Abstract
Background
Endoscopic therapy reduces the rebleeding rate and the need for surgery in patients with bleeding peptic ulcers.
Objectives
To determine whether a second procedure improves haemostatic efficacy or patient outcomes or both after epinephrine injection in adults with high‐risk bleeding ulcers.
Search methods
For our update in 2014, we searched the following versions of these databases, limited from June 2009 to May 2014: Ovid MEDLINE(R) 1946 to May Week 2 2014; Ovid MEDLINE(R) Daily Update May 22, 2014; Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations May 22, 2014 (Appendix 1); Evidence‐Based Medicine (EBM) Reviews—the Cochrane Central Register of Controlled Trials (CENTRAL) April 2014 (Appendix 2); and EMBASE 1980 to Week 20 2014 (Appendix 3).
Selection criteria
We included randomised controlled trials (RCTs) comparing epinephrine alone versus epinephrine plus a second method. Populations consisted of patients with high‐risk bleeding peptic ulcers, that is, patients with haemorrhage from peptic ulcer disease (gastric or duodenal) with major stigmata of bleeding as defined by Forrest classification Ia (spurting haemorrhage), Ib (oozing haemorrhage), IIa (non‐bleeding visible vessel) and IIb (adherent clot) (Forrest Ia‐Ib‐IIa‐IIb).
Data collection and analysis
We used standard methodological procedures as expected by The Cochrane Collaboration. Meta‐analysis was undertaken using a random‐effects model; risk ratios (RRs) with 95% confidence intervals (CIs) are presented for dichotomous data.
Main results
Nineteen studies of 2033 initially randomly assigned participants were included, of which 11 used a second injected agent, five used a mechanical method (haemoclips) and three employed thermal methods.
The risk of further bleeding after initial haemostasis was lower in the combination therapy groups than in the epinephrine alone group, regardless of which second procedure was applied (RR 0.53, 95% CI 0.35 to 0.81). Adding any second procedure significantly reduced the overall bleeding rate (persistent and recurrent bleeding) (RR 0.57, 95% CI 0.43 to 0.76) and the need for emergency surgery (RR 0.68, 95% CI 0.50 to 0.93). Mortality rates were not significantly different when either method was applied.
Rebleeding in the 10 studies that scheduled a reendoscopy showed no difference between epinephrine and combined therapy; without second‐look endoscopy, a statistically significant difference was observed between epinephrine and epinephrine and any second endoscopic method, with fewer participants rebleeding in the combined therapy group (nine studies) (RR 0.32, 95% CI 0.21 to 0.48).
For ulcers of the Forrest Ia or Ib type (oozing or spurting), the addition of a second therapy significantly reduced the rebleeding rate (RR 0.66, 95% CI 0.49 to 0.88); this difference was not seen for type IIa (visible vessel) or type IIb (adherent clot) ulcers. Few procedure‐related adverse effects were reported, and this finding was not statistically significantly different between groups. Few adverse events occurred, and no statistically significant difference was noted between groups.
The addition of a second injected method reduced recurrent and persistent rebleeding rates and surgery rates in the combination therapy group, but these findings were not statistically significantly different. Significantly fewer participants died in the combined therapy group (RR 0.50, 95% CI 0.25 to 1.00).
Epinephrine and a second mechanical method decreased recurrent and persistent bleeding (RR 0.31, 95% CI 0.18 to 0.54) and the need for emergency surgery (RR 0.20, 95% CI 0.06 to 0.62) but did not affect mortality rates.
Epinephrine plus thermal methods decreased the rebleeding rate (RR 0.49, 95% CI 0.30 to 0.78) and the surgery rate (RR 0.20, 95% CI 0.06 to 0.62) but did not affect the mortality rate.
Our risk of bias estimates show that risk of bias was low, as, although the type of study did not allow a double‐blind trial, rebleeding, surgery and mortality were not dependent on subjective observation. Although some studies had limitations in their design or implementation, most were clear about important quality criteria, including randomisation and allocation concealment, sequence generation and blinding.
Authors' conclusions
Additional endoscopic treatment after epinephrine injection reduces further bleeding and the need for surgery in patients with high‐risk bleeding peptic ulcer. The main adverse events include risk of perforation and gastric wall necrosis, the rates of which were low in our included studies and favoured neither epinephrine therapy nor combination therapy. The main conclusion is that combined therapy seems to work better than epinephrine alone. However, we cannot conclude that a particular form of treatment is equal or superior to another.
Plain language summary
Epinephrine injection versus epinephrine injection and a second endoscopic method in high‐risk bleeding ulcers
Background
Peptic ulcers develop when the usual protective mechanism of the body breaks down and digestive juices produced in the stomach, intestines and digestive glands damage the lining of the stomach or duodenum. Non‐steroidal anti‐inflammatory drugs and a bacterium called Helicobacter pylori are common causes of ulcers.
When ulcers in the stomach and small intestine (duodenum) start to bleed extensively (haemorrhage), the bleeding can be life threatening and requires emergency treatment.
Patients undergo an endoscopy so clinicians can locate the source of bleeding. Active bleeding or non‐bleeding visible blood vessels at endoscopy are deemed 'high risk,' in that further bleeding may occur even if the initial haemorrhage can be stopped. Once the source of bleeding has been identified, endoscopic therapy reduces rebleeding rate, need for surgery and deaths. Endoscopic therapy consists of an agent to stop the bleeding, which is injected into the bleeding area; epinephrine (adrenaline) is the most popular agent. Experts disagree on the need for a second procedure such as bipolar electrocoagulation, heater probe, sclerosant or clips immediately after epinephrine; although it seems to reduce further bleeding, the effects of a second procedure on continuing illness (morbidity), surgery rates and death remain unclear.
Review question
In bleeding peptic ulcers, does a second endoscopic method reduce further bleeding, the need for emergency surgery and death rates?
Study characteristics
We performed an extensive search for randomised trials comparing epinephrine alone versus epinephrine plus a second method. We found 19 clinical trials involving 2033 randomly assigned participants
Key results
We found that adding a second procedure reduced the further bleeding rate and the need for emergency surgery, but the effect of this approach on death rates has not been proven. In conclusion, additional endoscopic treatment after epinephrine injection reduces further bleeding and the need for surgery in patients with high‐risk bleeding peptic ulcer.
Quality of the evidence
Our risk of bias estimates show that the overall quality of the included studies was moderate or high. Although some studies had limitations in their design or implementation, most were clear about important quality criteria including randomisation and allocation concealment, sequence generation and blinding. We rated the quality of evidence as moderate for most outcomes. Further research is likely to have an impact on our confidence in the estimate of effect and may change the conclusions of this review.
Summary of findings
Summary of findings for the main comparison. Epinephrine versus epinephrine and any second endoscopic method in high‐risk bleeding ulcers.
| Epinephrine versus epinephrine and any second endoscopic method in high‐risk bleeding ulcers | ||||||
| Patient or population: patients with high‐risk bleeding ulcers Settings: secondary care (hospital) Intervention: epinephrine versus epinephrine and any second endoscopic method | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Epinephrine versus epinephrine and any second endoscopic method | |||||
| Recurrent and persistent bleeding overall rates with or without second‐look endoscopy Rebleeding (persistent or recurrent bleeding) | Study population | RR 0.57 (0.43‐0.76) | 1926 (19 studies) | ⊕⊕⊕⊝ moderatea | Favours combined therapy | |
| 223 per 1000 | 127 per 1000 (96‐170) | |||||
| Moderate | ||||||
| 222 per 1000 | 127 per 1000 (95‐169) | |||||
| Surgery rate Number requiring emergency surgery | Study population | RR 0.68 (0.5‐0.93) | 1841 (18 studies) | ⊕⊕⊕⊝ moderateb | Favours combined therapy | |
| 106 per 1000 | 72 per 1000 (53‐99) | |||||
| Moderate | ||||||
| 102 per 1000 | 69 per 1000 (51‐95) | |||||
| Mortality rate Number of deaths (30‐day mortality or in‐hospital mortality) | Study population | RR 0.64 (0.39‐1.06) | 1841 (18 studies) | ⊕⊕⊕⊝ moderateb | Favours combined therapy | |
| 47 per 1000 | 30 per 1000 (18‐49) | |||||
| Moderate | ||||||
| 32 per 1000 | 20 per 1000 (12‐34) | |||||
| Adverse effects of endoscopy therapy Adverse effects | Study population | RR 1.25 (0.4‐3.96) | 1281 (12 studies) | ⊕⊕⊕⊝ moderateb | No statistically significant difference between groups | |
| 8 per 1000 | 10 per 1000 (3‐31) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0‐0) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aModerate statistical heterogeneity was present. bRelatively few events.
Background
Description of the condition
Peptic ulcers develop when the usual protective mechanism of the body breaks down and digestive juices produced in the stomach, intestines and digestive glands damage the lining of the stomach or duodenum. Non‐steroidal anti‐inflammatory drugs and Helicobacter pylori are the most common causes of peptic ulcer.
Bleeding peptic ulcer is a serious condition in which ulcers in the upper digestive system (stomach) and the small intestine (duodenum) start to bleed extensively (haemorrhage). The bleeding can be life threatening and requires emergency treatment.
Patients usually require an endoscopy during the first 24 hours after admission so clinicians can locate the source of bleeding (Cooper 1999). Patients with active bleeding or non‐bleeding visible vessels at endoscopy are deemed 'high risk,' as this finding predicts risk of further bleeding and guides management decisions. Once the source of bleeding has been identified, endoscopic therapy reduces rebleeding rate, need for surgery and morbidity and mortality among patients bleeding from a peptic ulcer (Cook 1992).
Endoscopic therapy should be provided to patients with stigmata of high risk (Laine 2012). Such high‐risk patients receive endoscopic therapy consisting of an agent to stop the bleeding, which is injected into the bleeding area. Injection of epinephrine is the most popular method used to stop bleeding. Second endoscopic methods may include bipolar electrocoagulation, heater probe, sclerosant or clips. At the time of publication of the first version of this review, some experts disagreed on the need for a second procedure immediately after epinephrine; although it seems to reduce further bleeding, the effects of a second procedure on continuing illness (morbidity), surgery rates and death (mortality) remain unclear.
Description of the intervention
Many different endoscopic haemostatic techniques have been developed and studied over the past 25 years. Methods are based on injection of vasoconstrictor substances (epinephrine), sclerosant substances (polidocanol, absolute alcohol), clotting factors (thrombin, fibrin glue) or adhesives (cyanoacrylate). Thermal therapies include laser, monopolar electrocoagulation, argon plasma coagulation, bipolar probes and heater probe. More recently, use of mechanical devices to clip the bleeding vessel (haemoclip) has been incorporated. Epinephrine injection—alone or in combination with another technique—has become the most popular endoscopic method for emergency endoscopic haemostasis because of its safety, low cost and easy application (Savides 2000).
Previous guidelines suggested that no clear evidence shows that any technique is superior to injection of epinephrine alone for the endoscopic treatment of high‐risk bleeding peptic ulcers (British Society 2002; Feu 2003; Laine 2009). However, some individual studies have reported a significant reduction in further bleeding rates with the addition of a second endoscopic treatment (Chung 1997; Garrido 2002; Kubba 1996; Lin 1999; Lo 2006). Although absolute improvements in haemostatic efficacy were relatively small (from 10% to 20%), they represent a 30% to 60% reduction in the relative risk of recurrent haemorrhage.
At the time of publication of the first version of this review, although some randomised studies had established that epinephrine in combination with a second haemostatic technique is better than epinephrine alone, guidelines available at the time did not provide clear recommendations on this point (British Society 2002; Feu 2003). The American Society for Gastrointestinal Endoscopy (ASGE) suggests that combination therapy is better than epinephrine alone for the treatment of peptic ulcer bleeding (ASGE 2004). After publication of the first version of our review in The Cochrane Library (Vergara 2007), further reviews recommended combined treatment for high‐risk peptic ulcer (Kovacs 2008; Peter 2008). Since then, updated guidelines for acute non‐variceal bleeding have been produced. The ASGE has recommended that if "epinephrine is used to treat peptic ulcer bleeding with high‐risk stigmata, a second endoscopic treatment modality (co‐aptive thermal device, sclerosants, thrombin/fibrin glue or clips) should also be used" (Hwang 2012). This is also stated in UK National Institute for Health and Care Excellence (NICE) guideline, which indicates that epinephrine should not be used alone for the treatment of bleeding, but it should be used in combination with a mechanical method, thermal coagulation or fibrin or thrombin (NICE 2012). Repeat endoscopy, with treatment as appropriate, should be considered for all patients at high risk of rebleeding, particularly if doubt exists about adequate haemostasis at the first endoscopy. The American College of Gastroenterology (AGA) Guideline of 2012 (Laine 2012) suggested that patients with acute upper gastrointestinal bleeding should undergo endoscopy within 24 hours of admission, with stigmata of recent haemorrhage recorded, as they predict risk of further bleeding (using the Forrest classification). Endoscopic therapy should be provided to patients with active spurting or oozing bleeding and to those with a non‐bleeding visible vessel. A strong recommendation with high‐quality evidence suggests that epinephrine therapy should be used in combination, as epinephrine monotherapy is less effective than other monotherapies in preventing further bleeding. The second treatments recommended include thermal therapy and injection of a sclerosant. Clips were recommended, as they appear to decrease further bleeding and the need for surgery, although the guidance does state that "comparisons of clips versus other therapies yield variable results and currently used clips have not been well studied." The guidance goes further and states that "for the subset of patients with actively bleeding ulcers, thermal therapy or epinephrine plus a second modality may be preferred over clips or sclerosant alone to achieve initial haemostasis." Routine second‐look endoscopy was not recommended in this guideline.
Why it is important to do this review
Although use of a second endoscopic procedure for the treatment of high‐risk bleeding peptic ulcers is now accepted, the addition of a second endoscopic technique can increase cost and risk of complications of the procedure; therefore we investigated whether a reduction in further bleeding actually offsets these drawbacks.
In this updated review, we wanted to ensure that we included all recently published studies of epinephrine injection versus epinephrine and a second endoscopic method for treatment of high‐risk bleeding ulcers.
Objectives
To determine whether a second procedure improves haemostatic efficacy or patient outcomes or both after epinephrine injection in adults with high‐risk bleeding ulcers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials.
Types of participants
Patients with high‐risk bleeding peptic ulcers, that is, patients with haemorrhage from peptic ulcer disease (gastric or duodenal) with major stigmata of bleeding as defined by Forrest classification: Ia (spurting haemorrhage), Ib (oozing haemorrhage), IIa (non‐bleeding visible vessel) and IIb (adherent clot) (Forrest Ia‐Ib‐IIa‐IIb) (Forrest 1974).
Types of interventions
Epinephrine injection versus epinephrine injection and a second endoscopic method.
Types of outcome measures
Primary outcomes
Rebleeding rates (persistent bleeding and recurrent bleeding) as confirmed by endoscopy and further clinically significant bleeding as defined according to the criteria established in each study.
Secondary outcomes
Surgery rate.
Mortality rate.
Adverse effects.
Search methods for identification of studies
Electronic searches
For the first published version of this review, we searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (which includes the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (UGPD) Trials Register) (2006, Issue 1).
MEDLINE (1966 to February 2006).
EMBASE (1980 to February 2006).
We also searched the reference lists of articles.
We contacted experts in the field.
We searched the following databases in 2009.
Cochrane Central Register of Controlled Trials (CENTRAL) (which includes the Cochrane UGPD Group Trials Register) (2009, Issue 1).
MEDLINE (2006 to September 2009).
EMBASE (2006 to September 2009).
For our update in 2014, we searched the following versions of the databases, limited from June 2009 to May 2014.
Ovid MEDLINE(R) 1946 to May Week 2 2014 (Appendix 1).
Ovid MEDLINE(R) Daily Update May 22, 2014.
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations May 22, 2014.
EBM Reviews—Cochrane Central Register of Controlled Trials April 2014 (Appendix 2).
EMBASE 1980 to Week 20 2014 (Appendix 3).
The search strategy for this review was constructed by using a combination of medical subject headings (MeSH) and text words related to the use of epinephrine injection alone and epinephrine injection with a secondary endoscopic therapy for the treatment of bleeding peptic ulcers. Articles published in any language were included.
