Visual Abstract
Key Words: drug development, peripherally restricted, pulmonary arterial hypertension, serotonin
Highlights
-
•
5-HT2B has been identified previously as a potential therapeutic target for treatment of PAH; however, the potential of unwanted neural effects of targeting the receptor has prevented this strategy from advancing.
-
•
Here, the authors have developed a novel, selective, and systemically restricted 5-HT2B partial agonist and verified treatment efficacy of tool compound (VU6047534) in preclinical models of PAH.
-
•
The authors have also identified next-generation compounds in the chemical series that are predicted to be systemically restricted in humans via P-gp-mediated efflux to enable clinical translation.
Summary
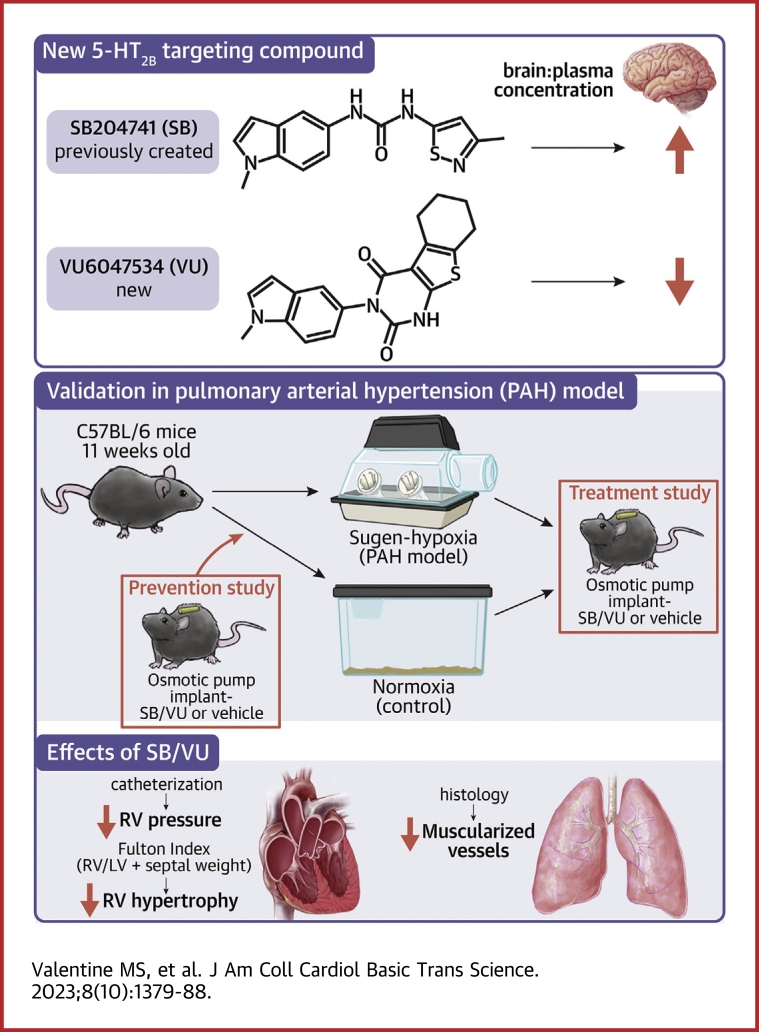
Ligands for the serotonin 2B receptor (5-HT2B) have shown potential to treat pulmonary arterial hypertension in preclinical models but cannot be used in humans because of predicted off-target neurological effects. The aim of this study was to develop novel systemically restricted compounds targeting 5-HT2B. Here, we show that mice treated with VU6047534 had decreased RVSP compared with control treatment in both the prevention and intervention studies using Sugen-hypoxia. VU6047534 is a novel 5-HT2B partial agonist that is peripherally restricted and able to both prevent and treat Sugen-hypoxia–induced pulmonary arterial hypertension. We have synthesized and characterized a structurally novel series of 5-HT2B ligands with high potency and selectivity for the 5-HT2B receptor subtype. Next-generation 5-HT2B ligands with similar characteristics, and predicted to be systemically restricted in humans, are currently advancing to investigational new drug–enabling studies.
