Abstract
Due to the close bond between humans and companion animals, a thorough understanding of the diversity of Cryptosporidium species and Giardia assemblages in cats and dogs is essential to determine the potential zoonotic risks. Analysis of molecular studies shows that C. felis and C. canis are the main species infecting cats and dogs, respectively. These species are largely host-specific, as despite intense association with humans, prevalence of C. felis and C. canis in humans is low and predominantly in immunocompromised individuals and low-income countries. There have been reports of C. parvum in cats and dogs and two reports of C. hominis in dogs. In most studies conducted to date, however, the prevalence of zoonotic species was low and may be associated with coprophagy and or/spillback, but this remains to be determined. Results of subtyping studies suggest that for C. felis and C. canis, some zoonotic transmission may occur but host-adapted subtypes also exist. Giardia duodenalis assemblages C and D are commonly reported in dogs, with assemblages F and A most common in cats. Assemblages C, D and F are largely host-specific as there are only a handful of reports of them in humans. Reports of assemblage A and B in cats and dogs may be due to coprophagy or spillback from owners. Despite the extent of pet ownership and the close contact between humans and companion animals worldwide, the overall risk of zoonotic transmission from cats and dogs to humans is uncertain but thought to be low due to C. canis, C. felis and G. duodenalis assemblages C, D and F being predominantly host-specific, the relatively low prevalence of C. parvum (and C. hominis) in cats and dogs (which may be due to mechanical carriage), and low oo/cyst shedding. Carefully designed epidemiological studies of cats and dogs and their owners using subtyping tools are essential to better quantify the extent of spillover and spillback of Cryptosporidium and Giardia between pets and their owners.
Keywords: Cryptosporidium, Giardia, Cats, Dogs, Zoonotic potential, Zoonosis, Molecular typing
Graphical abstract
Highlights
-
•
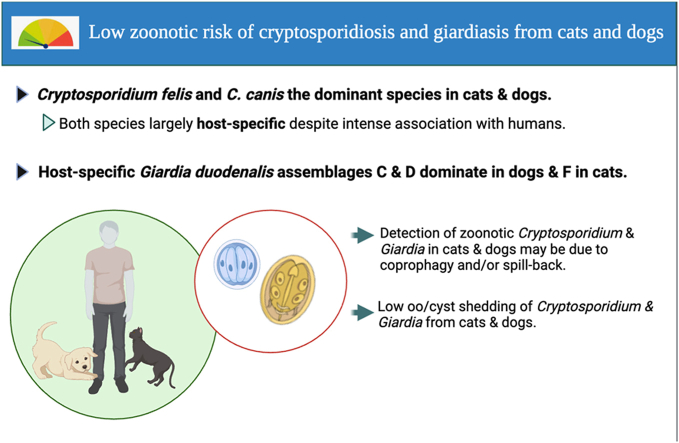
Cryptosporidium felis and Giardia assemblages F and A are dominant in cats.
-
•
Cryptosporidium canis and Giardia assemblages C and D dominate in dogs.
-
•
Prevalence of C. felis and C. canis in immunocompetent humans is low.
-
•
Low oo/cyst shedding of Cryptosporidium and Giardia from cats and dogs.
-
•
Overall zoonotic risk of Cryptosporidium and Giardia in cats and dogs is uncertain but likely to be low.
1. Introduction
Humans and their companion animals, particularly cats and dogs, have had a long and close association over many centuries and provide a wide variety of benefits to their owners and foster carers including increased physical activity, companionship and improvements in mental health (Martins et al., 2023; Zablan et al., 2023). Cat and dog ownership also increases connections with family members and the wider community (Wood et al., 2015), with higher wellbeing and trust, reduced anxiety and reduced screen time particularly in adolescents (Charmaraman et al., 2022; Koyasu et al., 2023).
