Abstract
Flux into the glycolytic pathway of most cells is controlled via allosteric regulation of the irreversible, committing step catalyzed by ATP-dependent phosphofructokinase (PFK) (ATP-PFK; EC 2.7.1.11), the key enzyme of glycolysis. In some organisms, the step is catalyzed by PPi-dependent PFK (PPi-PFK; EC 2.7.1.90), which uses PPi instead of ATP as the phosphoryl donor, conserving ATP and rendering the reaction reversible under physiological conditions. We have determined the enzymic properties of PPi-PFK from the anaerobic, hyperthermophilic archaeon Thermoproteus tenax, purified the enzyme to homogeneity, and sequenced the gene. The ∼100-kDa PPi-PFK from T. tenax consists of 37-kDa subunits; is not regulated by classical effectors of ATP-PFKs such as ATP, ADP, fructose 2,6-bisphosphate, or metabolic intermediates; and shares 20 to 50% sequence identity with known PFK enzymes. Phylogenetic analyses of biochemically characterized PFKs grouped the enzymes into three monophyletic clusters: PFK group I represents only classical ATP-PFKs from Bacteria and Eucarya; PFK group II contains only PPi-PFKs from the genus Propionibacterium, plants, and amitochondriate protists; whereas group III consists of PFKs with either cosubstrate specificity, i.e., the PPi-dependent enzymes from T. tenax and Amycolatopsis methanolica and the ATP-PFK from Streptomyces coelicolor. Comparative analyses of the pattern of conserved active-site residues strongly suggest that the group III PFKs originally bound PPi as a cosubstrate.
As first discovered in Entamoeba histolytica (27), in some members of all three domains of life (Bacteria, Eucarya, and Archaea), the first committing step of glycolysis, the phosphorylation of fructose 6-phosphate (Fru 6-P), is catalyzed not by common ATP-dependent phosphofructokinase (PFK) (ATP-PFK; EC 2.7.1.11) but by an enzyme which uses PPi as a phosphoryl donor (PPi-PFK; EC 2.7.1.90) (22–34). The only archaeal PPi-PFK described so far is the enzyme of Thermoproteus tenax, a hyperthermophilic, anaerobic archaeon which is able to grow chemolithotrophically with CO2, H2, and S0, as well as chemo-organothrophically in the presence of S0 and carbohydrates (11, 41). As shown by enzymatic and in vivo studies (pulse-labeling experiments), T. tenax metabolizes glucose mainly via a variation of the Embden-Meyerhof-Parnas pathway distinguished by the reversible PPi-PFK reaction (34, 35).
In contrast to the virtually irreversible reaction catalyzed by the ATP-PFK, the phosphorylation by PPi is reversible. Thus, for thermodynamic reasons, the PPi-PFK should be able to replace the enzymes of both the forward (ATP-PFK) and reverse (fructose-bisphosphatase [FBPase]) reactions. Consistent with the presumed bivalent function of the PPi-dependent enzyme, in prokaryotes and parasitic protists which possess PPi-PFK, little, if any, ATP-PFK or FBPase activity is present. Strikingly, the PPi-PFKs of these organisms proved to be nonallosteric, suggesting that the control of the carbon flux through the pathway is no longer exerted by the PFK in these organisms. A considerably different situation has been described for higher plants and the green alga Euglena gracilis, showing comparable ATP-PFK, FBPase, and PPi-PFK activities and allosteric regulation of their PPi-dependent enzyme by fructose 2,6-bisphosphate (12, 22). However, in most cases it is not obvious which physiological role PPi-PFK performs: reversible catalysis of glycolysis/gluconeogenesis, increase of the energy yield of glycolysis under certain conditions in which the energy charge is low, or ATP-conservation in obligately fermentative organisms (22).
Closely related to questions concerning the biological function of PPi-PFKs is the matter of their evolutionary origin: are these enzymes the result of a secondary adaptation from ATP-PFKs, or do they represent an original phenotype, as suggested by their specificity for PPi, which is thought to be an ancient source of metabolic energy (9, 18, 19, 26). Indicated by sequence similarity (3, 4), all known PPi- and ATP-PFKs are homologous and therefore originated from a common ancestral root. From more recent studies of Streptomyces coelicolor PFK (4), the previous assumption of a single event which separated PPi- and ATP-PFKs had to be revised in favor of a multiple differentiation, leaving open, however, the question of the primary or secondary origin of PPi-PFK.
Understanding of PFK evolution has been impaired by a lack of knowledge concerning archaeal PFK, although the existence of ATP-PFK (31), PPi-PFK (34), and also ADP-dependent PFK (16, 31) in Archaea has been described. To address the evolution of PFK, we describe the PPi-PFK from T. tenax and compare its sequence and structure to those of known bacterial and eucaryal PFK enzymes.
MATERIALS AND METHODS
Organism and growth conditions.
T. tenax Kra 1 (41), DSM 2078, was cultivated as previously described (34, 35). Escherichia coli strains were grown at 37°C in Luria-Bertani medium in the presence or absence of ampicillin (100 μg/ml) (29). Methods for measuring enzyme activity and protein content and performance of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), N-terminal sequencing, and molecular mass determination of the native protein were described previously (34, 35).
Purification of PPi-dependent PFK.
