Abstract
Background
Little is known about the prevalence or chronicity of prescriptions of central nervous system-active (CNS-active) medications in older Veterans.
Objective
We sought to describe (1) the prevalence and trends in prescription of CNS-active medications in older Veterans over time; (2) variation in prescriptions across high-risk groups; and (3) where the prescription originated (VA or Medicare Part D).
Design
Retrospective cohort study from 2015 to 2019.
Participants
Veterans age ≥ 65 enrolled in the Medicare and the VA residing in Veterans Integrated Service Network 4 (incorporating Pennsylvania and parts of surrounding states).
Main Measures
Drug classes included antipsychotics, gabapentinoids, muscle relaxants, opioids, sedative-hypnotics, and anticholinergics. We described prescribing patterns overall and in three subgroups: Veterans with a diagnosis of dementia, Veterans with high predicted utilization, and frail Veterans. We calculated both prevalence (any fill) and percent of days covered (chronicity) for each drug class, and CNS-active polypharmacy (≥ 2 CNS-active medications) rates in each year in these groups.
Key Results
The sample included 460,142 Veterans and 1,862,544 person-years. While opioid and sedative-hypnotic prevalence decreased, gabapentinoids exhibited the largest increase in both prevalence and percent of days covered. Each subgroup exhibited different patterns of prescribing, but all had double the rates of CNS-active polypharmacy compared to the overall study population. Opioid and sedative-hypnotic prevalence was higher in Medicare Part D prescriptions, but the percent of days covered of nearly all drug classes was higher in VA prescriptions.
Conclusions
The concurrent increase of gabapentinoid prescribing paralleling a decrease in opioid and sedative-hypnotics is a new phenomenon that merits further evaluation of patient safety outcomes. In addition, we found substantial potential opportunities for deprescribing CNS-active medications in high-risk groups. Finally, the increased chronicity of VA prescriptions versus Medicare Part D is novel and should be further evaluated in terms of its mechanism and impact on Medicare-VA dual users.
Supplementary Information:
The online version contains supplementary material available at 10.1007/s11606-023-08250-z.
INTRODUCTION
The age-friendly health system (AFHS) model seeks to redesign care delivery to make it safer and more aligned with the needs and goals of older adults.1–3 This model centers on four “M”s that represent core components of high-quality care for older adults. One of the “M”s of the AFHS model is Medications, with interconnectedness to the other “M”s: Mentation, Mobility, and what Matters. Growing evidence suggests central nervous system-active (CNS-active) medications are commonly prescribed to older adults. For example, 74% of Medicare beneficiaries with dementia received CNS-active medications in 2014–2015, and 14% received 3 or more.4 These prescriptions have the potential to result in harm through influence on Mentation and Mobility, for example, resulting in falls and/or injury. As a result, there is increasing interest in developing effective interventions to reduce potentially inappropriate prescribing.5,6 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) direct-to-consumer intervention — alone or paired with providing feedback to the prescriber — is effective in reducing prescribing of CNS-active drug classes in several randomized, controlled trials.7,8
In contrast to the Medicare population4,9, data regarding CNS-active prescribing to older Veterans is incomplete.10 Several studies suggest evaluating “dual” prescribing from VA and Medicare Part D is essential, since Veterans often receive prescriptions from both sources, increasing the potential for adverse drug events.11–14 In addition, evaluating prescribing in high-risk groups for adverse drug events (such as Veterans with a diagnosis of dementia) may be particularly important.15 However, the existing literature includes data prior to significant efforts in the VA to reduce prescriptions of opioids and benzodiazepines, does not include other high-risk patient populations (such as frail older Veterans or those with high comorbidity burden), and has focused on isolated drug classes rather than psychoactive medications as a whole.
As part of a quality improvement initiative called the Safer Aging through Geriatrics-Informed Evidence-Based Practices (SAGE) program, funded by the VA Quality Enhancement Research Initiative (QUERI), our team seeks to adapt and implement the EMPOWER intervention across Veterans Integrated Service Network 4 (VISN 4). VISN 4 encompasses a geographic area comprising the whole of Pennsylvania plus parts of neighboring states, and serves more than 180,000 Veterans age 65 and older annually, across 9 VA Medical Centers and 45 outpatient clinics.