Pet owner behaviours such as sleeping with pets, allowing pets to lick faces and hands, insufficient/infrequent hand-washing, and feeding raw meat to cats and dogs, can promote the transmission of a variety of zoonotic pathogens from pets to humans. These include Salmonella (Davies et al., 2019), antimicrobial resistant bacteria (Jin et al., 2023), ectoparasites and associated zoonotic pathogens (Tahir et al., 2019; Saleh et al., 2021; Mateo et al., 2023), Toxocara (Patterson, 2023) and other helminths (Overgaauw et al., 2020; Mateo et al., 2023), and enteric protozoa such as Cryptosporidium and Giardia (Cai et al., 2021; Meng et al., 2021).
Cryptosporidium oocysts and Giardia cysts are immediately infectious when shed in faeces and are environmentally robust (Cai et al., 2021; Ryan et al., 2021a). Transmission of Cryptosporidium and Giardia in cats and dogs is via faecal-oral contamination through coprophagy or ingestion of contaminated food or water. Although frequently asymptomatic in adult cats and dogs, Cryptosporidium and Giardia can both cause watery diarrhoea, abdominal pain and vomiting in both humans and young pets (Cai et al., 2021; Li et al., 2021a; Ryan et al., 2021a). Fenbendazole and metronidazole are commonly used to treat clinical giardiasis in cats and dogs, but have poor efficacy (Kaufmann et al., 2022). There is no registered treatment available for cryptosporidiosis in cats and dogs (ESCCAP, 2018). Mixed infections with Giardia and Cryptosporidium and other enteropathogens are common in both dogs and cats, particularly in dogs (Hamnes et al., 2007; Pallant et al., 2015; Xu et al., 2016; Gil et al., 2017; Sommer et al., 2018; López-Arias et al., 2019; Nagamori et al., 2020; Mateo et al., 2023; Murnik et al., 2023).
The 18S ribosomal RNA (rRNA) is the most commonly used locus for identifying Cryptosporidium to species level, while the glycoprotein 60 gene (gp60) is most widely used for subtyping (Ryan et al., 2021a; Yang et al., 2021). Over 49 Cryptosporidium species have been identified to date, with C. hominis and C. parvum responsible for ∼95% of human infections (Ryan et al., 2021a).
Human infections with Giardia are caused by Giardia duodenalis, a multi-species complex, consisting of eight assemblages. Assemblages A and B predominantly infect humans, assemblages C and D infect mostly dogs, assemblage F infects mainly cats, assemblage G infects rodents and assemblage H infects pinnipeds (Cai et al., 2021; Ryan et al., 2021a). The most commonly used typing loci for Giardia are the betagiardin (bg), glutamate dehydrogenase (gdh), triosephosphate isomerase (tpi) genes, and the 18S rRNA locus (Cai et al., 2021; Ryan et al., 2021a). Multilocus sequence typing (MLST) of the bg, gdh and tpi genes has identified three sub-assemblages within assemblage A: sub-assemblage AI (predominantly in livestock, companion animals and some humans); AII (predominantly in humans); and AIII (in wild ruminants) (Cai et al., 2021). In contrast, sub-assemblages within assemblage B, cannot be reliably determined with the currently used loci (Cai et al., 2021).
Due to the close relationship between humans and companion animals, a thorough understanding of the diversity of Cryptosporidium species and Giardia assemblages in cats and dogs is essential to determine their zoonotic risk and public health implications. This review analyses Cryptosporidium species and Giardia assemblages reported in cats and dogs globally, and discusses the zoonotic risk posed by the Cryptosporidium species and Giardia assemblages identified. The majority of the > 350 publications on Cryptosporidium and Giardia in cats and dogs have been epidemiological surveys, with molecular tools used in more recent years, enabling researchers to type the pathogens to species and subtype level. This review centers on the findings and discussions emerging from these molecular studies.