T. tenax cells (15 g [wet weight]) were resuspended in 25 ml of 0.1 M HEPES-KOH buffer (pH 7.5) containing 0.3 M 2-mercaptoethanol. The cells were disrupted by passing the suspension four times through a French pressure cell (35-ml volume, 200 MPa). Cell debris was removed by centrifugation at 40,000 × g for 30 min (4°C). The supernatant was incubated for 30 min at 80°C, centrifuged again, and applied to a Q-Sepharose fast-flow column (volume, 90 ml; diameter, 2.5 cm; Pharmacia) equilibrated with 50 mM HEPES-KOH (pH 7.5) containing 30 mM 2-mercaptoethanol and 100 mM KCl. Protein was eluted with a linear salt gradient (100 to 400 mM KCl; 400 ml). The fractions containing PPi-PFK activity were pooled, (NH4)2SO4 was added to a final concentration of 0.4 M, and the mixture was applied to a phenyl-Sepharose CL4B column (volume, 20 ml; diameter, 2 cm; Pharmacia) pre-equilibrated in 50 mM HEPES-KOH (pH 7.5) containing 7.5 mM 2-mercaptoethanol, 200 mM KCl, and 0.4 M (NH4)2SO4. The column was washed with 3 volumes of the same buffer, and the protein was eluted with a linear gradient (120 ml) with a decreasing ion concentration [0.4 to 0.0 M (NH4)2SO4]) and an increasing ethylene glycol concentration (0.0 to 50%). Pooled fractions with PPi-PFK activity were dialyzed against 50 mM HEPES-KOH (pH 7.5) containing 7.5 mM 2-mercaptoethanol and 300 mM KCl, concentrated by membrane filtration (Centricon 30; Amicon), and subjected to gel filtration on HiLoad 26/60 Superdex 200 prep grade (volume, 325 ml; diameter, 2.6 cm; Pharmacia) preequilibrated in the same buffer. Fractions containing the homogeneous enzyme solution were pooled.
Cloning and sequencing of the PPi-PFK gene pfp.
The genomic DNA of T. tenax was prepared by the method of Weil et al. (38) modified as described by Meakin et al. (21). Southern hybridizations (8) were performed with an oligonucleotide probe (PFK 1: AT[ACT] TA[CT] CA[CT] GG[AGCT] TGG [AC]G) derived from the hexapeptide IYHGWR, corresponding to positions 35 to 40 of the N-terminal amino acid sequence determined by Edman degradation. Hybridization and signal detection were performed with the digoxigenin kit from Boehringer in accordance with the instructions of the manufacturer. A 5.2-kb HindIII fragment was labelled with the oligonucleotide probe. After elution from the agarose gel, fragments in that size range were cloned into pBluescript II KS+ (Stratagene) by using E. coli KL1 Blue (Stratagene) as the host (29). Positive clones were analyzed by restriction analysis and sequencing. The sequence of the gene was determined in both directions by the chain termination method (30) as modified by Wiemann et al. (39) with the aid of an Automated Laser Fluorescent DNA Sequencer (Pharmacia).
Tree construction.
Database searching for homologous amino acid sequences was performed by running the BLASTP program (1) with the amino acid sequence database at the National Center for Biotechnology Information. Sets of amino acid sequences were first aligned with the help of the CLUSTAL program (15) by using the default parameters and then edited by eye. Regions of uncertain alignment were deleted from the final alignment. Phylogenetic trees were constructed by using both the maximum-parsimony and distance matrix methods. The maximum-parsimony method was performed with the PAUP (37) and PHYLIP (10) software packages. For the distance matrix method, we used the matrix options Dayhoff and George-Hunt-Barker to create the distance matrices in PROTDIST. The distance trees were inferred by using the NEIGHBOR program (10). The SEQBOOT, PROTPARS, PROTDIST, NEIGHBOR, and CONSENSE programs of the PHYLIP package were used to derive confidence limits, estimated by 100 bootstrapping replicates.
Nucleotide sequence accession number.
The pfp nucleotide and amino acid sequence data reported in this study has been submitted to the EMBL database under accession no. Y14655.
RESULTS AND DISCUSSION
Purification and enzymic properties of PPi-PFK.
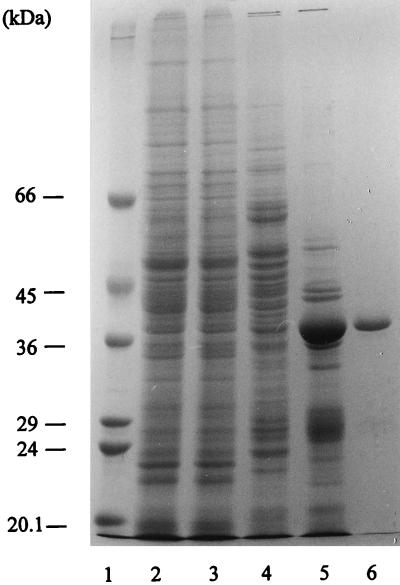
PPi-PFK was purified to homogeneity, as judged by SDS-PAGE (Fig. 1), from heterotrophically grown cells. The protein proved to be insensitive to oxygen but highly sensitive to low ionic strength. To prevent inactivation, almost all purification steps were performed at KCl concentrations of 100 to 300 mM. From 15 g of wet cells, 2.6 mg of homogeneous protein with a specific activity of 3.5 U/mg was recovered, corresponding to a yield of 23%. The comparatively low specific activity of the PPi-PFK of T. tenax (for comparison: Amycolatopsis methanolica, 107 U/mg [2]; E. histolytica, 45.7 U/mg [27]) is, however, mainly due to the low assay temperature of 50°C, which was chosen for the use of mesophilic auxiliary enzymes in the coupled optical test.