Pulmonary arterial hypertension (PAH) is a progressive lethal disease characterized by widespread obstruction in the smallest arteries of the lungs. Pulmonary vascular obstruction leads to increased pulmonary vascular resistance, which subsequently causes right heart failure. Prevalence of PAH is 25 cases per million,1 which represents more than 8,200 PAH patients in the United States. Its notorious mortality continues in the current era, as one-third of all patients die within 3 years. Vigorous debate surrounds whether current therapies have any impact on overall survival.2,3 Currently, there are 13 U.S. Food and Drug Administration–approved drugs available for patients with PAH; however, most have significant adverse side effects, they cost over $100,000/y, and none modify the pathophysiology of the disease.4
Despite the existing need for disease-modifying therapeutics, the rational selection of targets has been significantly hampered by the poor understanding of the cellular and molecular mechanisms that mediate PAH pathogenesis. Small vessel remodeling, an active pathologic process that results in thickened, muscularized arteriole walls and obliteration of the vessel lumen, is ultimately responsible for the clinical symptoms associated with PAH. Serotonin signaling is a known contributor to PAH because of appetite suppressants,5 and serotonin 2B receptor (5-HT2B) is considered to be one of the primary culprits.6,7 Of note, genetic deletion of 5-HT2B in mice prevents them from developing elevated right ventricular systolic pressure (RVSP) and right ventricle (RV) hypertrophy caused by hypoxia and dexfenfluramine treatments.7 Moreover, in an elegant follow-up study, bone marrow (BM) transplants between WT and 5-HT2B null mice revealed that the presence of 5-HT2B in the BM compartment was the key initiator of PAH in the hypoxia disease model.8 We have shown that targeting 5-HT2B with antagonists in the heritable mouse model of PAH (BMPR2 mutation9) prevents increased RVSP, microvessel muscularization, and Src tyrosine signaling.10
Expanding on the previous study,8 we recently demonstrated that bone marrow (BM)-derived proangiogenic cells (PACs), which are a subtype of myeloid cells, are the direct mediators of small vessel remodeling in the Sugen-hypoxia (Su/Hx) mouse model of PAH through a 5-HT2B-dependent mechanism.11 By selectively ablating PACs utilizing a transgenic mouse model, we successfully prevented and reversed the development of elevated pulmonary pressures. PAC ablation also reduced markers of small vessel remodeling and restored vessel wall compliance to normal levels. We then illustrated the effectiveness of 5-HT2B antagonism in preventing experimental PAH in the same disease model and, through lineage tracing of hematopoietic cells, showed reduced recruitment and altered gene expression profiles of PACs in animals treated with a pharmacological inhibitor of 5-HT2B.
Mechanistically, we have previously shown that 5-HT2B antagonism prevents PAH and disease by suppressing Src tyrosine trafficking and signaling.10,12,13 While 5-HT2B antagonism shows tremendous promise for PAH therapy, current embodiments penetrate the blood-brain barrier. 5-HT2B inhibition, through genetic mutation, in the central nervous system is associated with several adverse consequences, such as depression, aggression, impaired sleep, and suicidality that limit the clinical potential of this treatment.14, 15, 16 Therefore, to overcome this limitation, we have developed novel 5-HT2B ligands that are both selective and potent, but also systemically restricted such that they will not cause neurological side effects.
Methods
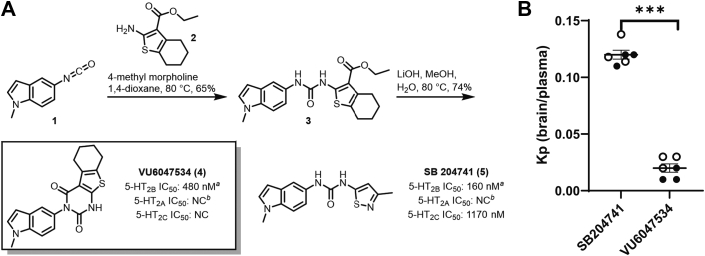
Drawing on a previously disclosed report by Forbes et al17 detailing a series of indole ureas as high affinity 5-HT2B antagonists, we sought to identify next-generation molecules with improved selectivity for the 5-HT2B subtype that would also be peripherally restricted in rodent models of PAH. Toward this end, our initial strategy focused on modifications to the isothiazole pendant (as drawn) of SB204741 ([5] in Figure 1A). We envisioned that the addition of polar modifications to the molecule at this position would likely lead to peripheral restriction through P-glycoprotein (P-gp) efflux mechanisms and/or low membrane permeability. Accordingly, we first examined an array of commercially available substituted thiophenes (see Supplemental Figures 1A and 1B) with the intention of modifying the eastern carboxylate (as drawn) with various polar pendants through standard amide bond-forming chemistry. Ultimately, we found that spontaneous cyclization under basic hydrolysis conditions yielded the VU6047534 chemotype (see Supplemental Figure 1). This compound was selected for further profiling, out of an initial ∼200 compounds assessed, based on its potency and high subtype selectivity relative to 5-HT2A and 5-HT2C.