In this study, in order to implement the EMPOWER intervention effectively, we sought to understand (1) the prevalence and trends in prescribing of different CNS-active drug classes in older Veterans; (2) variation in prevalence and trends across Veteran populations at high risk of adverse drug events; and (3) variation by where the CNS-active drug prescription originates (i.e., VA or Medicare Part D).
METHODS
Population Description
We created a person-year dataset that included Veterans age ≥ 65 enrolled in VA from 2015 to 2019 and were assigned to a VA hospital or clinic in Veterans Integrated Service Network 4 (VISN 4). To include Medicare Part D prescribing, we excluded Veterans who were not enrolled in Medicare parts A and B or a Medicare Advantage plan during each particular year of the study (N = 228,756 person-years or 10.9%). We did not exclude Veterans without Part D (prescription drug) coverage, since they may be obtaining their medications from the VA (51% of our overall sample of person-years included Part D coverage).
Data Sources
We linked VA and non-VA data sources for each Veteran using scrambled social security numbers. We used the VA Corporate Data Warehouse to identify all VA outpatient prescription dispensing records in a given year. These records provide information on date, quantity, and days’ supply dispensed, drug name, and VA drug class16 for medications dispensed from a VA pharmacy (brick-and-mortar or mail-order). We obtained matched Medicare Part D prescription drug claims from the VA Medicare-Medicaid Analysis Center. We identified prescription dispensing records using analogous methods. This file includes prescriptions in Medicare fee-for-service and Medicare Advantage enrollees. Inpatient hospital and nursing facility prescriptions were excluded because we did not have access to these data in the Medicare population.
Dependent Variables — CNS-Active Medications
Using the VA drug classification system, we identified prescriptions for specific medications within the following drug classes of interest: antipsychotics, gabapentinoids, muscle relaxants, opioids, and sedative-hypnotics. We also identified prescribing rates of medications with high anticholinergic burden, using the Anticholinergic Cognitive Burden scale.17 Medications in level 3 of the scale (highest cognitive burden) were included. To avoid double-counting, we only included anticholinergic medications that did not fall into one of the other categories. For example, an anticholinergic medication for an upper respiratory tract infection or bladder spasm would be counted as an anticholinergic, but an antipsychotic with high anticholinergic burden was classified as an antipsychotic. We also calculated central nervous system (CNS)-active polypharmacy rates, identifying individuals with two or more CNS-active drug classes defined above prescribed concurrently (a minimum of 1 day of overlap) over the course of a single calendar year.
Subgroup Analyses
In addition to the overall population, we were interested in prescribing in specific subgroups who may be more susceptible to adverse drug events.
First, we identified a prevalent cohort of Veterans with a diagnosis of dementia. We mirrored the approach of the Centers for Medicare and Medicaid Services (CMS) 27-item Chronic Conditions file.18 This method relies on a validated list of ICD-9/-10 codes found in any outpatient or inpatient visit prior to or within a specific year (in our case, in VA or Medicare data) to identify older adults with a diagnosis of dementia due to Alzheimer’s disease or other causes.
Second, we identified those with high comorbidity using a high Care Assessment Needs score (CAN score > 90 at any point during the study year).19 We used the maximum CAN score for each Veteran within each year of the study. The CAN score is an algorithm developed internally to the VA to predict 90-day and 1-year hospitalizations and mortality on a scale of 1–100, with 100 connoting highest risk. The CAN score is used for risk stratification in the VA, identifying a cohort of medically ill older Veterans. While it includes dementia as a covariate, it also includes more than thirty other variables including demographics, vital signs, prior utilization, comorbidities, laboratory results, and prescription data. It does not include measures of frailty or nursing home utilization.
Third, we identified a population of frail Veterans using the Jen Frailty Index (JFI), a validated claims-based measure of frailty.20 Veterans with a JFI of 5 or more (corresponding to 1–2 Activity of Daily Living deficiencies) at any point in the year of study were included in the frail cohort. Both the CAN score and JFI require one VA face-to-face visit (outpatient or inpatient) to be calculated; Veterans who did not have a face-to-face visit in a particular year thus had missing data. Since missingness in these scores and prescriptions might be related to a Veteran not regularly engaging with VA care, we conducted a sensitivity analysis removing Veterans from the analysis who did not have any VA visits in that calendar year. We otherwise included Veterans missing a CAN or JFI score in the overall analysis and subgroup analyses not related to CAN or JFI score.
