Abstract
Background
Anticoagulants including direct oral anticoagulants (DOACs) are among the highest-risk medications in the United States. We postulated that routine consultation and follow-up from a clinical pharmacist would reduce clinically important medication errors (CIMEs) among patients beginning or resuming a DOAC in the ambulatory care setting.
Objective
To evaluate the effectiveness of a multicomponent intervention for reducing CIMEs.
Design
Randomized controlled trial.
Participants
Ambulatory patients initiating a DOAC or resuming one after a complication.
Intervention
Pharmacist evaluation and monitoring based on the implementation of a recently published checklist. Key elements included evaluation of the appropriateness of DOAC, need for DOAC affordability assistance, three pharmacist-initiated telephone consultations, access to a DOAC hotline, documented hand-off to the patient’s continuity provider, and monitoring of follow-up laboratory tests.
Control
Coupons and assistance to increase the affordability of DOACs.
Main measure
Anticoagulant-related CIMEs (Anticoagulant-CIMEs) and non-anticoagulant-related CIMEs over 90 days from DOAC initiation; CIMEs identified through masked assessment process including two physician adjudication of events presented by a pharmacist distinct from intervention pharmacist who reviewed participant electronic medical records and interview data.
Analysis
Incidence and incidence rate ratio (IRR) of CIMEs (intervention vs. control) using multivariable Poisson regression modeling.
Key Results
A total of 561 patients (281 intervention and 280 control patients) contributed 479 anticoagulant-CIMEs including 31 preventable and ameliorable ADEs and 448 significant anticoagulant medication errors without subsequent documented ADEs (0.95 per 100 person-days). Failure to perform required blood tests and concurrent, inappropriate usage of a DOAC with aspirin or NSAIDs were the most common anticoagulant-related CIMEs despite pharmacist documentation systematically identifying these issues when present. There was no reduction in anticoagulant-related CIMEs among intervention patients (IRR 1.17; 95% CI 0.98–1.42) or non-anticoagulant-related CIMEs (IRR 1.05; 95% CI 0.80–1.37).
Conclusion
A multi-component intervention in which clinical pharmacists implemented an evidence-based DOAC Checklist did not reduce CIMEs.
NIH Trial Number
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08315-z.
KEY WORDS: adverse events, epidemiology and detection, medication safety, transitions in care, ambulatory care, pharmacists
Prior research identifies anticoagulants, including direct oral anticoagulants (DOACs), as the drug class most associated with medication harm-related emergency visits across the United States.1 In one prior study of DOACs, problems with dosing and medication appropriateness occurred in 60% of ambulatory care patients prescribed a DOAC in outpatient practices.2 Yet, while patients frequently initiate DOACs in ambulatory settings, few studies address the risks associated with them in this setting, and still fewer rigorously test interventions in this setting to ameliorate these risks.3
In hospital settings, clinical pharmacists are routinely engaged in the process of initiating DOACs. By contrast, in ambulatory care settings, consultation with a clinical pharmacist is not routinely available and frequently does not occur.4 Previous research5 suggests that clinical pharmacist engagement in ambulatory care medication management can be helpful but has seldom focused specifically on anticoagulants. Recently, the Anticoagulation Forum (AC Forum), a multidisciplinary nonprofit organization focused on anticoagulation issues, endorsed the DOAC Checklist for Optimal Care Transitions (DOAC Checklist) to delineate the steps required to ensure a safe transition of care in patients prescribed DOACs.6 These steps include evaluation of the appropriateness of DOAC, confirmation of the affordability and access of DOAC prescribed, patient education, telephone access to anticoagulation expertise, documentation of key information with hand-off to continuity provider, and renal and hepatic function monitoring.
In undertaking this study, we postulated that implementing the DOAC Checklist in busy ambulatory care settings would benefit from systematic pharmacist involvement focused on each checklist step for patients starting or restarting DOACs in an ambulatory setting. Those diagnosed with deep vein thrombosis (DVT) predominate in this group, but growing numbers of patients diagnosed with pulmonary embolism (PE)—i.e., the more severe form of venous thromboembolism (VTE)—are being discharged from the emergency department or after short-stay hospitalization (i.e., both of which represent ambulatory care settings with more limited resources and reimbursement for services).7 In addition, many patients with new-onset or persistent atrial fibrillation (AF) require ambulatory anticoagulant initiation. Finally, some patients previously prescribed DOACs but stopping them due to a complication (including stroke or other emboli, worsening of existing VTE, or bleeding) may decide to re-initiate a DOAC.