To identify RCTs, the search strategy in Appendix 1 (MEDLINE) was combined with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Searching other resources
We handsearched reference lists from trials selected by electronic searching to identify further relevant trials and published abstracts from conference proceedings from United European Gastroenterology Week (published in Gut) and Digestive Disease Week (published in Gastroenterology).
We also contacted members of the Cochrane UGPD Group and experts in the field to provide details of outstanding clinical trials and relevant unpublished materials.
Data collection and analysis
Selection of studies
Two review authors separately evaluated potentially included studies, that is, studies designed to compare the efficacy of different endoscopic methods to achieve definitive haemostasis in patients with peptic ulcer.
Data extraction and management
Two review authors (XC and MV) extracted data, which were reviewed by a third review author (JPG). When results were discordant, papers were jointly reviewed until differences were resolved. For this update, CB and MV reviewed updated search results, selected new trials for inclusion, extracted data separately and conferred over discrepancies until consensus was achieved. CB and MV updated the text of the review.
Assessment of risk of bias in included studies
In this updated version of the review, we introduced the risk of bias table to assess study quality. Consistent with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), this version of the review incorporates additional elements into 'Risk of bias' tables that were not included in the previous published review. Two review authors (MV with XC, MV with CB) independently assessed study quality. Discrepancies in interpretation were resolved by consensus.
Measures of treatment effect
Risk ratios (RRs) and 95% confidence intervals (CIs) were used in comparisons. We used a random‐effects model for analyses. We performed subanalysis for further bleeding to examine the efficacy of different techniques (sclerosant agents, mechanical haemostasis and thermal devices) associated with epinephrine injection versus epinephrine alone.
Unit of analysis issues
Randomisation of clusters can result in overestimation of the precision of results (with higher risk of a Type I error) when their use has not been compensated for in the analysis. None of the included studies employed cluster randomisation. For studies that included more than one active intervention group and only one control group, we selected the interventions that most closely matched our inclusion criteria and excluded the others (Chapter 16.5.4, Higgins 2011).
Dealing with missing data
When data were not available in published trial reports, or when clarification was needed, we contacted trial investigators to request missing information.
Assessment of heterogeneity
The extent to which variations were noted in methods, populations, interventions or outcomes was assessed. Consistency of results was assessed by visual inspection of the forest plot and by examination of I2 (Higgins 2002), a quantity that describes the approximate proportion of variation in point estimates that is due to heterogeneity rather than to sampling error.
Some clinical heterogeneity was noted across the included studies (see Description of studies), as was some statistical heterogeneity for outcomes for which it was possible to combine study data. Quantitative syntheses of the data therefore were undertaken using a random‐effects model.
Data synthesis
Main comparisons contrasted epinephrine injection versus epinephrine injection plus another haemostatic method. The primary outcome variable was further bleeding, defined as persistence or recurrence of bleeding during follow‐up. We analysed emergency surgery during hospitalisation and morbidity and mortality rates. All results were obtained using Review Manager 5 (RevMan 2014). The statistical tests and formulae implemented in RevMan are described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Subanalysis was also performed according to the type of peptic ulcer haemorrhage observed: active spurting or oozing (Forrest Ia or Ib), non‐bleeding visible vessel (Forrest IIa) or adherent clot (Forrest IIb). We split further bleeding rates into failure to achieve initial haemostasis and recurrence during follow‐up and analysed the data separately. Finally, to ascertain the influence of second‐look endoscopy on the results, we analysed separately studies that performed this procedure. We also conducted separate analyses depending on the second type of endoscopic technique associated with epinephrine (thermal, sclerosant or mechanical).
Results
Description of studies
Results of the search
Previous searches in 2006 and 2009 identified 18 included studies and 13 excluded studies.The updated search in May 2014 identified 116 new citations (MEDLINE 45, EMBASE 83, CENTRAL 19); eight potentially relevant records were obtained and scrutinised; seven of these reports did not meet the inclusion criteria (see Characteristics of excluded studies) and had to be excluded. One new trial met the inclusion criteria and was included (Figure 1). Review authors identified a total of 19 included studies and 20 excluded studies.
1.
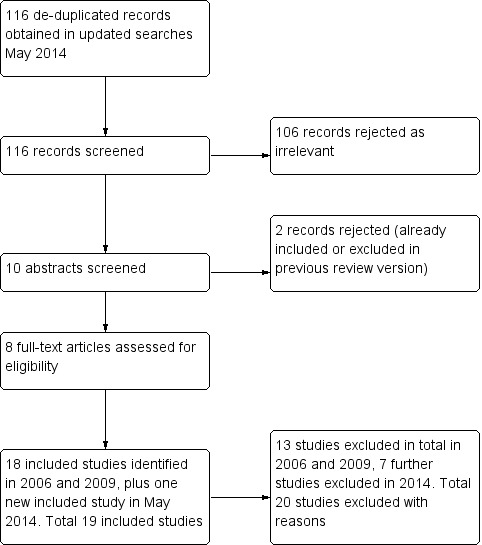
Study flow diagram.
Included studies
For this update, we included one new study (Grgov 2012). Nineteen articles compared epinephrine injection versus epinephrine plus any other endoscopic method for the endoscopic treatment of bleeding peptic ulcers (Balanzo 1990; Lo 2006; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Garrido 2002; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Loizou 1991; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993; Villanueva 1996). A total of 2033 initially randomly assigned participants were included. Grgov 2012 was published in Serbo‐Croatian, and Garrido 2002 in Spanish. Two studies were published as abstracts only (Lee 1997; Villanueva 1996).
Design
All included studies were RCTs, as specified in our inclusion criteria. Two studies were published as abstracts (not as papers). Sixteen studies used a two‐arm trial design (Balanzo 1990; Lo 2006; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Garrido 2002; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Loizou 1991; Park 2004; Pescatore 2002; Villanueva 1993; Villanueva 1996); only three studies (Chung 1999; Lin 1999; Sollano 1991) used a three‐arm trial design.
Participants
Characteristics of the participants in each study were recorded, including numbers of participants, age and gender of participants, percentage of duodenal and gastric ulcers and Forrest type.
The number randomly assigned varied from 276 participants in Chung 1997 to 42 participants in Loizou 1991 (the smallest trial). Eleven studies initially randomly assigned fewer than 100 participants (Balanzo 1990; Garrido 2002; Grgov 2012; Lee 1997; Lin 1993; Lin 1999; Loizou 1991; Park 2004; Sollano 1991; Villanueva 1993; Villanueva 1996).
Study participants were adults with a mean age greater than 54 years. Age was not reported in Lee 1997, Sollano 1991 or Villanueva 1996. A predominance of male participants was noted in all included studies. Lee 1997, Sollano 1991 and Villanueva 1996 did not report the male‐to‐female ratio. More duodenal than gastric ulcers were included in the trials that reported this characteristic; Lee 1997 and Villanueva 1996 did not report this characteristic.
The Forrest classification of type of bleeding group was reported in all studies, but only 13 studies (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Garrido 2002; Kubba 1996; Lin 1993; Loizou 1991; Park 2004; Pescatore 2002; Villanueva 1993) reported ulcers categorised by type of bleeding.
Further details of each study can be found in Characteristics of included studies.
Interventions
We recorded any dosage of epinephrine and second endoscopic treatment applied. Medical treatments given as adjuncts were different in each study or were not specified. However, the best treatment for haemorrhage from peptic ulcer is the endoscopic treatment that we analysed in the meta‐analysis.
Twelve studies compared epinephrine versus epinephrine plus a second injected agent. The second injected agents were thrombin in Balanzo 1990 and Kubba 1996; fibrin glue in Pescatore 2002; ethanolamine in Choudari 1994; sodium tetradecyl sulphate in Chung 1993; ethanol in Chung 1996, Lee 1997 and Lin 1993; polidocanol in Garrido 2002, Villanueva 1993 and Villanueva 1996; and ethoxy sclerol in Sollano 1991.
Three studies compared epinephrine versus epinephrine plus heat: Chung 1997 used a heat probe, Lin 1999 used bipolar electrocoagulation and Loizou 1991 employed neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser photocoagulation. The remaining four studies used a mechanical method such as haemoclips (Lo 2006; Chung 1999; Grgov 2012) or band ligation (Park 2004). The dosage of epinephrine and the method of injection used were stated in the study reports. Two studies used the same dosage of epinephrine in both groups—epinephrine alone or combined treatment—independently of whether the haemorrhage stopped (7.5 mL Choudari 1994; 10 mL Loizou 1991). Another study (Lo 2006) injected epinephrine until haemostasis or to a maximum dose of 20 mL of epinephrine in both groups, independently of achieving haemostasis. The remaining included studies investigated the use of injected epinephrine until haemostasis was achieved. Seven included studies did not specify the dosage (Balanzo 1990; Chung 1993; Chung 1996; Chung 1999; Garrido 2002; Park 2004; Villanueva 1996). In the remaining studies (Chung 1997; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Pescatore 2002; Sollano 1991; Villanueva 1993), the volume of epinephrine used was reported (Characteristics of included studies).
Ten studies performed one or more scheduled second‐look endoscopies 24 to 72 hours after the initial technique was applied (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993) (n = 1203). When active bleeding or persistent high‐risk stigmata (Forrest Ia‐Ib‐IIa‐IIb) were observed, a second therapeutic procedure was performed. Nine studies did not schedule second‐look endoscopy (Lo 2006; Garrido 2002; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Loizou 1991; Park 2004; Villanueva 1996).
Outcomes
Primary outcome
Persistent and recurrent bleeding
We used the following terms: Persistent bleeding occurs when no initial haemostasis was achieved, and recurrent bleeding is a new bleed after initial haemostasis; in trials in which all participants had successful initial haemostasis, the number relates only to the number with recurrent bleeding (as persistent bleeding cannot occur if initial haemostasis is successful).
In most studies, the primary endpoint was defined as endoscopic therapy failure, that is, a combination of persistent haemorrhage and recurrence during follow‐up (further bleeding). Clinical criteria used for presuming further bleeding differed between studies (Characteristics of included studies). Recurrent bleeding in the outcome 'with second‐look endoscopy' was confirmed endoscopically, and 10 studies reported this outcome (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Lin 1993; Pescatore 2002; Sollano 1991; Villanueva 1993); otherwise rebleeding rates included both endoscopic and clinically evident rebleeding. Analyses were also performed on included studies that reported data for persistent haemorrhage and recurrence separately during follow‐up.
Fourteen studies reported initial failure of haemostasis and recurrent bleeding separately (Balanzo 1990; Lo 2006; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Lee 1997; Lin 1993; Lin 1999; Loizou 1991; Park 2004; Sollano 1991; Villanueva 1993). The remaining five studies did not distinguish between persistent and recurrent bleeding (Choudari 1994; Garrido 2002; Kubba 1996; Pescatore 2002; Villanueva 1996).
Fourteen studies randomly assigned participants who reported bleeding rates when peptic ulcers were actively bleeding (spurting Forrest Ia or oozing Forrest Ib) (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Garrido 2002; Kubba 1996; Lee 1997; Lin 1993; Loizou 1991; Park 2004; Pescatore 2002; Villanueva 1993).
Nine studies with a total of 448 participants provided data on peptic ulcers with a non‐bleeding visible vessel (Forrest IIa) (Balanzo 1990; Choudari 1994; Chung 1993; Garrido 2002; Kubba 1996; Loizou 1991; Park 2004; Pescatore 2002; Villanueva 1993). Only four studies (Lo 2006; Garrido 2002; Grgov 2012; Pescatore 2002) included participants with adherent clot (Forrest IIb), although Lo 2006 and Grgov 2012 did not report results according to Forrest category, and therefore results from only 30 participants appear in our analyses.
Secondary outcomes
Surgery and mortality
Criteria for emergency surgery were not specified in most studies. Thirty‐day mortality and in‐hospital mortality were the criteria used most often for defined mortality (Characteristics of included studies).
The need for emergency surgery was evaluated in 18 studies (1841 participants) (Balanzo 1990; Lo 2006; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Loizou 1991; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993; Villanueva 1996). Garrido 2002 did not specify surgery rates in each group, only total rate.
The criteria for mortality rate were also different between studies. Some studies used hospital mortality; others used 30‐day mortality or did not specify the criteria for mortality. The mortality rate was evaluated in 18 studies (Balanzo 1990; Lo 2006; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Loizou 1991; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993; Villanueva 1996); only Garrido 2002 did not report mortality.
In addition, 12 studies reported complications that occurred in the study population (Lo 2006; Chung 1993; Chung 1997; Chung 1999; Grgov 2012; Kubba 1996; Lin 1993; Lin 1999; Loizou 1991; Pescatore 2002; Sollano 1991; Villanueva 1993).
Excluded studies
A total of 20 studies were excluded from the review; 13 were excluded before this update was performed (Buffoli 2001; Chua 2001; Chung 1990; Chung 1992; Chung 1997a; De Goede 1998; Dedeu 2003; Ell 2002; Gevers 2002; Male 1999; Pescatore 1999; Sabat 1998; Wehrmann 1994), and we excluded a further seven studies in 2014 (Chittmittrapap 2010; Grgov 2013; Karaman 2011; Lecleire 2009; Ljubicic 2012; Taghavi 2009; Tsoi 2009). The main reasons for exclusion of studies were as follows: non‐randomised studies (Buffoli 2001; Chittmittrapap 2010; Chua 2001; Tsoi 2009); preliminary results published later in another article (Chung 1990; Chung 1992; Chung 1997a; De Goede 1998; Male 1999; Pescatore 1999); impossible to extract data (Dedeu 2003; Ell 2002); did not fit our criteria for intervention, that is, did not compare epinephrine alone versus epinephrine plus a second agent (Gevers 2002; Grgov 2013; Karaman 2011; Ljubicic 2012; Taghavi 2009); participants did not fit our inclusion criteria (Lecleire 2009); or important methodological problems were noted within the study (Sabat 1998; Wehrmann 1994) (see Characteristics of excluded studies).
Risk of bias in included studies
A summary of the risk of bias across studies is given in Figure 2 and Figure 3.
2.
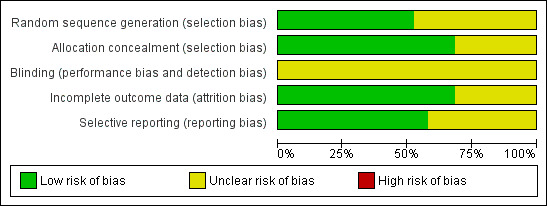
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
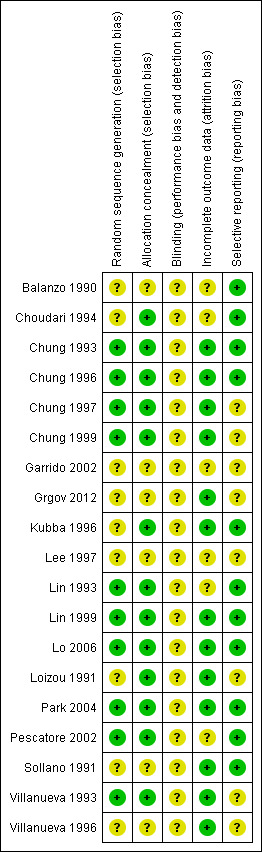
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was adequately described in only 10 of the included studies (Lo 2006; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Lin 1993; Lin 1999; Park 2004; Pescatore 2002; Villanueva 1993) and was judged as unclear in the remaining studies. Allocation concealment was adequate in 13 studies and was judged as unclear in six studies (Balanzo 1990; Garrido 2002; Grgov 2012; Lee 1997; Sollano 1991; Villanueva 1996).
Blinding
The studies were not double blind because the endoscopist must know the technique to be applied.
Incomplete outcome data
Only six studies were judged as unclear for incomplete outcome data (Balanzo 1990; Choudari 1994; Garrido 2002; Lee 1997; Lin 1993; Pescatore 2002); all remaining studies were judged as having low risk of bias.