Figure 1.
Design and Evaluation of VU6047534
(A) Radioligand binding assays for 5-HT2B, 5-HT2A and 5-HT2C subtypes for VU6047534 compared to SB204741 by Eurofins Panlabs Discovery Services (Taiwan) according to standard protocols. NC = not calculated; all % inhibition < 50. Details can be found in Supplemental Figure 1. (B) Comparison between the ratio of total brain concentration to total plasma concentration (Kp) for SB204741 and VU6047534 in C57BL/6 mice 15 minutes post administration (i.p.). Open circles are female mice and filled circles are male mice. Data presented as mean ± SEM and compared with unpaired t-test, ∗∗∗P < 0.001.
We then utilized a common preclinical model of PAH—Su/Hx—to assess the efficacy of VU6047534 comparatively with SB204741, realizing that no animal model completely mimics the human form of PAH.18 For all studies, VU6047534 was given at 10 mg/kg/d and SB204741 was given at 1 mg/kg/d via osmotic pumps; the rationale for this dosing was based off of previously reported IC50 for SB204741 of 39.3 nmol/L.19
Eleven-week-old male C57BL/6 mice were purchased from Jackson Laboratory. Two Su/Hx PAH studies were done with either 5-HT2B compound given preventatively at the time of disease induction or therapeutically after the development of disease (Supplemental Figures 2 and 3). At the conclusion of each study, RVSP was measured in a closed-chest procedure, and RV hypertrophy was assessed with Fulton Index (FI = RV weight/[left ventricle weight + septum]). Partially and fully muscularized vessels in the lung were quantified from immunofluorescent images.
Finally, in a separate cohort of mice, osmotic pumps were implanted for 2 weeks and then mice were assessed for a battery of neurobehavioral tests to determine if either VU6047534 or SB204741 resulted in off-target CNS effects.
All animal experiments complied with institutional and national requirements for the care and use of laboratory animals with full Institutional Animal Care and Use Committee approval. All results are presented as mean ± SEM. GraphPad Prism was used for all statistical analyses. Comparisons between 2 groups were performed using an unpaired Student’s t-test, whereas comparisons among multiple groups (± Su/Hx or ± PAB with ± drug treatment) used a 1-way analysis of variance followed by Tukey’s post hoc test for multiple pairwise comparisons. A P value ≤0.05 was considered statistically significant.
Results
VU6047534 is selective for 5-HT2B and systemically restricted in rodents
After synthetic optimization and an initial round of medicinal chemistry (∼200 compounds in total, Supplemental Figures 1A and 1B), VU6047534 was chosen for further profiling based on its good 5-HT2B potency (480 nmol/L) and high selectivity relative to 5-HT2A and 5-HT2C (no significant inhibition demonstrated up to 10 μmol/L concentration). Additionally, an ancillary pharmacology screen revealed no significant off-target responses for VU6047534 across a panel of 68 common membrane proteins, ion channels, and transporters at 10 μmol/L concentration, including the human ether-a-go-go related gene potassium channel (Supplemental Table 1). Additionally, VU6047534 displayed no cytochrome P450 inhibition up to 30 μmol/L across 4 major isoforms (1A2, 2C9, 2D6, and 3A4).20
Encouraged by the remarkable selectivity of VU6047534 (Figure 1A), we next sought to assess the pharmacokinetic profile and level of CNS-penetration in mice. In a study of brain to plasma levels in male and female C57BL/6 mice, VU6047534 was found to have minimal brain concentrations across all time points tested after intraperitoneal administration at 10 mg/kg (Supplemental Figure 4, Supplemental Tables 2 and 3) and the total brain to total plasma partition coefficient (Kp) was 0.02 after 15 minutes (Figure 1B), which was Tmax. In a similar study in mice, SB204741 was found to have significantly higher brain penetration with a Kp of 0.12 (Figure 1B, Supplemental Figure 5, Supplemental Tables 4 and 5). Neither compound altered mouse neurobehavioral outcomes after chronic exposure for 2 weeks (Supplemental Figures 6 and 7).