FIG. 1.
SDS-polyacrylamide gel electropherogram of the fractions of the following purification steps of PPi-PFK: molecular size standard (lane 1), crude extract (40 μg of protein; lane 2), heat-treated extract (40 μg of protein; lane 3), anion-exchange chromatography product (20 μg of protein; lane 4), hydrophobic interaction chromatography product (20 μg of protein; lane 5), and gel filtration product (3 μg of protein; lane 6).
Molecular mass determinations of the PPi-PFK of T. tenax yielded 37 kDa for the subunit (SDS-PAGE) and 100 kDa for the native enzyme (gel filtration). Thus, the quaternary structure of the enzyme (homodimer or homotrimer) could not be deduced unequivocally from the mass ratio.
The enzymic properties characterized the PPi-PFK of T. tenax as a bidirectionally working enzyme with, however, a slight preference for the phosphorylating direction (Table 1). The enzyme displayed Michaelis-Menten kinetics in the catabolic and anabolic directions with similar affinities for PPi, Fru 6-P, and fructose 1,6-bisphosphate (Fru 1,6-P2) (Km values of 23, 53, and 33 μM, respectively). The affinity for Pi was significantly lower (Km = 1.43 mM), which might be explained by an adaptation to higher intracellular Pi concentrations, as discussed by Reeves et al. (27) for the PPi-PFK of E. histolytica.
TABLE 1.
Kinetic parameters of PPi-PFKa
| Variable substrate | Km (mM) | Vmaxf (U/mg of protein) |
|---|---|---|
| PPib | 0.023 | 2.7 |
| Fru 6-Pc | 0.053 | 2.9 |
| Pid | 1.43 | 2.1 |
| Fru 1,6-P2e | 0.033 | 2.0 |
Assay temperature, 50°C.
With 10 mM Fru 6-P and 1 mM MgCl2.
With 5 mM PPi and 1 mM MgCl2.
With 20 mM Fru 1,6-P2 and 5 mM MgCl2.
With 5 mM Pi and 5 mM MgCl2.
Vmax, maximum velocity.
Like other PPi-PFKs described so far (those of A. methanolica [2], E. histolytica [27], and Propionibacterium freudenreichii [23]), the enzyme of T. tenax showed high specificity for its substrates. In the phosphorylating reaction, PPi could not be replaced by ATP or ADP. Glucose 6-phosphate (forward direction) and fructose 2,6-bisphosphate (reverse reaction) were not used as substrates. The activity of PPi-PFK in both directions depended on the presence of Mg2+ ions, as shown by inhibition in the presence of EDTA.
The PPi-PFK of T. tenax is not regulated by the known allosteric modulators of PFKs such as adenine nucleotides (ATP, ADP, and AMP; concentrations tested, 2, 5, and 10 mM), metabolites (glucose, pyruvate, phosphoenolpyruvate, and citrate; concentration tested, 5 mM), and fructose 2,6-bisphosphate (concentrations tested, 0.1 and 1 mM).
Current knowledge indicates that nonallosteric PPi-PFK is only present in organisms which integrated this enzyme into their basic carbohydrate metabolism, replacing ATP-PFK and FBPase (P. freudenreichii, E. histolytica, etc.). Since one of the main control points of the Embden-Meyerhof-Parnas pathway is lost with this substitution, the flux through the pathway must be regulated in different ways. As reported previously, T. tenax does not show any ATP-PFK or FBPase activity (34). In this respect, the nonallosteric phenotype of PPi-PFK corresponds to its proposed function as an integral constituent of basic carbohydrate metabolism. Obviously, in this organism the main control point of the pathway, besides pyruvate kinase, is an irreversible, nonphosphorylating glyceraldehyde 3-phosphate dehydrogenase (33).
Nucleotide sequence of the pfp gene.
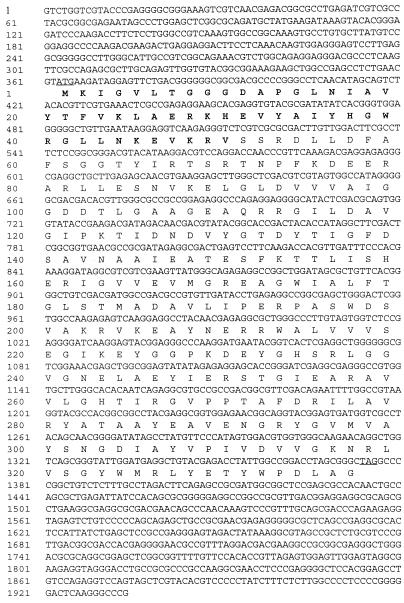
A clone containing a 5.2-kb HindIII fragment of the genomic DNA of T. tenax was selected by hybridization with an oligonucleotide probe derived from the N-terminal sequence of the purified protein. Sequence analysis revealed an open reading frame whose deduced amino acid sequence corresponds to the determined N-terminal protein sequence. The complete sequence of the T. tenax pfp gene (Fig. 2) comprises 1,011 bp coding for 337 amino acids with a calculated molecular mass of 36.8 kDa, which corresponds to the subunit molecular mass of 37 kDa determined by SDS-PAGE.