Statistical Analysis
We calculated both prevalence and percent of days covered (PDC) of prescriptions of specific drug classes across the population and our specific subgroups in each calendar year. Prevalence refers to the proportion of Veterans in our cohort who had at least one prescription dispensed of one of our medication classes of interest during one of the years of the study. PDC is a measure of chronicity, defined as the percent of days of each year covered by dispensed medication within each drug class. The sample used for the PDC calculation was the prevalent sample — that is, only those who received a prescription for the specific medication in question. We reported trends over the 5 years of the sample in both prevalence and PDC of specific medication classes in our overall cohort and subgroups, including both VA and non-VA prescriptions. We also calculated the prevalence of CNS-active polypharmacy overall and in our specific subgroups. This study was determined to be quality improvement and IRB approval was not required.
RESULTS
Our overall sample included 460,142 Veterans and 1,862,544 person-years. Veterans had a mean age of 76 (standard deviation, SD, 8.0), 98% were male, and 91% were white. The mean CAN score was 55 (SD 29.0), the mean JFI score was 3.5 (SD 2.1), and 14% had a diagnosis of dementia.
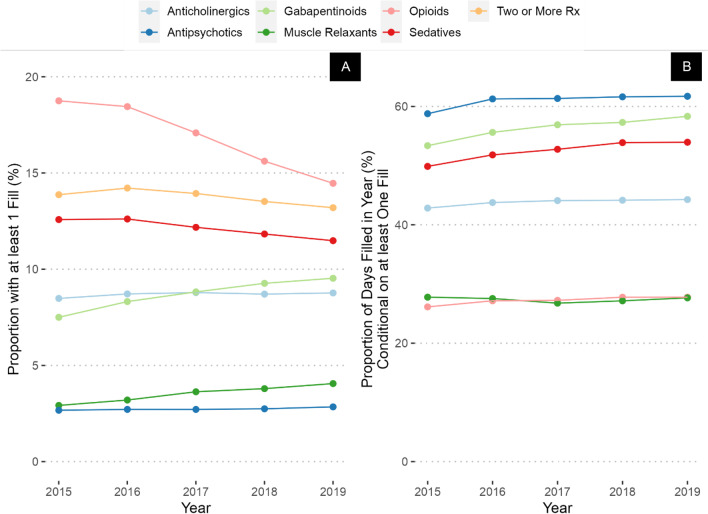
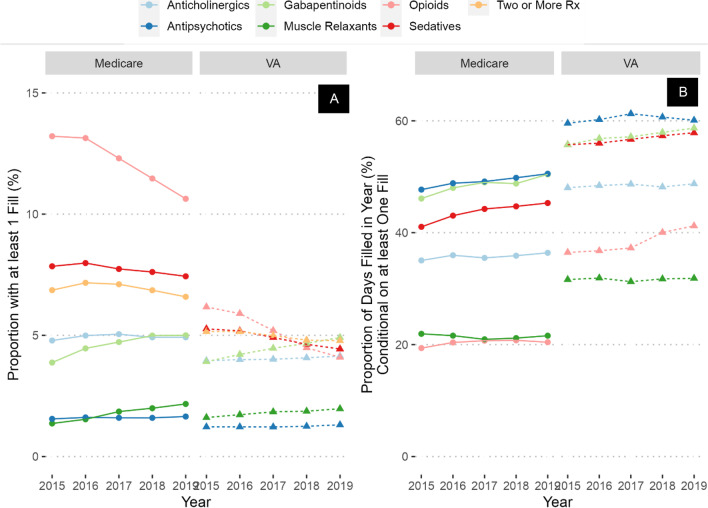
In 2015, opioids were the most prevalent CNS-active medications prescribed (19%) followed by sedative-hypnotics (13%), anticholinergics (8.5%), gabapentinoids (7.5%), muscle relaxants (2.9%), and antipsychotics (2.7%; Fig. 1). However, among users of these classes, medications with highest PDC were antipsychotics (59% of days), followed by gabapentinoids (53%), sedative-hypnotics (50%), anticholinergics (43%), muscle relaxants (28%), and opioids (26%).
