Abstract
Snake venoms contain various molecules known for activating innate immunity and causing local effects associated with increased vascular permeability, such as vascular leakage and edema, common symptoms seen in snakebite envenomings. We have demonstrated that snake venom cysteine-rich secretory proteins (svCRiSPs) from North American pit vipers increase vascular permeability. This study aimed to explore the functional role of CRiSP isolated from Mojave rattlesnake (Crotalus scutulatus scutulatus) venom (Css-CRiSP) on the activation of inflammatory responses in different models. We measured the release of inflammatory mediators in cultured human dermal blood endothelial cells (HDBEC), lymphatic endothelial cells (HDLEC) and monocyte-derived macrophages (MDM) at 0.5, 1, 3, 6, and 24 h after treatment with Css-CRiSP (1 μM). We also determined the acute inflammatory response in BALB/c mice 30 min after intraperitoneal injection of the toxin (2 μg/mouse). Css-CRiSP induced the production of IL-8 and IL-6, but not TNF-α, in HDBEC and HDLEC in a time-dependent manner. In addition, Css-CRiSP significantly enhanced the production of IL-6, TNF-α, IL-8, and IL-1β in MDM. Moreover, it caused a remarkable increase of chemotactic mediators in the exudates of experimental mice. Our results reveal that Css-CRiSPs can promote a sustained release of inflammatory mediators on cell lines and an acute activation of innate immunity in a murine model. These findings contribute to the growing body of evidence supporting the involvement of svCRiSPs in the augmentation of envenomation effects, specifically, the role of svCRiSPs in inducing vascular dysfunction, initiating early inflammatory responses, and facilitating the activation of leukocytes and releasing mediators. These findings will lead to a better understanding of the pathophysiology of envenoming by Mojave rattlesnakes, allowing the development of more efficient therapeutic strategies.
Keywords: Snake venom cysteine-rich secretory protein (svCRiSP), Mojave rattlesnake (Crotalus scutulatus scutulatus), Inflammatory response, Endothelial cells, Monocyte-derived macrophages (MDM), Cytokine
Graphical abstract
Highlights
-
•
A svCRiSP from Mojave rattlesnake triggered the release of key pro-inflammatory cytokines and chemokines in cell lines.
-
•
Early release of pro-inflammatory mediators was observed locally and systemically in mice treated with Css-CRiSP.
-
•
This study provides further evidence of the pro-inflammatory effects of crotalid CRiSP.
-
•
Css-CRiSP may modulate the vascular dysfunction and persistent inflammation seen in envenomings by Mojave rattlesnakes.
1. Introduction
Snake venoms are diverse and complex mixtures of chemical components such as proteins, peptides, and organic and inorganic compounds. This diverse and intricate amalgamation possesses the ability to induce toxic effects synergistically (Minutti-Zanella et al., 2021). Most snake venom toxins can be classified into one of 42 families based on their prevalence and abundance (Tasoulis and Isbister, 2017, 2023). These families include dominant toxins such as phospholipase A2s (PLA2), snake venom metalloproteinases (SVMP), snake venom serine proteases (SVSP), and three-finger toxins. There are also secondary families like L-amino acid oxidases (LAAO), C-type lectins (CTL), disintegrins, snake venom cysteine-rich secretory proteins (svCRiSPs), kunitz peptides, and natriuretic peptides. Additionally, there are minor and rare protein families within snake venoms. Nonetheless, intraspecific and interspecific variations can occur, altering their prevalence and ultimately impacting their effects on the afflicted individual (Tasoulis and Isbister, 2017). These toxins target a wide range of important molecules, disturbing the homeostasis of the victim and producing life-threatening alterations such as hemorrhage, swelling, edema, tissue damage, myonecrosis, irreversible kidney failure, paralysis, and respiratory failure, resulting in permanent disability or death (World Health Organization, 2019).
The pathophysiology of snakebites does not rely solely on the effects of venom toxins, but also on the victims’ response to the toxins (Bickler, 2020). Snake venoms can promote a disproportional activation of key molecules, thus triggering physiological pathways in the victims that allow an increase in venom toxicity (Cavalcante et al., 2022a). The exacerbated up-regulation of the molecular cascades related to the inflammatory response is among the most affected endogenous processes (Teixeira et al., 2019; Cavalcante et al., 2022b). Under physiological conditions, inflammation is part of the innate immune response towards snake venoms. Recognition of damage-associated molecular patterns (DAMPs) or alarmins and venom-associated molecular patterns (VAMPs) by cells belonging to innate immunity, promotes their activation and initiates the inflammatory response as an initial mechanism to protect, neutralize and resolve the symptoms (Zuliani, 2023). However, the snake venom toxins can sustain pathological inflammation in the victim, leading to venom-induced, immune-mediated tissue damage that ultimately can cause organ system failure (Bickler, 2020; Ryan et al., 2021).
Several studies using isolated toxins such as SVMP, PLA2, LAAO and CTL support their role promoting inflammation by directly stimulating immune cells to release key mediators of this response (Bickler, 2020; Zuliani et al., 2020; Zuliani, 2023). In addition, other protein families are capable of triggering the inflammatory response, such as hyaluronidases and svCRiSPs. However, their role in innate immunity modulation during snakebites has yet to be thoroughly studied (Bickler, 2020; Deka et al., 2020). svCRiSPs are a widely distributed family of non-enzymatic proteins with a molecular weight of 24–28 kDa isolated from numerous animals, including reptile venoms (Matsunaga et al., 2009). Despite being widely recognized as ion channel blockers, increasing data have shown that svCRiSPs can display other biological properties such as pro-inflammatory effects by inducing cell activation, cytokine release, chemotaxis, and increase of vascular permeability (Wang et al., 2010; Lodovicho et al., 2017; Bernardes et al., 2019; Suntravat et al., 2019; Deka et al., 2020).
Our recent study investigated the signaling pathways responsible for the endothelial and vascular permeability induced by svCRiSPs purified from the most medically important snakes in North America (Suntravat et al., 2021). Our findings revealed that a CRiSP isolated from Crotalus scutulatus scutulatus venom (Css-CRiSP) exhibited the most pronounced permeability both in vivo and in vitro by modulating specific signaling pathways associated with the expression of adhesion proteins. Herein, we further examined the effect of this toxin on the release of pro-inflammatory mediators utilizing three types of cultured cells, namely human dermal blood endothelial cells (HDBEC), human dermal lymphatic endothelial cells (HDLEC), and monocyte-derived macrophages (MDM) from differentiation of U-937 cells. In addition, biofluids of BALB/c mice treated intraperitoneally with Css-CRiSP were studied to assess the acute inflammatory response induced by this toxin. This work provides new findings on the role of svCRiSPs in the induction of inflammatory responses that can contribute to the vascular dysfunction (local and systemic alterations) seen in snake envenoming by North American pit vipers.
2. Materials and methods
2.1. Snake venom collection and isolation of Css-CRiSP
Crude venom of type A C. s. scutulatus (Mojave rattlesnake) was obtained from a single adult snake housed in the serpentarium at the National Natural Toxins Research Center (NNTRC) in Texas A&M University-Kingsville (TAMUK), Kingsville, TX, USA. The venom was extracted from the snake, allowing it to bite into a disposable plastic cup covered with parafilm. The venom was centrifuged at 10,000×g for 5 min at 4 °C using a Beckman Coulter Avanti 30 centrifuge, filtered through a 0.45 μm MillexHV syringe filter unit (Millipore, Billerica, MA, USA) under positive pressure, lyophilized, and stored at −80 °C until use.