Selective reporting
A total of 11 studies were rated as having low risk of bias for selected outcome reporting (Balanzo 1990; Lo 2006; Choudari 1994; Chung 1993; Chung 1996; Kubba 1996; Lin 1993; Lin 1999; Park 2004; Pescatore 2002; Sollano 1991). The remaining studies were rated as having unclear risk of bias. However in the absence of initial study protocols, it is not clear whether all studies reported outcomes as prespecified in the trial protocol.
Other potential sources of bias
Other potential sources of bias were noted in the design of studies in terms of interventions used and outcomes assessed. The different treatment strategies used could bias results, as some may appear more favourable in terms of achieving haemostasis, such as the technique used by Lo 2006, in which epinephrine was injected until haemostasis was achieved or until a maximum dose of 20 mL of epinephrine was given in both groups, independently of achieving haemostasis. It is difficult, however, to establish whether this method could decrease the frequency of recurrence.
Some studies used different definitions of haemorrhage. However, all studies included in the meta‐analysis that reported rebleeding criteria were very similar, suggesting that the definition of rebleeding was not a source of bias. As explained, outcome criteria in all studies were very similar, so we believe that these data did not bias study results.
The mortality rate was also different between studies using different criteria. Some studies used hospital mortality, while others used 30‐day mortality or did not specify the criteria for mortality. This made comparison of mortality rates difficult and hindered conclusions about the rate of mortality resulting from haemorrhage in people with ulcers. Moreover, data on previous morbidity among study participants often were not provided in study reports.
Effects of interventions
See: Table 1
We use the following terms: Persistent bleeding occurs when no initial haemostasis is achieved, and recurrent bleeding is a new bleed after initial haemostasis. In trials in which all had successful haemostasis, the number relates only to the number with recurrent bleeding (as bleeding cannot be persistent if the initial haemostasis is successful).
In the text below, an I² statistical value for heterogeneity is reported as follows: 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: shows considerable heterogeneity.
Numbers given show the total numbers of participants in the analysis. When it was possible to calculate an effect size, these values were reported with 95% confidence intervals. When the calculated effect size was statistically significant (P value < 0.05), we stated whether the result favours the intervention or the control condition.
We have summarised results below under headings corresponding to the primary and secondary outcomes outlined in the section entitled Types of outcome measures.
COMPARISON 1. Epinephrine versus epinephrine and any second endoscopic method
Initial failure of haemostasis (persistent bleeding)
For this outcome, we found 14 relevant trials that provided separate data on participants who did not achieve initial haemostasis (Balanzo 1990; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Lee 1997; Lin 1993; Lin 1999; Lo 2006; Loizou 1991; Park 2004; Sollano 1991; Villanueva 1993) (n = 1380). No significant difference was noted between epinephrine and epinephrine and any second endoscopic method (RR random 0.77, 95% CI 0.42 to 1.4; Analysis 1.1).
1.1. Analysis.
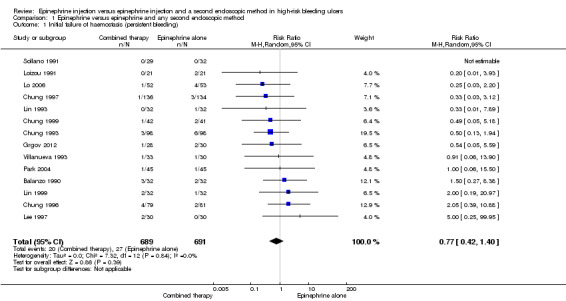
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 1 Initial failure of haemostasis (persistent bleeding).
Recurrent bleeding only (bleeding after initial haemostasis)
For this outcome, we found 14 relevant trials (Balanzo 1990; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Lee 1997; Lin 1993; Lin 1999; Lo 2006; Loizou 1991; Park 2004; Sollano 1991; Villanueva 1993) (n = 1333). A statistically significant difference was reported between epinephrine and epinephrine and any second endoscopic method favouring combined therapy (RR random0.53, 95% CI 0.35 to 0.81, P value 0.03; Analysis 1.2). This outcome had moderate levels of heterogeneity (Chi2 = 21.92, df = 13, P value 0.06, I2 = 41%).
1.2. Analysis.
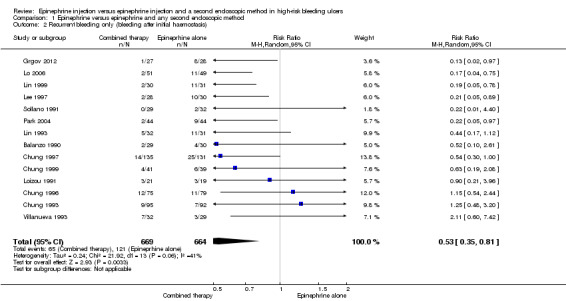
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 2 Recurrent bleeding only (bleeding after initial haemostasis).
Recurrrent and persistent bleeding overall rates with or without second‐look endoscopy
All articles that compared substances administered by endoscopic injection (sclerosants such as ethanol, polidocanol, ethanolamine or tetradecyl sulphate; adhesive agents such as cyanoacrylate; and thrombotic substances such as fibrin glue or thrombin), epinephrine plus thermal agents and epinephrine plus a mechanical method such as clips were analysed together. All studies reported this outcome, that is, clinically diagnosed and/or endoscopically confirmed rebleeding (19 relevant trials; Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Garrido 2002; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Lo 2006; Loizou 1991; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993; Villanueva 1996) (n = 1926). A statistically significant difference between epinephrine and epinephrine and any second endoscopic method favoured combined therapy (RR random 0.57, 95% CI 0.43 to 0.76, P value 0.0001; Analysis 1.3). This outcome had moderate levels of heterogeneity (Chi2 = 29.11, df = 18, P value 0.05, I2 = 38%).
1.3. Analysis.
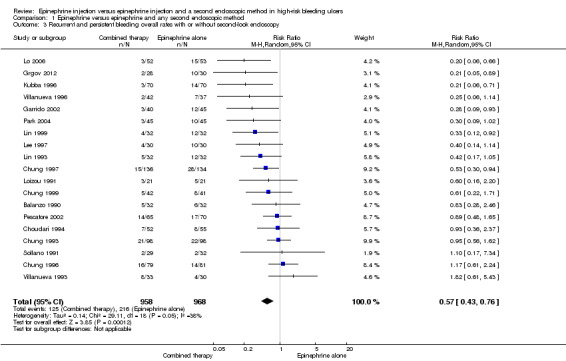
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 3 Recurrent and persistent bleeding overall rates with or without second‐look endoscopy.
Recurrent and persistent bleeding and second‐look endoscopy
With second‐look endoscopy (endoscopically confirmed persistent or recurrent bleeding)
Ten studies performed one or more scheduled second‐look endoscopies 24 to 72 hours after the initial technique was applied (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993) (n = 1203). When active bleeding or persistent high‐risk stigmata (Forrest Ia‐Ib‐IIa‐IIb) were observed endoscopically, a second therapeutic procedure was performed. No significant difference was noted between the numbers with endoscopically confirmed rebleeding in the epinephrine group and in the epinephrine and second endoscopic method groups (RR 0.82, 95% CI 0.64 to 1.06; Analysis 1.4).
1.4. Analysis.
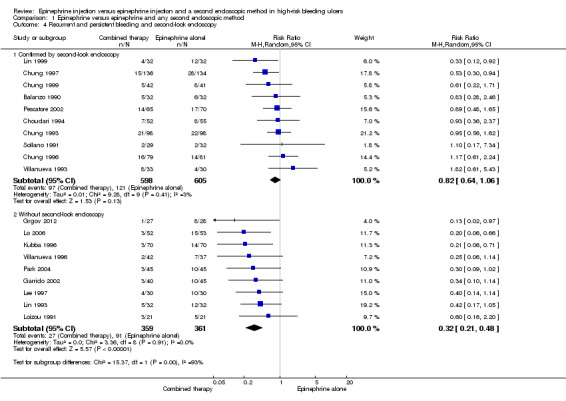
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 4 Recurrent and persistent bleeding and second‐look endoscopy.
Without second‐look endoscopy
Nine studies did not schedule second‐look endoscopy (Lo 2006; Garrido 2002; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Loizou 1991; Park 2004; Villanueva 1996) (n = 720). A statistically significant difference in the numbers of participants with rebleeding (clinically diagnosed and endoscopically confirmed) between epinephrine and epinephrine and any second endoscopic method favoured combined therapy (RR random 0.32, 95% CI 0.21 to 0.48, P value < 0.00001; Analysis 1.4).
Surgery rate
For this outcome, we found 18 relevant trials that reported the numbers of participants who needed surgical intervention on an emergency basis because initial haemostasis could not be obtained, or because rebleeding occurred (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Lo 2006; Loizou 1991; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993; Villanueva 1996) (n = 1841). A statistically significant difference between epinephrine and epinephrine and any second endoscopic method favoured combined therapy (RR random 0.68 95% CI 0.50 to 0.93, P value 0.02; Analysis 1.5).
1.5. Analysis.
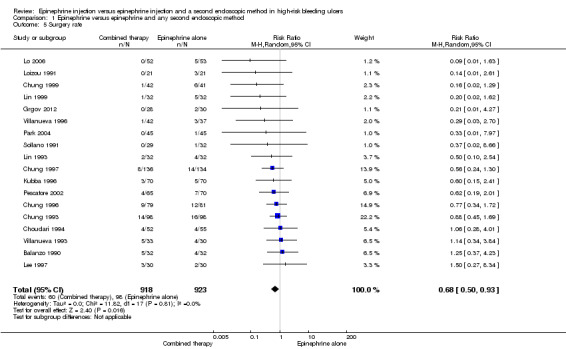
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 5 Surgery rate.
Mortality rate
For this outcome, we found 18 relevant trials reporting mortality as death in hospital or as a result of bleeding peptic ulcer (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012; Kubba 1996; Lee 1997; Lin 1993; Lin 1999; Lo 2006; Loizou 1991; Park 2004; Pescatore 2002; Sollano 1991; Villanueva 1993; Villanueva 1996) (n = 1841). No significant difference was noted between epinephrine and epinephrine and any second endoscopic method (RR random 0.64, 95% CI 0.39 to 1.06; Analysis 1.6).
1.6. Analysis.
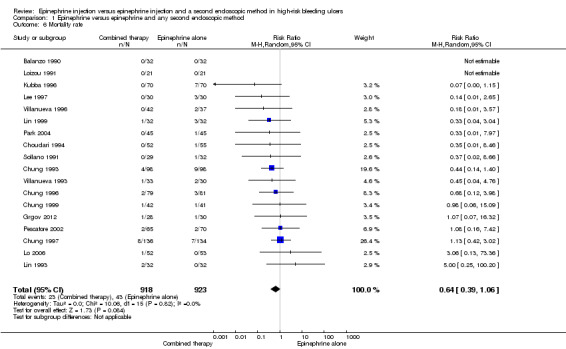
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 6 Mortality rate.
Adverse effects of endoscopic therapy
For this outcome, we found 12 relevant trials that reported whether complications had occurred and the types of complications reported (Chung 1993; Chung 1996; Chung 1997; Chung 1999; Grgov 2012, Kubba 1996; Lin 1993; Lin 1999; Lo 2006; Pescatore 2002; Sollano 1991; Villanueva 1993) (n = 1281). No significant difference was noted between epinephrine and epinephrine and any second endoscopic method (RR random 1.25, 95% CI 0.4 to 3.96; Analysis 1.7). Details of adverse effects encountered (found in Table 2) included perforations, mucosal injury and necrosis. Induction of massive bleeding requiring surgery was more frequent in the epinephrine only group (n = 5); necrosis appeared in three participants (two in the combined therapy group and one in the epinephrine alone group), and perforation (three participants) was observed in the combined therapy group. Thus, adverse events were slightly more frequent in the combined therapy group (6/610 participants) than in the epinephrine alone group (5/648 participants), although this difference did not reach statistical significance.
1.7. Analysis.
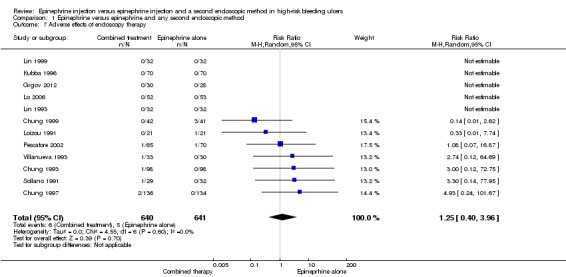
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 7 Adverse effects of endoscopy therapy.
1. Procedure‐related adverse effects.
| Study ID | Adverse effects in epinephrine plus second agent group (combined therapy) | Adverse effects in epinephrine group |
| Lo 2006 | None | None |
| Chung 1993 | Quote, page 613: "A 75‐year‐old man in the epinephrine plus STD group had abdominal pain after injection therapy. An actively bleeding pre‐pyloric ulcer had been injected with 8 ml of epinephrine followed by 1 ml of STD. Signs of upper abdominal peritonitis developed 36 hours later. At operation extensive infarction and necrosis were seen along the lesser curvature of the stomach. The right gastric artery was cord‐like and thrombosed along its course. Extensive coagulative necrosis was observed in the arteries in the muscularis propria. It is likely that the STD was injected directly into the right gastric artery and travelled in the artery along the lesser curvature. It caused coagulative necrosis in the smaller arteries and necrosis along the lesser curvature of the stomach. Fortunately, the patient made an uneventful recovery after a subtotal gastrectomy" | No untoward cardiovascular events were observed during endoscopic injection therapy in either group. |
| Chung 1997 | Two participants underwent surgery for perforations related to heat probe application | None |
| Chung 1999 | No 'notable' complications were reported in the haemoclip and combined therapy groups | Three participants. In 1 case, bleeding was aggravated during treatment of a non‐bleeding visible vessel, and surgical intervention for control of bleeding ultimately was required. In the other 2 cases, submucosal haematoma developed |
| Grgov 2012 | None | None |
| Kubba 1996 | None | None |
| Lin 1993 | None | None |
| Lin 1999 | None | None |
| Loizou 1991 | None | Text states no procedure‐related complications, but in 1 participant in the epinephrine group, injection provoked spurting haemorrhage |
| Pescatore 2002 | Perforation (leading to surgical intervention) (n = 1) Non–procedure‐related complications: pneumonia (n = 1) |
Ulcer haemorrhage (n = 1 patient in group E) induced by epinephrine injection that led to surgical intervention. Non–procedure‐related complications were reported: pneumonia (n = 3); stroke (n = 1) |
| Sollano 1991 | Mucosal injury or necrosis (n = 1) | Mucosal injury or necrosis (n = 1) |
| Villanueva 1993 | Size of ulcer increased 5‐fold after infection, developed pneumoperitoneum, resolved spontaneously | None |
Recurrent and persistent bleeding according to type of haemorrhage
Forrest Ia‐Ib (spurting or oozing haemorrhage)
Thirteen studies with a total of 1019 participants reported bleeding rates when peptic ulcers were actively bleeding (spurting Forrest Ia; oozing Forrest Ib) We included participants who had confirmed bleeding by endoscopy and those who clinically rebled in Analysis 1.8) (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Chung 1997; Chung 1999; Garrido 2002; Kubba 1996; Lin 1993; Loizou 1991; Park 2004; Pescatore 2002; Villanueva 1993 (n = 1019). A statistically significant difference favoured the combined therapy group (RR 0.66, 95% CI 0.49 to 0.88, P value 0.005, I2 = 15%)
1.8. Analysis.
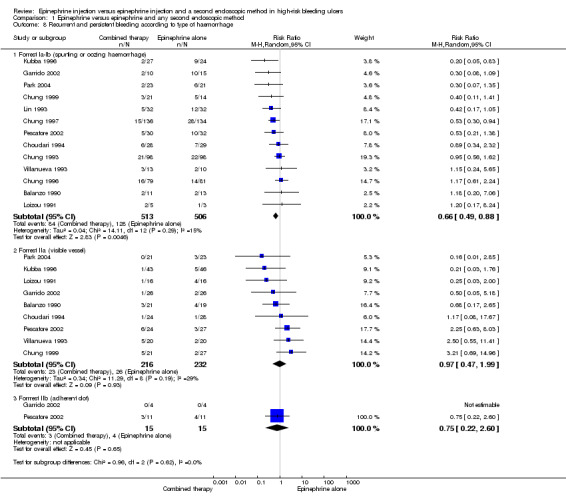
Comparison 1 Epinephrine versus epinephrine and any second endoscopic method, Outcome 8 Recurrent and persistent bleeding according to type of haemorrhage.