Given the diversity of indications for DOACs, ensuring the appropriateness of DOAC use requires a careful review of patient factors that may affect DOAC safety. After making the decision to start a DOAC, patients and their families require support as they learn to use DOACs. Clinical pharmacists have the training to assess the appropriateness of DOACs and can assist ambulatory care providers in assessing the appropriateness of DOAC initiation. Pharmacists are also trained in providing patient education and support for medication initiation and in assuring needed laboratory monitoring. Finally, pharmacist scopes of practice include coordination of medication care between patients and ambulatory care providers. These critical pharmacist skills have the potential to enhance the implementation of the DOAC Checklist.
In summary, the goal of this study was to rigorously test pharmacist-supported implementation of the DOAC Checklist as a method for reducing DOAC-related adverse events among ambulatory care patients. Our objectives were to develop and implement an intervention to reduce clinically important medication errors (CIMEs) among patients who newly initiated DOACs or who restarted them after a complication. CIMEs include preventable adverse drug events (ADEs), ameliorable ADEs, and medication errors. To accomplish this, we adapted and operationalized the DOAC Checklist to create a feasible, comprehensive, standardized intervention led by a clinical pharmacist, and compared CIMEs between patients randomized to our intervention and control patients.
METHODS
Ethics Review
The UMass Chan Medical School institutional review board reviewed and approved this study.
Population/Setting
We enrolled ambulatory patients within four days of an ambulatory encounter in which a provider initiated a DOAC or resumed one after a complication. Eligible encounters included those occurring in an office or emergency department or as a short-stay hospitalization (< 3 midnights) within our health care system in Massachusetts. Complications included a worsening or lengthening of a DVT or propagation of a DVT into a PE, stroke, transient ischemic attack (TIA), systemic embolism, or bleeding following established definitions for major and clinically relevant major hemorrhage.8,9 We included patients speaking English, Spanish, or Portuguese and excluded individuals under the age of 18, prisoners, and pregnant patients.
Recruitment
To recruit patients, we advertised widely and offered providers multiple ways to refer patients to us. We also screened our electronic health record (EHR) daily for positive imaging results consistent with VTE and medication orders consistent with new starts of DOACs and recruited all those initiating a DOAC between 03-11-2020 and 05-25-2021. In order to attract provider referrals and patient participation, we distributed manufacturer-based 30-day free trial coupons along with our other recruitment materials (introductory letter and fact sheet to facilitate verbal consent) to all potential patients. We also offered to fill out manufacturer-based medication payment assistance forms.
Intervention Pharmacist Training
The PI (AK) trained each pharmacist on the intervention protocol and observed a minimum of five consultations before allowing the pharmacist to work independently.
Baseline Interview
After calling patients and obtaining consent, we conducted a structured interview to collect information about demographics, health literacy, and prior anticoagulant use.
Randomization
Following precedence,10 we randomized patients to our intervention or control groups using a table of randomly permuted blocks of 2, 4, 6, or 8 based on qualifying condition for entry into the study including (1) participants with new or worsening episodes of VTE and (2) those with atrial fibrillation, stroke, TIA, systemic embolism, or bleeding. We also stratified patients based on whether they were referred or had already been a patient within a dedicated anticoagulation clinic at the time of randomization. The anticoagulation clinic within our health system provided medication supervision for patients receiving DOACs but without pharmacist consultation. Providers were free to refer patients to the clinic, but there was no specific prompt or protocol to make a referral.
Study Intervention
Table 1 contains the components of our intervention.
Table 1.