Css-CRiSP was isolated from crude venom as previously described (Suntravat et al., 2021). Briefly, whole venom was first fractionated by reverse phase chromatography using a Higgins Analytical PROTO 300 C18, 250 × 4.6 mm, 5 μm column (Higgins Analytical, Inc., CA, USA). Fractions were eluted using a 0.1% Trifluoroacetic acid (TFA), and 80% Acetonitrile (ACN) in 0.1% TFA gradient over a time period of 70 min with a flow rate of 1 mL/min. Then, the fraction containing Css-CRiSP, was purified by cation-exchange chromatography using a SP- 5PW Waters Protein-Pak™ column (7.5 × 75 mm2) (Waters Corp., MA, USA). Fractions were eluted using a 0.02 M Tris buffer, pH 7.0 and 0.02 M Tris, 0.5 M NaCl, pH 7.0 gradient, over a time period of 90 min with a flow rate of 1 mL/min. A Waters 2487 tunable detector was used to monitor the absorbance at 280 nm. Purified Css-CRiSP was confirmed by SDS-PAGE and N-terminal amino acid sequencing using an Edman degradation on a PPSQ-33B protein sequencer (SHIMADZU, Kyoto, Japan).
We determined the endotoxin content of Css-CRiSP using Pierce™ Chromogenic Endotoxin Quant Kit (Thermo Scientific, IL). The sample showed a value of 0.1 endotoxin unit (EU)/mL, which was within the acceptable threshold of 1 EU/m.
2.2. Cell lines and culture conditions
HDBEC and HDLEC were obtained from PromoCell (PromoCell GmbH, Heidelberg, Germany). Cells were cultured in gelatin-coated 75 cm2 flasks under constant humidity (5% CO2, 37 °C) and supplemented with endothelial cell growth media MV and MV2 (PromoCell GmbH), respectively, containing 5% fetal bovine serum (FBS) following the manufacturer's instructions. All experiments were conducted in passages 3–6 to obtain consistent data. HDBEC and HDLEC were seeded into 6-well plates at 5 × 104 cells/well and maintained until reaching a confluency of 90%.
The human monocytic U-937 cell line was obtained from the American Type Culture Collection (ATCC® CRL1593.2™, VA). Cells were cultured in 75 cm2 flasks under constant humidity (5% CO2, 37 °C) and maintained with RPMI-1640 medium (ATCC®, VA, USA), containing 10% FBS, 50 U/mL penicillin, and 50 μg/mg streptomycin. For the experiments, cells at passages 3–6 were seeded into 6-well plates at 3 × 106 cells/well and incubated for 24 h with 20 nM of phorbol 12-myristate 13-acetate (PMA, Sigma, MO, USA), to induce the differentiation into a macrophagic phenotype (monocyte-derived macrophages, MDM). After this period, cells rested by incubating them in RPMI-1640 containing 2% FBS for 48 h before conducting the studies (Dias-Netipanyj et al., 2016).
2.3. Cell viability assay
MDM were seeded in a 96-well assay plate at 2 × 105 cells/well and treated with Css-CRiSP at various concentrations (0.3–10 μM) for 24 h at 37 °C in 5% CO2. After this, cells were then incubated with CellTiter-Blue® reagent (Promega, WI, USA) for 3 h at 37 °C. Fluorescence was recorded at 560–590 nm using a Fluoroskan Ascent FL (Thermo Scientific, IL, USA). Cells treated only with sterile PBS were used as a negative control whereas cells treated with 1% Triton X-100 were considered as positive control for toxicity comparison. The percentage of cell viability was calculated relative to the negative control, which was defined as 100% viability.
2.4. Cell activation assays
Cell cultures were treated with Css-CRiSP (1 μM) or 0.25 μg/mL of lipopolysaccharide (LPS) as a positive control. Phosphate buffered solution (PBS) was used as a negative control. At 0.5, 1, 3, 6, and 24 h, supernatants were collected and subsequently stored at −80 °C until further analysis to evaluate the release of inflammatory mediators.
2.5. Animals
BALB/c mice (male & female, 18–20 g body weight) were housed in the serpentarium at the NNTRC, and kept in temperature-controlled rooms with 12 h dark–light cycles and received water and food ad libitum. The animal studies were conducted according to the institutional guidelines and after prior approval from the Institutional Animal Care and Use Committee of Texas A&M University-Kingsville, Texas, USA (Viper Resource Center at Texas A&M University-Kingsville, IACUC #: 2021-11-29/1474).
2.6. Experimental treatment with Css-CRiSP
Groups of 10 mice were intraperitoneally (i.p.) injected with 100 μL of Css-CRiSP (2 μg/mouse), a sub-lethal dose of C. s. scutulatus whole venom (68.5 μg/mouse) or PBS (negative control). After 30 min, mice were euthanized by cervical dislocation, and blood was collected by cardiac puncture and treated with 1% EDTA as an anticoagulant. Ten microliters of whole blood were used to determine the hematological profile, while the rest was centrifuged for 10 min at 2500 g, and the plasma was collected and stored at −80 °C until use. In the same groups of mice, a midline incision was done, and the peritoneal exudates were collected by washing the cavities with 1 mL of cold, sterile PBS (pH 7.2) supplemented with 2% L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 2% FBS, 5 IU/mL heparin, and 15 μg/mL of polymyxin B (a well-established pharmacologic antagonist of LPS). The peritoneal fluids were collected and centrifuged at 740 g for 10 min. The supernatants were collected and stored at −80 °C until use, while the pellets were used to do the differential cell count.
2.7. Differential cell counting
Leukocyte counts were performed with Turk's blood diluent (Azer Scientific, PA, USA) in a Neubauer chamber, and values were expressed as mean ± standard error of the mean (SEM). In addition, cell differential was determined on blood and exudate smears with VWR® hematology quick stain (single step Wright's staining; VWR International, PA, USA) and expressed in percentage as mean ± SEM.
2.8. Quantification of inflammatory mediators
The levels of inflammatory mediators (IL-6, IL-8/the mouse IL-8 paralog keratinocyte-derived chemokine (KC), TNF-α, IL-1β, IL-10, and MMP-9) were determined in cultured supernatants, and mouse plasma and peritoneal cavity exudate by enzyme-linked immunosorbent assay (ELISA) using commercial kits (Thermo Scientific, IL, USA; Peprotech, NJ, USA; R&D systems, MN, USA). The samples were evaluated in duplicates and at different dilutions (the dilution level was determined for each sample to better fit within the detection range of the ELISA). The absorbances were read at 405 or 450 nm at the completion of every assay, according to the manufacturer's protocol. The concentrations were calculated employing a standard curve with recombinant cytokines/chemokines and expressed as pg/mL.
2.9. Data analysis
All results were obtained from two or more independent experiments. The results were expressed as mean ± standard deviation (SD) or SEM according to the assay performed. The student's t-test was estimated to evaluate significant differences between the control and experimental groups. We also employed one-way ANOVA followed by Tukey's post-hoc test to evaluate significant differences between the control and experimental groups in the release of inflammatory mediators on MDM. Values of p < 0.05 and 0.01 were considered statistically significant for all experiments.
