Abstract
Background
Keratoconus is an ectatic (weakening) disease of the cornea, which is the clear surface at the front of the eye. Approximately 10% to 15% of patients diagnosed with keratoconus require corneal transplantation. This may be full‐thickness (penetrating) or partial‐thickness (lamellar).
Objectives
To compare visual outcomes after deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty for keratoconus, and to compare additional outcomes relating to factors which may contribute to poor visual outcomes (e.g. astigmatism, graft rejection and failure).
Search methods
We searched a number of electronic databases including CENTRAL, PubMed and EMBASE without using any date or language restrictions. We last searched the electronic databases on 31 October 2013. We also handsearched the proceedings of several international ophthalmic conferences.
Selection criteria
We included all randomised controlled trials (RCTs) comparing the outcomes of DALK and penetrating keratoplasty in the treatment of keratoconus.
Data collection and analysis
Two authors assessed trial quality and extracted data independently. For dichotomous data (graft failure, rejection, achievement of functional vision) results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). For continuous data (postoperative best corrected visual acuity (BCVA), uncorrected visual acuity (UCVA), keratometric astigmatism and spherical equivalent) results were expressed as mean differences (MDs) and 95% CIs.
Main results
We identified two completed studies, with a total of 111 participants (n = 30 and n = 81), both conducted in Iran, that met our inclusion criteria. Participants had moderate to severe keratoconus pre‐operatively and were randomly allocated to receive either DALK or penetrating keratoplasty. Only one eye of each participant was treated as part of the trials. The smaller study had 12 month follow‐up data for all participants. For the larger study, four DALK surgeries had to be abandoned due to technical failure and visual and refractive outcomes were not measured in these participants. Follow‐up length for the remaining 77 participants ranged from 6.8 to 36.4 months, with all 77 followed for at least three months post‐suture removal. Details of the randomisation procedure were unavailable for the smaller study and so sensitivity analyses were conducted to determine if the results from this study had affected the overall results of the review.
Neither of the included studies reported a difference between groups on any of the measures of post‐graft visual achievement, keratometric astigmatism or spherical equivalent. A single case of graft failure in a penetrating keratoplasty was reported. No postoperative graft failures were reported in the DALK group of either study.
Instances of graft rejection were reported in both groups, in both studies. The majority of these cases were successfully treated with steroids. The data, which related to all cases in each study ‐ given that the four cases that did not go ahead as planned had already technically failed without presence of rejection ‐ showed that rejection was less likely to occur in DALK (odds ratio (OR): 0.33, 95% confidence interval (CI) 0.14 to 0.81, GRADE rating: moderate).
Results of the sensitivity analysis indicated that inclusion of the Razmju 2011 study did not bias the results with regards to rejection episodes. While sensitivity analysis showed altered results with regards to failure rates, the data available from the Javadi 2010 study alone had a very wide 95% CI, suggesting an imprecise estimate. Therefore, even after removal of the Razmju 2011 data, it is still difficult to draw conclusions regarding superiority of one technique over another with regards to graft failure.
DALK was unable to be completed as planned in four cases and in a further three cases, complications during dissection required further intervention. Other adverse events, of varying severity, were reported in both intervention groups with similar frequency. For both types of surgery, these included postoperative astigmatism, steroid induced ocular hypertension and persistent epithelial defects. In recipients of DALK, one participant had interface neovascularisation (a proliferation of blood vessels where the host and donor cornea come together) and one had wrinkling of Descemet's membrane, the basement membrane separating the corneal stroma from the corneal endothelium. In the penetrating keratoplasty groups, one participant required graft resuturing and one had an atonic pupil, a condition in which the pupil dilates and is non‐reactive.
Overall, the quality of the evidence was rated as very low to moderate, with methodological limitations, incomplete data analysis and imprecision of findings, as well as high risk of bias in several areas for both studies.
Authors' conclusions
We found no evidence to support a difference in outcomes with regards to BCVA at three months post‐graft or at any of the other time points analysed (GRADE rating: very low). We also found no evidence of a difference in outcomes with regards to graft survival, final UCVA or keratometric outcomes. We found some evidence that rejection is more likely to occur following penetrating keratoplasty than DALK (GRADE rating: moderate). The small number of studies included in the review and methodological issues relating to the two, mean that the overall quality of the evidence in this review is low. There is currently insufficient evidence to determine which technique may offer better overall outcomes ‐ final visual acuity and time to attain this, keratometric stabilisation, risk of rejection or failure, or both, and risk of other adverse events ‐ for patients with keratoconus. Large randomised trials comparing the outcomes of penetrating keratoplasty and DALK in the treatment of keratoconus are needed.
Plain language summary
Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus
Review question We reviewed the evidence about the effect of deep anterior lamellar keratoplasty (DALK) (new technique) versus penetrating keratoplasty (standard technique) in people with keratoconus.
Background Keratoconus is a disease of the cornea, which is the clear surface at the very front of the eye. In eyes with keratoconus, the cornea becomes weak and cannot keep the spherical shape needed to provide "normal" vision. While the majority of people diagnosed with keratoconus can be treated with rigid contact lenses, in approximately 10% to 15% of patients this treatment is not sufficient and they require a corneal graft. This may be full‐thickness (penetrating) or partial‐thickness (lamellar). While the nature of each of these two types of transplantation suggest pros and cons for both, the clinical and practical outcomes have not previously been systematically reviewed.
Study characteristics We included two randomised controlled trials (RCTs) which involved a total of 111 participants in this review. Both trials were conducted in single medical centres in Iran and compared the outcomes, at least three months post‐suture removal (for a minimum of 12 months in the newer study, and for a range of 6.8 to 36.4 months in the older study), of participants with keratoconus who had received DALK to those who had received penetrating keratoplasty. The evidence is current to October 2013.
Key results The results suggested that graft rejection is more likely to occur following penetrating keratoplasty, however likelihood of graft failure was similar in both groups, as were visual and structural results.
DALK was unable to be completed as planned in four cases and in a further three cases complications during dissection required further intervention. Other adverse events, of varying severity, were reported in both intervention groups. For both types of surgery, these included postoperative astigmatism (when the cornea is no longer perfectly curved), raised pressure in the eye following steroid use, and a failure of the epithelium, the front layer of the eye, to heal properly. In recipients of DALK, one participant had interface neovascularisation (a growth of blood vessels where the host and donor cornea come together) and one had wrinkling of Descemet's membrane, a structural element of the cornea. In the penetrating keratoplasty groups, one participant required graft resuturing and one had an atonic pupil, a condition in which the pupil dilates and is non‐reactive. The included studies reported adverse events thoroughly.
Quality of the evidence The evidence remains weak, as the quality of evidence is rated very low to moderate.
Large trials comparing the outcomes of DALK and penetrating keratoplasty for the treatment of keratoconus, are needed. These should be randomised single‐masked trials, in which graft recipients are unaware of their group allocation. Because of the nature of the surgery, it is not likely to be possible to conduct double‐masked trials as practitioners who are qualified to undertake outcomes assessments would be able to see which graft a participant had received. Future trials should include regular, long‐term follow‐up and consistent methods must be used.
Summary of findings
for the main comparison.
| Deep anterior lamellar keratoplasty (DALK) compared with penetrating keratoplasty for keratoconus | ||||||
|
Patient or population: Patients with keratoconus undergoing corneal grafting Settings: Hospital Intervention: Deep anterior lamellar keratoplasty (DALK) Comparison: Penetrating keratoplasty | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Penetrating keratoplasty | DALK | |||||
| Postoperative BCVA (3 months) (LogMAR) | No usable data were available on this measure in either of the identified studies | |||||
|
Postoperative functional BCVA achieved (3 months) Functional BCVA = vision of 0.30 LogMAR or better. As at, 3 months post‐graft |
No usable data were available on this measure in either of the identified studies | |||||
|
Postoperative BCVA (12 months) (LogMAR) As at, 12 months post‐graft |
The mean postoperative BCVA in the control group was 0.08 LogMAR | The mean postoperative BCVA in the intervention group was 0.06 LogMAR higher (95%CI ‐0.02 to 0.14) |
N/A | 30 (1) | ⊕⊝⊝⊝ very low1 | |
|
Postoperative BCVA (post‐suture removal, varied follow‐up times) (LogMAR) As at, at least 3 months post‐suture removal |
The mean postoperative BCVA in the control group was 0.15 LogMAR | The mean postoperative BCVA in the intervention group was 0.03 LogMAR higher (95%CI ‐0.01 to 0.07) |
N/A | 77 (1) | ⊕⊝⊝⊝ very low1 | |
|
Postoperative functional BCVA achieved (post‐suture removal, varied follow‐up times) Functional BCVA = vision of 0.30 LogMAR or better As at, at least 3 months post‐suture removal |
670 per 1000 |
545 per 1000 (92 to 932) |
OR 0.59 (0.05 to 6.77) | 77 (1) | ⊕⊝⊝⊝ very low1 | |
|
Presence of at least one rejection episode As at, post‐suture removal |
180 per 1000 | 68 per 1000 (30 to 151) | OR 0.33 (0.14 to 0.81) | 111 (2) | ⊕⊕⊕⊝ moderate2 | |
|
Failed graft Including cases where the DALK or PK procedure was unable to be successfully completed as outlined in the methodology for the study |
In Javadi 2010 4/46 people in the DALK group had failed graft compared to 0/35 in PK group (OR 7.52, 95% 0.39 to 144.43); in Razmju 2011 0/15 people in DALK group had failed graft compared to 1/15 in PK group (OR 0.31, 0.01 to 8.28) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; BCVA: Best corrected visual acuity; UCVA: Uncorrected visual acuity; LogMAR: Logarithm of the minimum angle of resolution (see footnotes) | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
LogMAR: Each line of a LogMAR chart has 5 letters. Each letter is worth 0.02 on the LogMAR scale. Normal vision (6/6 or 20/20) is a logMAR score of 0. A person with vision one full line (five letters) worse than normal will score 0.10, while a person with vision one line better than normal will score ‐0.10.
Assumed risks for frequency of graft failure, rejection episodes and achievement of functional BCVA in penetrating keratoplasty performed for keratoconus, are based on figures from the 2012 Australian Corneal Graft Registry report. The assumed risk for achievement of functional UCVA is based on the assertion that half of keratoconus patients who undergo penetrating keratoplasty need to wear visual aids to achieve their BCVA.
1Downgraded for risk of bias, imprecision and inconsistency (not possible to assess consistency as only one small trial).
2Downgraded for risk of bias.
Summary of findings 2. Summary of findings ‐ Javadi findings only.
| Deep anterior lamellar keratoplasty (DALK) compared with penetrating keratoplasty for keratoconus | ||||||
|
Patient or population: Patients with keratoconus undergoing corneal grafting Settings: Hospital Intervention: Deep anterior lamellar keratoplasty (DALK) Comparison: Penetrating keratoplasty | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Penetrating keratoplasty | DALK | |||||
|
Postoperative BCVA (3 months) (LogMAR) Measured on LogMar scale where normal vision is 0.00. Each line of a LogMAR chart has 5 letters. Each letter is worth 0.02 on the LogMAR scale. A person with vision one full line (five letters) worse than normal will score 0.10, while a person with vision one line better than normal will score ‐0.10. As at, 3 months post‐graft |
N/A | N/A | N/A | N/A | N/A | No usable data were available on this measure in the identified study |
|
Postoperative functional BCVA achieved (3 months) Functional BCVA = vision of 0.30 LogMAR or better. As at, 3 months post‐graft |
N/A | N/A | N/A | N/A | N/A | No usable data were available on this measure in the identified study |
|
Postoperative BCVA (post‐suture removal, varied follow‐up times) Measured on LogMar scale where normal vision is 0.00. Each line of a LogMAR chart has 5 letters. Each letter is worth 0.02 on the LogMAR scale. A person with vision one full line (five letters) worse than normal will score 0.10, while a person with vision one line better than normal will score ‐0.10. As at, at least 3 months post‐suture removal |
The mean postoperative BCVA in the control group was 0.15 LogMAR | The mean postoperative BCVA in the intervention group was 0.03 LogMAR higher (95%CI ‐0.01 to 0.07) |
N/A | 77 (1) | ⊕⊝⊝⊝ very low1,2,3 | |
|
Postoperative functional BCVA achieved (post‐suture removal, varied follow‐up times) Functional BCVA = vision of 0.30 LogMAR or better. As at, at least 3 months post‐suture removal |
Study population | OR 0.59 (0.05 to 6.77) | 77 (1) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 670 per 1000 |
545 per 1000 (92 to 932) |
|||||
|
Presence of at least one rejection episode As at, at least 3 months post‐suture removal |
Study population | OR 0.37 (0.14 to 0.98) | 81 (1) | ⊕⊕⊝⊝ low1,3 | ||
| 180 per 1000 | 75 per 1000 (30 to 177) | |||||
|
Failed graft Including cases where the DALK or PK procedure was unable to be successfully completed as outlined in the methodology for the study. As at, at least 3 months post‐suture removal |
Study population | OR 7.52 (0.39 to 144.43) | 81 (1) | ⊕⊝⊝⊝ very low1,3,4 | ||
| 33 per 1000 | 204 per 1000 (13 to 831) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; BCVA: Best corrected visual acuity; UCVA: Uncorrected visual acuity; LogMAR: Logarithm of the minimum angle of resolution | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Assumed risks for frequency of graft failure, rejection episodes and achievement of functional BCVA in penetrating keratoplasty performed for keratoconus, are based on figures from the 2012 Australian Corneal Graft Registry report. The assumed risk for achievement of functional UCVA is based on the assertion that half of keratoconus patients who undergo penetrating keratoplasty need to wear visual aids to achieve their BCVA.