2. Cryptosporidium in cats and dogs
Generally, the overall prevalence of Cryptosporidium in cats and dogs globally is < 10%. However, some studies using microscopy and immunological-based detection methods have reported higher prevalences (> 20%) (e.g. Cirak and Bauer, 2004; Hamnes et al., 2007; Ferreira et al., 2017). A recent systematic review of the prevalence of Cryptosporidium in cats reported the mean prevalence by microscopy, coproantigens and molecular tools to be 4.2%, 8.2%, and 5.0%, respectively, with an overall mean prevalence of 6% (Meng et al., 2021). In dogs, the overall mean prevalence has been reported at 8% using microscopic detection, 7% using coproantigen detection and 6% using molecular diagnostic methods (Taghipour et al., 2020). For a more complete overview of all studies including microscopy-based analyses, refer to Li et al. (2021a).
Cryptosporidium felis and C. canis are the main species infecting cats and dogs globally, respectively (Lucio-Forster et al., 2010; Li et al., 2021a; Meng et al., 2021; Ryan et al., 2021b) (Table 1, Table 2). Cryptosporidium felis and C. canis are also among the top five Cryptosporidium species infecting humans, predominantly in low-income countries (Yang et al., 2021). In cats, in addition to C. felis, there have also been some reports of C. parvum, C. canis, C. muris, C. ryanae, and rat genotypes III and IV. In dogs, C. hominis, C. parvum, C. andersoni, C. muris, C. scrofarum, C. meleagridis, C. ubiquitum, and rat genotypes III and IV have been reported occasionally, in addition to C. canis. Many of these species are zoonotic and are listed in Table 1, Table 2. Cryptosporidium ryanae and rat genotype III were reported in one and six cats, respectively, in Australia (Yang et al., 2015), most likely due to coprophagy, and rat genotype IV was reported in a single cat in China (Li et al., 2019a). The zoonotic status of C. andersoni is uncertain (Yang et al., 2021) and it has not been included in Table 2, but there has been a single report of C. andersoni (0.1%; 1/676) in a shelter dog in the UK (Rosanowski et al., 2018). Young age, presence of diarrhoea, and living in pet shops and shelters are risk factors associated with Cryptosporidium infection in cats and dogs (Li et al., 2021a; Mateo et al., 2023).
Table 1.
Zoonotic Cryptosporidium and Giardia species and assemblages identified in cats globally.
| Species/Assemblage | Hosts | Prevalence | Country | Reference |
|---|---|---|---|---|
| Cryptosporidium | ||||
| C. felis | Domestic cats | 1.3% (2/162) | Australia | Sargent et al. (1998) |
| Domestic cat | – | Australia | Morgan et al. (1998) | |
| Shelter & domestic cats | 1.7% (18/1063) | Australia | Palmer et al. (2008a, b) | |
| Various | 2.3% (8/345) | Australia | Yang et al. (2015) | |
| Domestic cats | 1.7% (5/298) | Austria | Hinney et al. (2015) | |
| Shelter cats | 1.5% (2/130) | Austria | Joachim et al. (2023) | |
| Cryptosporidium-positive domestic cats | 100% (7/7) | Brazil | Thomaz et al. (2007) | |
| Domestic cats | 6.1% (3/49) | Brazil | Alves et al. (2018) | |
