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishto M, et al. An in silico—in vitro pipeline identifying an hla-a*02:01+ kras g12v+ spliced epitope candidate for a broad tumor-immune response in cancer patients. Front. Immunol. 2019;10:2572. doi: 10.3389/fimmu.2019.02572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim MJW, et al. High-affinity oligoclonal TCRs define effective adoptive T cell therapy targeting mutant KRAS-G12D. Proc. Natl Acad. Sci. USA. 2020;117:12826–12835. doi: 10.1073/pnas.1921964117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao AA, et al. ProTECT-prediction of T-Cell epitopes for cancer therapy. Front. Immunol. 2020;11:483296. doi: 10.3389/fimmu.2020.483296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linard B, et al. A ras-mutated peptide targeted by ctl infiltrating a human melanoma lesion. J. Immunol. 2002;168:4802–4808. doi: 10.4049/jimmunol.168.9.4802. [DOI] [PubMed] [Google Scholar]
- 26.Toor JS, et al. A recurrent mutation in anaplastic lymphoma kinase with distinct neoepitope conformations. Front. Immunol. 2018;9:99. doi: 10.3389/fimmu.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peri A, et al. Combined presentation and immunogenicity analysis reveals a recurrent RAS.Q61K neoantigen in melanoma. J. Clin. Invest. 2021;131:e129466. doi: 10.1172/JCI129466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hélias-Rodzewicz Z, et al. Variation of mutant allele frequency in NRAS Q61 mutated melanomas. BMC Dermatol. 2017;17:9. doi: 10.1186/s12895-017-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Z, Liu F, Zhang J. Targeting NRASQ61K mutant delays tumor growth and angiogenesis in non-small cell lung cancer. Am. J. Cancer Res. 2017;7:831–844. [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y, et al. Inhibition of melanoma development in the Nras(Q61K)::Ink4a−/− mouse model by the small molecule BI-69A11. Pigment Cell Melanoma Res. 2013;26:136–142. doi: 10.1111/pcmr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjorkman PJ, et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 32.Apostolopoulos V, et al. Crystal structure of a non-canonical low-affinity peptide complexed with MHC class I: a new approach for vaccine design. J. Mol. Biol. 2002;318:1293–1305. doi: 10.1016/S0022-2836(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 33.Antunes DA, Abella JR, Devaurs D, Rigo MM, Kavraki LE. Structure-based methods for binding mode and binding affinity prediction for peptide-MHC complexes. Curr. Top. Med. Chem. 2018;18:2239–2255. doi: 10.2174/1568026619666181224101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nerli, S. & Sgourakis, N. G. Structure-based modeling of SARS-CoV-2 peptide/HLA-A02 antigens. Front. Med. Technol.2, 3478 (2020). [DOI] [PMC free article] [PubMed]
- 35.Gupta S, Nerli S, Kutti Kandy S, Mersky GL, Sgourakis NG. HLA3DB: comprehensive annotation of peptide/HLA complexes enables blind structure prediction of T cell epitopes. Nat. Commun. 2023;14:6349. doi: 10.1038/s41467-023-42163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc. Natl Acad. Sci. USA. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellman LM, et al. Differential scanning fluorimetry based assessments of the thermal and kinetic stability of peptide-MHC complexes. J. Immunol. Methods. 2016;432:95–101. doi: 10.1016/j.jim.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, et al. Universal open MHC-I molecules for rapid peptide loading and enhanced complex stability across HLA allotypes. Proc. Natl Acad. Sci. USA. 2023;120:e2304055120. doi: 10.1073/pnas.2304055120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun, Y. et al. Xeno interactions between MHC-I proteins and molecular chaperones enable ligand exchange on a broad repertoire of HLA allotypes. Sci Adv.9, eade7151 (2023). [DOI] [PMC free article] [PubMed]
- 40.Buchli R, et al. Development and validation of a fluorescence polarization-based competitive peptide-binding assay for HLA-A*0201–a new tool for epitope discovery. Biochemistry. 2005;44:12491–12507. doi: 10.1021/bi050255v. [DOI] [PubMed] [Google Scholar]