1Not specified whether allocation concealment and blinding was used for either participants or outcome assessors.
2Incomplete data analysed. High risk of attrition bias as the outcomes from the four failed grafts were likely to be poor.
3Inability to assess consistency due to inclusion of just one trial.
4Very wide confidence interval, suggesting an imprecise estimate, which crosses the line of no difference (OR 7.52, 95% CI 0.39 to 144.43).
Background
Description of the condition
Keratoconus is an ectatic (weakening) disease of the cornea, which is the clear surface at the front of the eye (Coster 2002). Weakness in the tensile strength of the cornea results in distortion of the anterior refractive surface of the eye. The weakened cornea is unable to stand the intraocular pressure and protrudes in a conical shape (Ertan 2008a). The increased curvature of the conical cornea results in myopia (short‐sightedness), and irregularities of the cone produce astigmatism, which causes blurred vision.
Keratoconus is usually bilateral and manifests early, in the first two decades of life (Ertan 2008a; Espandar 2010). Progression is uncommon after the age of 35 years (Romero‐Jimenez 2010). For most people, progression is slow. Early onset of the condition is often associated with more rapid progression.
Corneal curvature is used to assess the severity and progression of keratoconus. Central corneal curvature is adequately measured by keratometry. More extensive assessment of corneal shape requires videokeratography (Gobbe 2005; Jafri 2007).
Various systems have been developed to classify the progression of keratoconus into different stages of the disease (Romero‐Jimenez 2010). One such system which is widely accepted and utilised is the Amsler‐Krumeich classification system, separating the disease into four stages based on level of myopia and astigmatism, central keratometric readings, scarring and corneal thickness (Ertan 2008b). A variation on this system has been developed more recently by Alió and Shabayek (Alió 2006) to incorporate diagnostic information relating to the severity of higher order corneal aberrations, the visual distortion created by a wavefront of light passing through an irregular eye.
Advanced keratoconus is also accompanied by an increased incidence of hydrops. This is an acute stromal oedema caused by breaks in Descemet’s membrane through which aqueous humour, the substance filling the space between the lens and cornea, enters and swells the stroma, the major component of the cornea. Hydrops generally results in scarring of the cornea, which can have a continued impact on visual potential (Rabinowitz 1998; Romero‐Jimenez 2010), however it is a rare complication (Bilgin 2009).
Keratoconus is common. It has been reported to affect approximately one person in 2000 in the North American population (Rabinowitz 1998). Although some suggest that the prevalence could actually be as high as one per 500 people (Ertan 2008a; Espandar 2010), other studies have found lower prevalence rates, sometimes as low as one per 70,000 people. These studies were conducted in a number of different countries and although the disease affects all ethnic groups, findings suggest that people of various ethnic backgrounds, particularly those of Asian descent, may be more prone to the disease than Caucasian populations (Georgiou 2004). The inconsistency in reported prevalence across studies is probably also due to the wide range of definitions and diagnostic criteria utilised by practitioners and researchers (Rabinowitz 1998; Romero‐Jimenez 2010).
The disease affects both men and women, however some studies have found variations in prevalence across the sexes. Genetics have been found to play a role in the disease, with a family history reported in approximately 6% to 10% of cases and an increased risk in first degree relatives also documented. A recent study on keratoconus in monozygotic and dizygotic twins supports the premise that genetic background makes an important contribution to disease severity, but also suggests an environmental effect on its expression (Tuft 2012). A review of the genes thus far implicated in keratoconus has identified at least six coding mutations worthy of further investigation (Wheeler 2012), and new candidate genes continue to be pulled from genome‐wide association studies (Lu 2013). The definitive cause of keratoconus is still unknown (Rabinowitz 1998; Wang 2000).
Description of the intervention
The treatment of keratoconus relies on the use of a hard or semi‐rigid contact lens to cover the irregular cornea and provide a new, appropriately curved anterior refractive surface (Bilgin 2009; Rabinowitz 1998). If the surface of the conical cornea is too steep or too irregular to bear a contact lens, or if the eye is too sensitive to tolerate a lens, surgery becomes necessary; approximately 10% to 15% of patients diagnosed with keratoconus require surgery (Rabinowitz 1998; Romero‐Jimenez 2010). Corneal transplantation is the procedure employed. The purpose of corneal transplantation for keratoconus is to replace the abnormal anterior refracting surface of the eye with a donor cornea that has a normal anterior surface shape. Corneal transplantation for keratoconus may be full‐thickness (penetrating) or partial‐thickness (lamellar).
Penetrating keratoplasty
Penetrating keratoplasty has been performed as a treatment for keratoconus for over 70 years (Castroviejo 1948) and remains the leading treatment for those sufferers with contact lens intolerance (Jhanji 2010; Rabinowitz 1998). Existing longitudinal data show that keratoconus is one of the most common indications for penetrating keratoplasty and that these recipients have higher graft survival rates than those undergoing the surgery for other conditions (Jaycock 2008; Williams 2007). An analysis conducted in 2006 concluded that it was a cost‐effective treatment for severe cases of keratoconus (Roe 2008).
Penetrating keratoplasty involves the replacement of a full‐thickness portion of the cornea (Coster 2002; Romero‐Jimenez 2010). There are many variations in technique, however a recent review of the published evidence concluded that there was “no evidence for the superiority of any specific technique” (Frost 2006).
Deep anterior lamellar keratoplasty (DALK)
More recently, lamellar transplantation, in which only a partial‐thickness of the cornea is replaced, has been reintroduced as a surgical treatment for keratoconus (Romero‐Jimenez 2010). This form of transplantation has been used for decades, however poor visual outcomes resulted in a decline in its use (Trimarchi 2001). Newer techniques in which the interface of the donor and host is at the level of Descemet's membrane have reinvigorated the use of this form of surgery (Karimian 2010; Sugita 1997).
Deep anterior lamellar keratoplasty (DALK) is frequently used for keratoconus. Advocates claim that this procedure is preferable to penetrating keratoplasty for eyes that are free from corneal scarring or hydrops (Jhanji 2010). The premise is that, because the endothelial cell layer of the recipient is left intact during DALK, the prospect of endothelial rejection is precluded (Romero‐Jimenez 2010; Tan 2010b).
Various techniques have been used to dissect the stroma from the underlying Descemet’s membrane (Jhanji 2010; Tan 2010b). Common approaches include manual dissection (Anwar 2002; Karimian 2010), which may be enhanced by injection of air into the anterior chamber and stroma (Archila 1984); dissection with a visco‐elastic substance, as advocated by Melles (Melles 2000); or the big‐bubble dissection technique advocated by Anwar et al (Anwar 2002). Dissection with the femtosecond laser is also gaining some currency (Buzzonetti 2010; Farid 2009). Each approach has its proponents.
How the intervention might work
To reiterate, the purpose of corneal transplantation for keratoconus is to replace the abnormal anterior refractive surface of the eye with a cornea that has a normal shape. In penetrating keratoplasty the full‐thickness of the cornea is replaced, while in DALK the corneal stroma is replaced down to the Descemet's membrane, so that the diseased portion of the eye is still replaced, while the healthy endothelium of the recipient is left intact.
Why it is important to do this review
While the desired therapeutic outcomes are identical, the benefit and risk profile of the two procedures may be different and disparate outcomes have been reported. Replacing fewer layers of the cornea may reduce the likelihood of rejection and subsequent failure as the endothelium, the layer involved in many incidences of rejection, is no longer replaced during the surgery. However, other complications may arise due to problems at the donor/host junction. Visual outcomes may be affected by this or by the reduction in time from transplantation to suture removal. It is important that outcomes from the newer treatment, DALK, be compared to those achieved using the traditional penetrating technique in terms of visual outcome and graft survival. The results will help to inform corneal surgeons and keratoconus sufferers of the appropriateness of each treatment for this condition. This will aid in the clinical decision making process with regard to the selection of treatment for individuals with this condition.
Objectives
To compare visual outcomes after deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty for keratoconus, and to compare additional outcomes relating to factors which may contribute to poor visual outcomes (e.g. astigmatism, graft rejection and failure).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that met the stated inclusion criteria. The review included all RCTs in which one arm received treatment with penetrating keratoplasty and the other with DALK. Where a study was defined as being randomised but the details were not included in the published literature, we attempted to gain this information from the authors.
Types of participants
Participants of any age could be included in selected trials. We excluded studies that included participants with other confounding related disorders, such as pellucid marginal degeneration (PMD) (the thinning of the periphery rather than the central area of the cornea) or keratoglobus in which the entire corneal surface is involved. We only included in the review studies which specified an objective method of diagnosis of the keratoconus (slit lamp examination, corneal topography, wave front analysis). Participants could be at any stage of the disease and there could be a mixture of stages of progression amongst participants, as long as this was specified. Participants must not have had a history of corneal scarring or hydrops. With regards to keratoconus prevalence or progression, no significant differences across cultural and racial backgrounds have been confirmed in the scientific literature. Thus, studies from anywhere in the world were eligible for inclusion.
Types of interventions
We included trials in which the outcomes of penetrating keratoplasty and DALK, as treatments for keratoconus, were directly compared to one another. We also included studies in which both treatments were compared to one another as well as a third treatment or a control group. We excluded studies comparing either one of these treatments alone to a third treatment or a control group.
Types of outcome measures
Studies which reported at least one clinical outcome were eligible for inclusion.
Primary outcomes
The vast majority of keratoconus patients who undergo corneal graft surgery do so in order to gain improved vision (Williams 2007). In some cases, uncorrected post‐graft vision may improve to a functional level but for others, approximately half of recipients, correction with contact lenses or spectacles will still be necessary in order to achieve optimal, useful vision. Therefore, we used post‐graft best corrected visual acuity (BCVA) as the primary outcome measure.
At three months post‐graft, the initial healing of the eye should have concluded and BCVA results start to become more reliable. Three months was therefore selected as the time point at which the balance between the likelihood that BCVA results would be valid were balanced with the need to minimise the likelihood of attrition bias, and so this time point was selected for the primary outcome measure.
For inclusion in analyses, BCVA needed to be provided (mean and range) in terms of either Snellen or LogMAR measurements (measurements given in either of these two forms can be easily converted into the other for the relevant analyses). Where necessary, Snellen measurements were converted into LogMAR. LogMAR measurements were to be obtained using the Bailey‐Lovie chart, not the adapted ETDRS chart, developed as part of the Early Treatment of Diabetic Retinopathy Study (Kaiser 2009), which gives a somewhat different result and is not accurate for measuring visual acuity in people without disorders of the retina.
Where recorded, post‐graft BCVA was to be considered in two ways, firstly in terms of the level of BCVA achieved (functional vision of 6/12 or better versus non‐functional vision of 6/15 or worse) and also in terms of change from pre‐graft BCVA (improvement of >1 line of Snellen acuity, ≤1 line of change in Snellen acuity in either direction, deterioration of > 1 line of Snellen acuity).
Secondary outcomes
Analyses of BCVA at six months, 12 months and 24 months were planned as secondary outcomes. Final BCVA is often not achieved until after all sutures have been removed and the eye has had a chance to settle. As such, analysis of BCVA at least three months post‐suture removal were also planned as a secondary outcome measure.
Uncorrected visual acuity (UCVA) was a secondary outcome measure. Again, it was planned that this would be analysed for measurements at three, six, 12 and 24 months post‐surgery, plus at least three months post‐suture removal, where provided (mean and range) in terms of either Snellen or LogMAR measurements. As with BCVA, these data were to be considered both in terms of the final UCVA achieved and with regards to the change in UCVA pre‐ to post‐graft.
We also considered the method of visual correction (in order to be able to achieve a desirable BCVA) and keratometry readings (to determine level of astigmatism) as secondary outcome measures.
Other secondary outcome measures were the frequency of rejection episodes and graft failure.
Timing of outcome assessment
As visual recovery from corneal grafting can continue for a long time, outcome measures were to be analysed primarily at three months post‐graft; as well as in the context of secondary outcome measures at the following periods of time post‐graft, where possible: six, 12 and 24 months. In addition, the removal of sutures has been shown to impact on final visual acuity and so, where available, comparisons were made between both BCVA and UCVA at least three months after final suture removal.
We contacted authors in an effort to ascertain these measurements where they were not given in the published literature.
Adverse outcomes
It was anticipated that other adverse effects may have been reported, including intraoperative complications, such as perforation or need for re‐bubbling, and postoperative events such as scarring, infection, cataract or pain. All adverse events reported were considered important. The frequency of these events was also compared across treatment groups in a qualitative manner.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2013, Issue 9), the Database of Abstracts of Reviews of Effects (DARE) (2013, Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2013), PubMed (1966 to October 2013), EMBASE (January 1980 to October 2013), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to October 2013), OpenGrey, Web of Science ‐ Science Citation Index (SCI) (January 1970 to October 2013), Health Collection ‐ Informit (January 1977 to October 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 31 October 2013.
See: Appendices for details of search strategies for CENTRAL and DARE (Appendix 1), MEDLINE (Appendix 2), PubMed (Appendix 3), EMBASE (Appendix 4), LILACS (Appendix 5), CINAHL (Appendix 5), OpenGrey (Appendix 7), SCI (Appendix 8), Informit (Appendix 9), mRCT (Appendix 10), ClinicalTrials.gov (Appendix 11) and the ICTRP (Appendix 12).
