Abstract
Objective To investigate the effect of patients' preferences in the treatment of atrial fibrillation by using individualized decision analysis in which probability and utility assessments are combined into a decision tree. Design Observational study based on interviews with patients. Setting 8 general practices in Avon, England. Participants 260 randomly selected patients aged 70 to 85 years with atrial fibrillation. Main outcome measures Patients' treatment preferences regarding anticoagulation treatment (warfarin sodium) after individualized decision analysis; comparison of these preferences with treatment guidelines on the basis of comorbidity and absolute risk and compared with current prescription. Results Of 195 eligible patients, 97 participated in decision making using decision analysis. Among these 97, the decision analysis indicated that 59 (61%; 95% confidence interval, 50%-71%) would prefer anticoagulation treatment, considerably fewer than those who would be recommended treatment according to guidelines. There was marked disagreement between the decision analysis and guideline recommendations (κ≥0.25). Of 38 patients whose decision analysis indicated a preference for anticoagulation, 17 (45%) were being prescribed warfarin; on the other hand, 28 (47%) of 59 patients were not being prescribed warfarin, although the results of their decision analysis suggested they wanted to be. Conclusions In the context of shared decision making, individualized decision analysis is valuable in a sizable proportion of elderly patients with atrial fibrillation. Taking account of patients' preferences would lead to fewer prescriptions for warfarin than under published recommendations. Decision analysis as a shared decision-making tool should be evaluated in a randomized controlled trial.
Atrial fibrillation is an independent risk factor for stroke. Randomized trials have established that anticoagulation with warfarin sodium is associated with a relative reduction in the risk of stroke of 68%.1 Community-based studies that estimated the prevalence of atrial fibrillation, however, show underdiagnosis and undertreatment.2,3 Commentators from general practice attribute the poor incorporation of this treatment in clinical practice to a lack of representativeness of patients enrolled in clinical trials. In particular, patients managed in primary care may be more likely than patients in clinical trials to find “minor” side effects from anticoagulation problematic.4,5
Decision analysis is a form of shared decision making that explicitly combines the probabilities of events resulting from treatment decisions with quantitative estimates of patients' perceptions (utilities) regarding the consequences of treatment.6 The increasing computerization in general practices, along with the development of user-friendly software, means that utility assessment with decision analysis is a realistic aim for decision making with individual patients.7
Qualitative research has established that patients' health beliefs are important factors in determining whether they accept or decline anticoagulation treatment for atrial fibrillation.8 We examined the effects of patients' preferences, measured by utility assessment, on treatment choices and compared this method of decision making with evidence-based recommendations based on age and comorbidity or absolute risk of stroke.9,10,11
METHODS
Selection of participants
We invited 17 general practices to take part in the study, of which 13 accepted. Owing to time constraints, only the first 8 practices on an unordered list were included. We identified patients with atrial fibrillation by a diagnostic code on the practices' computer records and repeated prescriptions for digoxin. We used random-number tables to select patients between 70 and 85 years old. We sampled 30 or 40 patients per practice, depending on the list size, yielding a total sample size of 260 patients. In each practice, the list of sampled patients was shown to the general practitioners, and unsuitable patients were excluded (see the figure on wjm's web site). We sent letters and an information sheet to the remaining patients inviting them to take part in the study. We then telephoned patients to arrange an interview (with J P) at their practice; written consent was obtained at the start of the interview.