3. Results
3.1. Effects of Css-CRiSP on HDBEC and HDLEC activation
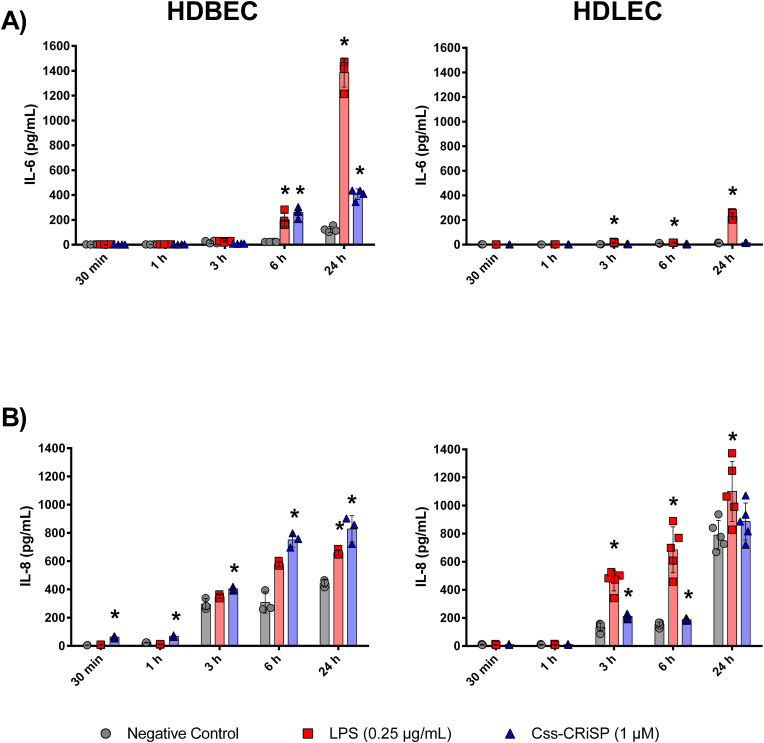
Css-CRiSP induced a remarkable release of IL-6 and IL-8 on HDBEC compared to HDLEC (Fig. 1). Our toxin induced the release of IL-6 in HDBEC after 6 h and 24 h of treatment but not in HDLEC. The higher value obtained was detected at 24 h (427 ± 16 pg/mL), compared to the negative control (134 ± 31 pg/mL) (p < 0.05) (Fig. 1A). In addition, the release of IL-8 was detected in HDBEC starting at 30 min after treatment with Css-CRiSP. After 3 h, the values obtained were comparable to those seen with the positive control LPS, especially at 24 h post-treatment (673 ± 37 pg/mL vs. 667 ± 27 pg/mL, respectively). Meanwhile, the release of this chemokine was seen at 3 h and 6 h in HDLEC incubated with Css-CRiSP, being the response lower than the one seen with LPS (210 ± 16 pg/mL vs. 465 ± 72 pg/mL at 3 h, and 188 ± 8 pg/mL vs. 741 ± 163 pg/mL at 6 h, respectively) (Fig. 1B). No release of other inflammatory mediators (TNF-α, IL-1β, IL-10, and MMP-9) was detected in endothelial cells (data not shown).
Fig. 1.
Release of inflammatory mediators in HDBEC and HDLEC. Cells were treated with Css-CRiSP (1 μM) or LPS (0.25 μg/mL). At different time points, supernatants were collected and IL-6 (A) and IL-8 (B) were determined by ELISA. Results represent 3 independent experiments in duplicate (mean ± SD). *p < 0.05 vs. negative control. Negative control: cells treated with PBS. Positive control: cells treated with LPS.
3.2. Effects of Css-CRiSP on MDM activation
We performed a cell viability assay on MDM to confirm the findings seen in supernatants were not due to loss of viability. There was no evidence of detectable morphological changes or loss of cell viability as measured by a CellTiter-Blue® assay (Data not shown).
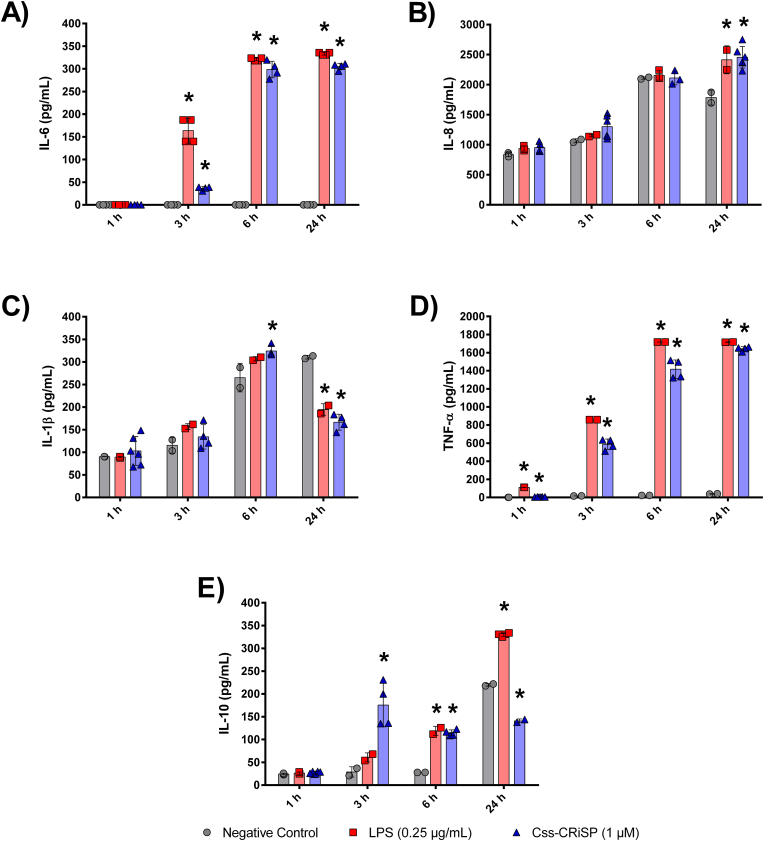
The treatment with 1 μM of Css-CRiSP promoted macrophagic activation by inducing the release of inflammatory mediators in our model of MDM. Our results showed the production and secretion of the pro-inflammatory cytokines IL-6, IL-8, TNF-α, and IL-1β. TNF-α was significantly increased after 1 h of treatment, with values similar to those obtained with the positive control. The highest levels of IL-6 (305 ± 7 pg/mL), IL-8 (2456 ± 179 pg/mL), and TNF-α (1642 ± 23 pg/mL) were seen at 24 h, while the significant increase of IL-1β was at 6 h post-treatment (324 ± 12 pg/mL) (Fig. 2). Regarding the anti-inflammatory cytokine IL-10, the highest level was detected at 3 h post-treatment (176 ± 48 pg/mL) in macrophages incubated with Css-CRiSP. However, it decreased to 115 ± 10 pg/mL at 6 h. There were no significant alterations observed at 24 h, whit the values obtained (141 ± 4 pg/mL), being lower to those seen with the negative control (220 ± 2 pg/mL) and LPS (330 ± 4 pg/mL) (Fig. 2E).
Fig. 2.
Release of inflammatory mediators in MDM. Cells were treated with Css-CRiSP (1 μM) or LPS (0.25 μg/mL). At different time points, supernatants were collected and IL-6 (A), IL-8 (B), IL-1β (C), TNF-α (D), and IL-10 (E) were determined by ELISA. Results represent 3 independent experiments in duplicate (mean ± SD). *p < 0.05 vs. negative control. Negative control: cells treated with PBS. Positive control: cells treated with LPS.
3.3. Effects of Css-CRiSP in an experimental model of mice
In order to assess the acute effects of Css-CRiSP on local and systemic inflammatory responses, and compare it to the response seen with whole venom, BALB/c mice were i.p. injected with either PBS, 68.5 μg of C. s. scutulatus crude venom, or 2 μg of Css-CRiSP. Blood and peritoneal exudates were collected 30 min after the injection for hematological analysis and the production of inflammatory mediators. The whole blood of mice treated with Css-CRiSP showed a significant decrease in the total number of white blood cell (WBC) count, with a lower percentage of neutrophils, and a higher proportion of monocytes and eosinophils, compared to the negative control of mice injected with PBS. In contrast, the experimental group injected with whole venom showed a remarkable increase in WBC count, with a higher proportion of neutrophils, compare to the negative control and the Css-CRiSP group (Table 1).
Table 1.
Effect of Css-CRiSP (2 μg/mouse) and C. s. scutulatus crude venom (68.5 μg/mouse) on leukocytes in BALB/c mice whole blood, 30 min post-injection i.p.
| Group | Total WBC (x 103/mm3) | Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) | Basophils (%) |
|---|---|---|---|---|---|---|
| Control | 3.2 ± 0.3 | 17 ± 3 | 78 ± 3 | 3 ± 1 | 2 ± 0 | 1 ± 0 |
| Crude venom | 5.0 ± 0.6** | 24 ± 3* | 69 ± 2** | 5 ± 1 | 2 ± 0 | 0 ± 0 |
| Css-CRiSP | 1.9 ± 0.2** | 9 ± 1* | 80 ± 2 | 6 ± 1** | 5 ± 1* | 0 ± 0 |
n = 10; bold = statistically significant difference compared to control group (injected with PBS); *p < 0.05; **p < 0.01.