Searching other resources
We handsearched the following international conference proceedings in order to identify further, unpublished studies.
American Society of Cataract and Refractive Surgery Symposium and Congress: 2007 to 2012
Asia Pacific Academy of Ophthalmology Congress: 2012
European Society of Cataract and Refractive Surgeons Congress: 2009 to 2012
International Congress of Eye Research: 2012
Royal Australian and New Zealand College of Ophthalmology Congress: 2011 to 2012
Royal College of Ophthalmologists Congress: 2011 to 2012
World Ophthalmology Congress: 2008 to 2012
We attempted to contact the authors of any studies identified in this way to gain further information where this was required.
We also handsearched the reference lists of selected studies in order to identify other relevant articles, conference presentations or book chapters.
Data collection and analysis
Selection of studies
Two review authors (MK and KW) independently assessed all citations gathered using the outlined parameters. Each independently classified each citation as either 'definitely not relevant' or 'potentially relevant'. Where a study was judged by both authors at this time to be 'definitely not relevant', it was excluded from further analysis. In cases where one author believed a study to be 'definitely not relevant' while the other classified it as 'potentially relevant' the final classification of the study was determined by consensus.
Where citations were classified as 'potentially relevant', full copies of related publications were obtained by MK and each study was assigned an identification number (ID). Where a study published in a language other than English was identified as being potentially relevant, we initially arranged a translation of the methods and results sections of the study. If the study appeared to meet the selection criteria based on the methods and results, we then sought a full English translation of the study. Where the published data were felt by at least one author (either MK or KW) to be insufficient to determine the relevance of the study, MK contacted the trial investigators to request the necessary further information.
Having read the full articles and considered any further information gathered from trial investigators, the two review authors (MK and KW) classified them as either 'relevant' or 'not relevant'. These authors compared both lists, and excluded those that were classified as 'not relevant' by both review authors. The reasons for their exclusion were recorded and these are documented in the review. Those that were classified as 'relevant' by both review authors were included in the review. We recorded the reasons for any further exclusions and documented them in the review. MK entered data into RevMan (RevMan 2012) at each step in the review process.
Data extraction and management
Two review authors (MK and KW) independently extracted the data using a form based on one developed by the Cochrane Eyes and Vision Group. This included information on the following.
The age, gender, race, geographical location and grade of keratoconus of the participants, as well as the number in each treatment group and the comparability of the two groups on the aforementioned parameters at baseline.
The methods used in each intervention group.
Information on missing data and participants who did not complete the trial.
The outcomes of the treatments. We collected dichotomous data in terms of number at risk and number of events, and used means and standard deviations for continuous data. We extracted data for the outcome measures outlined for this review.
MK entered the data into RevMan (RevMan 2012), with KW checking the entered data for inconsistencies or errors. Where there were missing data, we identified this, along with any reasons given, and we conducted analysis utilising the available data.
Assessment of risk of bias in included studies
Two authors (MK and KW) independently assessed the risk of bias for the included studies as per the methods given in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Methodological quality was assessed for the following parameters.
Potential issues relating to selection bias
1.Randomisation technique: randomisation via a random number generator, random number table, shuffled cards or shuffled envelopes, where the assignment of treatment groups is conducted and confirmed prior to specific participant allocation and cannot be changed after this point, was considered appropriate. Inadequate techniques included alternation, assignment based on variables such as record numbers, dates of birth or days of the week, or any form of randomisation in which participant assignment could be altered or affected by the treating surgeon after the initial assignment. We excluded studies with inadequate randomisation techniques and recorded the reasons for this.
2. Allocation concealment: we considered methods such as central telephone randomisation and numbered, sealed, opaque envelopes as adequate methods of concealing the allocation from people recruiting participants.
Potential issues relating to performance bias
3 Blinding/masking: recipients should ideally be masked.
Potential issues relating to detection bias
4. Outcome measurements: despite differences in the techniques, outcomes of the interventions should be measured in the same way for both. Those administering the assessments of outcomes should ideally be masked.
Potential issues relating to attrition bias
5. Completion of follow‐up: was this equal across treatment groups, what were the reasons for this and were there adequate numbers remaining for the results to be meaningful?
Two review authors (MK and KW) graded the studies on each of these five parameters, providing a determination of low risk of bias, unclear risk of bias, or high risk of bias. They discussed any disagreements in classification. If necessary, the authors of the trials in question were contacted by MK in an attempt to clarify any unclear information.
Measures of treatment effect
Outcome measures comprised two types of data: continuous and dichotomous. We used mean differences to ana lyze continuous data (BCVA and UCVA measured in LogMAR, as well as change in BCVA and UCVA LogMAR results). For outcomes with dichotomous data (functional level of BCVA or UCVA, rejection episodes, graft failure), we measured the effect size using the odds ratio (OR).
Unit of analysis issues
There were no unit of analysis issues because people were randomised to treatment and one eye included in the included studies. Future updates of this review may include trials that include both eyes, either as within‐person studies, or where both eyes receive the same treatment. We will address these issues as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), especially in light of the fact that keratoconus may not be bilaterally symmetrical and outcome in one eye may affect outcome in the other. See also Differences between protocol and review.
Dealing with missing data
We considered the potential impact of any missing data. While Javadi 2010 did have missing data, no intention‐to‐treat (ITT) analyses were possible as data for the missing cases were not available. We documented the cases lost to follow‐up in this study and reported the available case analysis. We discussed the likely impact of these missing data in the text and we rated the study as being at high risk of attrition bias.
Assessment of heterogeneity
We assessed the heterogeneity of the included studies in order to determine whether it was appropriate to carry out a meta‐analysis on the data. This was done by examining the characteristics of the studies and the forest plot of study results, and by conducting a Chi² test of statistical heterogeneity and determining the I² statistic. Where the I² statistic indicated less than 30% variability due to heterogeneity, this was considered to be insubstantial, while over 50% was considered to indicate substantial heterogeneity. For cases in which 30% to 50% heterogeneity was estimated, we considered the magnitude and direction of the effect, as well as the P value of the Chi² test, when making a final decision about the substantiality. For the outcomes where we determined that the heterogeneity of the studies was substantial, we did not combine the results in a meta‐analysis but rather reported them in a descriptive summary. Where substantial heterogeneity was not present, we conducted meta‐analyses using the fixed‐effect model, as only a small number of studies were included.
Assessment of reporting biases
In order to assess bias relating to selective outcome reporting, we compared the intended outcome measures for each included trial, as recorded in the methods sections of resulting articles, to those reported in the results sections.
Data synthesis
For the majority of outcome measures, we identified an insufficient number of trials to conduct meta‐analyses, and so we have provided results in a descriptive summary form. Data were available from two studies for the outcome measures of "presence of rejection episodes" and "failed graft" and so we examined these results using meta‐analyses as well as presenting them in a descriptive summary form. We used fixed‐effect models as we only identified two studies.
Subgroup analysis and investigation of heterogeneity
In cases where we determined heterogeneity of the studies to be significant, we decided not to combine the results in a meta‐analysis, but rather report them in a descriptive summary. We judged heterogeneity to be substantial with regards to the variable "failed graft" and so judged the quantitative analysis of the data from the two studies to be unreliable, and we provided a descriptive summary instead.
Sensitivity analysis
We conducted one sensitivity analysis, in which we removed the data from the Razmju 2011 study, as the risk of allocation bias ‐ encompassing issues related to the randomisation process, including sequence generation and allocation concealment ‐ was judged to be high. In this way, it was possible to assess how strongly the results of our review are related to the decisions and assumptions that we have made throughout the review process.
Methods for creating Summary of Findings tables
In Table 1, we presented data for seven outcome measures. Firstly, the primary outcome measures of BCVA and achievement of functional BCVA, at three months post‐graft. As the primary outcome of interest was BCVA, we then included outcomes relating to BCVA at other time points that were reported in either of the studies, these being BCVA achieved 12 months post‐graft and three months post‐suture removal, and achievement of functional BCVA post‐suture removal. In addition, we included the two outcomes for which data were available from both studies, presence of rejection and graft failure. We did not include outcomes for BCVA at 12 months post‐graft in Table 2 as these data were not given in the Javadi 2010 paper.
For the two continuous outcome measurements included in the 'Summary of findings' table for which data were available (BCVA at 12 months post‐graft and BCVA post‐suture removal) the assumed risk was the mean value in the penetrating keratoplasty group, while the corresponding risk was the mean value in the DALK group. For the three dichotomous outcomes (graft failure, rejection episodes and achievement of functional BCVA) we calculated the assumed risk based on figures from the 2012 Australian Corneal Graft Registry report (Williams 2012). We based the corresponding risks (and 95% confidence intervals) for the DALK group on the assumed risk in the comparison group (ACR) and the relative effect (OR) of the intervention (and its 95% CI) in that corresponding risk (CR) = 1000*((OR*ACR)/(1‐ACR+(OR*ACR))). Two review authors (MK and KW) independently evaluated the quality of evidence using the GRADE system, as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Results
Description of studies
Results of the search
The electronic searches yielded a total of 206 references (Figure 1). The Trials Search Co‐ordinator scanned the search results, removed duplicates and removed 95 references which were not relevant to the scope of the review. We screened the remaining 72 records from the electronic search, and we also found a further five records identified through handsearching of conference proceedings, resulting in 77 records to be screened. We excluded 31 records as not relevant after evaluation of their abstract.
1.
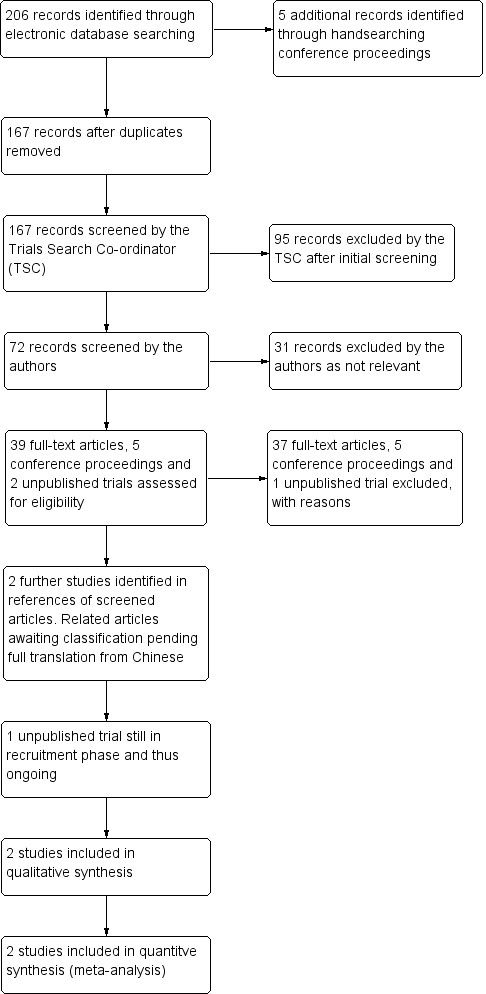
Results from searching for studies for inclusion in the review.
We assessed 39 full‐text articles, five abstracts from conference proceedings and two unpublished trials identified via ClinicalTrials.gov for eligibility.
We judged 37 full‐text articles and two conference abstracts as not eligible for inclusion and so we excluded them with reasons documented. After several attempts to contact the authors of three of the unpublished trials identified from the conference proceedings, we were unable to source any further information on these studies and therefore excluded them also. We were unable to obtain contact details for the authors of one of the trials registered on ClinicalTrials.gov and so excluded this.
We identified two additional potentially relevant articles (Jiang 2006; Liu 2008) in the reference list of one of the screened articles. These articles are both in Chinese and are currently awaiting classification, pending full translation. See the Characteristics of studies awaiting classification table for further information.
One unpublished trial (NCT01901614) meets the inclusion criteria for this review, but is still in the recruitment phase and is thus categorized as ongoing.
Two studies met all eligibility criteria and we included them in the qualitative synthesis and meta‐analyses. However, details of the randomisation procedure were unavailable for one of these studies and so we conducted sensitivity analysis to determine the effect this might have on the overall findings.
Included studies
We included two studies that met our inclusion criteria (Javadi 2010; Razmju 2011). We also conducted a sensitivity analysis in which we removed the data from the Razmju 2011 study and considered the data in the Javadi 2010 study alone to see what impact this might have on the results and conclusions.
Study design
Both studies were randomised single centre trials, with participants allocated to receive either penetrating keratoplasty or DALK. Only one eye of each participant was treated as part of the trials.
Participants
Both studies were conducted in Iran. Razmju 2011 included 30 participants, with 15 allocated to each treatment group, while Javadi 2010 included 81 participants, with 46 undergoing DALK and 35 penetrating keratoplasty. Participants had moderate to severe keratoconus pre‐operatively.
Interventions
Details of the specific interventions used were not given by Razmju 2011, with their trial report simply stating that one arm of the study received penetrating keratoplasty, and the other deep lamellar keratoplasty.