FIG. 2.
The nucleotide sequence of the pfp gene of T. tenax and its flanking regions. The deduced amino acid sequence is shown beneath the nucleotide sequence. The ATG start and TGA stop codons are underlined, and the determined N-terminal amino acid sequence of the purified protein is in boldface.
No convincing motifs for transcription or translation signals were recognized in the flanking regions. A partial open reading frame upstream (position 0 to 363) and a short open reading frame downstream (position 1485 to 1709) of the pfp gene were identified with, however, no significant similarities to known proteins. Whether the pfp gene is part of an operon, as found for the pfp and pfk genes of several bacterial species (3, 5, 20, 28), remains to be determined.
Overall sequence similarity of PPi-PFK and functional residues.
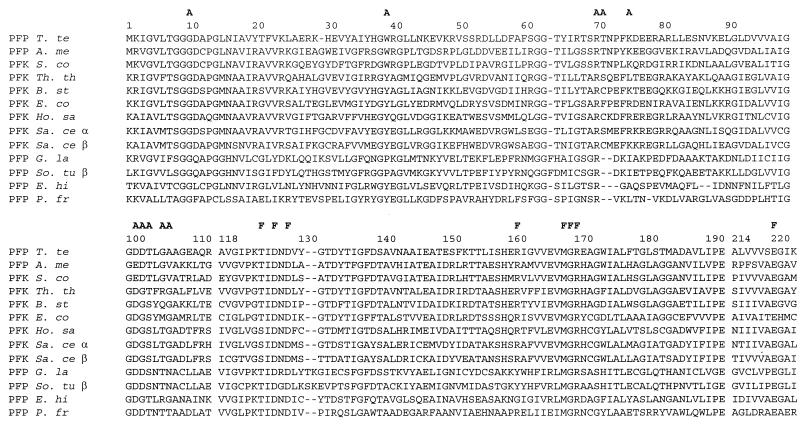
To determine the overall sequence similarities of the T. tenax enzyme with known PFKs and to identify structural features correlated with its PPi dependence, we aligned the T. tenax PFK with 30 sequences of enzymes whose cosubstrate specificity was clearly defined. The sequence data set comprises 10 sequences of PPi-PFKs (7 eucaryal sequences, 2 bacterial sequences, and 1 archaeal sequence) and 21 sequences of ATP-PFKs (12 eucaryal and 9 bacterial sequences). To ensure reliable assignment of homologous positions, we selected three well-conserved sequence fragments corresponding to positions 1 to 112, 118 to 192, and 214 to 223 of the T. tenax sequence, comprising 201 residues (Fig. 3).
FIG. 3.
Multiple alignment of ATP- and PPi-PFKs. The amino acid sequence of the PPi-PFK of T. tenax was aligned with 12 amino acid sequences of PPi- and ATP-PFKs in the EMBL and SwissProt databases. Gaps introduced for optimal alignment are marked by hyphens, and functionally important residues are marked by the letters F (Fru 6-P binding site) and A (ATP or PPi binding site). Abbreviations: T. te, T. tenax; A. me, A. methanolica; S. co, S. coelicolor; Th. th, Thermus aquaticus subsp. thermophilus; B. st, B. stearothermophilus; E. co, E. coli; Ho. sa, Homo sapiens; Sa. ce, Saccharomyces cerevisiae (α and β subunits); G. la, Giardia lamblia; So. tu, Solanum tuberosum (β subunit); E. hi, E. histolytica; P. fr, P. freudenreichii subsp. shermanii.
As shown by the obvious sequence similarity (Fig. 3), all of the PFKs, irrespective of cosubstrate specificity, are homologous and thus have evolved from a common ancestor (3, 4). Although the overall sequence similarity does not reflect basic structural differences between PPi- and ATP-PFKs, the ATP-dependent enzymes are more similar to each other (42 to 72% identity) than are the PPi-dependent enzymes, which exhibit considerable heterogeneity (24 to 51% identity). Strikingly, the PPi-PFK of T. tenax showed, on average, higher similarity to ATP-PFK (39 to 41% identity) than to the PPi-dependent homologs (20 to 30% identity). The highest similarities found were to the ATP-PFK of S. coelicolor (46.8% identity) and to the PPi-dependent enzyme of A. methanolica (46.3% identity).
To identify sequence motifs correlated with the cosubstrate specificity and thus suitable for obtaining closer insights into the structural basis of its differentiation, we focussed on active-site residues assigned by homology on the basis of the resolved three-dimensional structure of the bacterial ATP-PFKs of E. coli and Bacillus stearothermophilus (14, 32). As demonstrated by experimental verification, the prediction of functionally important residues by homology proved to be reliable even for distantly related PFK species (e.g., PPi-PFK from P. freudenreichii [40]), indicating a highly conserved spatial structure in the active-site region of ATP- and PPi-PFKs.