- 41.Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48:W449–W454. doi: 10.1093/nar/gkaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tugarinov V, Choy W-Y, Orekhov VY, Kay LE. Solution NMR-derived global fold of a monomeric 82-kDa enzyme. Proc. Natl Acad. Sci. USA. 2005;102:622–627. doi: 10.1073/pnas.0407792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natarajan K, et al. An allosteric site in the T-cell receptor Cβ domain plays a critical signalling role. Nat. Commun. 2017;8:15260. doi: 10.1038/ncomms15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgourakis, N. G. et al. A Novel MHC-I Surface Targeted for Binding by the MCMV m06 Immunoevasin Revealed by Solution NMR. J Biol Chem.290, 28857–28868 (2015). [DOI] [PMC free article] [PubMed]
- 45.McShan, A.C. et al. TAPBPR promotes antigen loading on MHC-I molecules using a peptide trap. Nat Commun.12, 3174 (2021). [DOI] [PMC free article] [PubMed]
- 46.McShan, A. C. et al. TAPBPR employs a ligand-independent docking mechanism to chaperone MR1 molecules. Nat Chem Biol. 18, 859–868 (2022). [DOI] [PMC free article] [PubMed]
- 47.Jiang, J. et al. Structural mechanism of tapasin-mediated MHC-I peptide loading in antigen presentation. Nat Commun.13, 5470 (2022). [DOI] [PMC free article] [PubMed]
- 48.Papadaki, G. F. et al. A Chicken Tapasin ortholog can chaperone empty HLA-B∗37:01 molecules independent of other peptide-loading components. J Biol Chem.299, 105136 (2023). [DOI] [PMC free article] [PubMed]
- 49.McShan AC, et al. Peptide exchange on MHC-I by TAPBPR is driven by a negative allostery release cycle. Nat. Chem. Biol. 2018;14:811–820. doi: 10.1038/s41589-018-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar A, Wagner G, Ernst RR, Wüthrich K. Studies of J-connectivities and selective 1H-1H Overhauser effects in H2O solutions of biological macromolecules by two-dimensional NMR experiments. Biochem. Biophys. Res. Commun. 1980;96:1156–1163. doi: 10.1016/0006-291X(80)90073-X. [DOI] [PubMed] [Google Scholar]
- 51.Eisen HN, et al. Promiscuous binding of extracellular peptides to cell surface class I MHC protein. Proc. Natl Acad. Sci. USA. 2012;109:4580–4585. doi: 10.1073/pnas.1201586109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saini SK, et al. Empty peptide-receptive MHC class I molecules for efficient detection of antigen-specific T cells. Sci. Immunol. 2019;4:eaau9039. doi: 10.1126/sciimmunol.aau9039. [DOI] [PubMed] [Google Scholar]
- 53.Anjanappa R, et al. Structures of peptide-free and partially loaded MHC class I molecules reveal mechanisms of peptide selection. Nat. Commun. 2020;11:1314. doi: 10.1038/s41467-020-14862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khilko SN, et al. Direct detection of major histocompatibility complex class I binding to antigenic peptides using surface plasmon resonance. Peptide immobilization and characterization of binding specificity. J. Biol. Chem. 1993;268:15425–15434. doi: 10.1016/S0021-9258(18)82275-X. [DOI] [PubMed] [Google Scholar]
- 55.Elliott T, Williams A. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol. Rev. 2005;207:89–99. doi: 10.1111/j.0105-2896.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 56.Lan H, et al. Exchange catalysis by tapasin exploits conserved and allele-specific features of MHC-I molecules. Nat. Commun. 2021;12:4236. doi: 10.1038/s41467-021-24401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurimoto E, et al. Structural and functional mosaic nature of MHC class I molecules in their peptide-free form. Mol. Immunol. 2013;55:393–399. doi: 10.1016/j.molimm.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Springer S, Doring K, Skipper JC, Townsend AR, Cerundolo V. Fast association rates suggest a conformational change in the MHC class I molecule H-2Db upon peptide binding. Biochemistry. 1998;37:3001–3012. doi: 10.1021/bi9717441. [DOI] [PubMed] [Google Scholar]
- 59.Gakamsky DM, Davis DM, Strominger JL, Pecht I. Assembly and dissociation of human leukocyte antigen (HLA)-A2 studied by real-time fluorescence resonance energy transfer. Biochemistry. 2000;39:11163–11169. doi: 10.1021/bi000763z. [DOI] [PubMed] [Google Scholar]
- 60.Binz A-K, Rodriguez RC, Biddison WE, Baker BM. Thermodynamic and kinetic analysis of a peptide-class I MHC interaction highlights the noncovalent nature and conformational dynamics of the class I heterotrimer. Biochemistry. 2003;42:4954–4961. doi: 10.1021/bi034077m. [DOI] [PubMed] [Google Scholar]
- 61.Hateren, A. van. et al. Direct evidence for conformational dynamics in major histocompatibility complex class I molecules. J. Biol. Chem. 292, 20255–20269 (2017). [DOI] [PMC free article] [PubMed]