Decision analysis
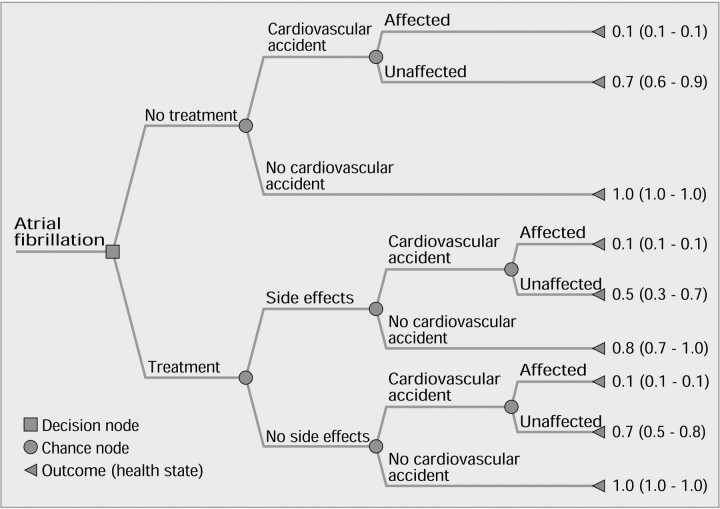
Details of each participant's risk factor profile were abstracted from medical records and verified with the participant. Absolute annual risks of a thromboembolic event were derived from a literature search and tailored to each participant according to his or her age and comorbidity (table 1).1,11,12,13,14,15 The relative risk reduction and the probability of adverse effects if warfarin treatment was given were also obtained from the literature, as was the likelihood of functional independence after a stroke (table 1). The treatment alternatives and their possible consequences were then mapped out by a decision tree (figure 1). The 9 health states (outcomes) from the decision tree were shown on laminated cards to the participant, who then ranked them in order of preference. Utilities for each health state were elicited using the “time trade-off” method, which quantifies the length of time in perfect health that is viewed by the patient to be equivalent to a given period of ill health (box). Participants were also asked to complete a short questionnaire to assess how they felt about the interview process.
Table 1.
| Chance node | Absolute annual risk, % (95% CI) |
|---|---|
| Risk of having a thromboembolic event (stroke) among patients with untreated atrial fibrillation1,11,12,13 | |
| Age 65-75 yr | |
| No risk factor | 4.3 (2.7-7.1) |
| ≥1 risk factor | 5.7 (3.9-8.3) |
| Congestive cardiac failure, no risk factor | 8.4 (2.1-33.0) |
| Congestive cardiac failure, ≥1 risk factor | 11.7 (5.3-26.0) |
| Age > 75 yr | |
| No risk factor | 3.5 (3.5-26.0) |
| ≥1 risk factor | 8.1 (4.7-13.9) |
| Congestive cardiac failure, no risk factor | 10.9 (1.4-78.0) |
| Congestive cardiac failure, ≥1 risk factor | 19.7 (7.4-52.0) |
| Previous cardiovascular accident or TIA | 12.0‡ |
| Relative risk reduction when treated with warfarin sodium1 | |
| Annual risk reduction, % | 68 |
| Probability of outcome after thromboembolic event (stroke)14 | |
| Affected (functionally dependent) | 35 (30.0-39.0) |
| Unaffected (functionally independent) | 65 (61.0-70.0) |
| Probability of side effects with warfarin treatment15 | |
| Minor side effect | 11.8 (8.8-16.0) |
| Major side effect | 1.1 (0.5-1.5) |
| Any side effect | 12.9‡ |
| Risk factor = diabetes mellitus or hypertension (but not congestive cardiac failure). | |
| CI = confidence interval; TIA = transient ischemic attack. | |
Figure 1.
Decision tree of health states resulting from having atrial fibrillation with utility values (median [interquartile range]) for each health state
Data analysis
The probabilities (risks) and utilities were assigned to each person's decision tree. These were then multiplied and summed to give expected utility values for the 2 main branches of the tree—treatment and no treatment. After this, a participant was to accept treatment if the expected utility of “treatment” exceeded that of “no treatment.” In the primary analysis, the probability of “any” side effects was used; the probabilities for “major” and “minor” side effects were incorporated into a sensitivity analysis.
Each participant's preference about warfarin treatment from the decision analysis was compared with recommendations by using 2 sets of published criteria based only on comorbidity and age.9,11 The first of these was from a consensus conference, which included recommending treatment for all patients older than 75 years, regardless of risk factors.9 The second recommendation was based on absolute annual risk for all patients, using risks derived from the literature, as in table 1. In this study, warfarin treatment was assumed to be recommended if the participant's absolute annual risk was greater than 5%; this is consistent with recent guidelines based on absolute risk.11 The result of the decision analysis was also compared with whether the participant was receiving anticoagulation treatment at the time of interview.