Intervention Protocol Adapted from Direct Oral Anticoagulant (DOAC) Checklist
| Checklist feature | Description of feature | Staff member delivering feature | Timing of delivery |
|---|---|---|---|
| Assess DOAC appropriateness | • Reviewed patient charts for appropriateness | Intervention pharmacist (a clinical pharmacist) | Within seven days of randomization |
| Ensure access and affordability |
• Distributed coupons and information about manufacturer-based medication payment assistance programs* • Offered help with completing these applications* |
Study staff | During the 90-day study period |
| Provide DOAC education |
• Provided intervention patients with education regarding medication interactions, especially those related to concomitant use of DOACs with aspirin or nonsteroidal anti-inflammatory drugs (NSAID). The pharmacist’s approach was to solicit patient understanding and then remediate as required. (See Appendix A.) • Mailed the patient educational materials after the first tele-visit. (See Appendix B.) * |
Intervention pharmacist | In up to 3 telephone consultations (tele-visits). First call within 1 week of randomization. The subsequent two calls occurring 31–60 and 61–90 days from randomization, respectively |
| Hotline | • Provided patients with a number to call to discuss any medication issue from 6AM-10 PM, seven days a week | Intervention pharmacist | During the 90-day study period |
| Dose de-escalation | • Instructed patients about the dose de-escalation required for apixaban and rivaroxaban recorded this instruction in the EHR | Intervention pharmacist | During the 90-day study period |
| Communication/handoff to continuity provider |
• Documented in a progress note if she had any concerns and then routed the note for review by the patient’s continuity care provider in our institution’s EHR • Notified the patient’s prescriber in a brief staff message composed when routing the note of the visit to the provider if the patient was missing any lab work • Included instructions for duration and monitoring of DOAC in EHR documentation for the third tele-visit |
Intervention pharmacist | After each tele-visit |
| Perioperative support/lab work |
• Made recommendations on the interruption and resumption of anticoagulant for surgery • Made recommendations for the timing of follow-up bloodwork in the third tele-visit note |
Intervention pharmacist | During the 90-day study period |
*We offered coupons, assisted with completing manufacturer-based medication payment assistance applications, and mailed educational materials to both intervention and control patients in order to attract patients and providers
Control Patients
All enrolled patients had previously received recruitment materials (DOAC coupons and our offer to complete manufacturer-based medication payment assistance forms). After enrollment, we mailed patients in both study arms the same educational materials. Control patients had access to standard pharmacy resources within our system but no access to study intervention-trained pharmacists.
Exit Interview
We conducted exit interviews with patients after 90 days had elapsed from the day of randomization. In the interview, we followed the approach of Forster and colleagues 11 and others, 12–14 assessing the patient’s condition since randomization by using a full review of organ systems.
Chart Review by Research Pharmacists
One of the research pharmacists (CC, WT, and CB), distinct from the intervention pharmacists, reviewed each enrolled patient’s chart (electronic medical record) and interview data over the 90 days post-randomization to identify DOAC-related medication errors. We instructed them to ignore notes by intervention pharmacists when performing their review. We masked the allocation status of control patients by uploading dummy intervention pharmacist notes into control patient records prior to the reviews. Pharmacist reviewers relied on the following established definitions:15
Clinically Important Medication Errors
Medication errors may or may not result in ADEs. Clinically important medication errors (CIMEs) included the combination of preventable and ameliorable ADEs and medication errors as defined below.
Preventable ADEs
Preventable ADEs include adverse events directly related to a medication error that caused the patient any degree of harm.
Ameliorable ADEs
Ameliorable ADEs include adverse events that worsened (increased in duration or severity) because of an error, although reviewers judged that the ADE occurred in the absence of an error.
Medication Error
Any error in prescribing, filling, or monitoring a medication or patient adhering to a medication that was not associated with a subsequent ADE during the 90-day follow-up.
Outcome Adjudication
Two of the faculty physicians (AK, SJ, CD, MI, or JG) reviewed each event presented by a research pharmacist during teleconferences. As training, each physician reviewed a minimum of four practice charts with the principal investigator (AK). From these practice sessions, we developed a quick reference sheet to guide physician adjudications in common scenarios. (Appendix C) After listening to the pharmacist’s presentation of an event, each physician recorded their adjudication for the presence of an event, its severity, and its preventability. They also recorded whether the event involved an anticoagulant, typically the DOAC prescribed. Then, they discussed their responses to achieve consensus. Physicians were masked to the treatment allocation of enrolled patients.
Measures
Our primary study outcome measure was the frequency of anticoagulant-related CIMEs as described above. Secondarily, we tracked the total number of non-anticoagulant CIMEs. We measured the covariates, listed in Table 1, from the baseline interview or from electronic health records.
Analysis
We estimated the mean incidence rates of primary and secondary outcomes within each group (intervention and control). Denominators included up to 90 person-days after randomization excluding days after patients died. We then calculated incidence rates as events per 100 person-days. For the primary and secondary outcomes, we calculated the ratio of the CIME incidence rates for the intervention and control groups (i.e., incidence rate ratios) by constructing multivariable Poisson regression models which accounted for the number of days each participant contributed, adjusted for several covariates including age, gender, race-ethnicity as a composite variable, income, educational level, health literacy, qualifying indication for entry into the study (venous versus non-venous), previous anticoagulant use, referral to anticoagulation clinic, chronic kidney disease, and anemia. We also included the number of medications in a patient’s active list in the electronic health record at the time of randomization. We also examined the interaction of our intervention with two covariates—qualifying condition for entry into study and health literacy—to see if the effect of our intervention varied in subsets based on those variables.