Javadi 2010 conducted DALK using the big‐bubble technique with the recipient cornea trephined 7.75 mm or 8 mm, depending on the recipients vertical corneal diameter (greater than or equal to versus less than 10.5 mm). Air was injected into the mid‐stroma to form a big‐bubble extending to the trephination site. The stroma was then removed. In penetrating keratoplasty, the recipient trephination size (7.75 mm or 8 mm) was again dependent on the recipients vertical corneal diameter (greater than or equal to versus less than 10.5 mm). Trephination was conducted with a Hessburg‐Barron suction trephine and the excision was completed using right and left corneal scissors. For these donor corneas, trephination was conducted from the endothelial side. In all surgeries, the size of the corneal donor button was dependent on the recipient trephine size and was 0.25 mm or 0.5 mm larger, depending on vitreous length (greater than or equal to versus less than 16 mm). The donor cornea was sutured to the recipient in one of three different ways, all using 10‐0 nylon sutures: 16 interrupted sutures, one single 16‐ to 18‐bite running suture, or a single 16‐bite running suture plus an 8‐bite interrupted suture. This was based on surgeon preference and the condition of the eye. Suture tension was adjusted via intraoperative keratoscopy. Cefazolin 100 mg and betamethasone 4 mg were injected subconjunctivally. Participants received topical chloramphenicol every six hours for 30 days and topical betamethasone 0.1% every six hours, tapered over two to three months.
Outcome measures
Razmju 2011 reported outcomes relating to the level achieved and change from postoperative levels for both BCVA and spherical equivalent. They also reported on intraoperative and postoperative complications, including rejection and failure. Outcomes were measured at 12 months.
Javadi 2010 reported outcomes on measures of visual acuity (UCVA, BCVA) and refraction (spherical equivalent, keratometric astigmatism). They also reported on intraoperative and postoperative complications (failure and rejection). Follow‐up examinations were performed at one, three, seven and 30 days; three, six and 12 months; and three to six months after complete suture removal. Outcome data were provided regarding overall failure and rejection rates. Outcomes post‐suture removal (variable follow‐up lengths) were given for keratometric astigmatism (final level and change from postoperative), spherical equivalent (final level and change from postoperative), and final BCVA. Graphs showed results for BCVA, UCVA, spherical equivalent and keratometric astigmatism pre‐operatively, at one, three, six, 12 and 24 months, and at a minimum three months post‐suture removal. The majority of these data were not specified in‐text, and so the exact figures at each time‐point are uncertain.
Excluded studies
We excluded 30 studies (Acar 2013; Akdemir 2012; Amayem 2013; Arenas 2005; Behrens 2000; Birnbaum 2008; Borderie 2012; Busin 1991; Cardoso da Silva 2007; Cohen 2010; Farias 2008; Funnell 2006; Hara 2013; Haugen 2001; Javadi 2006; Jiang 2011; Jones 2009; Koo 2011; Kubaloglu 2012; McDonald 1987; Motlagh 2012; Panda 1999; Salvetat 2013; Sari 2012; Serdarevic 1996; Shimmura 2005; Shoja 2007; Tan 2010a; Watson 2004; Yamaguchi 2011) relating to 32 papers because they were not RCTs. We excluded a further four papers (Frost 2006; Reinhart 2011; Shi 2012; Tan 2007) as they turned out to be review articles rather than original studies. We excluded three studies (Panda 2012; Kandemir 2011; Mahjoub 2011), all identified via handsearching of conference proceedings, as it was unclear whether they were RCTs and what, if any, exclusions of participants were undertaken. In each case, we attempted to contact the authors of these studies to gather further information, a minimum of two times, without success. One study (Cheng 2011), for which there were two published articles and two conference abstracts available, appears to meet all criteria except that only a subgroup of participants had keratoconus. Communication with the study authors suggests that data for this subgroup only may be available, however it has not been provided as yet and so we have excluded this study at this time, but we could include it in future updates if the data become available. We excluded one study (NCT00371202) registered on ClinicalTrials.gov, as it was unclear whether the trial was completed and we were unable to obtain contact details for the study author.
Risk of bias in included studies
The assessed risk of bias of the included studies is shown graphically in Figure 2 and Figure 3.
2.
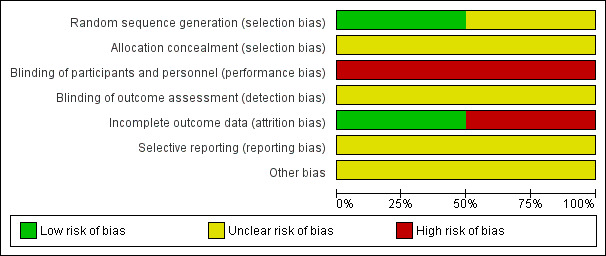
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Both studies reported that the participants were randomly allocated to the two groups. Razmju 2011 did not specify their method of randomisation and we therefore judged this study to have an unclear risk of bias. Javadi 2010 indicated that they used a random number table and therefore we judged their study to have a low risk of bias.
Allocation concealment
No information was provided by Razmju 2011 or Javadi 2010 about whether there was adequate allocation concealment (though Javadi 2010 did reveal that the random allocation was carried out at the same clinic where the surgery was performed) and therefore there was unclear risk of bias for both studies.
Blinding
Due to the differences in the two treatments being studied, it is not possible to mask surgeons to which procedure is being performed. No information was provided by Razmju 2011 or Javadi 2010 about whether participants or follow‐up personnel were masked. It was also not stated whether follow‐up was conducted by the surgeons themselves, or others. Thus the risk of bias was judged to be high for both studies in terms of masking of participants and personnel, and unclear with regards to masking of outcome assessment.
Incomplete outcome data
For Razmju 2011, it appears that all outcome data are based on the 30 participants reported to have been included in the study and therefore we deemed risk of bias to be low for this factor.
Data for four eyes in the DALK group were excluded from several of the follow‐up analyses in Javadi 2010 because of failed air injection. The exclusion of these four participants from the outcomes relating to post‐suture removal measurements of visual and refractive outcomes has the potential to have skewed the data. The outcomes for these participants would be likely to be negative if no further treatment was undertaken, and this would bias the findings in favour of the DALK group. However, it is not specified within the paper what further treatments these participants went on to have, and it is highly likely that they underwent an alternative or additional grafting procedure. It is therefore possible that they had positive outcomes due to these further treatments. No intention‐to‐treat (ITT) analyses incorporating the results from these four participants were conducted by Javadi 2010 and it was not possible to conduct them as part of this review due to a lack of available data for the four missing cases. Although all participants (except the four excluded at the time of surgery) completed a minimum three‐month post‐suture removal follow‐up, there was great variation in the length of follow‐up reported. Therefore, we judged the study to be at high risk of attrition bias.
Selective reporting
Results for all participants appear to be reported in both Razmju 2011 and Javadi 2010, though in both cases it is unclear whether the outcome measures reported were what was planned, as a protocol is not available for either study. Therefore, we judged the risk of selective reporting bias to be unclear for both studies.
Other potential sources of bias
We did not detect any other potential sources of bias in either study.
Effects of interventions
All data included in the review were from published sources. Correspondence with the authors did not result in the provision of further usable unpublished data at this time.
Each included study only had each outcome measure reported on a single scale. Razmju 2011 only presented data for 12 months post‐graft. While Javadi 2010 made reference to results at three, six, 12 and 24 months in their figures, usable data were only available for three or more months post‐suture removal. We made efforts to obtain further data from the authors, however we did not receive any. We presented all usable data from both included studies in this review.
Primary outcome
Neither of the included studies reported data relating to the primary outcome measure of the review, BCVA at three months post‐graft. Javadi 2010 appeared to have this data as they showed "changes in BCVA" at three months within their Figure 1, however a request to the authors for this information in a usable format did not result in provision of the data.
Secondary outcomes
Javadi 2010 reported post‐suture removal outcomes for 77 of their 81 randomised participants. Data on these 77 participants were available for each of the visual and refractive outcomes mentioned below. In the other four participants, the initial operation did not proceed as planned. As this review aimed to compare the effectiveness of each technique, these grafts were treated as a graft failure for the purpose of analysis. As such, data were available with regards to the outcomes "failed graft" and "presence of at least one rejection episode" for all 81 recipients. Other adverse events were reported for the 77 participants who underwent the planned procedure. Razmju 2011 reported 12 month postoperative outcomes for all 30 of their participants on each of the outcomes mentioned below.
Visual acuity outcomes
With regards to secondary outcomes relating to visual acuity, Razmju 2011 reported a significant improvement in BCVA in both groups 12 months following surgery, however there was no significant difference in this change between the two groups with a small estimate of effect that crossed the line of no difference (mean difference (MD) 0.06 LogMAR, 95% CI ‐0.02 to 0.14; Analysis 1.1), indicating no superiority of either technique at this time. Javadi 2010 also found a non‐significant difference in the BCVA of participants in each intervention group at their final, post‐suture removal, follow‐up, with a small estimate of effect that crossed the line of no difference (MD 0.03 LogMAR, 95% CI ‐0.01 to 0.07; Analysis 1.2). A comparison of the changes from pre‐graft was not given at this time point. The percentages of participants reporting functional best corrected vision (>= 20/40) at this time, were also not significantly different across groups (odds ratio (OR) 0.59, 95% CI 0.05 to 6.77; Analysis 1.3). There was also a non‐significant estimate of effect across groups in the Javadi 2010 study at the final follow‐up for UCVA (MD 0.09 LogMAR, 95% CI ‐0.07 to 0.25; Analysis 1.4). The percentage of participants reporting functional uncorrected vision (>= 20/40) at this time, was also not significantly different across groups (OR 1.48, 95% CI 0.58 to 3.81; Analysis 1.5).
1.1. Analysis.
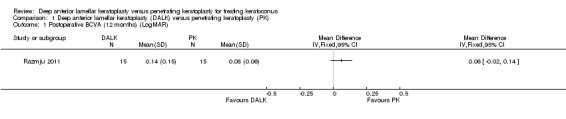
Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 1 Postoperative BCVA (12 months) (LogMAR).
1.2. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 2 Postoperative BCVA (varied follow‐up times, post‐suture removal) (LogMAR).
1.3. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 3 Postoperative functional BCVA achieved (varied follow‐up times, post‐suture removal).
1.4. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 4 Postoperative UCVA (varied follow‐up times, post‐suture removal) (LogMAR).
1.5. Analysis.
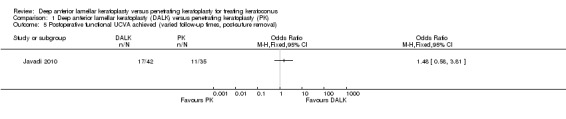
Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 5 Postoperative functional UCVA achieved (varied follow‐up times, post‐suture removal).
Refractive outcomes
Javadi 2010 reported no significant differences in mean keratometric astigmatism across groups, at least three months post‐suture removal, and we found a non‐significant estimate of effect (MD 0.47D, 95% CI ‐1.36 to 0.42; Analysis 1.6). No significant differences were found by Javadi 2010 pre‐operatively or at one, three, six, 12, or 24 months follow‐up. No significant differences in spherical equivalent across groups at any time point (pre‐operation, one, three, six, 12, 24 months or three months post‐suture removal) were observed, with a non‐significant estimate of effect at least three months post‐suture removal (MD 0.60D, 95% CI ‐1.52 to 0.32; Analysis 1.7). Razmju 2011 reported improvements on measurements of both astigmatism and spherical equivalent, measured in dioptres (D), in both intervention groups at 12 months. A comparison of the improvement across the two intervention groups showed no significant difference for either spherical equivalent, or astigmatism, with a non‐significant estimate of effect for either (MD 0.69D, 95% CI ‐1.19 to 2.57; Analysis 1.8, MD 1.26D, 95% CI ‐0.88 to 3.40; Analysis 1.9). Neither study reported on the methods of visual correction used by participants to achieve BCVA at any time point.
1.6. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 6 Postoperative keratometric astigmatism (varied follow‐up times, post‐suture removal) (D).
1.7. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 7 Postoperative spherical equivalent (varied follow‐up times, post‐suture removal) (D).
1.8. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 8 Postoperative keratometric astigmatism (12 months) (D).
1.9. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 9 Postoperative spherical equivalent (12 months) (D).
Adverse events
With regards to adverse events, Razmju 2011 reported four cases of endothelial rejection in the penetrating keratoplasty group, three of which resolved with steroid treatment and one of which ended in graft failure. They also reported a case of stromal rejection in one participant who had received a DALK, which was successfully treated with steroids. Javadi 2010 reported 11 cases of endothelial rejection in the penetrating keratoplasty group, and four cases of subepithelial rejection. All cases resolved with steroid treatment. There were also 10 cases of subepithelial rejection in the DALK group which were also successfully treated with steroids. Our analyses showed a significant estimate of effect, with rejection just a third as likely to result from a DALK procedure than a penetrating keratoplasty (P = 0.02, OR 0.33, 95% CI 0.14 to 0.81; Analysis 1.10).
1.10. Analysis.

Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 10 Presence of at least one rejection episode.
Razmju 2011 reported no intraoperative complications in either group. Javadi 2010 reported that in four planned DALK procedures, the eyes required manual dissection to Descemet's membrane due to failed air injection. It is unclear whether these participants still went on to undergo a DALK or if their surgery had to be converted to a penetrating keratoplasty, however the surgeries could be considered a technical failure and their results were excluded from all analyses of visual and keratometric outcomes. There was no significant difference in the likelihood of technical failure across groups in this study (OR 7.52, 95% CI 0.39 to 144.43; Analysis 1.11). No postoperative failures were reported in this study, while one was reported in the Razmju 2011 study. There was no significant difference in the likelihood of postoperative failure (OR 0.31, 95% CI 0.01 to 8.28; Analysis 1.11). A meta‐analysis was not conducted on these data due to significant heterogeneity between the two studies.
1.11. Analysis.
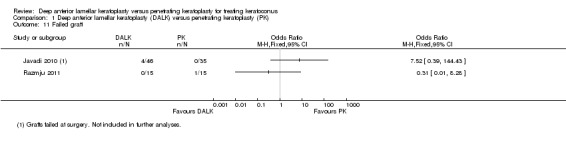
Comparison 1 Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK), Outcome 11 Failed graft.