| Domestic cats | 5.4% (3/55) | Brazil | de Oliveira et al. (2021) | |
| Various | 1.9% (1/52) | China | Li et al. (2015) | |
| Domestic cats | 3.8% (6/160) | China | Xu et al. (2016) | |
| Domestic cats | 2.3% (8/346) | China | Li et al. (2019b) | |
| Wide range | 5.0% (21/418) | China | Li et al. (2019a) | |
| Domestic cats | 0.6% (1/171) | China | Wang et al. (2021) | |
| Domestic cat | 10.9% (5/46) | Colombia | Santín et al. (2006) | |
| Domestic cats | 100% (1/1) | Czech Republic | Hajdušek et al. (2004) | |
| Domestic & stray cats | 4.3% (11/255) | Czech Republic, Poland, Slovakia | Kváč et al. (2017) | |
| Domestic cats | 2.8% (9/317) | Denmark | Enemark et al. (2020) | |
| Domestic cats | 0.7% (1/134) | Egypt | Elmahallawy et al. (2023) | |
| Domestic & shelter cats | 1.5% (4/264) | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 1.4% (2/140) | Iran | Ranjbar et al. (2018) | |
| Domestic dogs | 0.6% (2/300) | Iran | Homayouni et al. (2019) | |
| Stray cats | 5.3% (7/132) | Iran | Karimi et al. (2023) | |
| Shelter cats | 1.5% (1/65) | Italy | Gil et al. (2017) | |
| Domestic dogs | 0.1% (1/705) | Italy | Simonato et al. (2017) | |
| Domestic cats | 1.8% (19/1079) | Japan | Yamamoto et al. (2009) | |
| Domestic cats | 12.7% (7/55) | Japan | Yoshiuchi et al. (2010) | |
| Domestic & pet shop cats | 1.4% (4/286) | Japan | Ito et al. (2017) | |
| Domestic cats | 1.0% (1/101) | Poland | Piekara-Stępińska et al. (2021) | |
| Shelter cats | 0.6% (1/158) | South Korea | Kwak and Seo (2020) | |
| Domestic cats | 2.9% (1/34) | Spain | de Lucio et al. (2017) | |
| Temple cats | 2.5% (2/80) | Thailand | Koompapong et al. (2014) | |
| Domestic & shelter cats | 1.5% (1/66) | Thailand | Tangtrongsup et al. (2020) | |
| Stray cats | 3.0% (12/399) | Turkey | Köseoğlu et al. (2022) | |
| Cat colony cats | 100% (18/18) | USA | Fayer et al. (2006) | |
| Domestic cats | 4.8% (12/250) | USA | Ballweber et al. (2009) | |
| C. parvum | Domestic cats | 2.0% (1/49) | Brazil | Alves et al. (2018) |
| Domestic cat | 100% (1/1) | Chile | Neira et al. (2010) | |
| Various | 1.9% (1/52) | China | Li et al. (2015) | |
| Wide range | 0.7% (3/418) | China | Li et al. (2019a) | |
| Domestic cats | 14.3% (1/7) | Costa Rica | Scorza et al. (2011) | |
| Domestic cats | 3.7% (5/134) | Egypt | Elmahallawy et al. (2023) | |
| Domestic cats | 5.3% (1/19) | Germany | Sotiriadou et al. (2013) | |
| Domestic & shelter cats | 1.5% (1/66) | Thailand | Tangtrongsup et al. (2020) | |
| C. canis | Domestic cats | 3.0% (1/133) | Iran | Karimi et al. (2023) |
|
C. muris |
Domestic cat | 100% (1/1) | Australia | FitzGerald et al. (2011) |
| Various | 0.3% (1/345) | Australia | Yang et al. (2015) | |
| Wide range | 0.3% (1/418) | China | Li et al. (2019a) | |
| Domestic cat | 2.2% (1/46) | Colombia | Santín et al. (2006) | |
| Domestic cat |
100% (1/1) |
Czech Republic |
Pavlásek and Ryan (2007) |
|
| Giardia | ||||
| Assemblage A | Giardia-positive cats | 33.3% (6/18) | Australia | Read et al. (2004) |
| Various | 0.3% (1/345) | Australia | Yang et al. (2015) | |
| Domestic cats | 1.3% (4/298) | Austria | Hinney et al. (2015) | |