Figure 1.
Prevalence (A) and percent of days covered (B) of CNS-active medication prescribing across the entire study period and population.
The largest increase in prevalence between 2015 and 2019 was in gabapentinoids (increased by 2 percentage points to 9.5%), followed by muscle relaxants (increased by 1.2 percentage points to 4.1%), and anticholinergics (increased by 0.3 percentage points to 8.8%). Antipsychotic prevalence did not change, while opioids exhibited the largest decrease (decreased by 5 percentage points to 14%), followed by sedative-hypnotics (decreased by 2 percentage points to 11%). However, the PDC of nearly all medication classes increased over the time period, with the largest change in gabapentinoids (increased by 5 percentage points to 58%), sedative-hypnotics (increased by 4 percentage points to 54%), and opioids (increased by 2 percentage points to 28%). Thus, by 2019, among patients receiving antipsychotics, sedative-hypnotics, or gabapentinoids, the majority of days were covered by these medications. CNS-active polypharmacy rates decreased slightly during the time period, from 12 to 11% of the sample. In a sensitivity analysis restricted to only those who had at least one VA visit annually, rates and trends of prevalence and PDC were similar (Appendix Table 1, Fig. 1).
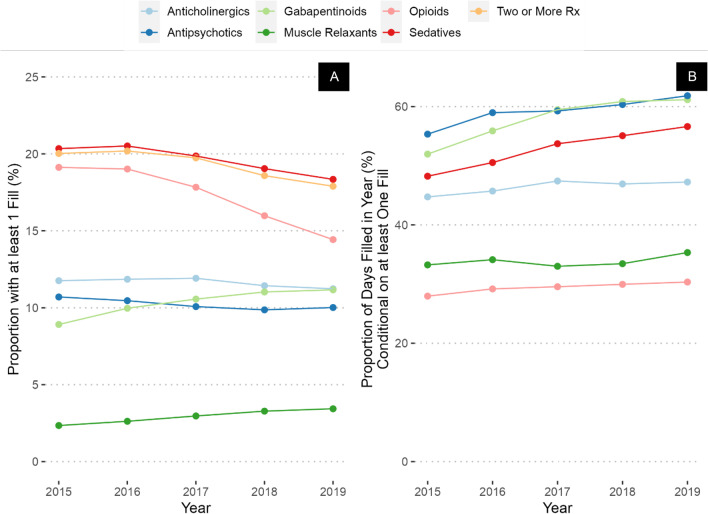
In Veterans with a diagnosis of dementia, CNS-active polypharmacy was nearly twice as common compared to Veterans without this diagnosis (20% in 2015 to 18% in 2019, compared to 11% and 10%, respectively, Fig. 2A and Appendix Table 2). The largest differences in prevalence between Veterans with a diagnosis of dementia compared to those without were in antipsychotics (11% among Veterans with a diagnosis of dementia in 2015 compared to 1.5% in those without a diagnosis), followed by sedative-hypnotics (20% vs. 11%), and anticholinergics (12% vs 8%). Rates of prescribing of opioids were the same in the two groups in 2015. Trends over the study period were like those in the overall population, with small increases in the prevalence of gabapentinoids and muscle relaxants and similar decreases in opioid and sedative-hypnotic prevalence. PDC was stable or increased in every drug class, with the largest increase in gabapentinoids (increased from 52 to 61% of days covered — Fig. 2B), sedative-hypnotics (increased from 48 to 57%), and antipsychotics (increased from 55 to 62% of days covered).
Figure 2.
Prevalence (A) and percent of days covered (B) of CNS-active medication prescribing in Veterans with a diagnosis of dementia.