RESULTS
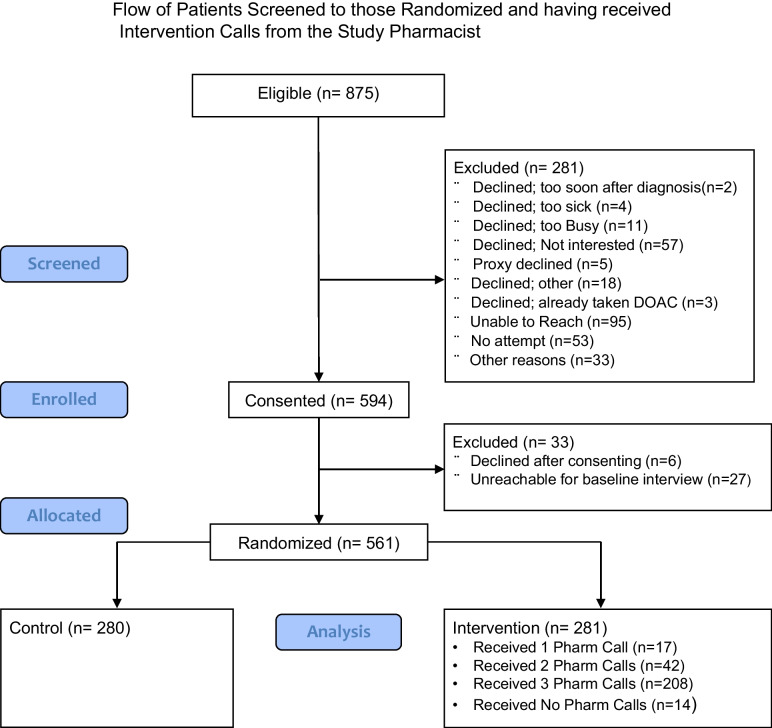
We identified 875 eligible patients and randomized 561 patients of them including 281 intervention and 280 control patients (Fig. 1) For health literacy, we found 74.2% had high health literacy based on confidence in filling out medical forms. Most patients had VTE (69.7%) as the qualifying condition for enrollment in our study. Few people received a referral to our anticoagulation clinic (4.1%). Across intervention and control groups, there was a good balance in all demographic and patient characteristics with the exception of a household income of less than $20,000 per year being more common among control patients (14.3%) versus intervention patients (6.8%). (Table 2) Within the stratum of patients with venous qualifying conditions for entry into the study, there were trends toward greater imbalance in age and income with more patients with age 76 + in the intervention group (14% versus 11%) and fewer patients with income less than $20,000 per year (Appendix D).
Fig. 1.
Flow of patients screened to those randomized and having received intervention calls from the study pharmacist.
Table 2.
Key Characteristics for Enrolled Patients Stratified by Intervention vs. Control Allocation
| Category | All Frequency (% out of 561) |
Intervention group Frequency (% out of 281) |
Control group Frequency (% out of 280) |
|---|---|---|---|
| Demographics | |||
| Age | |||
| < 50 | 125 (22.3) | 72 (25.6) | 53 (18.9) |
| 50–65 | 195 (34.8) | 92 (32.7) | 103 (36.8) |
| 66–75 | 134 (23.9) | 62 (22.1) | 72 (25.7) |
| 76 + | 107 (19.1) | 55 (19.6) | 52 (18.6) |
| Female sex | 251 (44.7) | 127 (45.2) | 124 (44.3) |
| Race/ethnicity | |||
| Asian/other | 14 (2.5) | 7 (2.5) | 7 (2.5) |
| Hispanic | 54 (9.6) | 24 (8.5) | 30 (10.7) |
| Non-Hispanic Black | 24 (4.3) | 11 (3.9) | 13 (4.6) |
| Non-Hispanic White | 469 (83.6) | 239 (85.1) | 230 (82.1) |
| Income | |||
| < 20,000 | 59 (10.5) | 19 (6.8) | 40 (14.3)* |
| 20–49,999 | 120 (21.4) | 59 (21.0) | 61 (21.8) |
| 50–99,999 | 122 (21.8) | 71 (25.3) | 51 (18.2) |
| > 100,000 | 132 (23.5) | 69 (24.6) | 63 (22.5) |
| Prefer not to answer/don’t know/missing | 128 (22.8) | 63 (22.4) | 65 (23.2) |
| Education | |||
| High school or below** | 198 (35.3) | 96 (34.2) | 102 (36.4) |
| Beyond high school† | 363 (64.7) | 185 (65.8) | 178 (63.6) |
| Health Literacy | |||
| Confidence filling out medical forms | |||
| Low health literacy‡ | 145 (25.8) | 69 (24.6) | 76 (27.1) |
| High health literacy§ | 416 (74.2) | 212 (75.4) | 204 (72.9) |
| Hard time understanding when people speak quickly | |||
| Low health literacy‖ | 210 (37.4) | 104 (37.0) | 106 (37.9) |
| High health literacy¶ | 351 (62.6) | 177 (63.0) | 174 (62.1) |
| Anticoagulation prescribing related issues | |||
| Qualifying ConditionΩ | |||
| Non-venous condition | 170 (30.3) | 86 (30.6) | 84 (30.0) |
| Venous thromboembolism | 391 (69.7) | 195 (69.4) | 196 (70.0) |
| Referral to Anticoagulation Clinic | 23 (4.1) | 12 (4.3) | 11 (3.9) |
| Prior use of anticoagulation | 147 (26.2) | 81 (28.8) | 66 (23.6) |