All these comparisons were performed by using crude percentages of disagreements between the classifications, both overall and by type of disagreement. The level of agreement that would be expected by chance was corrected for using κ statistics.16 Ethical approval was obtained from our local research ethics committee before the start of the study.
RESULTS
Representativeness
In all, 97 participants completed the decision analysis. Table 2 shows the characteristics of these participants. The sex ratio (55% female) was similar to that for the original sample of 260 patients. The participants were also comparable in several respects to those recruited in the 5 randomized controlled trials that evaluated the use of warfarin or aspirin in atrial fibrillation, except that women were underrepresented in the trials.1
Table 2.
Characteristics of study participants (n = 97)*
| Characteristics | Participants |
|---|---|
| Mean (SD) age, yr | 77 (3.9) |
| Women | 48 |
| Diabetes | 12 |
| Previous cardiovascular accident or TIA | 21 |
| Hypertension | 47 |
| Congestive cardiac failure | 22 |
| Angina or previous MI | 25 |
| ≥1 risk factors | 46 |
| Taking warfarin sodium | 48 |
| Taking aspirin | 39 |
| Taking digoxin | 60 |
| TIA = transient ischemic attack; MI = myocardial infarction. | |
Values are number of participants except as stated otherwise.
Table 2.
| Example of time trade-off method |
|---|
| To assess the utility of the health state “treated with warfarin, experienced side effects, has had cardiovascular accident, unaffected afterwards” (see figure 1) in a 75-year-old woman |
| The patient is asked to choose between 2 alternatives: living in the health state in question until age 80, or living in perfect health for a shorter time. The options are presented on laminated sheets, and the age to which the patient could live in perfect health is varied until she is unable to choose between the 2 alternatives. Let us suppose that she regards living until age 77 in perfect health as “equivalent to” living until age 80 in the health state in question—that is, she would be willing to give up 3 of her remaining 5 years of life to have perfect health. Utility of the health state in question is then calculated as: |
| 1-[number of years willing to give up/(80 - current age)] |
| This would be 1 - (3/5)=0.4, with 0.4 representing the value that this patient places on this state of health |
Proportions recommended for warfarin treatment according to various criteria
Individual utility values varied little within each health state (figure 1). According to the decision analysis, of the 97 participants, 59 (61%; 95% confidence interval [CI], 50%-71%) preferred treatment with warfarin; the corresponding figures for the other 2 recommendations were 89 (92%; 95% CI, 84%-96%) for the consensus conference9 and 70 (72%; 95% CI, 62%-81%) when based on absolute risk.11
Comparison of decision analysis with recommendations and current treatment
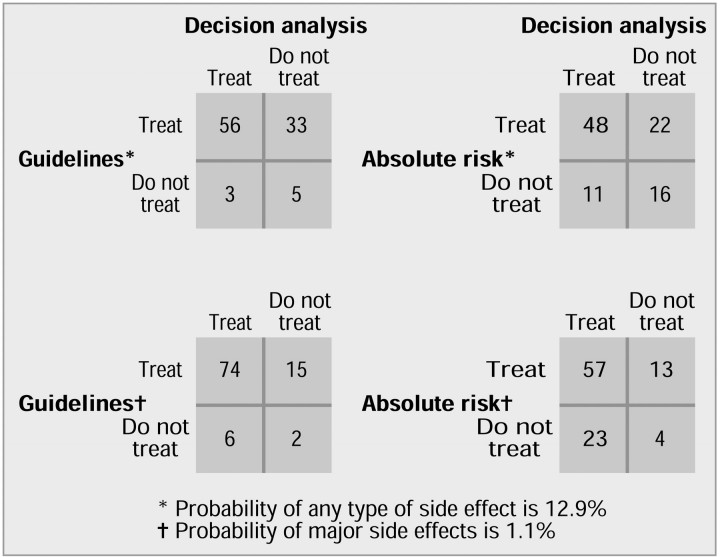
The primary comparison of the decision analysis based on any side effects with the other recommendations shows a high level of disagreement (figure 2). Moreover, most of the discrepancies are “false-positives”; for example, of the 38 participants whose decision analysis indicated that they preferred not to be treated with warfarin, 33 (87%) would have been recommended for treatment according to the consensus conference's guidelines (figure 2). Because the chance of minor side effects is closely similar to that of any side effects, the results of this part of the sensitivity analysis are not shown. Indeed, even though the risk of major side effects is considerably lower, using this probability in the calculations for the decision analysis had no appreciable effect on the results (figure 2). The measures of agreement (κ statistic) for treatment preferences based on decision analysis and corrected for chance compared with the consensus guidelines and absolute risk recommendations were 0.09 and 0.25 when any side effects were considered and 0.05 and -0.04, respectively, when major side effects were considered, indicating “poor” levels of agreement.