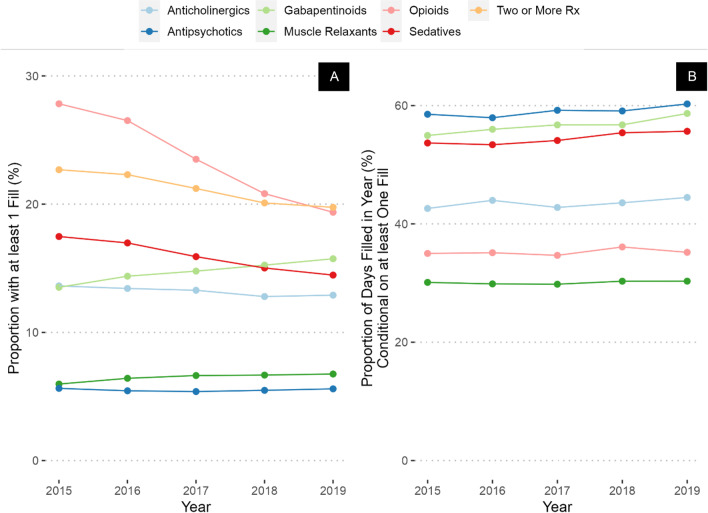
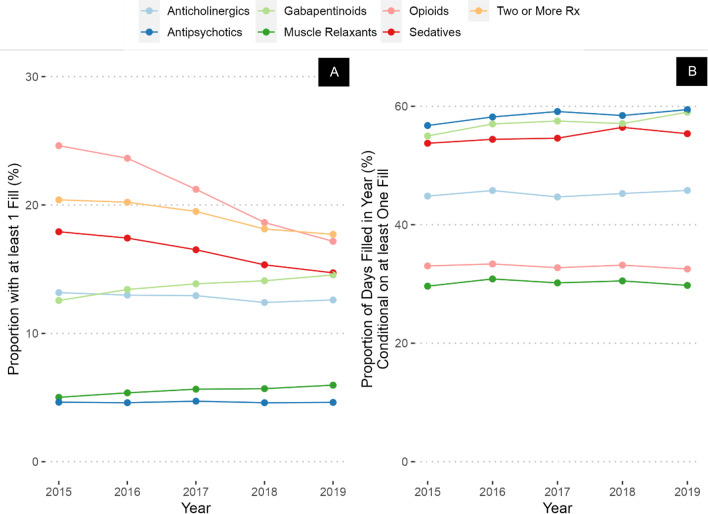
Veterans with a high CAN score had higher prevalence of prescriptions of all drug classes compared to the overall sample (Fig. 3A), but there were notable differences compared to Veterans with a diagnosis of dementia. For example, in 2015, Veterans with high CAN score had lower rates of antipsychotic (5.6% in Veterans with high CAN score vs 11% in Veterans with diagnosis of dementia) and sedative-hypnotic prescribing (17% vs 20%), but higher rates of prescribing of opioids (28% vs 19%), gabapentinoids (14% vs. 7.3%), and anticholinergics (14% vs. 8.0%) compared to Veterans with a diagnosis of dementia. However, the only classes increasing in prevalence over time in Veterans with high CAN score were gabapentinoids (increased from 14% in 2015 to 16% in 2019) and muscle relaxants (increased from 6.0 to 6.8%, Appendix Table 3), while similar large decreases were noted in opioid prevalence (decreased from 28 to 19%) and sedative-hypnotics (decreased from 17 to 14%). Gabapentinoids, antipsychotics, and sedatives had the highest PDC (including a majority of days each year), with the largest difference in gabapentinoids (55 to 59%). Veterans with high JFI had prevalence, PDC, and trends over time like Veterans with high CAN score (Fig. 4A and B, Appendix Table 4).
Figure 3.
Prevalence (A) and percent of days covered (B) of CNS-active medication prescribing in Veterans with a high Care Assessment Needs (CAN) score.
Figure 4.
Prevalence (A) and percent of days covered (B) of CNS-active medication prescribing in Veterans with high frailty score.
When comparing VA prescriptions versus those covered by Medicare Part D, the most striking differences in prevalence of prescribing were in opioids and sedative-hypnotics, where substantially more prescriptions were dispensed through Medicare Part D (Fig. 5A). However, all medication classes demonstrated higher PDC when dispensed through the VA across all the years of the study period (Fig. 5B).
Figure 5.
Prevalence (A) and percent of days covered (B) of CNS-active medication prescribing for Veterans, stratified by Medicare versus VA prescribers.