With respect to other complications, Razmju 2011 reported a case of interface neovascularisation in one DALK participant.Descemet's membrane was perforated in three cases in the DALK group in the Javadi 2010 study, resulting in double chamber formation in two eyes. In one case this resolved spontaneously while the other required treatment with an intracameral air injection. It is not stated what happened in the third case. Javadi 2010 reported nine cases of intolerable postoperative astigmatism requiring refractive surgery, five in the DALK group and four in the penetrating keratoplasty group. There were two cases in each group of steroid induced ocular hypertension. Five participants in the DALK group and two in the penetrating keratoplasty group suffered from persistent epithelial defects. In the DALK group, one participant also had wrinkling of Descemet's membrane. In the penetrating keratoplasty group, one participant required graft resuturing and one had an atonic pupil, a condition in which the pupil dilates and is non‐reactive.
Discussion
Summary of main results
No evidence was available with regards to the effect of deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty on best corrected visual acuity (BCVA) at three months post‐surgery.
The evidence of the review suggests that both DALK and penetrating keratoplasty are successful in improving BCVA, spherical equivalent and keratometric astigmatism of patients with keratoconus at the time points of 12 months post‐surgery and at least three months post‐final suture removal. The review did not find any evidence for a significantly better result from either procedure based on the data from the two studies included.
Instances of graft rejection were more frequent in participants undergoing penetrating keratoplasty in both included studies. The majority of these complications were resolved with steroid treatment.
Graft failure was reported in one penetrating keratoplasty recipient in Razmju 2011, while four intended DALK recipients had to undergo an adjusted procedure, and three others experienced tearing of Descemet's membrane in Javadi 2010.
Other adverse events, of varying severity, were reported in both intervention groups with similar frequency. For both types of surgery, these included postoperative astigmatism, steroid induced ocular hypertension and persistent epithelial defects. Individual cases of neovascularisation and wrinkling of Descemet's membrane were reported in DALK recipients, while the need for graft resuturing and atonic pupil, a condition in which the pupil dilates and is non‐reactive, were reported following penetrating keratoplasty.
Overall completeness and applicability of evidence
The included studies failed to address the main objective of the review, to evaluate the quality of BCVA three months post‐graft. While information on post‐graft BCVA were available in both studies, these data were only available at 12 months follow‐up in Razmju 2011 and at various time points post‐suture removal in Javadi 2010. Razmju 2011 did not address the question of the number of participants who achieved functional levels of vision post‐graft. Data on the primary outcome measure of BCVA at three months post‐graft were shown in a graph in Javadi 2010; however, the number of participants achieving functional BCVA and the mean BCVA achieved by each group at this point, with the corresponding P value for the difference between the two groups in terms of the change from pre‐operative levels, were not given. The same issues were present for the secondary outcome measurements of UCVA, spherical equivalence and astigmatism.
Both studies provided adequate information on the number of rejection episodes and graft failures, plus any other adverse events, at least three months post‐suture removal.
The low number of identified studies, the lack of data relating to the primary outcome measure and the inability to confirm the randomisation technique used in Razmju 2011, reduced the ability to conduct quantitative synthesis. Further studies are needed, to increase the completeness and applicability of the evidence. The available evidence does however suggest that rejection episodes are more likely to occur in penetrating keratoplasty procedures. From an ethical perspective, researchers may need to discuss this possible increased risk with any patients included in future studies which aim to compare outcomes from the two techniques, and highlight the need to balance this risk with the need to determine whether other outcomes (visual and keratometric) are comparable.
Quality of the evidence
Overall, the quality of the evidence is rated as very low to moderate, with methodological limitations, incomplete data analysis and imprecision of findings leading to the results being downgraded in the Table 1 A sensitivity analysis, in which the findings of Javadi 2010 alone were considered in order to assess the impact that the potential lack of adequate randomisation in Razmju 2011 may have had, resulted in evidence rated very low to low, as shown in the Table 2. Removing the results of Razmju 2011 in the sensitivity analysis did not result in a large change to the findings with regards to rejection episodes. However, the findings with regards to failed grafts were heterogeneous between the two included studies, and removing the Razmju 2011 data resulted in a higher corresponding risk for DALK. This has a very wide 95% CI, suggesting an imprecise estimate, which crosses the line of no difference (OR 7.52, 95% CI 0.39 to 144.43). The quality of evidence for these outcomes was judged to be low for presence of rejection episodes and very low for graft failure, primarily because the inclusion of just one trial led to an inability to assess consistency. It does not appear that inclusion of Razmju 2011 biased the results with regards to rejection episodes, however it may have done so for failure rates.
Methodological quality
The review included two studies, both conducted in Iran. One study (Javadi 2010) was well designed, conducted and reported, and was of moderate methodological quality. No ITT analyses were undertaken, creating a high risk of attrition bias. This study randomised participants through an eligible manner but did not specify whether allocation was concealed from people recruiting participants to the trial.
The second study (Razmju 2011) was rated as having poor methodological quality. While stating that it was randomised, the authors provided no details of the randomisation technique employed and also did not specify whether allocation concealment was undertaken. It appears that all participants completed follow‐up, so attrition bias was rated as low risk. A sensitivity analysis was undertaken to see whether the inclusion of this study impacted on the results of the quantitative analysis. Removal of these results appeared to impact the findings with regards to graft failure, resulting in a higher corresponding risk for DALK. However, this had a very wide 95% CI, suggesting an imprecise estimate. Removal of these data did not change the findings regarding rejection episodes.
Treatment
Javadi 2010 was clear about the techniques used, so that the applicability of the results to this review was confirmed.
Razmju 2011 did not give specifics of the operative techniques used. These can vary greatly, particularly for DALK procedures and so the lack of this information also lowers the level of confidence that can be had in the applicability of the evidence from this trial to the review.
Outcomes
The small number of eligible studies identified and the poor applicability of the outcomes reported in both included studies, meant that there was inadequate evidence to be able to evaluate and compare the effectiveness of the two interventions.
Potential biases in the review process
We consider that the search strategies used to identify relevant studies were adequate, with a wide range of databases and additional grey literature reviewed. However, we also acknowledge that we identified one potentially relevant trial (for which we were unable to obtain contact information for the study co‐ordinator), and several unpublished, potentially relevant trials, for which our requests for further information from the authors did not receive replies. Why these trials are unpublished remains unclear, and thus bias due to the exclusion of data from these studies is possible. Due to the identification of just two trials to include in the review, we were unable to use a funnel plot to investigate publication bias.
The review of each potential study for relevance by two review authors, independently, reduced the risk that relevant studies would be excluded due to personal bias or human error on the part of the review authors. Likewise, the independent extraction of data from the included studies by the two review authors, and the review of the final included results by all review authors, reduced the risk of bias in these steps of the process.
Agreements and disagreements with other studies or reviews
The review by Reinhart 2011 identified 11 non‐randomised (case‐control, cohort and case series) studies, which compared DALK and penetrating keratoplasty for any indication (not specifically for keratoconus). Their conclusions were that the two techniques lead to equivalent outcomes in terms of BCVA and refractive measurements. The present review found insufficient evidence to form conclusions regarding the comparison of outcomes from the two techniques for these outcome measures.
The review by Shi 2012 included three studies which they identified as RCTs, as well as eight non‐randomised (two case‐control and six cohort) studies, comparing the results of DALK and penetrating keratoplasty for keratoconus. One of the RCTs identified was that of Javadi 2010. They did not identify Razmju 2011. The other two RCTs identified were Liu 2008 and Jiang 2006. We are still awaiting full translation of the articles relating to these two studies, and are yet to confirm that they were appropriately randomised for inclusion in the present review. The conclusions of Shi 2012, based on the results of the three randomised studies, were that penetrating keratoplasty lead to greater improvement in BCVA, that there were no significant differences in the outcomes relating to refractive measurements, and that DALK lead to a significantly lower likelihood of rejection. This conclusion regarding rejection is in agreement with the findings of the present review. The present review found insufficient evidence to form conclusions surrounding the comparison of outcomes from the two techniques regarding improvement in BCVA and refractive measurements.
Authors' conclusions
Implications for practice.
We found no evidence to support a difference in outcomes with regards to BCVA, either three months post‐graft or at any other time point included in the review, in participants undergoing either DALK or penetrating keratoplasty for keratoconus.
We found some evidence, of low to moderate quality, that rejection is more likely to occur in this population following penetrating keratoplasty than DALK.
We found no evidence to support a difference in outcomes with regards to graft survival, final UCVA or keratometric outcomes.
There is currently insufficient evidence to determine which technique may offer better overall outcomes ‐ final visual acuity and time to attain this, keratometric stabilisation, risk of rejection or failure, or both, and risk of other adverse events ‐ for patients with keratoconus.
Implications for research.
Large randomised trials, comparing the outcomes of penetrating keratoplasty and DALK in the treatment of keratoconus, are needed. Necessary sample sizes to detect a clinically significant difference between the two groups should be calculated and utilised in the trial design. Selection criteria need to be consistent and reported, including the method of diagnosis used. People receiving the intervention for an indication other than keratoconus should not be included in the trial. Randomisation procedures need to be acceptable and reported. Details of the interventions need to be documented and reported. Recipients need to be masked to the intervention administered and this should be reported. Because of the nature of the surgery, it is not likely to be possible to conduct double‐masked trials as practitioners who are qualified to undertake outcomes assessments would be able to see which graft a participant had received. Outcome measures need to include: rates of rejection and failure, visual outcomes, refractive outcomes, and adverse events. Planned comparisons need to be documented and carried out, with intention‐to‐treat (ITT) analyses applied in instances where participants have missing data. Follow‐up should be at regular time periods, with follow‐up extending to at least 12 months, or three months post‐suture removal, whichever is longer. Appropriate analyses need to be utilised to compare the data across both groups and time periods. Baseline data for participants needs to be reported and any differences across groups controlled for in analyses.
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) created and ran the electronic search strategies. We would like to thank Catey Bunce and Vishal Jhanji for peer reviewing the protocol and full review and Jennifer Evans and Anupa Shah for their assistance with the review.
We would like to thank Xue Wang for translating Chinese papers.
Appendices
Appendix 1. CENTRAL and DARE search strategy
#1 MeSH descriptor Keratoconus #2 keratocon* #3 ectatic* or ectasia #4 (#1 OR #2 OR #3) #5 MeSH descriptor Keratoplasty, Penetrating #6 (penetrating or perforating) near/2 (keratoplast*) #7 full near/3 thickness near/3 cornea* #8 (#5 OR #6 OR #7) #9 deep anterior lamellar keratoplast* #10 deep lamellar keratoplast* #11 partial near/3 thickness near/3 cornea* #12 big near/2 bubble #13 DALK #14 (#9 OR #10 OR #11 OR #12 OR #13) #15 (#4 AND #8 AND #13)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp Keratoconus/ 14. keratocon$.tw. 15. (ectatic$ or ectasia).tw. 16. or/13‐15 17. Keratoplasty, Penetrating/ 18. ((penetrating or perforating) adj2 keratoplast$).tw. 19. (full adj3 thickness adj3 cornea$).tw. 20. or/17‐19 21. deep anterior lamellar keratoplast$.tw. 22. deep lamellar keratoplast$.tw. 23. (partial adj3 thickness adj3 cornea$).tw. 24. (big adj2 bubble).tw. 25. DALK.tw. 26. or/21‐25 27. 16 and 20 and 26 28. 12 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. PubMed search strategy
(((randomized controlled trial[pt] OR controlled clinical trial[pt]) OR (randomized OR randomized OR randomly OR placebo[tiab]) OR (trial[ti]) OR ("Clinical Trials as Topic"[MeSH Major Topic])) NOT (("Animals"[Mesh]) NOT ("Humans"[Mesh] AND "Animals"[Mesh]))) AND (((keratoconus[MeSH Terms]) OR (keratocon*) OR (ectatic* OR ectasia)) AND ((keratoplasty, penetrating[MeSH Terms]) OR (keratoplast*) OR (DALK)))
Appendix 4. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp keratoconus/ 34. keratocon$.tw. 35. (ectatic$ or ectasia).tw. 36. or/33‐35 37. exp penetrating keratoplasty/ 38. ((penetrating or perforating) adj2 keratoplast$).tw. 39. (full adj3 thickness adj3 cornea$).tw. 40. or/37‐39 41. deep anterior lamellar keratoplast$.tw. 42. deep lamellar keratoplast$.tw. 43. (partial adj3 thickness adj3 cornea$).tw. 44. (big adj2 bubble).tw. 45. DALK.tw. 46. or/41‐45 47. 36 and 40 and 46 48. 32 and 47
Appendix 5. LILACS search strategy
keratocon$ and keratoplast$ or DALK
Appendix 6. CINAHL (EBSCO) search strategy
S7 S3 AND S6 S6 S4 OR S5 S5 DALK S4 keratoplasty or keratoplasties S3 S1 OR S2 S2 ectatic or ectasia S1 Keratoconus
Appendix 7. OpenGrey search strategy
keratoconus and keratoplasty
Appendix 8. Web of Science SCI search strategy
#9 #7 AND #8 #8 TS=random* #7 #3 AND #6 #6 #4 OR #5 #5 TS=DALK #4 TS=keratoplasty #3 #1 OR #2 #2 TS=ectatic #1 TS=keratoconus
Appendix 9. Health Collection (Informit) search strategy
SUBJECT=(keratoconus) AND SUBJECT=(keratoplasty)
Appendix 10. metaRegister of Controlled Trials search strategy
keratoconus AND keratoplasty
Appendix 11. ClinicalTrials.gov search strategy
Keratoconus AND Keratoplasty
Appendix 12. ICTRP search strategy
keratoconus AND keratoplasty
Data and analyses
Comparison 1. Deep anterior lamellar keratoplasty (DALK) versus penetrating keratoplasty (PK).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative BCVA (12 months) (LogMAR) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Postoperative BCVA (varied follow‐up times, post‐suture removal) (LogMAR) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Postoperative functional BCVA achieved (varied follow‐up times, post‐suture removal) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Postoperative UCVA (varied follow‐up times, post‐suture removal) (LogMAR) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Postoperative functional UCVA achieved (varied follow‐up times, post‐suture removal) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Postoperative keratometric astigmatism (varied follow‐up times, post‐suture removal) (D) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Postoperative spherical equivalent (varied follow‐up times, post‐suture removal) (D) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Postoperative keratometric astigmatism (12 months) (D) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Postoperative spherical equivalent (12 months) (D) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Presence of at least one rejection episode | 2 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.81] |
| 11 Failed graft | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Javadi 2010.