| Anticoagulant Prescribed | |||
| Apixaban | 486 (86.6) | 245 (87.2) | 241 (86.1) |
| Dabigatran | 1 (0.2) | 0 (0) | 1 (0.36) |
| Rivaroxaban | 74 (13.2) | 39 (13.6) | 35 (12.5) |
| Chronic Kidney Disease± | |||
| Stage 1 | 320 (57.0) | 156 (55.5) | 164 (58.6) |
| Stage 2 | 132 (23.5) | 70 (24.9) | 62 (22.1) |
| Stage 3 | 74 (13.2) | 35 (12.5) | 39 (13.9) |
| Stage 4/5 | 8 (1.4) | 6 (2.1) | 2 (0.7) |
| Anemia# | 187 (33.3) | 107 (38.1) | 80 (28.6) |
| Count of total medications | |||
| 0–5 | 115 (20.5) | 55 (19.6) | 60 (21.4) |
| 6–10 | 138 (24.6) | 71 (25.3) | 67 (23.9) |
| 11–15 | 100 (17.8) | 47 (16.7) | 53 (19.0) |
| 16 + | 88 (15.7) | 44 (15.6) | 44 (15.7) |
| Missing | 120 (21.4) | 64 (22.8) | 56 (20.0) |
*Comparison between intervention and control had a chi-square p value < 0.05; for all other comparisons p value > 0.05
†Beyond high school includes: 1–3 years college/post high school trade or technical, college graduate, post graduate education
‡Patients with low confidence rated their confidence as a little bit, somewhat, not at all, prefer not to answer, and missing responses
§Patients with high confidence rated their confidence as: extremely, quite a bit
‖Patients with low health literacy answered: agree, strongly agree, and prefer not to answer in response to the statement: I have a hard time understanding when people speak quickly). Missing responses were counted as low health literacy
¶ Patients with high health literacy answered: strongly disagree and disagree in response to the statement: I have a hard time understanding when people speak quickly)
#Hemoglobin < 13 g/dL (Male), < 12 g/dL (Female). Percentages based on the original patient count
**High school or below includes high school graduate, ≤ high school, prefer not to answer, missing
ΩNon-venous condition includes atrial fibrillation, systemic embolism/acute limb ischemia, resumption of anticoagulation after
bleeding; venous indication included new or worsened episode (e.g., extension or propagation of previously diagnosed DVT)
±Defined by established criteria2as a creatinine clearance calculated in mL/min/1.73m2 units for each stage: > 90 (stage 1), 60–80 (stage 2), 30–59 (stage 3), 15–29 (stage 4), < 15 (stage 5)
Physician reviewers identified 479 anticoagulant-related clinically important CIMEs for an incidence rate of 0.95 errors per 100 person-days. Medication errors without a resulting ADE constituted the vast majority of errors with 448 for an incidence rate of 0.89 per 100 person-days. There was no significant difference in the incidence rate for the intervention and control patients with an adjusted incidence rate ratio of 1.17 (95% CI 0.98–1.42). (Appendix E for full model results.) Physician reviewers identified 230 non-anticoagulant-related CIMEs for an incidence rate of 0.46 per 100 person-days with no difference between intervention and control patients (Table 3). We did not observe any significant interaction between the effect of our intervention and the qualifying condition for entry into the study or health literacy (p value 0.80 and 0.49, respectively).
Table 3.