DISCUSSION
There are several novel and notable findings from this evaluation conducted to support a quality improvement deprescribing intervention for older Veterans in VISN 4. First, while the prevalence of opioids and sedative-hypnotics — which have received substantial national attention — has declined, we observed a commensurate increase in gabapentinoid prescribing. Second, even medication classes that are decreasing in prevalence may be increasing in PDC, increasing the total amount of medications dispensed in each medication class over time. This is a substantial difference when comparing medications dispensed from VA versus Medicare Part D, demonstrating much higher PDC across all medication classes in the VA. Third, the prevalence and PDC of prescribing vary across the overall population and different high-risk subgroups, informing potential quality improvement opportunities in select populations.
These findings build on those of others demonstrating an increase in prescribing of gabapentinoids, perhaps in response to the significant focus on opioid prescribing for pain. For example, in another study of older Veteran nursing home residents with dementia, rates of antipsychotic prescribing decreased — only to be mirrored by increases in gabapentinoid, opioid, and antidepressant prescribing.21 Similar trends are seen in the Medicare population with a diagnosis of dementia, where gabapentin was the most common component of CNS-active polypharmacy.4 However, data about relative harms of gabapentinoids — compared to opioids or sedative-hypnotics — is more nascent.22–25 This recurrent finding merits further research to determine if — as suspected — the increased prescription of gabapentin is a substitution effect for other better-known yet potentially harmful medications, and whether gabapentin is indeed “safer.” This finding — in concert with our finding that opioid and sedative-hypnotic prescriptions are now more commonly derived from Medicare Part D — illustrates a known concern with “substitution” effects associated with deprescribing efforts (getting the medication from other providers, or a different medication altogether).
To our knowledge, our finding about different patterns of PDC in VA versus Part D is novel. The etiology of this finding is not clear and merits further investigation. We suspect it may be due to financial incentives (Veterans may transfer chronic medications from Part D to VA if cheaper) or VA prescribing practice, which is oriented towards 90-day mail-order prescriptions for chronic medications. It is important to emphasize that VA and Part D prescriptions are not synonymous with a VA versus non-VA prescriber. VA prescribers can send prescriptions to non-VA pharmacies which are covered by Part D, and VA prescribers may be asked to transfer a prescription previously covered by Part D into the VA. Regardless of mechanism, this finding suggests while efforts to limit initial prescriptions may be important, deprescribing efforts may be even more impactful in reducing the total burden of CNS-active medications in this population. We are not aware of broad policy or payment initiatives in the USA to support deprescribing outside of specific drug classes (e.g., opioids), but such initiatives may also merit testing if they carefully balance the intended and potential unintended consequences of overprescribing.
Strengths of this study include the robust ascertainment and characterization of prescribing in both VA and Medicare Part D in an older Veteran population, as well as in important high-risk subpopulations. However, there are also several important limitations. We were unable to identify over-the-counter prescriptions, which are common in some medication classes (e.g., anticholinergics). We likely undercounted the extent of anticholinergic burden by only capturing medications that were not included in drug classes we studied. We only identified prescriptions in VISN 4, which may exhibit different regional prescribing trends than other areas. We could only identify medications dispensed, not whether the patient was taking the medication. Finally, we did not include data on patient outcomes, which is beyond the scope of the current analysis.
These findings reinforce the positive correlation seen in other studies between higher comorbidity burden or frailty and higher rates of CNS-active prescribing.26 Since it is likely CNS-active medications were started in response to a clinical diagnosis or symptoms, these findings challenge the idea that deprescribing without implementing another intervention with lower associated harms is likely to be successful. For example, when asked, medications older adults find most helpful are often those considered “potentially inappropriate” such as pain medications and sedatives.27 The age-friendly health system model at the foundation of our SAGE QUERI program is a useful paradigm for considering the value of medications and ways to substitute with non-pharmacologic treatments. Reviewing how a medication is affecting Mentation or Mobility can empower patients and prescribers to take action to deprescribe, while focusing on What Matters places the medication into the overall context of goals.1,2 For example, in our SAGE QUERI program, we are implementing the tailored activities program (TAP), an evidence-based practice that improves outcomes for Veterans with dementia and their caregivers.28–30 Being able to refer to the TAP provides a mechanism to support potential long-term deprescribing of antipsychotics in Veterans with a diagnosis of dementia.