Forrest IIa (visible vessel)
Nine studies with a total of 440 participants provided data on peptic ulcers with a non‐bleeding visible vessel (Forrest IIa) (Balanzo 1990; Choudari 1994; Chung 1993; Garrido 2002; Kubba 1996; Loizou 1991; Park 2004; Pescatore 2002; Villanueva 1993). No significant difference was reported between epinephrine and epinephrine and any second endoscopic method (RR 0.97, 95% CI 0.47 to 1.99, I2 = 29%; Analysis 1.8).
Forrrest IIb (adherent clot)
Only four studies (Lo 2006; Garrido 2002; Grgov 2012; Pescatore 2002) included participants with adherent clot (Forrest IIb). Lo 2006and Grgov 2012 did not provide results on different Forrest groups included in the study and so were not included in this analysis. In the epinephrine alone group, 4/15 (26.7%) presented further bleeding versus 3/15 (20%) in the combined therapy group. In this subgroup, we found two relevant trials (n = 30). No significant difference between epinephrine and epinephrine and any second endoscopic method was noted (RR random 0.75, 95% CI 0.22 to 2.6; Analysis 1.8).
COMPARISON 2. Epinephrine plus epinephrine and a second injected agent
Recurrent and persistent bleeding
For this outcome, we found 11 RCTs (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Garrido 2002; Kubba 1996; Lee 1997; Lin 1993; Pescatore 2002; Sollano 1991; Villanueva 1993) (n = 1135); no statistically significant difference favoured combination therapy (RR 0.74, 95% CI 0.53 to 1.04, P value 0.08; Analysis 2.2). This outcome had moderate levels of heterogeneity (Chi2 = 15.66, df = 10, P value 0.11, I2 = 36%).
2.2. Analysis.
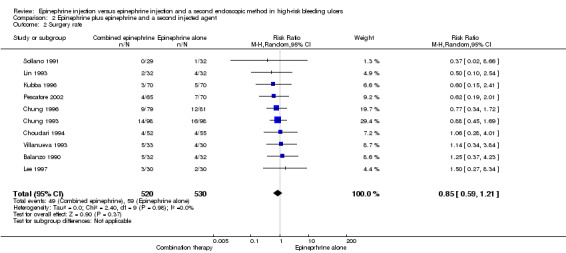
Comparison 2 Epinephrine plus epinephrine and a second injected agent, Outcome 2 Surgery rate.
Surgery rate
For this outcome, we found 10 RCTs (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Kubba 1996; Lee 1997; Lin 1993; Pescatore 2002; Sollano 1991; Villanueva 1993) (n = 1050) (RR 0.85, 95% CI 0.59 to 1.21; Analysis 2.2). No significant differences were noted between epinephrine and epinephrine and any second injected agent.
Mortality rate
For this outcome, we found 10 RCTs (Balanzo 1990; Choudari 1994; Chung 1993; Chung 1996; Kubba 1996; Lee 1997; Lin 1993; Pescatore 2002; Sollano 1991; Villanueva 1993) (n = 1050) (RR 0.5, 95% CI 0.25 to 1.0; Analysis 2.3). A statistically significant difference was reported between epinephrine and epinephrine and any second injected agent.
2.3. Analysis.
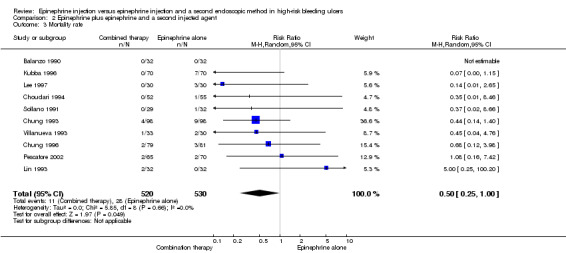
Comparison 2 Epinephrine plus epinephrine and a second injected agent, Outcome 3 Mortality rate.
COMPARISON 3. Epinephrine versus epinephrine and mechanical endoscopic methods
Recurrrent and persistent bleeding
For this outcome, we found five relevant trials that reported the efficacy of adding a mechanical haemoclip to epinephrine injection (Chung 1999; Grgov 2012; Lo 2006; Park 2004; Villanueva 1996) (n = 415). A statistically significant difference between epinephrine and epinephrine and mechanical endoscopic methods favoured epinephrine plus a mechanical method (RR random 0.31, 95% CI 0.18 to 0.55, P value < 0.0001; Analysis 3.1).
3.1. Analysis.
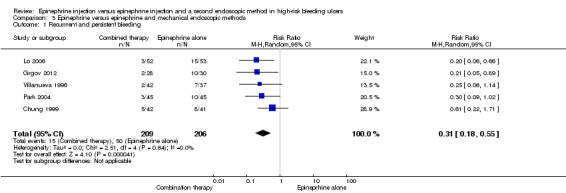
Comparison 3 Epinephrine versus epinephrine and mechanical endoscopic methods, Outcome 1 Recurrrent and persistent bleeding.
Surgery rate
Five RCTs provided data on surgery rate (Chung 1999; Grgov 2012; Lo 2006; Park 2004; Villanueva 1996) (n = 415). A statistically significant difference between epinephrine and epinephrine and mechanical endoscopic methods favoured epinephrine plus a mechanical method (RR random 0.2, 95% CI 0.06 to 0.62, P value 0.005; Analysis 3.2).
3.2. Analysis.
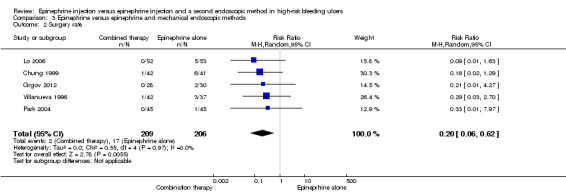
Comparison 3 Epinephrine versus epinephrine and mechanical endoscopic methods, Outcome 2 Surgery rate.
Mortality rate
Mortality rate was provided in five RCTs (Chung 1999; Grgov 2012; Lo 2006; Park 2004; Villanueva 1996) (n = 415). No significant difference was noted between epinephrine and epinephrine and mechanical endoscopic methods (RR random 0.73, 95% CI 0.19 to 2.71; Analysis 3.3).
3.3. Analysis.
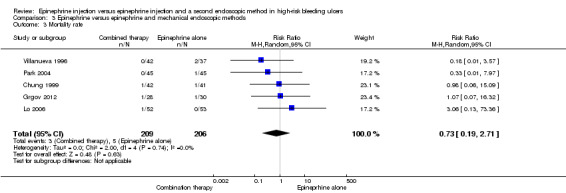
Comparison 3 Epinephrine versus epinephrine and mechanical endoscopic methods, Outcome 3 Mortality rate.
COMPARISON 4. Epinephrine plus epinephrine and thermal methods
Recurrent and persistent bleeding
Three studies compared epinephrine alone versus epinephrine combined with thermal haemostatic methods (contact heat probe, Nd:YAG laser or bipolar electrocoagulation) (Chung 1997; Lin 1999; Loizou 1991) (n = 376) (RR random 0.49, 95% CI 0.30 to 0.78, P value 0.003; Analysis 4.1). A statistically significant difference was observed between epinephrine and epinephrine and thermal endoscopic methods.
4.1. Analysis.
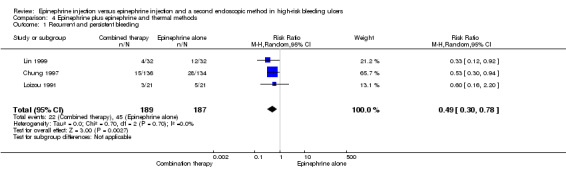
Comparison 4 Epinephrine plus epinephrine and thermal methods, Outcome 1 Recurrent and persistent bleeding.
Surgery rate
Surgery rate was given in three trials (Chung 1997; Lin 1999; Loizou 1991) (n = 376), A statistically significant difference between epinephrine and epinephrine and thermal endoscopic methods favoured the combination therapy group (RR random 0.45, 95% CI 0.21 to 0.95, P value 0.04; Analysis 4.2).
4.2. Analysis.
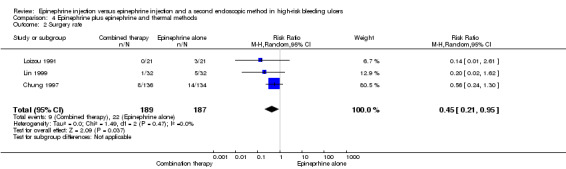
Comparison 4 Epinephrine plus epinephrine and thermal methods, Outcome 2 Surgery rate.
Mortality rate
For this outcome, we found three relevant trials (Chung 1997; Lin 1999; Loizou 1991) (n = 376) (RR random 0.92, 95% CI 0.37 to 2.26; Analysis 4.3). No significant difference was observed between epinephrine and epinephrine and thermal endoscopic methods.
4.3. Analysis.
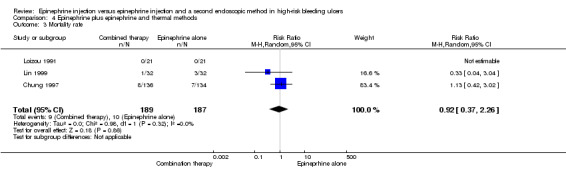
Comparison 4 Epinephrine plus epinephrine and thermal methods, Outcome 3 Mortality rate.
Discussion
Summary of main results
Nineteen studies including a total of 2033 initially randomly assigned participants were included, of which 11 used a second injected agent, five used a mechanical method (haemoclips) and three employed thermal methods.
For the comparison of epinephrine versus any second agent, we produced meta‐analyses for eight outcomes. Few participants failed initial haemostasis, and analyses of epinephrine versus epinephrine plus any second method failed to show differences in achieving initial haemostasis between epinephrine alone and epinephrine with a second endoscopic method.
Adding any second procedure significantly reduced rebleeding rates in three analyses, that is, fewer participants in the combined therapies groups experienced recurrent bleeding after initial haemostasis (RR 0.53, 95% CI 0.35 to 0.81); fewer participants had persistent and recurrent bleeding (with or without second‐look endoscopy) (RR 0.57, 95% CI 0.43 to 0.76) and fewer had persistent or recurrent bleeding (without scheduled second‐look endoscopy) (RR 0.32, 95% CI 0.21 to 0.48). No difference in rebleeding rates was seen between groups in studies that did schedule second‐look endoscopy.
The numbers needing emergency surgery were significantly lower in the combined group (RR 0.68, 95% CI 0.50 to 0.93), but mortality rates were not significantly different with either method. Adverse events included perforations, mucosal injury and necrosis; these events were few, and no statistically significant difference between groups was noted.
For ulcers of the Forrest Ia or Ib type (oozing or spurting), the addition of a second therapy significantly reduced the rebleeding rate (RR 0.66, 95% CI 0.49 to 0.88); this difference was not seen for type IIa (visible vessel) or type IIb (adherent clot) ulcers.
Our analyses therefore showed that the risk of further bleeding was lower in the combination therapy groups than in the epinephrine alone groups, regardless of which second procedure was applied. Failure of endoscopic therapy is the main predictor of the need for surgery and of morbidity and mortality in patients with bleeding peptic ulcer (Brullet 1996). Therefore, it seems highly likely that reduction of further bleeding rates decreased the need for surgery and improved survival.
We also compared epinephrine versus epinephrine plus injected agents, versus epinephrine plus a mechanical method and versus epinephrine plus thermal methods separately.
For the comparison of epinephrine versus epinephrine plus any injected method, we produced meta‐analyses for three outcomes. Fewer participants in the combination group experienced recurrent and persistent bleeding, but this finding was not statistically significant. Fewer participants in the combination therapy group died and this was statistically significant (RR 0.50, 95% CI 0.25 to 1.00), but no difference between groups was noted for the number needing emergency surgery.
For the comparison of epinephrine versus epinephrine plus a mechanical method such as haemoclips, results favoured the combination therapy group for recurrent or persistent bleeding (RR 0.31, 95% CI 0.18 to 0.55); fewer participants in the combination group needed surgery (RR 0.20, 95% CI 0.06 to 0.62), but no statistically significant difference was reported for mortality.
For the comparison of epinephrine versus epinephrine plus a thermal method, results favoured combined therapy for recurrent and persistent bleeding (RR 0.49, 95% CI 0.30 to 0.78) and for surgery (RR 0.92, 95% CI 0.37 to 2.26), but no statistically significant difference was reported for mortality.
Our updated review with new data confirms the results published in our previous review (Vergara 2007).
Overall completeness and applicability of evidence
One limitation of this review is that definitions for haemorrhage, surgery and death were not the same in the different studies (Characteristics of included studies). Marked heterogeneity observed in defining further bleeding among studies precluded the definition of a homogeneous, predetermined criterion for the primary endpoint. We therefore accepted the definition established for each study. Further bleeding definitions are shown in Characteristics of included studies. As the analysis includes only randomised, comparative studies, the criteria were similar for the two groups (epinephrine alone and combined therapy) in each study, thus allowing further comparison. Furthermore, endoscopic confirmation of bleeding was required for studies that reported second‐look endoscopy, thus reducing heterogeneity. Criteria for mortality rate were different between studies. Some studies used hospital mortality as a key, and others used 30‐day mortality or did not specify the mortality criteria applied. This makes it difficult to extract definitive conclusions about mortality rates in haemorrhage from those in ulcer disease. Moreover, characteristics of the co‐morbidities of participants were not included in the results of most of the studies.
Medical treatments were also different between studies or were not specified. However, the best treatment for bleeding from a peptic ulcer is the endoscopic treatment that we analysed in the present meta‐analysis.
One important point to state involves the dosage of epinephrine and the methodological process used in each study. The dosage of epinephrine used was detailed in Characteristics of included studies. Seven included studies did not specify the dosage (Balanzo 1990; Chung 1993; Chung 1996; Chung 1999; Garrido 2002; Park 2004; Villanueva 1996). Two studies used the same dosage of epinephrine for both groups independently of whether haemorrhage stopped (Choudari 1994; Loizou 1991). Another study (Lo 2006) injected epinephrine until haemostasis or to a maximum dose of 20 mL of epinephrine in both groups, independently of achieving haemostasis.
Comparisons of epinephrine versus epinephrine plus a specific second method also raise some unanswered questions. First, although one might consider that the efficacy of injecting a second agent is similar to that achieved with thermal and mechanical methods, this interpretation should be treated with extreme caution. The only conclusion that can be drawn from our meta‐analysis is that, whichever second treatment is used, combined therapy seems to work better than epinephrine alone. However, we cannot conclude that a particular form of treatment is equal or superior to another. Indeed, an earlier meta‐analysis evaluating endoscopic therapy for bleeding peptic ulcers demonstrated that all methods of controlling bleeding in peptic ulcers (thermal devices, injectable agents such as sclerosants and thrombin/fibrin glue and haemoclips) were effective, with no single modality determined to be superior (Laine 2009). There are two main reasons for this. First, the subgroups (further injection, thermal methods or mechanical methods) include different procedures that may present heterogeneous activity. In fact, each endoscopic treatment presents different characteristics: Epinephrine produces vasoconstriction, vessel compression and platelet aggregation, but it does not seem to induce permanent thrombosis in blood vessels (Lin 2002). Sclerosant agents such as polidocanol or ethanol can produce thrombosis of vessels favouring haemostasis, although they may also induce significant tissue injury (Randall 1989; Ritgeers 1989). Whether human thrombin injection could reduce the risk of tissue damage remains unclear, and thrombin is more expensive than other additional treatments (Laine 2003). Thermal agents produce thrombosis of vessels and risk damaging tissue. Among them, laser photocoagulation seems to be associated with higher risks of perforation, optical hazard, high cost and imperfect haemostatic effect. Multi‐polar electrocoagulation and heater probe thermocoagulation have been reported to produce excellent results; they are also less expensive and are more easily portable than laser methods (Llach 1996). Mechanical methods close the vessel. They were also associated with few complications, but technical difficulty was associated with applying the haemoclip to the posterior wall of the proximal body and cardia of the stomach (Grgov 2012), and to the posterior wall of the duodenum, because of the requirement that the haemoclip meet the lesion at a right angle (Chung 1999; Simoens 2001). A meta‐analysis compared haemoclip versus other methods to investigate the possible benefits of haemoclips versus other endoscopic methods. The evidence showed no differences between them (Yuan 2008).