Based on its structural similarity to SB204741 and potent activity in the 5-HT2B radioligand binding assay, we initially surmised that the profile of VU6047534 in functional assays would be comparable to SB204741. Interestingly, in several 5-HT2B functional readouts, VU6047534 appears to behave as a weak partial agonist at high concentrations, whereas SB204741 behaves as a true antagonist in the same assays (Supplemental Figure 6). Because of the robust potency of VU6047534 in the context of radioligand binding, it is reasonable to assume that the compound has inhibitory actions across the physiologically relevant concentrations for the present study, and any agonist activity would only manifest at much higher concentrations.
Systemically restricted 5-HT2B ligand prevents experimental PAH
C57BL6/J mice received subcutaneous osmotic pump implants delivering the 5-HT2B ligands, SB204741 (1 mg/kg/d) or VU6047534 (10 mg/kg/d), whereas control mice received vehicle control (50% DMSO/50% PEG300) before being placed in either normoxia or hypoxia (10% O2) conditions for a duration of 3 weeks (Supplemental Figure 2). Rationale for the dosing regimen is detailed in the Methods section. The hypoxia mice also received Sugen (SU5416), which is a VEGFR2 inhibitor, at 20 mg kg−1 week−1 intraperitoneally while being maintained in the hypoxia environments to induce experimental PAH.
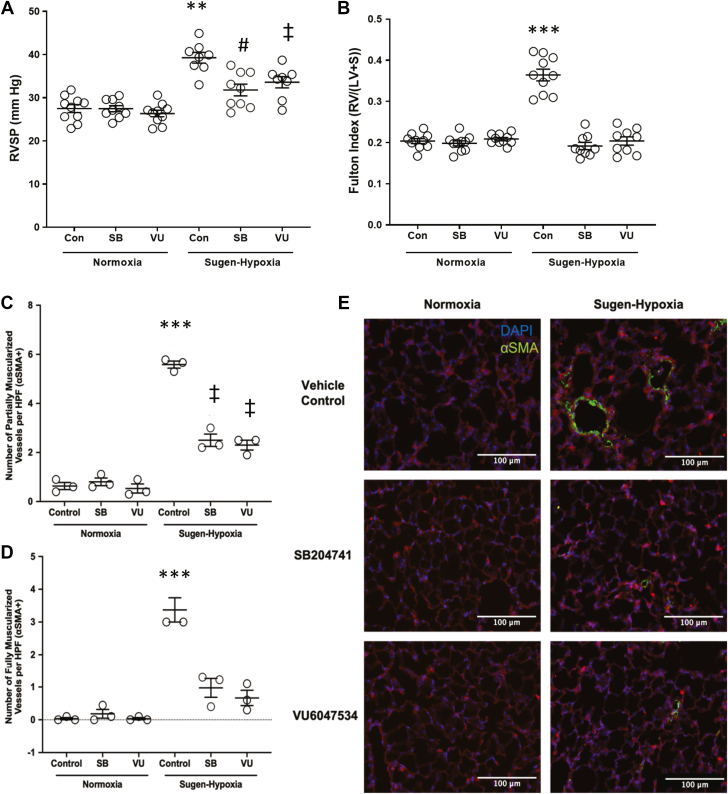
Following right heart catheterization at the termination of experimental PAH induction, mice that received either SB204741 or VU6047534 had significantly reduced RVSP compared with vehicle control-treated Su/Hx mice (Figure 2A). Furthermore, there was no difference between the 2 5-HT2B ligands administered on changes to RVSP. Similarly, RV hypertrophy was considerably diminished in Su/Hx mice that were treated with SB204741 or VU6047534 when compared with vehicle control-treated Su/Hx mice and were comparable to the normoxia groups (Figure 2B). As with the pressures measured, there was also no detectable difference in RV hypertrophy between SB204741 or VU6047534.
Figure 2.