Frequency, Incidence Rate, and Incidence Rate Ratio of Anticoagulant and Non-Anticoagulant-Related Clinically Important Medication Errors for Intervention versus Control Patients
| Total | Intervention | Control | IRR (95% CI)** | |||||
|---|---|---|---|---|---|---|---|---|
| Frequency | IR* | Frequency | IR | Frequency | IR | Unadjusted | Adjusted¥ | |
| AC-CIMEs | 479 | 0.95 | 253 | 1.05 | 226 | 0.91 | 1.11 (0.93– 1.32) | 1.17 (0.98–1.42) |
| Preventable ADEs | 27 | 0.05 | 16 | 0.06 | 11 | 0.04 | ||
| Ameliorable ADEs | 4 | 0.01 | 3 | 0.01 | 1 | 0.004 | ||
| Medication errors | 448 | 0.89 | 234 | 0.93 | 214 | 0.86 | ||
| Non-AC-CIMEs | 230 | 0.46 | 121 | 0.48 | 109 | 0.44 | 1.09 (0.85–1.42) | 1.05 (0.80–1.37) |
| Preventable ADEs | 28 | 0.06 | 14 | 0.06 | 14 | 0.06 | ||
| Ameliorable ADEs | 8 | 0.02 | 4 | 0.02 | 4 | 0.02 | ||
| Medication Errors | 194 | 0.39 | 103 | 0.41 | 91 | 0.36 | ||
Abbreviations: AC = anticoagulation, ADE = adverse drug event, CIMEs = clinically important medication errors, CI = confidence interval, IR = incidence rate, IRR = incidence rate ratio
*Frequency of event per 100 person-days
**Given small number of preventable and ameliorable ADEs, we only report IRR for composite outcomes of AC CIMEs and
non-AC CIMEs
¥Adjusted for age, gender, race/ethnicity, income, education, health literacy, qualifying condition, referral to the anticoagulation clinic, prior AC use, AC medication, chronic kidney disease, anemia, and total number of medications
We also note several features of the 709 total CIMEs we identified (Table 4). One-third (235/709) occurred within the first 7 days after randomization, and another 235 within the first 28 days. In 43.5% of the 709 CIMEs, only an anticoagulant was involved. Of these cases, failure to order routine laboratory tests recommended by our facility when initiating DOACs was the most common (9.5%). Concurrent usage of aspirin or NSAID made up another 21.1% of errors. When considering just the 67 adverse drug events (i.e., ameliorable or preventable ADEs), most were less serious in nature (65.7%) and constituted temporary harm (85.1%) not requiring hospitalization or intervention to sustain life. In summary, the CIMEs we identified, in general, occurred very soon after the index event and mostly comprised of medication errors (as opposed to actual ADEs); failure to order bloodwork and discontinuation of aspirin and NSAIDs predominated among these errors.
Table 4.
Characteristics and Frequency of Clinically Important Medication Errors
| Characteristic of CIMEs | Frequency (% out of n = 709) |
|---|---|
| All CIMEs | |
| Type | |
| Preventable ADE | 55 (7.8) |
| Ameliorable ADE | 12 (1.7) |
| Medication errors | 642 (90.5) |
| Days elapsed from randomization | |
| 0–7 | 235 (33.1) |
| 8–28 | 235 (33.1) |
| 29–90 | 197 (27.8) |
| Unclear timing* | 42 (6.0) |
| Medication regimens involved | |
| Anticoagulant alone | 302 (43.5) |
| Anticoagulant + aspirin | 85 (12.2) |
| Anticoagulant + NSAIDs | 62 (8.9) |
| CardiovascularΩΩ | 41 (5.9) |
| All remaining regimens | 204 (29.4) |
| CIMEs excluding medication errors | Frequency (% out of n = 67) |
| Severity of ADE** | |
| Less serious event | 44 (65.7) |
| Serious event | 20 (29.8) |
| Life-threatening | 3 (4.5) |
| Fatal event | 0 |
| NCC MERP harm category¥ | |
| E: Temporary harm | 57 (85.1) |
| F: Harm leads to hospitalization | 9 (13.4) |
| G: Harm contributes to permanent injury | 0 |
| H: Harm requires intervention to sustain life | 1 (1.5) |
| I: Harm contributes to death | 0 |
Abbreviations: ADE = adverse drug event, CIMEs = clinically important medication error
*Reported by the patient at the time of exit interview without specification of date
**Examples of each severity category: Less serious (non-urticarial rash, fall no fracture, bleeding without transfusion), serious (urticaria, falls with fracture, bleeding with transfusion); life-threatening (bleeding with hypotension, hypoglycemic encephalopathy)
ΩΩCategory included blood pressure and medications to control heart rate and arrhythmia
¥National Coordinating Council for Medication Error Reporting and Prevention
DISCUSSION
Our study results are in contrast to other published work. Having pharmacists participate in the care of ambulatory patients has been shown to reduce the rate of ADEs by as much as 50%.5 In a previous study we conducted, having a pharmacist conduct an intervention that included in-person, home-based consultation and a follow-up phone call did not find a reduction in clinically important medication errors14 for older adults hospitalized and subsequently discharged on high-risk medications including anticoagulants.16 Our current study showed a higher overall incidence of CIMEs (1.41 vs. 0.60 per 100 person-days) than the previous study but a lower incidence of preventable and ameliorable ADEs (0.12 vs. 0.44 per 100 person-days). In the previous study, patients were sicker by virtue of all requiring hospitalization and being 65 years and older. Our current intervention for ambulatory patients extended the participation of a pharmacist beyond the immediate post-discharge period to 90 days. In addition, we focused on a distinct group of patients prescribed the highest-risk class of medication transitioning from the ambulatory setting back to home. To our knowledge, no one has analyzed this particular group of patients or assessed the effectiveness of the DOAC Checklist. Miele et al. found that the use of pharmacists following a DOAC dosing guide led to a reduction in inappropriate dosing from 32.4% down to 13.8%.17 We did not find as many dosing errors. Rather, more of the errors we found came from the concomitant use of DOACs with aspirin or NSAIDs and from failure to order baseline laboratories.