In addition, peritoneal exudates from both experimental groups exhibited a significant increase in the total number of cells counted, along with a higher proportion of polymorphonuclear cells, compared to the negative control group. The highest cell count was seen in the group treated with crude venom (Table 2).
Table 2.
Effect of Css-CRiSP (2 μg/mouse) and C. s. scutulatus crude venom (68.5 μg/mouse) on leukocytes in BALB/c mice peritoneal exudate, 30 min post-injection i.p.
| Group | Cell Count (x 103/mm3) | Polymorphonuclear Cells (%) | Mononuclear Cells (%) |
|---|---|---|---|
| Control | 1.3 ± 0.3 | 12 ± 2 | 88 ± 2 |
| Crude venom | 4.6 ± 0.7** | 19 ± 2* | 82 ± 2* |
| Css-CRiSP | 2.5 ± 0.4* | 20 ± 2* | 80 ± 2* |
n = 10; bold = statistically significant difference compared to control group (injected with PBS); *p < 0.05.
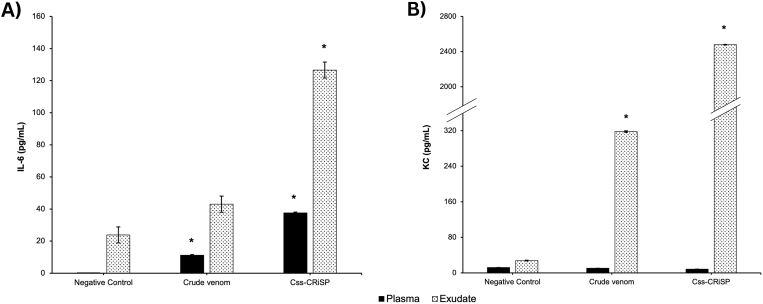
To further analyze the inflammatory events in vivo, we measured the levels of pro-inflammatory cytokines TNF-α, IL-6, and KC in plasmas and peritoneal exudate samples (Fig. 3). Css-CRiSP caused a remarkable increase of IL-6 and KC concentrations 30 min post-injection in the exudates of mice, with values of 127 ± 1 pg/mL and 2563 ± 3 pg/mL, respectively. On the other hand, exudates from mice treated with crude venom only showed a significant increase in KC levels (318 ± 2 pg/mL). Furthermore, a modest increase of IL-6 was detected in the plasma of mice treated with Css-CRiSP (38 ± 1 pg/mL) and crude venom (13 ± 1 pg/mL) in comparison to the negative control (0 ± 1 pg/mL) (Fig. 3A). TNF-α was not detected in the samples analyzed (results not shown).
Fig. 3.
Effect of Css-CRiSP and C. s. scutulatus crude venom in the production pro-inflammatory mediators. BALB/c mice were treated with Css-CRiSP (2 μg/mouse), C. s. scutulatus crude venom (68.5 μg/mouse) or PBS (negative control) for 30 min. Then, samples were collected and levels of IL-6 (A) and KC (B) in mouse plasma (black bars) and peritoneal cavity exudate (dotted bars) were determined using ELISA kits. Results represent 2 independent experiments in duplicate (mean ± SD). n = 10. *p < 0.05 vs. negative control.
4. Discussion
The Mojave rattlesnake (C. s. scutulatus) is considered a medically important species in North America, having a distribution that comprises the southwestern region of the United States, specifically in southern California, southern Nevada, southwestern Utah, most of Arizona, southern New Mexico, the Trans-Pecos region of Texas, and extending into areas of Mexico with desertic and xeric vegetation (Campbell et al., 2004; Dobson et al., 2018). It has been well described the geographic variation within the species according to its biochemical properties, with two distinct populations being described: a population displaying neurotoxic properties with low proteolytic activity, named type A, and the second that lacks the neurotoxic component and shows high proteolytic/hemorrhagic activity, designated as type B (Glenn and Straight, 1978; Dobson et al., 2018). This distinction is based on the presence of the Mojave toxin, a dimeric complex exhibiting pre-synaptic neurotoxicity and phospholipase A2 (PLA2) activity (type A), and snake venom metalloproteinases (SVMPs) of the PI and PIII subfamilies (type B). However, a third group has been described showing both activities (designated as type A + B), and more recently, new phenotypes have been discovered based on the predominance of myotoxin-A (M) and named as type A + M, type B + M, and type A/B + M (Massey et al., 2012; Dobson et al., 2018).
Snakebite envenomings (SBE) caused by this particular species exhibit a notable phenotypical variation, leading to diverse clinical manifestations. SBE resulting from type A group snakes typically lack the common local tissue effects observed in most crotalid envenomings. On the other hand, SBE caused by type B group individuals display hemorrhagic manifestations and exhibit the classic local tissue damage seen in other North American rattlesnake SBE, such as ecchymosis and necrosis (Hardy, 1983; Farstad et al., 1997). However, regardless of the type, swelling and local edema are commonly described symptoms in most SBE caused by Mojave rattlesnakes (Farstad et al., 1997). In some cases, immediate angioedema or anaphylactic/anaphylactoid reactions can occur, probably due to immune activation and the release of various mediators from mast cells. These severe reactions may necessitate prompt intubation for proper management (Smelski et al., 2023).
Swelling and local edema are also hallmarks of North American pit viper SBE (Lavonas et al., 2011; Kanaan et al., 2015). In severe cases, major swelling can produce ischemia and neural compression, causing permanent disability in the victims (Teixeira et al., 2009). In addition, it has been shown that antivenom therapy does not reverse the local alterations of snake venoms, and several animal studies with viper venoms have demonstrated that pre-incubation with antivenom has poor neutralizing capacity on edema formation, suggesting the involvement of inflammatory mediators (Teixeira et al., 2009, 2019).
Snake venom toxins disrupt the homeostasis of the victim through various mechanisms, triggering the activation and development of acute inflammatory responses. This leads to the recruitment and activation of immune cells, and the release of chemical mediators (Menaldo et al., 2017; Mamede et al., 2020). While inflammation is typically involved in tissue repair processes, it has been observed that it plays a preeminent role in the pathophysiology of local effects seen in SBE, exacerbating local tissue damage and resulting in critical outcomes such as sustained edema, impaired tissue function, myonecrosis, and systemic effects (Mamede et al., 2020). Traditionally, these effects have been attributed to major toxin families in snake venoms, such as PLA2 and SVMPs. However, other toxin families ubiquitously present in these venoms, such as CRiSP, can also play a significant role in the inflammatory process during SBE (Bernardes et al., 2019; Bickler, 2020; Deka et al., 2020). For example, Natrin, a svCRiSP isolated from Naja atra venom, has been shown to act as an inflammatory modulator on endothelial cells. It induces the expression of cell adhesion molecules by activating MAPK and NF-κB signaling pathways (Wang et al., 2010).
The endothelial barrier plays a critical role in acting as sentinel in the early events of innate immunity. Upon activation by foreign agents and detrimental endogenous materials, a disruption in the balance between the cell-cell junction and the release of chemical mediators will allow the activation and trafficking of leukocytes to the site of injury (Mai et al., 2013). In our previous studies, we have demonstrated that svCRiSPs derived from North American snakes can increase vascular permeability in vivo and enhance endothelial permeability in HDBEC and HDLEC (Suntravat et al., 2019, 2021). These findings suggest the involvement of specific cell signaling pathways that may contribute to the initiation or progression of the inflammatory response.