To assign residues involved in substrate and cosubstrate binding, we considered only residues in unequivocally homologous sequence regions. Residues located in the vicinity of gaps introduced for optimal alignment, indicating major spatial deviations, were disregarded (e.g., residues 70 and 71 in the T. tenax PFK, corresponding to residues 72 and 73 in the B. stearothermophilus PFK). For the alignment, only the catalytically active β subunits of the PPi-dependent plant enzymes were considered. The residues assigned to the Fru 6-P and ATP or PPi binding of the various PFKs are listed in Table 2.
TABLE 2.
Active-site residues of PFKs.
| Site and positiona | ATP-PFK
|
PPi-PFK
|
|||||
|---|---|---|---|---|---|---|---|
| Eucarya (12 species) |
Bacteria
|
Eucarya (7 species) |
Bacteria
|
Archaea (T. tenax) | |||
| 8 species | S. coelicolor | P. freudenreichii | A. methanolica | ||||
| Fru 6-P binding | |||||||
| 125 (124) | S (D) | T | T | T | T | T | T |
| 127 (126) | D (Q) | D | D | D | D | D | D |
| 129 (128) | D | D | D | D | D | D | D |
| 162 (161) | R | R | R | Y (R, G) | R | R | R |
| 169 (168) | M (Q) | M | M | M | M | M | M |
| 170 (169) | G (V) | G | G | G | G | G | G |
| 171 (170) | R (S) | R (H) | R | R | R | R | R |
| 222 (220) | E | E | E | E | E | E | E |
| ATP or PPi binding | |||||||
| 11 (10)b | G | G | G | G | G | G | G |
| 41 (39)c | Y | Y (F) | W | P (F, Y) | Y | W | W |
| 77 (75)d | R (K) | Kf | K | I (T, A) | L | K | K |
| 103 (101)e | D | D (N) | E | D | D | E | D |
| 104 (102)b | G | G | D | D (G) | D | D | D |
| 105 (103)b | S | S (T) | T | S (T)g | T | T | T |
| 107 (105)d | T (I) | Hf | G | T (F, R) | T | G | G |
| 108 (106)d | G | G | V | N (S, G) | T | V | A |
The residues involved in substrate and cosubstrate binding were assigned on the basis of the resolved three-dimensional structures of the ATP-PFKs of E. coli and B. stearothermophilus. The functions of the residues in E. coli and B. stearothermophilus ATP-PFKs are described in references 14 and 32. Numbering is according to the B. stearothermophilus sequence; the numbers in parentheses refer to the T. tenax sequence. Dominant residues are in boldface, and residues in parentheses occur in one species.
Bonds to β-P.
Contacts ribose.
Contacts adenine.
Binds to Mg2+.
Deviation in more than two species.
Occurs in two species.
Virtually all of the residues assigned to Fru 6-P binding are conserved, irrespective of the type and origin of the enzyme, indicating highly similar spatial arrangements of the substrate binding site throughout all PFKs. On the contrary, the conservation of the residues predicted for binding of the phosphoryl donor is significantly lower. Only a Gly at position 11 is common to all of the PFKs. At position 103, an acidic residue (Asp or Glu) is dominant. Strikingly, the PPi-dependent enzymes and the ATP-PFK of S. coelicolor showed systematic deviations at residues 41, 104, 105, and 108, which were identified as important for ATP binding in the E. coli and B. stearothermophilus enzymes (14, 32), suggesting that these changes correlate with differences in cosubstrate binding.
Although the residues at positions 77 and 107 have been assigned important functions in adenine binding, their variability in ATP-dependent enzymes is too high for them to be indicative of cosubstrate binding specificity. The ATP-PFKs, except for the ATP-PFK of S. coelicolor, are characterized by Tyr at position 41, Gly at positions 104 and 108, and Ser at position 105. The high conservation of these residues is ascribed to their specific contact with ribose (Tyr 41) or adenine (Gly 108) or just the limited space left between the polypeptide chain and the bulky ligand (Gly 104 and Ser 105).
The assigned residues in the PPi-PFKs deviating from the respective pattern of the ATP-dependent enzymes are a hydrophobic—mostly aromatic—residue at position 41, Asp at position 104, Ser or Thr at position 105, and an unspecified residue at position 108. As shown in Table 2, the T. tenax PFK fits very well into the common pattern of PPi-PFKs.
Surprisingly, at four of the eight listed positions, the ATP-dependent enzyme of S. coelicolor shows the characteristic residues of PPi-PFKs. Furthermore, six of the eight residues are identical in the ATP-dependent enzyme of S. coelicolor and the PPi-dependent enzymes of T. tenax and a complete accordance could be observed with the PPi-PFK of A. methanolica. This close resemblance—despite the difference in cosubstrate specificity—hinted that structural determinants other than the predicted active-site residues govern the cosubstrate specificity of this enzyme. The high level of correspondence to the structural features characteristic of the PPi-dependent enzymes, along with the preferred overall sequence similarity to the PPi-PFKs from A. methanolica (74.1% identity) and T. tenax (46.8% identity), strongly suggests that the S. coelicolor enzyme evolved from a PPi-dependent precursor and converted more recently to the ATP-dependent phenotype.
Phylogenetic analyses.