Figure 2.
Levels of disagreement between treatment preferences based on decision analysis and recommendation of treatment guidelines from a consensus conference9 and absolute risk
Of the 38 participants whose decision analysis indicated that they preferred not to be treated with warfarin, 17 (45%) were in fact being prescribed warfarin. Of the remaining 59 participants, 28 (47%) were not being prescribed warfarin—that is, contrary to their decision.
Questionnaire responses
Altogether, 82 participants stated a preference to be involved in shared decision making about their medical care; 67 reported current involvement. Ninety participants thought that the decision analysis interview could be performed in general practice by either their general practitioner or a practice nurse. When asked whether they found it unsettling to discuss the possibility of having a stroke or side effects from treatment, 73 said “no,” 22 said “a little,” and 2 said that they found it “very” unsettling.
DISCUSSION
Interpretation of findings
When incorporated by decision analysis, patients' preferences could have an important effect on treatment choice in elderly patients, with nearly 40% of patients with atrial fibrillation in this study preferring not to receive anticoagulation. Furthermore, when the results of decision analysis are compared with guidelines based on the absolute risk of stroke, there is marked disagreement (figure 2). Guidelines ignoring patients' preferences would recommend treatment of a higher proportion of patients.
A large proportion of elderly people were either unwilling or too unwell to participate in shared decision making—at least in the context of a research study (see figure on wjm's web site). This may act as a barrier to using any form of shared decision-making tool in clinical practice.17 On the other hand, questionnaire responses from those who participated in decision analysis accord with previous findings that decision analysis is well accepted by patients, and most (85%) interviewees would prefer to be involved in clinical decision making.18
Table 2 shows that apart from an underrepresentation of women, the participants in this study were not substantially different from participants in clinical trials of the treatment of atrial fibrillation.1 This suggests that a reluctance to apply results of randomized trials may not be justifiable purely on the basis of differences in patients' characteristics.4
Comparison with guidelines
Guidelines for anticoagulation in atrial fibrillation based on absolute risk or clinical criteria have been widely promulgated.9,11 Glasziou and Irwig equate 1 death from intracranial hemorrhage with the prevention of 4 cases of thromboembolic stroke and suggest that when the annual risk of stroke exceeds 2%, the benefits start to outweigh the potential harm induced by anticoagulation treatment.10 This form of absolute risk assessment has been used as the criterion for judging evidence-based treatment in the community.9,11 Wide concern has been expressed that when such criteria are used, atrial fibrillation is being undertreated in elderly people.2,3,19 Our results suggest that treatment choice among elderly people is more complex than simply applying absolute risk standards of treatment. Factors relating to individual patients have been described and attributed as one of the reasons for the poor incorporation of anticoagulation treatment in clinical practice.15 Among patients who are willing and able to participate in shared decision making, individual preferences and probabilities seemingly combine to make some patients more averse to the consequences of anticoagulation than to those of atrial fibrillation. The findings suggest that guidelines for the management of atrial fibrillation should be modified to incorporate patients' preferences in treatment decisions, particularly with regard to the consequences of anticoagulation treatment.20,21
Previous studies
Qualitative research has established the importance of patients' preferences as a major factor in determining choice of treatment.8 A randomized trial evaluating the efficacy of anticoagulation treatment shows that quality of life is substantially reduced when patients experience even minor side effects.22 Sensitivity analyses in the current study show that variation in the severity and likelihood of the side effects for individual patients has an effect on treatment choice and confirms the importance of eliciting patients' preferences.23