In this study, we investigated the release of key inflammatory mediators in the supernatants of HDBEC and HDLEC following treatment with Css-CRiSP (1 μM) at various time points. Our findings demonstrate that Css-CRiSP induces the release of pro-inflammatory mediators in both HDBEC and HDLEC (Fig. 1). Notably, the activation of HDBEC was more pronounced compared to HDLEC, as significant levels of IL-6 were detected at 6 h post-treatment. Furthermore, we observed a rapid and robust release of the chemotactic factor IL-8 in HDBEC, starting as early as 30 min after adding the toxin. These distinct responses are probably related to the heterogenicity among endothelial cells based on their origin, which may induce different responses to physical, biochemical and environmental stimuli (Potente and Mäkinen, 2017; Krüger-Genge et al., 2019; Przysinda et al., 2020). Our results agree with our previous data showing the upregulation of adhesion molecules and the phosphorylated mechanistic target of rapamycin (mTOR-pS2448) in HDBEC treated for 30 min with Css-CRiSP (Suntravat et al., 2021). mTOR has a complex and variable role in cells, being involved in the regulation of various processes such as energy metabolism, autophagy, protein synthesis, and inflammation (Weichhart et al., 2015; Saxton and Sabatini, 2017; Soltani et al., 2018). In this sense, the mTOR signaling pathway is generally linked to the activation of cytokine receptors, tyrosine kinase receptors, growth factor receptors, and Toll-like receptors (TLRs) signaling, thus modulating the immune response (Weichhart et al., 2015; Saxton and Sabatini, 2017; Soltani et al., 2018; Li et al., 2018). Our findings suggest Css-CRiSP could not only promote endothelial permeability but also trigger the release of cytokines in these cells through the crosstalk between PI3K/Akt/mTOR and TLRs/NF-κB signaling pathways, which have been reported to promote inflammation against specific antigens (Liu et al., 2017; Li et al., 2018).
In agreement with previous reports showing no cytotoxic effects of Css-CRiSP on HDBEC and HDLEC (Suntravat et al., 2021), we confirmed this toxin did not induce loss of cell viability on MDM. Moreover, our findings demonstrated that Css-CRiSP induces macrophage activation as early as 1 h post-treatment, promoting the early release of TNF-α. This activation is accompanied by the release of other pro-inflammatory cytokines including IL-1β, IL-8 and IL-6. Additionally, Css-CRiSP promotes the secretion of the immunoregulatory cytokine IL-10. However, this release decreased over time, with values lower than those seen in the negative control at 24 h. Macrophages are responsible for antigen presentation and phagocytosis and modulate the immune response through the induction of cytokine, chemokine, and growth factor production (Fujiwara and Kobayashi, 2005; Boda et al., 2018). Deka et al. (2020) investigated the effects of Nk-CRISP, a CRiSP isolated from Naja kaouthia venom, on macrophages. They found that after a 2 h treatment, Nk-CRISP led to the upregulation of the mRNA expression of pro-inflammatory mediators such as TNF-α, IL-1β, IL-6, and COX-2. They also demonstrated the possible interaction between the cysteine-rich domain region (CDR) of natrin (a CRiSP from Naja atra) with the TLR4-MD2 receptor using molecular docking studies. These findings provide further evidence of the role of svCRiSP in the innate immune response and suggest a possible mechanism of action similar to other toxins that act as venom-associated molecular patterns (VAMPs) (Zoccal et al., 2014; Deka et al., 2020). Here, we confirm that Css-CRiSP acts not only on endothelial cells but also in macrophages, possibly through a common pathway involving the same receptors, such as the TLRs, that turns these cell lines into a pro-inflammatory profile. However, additional research is essential to identify the signaling pathways activated in these cell lines and to fully understand the local and inflammatory effects at different doses on the cellular level. This inquiry is crucial for gaining insights into the pathological and functional impact on recipient cell profiles and behavior.
The injection of snake venom triggers the activation of resident cells, leading to the synthesis and release of molecules such as cytokines, histamine, nitric oxide, and eicosanoids (Teixeira et al., 2009; Menaldo et al., 2017; Gutiérrez et al., 2018). These molecules, in turn, play a crucial role in mediating diverse effects, including chemotaxis, vasodilation, and increased vascular permeability (Zamora et al., 2000; Kunder et al., 2011; Ashina et al., 2015; Yamaguchi et al., 2022). Based on the important role of CRiSPs from North American rattlesnakes appear to have in the acute effect on vascular and endothelial permeability both in vitro and in vivo, regulation of endothelial permeability, and modulation of key signaling pathways, we conducted in vivo studies to demonstrate how Css-CRiSP could exert its acute pro-inflammatory effects locally and systemically using a mouse model. These experiments were performed using a dose of 2 μg/mouse, which represented the proportion of svCRiSP (∼3%) found in the proteome of Mojave rattlesnake venom. In addition, we included a group of mice treated with a sub-lethal dose of C. s. scutulatus crude venom (68.5 μg/mouse) to compare the responses and estimate the possible contribution of Css-CRiSP in the inflammatory manifestations induced by the whole venom (Suntravat et al., 2021). The careful selection of this dosage aimed to secure the survival of all animals in the early stages of snake envenomation. Still, it produced acute local and systemic effects at the molecular level during the initial stage of snake envenomation in animals, as elucidated in our recent publication by Reyes et al. (2023).
It is also important to highlight the quantity of C. s. scutulatus crude venom and Css-CRiSP used in our in vivo experiment correspond to 216 mg/60 kg human body mass and 6.48 mg/60 kg human body mass, respectively. When considering the venom amount, factors like snake size play a role, affecting the potential quantity obtainable through manual venom extraction or injection in a single snakebite (de Roodt et al., 2016). However, this aspect, particularly concerning most North American Rattlesnakes, lacks systematic study. Notably, most C. atrox adults share a similar size with C. scutulatus (Schuett et al., 2016; Cardwell et al., 2022). Reports indicate that the amount of venom collected from C. atrox through electrical venom extraction ranged from 173 to 330 mg (Glenn et al., 1972). Therefore, the dosage of C. s. scutulatus crude venom employed in our in vivo experiment falls within the potential range of a single snakebite injected, possibly mimicking the high dose of C. s. scutulatus crude venom and Css-CRiSP injected in humans.
The i.p. administration of Css-CRiSP induced an early decrease of leukocytes in the blood and an influx of cells into the peritoneal cavity of the experimental group, being the proportion of polymorphonuclear cells, specifically neutrophils, the main population of leukocytes being significantly shifted in both biofluids (Table 1, Table 2). Previous studies conducted with different doses of Bj-CRP, a svCRiSP isolated from Bothrops jararaca venom, revealed similar results at later times (1 and 4 h post-treatment), showing an increase of neutrophil count in peritoneal exudate with no changes in the mononuclear cell count, being the most remarkable effects with the highest dose tested (10 μg/mouse) (Lodovicho et al., 2017). Likewise, 10 μg/mouse of a svCRiSP from Bothrops alternatus, BaltCRP also caused a major influx of neutrophils into the peritoneum, with a higher count of leukocytes after 4 h (Bernardes et al., 2019). We showed that a low dose of Css-CRiSP (2 μg/mouse) promotes the migration of neutrophils from blood circulation to the site of injection as early as 30 min post-treatment, significantly altering the proportion of these population in peritoneal exudate in a fashion that also affects the ratio of mononuclear cells and the total cell count.
On the other hand, mice treated with crude venom also showed a significant shift in the proportion of neutrophils in blood and exudate, but this group displayed a remarkable increase of leukocyte count in both biofluids, indicating a possible local and systemic activation of immune responses at early times. Studies conducted with Bothrops sp. venoms have shown the ability of this genus to induce local inflammatory effects through changes in vascular permeability, edema and the acute accumulation of leukocytes into the site of injection (Zamuner et al., 2001;Zamuner and Teixeira, 2002; Zuliani et al., 2005; Fernandes et al., 2006). In this sense, these events were attributed to the main toxin families found in this genus, SVMPs, SVSPs and PLA2s, capable of promoting specific molecular signaling pathways that in turn could lead to the release of metabolites known for their proinflammatory and vasoactive effects (Moreira et al., 2012; Bernardes et al., 2015; Menaldo et al., 2017; Echeverría et al., 2018; Mamede et al., 2020). In this study, we showed that besides the main toxin families found in type A Mojave rattlesnake venom, such as SVSPs and PLA2s, CRiSP could also contribute to the extravasation of leukocytes to the site of injection; however, this toxin does not appear to affect the differential cell counting changes seen in blood circulation in mice treated with crude venom, where an important increase of the proportion of neutrophils was seen with a relevant decrease of the lymphocyte population.