| Methods | Randomised comparative clinical trial in a single private clinic in Iran. Randomisation based on a random number table | |
| Participants | 81 eyes of 81 participants: 46 undergoing DALK and 35 undergoing penetrating keratoplasty. All 81 had data available with regards to graft failure and presence of rejection episodes. Four DALK recipients were excluded from analyses relating to visual and refractive outcomes as they failed to achieve bared Descemet's membrane, leaving 42 DALK recipients and 77 total recipients in these analyses. Age and gender breakdown are provided only for these 77 recipients. DALK: 29 males, 13 females; mean age = 26.91 ± 7.9 years, range 15‐44; penetrating keratoplasty: 28 males, 7 females; mean age = 30.89 ± 10.3 years, range 17‐50. Study dates not reported, but pre‐2009 | |
| Interventions | DALK was conducted using the big‐bubble technique with the recipient cornea trephined 7.75 mm or 8 mm, depending on the recipients' vertical corneal diameter (greater than or equal to versus less than 10.5 mm). Air was injected into the mid‐stroma to form a big‐bubble extending to the trephination site. The stroma was then removed In penetrating keratoplasty, the recipient trephination size (7.75 mm or 8 mm) was again dependent on the recipients' vertical corneal diameter (greater than or equal to versus less than 10.5mm). Trephenation was conducted with a Hessburg‐Barron suction trephine and the excision was completed using right and left corneal scissors. For these donor corneas, trephination was conducted from the endothelial side In all surgeries, the size of the corneal donor button was dependent on the recipient trephine size and was 0.25 mm or 0.5mm larger depending on vitreous length (greater than or equal to versus less than 16 mm). The donor cornea was sutured to the recipient in one of three different ways, all using 10‐0 nylon sutures: 16 interrupted sutures, one single 16‐ to 18‐bite running suture, or a single 16‐bite running suture plus an 8‐bite interrupted suture. This was based on surgeon preference and the condition of the eye. Suture tension was adjusted via intraoperative keratoscopy. Cefazolin 100 mg and betamethasone 4 mg were injected subconjunctivally. Subjects received topical chloramphenicol every six hours for 30 days and topical betamethasone 0.1% every six hours, tapered over two to three months Follow‐up examinations were performed at 1, 3, 7 and 30 days; 3, 6, 12 and 24 months; and 3‐6 months after complete suture removal |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Specific outcome information regarding number of grafts with rejection episodes, attainment of BCVA/UCVA of 20/40 and mean keratometric astigmatism, was provided at 3‐6 months after complete suture removal. Data for some visual outcomes and refraction were shown at 3, 6, 12 and 24 months as well as post‐suture removal within figures, but not referred to in‐text, with no specific measurements provided |
|
| Notes | Some secondary procedures (e.g. resuturing) were carried out in some subjects. Study conducted by the Ophthalmic Research Centre at Shahid Beheshti University. No financial support or conflicts of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on a "random number table" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation occurred in the private clinic where the surgery was carried out. It is unclear whether the allocation was concealed from staff recruiting participants to the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was impossible to mask the surgeon to which surgery was being conducted. It is unclear whether participants were masked to which surgery they underwent |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear who conducted the outcome assessment or whether they were aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four eyes were excluded from the DALK group because of failed air injection. The outcomes data for these four eyes were excluded from follow‐up analyses. This may skew the results in favour of DALK as the outcomes for these participants were more likely to be negative. There was great variation in follow‐up length, though all participants (except the four excluded at time of surgery) completed 6 month follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear if outcome measures are what was planned as protocol not available |
| Other bias | Unclear risk | Not mentioned |
Razmju 2011.
| Methods | Randomised single centre trial conducted at a hospital in Iran. Method of randomisation not stated | |
| Participants | Consecutive participants at a single hospital in Iran. 15 assigned to receive penetrating keratoplasty and 15 assigned to receive DALK. DALK: 3 males, 12 females, Mean age = 29.4 ± 2.3 years; penetrating keratoplasty: 5 males, 10 females; Mean age = 30.9 ± 4.1 years. Age range for all participants = 20‐40 years. The data were collected from 2009‐2011 | |
| Interventions | Details of the specific interventions used were not given, with their paper simply stating that one arm of the study received penetrating keratoplasty and the other deep lamellar keratoplasty. Follow‐up was conducted at 12 months post‐graft |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcome data were provided at 12 months regarding number of grafts with rejection episodes, mean BCVA, change in BCVA from baseline, mean keratometric astigmatism and mean spherical equivalent |
|
| Notes | This paper was published in Farsi and was translated by one of the review authors (MZ). Study funded and conducted as part of a medical doctorate at the Isfahan Univeristy of Medical Sciences. No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation technique is not specified |
| Allocation concealment (selection bias) | Unclear risk | No information is provided about whether there was adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information is provided about whether participants or personnel were masked. It would not be possible to mask the surgeon to which treatment was being given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information is provided about whether the investigators conducting the outcome assessment were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that data from all 30 enrolled participants are included in the outcome measures reported |
| Selective reporting (reporting bias) | Unclear risk | Results for all participants appear to be reported. Unclear if outcome measures are what was planned as protocol not available |
| Other bias | Unclear risk | Not mentioned |
BCVA: best corrected visual acuity DALK: deep anterior lamellar keratoplasty UCVA: uncorrected visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acar 2013 | Not an RCT |
| Akdemir 2012 | Not a RCT |
| Amayem 2013 | Not a RCT |
| Arenas 2005 | Not a RCT |
| Behrens 2000 | No DALK group |
| Birnbaum 2008 | No DALK group, not just participants with keratoconus, no exclusion of participants with hydrops/scarring |
| Borderie 2012 | Not a RCT, no exclusion of participants with comorbid eye conditions, no exclusion of participants with hydrops/scarring |
| Busin 1991 | Not a RCT |
| Cardoso da Silva 2007 | Not a RCT, no exclusion of participants with hydrops/scarring |
| Cheng 2011 | Only a subgroup of the included population (15 in each group) had keratoconus, with the remainder having a mixture of other presenting diseases. Data on this keratoconus subgroup requested from authors but yet to be received |
| Cohen 2010 | Not a RCT |
| Farias 2008 | No penetrating keratoplasty group |
| Frost 2006 | Review article, not original research |
| Funnell 2006 | Not a RCT |
| Hara 2013 | Not a RCT |
| Haugen 2001 | Not a RCT, no exclusion of participants with comorbid eye conditions, no exclusion of participants with hydrops/scarring |
| Javadi 2006 | No DALK group, no exclusion of participants with hydrops/scarring |
| Jiang 2011 | Not a RCT |
| Jones 2009 | Not a RCT, no exclusion of participants with hydrops/scarring |
| Kandemir 2011 | Unclear if it was a RCT, or whether exclusions were undertaken. No response from author to enquiries |
| Koo 2011 | Not a RCT, no exclusion of participants with hydrops/scarring |
| Kubaloglu 2012 | Not a RCT |
| Mahjoub 2011 | Unclear if it was a RCT, or whether exclusions were undertaken. No response from author to enquiries |
| McDonald 1987 | No DALK group |
| Motlagh 2012 | Not a RCT |
| NCT00371202 | Unclear if completed. No results available. No response from author to enquiries |
| Panda 1999 | Not a RCT, no exclusion of participants with comorbid eye conditions, no exclusion of participants with hydrops/scarring |
| Panda 2012 | Unclear if it was a RCT, or whether exclusions were undertaken. No response from author to enquiries |
| Reinhart 2011 | Review article, not original research |
| Salvetat 2013 | Not a RCT |
| Sari 2012 | Unclear if it was a RCT, or whether exclusions were undertaken. No response from author to enquiries |
| Serdarevic 1996 | Quasi‐randomised trial, no DALK group, no exclusion of participants with hydrops/scarring |
| Shi 2012 | Review article, not original research |
| Shimmura 2005 | Not a RCT, no penetrating keratoplasty group |
| Shoja 2007 | No DALK group, no exclusion of participants with hydrops/scarring |
| Tan 2007 | Review article, not original research |
| Tan 2010a | Not a RCT |
| Watson 2004 | Not a RCT, no exclusion of participants with comorbid eye conditions |
| Yamaguchi 2011 | Not a RCT, no exclusion of participants with comorbid eye conditions, no exclusion of participants with hydrops/scarring |
DALK: deep anterior lamellar keratoplasty RCT: randomised controlled study
Characteristics of studies awaiting assessment [ordered by study ID]
Jiang 2006.
| Methods | Described as "randomised" but methods not described |
| Participants | 58 eyes of 47 participants: 30 undergoing DALK and 28 undergoing penetrating keratoplasty. Unclear whether results were reported for all participants. Mean age is provided for each group ‐ DALK: 28.6 years, penetrating keratoplasty: 31 years. The study time‐frame was 2001‐04. Participants were followed for a median of 28 months in the DLK group and 36 months in the penetrating group. |
| Interventions | Details not clear |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Only abstract available in English. Awaiting full translation from Chinese |
Liu 2008.
| Methods | Not clear |
| Participants | 48 eyes of 48 participants: 23 undergoing DALK and 25 undergoing penetrating keratoplasty. Unclear whether results were reported for all participants |
| Interventions | Details not clear |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Only abstract available in English. Awaiting full translation from Chinese |
BCVA: best corrected visual acuity DALK: deep anterior lamellar keratoplasty
Characteristics of ongoing studies [ordered by study ID]
NCT01901614.
| Trial name or title | OCT‐guided LALAK for KCN |
| Methods | Randomised comparative clinical trial in a single private hospital in Portland, Oregon, USA. Block randomisation by independent statistician based on a random number table |
| Participants | Estimated enrolment of 48 |
| Interventions | Laser assisted lamellar anterior keratoplasty (LALAK): A dovetail shaped cut will be made on the donor cornea tissue using femtosecond laser, proportional in depth to the central stromal thickness, and the section of cornea to be grafted then separated from the stroma, stored in preservation media and shipped to the surgeon. Topical anaesthesia is given to the recipient and a matching femtosecond laser cut will be made in the host cornea allowing the donor portion to be inserted in a tongue‐in‐groove manner. The graft is sutured in place and a protective eye shield placed in the eye. Intralase‐enabled keratoplasty (IEK): A full‐thickness graft is prepared from donor tissue using zigzag side cuts. The section of cornea to be grafted is then separated from the rim, stored in preservation media and shipped to the surgeon. Topical anaesthesia is given to the recipient and a matching femtosecond laser cut will be made in the host cornea creating a bridge 70‐100 microns in size for the donor button to rest on. The graft is sutured in place and a protective eye shield placed in the eye |
| Outcomes | Primary outcomes
|
| Starting date | August 2013 |
| Contact information | Janice Ladwig: ladwig@ohsu.edu; Denny Romfh: romfhd@ohsu.edu |
| Notes | Estimated completion date 2016 |
BCVA: best corrected visual acuity
Differences between protocol and review
1. MZ joined the author team for the review.
2. Despite our efforts we were unable to obtain the conference proceedings for the following conferences to handsearch.
American Society of Cataract and Refractive Surgery Symposium and Congress: pre‐2007
Asia Pacific Academy of Ophthalmology Congress: pre‐2012
Cambridge Ophthalmological Society Meeting: any
European Society of Cataract and Refractive Surgeons Congress: pre‐2009
International Congress of Eye Research: pre‐2012
Royal Australian and New Zealand College of Ophthalmology Congress: pre‐2011
Royal College of Ophthalmologists Congress: pre‐2011
World Cornea Congress: any
World Ophthalmology Congress: pre‐2008
3. In our methodology, the sentence "We only included in the review studies which specified a reliable method of diagnosis of the keratoconus (slit lamp examination, corneal topography, wave front analysis)" was changed to "We only included in the review studies which specified an objective method of diagnosis of the keratoconus (slit lamp examination, corneal topography, wave front analysis)" as we felt that "reliable" was a subjective word, and the classification of types of diagnosis as being "reliable" would be difficult to argue.
4. The seven points outlined under the "assessment of risk of bias" section of the protocol were condensed into five points that better reflected the risk of bias assessment guidelines outlined in the Cochrane Handbook and removed points that were actually factors relating to the inclusion criteria for the studies in the review, rather than the risk of bias (inclusion and exclusion criteria and methodology of keratoconus grading).