Decision analysis is usually used as a means of implementing evidence in practice, with preferences being elicited at a group level.6 With the development and increasing sophistication of computer software, however, individual decision analysis is likely to be more common in the future.17 This study suggests that elicitation and participation by means of decision analysis will enable patients to become more involved in the decision-making process. Decision aids improve knowledge and reduce decisional conflict without increasing anxiety.24 A recent randomized trial showed that an audiobooklet about atrial fibrillation improved patients' understanding of the benefits and risks of treatment choices.18 Although promising, most decision aids usually involve imparting knowledge to patients; they do not evaluate patients' own values about the consequences of treatment.25 To date, there has been only 1 randomized controlled trial of personalized decision analysis showing that it did influence clinical decision making in patients.26 If decision analysis is to be used as a shared decision-making tool, it will require “protected” time for the patient in the same way that videos and patient information leaflets are currently used as shared decision-making tools. Some software can elicit individual patients' preferences and can be used on a personal computer.
Study limitations
Utility assessment involves certain constraints, primarily in achieving a balance between keeping the decision tree as simple as possible and including all the relevant patient outcomes. It is possible to elicit and then aggregate complex utility states (such as severity of side effects), but this may be at the expense of better understanding for the patient and physician.27 In this study, therefore, separate utility values were not elicited for major and minor side effects; rather, all possible side effects were represented together.
All interviews were conducted face to face by the same researcher, and interviewer bias cannot be excluded in such circumstances. More objective utility assessment may soon be possible using interactive computer programs.17 Finally, only 8 of the original 17 practices participated in this study, and in those practices, only half of the eligible patients took part in decision analysis. Responses in these individuals may be systematically different from those that might have been given by nonrespondents, and further replication of these findings is required.
CONCLUSIONS
In this observational study, eliciting preferences and performing decision analysis seem to have important implications for clinical practice. Decision analysis as a shared decision-making tool, and in particular its effect on patients' knowledge, satisfaction, and uptake of and adherence to anticoagulation, should be examined in a randomized controlled trial.24
Summary points
Qualitative research has established patients' preferences as a major factor in determining choice of anticoagulation treatment in patients with atrial fibrillation
Decision analysis is a form of shared decision making that explicitly combines the probabilities of events resulting from treatment decisions with quantitative estimates of the patients' preferences
This study shows that eliciting patients' preferences and performing decision analysis has a major effect on a patient's preference for anticoagulation treatment
Evaluation of decision analysis as a shared decision-making tool and its effect on patients' knowledge, satisfaction, uptake, and adherence to anticoagulation treatment should be examined in a randomized controlled trial
Acknowledgments
We thank all 13 Avon, England practices and 260 patients for participating in this study. Both the division of primary health care and the department of social medicine are part of the Medical Research Council Health Services Research Collaboration.
Funding: This study was funded as part of a Wellcome entry-level training fellowship (J P). Some funding provided by a National Health Service research and development primary care career scientist award (T F).
Competing interests: None declared
This article was published in BMJ 2000;320:1380-1384
Authors Tom Fahey is senior lecturer and Alan Montgomery is MRC health services research training fellow, division of primary health care; Tim Peters is reader in medical statistics, department of social medicine, University of Bristol.