In addition, Css-CRiSP exhibited pro-inflammatory effects by inducing the acute release of IL-6 and KC in the exudates of the experimental group while also increasing IL-6 levels in the plasma of mice treated with the toxin (Fig. 3). In contrast, the whole venom only induced a significant release of KC in the exudate, and promoted a remarkable increase of IL-6 in blood, being these effects lower than those seen with Css-CRiSP. IL-6 is related to the early events of inflammation, promoting cell activation and regulating the recruitment of leukocytes into the site of injury (Fielding et al., 2008; Yu et al., 2013; Bernardes et al., 2019). This cytokine is involved in venom-induced leukocyte influx, mediating the maturation of neutrophils in bone marrow and increasing their levels in circulation, while also upregulating the expression of adhesion molecules (Fasshauer et al., 2003; Bernardes et al., 2015). KC is one of the major murine chemoattractants, a homolog of the human growth-related oncogene (GRO) chemokines that are functionally similar to IL-8. It is mainly produced by peritoneal macrophages but can be secreted by other cell types as well (De Filippo et al., 2008). KC plays a crucial role in promoting neutrophil recruitment and modulating vascular permeability by affecting down-regulation of tight junctions, a mechanism akin to the actions of IL-8 (De Filippo et al., 2008; Yu et al., 2013).
The high levels of KC found in the experimental group provide more insights about the relevant pro-inflammatory role of Css-CRiSP. It appears to directly or indirectly activate neighboring vascular endothelial cells, potentially through the activation of resident cells that secrete mediators. This activation may lead to increased vascular permeability, release of chemotactic factors, and expression of cell adhesion molecules. Consequently, these molecular events facilitate the activation and migration of cells into the peritoneal cavity, thereby intensifying the inflammatory response. By comparing the effects seen with Css-CRiSP only and the whole venom, the lower response seen after treatment with the latter could be a result of modulatory effects of other toxins present in the venom that could impact the development of the immune response at early times, exerting a regulatory effect on the release of inflammatory mediators or modifying the timeline of the immune response in our murine model. Regardless, more studies are needed to elucidate the dynamics between the toxins that make up the venom of this crotalid.
Lastly, similar to the findings observed with other snake venom toxins, our study did not observe significant variations in TNF-α levels with the whole venom or Css-CRiSP. This could be attributed to the regulatory effect of IL-6, which has been shown to downregulate the expression of TNF-α, thereby limiting the inflammatory process (Bernardes et al., 2019). Nevertheless, additional investigations are warranted to assess the presence of TNF-α at different time points and further elucidate its role in the context of our study.
In summary, inflammation is a pathophysiological response to injury and obnoxious stimulus. The inflammatory response caused by SBE can cause dysregulation between the pro-inflammatory mediators and their natural inhibitors, aiding the severity and persistence of the local effects and modulating the systemic clinical manifestations (Gutiérrez et al., 2018; Mamede et al., 2020). While the prominent toxin families found in snake venoms, such as SVMP and PLA2, are widely recognized as the primary contributors to this response, either independently or synergistically, our study contributes further evidence highlighting the significant pro-inflammatory role of svCRiSP. We demonstrate that crotalid CRiSP induces the release of key pro-inflammatory cytokines and chemokines in endothelial cells and macrophages and promotes local and systemic activation of innate immunity in a murine model, contributing to the inflammatory manifestations seen with Mojave rattlesnake venom. Our findings underscore the importance of svCRiSP in the complex interplay of snake venom toxins and their impact on the immune system. We also suggest a novel pathway that might involve mTOR and TLRs/NF-κB signaling to upregulate the release of pro-inflammatory cytokines on endothelial cells. Further investigations are necessary to uncover the precise mechanism of action of Css-CRiSP and identify the specific receptors it targets. This deeper understanding is crucial in comprehending the biological role of Css-CRiSP in vascular dysfunction and the persistent inflammatory response observed in snakebites caused by Mojave rattlesnakes. By unraveling these details, we can gain valuable insights into the pathophysiology of SBE and pave the way for developing more effective therapeutic strategies to alleviate the morbidity and mortality associated with these bites.
Funding
This research was supported by the NIH/AREA, NIH/NHLBI grant #2R15HL137134-02 (Texas A&M University-Kingsville, M. Suntravat), NIH/ORIP, Viper Resource Center grant #P40OD01960-18 (NNTRC, Texas A&M University-Kingsville, E.E. Sánchez), the Texas A&M University's Presidential Undergraduate Research Program and Ronald E. McNair Scholars Program, the 2021–2022 Research Supports, College of Arts and Sciences (Texas A&M University-Kingsville, M. Suntravat), the Spring 2023 Research Support, the Dick and Mary Lewis Kleberg College of Agriculture and Natural Resources (Texas A&M University-Kingsville, M. Suntravat), and the Robert A. Welch Foundation, grant #AC-0006 (TAMUK-Department of Chemistry).
Ethical statement
We, the authors, hereby submit our manuscript titled “Snake Venom Cysteine-Rich Secretory Protein from Mojave Rattlesnake Venom (Css-CRiSP) Induces Acute Inflammatory Responses on Different Experimental Models” to Toxicon: X for consideration of publication. We affirm that this manuscript represents original work and has not been previously published, nor is it under consideration for publication elsewhere.
We adhered to the highest ethical standards and guidelines. The animal research followed all applicable regulations and followed the ethical principles outlined in the Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee of Texas A&M University-Kingsville, Texas, USA, before commencing the experiments.
We conducted the research with integrity and transparency, ensuring accurate and reliable data collection, analysis, and interpretation. All necessary steps were taken to minimize potential conflicts of interest that could compromise the objectivity and impartiality of the findings.
Throughout the manuscript, we have appropriately acknowledged the contributions and ideas of other researchers by citing relevant references. Any potential conflicts of interest among the authors that could influence the interpretation or presentation of the results have been disclosed.
We have provided accurate and complete information in the manuscript, including methods, results, and discussion. Any instances of data manipulation or fabrication are absent from our research.
We are committed to responding to any queries or concerns raised by the editorial board or peer reviewers regarding the ethical conduct of our study. We are open to providing additional details or clarification as required.
By submitting this manuscript to Toxicon: X, we affirm that our research has adhered to rigorous ethical standards, ensuring the welfare of animals, maintaining privacy and confidentiality, and upholding academic integrity.
CRediT authorship contribution statement
Emelyn Salazar: Writing - original draft, Visualization, Investigation, Formal analysis, Data curation. Abcde Cirilo: Writing - original draft, Visualization, Investigation, Formal analysis. Armando Reyes: Visualization, Investigation. Martha Barrientos: Investigation. Jacob Galan: Writing - review & editing. Elda E. Sánchez: Writing - review & editing, Resources, Funding acquisition. Montamas Suntravat: Writing - review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge Dr. Peter Davies (Director, Center for Translational Cancer Research, Institute of Biosciences & Technology, Texas A&M University, Houston, TX) for contributing valuable suggestions to the overall scope of the program. We would also like to thank Nora Diaz DeLeon for her administrative assistance, Mark Höckmuller and Juan Salinas (NNTRC Serpentarium curator and animal room technician) and all the NNTRC personnel.
Handling editor: Ray Norton
Data availability
Data will be made available on request.