Even more important, the statistical treatments used in our meta‐analyses are not designed to compare additional treatments head to head. In fact, we lack randomised trials comparing different additional treatments after epinephrine injection, although some recent studies are available (e.g. Grgov 2013; Karaman 2011; Taghavi 2009), and we must await analysis of comparative studies to establish which is the best therapy when combined with epinephrine.
Our review did not determine whether combined therapy is better than sclerosants, thermal methods or mechanical methods alone. Little evidence in the literature suggests that this is the case. In a small study, Lin 1999 found that combined therapy seems better than bipolar electrocoagulation alone. Also, a recent meta‐analysis concluded that combined injection is superior to a sclerosant alone (Rollhauser 2000). In any event, the clinical relevance of this question may be minor.
One of the major fears associated with using combined therapy involves the possible risk of gastric wall necrosis or perforation or both. The present meta‐analysis shows that the risk of significant complications is very low. This review failed to find evidence of a difference in complication rates between groups. When the complications are examined in detail, it can be seen that induction of massive bleeding requiring surgery was more frequent in the epinephrine group, whereas gastric wall necrosis or perforation was more common in the combined therapy group. This possible small increase in the risk of perforation or necrosis is clearly compensated for by the benefits derived from reducing further bleeding, which result in a significant decrease in the need for surgery and in mortality; therefore this risk is not a reason for avoiding combined therapy.
Epinephrine injection is cheap, easy to perform and safe. In addition, according to our analysis, epinephrine seems as good as combined therapy for achieving initial haemostasis. By controlling active bleeding, it could allow a better endoscopic view and more accurate targeting of additional therapy. Therefore, medical or economic arguments against epinephrine injection are few.
Quality of the evidence
Of the 19 trials included in this review, some had limitations in design or implementation, but most were clear about important quality criteria including randomisation and allocation concealment, sequence generation and blinding. Our risk of bias estimates show that overall most studies were not at high risk of bias in any of the domains that we assessed. It should be noted that we did not rate performance bias because it is not possible to blind participants and personnel in studies of this nature. Although the type of study did not allow a double‐blind trial (Figure 2), rebleeding, surgery and mortality were not dependent on subjective observation.
The meta‐analysis involved a large number of well‐designed studies, but for most outcomes, the quality of the evidence was rated as moderate, and further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. We summarised the quality of the studies in Summary of findings table 1. When we downgraded the evidence to moderate quality, we did so because few events were reported; this was the case for the outcomes of surgery, mortality and adverse effects. The included studies involved relatively few participants, but larger populations are difficult to achieve in this type of trial. Some imprecision was noted (wide confidence intervals), and the results of some meta‐analyses showed low to moderate levels of statistical heterogeneity (inconsistency). One explanation for this may be the differences noted between trials (populations, definitions of outcomes such as in‐hospital or 30‐day mortality and definitions of rebleeding), as discussed above.
Potential biases in the review process
One limitation of our review is that despite exhaustive searching, it is difficult to be certain that every published and unpublished study was identified. We acknowledge that there is always a risk that some studies were not identified. However for the studies that we identified, the quality was rated as moderate for most outcomes, and because of the serious nature of this condition, incidences of adverse effects were reported in many trials.
Agreements and disagreements with other studies or reviews
The findings of our updated review are in agreement with the recommendations provided in three major clinical guidelines produced since the first published version of this review. Combined therapy of epinephrine plus a second endoscopic agent (e.g. bipolar electrocoagulation, heater probe, sclerosant, clips) for the treatment of patients with high‐risk bleeding peptic ulcers (Laine 2012; NICE 2012; Hwang 2012) is the main recommendation for active bleeding or non‐bleeding visible vessels.
As far as second‐look endoscopy is concerned, one meta‐analysis showed that although scheduled second‐look endoscopies reduced the rebleeding rate, they did not decrease the need for surgery nor mortality (Marmo 2003). It was also suggested that selective second‐look endoscopy for selected high‐risk patients could be a cost‐effective approach (Spiegel 2003). However, this strategy exposes patients to uncomfortable and somewhat risky procedures and increases the workload of the endoscopy unit. Results of the present study did not confirm that second‐look endoscopy diminished the risk of rebleeding. For all of these reasons, it remains unclear whether second‐look endoscopy offers any added benefit to combined therapy associated with proton pump inhibitor infusion. This finding is in agreement with current guidelines on the management of acute non‐variceal upper gastrointestinal bleeding. In the NICE 2012 guideline, the recommendation is to "consider a repeat endoscopy, with treatment as appropriate" (further endoscopic treatment or emergency surgery),"for all patients at high risk of re‐bleeding, particularly if there is doubt about initial haemostasis at the first endoscopy." The AGA guideline (Laine 2012) does not recommend routine second‐look endoscopy 24 hours after initial endoscopic haemostatic therapy but says that this should be offered for patients with clinical evidence of recurrent bleeding, and in these cases, haemostatic therapy should be applied for those with greater risk of stigmata of haemorrhage. Routine second‐look endoscopy, defined as a planned endoscopy performed within 24 hours of the initial endoscopy, in patients who have received adequate endoscopy is not recommended by the ASGE in its 2012 guideline (Chung 1999; Hwang 2012).
Authors' conclusions
Implications for practice.
The present study shows that adding a second endoscopic procedure after epinephrine injection reduces the rate of recurrence in patients with high‐risk bleeding peptic ulcer. This study also shows that adding any second endoscopic method reduces the need for surgery. In view of the evidence, and while we await the results of further studies, combined therapy should be considered the standard procedure in high‐risk peptic ulcer haemorrhage (Forrest Ia, Ib, IIa).
Implications for research.
The current evidence shows that addition of a second endoscopic method to epinephrine injection is better than epinephrine injection alone in high‐risk bleeding peptic ulcers, in terms of preventing both rebleeding and the need for emergency surgery. Future research should investigate the best endoscopic method that, when associated with epinephrine, achieves low rebleeding rates, reduced need for surgery and prevention of mortality and procedure‐related adverse effects when standardised outcome criteria such as use of a scheduled second endoscopy and criteria for rebleeding are used, and whether this was confirmed endoscopically. Further systematic reviews and meta‐analyses should assess such head‐to‐head comparisons to compare the efficacy of additional treatments.
Other therapeutic approaches, such as use of a high‐dose proton pump inhibitor or second‐look endoscopy, could influence the efficacy of combined therapy. Use of high‐dose proton pump inhibitors for patients with bleeding peptic ulcers is gaining acceptance. Evidence clearly suggests that these drugs reduce the risk of rebleeding (Gisbert 2001). However, many points remain unclear, such as the cost‐effectiveness of this approach, the ideal drug dosage to be used and whether this strategy should be reserved for patients at high risk of rebleeding. Both combined endoscopic therapy and proton pump inhibitor infusion are safe and comfortable for the patient. Therefore, although the extent of the benefit of combining the two approaches remains uncertain, this strategy seems reasonable until additional evidence becomes available.
What's new
| Date | Event | Description |
|---|---|---|
| 30 May 2014 | New search has been performed | Updated with new search results, 1 new trial added |
| 30 May 2014 | New citation required and conclusions have changed | New author (CB); new, updated results |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 5 November 2009 | New search has been performed | Updated. |
| 16 June 2008 | Amended | Converted to new review format. |
| 17 January 2007 | New citation required and conclusions have changed | Substantive amendment |
| 6 January 2007 | Amended | Minor update. |
| 14 February 2006 | Amended | New studies found and included or excluded. |
Notes
None.
Acknowledgements
Discussions by the group that developed the Catalan Guidelines for non‐variceal upper gastrointestinal bleeding were invaluable in helping to determine gaps in knowledge on bleeding peptic ulcer treatment. We are indebted to all participants, especially to Dr Faust Feu as the co‐ordinator of this group.
We thank Dr Nadja Smailagic, who provided translations of Grgov 2012 and Grgov 2013.
Appendices
Appendix 1. MEDLINE search
Ovid MEDLINE(R) 1946 to May Week 2 2014, Ovid MEDLINE(R) Daily Update May 22, 2014, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations May 22, 2014
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/1‐8
exp animals/ not humans.sh.
9 not 10
exp peptic ulcer/
exp peptic ulcer hemorrhage/
exp peptic ulcer perforation/
exp duodenal ulcer/
exp stomach ulcer/
(pep$ adj5 ulcer$).tw.
(stomach adj5 ulcer$).tw.
(duoden$ adj5 ulcer$).tw.
(gastr$ adj5 ulcer$).tw.
(bleed$ adj5 ulcer$).tw.
(rebleed$ adj5 ulcer$).tw.
(recurrent adj5 bleed$ adj5 ulcer$).tw.
(acute adj5 bleed$ adj5 ulcer$).tw.
exp gastrointestinal hemorrhage/
(gastrointestinal adj5 bleed$).tw.
(gastrointestinal adj5 rebleed$).tw.
(gastrointestinal adj5 hemorrhag$).tw.
(gastrointestinal adj5 haemorrhag$).tw.
(ulcer adj5 hemorrhag$).tw.
(ulcer adj5 haemorrhag$).tw.
(mucos$ adj5 injur$).tw.
(mucos$ adj5 ero$).tw.
(gastr$ adj5 ero$).tw.
(stomach adj5 ero$).tw.
or/12‐35
exp epinephrine/
epinephrine.tw.
exp vasoconstrictor agents/
or/37‐39
(argon adj5 plasma adj5 coagulat$).tw.
exp sclerotherapy/
sclerotherap$.tw.
exp electrocoagulation/
exp hemostasis/
exp hemostasis, endoscopic/
exp lasers/
exp endoscopy gastrointestinal/
electrocoagulat$.tw.
(therm$ adj5 coagulat$).tw.
(heater adj5 probe).tw.
laser$.tw.
endoclip$.tw.
hemoclip$.tw.
(monopolar adj5 coagulat$).tw.
(multipolar adj5 coagulat$).tw.
(bipolar adj5 coagulat$).tw.
exp sclerosing solutions/
sclerosant$.tw.
polidocanol.tw.
exp polyethylene glycols/
(endoscopic adj3 inject$).tw.
thrombin.tw.
fibrin glue.tw.
exp fibrin tissue adhesive/
cyanoacrylate.tw.
exp enbucrilate/
or/41‐67
36 and 40 and 68
11 and 69
limit 70 to ed=20090601‐20140523
Appendix 2. CENTRAL search strategy
EBM Reviews—Cochrane Central Register of Controlled Trials April 2014
exp peptic ulcer/
exp peptic ulcer hemorrhage/
exp peptic ulcer perforation/
exp duodenal ulcer/
exp stomach ulcer/
(pep$ adj5 ulcer$).tw.
(stomach adj5 ulcer$).tw.
(duoden$ adj5 ulcer$).tw.
(gastr$ adj5 ulcer$).tw.
(bleed$ adj5 ulcer$).tw.
(rebleed$ adj5 ulcer$).tw.
(recurrent adj5 bleed$ adj5 ulcer$).tw.
(acute adj5 bleed$ adj5 ulcer$).tw.
exp gastrointestinal hemorrhage/
(gastrointestinal adj5 bleed$).tw.
(gastrointestinal adj5 rebleed$).tw.
(gastrointestinal adj5 hemorrhag$).tw.
(gastrointestinal adj5 haemorrhag$).tw.
(ulcer adj5 hemorrhag$).tw.
(ulcer adj5 haemorrhag$).tw.
(mucos$ adj5 injur$).tw.
(mucos$ adj5 ero$).tw.
(gastr$ adj5 ero$).tw.
(stomach adj5 ero$).tw.
or/1‐24
exp epinephrine/
epinephrine.tw.
exp vasoconstrictor agents/
or/26‐28
(argon adj5 plasma adj5 coagulat$).tw.
exp sclerotherapy/
sclerotherap$.tw.
exp electrocoagulation/
exp hemostasis/
exp hemostasis, endoscopic/
exp lasers/
exp endoscopy gastrointestinal/
electrocoagulat$.tw.
(therm$ adj5 coagulat$).tw.
(heater adj5 probe).tw.
laser$.tw.
endoclip$.tw.
hemoclip$.tw.
(monopolar adj5 coagulat$).tw.
(multipolar adj5 coagulat$).tw.
(bipolar adj5 coagulat$).tw.
exp sclerosing solutions/
sclerosant$.tw.
polidocanol.tw.
exp polyethylene glycols/
(endoscopic adj3 inject$).tw.
thrombin.tw.
fibrin glue.tw.
exp fibrin tissue adhesive/
cyanoacrylate.tw.
exp enbucrilate/
or/30‐56
25 and 29 and 57
limit 58 to yr=2009‐2014
Appendix 3. EMBASE search strategy
EMBASE 1980 to Week 20 2014
random:.tw. or placebo:.mp. or double‐blind:.tw.
exp peptic ulcer/
exp peptic ulcer hemorrhage/
exp peptic ulcer perforation/
exp duodenal ulcer/
exp stomach ulcer/
(pep$ adj5 ulcer$).tw.
(stomach adj5 ulcer$).tw.
(duoden$ adj5 ulcer$).tw.
(gastr$ adj5 ulcer$).tw.
(bleed$ adj5 ulcer$).tw.
(rebleed$ adj5 ulcer$).tw.
(recurrent adj5 bleed$ adj5 ulcer$).tw.
(acute adj5 bleed$ adj5 ulcer$).tw.
exp gastrointestinal hemorrhage/
(gastrointestinal adj5 bleed$).tw.
(gastrointestinal adj5 rebleed$).tw.
(gastrointestinal adj5 hemorrhag$).tw.
(gastrointestinal adj5 haemorrhag$).tw.
(ulcer adj5 hemorrhag$).tw.
(ulcer adj5 haemorrhag$).tw.
(mucos$ adj5 injur$).tw.
(mucos$ adj5 ero$).tw.
(gastr$ adj5 ero$).tw.
(stomach adj5 ero$).tw.
or/2‐25
exp epinephrine/
epinephrine.tw.
exp vasoconstrictor agents/
or/27‐29
(argon adj5 plasma adj5 coagulat$).tw.
exp sclerotherapy/
sclerotherap$.tw.
exp electrocoagulation/
exp hemostasis/
exp hemostasis, endoscopic/
exp lasers/
exp endoscopy gastrointestinal/
electrocoagulat$.tw.
(therm$ adj5 coagulat$).tw.
(heater adj5 probe).tw.
laser$.tw.
endoclip$.tw.
hemoclip$.tw.
(monopolar adj5 coagulat$).tw.
(multipolar adj5 coagulat$).tw.