Targeting the 5-HT2B Receptor Prevents Elevated RVSP and Fulton Index in Experimental Pulmonary Arterial Hypertension
(A) 5-HT2B ligands, SB204741 (SB) and VU6047534 (VU) , prevent right ventricular systolic pressure (RVSP) elevation compared with nondrug-treated mice exposed to Sugen-hypoxia. (B) SB204741 and VU6047534 prevent elevated Fulton Index in mice exposed to Sugen-hypoxia. (C to E) Animals exposed to Sugen-hypoxia have significantly more muscularized vessels (0- to 25-μm diameter) than normoxic control animals, and SB204741 and VU6047534 significantly reduce the number of partially and fully muscularized vessels in Sugen-hypoxia exposed animals. Data presented as mean ± SEM. ∗∗P < 0.01 and ∗∗∗P < 0.001 vs all other experimental groups; #P < 0.05 vs control and VU6047534 normoxia groups; and ‡P < 0.01 vs normoxia groups. All statistics analyzed with 2-way analysis of variance followed by Tukey post hoc
test for multiple comparisons. HPF = high-powered field; LV = left ventricle; RV = right ventricle; S = septum; SMA = smooth muscle actin.
We also evaluated the number of muscularized small vessels to quantify PAH vascular remodeling in these experimental groups. We found that the number of partially and fully muscularized small vessels (0-25 μm in size) were increased in vehicle-treated Su/Hx mice following 3 weeks of disease induction (Figures 2C to 2E). Targeting of 5-HT2B with SB204741 or VU6047534 reduced the number of both partially and fully muscularized small vessels when administrating treatment before induction of experimental PAH, with a more modest effect on larger vessel sizes (25-50 μm and 50-100 μm) (Supplemental Figure 8).
Systemically restricted 5-HT2B ligand treats established experimental PAH
After investigating the therapeutic effects with the pharmacological inhibition of 5-HT2B using SB204741 and VU6047534 as a preventative measure, we assessed 5-HT2B targeting as an interventional treatment (Supplemental Figure 3). C57BL6/J mice were placed in either normoxia or hypoxia conditions for an initial duration of 2 weeks. Mice were then implanted with subcutaneous osmotic pumps delivering the 5-HT2B compounds SB204741 or VU6047534, whereas control mice received vehicle control. Following, mice were placed back into their respective normoxia or hypoxia chambers for an additional 2 weeks. The hypoxia mice also received Sugen at 20 mg kg−1 week−1 (intraperitoneally) while being maintained in the hypoxia environments for the total of 4 weeks to induce experimental PAH.
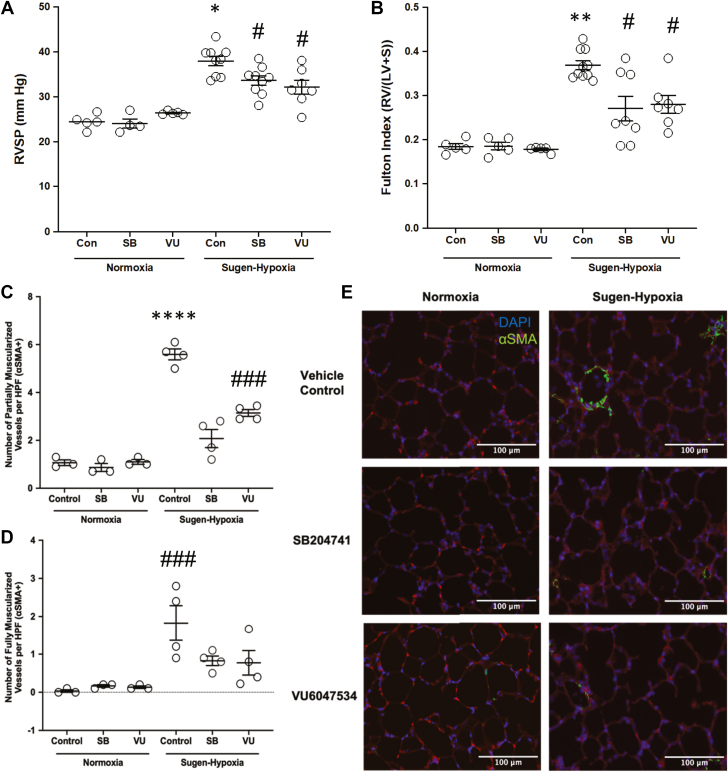
We found that mice receiving either SB204741 or VU6047534 after 2 weeks of experimental PAH induction had significantly reduced RVSP vs vehicle control-treated Su/Hx mice (Figure 3A). Additionally, there was also no detectable difference between SB204741 or VU6047534 on deviations to RVSP caused by Su/Hx. This effect was similar to the results from the preventative pharmacological inhibition using the 5-HT2B ligands shown in Figure 2A. RV hypertrophy was also reduced in Su/Hx mice that were treated with SB204741 or VU6047534 when compared with vehicle control-treated Su/Hx mice (Figure 3B).