Our findings raise questions about how best to utilize pharmacist time as part of efforts to reduce DOAC-related medication errors and adverse events. Rates of preventable and ameliorable ADEs were low in our health system over 90 days of follow-up, and the time spent on consultations was significant. Understanding the usefulness of our intervention would benefit from knowing how often medication errors detected within 90 days resulted in an ADE afterwards. One report found over 20% of patients followed up to 2 years developed renal dysfunction with DOAC dosing implications.18 Surveillance and stewardship strategies using informatics-based tools within the electronic health record have been proposed and may be able to detect, at lower cost, many of the errors we found.19 The CIME outcome including medication errors, preventable ADEs, and ameliorable ADEs comprise a set of modifiable outcomes that have been identified as a safety priority by the Agency for Healthcare Research and Quality.15 Several studies have already successfully implemented this outcome to understand the impact of various medication safety interventions.14,16,20
Our intervention consisting of pharmacist consultation delivered remotely over three months as part of a larger checklist to facilitate a transition in care from the ambulatory setting to home for patients taking DOACs does not appear to be able to reduce CIMEs in the first three months of use after initiation or complication. Errors related to not ordering proper laboratory tests and continuing aspirin or NSAID in the presence of a DOAC were the most common error types. Indirectly, they provide evidence that providers did not follow up or act on pharmacist recommendations despite being templated in pharmacist documentation. Reducing errors may require intervention beyond what pharmacists can provide without the active collaboration of providers. There is likely an acculturation phase during which providers and patients increasingly expect and value communication from pharmacists before being willing to consistently implement their recommendations. This is particularly true in the ambulatory setting where there may be less interaction between clinical pharmacists and providers. Empowering pharmacists to order labs and deprescribe medications would be another avenue toward decreasing CIMEs as demonstrated in a previous study.21
We acknowledge multiple limitations to our findings. Control patients received assistance with access and affordability which are items included on the DOAC Checklist. This limits our ability to compare the benefit of the entire checklist compared to usual care. Providing these services to control patients, however, likely increased our recruitment rate and thereby the representativeness of our sample. In a related limitation, we do not know the impact of local, community-based pharmacists or other community-based services. Our randomized design with more than 500 patients should have mitigated confounding by these external services. In addition, our study was a single-center trial which has known limitations.22 Also, although we met with the directors of each of the practices caring for study patients, we did not meet with individual providers, relying rather on practice leaders to disseminate information about our study. Further facilitation would likely have increased the readiness of providers to review and act on recommendations documented by pharmacists in the EHR. We tracked the frequency that providers did not adhere (but not the frequency that they did adhere) to a pharmacist’s recommendation to order bloodwork or discontinue aspirin or a NSAID. We also note that we allowed up to seven days for the first pharmacist visit and, thus, may have missed the benefit of early pharmacist consultation among some patients. There may also be differences in the effectiveness of the intervention in patients referred by providers who were aware of and engaged with our study goals compared with patients recruited through electronic reports. Small numbers of the former group limit our ability to discern the differential effect.
In conclusion, our intervention framed around the DOAC Checklist did not reduce CIMEs. Our findings raise questions about how best to utilize pharmacist time as part of efforts to reduce DOAC-related medication errors and adverse events. Low rates of ADEs in our health system, the absence of longer-term follow-up to understand the correlation of medication errors to downstream ADEs, and assistance provided to control patients limit the ability to extrapolate more generally about the impact of our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
N/A.
Funding
The Agency for Healthcare Research and Quality funded this study.