(bipolar adj5 coagulat$).tw.
exp sclerosing solutions/
sclerosant$.tw.
polidocanol.tw.
exp polyethylene glycols/
(endoscopic adj3 inject$).tw.
thrombin.tw.
fibrin glue.tw.
exp fibrin tissue adhesive/
cyanoacrylate.tw.
exp enbucrilate/
or/31‐57
26 and 30 and 58
1 and 59
limit 60 to em=200901‐201420
Data and analyses
Comparison 1. Epinephrine versus epinephrine and any second endoscopic method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Initial failure of haemostasis (persistent bleeding) | 14 | 1380 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.42, 1.40] |
| 2 Recurrent bleeding only (bleeding after initial haemostasis) | 14 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.81] |
| 3 Recurrent and persistent bleeding overall rates with or without second‐look endoscopy | 19 | 1926 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.76] |
| 4 Recurrent and persistent bleeding and second‐look endoscopy | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Confirmed by second‐look endoscopy | 10 | 1203 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.06] |
| 4.2 Without second‐look endoscopy | 9 | 720 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.21, 0.48] |
| 5 Surgery rate | 18 | 1841 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 6 Mortality rate | 18 | 1841 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.39, 1.06] |
| 7 Adverse effects of endoscopy therapy | 12 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.40, 3.96] |
| 8 Recurrent and persistent bleeding according to type of haemorrhage | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Forrest Ia‐Ib (spurting or oozing haemorrhage) | 13 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.88] |
| 8.2 Forrest IIa (visible vessel) | 9 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.47, 1.99] |
| 8.3 Forrrest IIb (adherent clot) | 2 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.22, 2.60] |
Comparison 2. Epinephrine plus epinephrine and a second injected agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent and persistent bleeding | 11 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.04] |
| 2 Surgery rate | 10 | 1050 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.21] |
| 3 Mortality rate | 10 | 1050 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
2.1. Analysis.
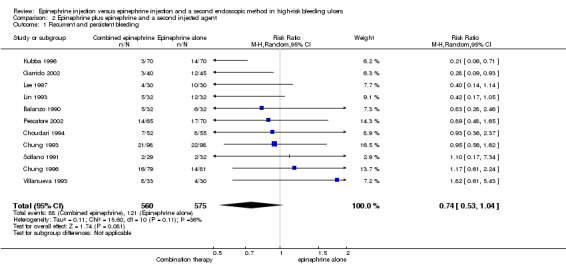
Comparison 2 Epinephrine plus epinephrine and a second injected agent, Outcome 1 Recurrent and persistent bleeding.
Comparison 3. Epinephrine versus epinephrine and mechanical endoscopic methods.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrrent and persistent bleeding | 5 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.18, 0.55] |
| 2 Surgery rate | 5 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.06, 0.62] |
| 3 Mortality rate | 5 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.19, 2.71] |
Comparison 4. Epinephrine plus epinephrine and thermal methods.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent and persistent bleeding | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.78] |
| 2 Surgery rate | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.95] |
| 3 Mortality rate | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Balanzo 1990.
| Methods | Randomised, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 64)• Age (years): 65 (mean) • Sex (male/female): 38/26 • Duodenal/gastric ulcer: 20/41 • Forrest group: Ia/Ib = 24, IIa = 40 | |
| Interventions | Interventions • Primary intervention: epinephrine injection vs epinephrine injection plus thrombin injection. No specification about how many different endoscopists participated in the intervention process • Medical treatment: not specified • Second‐look endoscopy: yes • Epinephrine volume: not specified | |
| Outcomes |
Outcomes assessed Rebleeding rates, surgery rate and mortality
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain the method used for sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not explain the method used for allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done. Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | n = 64 participants entered the study. For the outcome of haemostasis, all participants were accounted for. It is unclear why only 25 (group A) and 24 participants (group B) received an elective endoscopy; the outcome of participants not receiving a second (elective) endoscopy is also unclear |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
Choudari 1994.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 107) • Age (years): 68.5 (median) • Sex (male/female): 68/39 • Duodenal/gastric ulcer: 63/44 • Forrest group: Ia/Ib = 57, IIa = 50 (with high‐risk stigmata (Forrest Ia‐Ib‐IIa)) | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 55) vs epinephrine injection plus ethanolamine (n = 52). Only 1 endoscopist carried out the procedures • Medical treatment: H2 antagonists • Second‐look endoscopy: yes • Epinephrine volume: 7.5 mL in each group | |
| Outcomes |
Outcomes assessed
Rebleeding rate, surgery rate, units of blood transfused, duration of hospital admission and mortality
• Mortality criteria: 30‐day mortality
• Bleeding criteria: fresh hematemesis or melena plus shock or fall in haemoglobin > 2 g/dL
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Quote: "were then randomised by opening a sealed envelope to receive... " (pages 608‐609) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done. Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data: 107 participants were randomly assigned; however 5 patients who were actively bleeding from peptic ulcers were not included because the bleeding point was not identified, or initial haemostasis could not be achieved by epinephrine; a further 2 patients were excluded because their ulcers were inaccessible to injection. It is unclear whether these participants were randomly assigned but were not included in the analyses, or whether they were not randomly assigned. Outcomes were presented for all randomly assigned participants |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Chung 1993.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 200) • Age (years): 54.5 (mean) • Sex (male/female): 138/58 • Duodenal/gastric ulcer: 117/75 • Forrest group: Ia = 62, Ib = 134 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 99) vs epinephrine injection plus sodium tetradecyl sulphate (n = 101) • Medical treatment: ranitidine 50 mg/6 h intravenously • Second‐look endoscopy: yes • Epinephrine volume: not specified | |
| Outcomes |
Outcomes assessed
Rebleeding rate, surgery rate, hospital stay, transfusion requirements and mortality
• Mortality criteria: hospital mortality
• Bleeding criteria: fresh hematemesis or melena plus shock or fall in haemoglobin > 2 g/dL
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list generated by computer |
| Allocation concealment (selection bias) | Low risk | Sealed and numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: (abstract p 611): "After the procedure the patients were transferred to the surgical gastroenterology ward and were treated by surgeons who were unaware of the mode of treatment" Criteria: blinding was incomplete, as it is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 200 participants were randomly assigned. Outcomes were presented for all those randomly assigned, including details of n = 4 who were excluded after randomisation and those lost to follow up (n = 13) or who did not return for follow‐up to determine ulcer healing 4 weeks after discharge |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Chung 1996.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 160) • Age (years): 55.2 (mean) • Sex (male/female): 118/42 • Duodenal/gastric ulcer: 108/51 • Forrest group: (Forrest Ia‐Ib) Ia = 35, Ib = 125 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 81) vs epinephrine injection plus ethanol injection (n = 79) • Medical treatment: H2 antagonists • Second‐look endoscopy: yes • Epinephrine volume: not specified | |
| Outcomes |
Outcomes assessed
Rebleeding rate, surgery rate and mortality
• Mortality criteria: hospital mortality
• Bleeding criteria: not specified Emergency surgery (for uncontrolled arterial bleeding) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatment option had been determined by a computer generated random number list" (page 592) |
| Allocation concealment (selection bias) | Low risk | Quote: "We randomised patients into two groups by opening a sealed envelope containing the treatment option for each" (page 592) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 15 participants were excluded before randomisation (reasons given), 160 participants were randomly assigned. Outcomes were presented for all those randomly assigned, along with details of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Chung 1997.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 276) • Age (years): 58.5 (mean) • Sex (male/female): 187/89 • Duodenal/gastric ulcer: 176/87 • Forrest group: Ia = 58, Ib = 212 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 136) vs epinephrine injection and heat probe (n = 140) • Medical treatment: ranitidine • Second‐look endoscopy: yes • Epinephrine volume: 9.5 vs 10.1 | |
| Outcomes |
Outcomes assessed Rebleeding rate, length of hospital stay, requirement for blood transfusion, surgery rate and mortality
|
|
| Notes | Outcomes assessed Rebleeding rate, surgery rate and mortality • Mortality criteria: hospital mortality • Bleeding criteria: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisations took place at the time of endoscopy when actively bleeding ulcers were seen. The endoscopy nurse then opened a sealed envelope containing the treatment option, which had previously been determined by a random number list generated by a computer. Treatment was concealed from the endoscopist when the patient was admitted into the trial" (page 1308) Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisations took place at the time of endoscopy when actively bleeding ulcers were seen. The endoscopy nurse then opened a sealed envelope containing the treatment option, which had previously been determined by a random number list generated by a computer. Treatment was concealed from the endoscopist when the patient was admitted into the trial" (page 1308) Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: Randomisation took place at the time of endoscopy when actively bleeding ulcers were seen. 276 randomly assigned, Six patients were excluded after randomisation (n = 2 in epinephrine; n = 4 in combined therapy), as their ulcers were subsequently found to be malignant |
| Selective reporting (reporting bias) | Unclear risk | The published report includes all expected outcomes, including those that were prespecified |
Chung 1999.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 124) • Age (years): 55.5 (mean) • Sex (male/female): 69/14 • Duodenal/gastric ulcer: 20/61 • Forrest group: Ia = 8, Ib = 27, IIa = 48 | |
| Interventions |
Interventions
• Primary intervention: epinephrine injection (n = 41) vs epinephrine injection plus haemoclip (n = 42) vs haemoclip only group (n = 41)
• Medical treatment: not specified
• Second‐look endoscopy: yes
• Epinephrine volume: not specified
|
|
| Outcomes | Outcomes assessed Rebleeding rate, surgery rate and mortality • Mortality criteria: hospital mortality • Bleeding criteria: fresh hematemesis or melena plus shock or transfusion > 8 U | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The procedure performed was randomly chosen by means of drawing a single number (1 to 3) contained in sequentially numbered opaque envelopes" (page 14) |
| Allocation concealment (selection bias) | Low risk | Quote: "The procedure performed was randomly chosen by means of drawing a single number (1 to 3) contained in sequentially numbered opaque envelopes" (page 14) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 143 participants were randomly assigned. Nineteen participants could not undergo follow‐up endoscopic examinations because of personal objections or transfer to other hospitals |
| Selective reporting (reporting bias) | Unclear risk | The published report includes all expected outcomes, including those that were prespecified |
Garrido 2002.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 85) • Age (years): 63.1 (mean) • Sex (male/female): 50/35 • Duodenal/gastric ulcer: 27/58 • Forrest group: Ia/Ib = 25, IIa = 52, IIb = 8 | |
| Interventions | Interventions • Primary intervention: epinephrine injection vs epinephrine injection and polidocanol injection • Medical treatment: not specified • Second‐look endoscopy: no • Epinephrine volume: not specified | |
| Outcomes |
Outcomes assessed
Rebleeding rate
• Mortality criteria: not specified
• Bleeding criteria: haematemesis or fresh melena • Emergency surgery (assessed for whole group, but no separate data for the n = 85 cases randomly assigned) |
|
| Notes | Spanish language report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain sequence generation Comment: unclear |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not explain allocation concealment Comment: unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether n = 340 participants were randomly assigned or only the n = 85 reported in this publication who were treated endoscopically. Reporting of outcomes and numbers in each group at each time point is unclear, for example, 3 participants died as a result of haemorrhage, but it is not clear which therapy they received |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether all outcomes were reported |
Grgov 2012.
| Methods | Randomised, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 58) • Age (years): 62.2 (epinephrine); 65.0 (combination therapy) mean • Sex (male/female): 43/15 • Duodenal/gastric ulcer: 30/28 • Forrest group: Ia = 5, Ib = 47, IIa = 6 (epinephrine Ia n = 1, Ib n = 27, IIa n = 2; combination therapy Ia n = 4, Ib n = 20, IIa n = 4) | |
| Interventions | • Primary intervention: epinephrine injection (n = 30) vs epinephrine injection and haemoclip (n = 28) • Medical treatment: proton pump inhibitors (PPIs) as an intravenous bolus of 80 mg, followed by infusion of 8 mg/h for 72 hours, and after that oral PPI • Second‐look endoscopy: no (only if rebleeding suspected) • Epinephrine volume: epinephrine diluted in physiological saline solution (1:10,000), 0.5‐2 mL fractions into 4 quadrants around the ulcer. Epinephrine group mean 11 mL (4‐20 mL); epinephrine plus haemoclip 10.4 mL (4‐20 mL) (no statistically significant difference in volume) | |
| Outcomes |
Outcomes assessed
Rebleeding rate (initial and final), surgery rate, adequate clip placement and mortality
•Mortality criteria: mortality •Bleeding criteria: new hematemesis or melena after the appearance of fresh blood aspiration from a nasogastric probe and an increase in pulse rate over 100 per minute, decrease in systolic blood pressure by more than 30 mm Hg and a decrease in haemoglobin of at least 2 g/dL • Emergency surgery |
|
| Notes | Translated from Serbo‐Croatian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not explain allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 58 participants were randomly assigned. All participants were accounted for |
| Selective reporting (reporting bias) | Unclear risk | The published report includes all expected outcomes, including those that were prespecified |
Kubba 1996.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 140) • Age (years): 70 (median) • Sex (male/female): 93/47 • Duodenal/gastric ulcer: 78/52 • Forrest group: Ia/Ib = 51, IIa = 89 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 70) vs epinephrine injection and thrombin injection (n = 70) • Medical treatment: not specified • Second‐look endoscopy: no • Epinephrine volume: 10 mL median in epinephrine alone vs 7 mL median in combined therapy group | |
| Outcomes |
Outcomes assessed
Rebleeding rate, surgery rate and mortality
• Mortality criteria: 30‐day mortality • Bleeding criteria: fresh hematemesis or melena plus shock or fall in haemoglobin > 2 g/dL • Emergency surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized by opening a sealed envelope to be administered either injection with dilute epinephrine (group 1) or epinephrine plus human thrombin (group 2). The randomization was performed during the endoscopy" (page 634) Comment: probably adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 140 participants were randomly assigned (once participants/ulcers not meeting the inclusion criteria had been excluded at initial endoscopy). All participants were accounted for |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Lee 1997.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 60) • Age (years): not specified • Sex (male/female): not specified • Duodenal/gastric ulcer: not specified • Forrest group: Ia/Ib = 37, IIa = 23 | |
| Interventions | Interventions • Primary intervention: epinephrine injection vs epinephrine injection and ethanol injection • Medical treatment: not specified • Second‐look endoscopy: no • Epinephrine volume: 17.6 mL in epinephrine group alone vs 14.7 mL in combined therapy group | |
| Outcomes |
Outcomes assessed
Rebleeding rate, surgery rate and mortality
• Mortality criteria: not specified
• Bleeding criteria: not specified • Emergency surgery |
|
| Notes | Abstract report only; bleeding ulcer participants were a subgroup of n = 60 with active bleeding or a non‐bleeding visible vessel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain sequence generation (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not explain allocation concealment (abstract) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not possible to determine, short abstract report |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes were efficacy and safety of endoscopic treatment. All outcomes were reported but not by the intervention group |
Lin 1993.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 64) • Age (years): 59.6 (mean) • Sex (male/female): 57/7 • Duodenal/gastric ulcer: 34/29 • Forrest group: Ia = 21 Ib = 43 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 32) vs epinephrine injection and ethanol injection (n = 32) • Medical treatment: ranitidine or cimetidine • Second‐look endoscopy: no • Epinephrine volume: 6 mL in epinephrine group vs 5.5 mL in combined therapy group | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate, hospital stay, blood transfusions and mortality
• Mortality criteria: hospital mortality
• Bleeding criteria: unstable vital signs or continued bloody stools or hematemesis • Emergency surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two forms of injection were arranged by a statistician who was not involved in the study" (page 1182) Comment: probably adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "For every patient enrolled in the study, a sealed envelope was opened before performing an endoscopic injection to decide the injected solution. Two forms of injection were arranged by a statistician who was not involved in the study" (page 1182) Comment: probably adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Peptic ulcers with bleeding (oozing or spurting) were found in 100 participants. A total of 36 cases were excluded from the study, and it is unclear whether they were excluded after randomisation: 20 participants refused to give informed consent, 8 had bleeding gastric cancer and 8 had a bleeding tendency. 64 participants who had actively bleeding ulcer at initial endoscopy were randomly assigned, and all 64 participants were accounted for in each group |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Lin 1999.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 96) • Age (years): 67.7 (mean) • Sex (male/female): 57/7 • Duodenal/gastric ulcer: 25/37 • Forrest group: Ia = 6, Ib = 16, IIa = 42 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 32) vs epinephrine injection and bipolar electrocoagulation (n = 32) (vs bipolar electrocoagulation alone = 32) • Medical treatment: omeprazole 40 mg/6 h intravenously • Second‐look endoscopy: yes • Epinephrine volume: 7.1 mL vs 6.8 mL | |
| Outcomes |
Outcomes assessed
Bleeding rate, blood transfusions, surgery rate and mortality
• Mortality criteria: hospital mortality
• Bleeding criteria: unstable vital signs or continued bloody stools or hematemesis • Emergency surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by a statistician who was not involved in the study" (page 716) Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "For every patient enrolled in the study, a sealed envelope was opened before performing endoscopic treatment" (page 716) Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 96 randomly assigned, n = 1 in the epinephrine group; n = 2 in the gold probe group and n = 2 in the injection and gold probe group were excluded because no haemostasis was achieved. All other participants were accounted for in the groups to which they were randomly assigned |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Lo 2006.