References
- Ashina K., Tsubosaka Y., Nakamura T., Omori K., Kobayashi K., Hori M., Ozaki H., Murata T. Histamine induces vascular Hyperpermeability by increasing blood flow and endothelial barrier disruption in vivo. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes C.P., Menaldo D.L., Mamede C.C., Zoccal K.F., Cintra A.C., Faccioli L.H., Stanziola L., de Oliveira F., Sampaio S.V. Evaluation of the local inflammatory events induced by BpirMP, a metalloproteinase from Bothrops pirajai venom. Mol. Immunol. 2015;68(2 Pt B):456–464. doi: 10.1016/j.molimm.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Bernardes C.P., Menaldo D.L., Zoccal K.F., Boldrini-França J., Peigneur S., Arantes E.C., Rosa J.C., Faccioli L.H., Tytgat J., Sampaio S.V. First report on BaltCRP, a cysteine-rich secretory protein (CRISP) from Bothrops alternatus venom: effects on potassium channels and inflammatory processes. Int. J. Biol. Macromol. 2019;140:556–567. doi: 10.1016/j.ijbiomac.2019.08.108. [DOI] [PubMed] [Google Scholar]
- Bickler P.E. Amplification of snake venom toxicity by endogenous signaling pathways. Toxins. 2020;12(2) doi: 10.3390/toxins12020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda F., Banfai K., Garai K., Curticapean A., Berta L., Sipos E., Kvell K. Effect of Vipera ammodytes ammodytes snake venom on the human cytokine network. Toxins. 2018;10(7):259. doi: 10.3390/toxins10070259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.A., Lamar W.W., Brodie E.D. Comstock Pub. Associates; 2004. Venomous Reptiles of the Western Hemisphere. [Google Scholar]
- Cardwell M.D., Massey D.J., Smelski G., Wüster W. Mohave rattlesnake (Crotalus scutulatus) identification revisited. Wilderness Environ. Med. 2022;33(2):210–218. doi: 10.1016/j.wem.2022.01.003. [DOI] [PubMed] [Google Scholar]
- Cavalcante J.S., Brito I., De Oliveira L.A., De Barros L.C., Almeida C., Rossini B.C., Sousa D.L., Alves R.S., Jorge R., Santos L. Experimental Bothrops atrox envenomation: blood plasma proteome effects after local tissue damage and perspectives on thromboinflammation. Toxins. 2022;14(9):613. doi: 10.3390/toxins14090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante J., de Almeida C., Clasen M.A., da Silva E.L., de Barros L.C., Marinho A.D., Rossini B.C., Marino C.L., Carvalho P.C., Jorge R., Dos Santos L.D. A fingerprint of plasma proteome alteration after local tissue damage induced by Bothrops leucurus snake venom in mice. J. Proteonomics. 2022;253 doi: 10.1016/j.jprot.2021.104464. [DOI] [PubMed] [Google Scholar]
- De Filippo K., Henderson R.B., Laschinger M., Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 2008;180(6):4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- de Roodt A.R., Boyer L.V., Lanari L.C., Irazu L., Laskowicz R.D., Sabattini P.L., Damin C.F. Venom yield and its relationship with body size and fang separation of pit vipers from Argentina. Toxicon. 2016;121:22–29. doi: 10.1016/j.toxicon.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Deka A., Sharma M., Mukhopadhyay R., Devi A., Doley R. Naja kaouthia venom protein, Nk-CRISP, upregulates inflammatory gene expression in human macrophages. Int. J. Biol. Macromol. 2020;160:602–611. doi: 10.1016/j.ijbiomac.2020.05.169. [DOI] [PubMed] [Google Scholar]
- Dias-Netipanyj M.F., Boldrini-Leite L.M., Trindade E.S., Moreno-Amaral A.N., Elifio-Esposito S. Bjcul, a snake venom lectin, modulates monocyte-derived macrophages to a pro-inflammatory profile in vitro. Toxicol. Vitro: an international journal published in association with BIBRA. 2016;33:118–124. doi: 10.1016/j.tiv.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Dobson J., Yang D.C., Op den Brouw B., Cochran C., Huynh T., Kurrupu S., Sánchez E.E., Massey D.J., Baumann K., Jackson T.N.W., Nouwens A., Josh P., Neri-Castro E., Alagón A., Hodgson W.C., Fry B.G. Rattling the border wall: pathophysiological implications of functional and proteomic venom variation between Mexican and US subspecies of the desert rattlesnake Crotalus scutulatus. Comparative biochemistry and physiology. Toxicology & pharmacology: CB (Curr. Biol.) 2018;205:62–69. doi: 10.1016/j.cbpc.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría S., Leiguez E., Guijas C., do Nascimento N.G., Acosta O., Teixeira C., Leiva L.C., Rodríguez J.P. Evaluation of pro-inflammatory events induced by Bothrops alternatus snake venom. Chem. Biol. Interact. 2018;281:24–31. doi: 10.1016/j.cbi.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Farstad D., Thomas T., Chow T., Bush S., Stiegler P. Mojave rattlesnake envenomation in southern California: a review of suspected cases. Wilderness Environ. Med. 1997;8(2):89–93. doi: 10.1580/1080-6032(1997)008[0089:MREISC]2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Fasshauer M., Klein J., Lossner U., Paschke R. Interleukin (IL)-6 mRNA expression is stimulated by insulin, isoproterenol, tumour necrosis factor alpha, growth hormone, and IL-6 in 3T3-L1 adipocytes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2003;35(3):147–152. doi: 10.1055/s-2003-39075. [DOI] [PubMed] [Google Scholar]
- Fernandes C.M., Zamuner S.R., Zuliani J.P., Rucavado A., Gutiérrez J.M., Teixeira C.deF. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: leukocyte recruitment and release of cytokines. Toxicon. 2006;47(5):549–559. doi: 10.1016/j.toxicon.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Fielding C.A., McLoughlin R.M., McLeod L., Colmont C.S., Najdovska M., Grail D., Ernst M., Jones S.A., Topley N., Jenkins B.J. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008;181(3):2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Kobayashi K. Macrophages in inflammation. Curr. Drug Targets - Inflamm. Allergy. 2005;4(3):281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- Glenn J., Straight R. Mojave rattlesnake Crotalus scutulatus scutulatus venom: variation in toxicity with geographical origin. Toxicon. 1978;16(1):81–84. doi: 10.1016/0041-0101(78)90065-x. [DOI] [PubMed] [Google Scholar]
- Glenn J.L., Straight R., Snyder C.C. Yield of venom obtained from Crotalus atrox by electrical stimulation. Toxicon. 1972;10:575–579. doi: 10.1016/0041-0101(72)90118-3. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Rucavado A., Escalante T., Herrera C., Fernández J., Lomonte B., Fox J.W. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon: official journal of the International Society on Toxinology. 2018;148:123–131. doi: 10.1016/j.toxicon.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Hardy D.L. Envenomation by the Mojave rattlesnake (Crotalus scutulatus scutulatus) in southern Arizona. U.S.A. Toxicon : official journal of the International Society on Toxinology. 1983;21(1):111–118. doi: 10.1016/0041-0101(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Kanaan N.C., Ray J., Stewart M., Russell K.W., Fuller M., Bush S.P., Caravati E.M., Cardwell M.D., Norris R.L., Weinstein S.A. Wilderness medical society practice guidelines for the treatment of pitviper envenomations in the United States and Canada. Wilderness Environ. Med. 2015;26(4):472–487. doi: 10.1016/j.wem.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Krüger-Genge A., Blocki A., Franke R.P., Jung F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 2019;20(18):4411. doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunder C.A., St John A.L., Abraham S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118(20):5383–5393. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavonas E.J., Ruha A.M., Banner W., Bebarta V., Bernstein J.N., Bush S.P., Kerns W.P. 2nd, Richardson W.H., Seifert S.A., Tanen D.A., Curry S.C., Dart R.C. Rocky Mountain Poison and Drug Center, Denver Health and Hospital Authority. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg. Med. 2011;11:2. doi: 10.1186/1471-227X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Xi P., Wang Z., Han X., Xu Y., Zhang Y., Miao J. PI3K/Akt/mTOR signaling pathway participates in Streptococcus uberis-induced inflammation in mammary epithelial cells in concert with the classical TLRs/NF-ĸB pathway. Vet. Microbiol. 2018;227:103–111. doi: 10.1016/j.vetmic.2018.10.031. [DOI] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Targeted Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovicho M.E., Costa T.R., Bernardes C.P., Menaldo D.L., Zoccal K.F., Carone S.E., Rosa J.C., Pucca M.B., Cerni F.A., Arantes E.C., Tytgat J., Faccioli L.H., Pereira-Crott L.S., Sampaio S.V. Investigating possible biological targets of Bj-CRP, the first cysteine-rich secretory protein (CRISP) isolated from Bothrops jararaca snake venom. Toxicol. Lett. 2017;265:156–169. doi: 10.1016/j.toxlet.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Mai J., Virtue A., Shen J., Wang H., Yang X.F. An evolving new paradigm: endothelial cells--conditional innate immune cells. J. Hematol. Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamede C.C.N., de Sousa Simamoto B.B., da Cunha Pereira D.F., de Oliveira Costa J., Ribeiro M.S.M., de Oliveira F. Edema, hyperalgesia and myonecrosis induced by Brazilian bothropic venoms: overview of the last decade. Toxicon : official journal of the International Society on Toxinology. 2020;187:10–18. doi: 10.1016/j.toxicon.2020.08.016. [DOI] [PubMed] [Google Scholar]