5. We made a slight change to the way that BCVA and UCVA outcomes needed to be reported, as the outline in our protocol did not accurately reflect the ways in which visual acuity data can, and should, be reported in terms of Snellen lines or LogMAR.
6. We had said that studies for which the randomisation procedure was not clear would be excluded from meta‐analyses. Following reviewer feedback we felt that this was not the best course of action as it removed potentially relevant information from the review. We chose rather, to include them in meta‐analyses, but to also conduct sensitivity analyses to determine whether the results of these studies affect the overall results of the review.
7. We changed the title of our review from "Penetrating keratoplasty versus deep anterior lamellar keratoplasty for treating keratoconus" to "Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus" as it was felt that this better reflected that the outcomes from DALK were being compared to those for penetrating keratoplasty, which is the more established form of treatment for keratoconus.
8. It was planned that if the two authors assessing the citations for inclusion of studies in the review could not reach an agreement, a third author would be consulted. There were no instances where this was necessary and so a third author was not involved in these decisions. Similarly, following assessment of the full text articles, no studies were classified as 'relevant' by one author and 'not relevant' by the other, thus reference to a third author was not necessary or utilised. Finally, a third author was also to be consulted to resolve any disagreements between the two review authors (MK and KW) regarding the data entered into RevMan. No reference to a third review author was necessary and so this was not done.
9. Regarding outcome measures, where BCVA and UCVA were provided in terms of Snellen acuity, we had planned to transform these figures into LogMAR measurements for the purposes of these analyses. Both included studies reported BCVA and UCVA in LogMAR and so this was not necessary.
10. Where multiple studies were identified with data available on a common continuous variable, we had planned to assess the data relating to this variable to determine if it was skewed, as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If this was present we would then consider transformation of the data prior to inclusion in a meta‐analysis. As no continuous variables had data available for more than one included study, transformation was not necessary.
11. All unit of analysis issues were to be dealt with in the manner specified in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). As keratoconus is a bilateral condition, it was possible that some trials would involve one eye of each participant to be assigned to one treatment group (penetrating keratoplasty or DALK) and the other to the other treatment group and then the overall outcomes compared. Providing that adequate randomisation occurred in terms of which eye was assigned to which treatment group and all participants had both eyes included in the study, such studies would still meet the criteria for the review as these surgeries would be performed at different times. These trials were still eligible to be included in the analysis, with attention intended to be paid to this issue during sensitivity analysis. Neither of the included studies had subjects for which both eyes were included in the study and so this did not need to be done.
12. Where large amounts of data were missing, we planned to conduct intention‐to‐treat (ITT) analyses, where possible, to determine if this had had a significant impact on the results. If it was determined that it did have, the study would be excluded from further analyses. As Javadi 2010 was the only study identified with data available on the outcome measures for which data were missing, it was not possible to conduct sensitivity analyses to see if this impacted on the results.
13. We did not conduct any meta‐analyses using the random‐effects model as only a small number of studies were identified.
14. A funnel plot and sensitivity analysis were to be used to assess publication bias but, due to the low number of studies identified, this was not possible.
15. It was planned that, where reported in the same trials, we would conduct subgroup analyses for the two main DALK surgical techniques: the big‐bubble and the Melles. These analyses would determine whether the heterogeneity of the overall review was affected by the differences between these two techniques. Neither of the two trials identified involved multiple DALK surgical techniques, and so we did not conduct this subgroup analysis.
16. Additional sensitivity analyses were planned in which analyses would be re‐run on the data in which: 1) any trials for which the risk on any parameter was judged to be high were removed, 2) any trials that were funded by industry were removed, and 3) trials which are unpublished were removed. In this way, it would be possible to assess how strongly the results of our review are related to the decisions and assumptions that we have made throughout the review process. No trials fitting these criteria were included and so no such analysis was conducted.
In addition, it was also possible that some studies may have entered all eyes meeting the inclusion criteria into the study (including two eyes of one participant) and then randomised all of the eyes to a treatment group. This would mean that the same treatment may be received in both eyes of the same participant, or that some participants may have both eyes included in the trial while others have just one. The outcomes of corneal graft surgery in one eye have been shown to impact the outcomes of corneal graft surgery in the other eye of the same individual (Williams 2010). As such, the impact of multiple results from the same individual, whether they were in the same or different groups, need to be controlled for during analyses. If this had not been done, a sensitivity analysis was to be conducted, in which these trials would be removed, to check that this did not have a significant impact on the results. No trials fitting these criteria were included and so no such analysis was conducted.
Contributions of authors
Conceiving the review: KW, DC Designing the review: KW, MK, DC Co‐ordinating the review: MK Designing electronic search strategies: MK, KW, DC, Cochrane Eyes and Vision Group editorial team Undertaking searches: Cochrane Eyes and Vision Group editorial team, MK Screening search results: MK, KW Appraising quality of papers: MK, KW Writing to authors of papers for additional information: MK Translation of international papers: MZ, MK Data collection for the review: MK, KW Entering data into RevMan: MK Analysis of data: MK, KW Interpretation of data: MK, KW, DC, MZ Providing a methodological perspective: MK Providing a clinical perspective: DC, MZ Providing a policy perspective: DC, KW Writing the review: MK, KW Providing general advice on the review: DC, MZ Performing previous work that was the foundation of the current study: KW, DC
Sources of support
Internal sources
No sources of support supplied
External sources
-
DonateLife, Australia.
Funding of the Australian Corneal Graft Registry which employs MK
-
National Health and Medical Research Council, Australia.
KW is supported under a NHMRC Fellowship
-
National Institute of Health Research (NIHR), UK.
Richard Wormald (Co‐ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Declarations of interest
None known.
New
References
References to studies included in this review
Javadi 2010 {published data only}
- Javadi MA, Feizi S, Yazdani S, Mirbabaee F. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for keratoconus: a clinical trial. Cornea 2010;29(4):365‐71. [DOI] [PubMed] [Google Scholar]
Razmju 2011 {published data only}
- Razmju H, Shams M, Abtahi MA, Abtahi SH. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus: A clinical trial study. Journal of Isfahan Medical School 2011;29(144):798‐802. [Google Scholar]
References to studies excluded from this review
Acar 2013 {published data only}
- Acar BT, Akdemir MO, Acar S. Corneal biomechanical properties in eyes with no previous surgery, with previous penetrating keratoplasty and with deep anterior lamellar keratoplasty. Japanese Journal of Ophthalmology 2013;57(1):85‐9. [DOI] [PubMed] [Google Scholar]
Akdemir 2012 {published data only}
- Akdemir MO, Kandemir B, Sayman IB, Selvi C, Dogan OM. Comparison of contrast sensitivity and visual acuity between deep anterior lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. International Journal of Ophthalmology 2012;5(6):737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amayem 2013 {published data only}
- Amayem AF, Hamdi IM, Hamdi MM. Refractive and visual outcomes of penetrating keratoplasty versus deep anterior lamellar keratoplasty with hydrodissection for treatment of keratoconus. Cornea 2013;32(4):e2‐5. [DOI] [PubMed] [Google Scholar]
Arenas 2005 {published data only}
- Arenas E. Air deep lamellar keratoplasty best choice for keratoconus. Ocular Surgery News 2005;23(16):65‐6. [Google Scholar]
Behrens 2000 {published data only}
- Bahrens A, Seitz B, Langenbucher A, Kus MM, Küchle M, Naumann GO. Lens opacities after nonmechanical versus mechanical corneal trephination for keratoplasty in keratoconus. Journal of Cataract and Refractive Surgery 2000;26(11):1605‐11. [DOI] [PubMed] [Google Scholar]
Birnbaum 2008 {published data only}
- Birnbaum F, Schwartzkopff J, Böhringer D, Reinhard T. Penetrating keratoplasty with intrastromal corneal ring. A prospective randomized study [Perforierende keratoplastik mit intrastromalem hornhautring. Eine prospektive randomisierte studie]. Ophthalmologe 2008;105(5):452‐6. [DOI] [PubMed] [Google Scholar]
- Birnbaum F, Schwartzkopff J, Böhringer D, Reinhard T. The intrastromal corneal ring in penetrating keratoplasty ‐ long‐term results of a prospective randomized study. Cornea 2011;30(7):780‐3. [DOI] [PubMed] [Google Scholar]
Borderie 2012 {published data only}
- Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, Laroche L. Long‐term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology 2012;119(2):249‐55. [DOI] [PubMed] [Google Scholar]
Busin 1991 {published data only}
- Busin M, Bechrakis‐Böker I, Denninger U. Surgical therapy of keratoconus. Epikeratophakia versus penetrating keratoplasty [Chirurgische therapie des keratokonus. Epikeratophakie versus perforierende keratoplastik]. Fortschritte der Ophthalmologie 1991;88(6):794‐6. [PubMed] [Google Scholar]
Cardoso da Silva 2007 {published data only}
- Cardoso da Silva CA, Oliveira ES, Sena Júnior MP, Sousa LB. Contrast sensitivity in deep anterior lamellar keratoplasty versus penetrating keratoplasty. Clinics 2007;62(6):705‐8. [DOI] [PubMed] [Google Scholar]
- Cardoso de Silva CA, Oliveira ES, Sena Júnior MP, Souza LB. Contrast sensitivity in deep anterior lamellar keratoplasty versus penetrating keratoplasty [Comparação da sensibilidade ao contraste entre transplante lamelar anterior profundo e transplante penetrante para tratamento do ceratocone]. Arquivos Brasileiros De Oftalmologia 2008;71(1):71‐4. [DOI] [PubMed] [Google Scholar]
Cheng 2011 {published data only (unpublished sought but not used)}
- Cheng Y, Nuijts R. Visual outcomes and quality of vision in a randomized trial of deep anterior lamellar keratoplasty versus penetrating keratoplasty. European Society of Cataract and Refractive Surgery Congress Proceedings; 2010 Sept 4‐8; Paris. Paris: European Society of Cataract and Refractive Surgery, 2010.
- Cheng YY, Visser N, Schouten JS, Wijdh RJ, Pels E, Cleynenbreugel H, et al. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty versus penetrating keratoplasty: A randomized multicentre clinical trial. Ophthalmology 2011;118(2):302‐9. [DOI] [PubMed] [Google Scholar]
- Nuijts RM. Randomized trial of deep anterior lamellar keratoplasty versus penetrating keratoplasty. Proceedings of the American Society of Cataract and Refractive Surgery Symposium and Congress; 2009 Apr 3‐8; San Francisco. American Society of Cataract and Refractive Surgery, 2009.
- Biggelaar FJ, Cheng YY, Nuijts RM, Schouten JS, Wijdh RJ, Pels E, et al. Economic evaluation of deep anterior lamellar keratoplasty versus penetrating keratoplasty in the Netherlands. American Journal of Ophthalmology 2011;151(3):449‐59. [DOI] [PubMed] [Google Scholar]
Cohen 2010 {published data only}
- Cohen AW, Goins KM, Sutphin JE, Wandling GR, Wagoner MD. Penetrating keratoplasty versus deep anterior lamellar keratoplasty for the treatment of keratoconus. International Ophthalmology 2010;30(6):675‐81. [DOI] [PubMed] [Google Scholar]
Farias 2008 {published data only}
- Farias R, Barbosa L, Lima A, Mayumi E, Lourenço A, Freitas D, et al. Deep anterior lamellar transplant using lyophilized and Optisol corneas in patients with keratoconus. Cornea 2008;27(9):1030‐6. [DOI] [PubMed] [Google Scholar]
Frost 2006 {published data only}
- Frost NA, Wu J, Lai TF, Coster DJ. A review of randomized controlled trials of penetrating keratoplasty techniques. Ophthalmology 2006;113(6):942‐9. [DOI] [PubMed] [Google Scholar]
Funnell 2006 {published data only}
- Funnell CL, Ball J, Noble BA. Comparative cohort study of the outcomes of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Eye 2006;20(5):527‐32. [DOI] [PubMed] [Google Scholar]
Hara 2013 {published data only}
- Hara S, Kojima T, Dogru M, Uchino Y, Goto E, Matsumoto Y, et al. The impact of tear functions on visual outcome following keratoplasty in eyes with keratoconus. Graefe's Archive for Clinical and Experimental Ophthalmology 2013;251(7):1763‐70. [DOI] [PubMed] [Google Scholar]
Haugen 2001 {published data only}
- Haugen OH, Høvding G, Eide GE, Bertelsen T. Corneal grafting for keratoconus in mentally retarded patients. Acta Opthalmologica Scandinavia 2001;79(6):609‐15. [DOI] [PubMed] [Google Scholar]
Javadi 2006 {published data only}
- Javadi MA, Naderi M, Zare M, Jenaban A, Rabei HM, Anissian A. Comparison of the effect of three suturing techniques on postkeratoplasty astigmatism in keratoconus. Cornea 2006;25(9):1029‐33. [DOI] [PubMed] [Google Scholar]
Jiang 2011 {published data only}
- Jiang X, Hong J. Contrastive study of deep lamellar keratoplasty in treating keratoconus. International Journal of Ophthalmology 2011;11(9):1588‐90. [Google Scholar]
Jones 2009 {published data only}
- Jones MN, Armitage WJ, Ayliffe W, Larkin F, Kaye SB. Penetrating and deep anterior lamellar keratoplasty for keratoconus: A comparison of graft outcomes in the United Kingdom. Investigative Ophthalmology and Visual Science 2009;50(12):5625‐9. [DOI] [PubMed] [Google Scholar]
Kandemir 2011 {unpublished data only}
- Kandemir B, Sayman IB, Akdemir MO, Selvi C, Dogan OK. Comparison of visual acuity and contrast sensitivity between deep anterior lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. European Society of Cataract and Refractive Surgery Congress Proceedings; 2011 Feb 18‐20; Istanbul. Istanbul: European Society of Cataract and Refractive Surgery, 2011.