| Methods | Randomised, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 108) • Age (years): 63.5 (mean) • Sex (male/female): 81/24 • Duodenal/gastric ulcer: 51/54 • Forrest group: Ia/Ib = 42, IIa = 34, IIb = 30 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 53) vs epinephrine injection plus haemoclip (n = 52). Study was performed by 4 different endoscopists • Medical treatment: pantoprazole 40 mg intravenously • Second‐look endoscopy: no • Epinephrine volume: maximum 20 mL | |
| Outcomes |
Outcomes assessed Rebleeding rate, surgery, complications and 30‐day mortality
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Page 769: "Randomization was carried out during endoscopy by an individual not directly involved in the study who opened the sealed and numbered envelopes that contained the treatment assignments, which had been randomised with a computer program. NB re‐bleeders in the epinephrine group were then randomised again, but the results of this second randomised study are not added to the data from the original groups" |
| Allocation concealment (selection bias) | Low risk | Page 769: "Randomization was carried out during endoscopy by an individual not directly involved in the study who opened the sealed and numbered envelopes that contained the treatment assignments, which had been randomised with a computer program" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done. Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 108 participants randomly assigned, Three patients were excluded because of inability to obtain informed consent, gastric malignancy or multiple bleeding sites at endoscopy |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Loizou 1991.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 42) • Age (years): 60.5 (mean) • Sex (male/female): 31/11 • Duodenal/gastric ulcer: 22/20 • Forrest group: Ia/Ib = 8, IIa = 34 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 21) vs epinephrine injection and Nd:YAG laser photocoagulation (n = 21) • Medical treatment: ranitidine 300 mg/12 h oral intake • Second‐look endoscopy: no • Epinephrine volume: 10 mL in both groups | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate and mortality
• Mortality criteria: 30‐day mortality
• Bleeding criteria: fresh hematemesis or melena plus shock or fall in haemoglobin despite transfusion • Emergency surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain sequence generation Comment: unclear |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were stratified into two groups according to whether the visible vessel was actively bleeding at the time of endoscopy or not and separately randomised, by opening sealed numbered envelopes, to receive either epinephrine injection treatment or epinephrine injection + Nd:YAG laser photocoagulation" (page 1101) Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 42 participants were randomly assigned at endoscopy provided they fit the inclusion criteria. All participants were accounted for |
| Selective reporting (reporting bias) | Unclear risk | The published report includes all expected outcomes, including those that were prespecified |
Park 2004.
| Methods | Randomised trial, single blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 90) • Age (years): 61.8 (mean) • Sex (male/female): 76/14 • Duodenal/gastric ulcer: 18/72 • Forrest group: Ia = 18, Ib = 28, IIa = 44 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 45) vs epinephrine injection plus haemoclip application or band ligation (n = 45) • Medical treatment: ranitidine 50 mg/6 h intravenously • Second‐look endoscopy: no • Epinephrine volume: not specified | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate and mortality
• Mortality criteria: bleeding‐related deaths
• Bleeding criteria: fresh hematemesis, instability of vital signs and/or decrease in haemoglobin level of more than 2 g/dL/24 h • Emergency surgery |
|
| Notes | The published report includes all expected outcomes, including those that were prespecified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation of patients to treatment was by drawing sequentially numbered envelopes, each containing a previously determined, randomly selected assignment based on a table of random numbers" (page 174) Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of patients to treatment was by drawing sequentially numbered envelopes, each containing a previously determined, randomly selected assignment based on a table of random numbers" (page 174) Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single blind. Quote: "A prospective, randomized, single‐blind comparative trial was conducted in a university‐affiliated academic medical center" (page 173) Comment: It is unclear what single blinded means. It might be true that managing clinicians did not know what the endoscopic treatment had been, but this is not clearly specified in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 90 participants were randomly assigned. All participants were accounted for in a flow diagram showing outcomes for all. "Peptic ulcer with either an actively bleeding vessel (spurting or oozing) or a non‐bleeding visible vessel was found in 123 patients. Thirty‐three were excluded (before randomisation) because of inability to obtain informed consent, bleeding diathesis, gastric malignancy" (study report, page 175) |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Pescatore 2002.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 135) • Age (years): 68.7 (median) • Sex (male/female): 90/45 • Duodenal/gastric ulcer: 88/47 • Forrest group: Ia = 15, Ib = 47, IIa = 51, IIb = 22 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 70) vs epinephrine injection plus fibrin glue (n = 65) • Medical treatment: not specified • Second‐look endoscopy: yes • Epinephrine volume: 10.3 mL in epinephrine group vs 7.5 mL in combined therapy | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate, blood transfusion and mortality
• Mortality criteria: 30‐day mortality
• Bleeding criteria: fresh hematemesis or melena plus shock or fall in haemoglobin despite transfusion • Emergency surgery |
|
| Notes | The published report includes all expected outcomes, including those that were prespecified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed within each center by using a computer‐generated randomization list and sealed envelopes" (page 349) Comment: adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed within each center by using a computer‐generated randomization list and sealed envelopes" (page 349) Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing outcome data: 135 participants were randomly assigned. Unclear whether the following participants were excluded after randomisation: "Patients were excluded because of endoscopically uncontrollable bleeding; these patients underwent immediate surgery. Three of the latter patients had large bleeding vessels on the posterior aspect of the duodenal bulb, whereas 1 patient bled from an aorto‐duodenal fistula. Two further patients were excluded because of a suspicion that the bleeding gastric ulcer was neoplastic (later confirmed by histology)" (study report, page 350) Unclear whether all randomly assigned participants were accounted for, as some outcomes are reported as percentages |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Sollano 1991.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 83) • Age (years): not specified • Sex (male/female): not specified • Duodenal/gastric ulcer: 29/29 • Forrest group: Ia = 6, Ib = 35, IIa = 20 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 32) vs epinephrine injection and ethoxy sclerol (n = 29) (vs heater probe only n = 22) • Medical treatment: not specified • Second‐look endoscopy: yes • Epinephrine volume: 8.6 mL in epinephrine alone vs 11 mL in combined therapy | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate and mortality
• Mortality criteria: not specified
• Bleeding criteria: recurrence of hematemesis, melena or anaemia • Emergency surgery (after rebleeding) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: did not explain sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: did not explain allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done Criteria: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 83 were randomly assigned to epinephrine injection vs epinephrine injection and haemoclip application or band ligation |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were prespecified |
Villanueva 1993.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 63) • Age (years): 62 (mean) • Sex (male/female): 43/20 • Duodenal/gastric ulcer: 31/30 • Forrest group: Ia = 1, Ib = 22, IIa = 40 | |
| Interventions | Interventions • Primary intervention: epinephrine injection (n = 30) vs epinephrine injection and polidocanol injection (n = 33) • Medical treatment: ranitidine 50 mg/6 h intravenously • Second‐look endoscopy: yes • Epinephrine volume: Both groups received epinephrine in equal amounts. Those in group B also received polidocanol; accordingly, the total volume injected was greater (12 mL in epinephrine alone vs 15.2 mL in combined therapy—group B‐) | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate, blood transfusions and mortality
• Mortality criteria: hospital mortality
• Bleeding criteria: fresh hematemesis or melena plus hypovolaemia or fall in haemoglobin requiring transfusion • Emergency surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers Comment: adequate |
| Allocation concealment (selection bias) | Low risk | An opaque sealed envelope Comment: adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done: Blinding is virtually impossible in studies on endoscopic treatment. Review authors believe that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data: 63 participants were randomly assigned. 3 additional participants were excluded, as they were found not to fit with the inclusion criteria on endoscopy (reasons given). Outcomes were reported for all participants who remained in the study |
| Selective reporting (reporting bias) | Unclear risk | The published report includes all expected outcomes, including those that were prespecified |
Villanueva 1996.
| Methods | Randomised trial, not double blinded | |
| Participants | Participant characteristics • Number randomly assigned (n = 78) • Age (years): not specified • Sex (male/female): not specified • Duodenal/gastric ulcer: not specified • Forrest group: Ia/Ib = 25, IIa = 53 | |
| Interventions | Interventions • Primary intervention: epinephrine injection vs epinephrine injection and polidocanol injection • Medical treatment: ranitidine • Second‐look endoscopy: no • Epinephrine volume: not specified | |
| Outcomes |
Outcomes assessed
Bleeding rate, surgery rate and mortality
• Mortality criteria: hospital mortality
• Bleeding criteria: not specified • Emergency surgery |
|
| Notes | Abstract report only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors did not explain sequence generation (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not explain (abstract) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not done: Blinding is virtually impossible in studies on endoscopic treatment. Review authors that this did not introduce significant bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for in the groups to which they were randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Buffoli 2001 | Not randomised |
| Chittmittrapap 2010 | Non‐randomised (retrospective) study |
| Chua 2001 | Not randomised |
| Chung 1990 | Preliminary results published later in another article |
| Chung 1992 | Preliminary results published later in another article |
| Chung 1997a | Preliminary results published later in another article |
| De Goede 1998 | Preliminary results published later in another article |
| Dedeu 2003 | Does not specify number of participants in each group We contacted the author but did not receive an answer |
| Ell 2002 | Does not specify number of participants in each group It was not possible to contact this study author to clarify the results. The study was published only as an abstract, and no contact address was provided |
| Gevers 2002 | Combined epinephrine plus polidocanol in both groups |
| Grgov 2013 | Randomised,(n = 70) but compared (n = 34) participants with endoscopic haemoclip therapy versus (n = 36) participants with epinephrine injection plus endoscopic haemoclip therapy Compared haemoclip with and without epinephrine, does not fit inclusion criteria. Serbo‐Croatian |
| Karaman 2011 | Compares APC or heater probe. Epinephrine injection (5‐6 mL, 1:10,000 dilution) was applied around the ulcer in all participants before both of these 2 methods were applied. Does not fit our inclusion criteria (epinephrine vs epinephrine plus second method) |
| Lecleire 2009 | Mallory Weiss not in our inclusion criteria, not a comparison of epinephrine vs epinephrine plus second method |
| Ljubicic 2012 | Compares small volume epinephrine vs large volume epinephrine vs haemoclip alone |
| Male 1999 | Preliminary results published later in another article |
| Pescatore 1999 | Preliminary results published later in another article |
| Sabat 1998 | No definition of further clinically significant bleeding was established, only endoscopic rebleeding. The aim of the study was directed to endoscopic signs, not to clinical rebleeding |
| Taghavi 2009 | Randomised, but not epinephrine vs epinephrine plus second method (i.e. compared epinephrine injection plus argon plasma coagulation vs epinephrine injection plus haemoclips) |
| Tsoi 2009 | Review article, not trial report |
| Wehrmann 1994 | Study was excluded because primary endpoint was not achieved. Included studies must define clinically relevant rebleeding (confirmed by endoscopy), but in this study, investigators considered only endoscopic rebleeding. Results did not allow detection of which participants presented with clinical rebleeding |
Differences between protocol and review
We edited the background text of this review protocol after initial publication by providing updated information.
For studies with more than one active intervention and only one control group, we selected the intervention that most closely matched our inclusion criteria and excluded the others (see Higgins 2011, Chapter 16.5.4).
We completely updated and revised the methods section for clarity, although our methods remain unchanged from the previous version of the review.
We checked all data extracted and amended minor errors.
We updated the tables, content and presentation of the review to be consistent with MECIR reporting standards for systematic reviews. This included the addition of domains to the risk of bias tables and a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart of studies.
We clarified that persistent bleeding is failure of initial haemostasis, and recurrent bleeding is bleeding that recurs after initial haemostasis. Rebleeding rates included both endoscopic and clinical rebleeding.
Contributions of authors
Mercedes Vergara and Xavier Calvet developed the protocol, performed the main search strategy, assessed eligibility, extracted data, performed statistical analyses (meta‐analysis) and wrote the manuscript.
Javier P. Gisbert was involved in reviewing the final manuscript and providing relevant methodological support.
Cathy Bennett updated the review in May 2014, along with Mercedes Vergara.
Mercedes Vergara is responsible for data accuracy and is the guarantor of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
Mercedes Vergara, Xavier Calvet and Javier P Gisbert are investigators of the Centro de Investigación en Red de Enfermedades Hepáticas y Digestivas (CIBERhed). CIBERhed is funded by the Instituto de Salud Carlos III., Spain.
Declarations of interest
Dr Cathy Bennett is the proprietor of Systematic Research Ltd, a company that provides research services and is an employee of that company; Dr Bennett received a consultancy fee, as well as travel expenses for travel to work‐related meetings and conferences. Dr Bennett has received consultancy fees for other Cochrane reviews and for her work in evidence‐based medicine. Dr Bennett is a member of the data monitoring committee for the BOSS clinical trial; this work is not related to writing of systematic reviews.
Joint first author
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Balanzo 1990 {published data only}
- Balanzo J, Villanueva C, Sainz S, Espinos JC, Mendez C, Guarner C, et al. Injection therapy of bleeding peptic ulcer. A prospective randomized trial using epinephrine and thrombin. Endoscopy 1990;22:157‐9. [PUBMED: 2209496] [DOI] [PubMed] [Google Scholar]
Choudari 1994 {published data only}
- Choudari CP, Palmer KR. Endoscopic injection therapy for bleeding peptic ulcer: a comparison of adrenaline alone with adrenaline plus ethanolamine oleate. Gut 1994;35:608‐10. [PUBMED: 8200551] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 1993 {published data only}
- Chung SC, Leung JW, Leong HT, Lo KK, Li AK. Adding a sclerosant to endoscopic epinephrine injection in actively bleeding peptic ulcers: a randomized trial. Gastrointestinal Endoscopy 1993;39:611‐5. [PUBMED: 8224679] [DOI] [PubMed] [Google Scholar]
Chung 1996 {published data only}
- Chung SC, Leong HT, Chab AC, Lau JY, Yung MY, Leung JW, Li AK. Epinephrine or epinephrine plus alcohol for injection of bleeding ulcers: a prospective randomized trial. Gastrointestinal Endoscopy 1996;43:591‐5. [PUBMED: 8781939] [DOI] [PubMed] [Google Scholar]
Chung 1997 {published data only}
- Chung SS, Lau JY, Sung JJ, Chan AC, Lai CW, Ng EK, et al. Randomised comparison between adrenaline injection alone and adrenaline injection plus heat probe treatment for actively bleeding ulcers. BMJ 1997;314:1307‐11. [PUBMED: 9158465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 1999 {published data only}
- Chung IK, Ham JS, Kim HS, Park SH. Comparison of the hemostatic efficacy of the endoscopic hemoclip method with hypertonic saline epinephrine injection and a combination of the two for the management of bleeding peptic ulcers. Gastrointestinal Endoscopy 1999;49:13‐8. [PUBMED: 9869717] [DOI] [PubMed] [Google Scholar]
Garrido 2002 {published data only}
- Garrido A, Guerrero FJ, Perianes C, Arenas FJ, Palomo S. Local therapeutic injection in bleeding peptic ulcer: a comparison of adrenaline to adrenaline plus a sclerosing agent [Inyección local terapeutica en la ulcera gastroduodenal sangrante: estudio comparativo de adrenalina frente adrenalina más agente esclerosante]. Revista Española de Enfermedades Digestivas 2002;94(7):395‐400. [PUBMED: 12432836] [PubMed] [Google Scholar]
Grgov 2012 {published data only}
- Grgov S, Stamenković P, Janjić D. Comparison of haemostatic efficacy for endoscopic injection therapy of epinephrine and combination therapy of epinephrine and hemoclips for bleeding peptic ulcers. Srpski Arhiv za Celokupno Lekarstvo 2012; Vol. 140, issue 5‐6:299‐304. [PUBMED: 22826982] [DOI] [PubMed]
Kubba 1996 {published data only}
- Kubba AK, Murphy W, Palmer KR. Endoscopic injection for bleeding peptic ulcer: a comparison of adrenaline alone with adrenaline plus human thrombin. Gastroenterology 1996;111(3):623‐8. [PUBMED: 8780566] [DOI] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee MH, Jung HY, Yang SK, Kim HR, Homg WS, Min YI. Epinephrine alone versus epinephrine plus ethanol for injection therapy of bleeding peptic ulcers. Gastrointestinal Endoscopy 1997; Vol. 45:A283.