Based on these results, we have chosen to target two drug classes using EMPOWER: gabapentinoids and antipsychotics. We are using an internally derived risk score to identify Veterans at high risk for adverse drug events receiving gabapentin. In turn, we are identifying Veterans with a diagnosis of dementia receiving antipsychotics. We hope to provide new insights into how to implement evidence-based deprescribing interventions in the VA, as well as data about clinical effectiveness of deprescribing these medications.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The Safer Aging through Geriatrics-Informed Evidence-Based Practices (SAGE) QUERI program is funded by the VA Health Services Research & Development (HSR&D) Quality Enhancement Research Initiative (QUERI). The funder had no role in analysis or manuscript preparation.
Declarations
Disclaimer
The viewpoints expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Conflict of Interest
The authors have no relevant conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burke RE, Brown RT, Kinosian B. Selecting implementation strategies to drive Age-Friendly Health System Adoption. J Am Geriatr Soc. 2022;70(1):313–318. doi: 10.1111/jgs.17489. [DOI] [PubMed] [Google Scholar]
- 2.Burke RE, Ashcraft LE, Manges K, et al. What matters when it comes to measuring Age-Friendly Health System transformation. J Am Geriatr Soc. 2022;70(10):2775–2785. doi: 10.1111/jgs.18002. [DOI] [PubMed] [Google Scholar]
- 3.Fulmer T, Mate KS, Berman A. The Age-Friendly Health System Imperative. J Am Geriatr Soc. 2018;66(1):22–24. doi: 10.1111/jgs.15076. [DOI] [PubMed] [Google Scholar]
- 4.Maust DT, Strominger J, Kim HM, et al. Prevalence of Central Nervous System-Active Polypharmacy Among Older Adults With Dementia in the US. JAMA. 2021;325(10):952–961. doi: 10.1001/jama.2021.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomfield HE, Greer N, Linsky AM, et al. Deprescribing for Community-Dwelling Older Adults: a Systematic Review and Meta-analysis. J Gen Intern Med. 2020;35(11):3323–3332. doi: 10.1007/s11606-020-06089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomfield H, Linsky A, Bolduc J, Greer N, Naidl T, Vardeny O, MacDonald R, McKenzie L, Wilt TJ.Deprescribing for Older Veterans: A Systematic Review [Internet]. Washington (DC): Department of Veterans Affairs (US); 2019. Accessed November 1, 2022. http://www.ncbi.nlm.nih.gov/books/NBK561691/ [PubMed]
- 7.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–898. doi: 10.1001/jamainternmed.2014.949. [DOI] [PubMed] [Google Scholar]
- 8.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a Pharmacist-Led Educational Intervention on Inappropriate Medication Prescriptions in Older Adults: The D-PRESCRIBE Randomized Clinical Trial. JAMA. 2018;320(18):1889–1898. doi: 10.1001/jama.2018.16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maust DT, Gerlach LB, Gibson A, Kales HC, Blow FC, Olfson M. Trends in Central Nervous System-Active Polypharmacy Among Older Adults Seen in Outpatient Care in the United States. JAMA Intern Med. 2017;177(4):583–585. doi: 10.1001/jamainternmed.2016.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson CJ, Li Y, Jasuja GK, Keyhani S, Byers AL. Long-term Psychoactive Medications, Polypharmacy, and Risk of Suicide and Unintended Overdose Death Among Midlife and Older Women Veterans. J Gen Intern Med. 2022;37(Suppl 3):770–777. doi: 10.1007/s11606-022-07592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorpe JM, Thorpe CT, Schleiden L, Cashy J, Carico R, Gellad WF, Van Houtven CH. Association between dual use of department of veterans affairs and medicare part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019;179(11):1584-1586. doi:10.1001/jamainternmed.2019.2788 [DOI] [PMC free article] [PubMed]