Declarations
Conflict of Interest
Dr. Kapoor has received research grant support from Pfizer through its Independent Grants for Learning and Change funding mechanism and from Bristol Myers Squibb for independent medical education grants. Recently, he has received research grant support through a competitive process adjudicated and funded by the alliance, which is formed by both Pfizer and Bristol Myers Squibb. He has also been awarded a grant by Pfizer to examine conversations between patients and providers. Dr. McManus has received sponsored research support from Bristol Myers Squibb, Boehringer Ingelheim, Pfizer, Biotronik, and Philips Healthcare and has consulted for Bristol Myers Squibb, FlexCon, Samsung, Philips, and Pfizer. Dr. McManus has equity in Mobile Sense Technologies, LLC. Drs. Crawford, Mazor, and Gurwitz have also received research grant support from Bristol Meyers Squibb in the past 3 years (as coinvestigators on the grants secured by Dr. Kapoor). Dr. Gurwitz also serves as a consultant to United Healthcare.
Footnotes
Prior Presentations
N/A
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Budnitz DS, Shehab N, Lovegrove MC, Geller AI, Lind JN, Pollock DA. US emergency department visits attributed to medication harms, 2017–2019. JAMA. 2021;326(13):1299–1309. doi: 10.1001/jama.2021.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitworth MM, Haase KK, Fike DS, Bharadwaj RM, Young RB, MacLaughlin EJ. Utilization and prescribing patterns of direct oral anticoagulants. Int J Gen Med. 2017;10:87–94. doi: 10.2147/IJGM.S129235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairbanks RJ, Hildebrand JM, Kolstee KE, Schneider SM, Shah MN. Medical and nursing staff highly value clinical pharmacists in the emergency department. Emerg Med J. 2007;24(10):716–718. doi: 10.1136/emj.2006.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niznik JD, He H, Kane-Gill SL. Impact of clinical pharmacist services delivered via telemedicine in the outpatient or ambulatory care setting: a systematic review. Res Social Adm Pharm. 2018;14(8):707–717. doi: 10.1016/j.sapharm.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Nkansah N, Mostovetsky O, Yu C, et al. Effect of outpatient pharmacists' non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev. 2010;2010(7):Cd000336. doi: 10.1002/14651858.CD000336.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206–232. doi: 10.1007/s11239-015-1310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkaryoni A, Sirkar SD, Collins M, et al. Abstract 11434: emergency department disposition for patients with low-risk acute pulmonary embolism: a nationwide analysis. Circulation. 2021;144(Suppl_1):A11434–A11434. doi: 10.1161/circ.144.suppl_1.11434. [DOI] [Google Scholar]
- 8.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thrombos Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 10.Efird J. Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health. 2011;8(1):15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post-discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565–571. doi: 10.1007/s11606-014-3106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurwitz JH, Field TS, Ogarek J, et al. An electronic health record-based intervention to increase follow-up office visits and decrease rehospitalization in older adults. J Am Geriatr Soc. 2014;62(5):865–871. doi: 10.1111/jgs.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10. doi: 10.7326/0003-4819-157-1-201207030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Medication Errors and Adverse Drug Events. https://psnet.ahrq.gov/primer/medication-errors-and-adverse-drug-events#. Published 2019. Accessed February 6, 2023. [PubMed]
- 16.Gurwitz JH, Kapoor A, Garber L, et al. Effect of a multifaceted clinical pharmacist intervention on medication safety after hospitalization in persons prescribed high-risk medications: a randomized clinical trial. JAMA Intern Med. 2021;181(5):610–618. doi: 10.1001/jamainternmed.2020.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miele C, Taylor M, Shah A. Assessment of direct oral anticoagulant prescribing and monitoring pre- and post-implementation of a pharmacy protocol at a community teaching hospital. Hosp Pharm. 2017;52(3):207–213. doi: 10.1310/hpj5203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roldán V, Marín F, Fernández H, et al. Renal impairment in a "real-life" cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding) Am J Cardiol. 2013;111(8):1159–1164. doi: 10.1016/j.amjcard.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Barnes GD, Sippola E, Ranusch A, et al. Implementing an electronic health record dashboard for safe anticoagulant management: learning from qualitative interviews with existing and potential users to develop an implementation process. Implement Sci Commun. 2022;3(1):10. doi: 10.1186/s43058-022-00262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. National Library of Medicine. LEAVE Safe With DOACs. https://clinicaltrials.gov/ct2/show/NCT04068727. Published August 28, 2019. Updated January 12, 2023. Accessed May 26, 2023.
- 21.Johansson T, Abuzahra ME, Keller S, et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(2):532–548. doi: 10.1111/bcp.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;37(12):3114–3119. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.