- 62.Insaidoo FK, Zajicek J, Baker BM. A general and efficient approach for nmr studies of peptide dynamics in Class I MHC peptide binding grooves. Biochemistry. 2009;48:9708–9710. doi: 10.1021/bi9008787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi P, Xia Y, Khanra N, Veglia G, Kalodimos CG. 15N and 13C- SOFAST-HMQC editing enhances 3D-NOESY sensitivity in highly deuterated, selectively [1H,13C]-labeled proteins. J. Biomol. NMR. 2016;66:259–271. doi: 10.1007/s10858-016-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zwahlen C, et al. Methods for measurement of intermolecular NOEs by multinuclear NMR spectroscopy: Application to a bacteriophage λ N-peptide/boxB RNA. complex. J. Am. Chem. Soc. 1997;119:6711–6721. doi: 10.1021/ja970224q. [DOI] [Google Scholar]
- 65.Raveh B, London N, Zimmerman L, Schueler-Furman O. Rosetta FlexPepDockab-initio: simultaneous folding, docking and refinement of peptides onto their receptors. PLoS ONE. 2011;6:e18934. doi: 10.1371/journal.pone.0018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu T, et al. Subangstrom accuracy in pHLA-I modeling by Rosetta FlexPepDock refinement protocol. J. Chem. Inf. Model. 2014;54:2233–2242. doi: 10.1021/ci500393h. [DOI] [PubMed] [Google Scholar]
- 67.Kondo A, et al. Two distinct HLA-A*0101-specific submotifs illustrate alternative peptide binding modes. Immunogenetics. 1997;45:249–258. doi: 10.1007/s002510050200. [DOI] [PubMed] [Google Scholar]
- 68.Masson GR, et al. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nat. Methods. 2019;16:595–602. doi: 10.1038/s41592-019-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawse WF, et al. Peptide modulation of class I major histocompatibility complex protein molecular flexibility and the implications for immune recognition. J. Biol. Chem. 2013;288:24372–24381. doi: 10.1074/jbc.M113.490664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muñoz-Maldonado C, Zimmer Y, Medová M. A comparative analysis of individual RAS mutations in cancer biology. Front. Oncol. 2019;9:1088. doi: 10.3389/fonc.2019.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ayres CM, Corcelli SA, Baker BM. Peptide and peptide-dependent motions in MHC proteins: immunological implications and biophysical underpinnings. Front. Immunol. 2017;8:935. doi: 10.3389/fimmu.2017.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Douglass J, et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci. Immunol. 2021;6:eabd5515. doi: 10.1126/sciimmunol.abd5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bear AS, et al. Biochemical and functional characterization of mutant KRAS epitopes validates this oncoprotein for immunological targeting. Nat. Commun. 2021;12:4365. doi: 10.1038/s41467-021-24562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang QJ, et al. Identification of T-cell receptors targeting KRAS-mutated human tumors. Cancer. Immunol. Res. 2016;4:204–214. doi: 10.1007/s12026-015-8738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skora, A. D. et al. Generation of MANAbodies specific to HLA-restricted epitopes encoded by somatically mutated genes. Proc Natl Acad Sci USA112, 9967–9972 (2015). [DOI] [PMC free article] [PubMed]
- 77.Wright, K.M. et al. Hydrophobic interactions dominate the recognition of a KRAS G12V neoantigen. Nat Commun.14, 5063 (2023). [DOI] [PMC free article] [PubMed]
- 78.Poole, A. et al. Therapeutic high affinity T cell receptor targeting a KRASG12D cancer neoantigen. Nat Commun.13, 5333 (2022). [DOI] [PMC free article] [PubMed]
- 79.Lu, D. et al. KRAS G12V neoantigen specific T cell receptor for adoptive T cell therapy against tumors. Nat Commun.14, 6389 (2023). [DOI] [PMC free article] [PubMed]
- 80.Overall, S.A. et al. High throughput pMHC-I tetramer library production using chaperone-mediated peptide exchange. Nat Commun.11, 1909 (2020). [DOI] [PMC free article] [PubMed]
- 81.Ilca FT, Boyle LH. The glycosylation status of MHC class I molecules impacts their interactions with TAPBPR. Mol. Immunol. 2021;139:168–176. doi: 10.1016/j.molimm.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sagert L. et al. The ER folding sensor UGGT1 acts on TAPBPR-chaperoned peptide-free MHC I eLife. 12:e85432 (2023). [DOI] [PMC free article] [PubMed]
- 83.Domnick, A. et al. Molecular basis of MHC I quality control in the peptide loading complex. Nat Commun.13, 4701 (2022). [DOI] [PMC free article] [PubMed]