The enzyme of T. tenax is the first archaeal PFK available for phylogenetic studies. This offers the opportunity to gain insights into the differentiation of substrate specificity of these enzymes, as well as indications about the phenotype of the common PFK precursor.
As shown by the sequence similarity, the archaeal enzyme is a homolog of the known PFKs. This finding indicates once again that the central carbohydrate metabolism of glycolysis was established before the segregation of the three domains of life and that the present-day variations of the pathway observed in different phylogenetic lineages must be a result of divergence rather than convergence.
Phylogenetic trees were constructed by using a data set of 31 sequences with the aid of the maximum-parsimony and distance matrix methods. Only sequences of PFKs with defined cosubstrate specificity were included in the alignment. The different methods gave us the same tree topology, apart from a minor change. The ATP-PFK from Drosophila melanogaster, which forms a common stem with the ATP-PFK of Haemonchus contortus in the neighbor-joining tree, represents a separate lineage in the maximum-parsimony tree.
Surprisingly, the PFKs cluster into three different monophyletic groups (Fig. 4): (i) PFK group I, consisting of only ATP-PFKs; (ii) PFK group II, consisting of only PPi-PFKs; and (iii) PFK group III, comprising both ATP- and PPi-PFKs.
FIG. 4.
Phylogenetic tree of PFKs. The tree is based on distance analysis (neighbor-joining method) of sequences of PPi- and ATP-PFKs in the EMBL and SwissProt data banks. Only enzymes whose substrate specificity was clearly defined and sequence regions (positions 1 to 112, 118 to 192, and 214 to 223 of the T. tenax sequence) which show unequivocal similarity were included (Fig. 3). Bootstrap values (neighbor-joining and maximum-parsimony methods) are indicated at basal nodes (in percentages) and are based on 100 data sets. Groups: I, Dictyostelium discoideum, H. contortus, D. melanogaster, H. sapiens (muscle type), Rattus norvegicus (muscle type), Oryctolagus cuninculus (muscle type), Schistosoma mansoni, Aspergillus niger, S. cerevisiae, Klyveromyces lactis, T. aquaticus subsp. thermophilus, B. stearothermophilus, L. delbrueckii, L. lactis, B. macquariensis, C. acetobutylicum, Spiroplasma citri, and E. coli; II; P. freudenreichii subsp. shermanii, E. histolytica, Ricinus communis, S. tuberosum, G. lamblia, Naegleria fowleri; III, T. tenax, S. coelicolor, and A. methanolica.
The ATP-PFKs of group I form a coherent cluster divided into two branches of solely bacterial or eucaryal enzymes that are well separated from the other two monophyletic groups (PFK groups II and III) by a common stem. The branching order of the bacterial ATP-PFKs only partially follows the universal tree based on 16S rRNA sequences (24) and strongly suggests the presence of at least two paralogous lines (represented by the gram-positive bacteria [i] B. stearothermophilus, Lactococcus lactis, and Lactobacillus delbrueckii and [ii] Clostridium acetobutylicum and B. macquariensis). The branching point of the eucaryal PFKs is paralleled by a basal gene duplication leading to a more complex regulation capacity of these enzymes (12, 13, 25). Overall, the topology of this eucaryal subtree primarily reflects a vertical heritage of pfk genes.
The branching system of PFK group II includes bacterial and eucaryal PPi-dependent enzymes and is generally characterized by long branch lengths, probably due to either specific functional adaptation or release from functional constraints. There is no evidence for a gene duplication event between the bacterial nonallosteric PFK of P. freudenreichii and the allosteric eucaryal plant enzyme, as described for ATP-PFK group I. However, the α and β subunits of plants evolved by gene duplication (7, 12).
The PFK group III cluster is characterized by rather short branches comprising not only the PPi-dependent enzymes of A. methanolica and T. tenax but also the ATP-PFK of S. coelicolor. The topology of the tree is supported by fairly good bootstrap values: the separation of the group III PFKs from the group II enzymes is confirmed by bootstrap values of 86% (neighbor joining) and 89% (maximum parsimony) and its separation from the group I enzymes by bootstrap values of 71% (neighbor joining) and 50% (maximum parsimony), respectively. The affiliation of the T. tenax PFK and the enzymes of A. methanolica and S. coelicolor is ensured by an 82% (neighbor joining) or 70% (maximum parsimony) bootstrap value and further supported by unique sequence signatures (Fig. 3, GWRG [position 38 to 41], SRTNP [position 69 to 73], TLG [position 104 to 106], and AGW [position 172 to 174]).
The unexpectedly close clustering of a bacterial and archaeal PPi-PFK with a bacterial ATP-dependent enzyme apart from all of the other ATP-dependent representatives and the striking correspondence of the active-site residues lead to the assumption that the change of cosubstrate specificity happened more recently and independently from the basic event which separated the PFKs of groups I and II. Since—as outlined above—the pattern of the active-site residues of group III enzymes coincides with that of group II enzymes, classifying all group III enzymes as primarily PPi dependent, the change in cosubstrate specificity must have been from PPi to ATP in the S. coelicolor enzyme. We cannot estimate how complex these structural changes must be to switch the binding specificity from PPi to ATP. However, we assume that such changes occur more easily in a rather unspecified structure.