- 12.Carico R, Zhao X, Thorpe CT, et al. Receipt of Overlapping Opioid and Benzodiazepine Prescriptions Among Veterans Dually Enrolled in Medicare Part D and the Department of Veterans Affairs: A Cross-sectional Study. Ann Intern Med. 2018;169(9):593–601. doi: 10.7326/M18-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gellad WF, Zhao X, Thorpe CT, et al. Overlapping buprenorphine, opioid, and benzodiazepine prescriptions among veterans dually enrolled in Department of Veterans Affairs and Medicare Part D. Subst Abuse. 2017;38(1):22–25. doi: 10.1080/08897077.2016.1267071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gellad WF, Thorpe JM, Zhao X, et al. Impact of Dual Use of Department of Veterans Affairs and Medicare Part D Drug Benefits on Potentially Unsafe Opioid Use. Am J Public Health. 2018;108(2):248–255. doi: 10.2105/AJPH.2017.304174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorpe JM, Thorpe CT, Gellad WF, et al. Dual Health Care System Use and High-Risk Prescribing in Patients With Dementia: A National Cohort Study. Ann Intern Med. 2017;166(3):157–163. doi: 10.7326/M16-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VA.gov | Veterans Affairs. Accessed December 13, 2022. https://www.pbm.va.gov/nationalformulary.asp
- 17.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzheimer’s Disease and Related Disorders or Senile Dementia End-of-Year Indicator | ResDAC. Accessed December 13, 2022. https://resdac.org/cms-data/variables/mbsf-27-cc/alzheimers-disease-and-related-disorders-or-senile-dementia-end-year-indicator
- 19.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368–373. doi: 10.1097/MLR.0b013e31827da95a. [DOI] [PubMed] [Google Scholar]
- 20.Kinosian B, Wieland D, Gu X, Stallard E, Phibbs CS, Intrator O. Validation of the JEN frailty index in the National Long-Term Care Survey community population: identifying functionally impaired older adults from claims data. BMC Health Serv Res. 2018;18(1):908. doi: 10.1186/s12913-018-3689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach LB, Maust DT, Kales HC, et al. Evaluation of Antipsychotic Reduction Efforts in Patients With Dementia in Veterans Health Administration Nursing Homes. Am J Psychiatry. 2022;179(8):544–552. doi: 10.1176/appi.ajp.21060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peckham AM, Fairman KA, Sclar DA. All-Cause and Drug-Related Medical Events Associated with Overuse of Gabapentin and/or Opioid Medications: A Retrospective Cohort Analysis of a Commercially Insured US Population. Drug Saf. 2018;41(2):213–228. doi: 10.1007/s40264-017-0595-1. [DOI] [PubMed] [Google Scholar]
- 23.Daly C, Griffin E, Ashcroft DM, Webb RT, Perry IJ, Arensman E. Intentional Drug Overdose Involving Pregabalin and Gabapentin: Findings from the National Self-Harm Registry Ireland, 2007–2015. Clin Drug Investig. 2018;38(4):373–380. doi: 10.1007/s40261-017-0616-y. [DOI] [PubMed] [Google Scholar]
- 24.Piovezan RD, Kase C, Moizinho R, Tufik S, Poyares D. Gabapentin acutely increases the apnea-hypopnea index in older men: data from a randomized, double-blind, placebo-controlled study. J Sleep Res. 2017;26(2):166–170. doi: 10.1111/jsr.12495. [DOI] [PubMed] [Google Scholar]
- 25.Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, Moore RA. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938. doi:10.1002/14651858.CD007938.pub4 [DOI] [PMC free article] [PubMed]
- 26.Ouellet GM, Ouellet JA, Tinetti ME. Principle of rational prescribing and deprescribing in older adults with multiple chronic conditions. Ther Adv Drug Saf. 2018;9(11):639–652. doi: 10.1177/2042098618791371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tinetti ME, Costello DM, Naik AD, et al. Outcome Goals and Health Care Preferences of Older Adults With Multiple Chronic Conditions. JAMA Netw Open. 2021;4(3):e211271. doi: 10.1001/jamanetworkopen.2021.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. The Gerontologist. 2003;43(4):532–546. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- 29.Gitlin LN, Arthur P, Piersol C, et al. Targeting Behavioral Symptoms and Functional Decline in Dementia: A Randomized Clinical Trial. J Am Geriatr Soc. 2018;66(2):339–345. doi: 10.1111/jgs.15194. [DOI] [PubMed] [Google Scholar]
- 30.Gitlin LN, Winter L, Vause Earland T, et al. The Tailored Activity Program to reduce behavioral symptoms in individuals with dementia: feasibility, acceptability, and replication potential. The Gerontologist. 2009;49(3):428–439. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.