Figure 3.
Targeting the 5-HT2B Receptor Attenuates Elevated RVSP and Elevated Fulton Index in Experimental Pulmonary Arterial Hypertension
(A) 5-HT2B ligands, SB204741 and VU6047534, attenuate RVSP elevation compared with nondrug-treated mice exposed to Sugen-hypoxia. (B) SB204741 and VU6047534 prevent elevated Fulton Index in mice exposed to Sugen-hypoxia. (C to E) Animals exposed to
Sugen-hypoxia have significantly more muscularized vessels (0 to <25-μm diameter) than normoxic control animals, and SB204741 and VU6047534 significantly reduces the number of partially, but not fully muscularized vessels in Sugen-hypoxia exposed animals. Data presented as mean ± SEM. ∗P < 0.05 and ∗∗∗∗P < 0.0001 vs all other experimental groups: #P < 0.05, ##P < 0.01, and ###P < 0.001 vs normoxia groups. All statistics analyzed with 2-way analysis of variance followed by Tukey post hoc test for multiple comparisons. Abbreviations as in Figure 2.
The number of partially and fully muscularized small vessels (0-25 μm) were increased in vehicle-treated mice after 4 weeks of Su/Hx injury for disease induction (Figures 3C to 3E). 5-HT2B inhibition for 2 weeks with SB204741 or VU6047534 following 2 weeks of experimental PAH induction reduced the number of partially, but not fully, muscularized small vessels. Results of 5-HT2B inhibition on larger vessel sizes were similar (25-50 and 50-100 μm) (Supplemental Figure 9).
Next-generation, peripherally restricted 5-HT2B compounds for investigational new drug–enabling studies
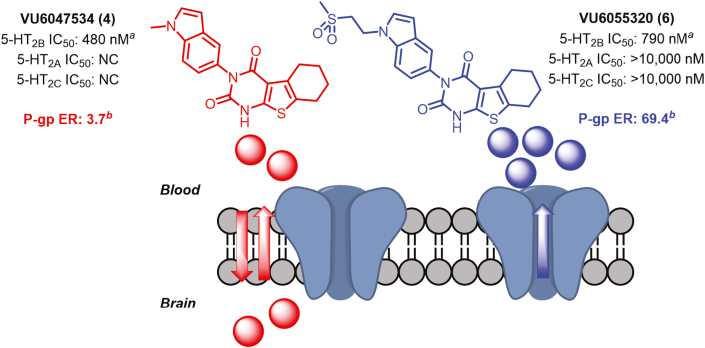
Despite the low brain exposure observed in rodents, we found that VU6047534 was predicted to have moderate brain penetration in MDR1-MDCK cell monolayers, indicating the potential for higher brain exposure in human subjects caused by lack of P-glycoprotein (P-gp)-mediated efflux (PappA-B [10−6 cm/s] = 18.0, PappB-A [10−6 cm/s] = 66.2; efflux ratio = 3.67). To engender this series with higher predicted P-gp efflux, indole N-1 modifications were explored (Figure 4). Briefly, N-substitutions with high polar surface area were found to yield molecules with similar potency and selectivity profiles to VU6047534, but with higher predicted P-gp efflux. Such compounds, as exemplified by VU6055320 (Figure 4), would be expected to have better clinical translatability to humans because of the likely lack of CNS exposure caused by robust P-gp efflux mechanisms.
Figure 4.
Next-Generation 5-HT2B Ligands for Human Translation
VU6055320 is a robust substrate for P-glycoprotein−mediated efflux and is predicted to be highly restricted to the periphery in human subjects. aRadioligand binding assays for 5-HT2B, 5-HT2A, and 5-HT2C subtypes by Eurofins Panlabs Discovery Services (Taiwan) according to standard protocols. NC = not calculated; all % inhibition <50. bP-gp efflux rate
(P-gp ER) data provided by Absorption Systems. Classification of brain penetration potential: high = P-gp ER <3.0; moderate = 10 > P-gp ER >3.0; low = P-gp ER >10.