| Shelter cats | 1.5% (2/130) | Austria | Joachim et al. (2023) | |
| Giardia-positive domestic cats | 42.1% (8/19) | Brazil | Souza et al. (2007) | |
| Domestic cats | 100% (1/1) | Brazil | Volotão et al. (2007) | |
| Giardia-positive domestic cats | 61.5% (8/13) | Canada | McDowall et al. (2011) | |
| Various | 3.6% (8/219) | Canada | Hoopes et al. (2015) | |
| Stray cats | 7.8% (8/102) | China | Zheng et al. (2015) | |
| Domestic cats | 1.2% (2/160) | China | Xu et al. (2016) | |
| Stray cats | 2.8% (3/104) | China | Pan et al. (2018) | |
| Wide range | 0.3% (1/418) | China | Li et al. (2019a) | |
| Domestic cats | 0.3% (1/346) | China | Li et al. (2019b) | |
| Domestic cats | 28.6% (2/7) | Costa Rica | Scorza et al. (2011) | |
| Giardia-positive domestic cats | 5.5% (1/18) | Czech Republic | Lecová et al. (2020) | |
| Domestic cats | 2.5% (8/317) | Denmark | Enemark et al. (2020) | |
| Giardia-positive cats | 43.0% (68/158) | Europe | Sprong et al. (2009) | |
| Domestic cats | 10.5% (2/19) | Germany | Sotiriadou et al. (2013) | |
| Domestic cats | 1.4% (2/145) | Germany | Sommer et al. (2018) | |
| Domestic & shelter cats | 2.3% (6/264) | Greece | Kostopoulou et al. (2017) | |
| Kennel cat | 100% (1/1) | Italy | Berrilli et al. (2004) | |
| Stray & domestic cats | 37.0% (10/27) | Italy | Papini et al. (2007) | |
| Domestic cats | 1.7% (3/181) | Italy | Paoletti et al. (2011) | |
| Domestic cats | 9.0% (14/156) | Italy | Zanzani et al. (2014) | |
| Sheltered cats | 1.5% (1/65) | Italy | Gil et al. (2017) | |
| Stray cats | 27.8% (37/133) | Italy | Guadano Procesi et al. (2022) | |
| Giardia-positive cats | 100% (3/3) | Italy, Croatia | Cacciò et al. (2008) | |
| Cats from cat café’s and petshops | 0.6% (2/321) | Japan | Suzuki et al. (2011) | |
| Giardia-positive domestic cat | 100% (1/1) | Mexico | Ponce-Macotela et al. (2002) | |
| Various | 1.0% (2/200) | Mexico | Veyna-Salazar et al. (2023) | |
| Domestic cats | 1.6% (1/60) | Netherlands | Overgaauw et al. (2009) | |
| Domestic cats | 3.0% (1/33) | Poland | Piekarska et al. (2016) | |
| Household & kennel cats | 9.0% (2/22) | Portugal | Ferreira et al. (2011) | |
| Giardia-positive cats | 27.7% (5/18) | Sweden | Lebbad et al. (2010) | |
| Domestic & shelter cats | 1.5% (1/66) | Thailand | Tangtrongsup et al. (2020) | |
| Domestic cats | 3.3% (4/122) | UK | Krumrie et al. (2022) | |
| Domestic cats | 2.4% (6/250) | USA | Vasilopulos et al. (2007) | |
| Giardia-positive domestic cats | 32.4% (12/37) | USA | Saleh et al. (2019) | |
| Assemblage B | Giardia-positive cats | 11.1% (2/18) | Australia | Read et al. (2004) |
| Giardia-positive domestic cats | 7.7% (1/13) | Canada | McDowall et al. (2011) | |
| Domestic cats | 3.7% (6/160) | China | Xu et al. (2016) | |
| Giardia-positive cats | 1.9% (3/158) | Europe | Sprong et al. (2009) | |
| Domestic & shelter cats | 0.4% (1/264) | Greece | Kostopoulou et al. (2017) | |
| Stray cats | 0.7% (1/132) | Iran | Karimi et al. (2023) | |
| Symptomatic cats | 29.4% (30/102) | Turkey | Sursal et al. (2020) | |
| Domestic cats | 8.0% (8/100) | Turkey | Önder et al. (2021) | |