Koo 2011 {published data only}
- Koo TS, Finkelstein E, Tan D, Mahta JS. Incremental cost‐utility analysis of deep anterior lamellar keratoplasty compared with penetrating keratoplasty for the treatment of keratoconus. American Journal of Ophthalmology 2011;152(1):40‐7. [DOI] [PubMed] [Google Scholar]
Kubaloglu 2012 {published data only}
- Kubaloglu A, Koytak A, Sari ES, Akyol S, Kurnaz E, Ozerturk Y. Corneal endothelium after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. Indian Journal of Ophthalmology 2012;60(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahjoub 2011 {unpublished data only}
- Mahjoub S. Penetrating versus deep lamellar femtosecond laser assisted keratoplasty in keratoconus. European Society of Cataract and Refractive Surgery Congress Proceedings; 2011 Sept 16; Vienna. Vienna: European Society of Cataract and Refractive Surgery, 2011.
McDonald 1987 {published data only}
- McDonald MB, Safir A, Waring GO III, Schlichtemeier WR, Kissling GE, Kaufman HE. A preliminary comparative study of epikeratophakia or penetrating keratoplasty for keratoconus. American Journal of Ophthalmology 1987;103(3 Pt 2):467. [DOI] [PubMed] [Google Scholar]
Motlagh 2012 {published data only}
- Motlagh BF, Sedghipoor MR, Abroon G, Sadigh AL. Outcomes of penetrating keratoplasty and deep anterior lamellar keratoplasty for keratoconus in a university teaching hospital. Iranian Journal of Ophthalmology 2012;24(2):52‐6. [Google Scholar]
NCT00371202 {unpublished data only}
- NCT00371202. Comparison of penetrating keratoplasty and deep lamellar keratoplasty with the big bubble technique for keratoconus. clinicaltrials.gov/ct2/show/NCT00371202 (accessed 7 May 2013).
Panda 1999 {published data only}
- Panda A, Bageshwar LM, Ray M, Singh JP, Kumar A. Deep lamellar keratoplasty versus penetrating keratoplasty for corneal lesions. Cornea 1999;18(2):172‐5. [DOI] [PubMed] [Google Scholar]
Panda 2012 {unpublished data only}
- Panda A. Deep anterior lamellar keratoplasty vs penetrating keratoplasty in keratoconus. Proceedings of the World Opthalmology Congress; 2012 Feb 16‐20; Abu Dhabi. Abu Dhabi: World Ophthalmology Congress, 2012.
Reinhart 2011 {published data only}
- Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman SC, Shtein RM. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty ‐ A report by the American Academy of Ophthalmology. Ophthalmology 2011;118(1):209‐18. [DOI] [PubMed] [Google Scholar]
Salvetat 2013 {published data only}
- Salvetat ML, Brusini P, Pedrotti E, Zeppieri M, Miani F, Marcigaglia M, et al. Higher order aberrations after keratoplasty for keratoconus. Optometry and Vision Science 2013;90(3):293‐301. [DOI] [PubMed] [Google Scholar]
Sari 2012 {published data only (unpublished sought but not used)}
- Sari ES, Kubaloglu A, Unal M, Llorens DP, Koytak A, Ofluoglu AN, et al. Penetrating keratoplasty versus deep anterior lamellar keratoplasty: comparison of optical and visual quality outcomes. British Journal of Ophthalmology 2012;96(8):1063‐7. [DOI] [PubMed] [Google Scholar]
Serdarevic 1996 {published data only}
- Serdarevic ON, Renard GJ, Pouliquen Y. Penetrating keratoplasty for keratoconus: Role of videokeratoscopy and trephine sizing. Journal of Cataract and Refractive Surgery 1996;22(9):1165‐74. [DOI] [PubMed] [Google Scholar]
Shi 2012 {published data only}
- Shi JL, Feng YF, Yu JG, Wang QM. Meta analysis of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Chinese Journal of Experimental Ophthalmology 2012;30(10):926‐31. [Google Scholar]
Shimmura 2005 {published data only}
- Shimmura S, Shimazaki J, Omoto M, Teruya A, Ishioka M, Tsubota K. Deep lamellar keratoplasty (DLKP) in keratoconus patients using viscoadaptive viscoelastics. Cornea 2005;24(2):178‐81. [DOI] [PubMed] [Google Scholar]
Shoja 2007 {published data only}
- Shoja M, Besharati MR. A comparison of the effect of donor‐recipient trephine size disparity on refractive error in keratoconus. Saudi Medical Journal 2007;28(9):1389‐92. [PubMed] [Google Scholar]
Tan 2007 {published data only}
- Tan DT, Por YM. Current treatment options for corneal ectasia. Current Opinion in Ophthalmology 2007;18(4):284‐9. [DOI] [PubMed] [Google Scholar]
Tan 2010a {published data only}
- Tan DT, Anshu A, Parthasarathy A, Htoon HM. Visual acuity outcomes after deep anterior lamellar keratoplasty: a case‐control study. British Journal of Ophthalmology 2010;94(10):1295‐9. [DOI] [PubMed] [Google Scholar]
Watson 2004 {published data only}
- Watson SL, Ramsay A, Dart JK, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology 2004;111(9):1676‐82. [DOI] [PubMed] [Google Scholar]
Yamaguchi 2011 {published data only}
- Yamaguchi T, Ohnuma K, Tomida D, Konomi K, Satake Y, Negishi K, et al. The contribution of the posterior surface to the corneal aberrations in eyes after keratoplasty. Investigative Ophthalmology and Vision Science 2011;52(9):6222‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jiang 2006 {published data only}
- Jiang W, Sun HM. Observation of curative effects of DLK and PK in patients with keratoconus. Chinese Journal of Practical Ophthalmology 2006;24(3):258‐60. [Google Scholar]
Liu 2008 {published data only}
- Liu P, Wang YQ, Wang X, Xue CL, Li HY. Observation of curative effects of DLKP and PKP in patients with keratoconus. Chinese Journal of Ocular Trauma and Occupational Eye Disease 2008;30(8):615‐8. [Google Scholar]
References to ongoing studies
NCT01901614 {unpublished data only}
- NCT01901614. Randomized clinical trial of OCT‐guided laser‐assisted lamellar anterior keratoplasty in adults for keratoconus. clinicaltrials.gov/ct2/show/NCT01901614 (accessed 30 April 2013).
Additional references
Alió 2006
- Alió JL, Shabayek MH. Corneal higher aberrations: A method to grade keratoconus. Journal of Refractive Surgery 2006;22(6):539‐45. [DOI] [PubMed] [Google Scholar]
Anwar 2002
- Anwar M, Teichmann MH. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet's membrane. Cornea 2002;21(4):374‐83. [DOI] [PubMed] [Google Scholar]
Archila 1984
- Archila EA. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea 1984‐1985;3(3):217‐8. [PubMed] [Google Scholar]
Bilgin 2009
- Bilgin LK, Yilmaz S, Araz B, Yuksel SB, Sezen T. 30 years of contact lens prescribing for keratoconic patients in Turkey. Contact Lens and Anterior Eye 2009;32(1):16‐21. [DOI] [PubMed] [Google Scholar]
Buzzonetti 2010
- Buzzonetti L, Laborante A, Petrocelli G. Standardized big‐bubble technique in deep anterior lamellar keratoplasty assisted by the femtosecond laser. Journal of Cataract and Refractive Surgery 2010;36(10):1631‐6. [DOI] [PubMed] [Google Scholar]
Castroviejo 1948
- Castroviejo R. Keratoplasty for the treatment of keratoconus. Transactions of the American Ophthalmology Society 1948;46:127‐53. [PMC free article] [PubMed] [Google Scholar]
Coster 2002
- Coster DJ. Fundamentals of Clinical Ophthalmology: Cornea. London: BMJ Books, 2002. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ertan 2008a
- Ertan A, Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea 2008;27(10):1109‐13. [DOI] [PubMed] [Google Scholar]
Ertan 2008b
- Ertan A, Kamburoglu G. Intacs implantation using a femtosecond laser for management of keratoconus: Comparison of 306 cases in different stages. Journal of Cataract and Refractive Surgery 2008;34(9):1521‐6. [DOI] [PubMed] [Google Scholar]
Espandar 2010
- Espandar L, Mayer J. Keratoconus: overview and update on treatment. Middle East African Journal of Ophthalmology 2010;17(1):15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farid 2009
- Farid M, Steinert RF. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. Journal of Cataract and Refractive Surgery 2009;35(5):809‐13. [DOI] [PubMed] [Google Scholar]
Georgiou 2004
- Georgiou T, Funnell CL, Cassels‐Brown A, O'Connor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye 2004;18(4):379‐83. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gobbe 2005
- Gobbe M, Guillon M. Corneal wavefront aberration measurements to detect keratoconus patients. Contact Lens and Anterior Eye 2005;28(2):57‐66. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jafri 2007
- Jafri B, Li X, Yang H, Rabinowitz YS. Higher order wavefront aberrations and topography in early and suspected keratoconus. Journal of Refractive Surgery 2007;23(8):774‐81. [DOI] [PubMed] [Google Scholar]
Jaycock 2008
- Jaycock PD, Jones MN, Males J, Armitage WJ, Cook SD, Tole DM, et al. Outcomes of same‐sizing versus oversizing donor trephines in keratoconic patients undergoing first penetrating keratoplasty. Ophthalmology 2008;115(2):268‐75. [DOI] [PubMed] [Google Scholar]
Jhanji 2010
- Jhanji V, Sharma N, Vajpayee RB. Management of keratoconus: Current scenario. British Journal of Ophthalmology 2011;95(8):1044‐50. [DOI] [PubMed] [Google Scholar]
Kaiser 2009
- Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Transactions of the American Ophthalmological Society 2009;107:311‐24. [PMC free article] [PubMed] [Google Scholar]
Karimian 2010
- Karimian F, Feizi S. Deep anterior lamellar keratoplasty: Indications, surgical techniques and complications. Middle East African Journal of Ophthalmology 2010;17(1):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2013
- Lu Y, Vitart V, Burdon KP, Khor CC, Bykhovskaya Y, Mirshahi A, et al. Genome‐wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nature Genetics 2013;45(2):155‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melles 2000
- Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. A quick surgical technique for deep, anterior lamellar keratoplasty using visco‐dissection. Cornea 2000;19(4):427‐32. [DOI] [PubMed] [Google Scholar]
Rabinowitz 1998
- Rabinowitz YS. Keratoconus. Survey of Ophthalmology 1998;42(4):297‐319. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roe 2008
- Roe RH, Lass JH, Brown MM. The value‐based medicine comparative effectiveness and cost effectiveness of penetrating keratoplasty for keratoconus. Cornea 2008;27(9):1001‐7. [DOI] [PubMed] [Google Scholar]
Romero‐Jimenez 2010
- Romero‐Jimenez M, Santodomingo‐Rubido J, Wolffsohn JS. Keratoconus: A review. Contact Lens and Anterior Eye 2010;33(4):157‐66. [DOI] [PubMed] [Google Scholar]
Sugita 1997
- Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. British Journal of Ophthalmology 1997;81(3):184‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2010b
- Tan DT, Anshu A. Anterior lamellar keratoplasty: 'Back to the Future' ‐ a review. Clinical and Experimental Ophthalmology 2010;38(2):118‐27. [DOI] [PubMed] [Google Scholar]
Trimarchi 2001
- Trimarchi F, Poppi E, Klersy C, Piacentini C. Deep lamellar keratoplasty. Ophthalmologica 2001;215(6):389‐93. [DOI] [PubMed] [Google Scholar]
Tuft 2012
- Tuft SJ, Hassan H, George S, Frazer DG, Willoughby CE, Liskova P. Keratoconus in 18 pairs of twins. Acta Ophthalmologica 2012;90(6):e482‐6. [DOI] [PubMed] [Google Scholar]
Wang 2000
- Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: Evidence for major gene determination. American Journal of Medical Genetics 2000;93(5):403‐9. [PubMed] [Google Scholar]
Wheeler 2012
- Wheeler J, Hauser MA, Afshari NA, Allingham RR, Liu Y. The genetics of keratoconus: a review. Reproductive System and Sexual Disorders 2012;3(Suppl 6):001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2007
- Williams KA, Lowe MT, Bartlett CM, Kelly L, Coster DJ. The Australian Corneal Graft Registry 2007 Report. Adelaide: Flinders University, 2007. [Google Scholar]
Williams 2010
- Williams KA, Kelly TL, Lowe MT, Coster DJ, all contributors to the Australian Corneal Graft Registry. The influence of rejection episodes in recipients of bilateral corneal grafts. American Journal of Transplantation 2010;10(4):921‐30. [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams KA, Lowe MT, Keane MC, Jones VJ, Loh RS, Coster DJ. The Australian Corneal Graft Registry 2012 Report. http://hdl.handle.net/2328/25859 (accessed 12 February 2014).
References to other published versions of this review
Keane 2012
- Keane M, Williams K, Coster D. Penetrating keratoplasty versus deep anterior lamellar keratoplasty for treating keratoconus. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009700] [DOI] [PMC free article] [PubMed] [Google Scholar]