- Massey D.J., Calvete J.J., Sánchez E.E., Sanz L., Richards K., Curtis R., Boesen K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteonomics. 2012;75(9):2576–2587. doi: 10.1016/j.jprot.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y., Yamazaki Y., Hyodo F., Sugiyama Y., Nozaki, M, Morita T. Structural divergence of cysteine-rich secretory proteins in snake venoms. J. Biochem. 2009;145:365–375. doi: 10.1074/jbc.M110.146290. [DOI] [PubMed] [Google Scholar]
- Menaldo D.L., Bernardes C.P., Zoccal K.F., Jacob-Ferreira A.L., Costa T.R., Del Lama M.P., Naal R.M., Frantz F.G., Faccioli L.H., Sampaio S.V. Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A2 from Bothrops atrox venom. Mol. Immunol. 2017;85:238–247. doi: 10.1016/j.molimm.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Minutti-Zanella C., Gil-Leyva E.J., Vergara I. Immunomodulatory properties of molecules from animal venoms. Toxicon : official journal of the International Society on Toxinology. 2021;191:54–68. doi: 10.1016/j.toxicon.2020.12.018. [DOI] [PubMed] [Google Scholar]
- Moreira V., Dos-Santos M.C., Nascimento N.G., Borges da Silva H., Fernandes C.M., D'Império Lima M.R., Teixeira C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon: official journal of the International Society on Toxinology. 2012;60(1):12–20. doi: 10.1016/j.toxicon.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Potente M., Mäkinen T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017;18(8):477–494. doi: 10.1038/nrm.2017.36. [DOI] [PubMed] [Google Scholar]
- Przysinda A., Feng W., Li G. Diversity of organism-wide and organ-specific endothelial cells. Curr. Cardiol. Rep. 2020;22(4):19. doi: 10.1007/s11886-020-1275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A., Hatcher J.D., Salazar E., Galan J., Iliuk A., Sanchez E.E., Suntravat M. Proteomic profiling of extracellular vesicles isolated from plasma and peritoneal exudate in mice induced by Crotalus scutulatus scutulatus crude venom and its purified cysteine-rich secretory protein (Css-CRiSP) Toxins. 2023;15:434. doi: 10.3390/toxins15070434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R.Y.M., Seymour J., Loukas A., Lopez J.A., Ikonomopoulou M.P., Miles J.J. Immunological responses to envenomation. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.661082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett G.W., Repp R.A., Spencer C.L., Beaman K.R., Painter C.W. In: Schuett G.W., Feldner M.J., Smith C.F., Reiserer R.S., editors. vol. 1. ECO Publishing; New Mexico: 2016. Species accounts: western diamond-backed rattlesnake (Crotalus atrox) pp. 333–394. (Rattlesnakes of Arizona). Rodeo. [Google Scholar]
- Smelski G., Cardwell M., Larsen J. Neurotoxic respiratory failure absent following Arizona rattlesnake bites. Toxicon : official journal of the International Society on Toxinology. 2023;224 doi: 10.1016/j.toxicon.2023.107034. [DOI] [PubMed] [Google Scholar]
- Soltani A., Bahreyni A., Boroumand N., Roshan M.K., Khazaei M., Ryzhikov M., Soleimanpour S., Avan A., Hassanian S.-M. Therapeutic potency of mTOR signaling pharmacological inhibitors in the treatment of proinflammatory diseases, current status, and perspectives. J. Cell Physiol. 2018;233(6):4783–4790. doi: 10.1002/jcp.26276. [DOI] [PubMed] [Google Scholar]
- Suntravat M., Cromer W.E., Marquez J., Galan J.A., Zawieja D.C., Davies P., Salazar E., Sánchez E.E. The isolation and characterization of a new snake venom cysteine-rich secretory protein (svCRiSP) from the venom of the Southern Pacific rattlesnake and its effect on vascular permeability. Toxicon : official journal of the International Society on Toxinology. 2019;165:22–30. doi: 10.1016/j.toxicon.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntravat M., Sanchez O., Reyes A., Cirilo A., Ocheltree J.S., Galan J.A., Salazar E., Davies P., Sanchez E.E. Evaluation of signaling pathways profiling in human dermal endothelial cells treated by snake venom cysteine-rich secretory proteins (svCRiSPs) from North American snakes using reverse phase protein array (RPPA) Toxins. 2021;13(9):613. doi: 10.3390/toxins13090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasoulis T., Isbister G.K. A review and database of snake venom proteomes. Toxins. 2017;9:E290. doi: 10.3390/toxins9090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasoulis T., Isbister G.K. A current perspective on snake venom composition and constituent protein families. Arch. Toxicol. 2023;97(1):133–153. doi: 10.1007/s00204-022-03420-0. [DOI] [PubMed] [Google Scholar]
- Teixeira C., Cury Y., Moreira V., Picolo G., Chaves F. Inflammation induced by Bothrops asper venom. Toxicon : official journal of the International Society on Toxinology. 2009;54(1):67–76. doi: 10.1016/j.toxicon.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Teixeira C., Fernandes C.M., Leiguez E., Chudzinski-Tavassi A.M. Inflammation induced by platelet-activating viperid snake venoms: perspectives on thromboinflammation. Front. Immunol. 2019;10:2082. doi: 10.3389/fimmu.2019.02082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L., Kuo J.H., Lee S.C., Liu J.S., Hsieh Y.C., Shih Y.T., Chen C.J., Chiu J.J., Wu W.G. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J. Biol. Chem. 2010;285(48):37872–37883. doi: 10.1074/jbc.M110.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T., Hengstschläger M., Linke M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2019. Snakebite Envenoming a Strategy for Prevention and Control. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Botta E., Holinstat M. Eicosanoids in inflammation in the blood and the vessel. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.997403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Huang X., Ma Y., Gao M., Wang O., Gao T., Shen Y., Liu X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013;9(9):966–979. doi: 10.7150/ijbs.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora R., Vodovotz Y., Billiar T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000;6(5):347–373. [PMC free article] [PubMed] [Google Scholar]
- Zamuner S.R., Teixeira C.F. Cell adhesion molecules involved in the leukocyte recruitment induced by venom of the snake Bothrops jararaca. Mediat. Inflamm. 2002;11(6):351–357. doi: 10.1080/0962935021000051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamuner S.R., Gutiérrez J.M., Muscará M.N., Teixeira S.A., Teixeira C.F. Bothrops asper and Bothrops jararaca snake venoms trigger microbicidal functions of peritoneal leukocytes in vivo. Toxicon. 2001;39(10):1505–1513. doi: 10.1016/s0041-0101(01)00123-4. [DOI] [PubMed] [Google Scholar]
- Zoccal K.F., Bitencourt C.daS., Paula-Silva F.W., Sorgi C.A., de Castro Figueiredo Bordon K., Arantes E.C., Faccioli L.H. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani J.P. Alarmins and inflammatory aspects related to snakebite envenomation. Toxicon. 2023;226 doi: 10.1016/j.toxicon.2023.107088. [DOI] [PubMed] [Google Scholar]
- Zuliani J.P., Fernandes C.M., Zamuner S.R., Gutiérrez J.M., Teixeira C.F. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45(3):335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Zuliani J.P., Soares A.M., Gutiérrez J.M. Polymorphonuclear neutrophil leukocytes in snakebite envenoming. Toxicon. 2020;187:188–197. doi: 10.1016/j.toxicon.2020.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.