Discussion
Through chemical optimization, we were able to demonstrate the synthesis of novel 5-HT2B ligands that maintain high potency and selectivity, while also exhibiting peripheral restriction in vivo. Interestingly, VU6047534 is best characterized as a weak partial agonist in 5-HT2B functional experiments, a profile that we had not anticipated at the outset of the present study. Despite this unanticipated finding in vitro, VU6047534 effectively functions as an antagonist in vivo and prevents experimental PAH with Su/Hx enhanced endovascular injury. We were also able to demonstrate that acute treatment targeting 5-HT2B is able to attenuate pressure changes and indications of vascular remodeling following initial experimental PAH induction with Sugen-increased endovascular injury with no adverse effects observed.
Prior evidence of pharmacological inhibition with potent and selective 5-HT2B antagonists have established the therapeutic potential of this treatment approach in several cardiovascular remodeling conditions, including as PAH10,11 and myocardial infarction.13 Additionally, previous studies have demonstrated that antagonism of 5-HT2B in the heart and lungs prevents activity of Src tyrosine kinase,10,13 which is directly involved with pathologic and noncanonical TGF-β1 signaling.12 Previous studies have shown that the 5-HT2B receptor is a critical mediator in experimental PAH with enhanced endovascular injury, and 5-HT2B antagonism was able to successfully prevent the development of disease and reverse established pathology.11 Snider et al13 demonstrated that 5-HT2B antagonism improves outcomes after myocardial infarction, which is believed to occur through blocking the fibrotic process of scar formation and border zone expansion that is driven particularly by the cardiac fibroblasts. Thus, 5-HT2B antagonism for treatment of PAH may function as a “2-hit” strategy because it prevents both the invasion of bone-marrow derived cells into the lung microvasculature and may also prevent fibrotic remodeling of ventricular fibroblasts. Further work is required to determine if 5-HT2B targeting similarly prevents fibrosis in the right ventricle.
Study Limitations
Although the profile and efficacy of these novel compounds are promising, there are additional studies that need to be completed. We found excellent systemic restriction with VU6047534 in mice, but we did not observe any difference in mouse neurobehavioral studies with either VU6047534 or SB204741. This indicates that targeting of 5-HT2B at the doses used here is not enough to alter behavior in mice. It should be noted that the previous reports of impulsivity, sleep impairment, and suicidality were caused by 5-HT2B genetic deletion or mutation in humans or model organisms. We are encouraged that the 10 mg/kg/d VU6047534 did not show any effects on the behavior of the mice; however, we had expected to observe behavior effects with SB204741, which we did not. We note here that the IC50 of SB204741 we quantified was 160 nmol/L (Figure 1A), which was greater than the reported value of 39.3 nmol/L that we based our studies on. Unfortunately, we had already begun the PAH studies and wanted to keep the dosage consistent throughout, so we did not alter the dose. This does indicate that that a more comparable dose for VU6047534 would be 3 mg/kg/d vs 1 mg/kg/d of SB204741.
In addition to these limitations of the current study, toxicology studies of other vital organs are warranted to confirm that there are no off-target effects. The gastrointestinal tracts of both mice and humans are abundant with the 5-HT2B receptor, and therefore, we speculate that a potent and selective antagonist or partial agonist may alter gut motility. It is worth noting, however, that a number of currently marketed psychoactive drugs (predominantly antipsychotics) display robust 5-HT2B activity in radioligand binding assays (<1 nmol/L in many cases), and thus, activity at this receptor need not necessarily preclude a compound from advancement to the clinic (see Supplemental Table 6). In addition to these limitations, experimental PAH mouse models do not fully recapitulate the human disease, and although we have used multiple injury models, evaluation of this compound in higher-order animals prior to humans is necessary.
Conclusions
In the current study, we have synthesized and characterized a series of structurally novel ligands with high potency and selectivity for the 5-HT2B receptor subtype. The 5-HT2B partial agonist VU6047534 is highly efficacious as a preventative treatment in preclinical mouse models of PAH, while also demonstrating interventional capacity as well. Finally, VU6055320 and other identified, selective 5-HT2B ligands within this chemical series that are also predicted to be peripherally restricted in humans should be further investigated and evaluated for their clinical impact.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: For the past 2 decades, there has been no new therapy for PAH, and moreover, none of the current treatments are disease modifying. The serotonin 2B receptor (5-HT2B) has a history of association with PAH, primarily caused by appetite suppressant drug use (ie, Fen-Phen) in the 1990s, which were potent 5-HT2B agonists. We have shown previously that antagonism of 5-HT2B prevents heritable and idiopathic PAH in preclinical models; however, the central penetration of these antagonists prevents their use in humans.