Lin 1993 {published data only}
- Lin MJ, Peung CL, Lee SD. Is sclerosant injection mandatory after an epinephrine injection for arrest of peptic ulcer hemorrhage? A prospective, randomised, comparative study. Gut 1993;34:1182‐5. [PUBMED: 8406150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 1999 {published data only}
- Lin HJ, Tseng GY, Perng CL, Lee FY, Chang FY, Lee SD. Comparison of adrenaline injection and bipolar electrocoagulation for the arrest of peptic ulcer bleeding. Gut 1999;44:715‐9. [PUBMED: 10205211] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2006 {published data only}
- Lo C‐C, Hsu P‐J, Lo G‐H, Lin C‐K, Chan H‐L, Tsai W‐L, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high‐risk bleeding ulcers. Gastrointestinal Endoscopy 2006;63(6):767‐73. [PUBMED: 16650535] [DOI] [PubMed] [Google Scholar]
Loizou 1991 {published data only}
- Loizou LA, Bown SG. Endoscopic treatment for bleeding peptic ulcers: randomized comparison of adrenaline injection and adrenaline injection + Nd YAG laser photocoagulation. Gut 1991;32(10):1100‐3. [PUBMED: 1955161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2004 {published data only}
- Park CH, Joo YE, Kim HS, Choi SK, Rew JS, Kim SJ. A prospective, randomized trial comparing mechanical methods of hemostasis plus epinephrine injection to epinephrine injection alone for bleeding peptic ulcer. Gastrointestinal Endoscopy 2004;60(2):173‐9. [PUBMED: 15278040] [DOI] [PubMed] [Google Scholar]
Pescatore 2002 {published data only}
- Pescatore P, Jormod P, Borovicka J, Pantoflickova D, Suter W, Meyenberger C, et al. Epinephrine versus epinephrine plus fibrin glue injection in peptic ulcer bleeding: a prospective randomized trial. Gastrointestinal Endoscopy 2002;55(3):348‐53. [PUBMED: 11868007] [DOI] [PubMed] [Google Scholar]
Sollano 1991 {published data only}
- Sollano SD, Ang VN, Moreno JA. Endoscopic hemostasis of bleeding peptic ulcers :1:10000 adrenalin injection vs 1:10000 adrenalin injection vs heater probe. Gastroenterologia Japonica 1991;26(Suppl 3):83‐5. [PUBMED: 1884968] [DOI] [PubMed] [Google Scholar]
Villanueva 1993 {published data only}
- Villanueva C, Balanzo J, Espinos JC, Fabrega E, Sainz S, Gonzalez D, et al. Endoscopic injection therapy of bleeding ulcer: a prospective and randomized comparison of adrenaline alone or with polidocanol. Journal of Clinical Gastroenterology 1993;17(3):195‐200. [PUBMED: 8228078] [DOI] [PubMed] [Google Scholar]
Villanueva 1996 {published data only}
- Villanueva C, Balanzo J, Sabat M, Gallego A, Ortiz J, Soriano G, et al. Injection therapy alone or with endoscopic hemoclip for bleeding peptic ulcer. Preliminary results of a randomized trial. Gastrointestinal Endoscopy 1996; Vol. 43:A281.
References to studies excluded from this review
Buffoli 2001 {published data only}
- Buffoli F, Graffeo M, Nicosia F, Gentile C, Cesar P, Rotti F, et al. Peptic ulcer bleeding comparison of two hemostatic procedures. American Journal of Gastroenterology 2001;96:89‐94. [DOI] [PubMed] [Google Scholar]
Chittmittrapap 2010 {published data only}
- Chittmittrapap S, Limmathurotsakul D, Rerknimitr R, Ridtitid W, Kongkam P, Treeprasertsuk S, et al. A retrospective study comparing the results of argon plasma coagulation versus bipolar probe coaptation as a treatment for high risk peptic ulcer bleeding. Gastrointestinal Endoscopy 2010; Vol. Conference, issue var.pagings:5.
Chua 2001 {published data only}
- Chua TS, Fock KM, Ng TM, Khor JL, Theo EK. Treatment of bleeding peptic ulcers with combination of epinephrine injection and endoscopic hemoclip application. Gastroenterology 2001; Vol. 120:A4370.
Chung 1990 {published data only}
- Chung SC, Leung JW, Leung HT, Lo KK, Griffin SM, Li AK. Does adding a sclerosant improve the results of endoscopic epinephrine injection in actively bleeding ulcers? An interim report of a randomized trial. Gastrointestinal Endoscopy 1992;38:A21. [DOI] [PubMed] [Google Scholar]
Chung 1992 {published data only}
Chung 1997a {published data only}
- Chung IK, Kim HS, Park SH, Lee MH, Kim SJ. The comparison of hemostatic efficacy of endoscopic hemoclip, hypertonic saline epinephrine injection and combined method in treatment for bleeding peptic ulcer. Gastrointestinal Endoscopy 1997;45(4):AB88. [DOI] [PubMed] [Google Scholar]
Dedeu 2003 {published data only}
- Dedeu JM, Villanueva C, Gomez C, Minana JM, Sola‐Vera J, Gallego A, et al. Endoscopic injection alone or combined with bipolar electrocoagulation for bleeding peptic ulcer. Preliminary results of a prospective and randomized trial. Gastrointestinal Endoscopy 2003; Vol. 49:A439.
De Goede 1998 {published data only}
- Goede E, Gevers AM, Simoens M, Hiele M, Rutgeerts P. Interim results of a randomized trial comparing injection therapy with epinephrine polidocanol 1% versus hemoclip vs injection combined with hemoclip for bleeding ulcers. Gastrointestinal Endoscopy 1998;53:A234. [DOI] [PubMed] [Google Scholar]
Ell 2002 {published data only}
- Ell C. Endoclip versus injection therapy in bleeding gastroduodenal ulcers: results of a prospective randomized multicenter trial. Gastroenterology 2002; Vol. 122:A1542.
Gevers 2002 {published data only}
- Gevers AM, Goede E, Simoens M, Hiele M, Ruteerts P. A randomized trial comparing injection therapy with hemoclip and with injection combined with hemoclip for bleeding ulcers. Gastrointestinal Endoscopy 2002;55:466‐9. [DOI] [PubMed] [Google Scholar]
Grgov 2013 {published data only}
- Grgov S, Radovanović‐Dinić B, Tasić T. Could application of epinephrine improve hemostatic efficacy of hemoclips for bleeding peptic ulcers? A prospective randomized study. Vojnosanitetski Pregled: Military Medical & Pharmaceutical Journal of Serbia & Montenegro 2013; Vol. 70, issue 9:824‐9. [0042‐8450] [DOI] [PubMed]
Karaman 2011 {published data only}
- Karaman A, Baskol M, Gursoy S, Torun E, Yurci A, Ozel BD, et al. Epinephrine plus argon plasma or heater probe coagulation in ulcer bleeding. World Journal of Gastroenterology. China: Department of Gastroenterology, Erciyes University, 38030 Kayseri, Turkey. drkaraman@hotmail.com, 2011; Vol. 17, issue 36:4109‐12. [1007‐9327] [DOI] [PMC free article] [PubMed]
Lecleire 2009 {published data only}
- Lecleire S, Antonietti M, Iwanicki‐Caron I, Duclos A, Ramirez S, Ben‐Soussan E, et al. Endoscopic band ligation could decrease recurrent bleeding in Mallory‐Weiss syndrome as compared to haemostasis by hemoclips plus epinephrine. Alimentary Pharmacology & Therapeutics. England: Gastroenterology Department, Digestive Endoscopy Unit, Rouen University Hospital, Rouen Cedex, France. stephane.lecleire@churouen.fr, 2009; Vol. 30, issue 4:399‐405. [1365‐2036] [DOI] [PubMed]
Ljubicic 2012 {published data only}
- Ljubicic N, Budimir I, Biscanin A, Nikolic M, Supanc V, Hrabar D, et al. Endoclips vs large or small‐volume epinephrine in peptic ulcer recurrent bleeding. World Journal of Gastroenterology. China: Department of Internal Medicine, 'Sestre milosrdnice' University Hospital Center, 10000 Zagreb, Croatia. neven.ljubicic@kbcsm.hr, 2012; Vol. 18, issue 18:2219‐24. [1007‐9327] [DOI] [PMC free article] [PubMed]
Male 1999 {published data only}
- Male GY, Lin H, Pergn CH, Lee FY, Chang FY, Lee SD. A prospective randomized trial comparing injection of epinephrine alone, bipolar electrocoagulation for the arrest of peptic ulcer bleeding. Gastrointestinal Endoscopy 1999; Vol. 49:A452.
Pescatore 1999 {published data only}
- Pescatore P, Jorned P, Borovicka J, Suter W, Delative J, Wissel P, et al. Randomized study of epinephrine versus epinephrine + fibrin injection in peptic ulcer bleeding. interim analysis. Gastrointestinal Endoscopy 1999;49:A448. [DOI] [PubMed] [Google Scholar]
Sabat 1998 {published data only}
- Sabat M, Villanueva C, Ortiz J, Sainz S, Torras X, Casso X, et al. Final results of a prospective and randomized trial evaluating endoscopic hemoclip for bleeding peptic ulcer. Gastroenterology 1998;114:G1118. [Google Scholar]
Taghavi 2009 {published data only}
- Taghavi SA, Soleimani SM, Hosseini‐Asl SMK, Eshraghian A, Eghbali H, Dehghani SM, et al. Adrenaline injection plus argon plasma coagulation versus adrenaline injection plus hemoclips for treating high‐risk bleeding peptic ulcers: a prospective, randomized trial. Canadian Journal of Gastroenterology and Hepatology. Canada: Gastroenterohepatology Research Centre, Nemazee Hospital, Shiraz, Iran. ataghavi@sums.ac.ir, 2009; Vol. 23, issue 10:699‐704. [0835‐7900] [DOI] [PMC free article] [PubMed]
Tsoi 2009 {published data only}
Wehrmann 1994 {published data only}
- Wehrmann T, Fremdling D, Leabcke B, Jong M. Epinephrine plus polidocanol versus epinephrine alone for endoscopic treatment of peptic ulcer haemorrhage. a prospective, randomized trial. European Journal of Gastroenterology and Hepatology 1994;6:1033‐8. [Google Scholar]
Additional references
ASGE 2004
- American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of endoscopy in acute non‐variceal upper GI hemorrhage. Gastrointestinal Endoscopy 2004;60:497‐504. [DOI] [PubMed] [Google Scholar]
British Society 2002
- British Society of Gastroenterology Endoscopy Committee. Non variceal upper gastrointestinal hemorrhage guidelines. Gut 2002;51:1‐6. [Google Scholar]
Brullet 1996
- Brullet E, Calvet X, Campo R, Rue M, Calot L, Donoso L. Factors predicting failure of endoscopic injection therapy in bleeding duodenal ulcer. Gastrointestinal Endoscopy 1996;43:111‐6. [DOI] [PubMed] [Google Scholar]
Cook 1992
- Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta‐analysis. Gastroenterology 1992;102:139‐48. [DOI] [PubMed] [Google Scholar]
Cooper 1999
- Cooper GS, Chak A, Way LE. Early endoscopy in upper gastrointestinal haemorrhage associations with recurrent bleeding, surgery, and length of hospital stay. Gastrointestinal Endoscopy 1999;49:145‐52. [DOI] [PubMed] [Google Scholar]
Feu 2003
- Feu F, Brullet E, Calvet X, Fernandez‐Llamazares J, Guardiola J, Moreno P, et al. Guidelines for the diagnosis and treatment of acute non‐variceal upper gastrointestinal bleeding. Gastroenterologia y Hepatologia 2003;26:70‐85. [DOI] [PubMed] [Google Scholar]
Forrest 1974
- Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet 1974;2:394‐7. [DOI] [PubMed] [Google Scholar]
Gisbert 2001
- Gisbert JP, Gonzalez L, Calvet X, Roque M, Gabriel R, Pajares JM. Proton pump inhibitors versus H2 antagonists: a meta‐analysis of their efficacy in treating bleeding peptic ulcer. Alimentary Pharmacology and Therapeutics 2001;15:917‐28. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hwang 2012
Kovacs 2008
- Kovacs TO. Management of gastroduodenal peptic bleeding. Current Gastroenterology Reports 2008;10:535‐42. [DOI] [PubMed] [Google Scholar]
Laine 2003
- Laine L. Endoscopic therapy for bleeding ulcers:room for improvement?. Gastrointestinal Endoscopy 2003;57:557‐60. [DOI] [PubMed] [Google Scholar]
Laine 2009
- Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence‐based approach based on meta‐analyses of randomized controlled trials. Clinical Gastroenterology and Hepatology 2009;7:33‐47. [DOI] [PubMed] [Google Scholar]
Laine 2012
- Laine L, Jensen DM. Management of patients with ulcer bleeding. American Journal of Gastroenterology 2012;107:345‐60. [DOI: 10.1038/ajg.2011.480;] [DOI] [PubMed] [Google Scholar]
Lin 2002
- Lin HJ, Hsleh YH, Tseng GY, Perng GL, Chang FY, Lee SD. A prospective, randomized trial of large‐ versus small‐volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointestinal Endoscopy 2002;55:615‐9. [DOI] [PubMed] [Google Scholar]
Llach 1996
- Llach L, Bordas JM, Salmeron JM, Panes J, Garcia Pagan JC, Feu Fet al. A prospective randomized trial of heater probe thermocoagulation versus injection therapy in peptic ulcer hemorrhage. Gastrointestinal Endoscopy 1996;43:117‐20. [DOI] [PubMed] [Google Scholar]
Marmo 2003
- Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding; is a second look necessary? A meta‐analysis. Gastrointestinal Endoscopy 2003;57:62‐7. [DOI] [PubMed] [Google Scholar]
NICE 2012
- NICE. Acute upper gastrointestinal bleeding: management. NICE clinical guideline 141, June 2012; Vol. guidance.nice.org.uk/cg141.
Peter 2008
- Peter S, Wilcox CM. Modern endoscopic therapy of peptic ulcer bleeding. Digestive Diseases 2008;20:291‐9. [DOI] [PubMed] [Google Scholar]
Randall 1989
- Randall GM, Jensen DM, Hirabayashi K, Machicado GA. Controlled study of different sclerosing agents for coagulation of canine gut arteries. Gastroenterology 1989;96:1274‐81. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ritgeers 1989
- Rutgeerts P, Geobes K, Vantrapen G. Experimental studies of injection therapy for severe nonvariceal bleeding in dogs. Gastroenterology 1989;97:610‐21. [DOI] [PubMed] [Google Scholar]
Rollhauser 2000
- Rollhauser C, Fleischer DE. Current status of endoscopic therapy for ulcer bleeding. Best Practice and Research. Clinical Gastroenterology 2000;14:391‐410. [DOI] [PubMed] [Google Scholar]
Savides 2000
- Savides TJ, Jensen DM. Therapeutic endoscopy for nonvariceal gastrointestinal bleeding. Gastroenterology Clinics of North America 2000;29:465‐87. [DOI] [PubMed] [Google Scholar]
Simoens 2001
- Simoens M, Rutgeerts P. Non variceal upper gastrointestinal bleeding. Best Practice and Research. Clinical Gastroenterology 2001;15:121‐33. [DOI] [PubMed] [Google Scholar]
Spiegel 2003
- Spiegel BM, Ofman JJ, Woods K, Vakil NB. Minimizing recurrent peptic ulcer haemorrhage after endoscopic hemostasis::the cost effectiveness of competing strategies. The American Journal of Gastroenterology 2003;98:86‐97. [DOI] [PubMed] [Google Scholar]
Yuan 2008
- Yuan Y, Wang C, Hunt RH. Endoscopic clipping for acute non variceal for upper GI bleeding: a meta‐analysis and critical appraisal of randomized controlled trials. Gastrointestinal Endoscopy 2008;68(2):339‐51. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Vergara 2007
- Vergara M, Calvet X, Gisbert J. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcer disease. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005584] [DOI] [PubMed] [Google Scholar]