Unfortunately, in all three main branches of the PFK tree, only members of two domains are present; thus, we cannot decide whether all three main lineages trace back to a common ancestor. Without further sequence information, we cannot rule out the possibility that the differentiation of the PFK occurred after the three domains had been segregated and the observed relationship is the result of lateral gene transfer events between domains. Under this aspect, the sequence information of other known archaeal ATP-PFKs and ADP-dependent PFKs would be of special relevance. The recently published archaeal genomes of Methanococcus jannaschii (6), Archaeoglobus fulgidus (17), and Methanobacterium thermoautotrophicum (36) comprise no open reading frames homologous to the known pfk and pfp genes.
Given the present stage of knowledge, the nonspecialized features of the PFK group III enzymes (from T. tenax, A. methanolica, and S. coelicolor), reflected by their limited regulatory capacity, their slow evolution rates, and their original specificity for PPi, which is assumed to be the primary phosphoryl donor in metabolic processes (9, 18, 19, 26), strongly suggest that these enzymes represent the descendants of the most ancient lineage within the known PFKs.
ACKNOWLEDGMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Thanks are due to W. Martin, Institute of Genetics, University of Braunschweig; H. Brinkmann, Université Paris Sud; and A. Siebers and I. Haidl, Max Planck Institute of Immunobiology, Freiburg, for stimulating discussion and critically reading the manuscript. B.S. thanks A. Schramm for experimental support.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Alves A M C R, Euverink G J W, Hektor H J, Hessels G I, Van der Vlag J, Vrijbloed J W, Hondmann D, Visser J, Dijkhuizen L. Enzymes of glucose and methanol metabolism in the actinomycete Amycolatopsis methanolica. J Bacteriol. 1994;176:6827–6835. doi: 10.1128/jb.176.22.6827-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves A M C R, Meijer W G, Vrijbloed J W, Dijkhuizen L. Characterization and phylogeny of the pfp gene of Amycolatopsis methanolica encoding PPi-dependent phosphofructokinase. J Bacteriol. 1996;178:149–155. doi: 10.1128/jb.178.1.149-155.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves A M C R, Euverink G J W, Bibb M J, Dijkhuizen L. Identification of ATP-dependent phosphofructokinase as a regulatory step in the glycolytic pathway of the actinomycete Streptomyces coelicolor A3(2) Appl Environ Microbiol. 1997;63:956–961. doi: 10.1128/aem.63.3.956-961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branny P, De La Torre F, Garel J-R. The genes for phosphofructokinase and pyruvate kinase of Lactobacillus delbrueckii subsp. bulgaricus constitute an operon. J Bacteriol. 1996;178:4727–4730. doi: 10.1128/jb.178.15.4727-4730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 7.Carlisle S M, Blakeley S D, Hemmingsen S M, Trevanion S J, Hiyoshi T, Kruger N J, Dennis D T. Pyrophosphate-dependent phosphofructokinase. Conservation of protein sequence between the a- and b-subunits and with the ATP-dependent phosphofructokinase. J Biol Chem. 1990;265:18366–18371. [PubMed] [Google Scholar]
- 8.Chomczynski P. One-hour downward alkaline capillary transfer for blotting of DNA and RNA. Anal Biochem. 1992;201:134–139. doi: 10.1016/0003-2697(92)90185-a. [DOI] [PubMed] [Google Scholar]
- 9.Dawes E A, Senior P. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 10.Felsenstein J. PHYLIP: phylogeny inference package, version 3.5.c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 11.Fischer F, Zillig W, Stetter K O, Schreiber G. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature. 1983;301:511–513. doi: 10.1038/301511a0. [DOI] [PubMed] [Google Scholar]
- 12.Fothergill-Gilmore L A, Michels P A M. Evolution of glucolysis. Biophys Mol Biol. 1993;59:105–235. doi: 10.1016/0079-6107(93)90001-z. [DOI] [PubMed] [Google Scholar]
- 13.Heinisch J, Ritzel R G, von Borstel R C, Aguilera A, Rodicio R, Zimmermann F K. The phosphofructokinase genes of yeast evolved from two duplication events. Gene. 1989;78:309–321. doi: 10.1016/0378-1119(89)90233-3. [DOI] [PubMed] [Google Scholar]
- 14.Hellinga H W, Evans P R. Nucleotide sequence and high-level expression of the major Escherichia coli phosphofructokinase. Eur J Biochem. 1985;149:363–373. doi: 10.1111/j.1432-1033.1985.tb08934.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins D G, Sharp P M. Computer applications in the biomedical sciences. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 16.Kengen S W M, de Bok F A M, van Loo N-D, Dijkema C, Stams A J M. Evidence for the operation of a novel Embden-Meyerhof-pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus. J Biol Chem. 1994;269:17537–17541. [PubMed] [Google Scholar]