TRANSLATIONAL OUTLOOK: We have developed a tool compound that shows systemic restriction and efficacy in preventing PAH in mouse models. Additionally, we have next-generation compounds that are predicted to be systemically restricted in humans and also similar receptor targeting characteristics. Future work is needed to de-risk these compounds for off-target effects, as well as general safety and toxicity in higher-order species as they progress through investigational new drug–enabling studies.
Funding Support and Author Disclosures
This work was supported by the National Institutes of Health (HL135790, HL095797, and HL087738) and Vanderbilt University Discovery Grant. Research undertaken in the Vanderbilt Mouse Neurobehavior Core was supported by the EKS NICHD of the National Institutes of Health under Award P50HD103537. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
Drs Bender and Lindsley thank the Warren Family and the WKWF for endowing the Warren Center for Neuroscience Drug Discovery.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Humbert M., Sitbon O., Chaouat A., et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M., Sitbon O., Yaici A., et al. for the French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 3.Macchia A., Marchioli R., Tognoni G., et al. Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J. 2010;159:245–257. doi: 10.1016/j.ahj.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Thenappan T., Ormiston M.L., Ryan J.J., Archer S.L. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abenhaim L., Moride Y., Brenot F., et al. International Primary Pulmonary Hypertension Study Group. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 6.Rothman R.B., Baumann M.H., Savage J.E., et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 7.Launay J.M., Herve P., Peoc'h K., et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 8.Launay J.M., Herve P., Callebert J., et al. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood. 2012;119:1772–1780. doi: 10.1182/blood-2011-06-358374. [DOI] [PubMed] [Google Scholar]
- 9.West J., Fagan K., Steudel W., et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 10.West J.D., Carrier E.J., Bloodworth N.C., et al. Serotonin 2B Receptor Antagonism Prevents Heritable Pulmonary Arterial Hypertension. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloodworth N.C., Clark C.R., West J.D., et al. Bone marrow-derived proangiogenic cells mediate pulmonary arteriole stiffening via serotonin 2B receptor dependent mechanism. Circ Res. 2018;123:e51–e64. doi: 10.1161/CIRCRESAHA.118.313397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheson J.D., Ryzhova L.M., Setola V., Merryman W.D. 5-HT(2B) antagonism arrests non-canonical TGF-beta1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol. 2012;53:707–714. doi: 10.1016/j.yjmcc.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snider J.C., Riley L.A., Mallory N.T., et al. Targeting 5-HT2B receptor signaling prevents border zone expansion and improves microstructural remodeling after myocardial infarction. Circulation. 2021;143:1317–1330. doi: 10.1161/CIRCULATIONAHA.120.051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bevilacqua L., Doly S., Kaprio J., et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitychoutis P.M., Belmer A., Moutkine I., Adrien J., Maroteaux L. Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology. 2015;40:2764–2773. doi: 10.1038/npp.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian Y., Cao Y., Deng B., et al. Sleep homeostasis regulated by 5HT2b receptor in a small subset of neurons in the dorsal fan-shaped body of drosophila. Elife. 2017;6 doi: 10.7554/eLife.26519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes I.T., Jones G.E., Murphy O.E., Holland V., Baxter G.S. N-(1-methyl-5-indolyl)-N'-(3-methyl-5-isothiazolyl)urea: a novel, high-affinity 5-HT2B receptor antagonist. J Med Chem. 1995;38:855–857. doi: 10.1021/jm00006a001. [DOI] [PubMed] [Google Scholar]
- 18.Boucherat O., Agrawal V., Lawrie A., Bonnet S. The latest in animal models of pulmonary hypertension and right ventricular failure. Circ Res. 2022;130:1466–1486. doi: 10.1161/CIRCRESAHA.121.319971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Euroscreenfast. Serotonin 5-HT2B Aequorin. https://euroscreenfast.com/assays/serotonin-5-ht2b-aequorin-fast-0506a
- 20.Wenthur C.J., Morrison R., Felts A.S., et al. Discovery of (R)-(2-fluoro-4-((-4-methoxyphenyl)ethynyl)phenyl) (3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM) J Med Chem. 2013;56:5208–5212. doi: 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.