- 84.O’Rourke S. M. et al. Production of soluble pMHC-I molecules in mammalian cells using the molecular chaperone TAPBPR. Protein Eng. Des. Sel.32, 525–532 (2019). [DOI] [PMC free article] [PubMed]
- 85.Nerli S, McShan AC, Sgourakis NG. Chemical shift-based methods in NMR structure determination. Prog. Nucl. Magn. Reson. Spectrosc. 2018;106–107:1–25. doi: 10.1016/j.pnmrs.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nerli S, et al. Backbone-independent NMR resonance assignments of methyl probes in large proteins. Nat Commun. 2023;12:691. doi: 10.1038/s41467-021-20984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evangelidis, T. et al. Automated NMR resonance assignments and structure determination using a minimal set of 4D spectra. Nat Commun.9, 384 (2018). [DOI] [PMC free article] [PubMed]
- 88.Hassan C, et al. Naturally processed non-canonical HLA-A*02:01 presented peptides. J. Biol. Chem. 2015;290:2593–2603. doi: 10.1074/jbc.M114.607028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ebrahimi-Nik H, et al. Reversion analysis reveals the in vivo immunogenicity of a poorly MHC I-binding cancer neoepitope. Nat. Commun. 2021;12:6423. doi: 10.1038/s41467-021-26646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amacher JF, Brooks L, Hampton TH, Madden DR. Specificity in PDZ-peptide interaction networks: Computational analysis and review. J. Struct. Biol. X. 2020;4:100022. doi: 10.1016/j.yjsbx.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans R. et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv. 2021.10.04.463034 (2021).
- 92.Sun, Y. et al. Structural principles of peptide-centric chimeric antigen receptor recognition guide therapeutic expansion. Sci Immunol.8, eadj5792 (2023). [DOI] [PMC free article] [PubMed]
- 93.Bai P, et al. Immune-based mutation classification enables neoantigen prioritization and immune feature discovery in cancer immunotherapy. Oncoimmunology. 2021;10:1868130. doi: 10.1080/2162402X.2020.1868130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tugarinov V, Kay LE. Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J. Am. Chem. Soc. 2003;125:13868–13878. doi: 10.1021/ja030345s. [DOI] [PubMed] [Google Scholar]
- 95.Delaglio F, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 96.Lee W, Tonelli M, Markley JL. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinforma. Oxf. Engl. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 98.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 99.Kan Z-Y, Ye X, Skinner JJ, Mayne L, Englander SW. ExMS2: an integrated solution for hydrogen-deuterium exchange mass spectrometry data analysis. Anal. Chem. 2019;91:7474–7481. doi: 10.1021/acs.analchem.9b01682. [DOI] [PubMed] [Google Scholar]
- 100.Styczynski MP, Jensen KL, Rigoutsos I, Stephanopoulos G. BLOSUM62 miscalculations improve search performance. Nat. Biotechnol. 2008;26:274–275. doi: 10.1038/nbt0308-274. [DOI] [PubMed] [Google Scholar]
- 101.Thomsen MCF, Nielsen M. Seq2Logo: a method for construction and visualization of amino acid binding motifs and sequence profiles including sequence weighting, pseudo counts and two-sided representation of amino acid enrichment and depletion. Nucleic Acids Res. 2012;40:W281–287. doi: 10.1093/nar/gks469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perez-Riverol Y, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The atomic coordinates for the NRASQ61K/HLA-A*01:01/hβ2m structure have been deposited in the Protein Data Bank with PDB ID 6MPP. Other crystal structures used in this study include 6AT9, 3BO8, and 4NQV. NMR chemical shift assignments of HLA-A*01:01 heavy chain have been deposited into the Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu) under accession number 27632 [doi:10.13018/BMR27632]. The HDX-MS data have been deposited to the ProteomeXchange Consortium via the PRIDE102 partner repository with the dataset identifier PXD044838. The protein sequences and sequence coverage maps for HDX-MS can be accessed from Figshare [10.6084/m9.figshare.24417373]. The kinetics plots for each peptide fragment of the HDX-MS biological triplicates can be accessed from Figshare for peptide-free [10.6084/m9.figshare.24415921], NRASQ61K[10.6084/m9.figshare.24415918], NRASQ61[10.6084/m9.figshare.24415942], NRASQ61H[10.6084/m9.figshare.24415939], NRASQ61L[10.6084/m9.figshare.24415945], and NRASQ61R[10.6084/m9.figshare.24415948] peptide-loaded HLA-A*01:01/hβ2m. Source data are provided with this paper.