- 17.Klenk H-P, Clayton R A, Tomb J-F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Loftus B, Peterson S, Reich C I, McNeil L K, Badger J H, Glodek A, Zhou L, Overbeek R, Gocayne J D, Weidman J F, McDonald L, Utterback T, Cotton M D, Spriggs T, Artiach P, Kaine B P, Sykes S M, Sadow P W, D’Andrea K P, Bowman C, Fujii C, Garland S A, Mason T M, Olsen G J, Fraser C M, Smith H O, Woese C R, Venter J C. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 18.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulaev I S, Vagabov V M. Polyphosphate metabolism in microorganisms. Adv Microb Physiol. 1983;24:83–171. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- 20.Llanos R M, Harris C J, Hillier A J, Davidson B E. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J Bacteriol. 1993;175:2541–2551. doi: 10.1128/jb.175.9.2541-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meakin S A, Nash J H E, Murray W D, Kennedy K J, Sprott G D. A generally applicable technique for the extraction of restrictable DNA from methanogenic bacteria. J Microbiol Methods. 1991;14:119–126. [Google Scholar]
- 22.Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991;285:1–5. doi: 10.1016/0014-5793(91)80711-b. [DOI] [PubMed] [Google Scholar]
- 23.O’Brian W, Bowien S, Wood H G. Isolation and characterization of a pyrophosphate-dependent phosphofructokinase from Propionibacterium shermanii. J Biol Chem. 1975;250:8690–8695. [PubMed] [Google Scholar]
- 24.Olsen G J, Woese C R, Overbeek R. The winds of (evolutionary) change: breathing new life into microbiology. J Bacteriol. 1994;176:1–6. doi: 10.1128/jb.176.1.1-6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poorman R A, Randolph A, Kemp R G, Heinrikson R L. Evolution of phosphofructokinase-gene duplication and creation of new effector sites. Nature. 1984;309:467–469. doi: 10.1038/309467a0. [DOI] [PubMed] [Google Scholar]
- 26.Reeves R. How useful is the energy in inorganic pyrophosphate? Trends Biochem Sci. 1976;March:53–55. [Google Scholar]
- 27.Reeves R E, South D J, Blytt H J, Warren L G. Pyrophosphate: d-fructose 6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function of 6-phosphofructokinase. J Biol Chem. 1974;149:7737–7741. [PubMed] [Google Scholar]
- 28.Sakai H, Ohta T. Molecular cloning and nucleotide sequence of the gene for pyruvate kinase of Bacillus stearothermophilus and the production of the enzyme in Escherichia coli. Evidence that the genes for phosphofructokinase and pyruvate kinase constitute an operon. Eur J Biochem. 1993;211:851–859. doi: 10.1111/j.1432-1033.1993.tb17618.x. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selig M, Kavier K B, Santos H, Schönheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol. 1997;167:217–232. doi: 10.1007/BF03356097. [DOI] [PubMed] [Google Scholar]
- 32.Shirakihara Y, Evans P R. Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products. J Mol Biol. 1988;204:973–994. doi: 10.1016/0022-2836(88)90056-3. [DOI] [PubMed] [Google Scholar]
- 33.Siebers B. Untersuchungen zum Kohlenhydrat-Metabolismus des hyperthermophilen Archaeums Thermoproteus tenax. Ph.D. thesis. Essen, Germany: University of Essen; 1995. [Google Scholar]
- 34.Siebers B, Hensel R. Glucose catabolism of the hyperthermophilic archaeum Thermoproteus tenax. FEMS Microbiol Lett. 1993;111:1–8. [Google Scholar]
- 35.Siebers B, Wendisch V F, Hensel R. Carbohydrate metabolism in Thermoproteus tenax: in vivo utilization of the non-phosphorylative Entner-Doudoroff pathway and characterization of its first enzyme, glucose dehydrogenase. Arch Microbiol. 1997;168:120–127. doi: 10.1007/s002030050477. [DOI] [PubMed] [Google Scholar]
- 36.Smith D R, Doucette-Stamm L A, Delroughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keaggle P, Lumm W, Pothier B, Qui D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, Church G, Daniels C, Mao J-I, Rice P, Nölling J, Reeve J. Complete genome sequence of Methanobacterium thermoautotrophicum delta H: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swofford D L. PAUP: phylogenetic analysis using parsimony, version 3.1.1. Champaign: Illinois National Historical Survey; 1993. [Google Scholar]
- 38.Weil C F, Cram D S, Sherf B A, Reeve J N. Structure and comparative analysis of the genes encoding component C of methyl coenzyme M reductase in the extremely thermophilic archaebacterium Methanothermus fervidus. J Bacteriol. 1988;170:4718–4726. doi: 10.1128/jb.170.10.4718-4726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiemann S, Rupp T, Zimmermann J, Voss H, Schwager C, Ansorge W. Primer design for automated sequencing utilizing T7 DNA polymerase and internal labeling with fluorescein-15-dATP. BioTechniques. 1995;18:688–697. [PubMed] [Google Scholar]
- 40.Xu J, Green P C, Kemp R G. Identification of basic residues involved in substrate binding and catalysis by pyrophosphate-dependent phosphofructokinase from Propionibacterium freudenreichii. J Biol Chem. 1994;269:15553–15557. [PubMed] [Google Scholar]
- 41.Zillig W, Stetter K O, Schäfer W, Janekovic D, Wunderl S, Holz I, Palm P. Thermoproteales: a novel type of extremely thermoacidophilic anaerobic archaebacteria isolated from icelandic solfatares. Zentralbl Bakteriol Hyg I Abt Orig C. 1981;2:205–227. [Google Scholar]