References
- 1.Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154: 1449-1457. [PubMed] [Google Scholar]
- 2.Sudlow M, Thomson R, Thwaites B, Rodgers H, Kenny RA. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet 1998;352: 1167-1171. [DOI] [PubMed] [Google Scholar]
- 3.Fahey T, Rimmer J, Godfrey P. Risk stratification in the management of atrial fibrillation in the community. Br J Gen Pract 1999;49: 295-296. [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney KG, Gray DP, Steele R, Evans P. Use of warfarin in non-rheumatic atrial fibrillation: a commentary from general practice. Br J Gen Pract 1995;45: 153-158. [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney KG, Pereira Gray DJ, Steele RJ, Evans PH. Caution needed in introducing warfarin treatment [commentary]. BMJ 1995;311: 560-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilford RJ, Pauker SG, Braunholtz DA, Chard J. Decision analysis and the implementation of research findings. BMJ 1998;317: 405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nease RF, Tsai R, Hynes LM, Littenberg B. Automated utility assessment of global health. Qual Life Res 1996;5: 175-182. [DOI] [PubMed] [Google Scholar]
- 8.Howitt A, Armstrong D. Implementing evidence based medicine in general practice: audit and qualitative study of antithrombotic treatment for atrial fibrillation. BMJ 1999;318: 1324-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laupacis A, Albers G, Dalen J, Dunn M, Feinberg W, Jacobson A. Antithrombotic therapy in atrial fibrillation. Chest 1995;108(suppl): 352S-359S. [DOI] [PubMed] [Google Scholar]
- 10.Glasziou P, Irwig L. An evidence based approach to individualising treatment. BMJ 1995;311: 1356-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lip GYH. Thromboprophylaxis for atrial fibrillation. Lancet 1999;353: 4-6 [erratum published in Lancet 1999;353:1978]. [DOI] [PubMed] [Google Scholar]
- 12.Atrial Fibrillation Investigators. Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med 1998;158: 1316-1320. [DOI] [PubMed] [Google Scholar]
- 13.EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342: 1255-1262. [PubMed] [Google Scholar]
- 14.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire community stroke project—1981-1986. 2. Incidence, case fatality rates and overall outcome of one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53: 16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000;160: 41-46. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991.
- 17.Coulter A. Partnerships with patients: the pros and cons of shared clinical decision-making. J Health Serv Res Policy 1997;2: 112-121. [DOI] [PubMed] [Google Scholar]
- 18.Man-Son-Hing M, Laupacis A, O'Connor AM, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA 1999;282: 737-743. [DOI] [PubMed] [Google Scholar]
- 19.English KM, Channer KS. Managing atrial fibrillation in elderly people [editorial]. BMJ 1999;318: 1088-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hlatky M. Patient preferences and clinical guidelines [editorial]. JAMA 1995;273: 1219-1220. [PubMed] [Google Scholar]
- 21.Thomson R, Partin D, Eccles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulation therapy to prevent stroke in patients with atrial fibrillation. Lancet 2000;355: 956-962 [erratum published in Lancet 2000;355:1466]. [DOI] [PubMed] [Google Scholar]
- 22.Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life: evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991;151: 1944-1949 [erratum published in Arch Intern Med 1992;152:825]. [PubMed] [Google Scholar]
- 23.Gullov AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation—the AFASAK 2 study. Atrial Fibrillation Aspirin and Anticoagulation. Arch Intern Med 1999;159: 1322-1328. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor AM, Rostom A, Fiset V, et al. Decison aids for patients facing health treatment or screening decisions: systematic review. BMJ 1999;319: 731-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards A, Elwyn G. The potential benefits of decision aids in clinical medicine [editorial]. JAMA 1999;282: 779-780. [DOI] [PubMed] [Google Scholar]
- 26.Clancy CM, Cebul RD, Williams SV. Guiding individual decisions: a randomized, controlled trial of decision analysis. Am J Med 1988;84: 283-288. [DOI] [PubMed] [Google Scholar]
- 27.Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 3—estimating probabilities and utilities. Med Decis Making 1997;17: 136-141. [DOI] [PubMed] [Google Scholar]