| Domestic cats | 3.3% (4/122) | UK | Krumrie et al. (2022) | |
| Assemblage C | Giardia-positive cats | 11.1% (2/18) | Australia | Read et al. (2004) |
| Stray cats | 1.0% (1/102)b | China | Zheng et al. (2015) | |
| Domestic cats | 1.3% (2/160) | China | Xu et al. (2016) | |
| Domestic cats | 9.0% (1/11) | China | Liu et al. (2017) | |
| Giardia-positive cats | 3.2% (5/158) | Europe | Sprong et al. (2009) | |
| Domestic & shelter cats | 0.4% (1/264) | Greece | Kostopoulou et al. (2017) | |
| Domestic cats | 1.4% (2/146) | Italy | Mancianti et al. (2015) | |
| Domestic & shelter cats | 6.1% (4/66) | Thailand | Tangtrongsup et al. (2020) | |
| Assemblage D | Giardia-positive cats | 38.8% (7/18) | Australia | Read et al. (2004) |
| Shelter & domestic cats | 0.1% (1/1063) | Australia | Palmer et al. (2008a, b) | |
| Domestic cats | 0.6% (1/160) | China | Xu et al. (2016) | |
| Giardia-positive cats | 1.9% (3/158) | Europe | Sprong et al. (2009) | |
| Domestic cats | 1.9% (3/156) | Italy | Zanzani et al. (2014) | |
| Domestic cats | 80.0% (8/10) | Romania | Adriana et al. (2016) | |
| Domestic & shelter cats | 1.5% (1/66) | Thailand | Tangtrongsup et al. (2020) | |
| Assemblage E | Giardia-positive cats | 5.5% (1/18) | Australia | Read et al. (2004) |
| Giardia-positive cats | 1.3% (2/158) | Europe | Sprong et al. (2009) | |
| Giardia-positive cats | 5.5% (1/18) | Sweden | Lebbad et al. (2010) | |
| Assemblage F | Shelter & domestic cats | 0.7% (7/1063) | Australia | Palmer et al. (2008a, b) |
| Various | 2.6% (9/345) | Australia | Yang et al. (2015) | |
| Domestic cats | 3.3% (10/298) | Austria | Hinney et al. (2015) | |
| Shelter cats | 7.7% (10/130) | Austria | Joachim et al. (2023) | |
| Giardia-positive domestic cats | 57.9% (11/19) | Brazil | Souza et al. (2007) | |
| Various | 1.9% (1/52) | China | Li et al. (2015) | |
| Stray cats | 1.0% (1/102) | China | Zheng et al. (2015) | |
| Domestic cats | 4.4% (7/160) | China | Xu et al. (2016) | |
| Domestic cats | 9.0% (1/11) | China | Liu et al. (2017) | |
| Stray cats | 2.8% (3/104) | China | Pan et al. (2018) | |
| Domestic cats | 2.6% (9/346) | China | Li et al. (2019b) | |
| Wide range | 3.3% (14/418) | China | Li et al. (2019a) | |
| Domestic cats | 1.2% (2/171) | China | Wang et al. (2021) | |
| Domestic cats | 6.3% (1/16) | China | Wu et al. (2022) | |
| Domestic cats | 6.5% (3/46) | Columbia | Santín et al. (2006) | |
| Giardia-positive domestic cats | 88.8% (16/18) | Czech Republic | Lecová et al. (2020) | |
| Domestic & stray cats | 6.3% (16/255) | Czech Republic, Poland, Slovakia | Kváč et al. (2017) | |
| Domestic cats | 0.6% (2/317) | Denmark | Enemark et al. (2020) | |
| Giardia-positive cats | 48.7% (77/158) | Europe | Sprong et al. (2009) | |
| Domestic cats | 9.6% (14/145) | Germany | Sommer et al. (2018) | |
| Domestic & shelter cats | 2.3% (6/264)a | Greece | Kostopoulou et al. (2017) | |
| Domestic cats | 1.0% (3/300) | Iran | Homayouni et al. (2019) | |
| Stray cats | 0.7% (1/132) | Iran | Karimi et al. (2023) | |
| Giardia-positive cat | 100% (1/1) | Italy | Lalle et al. (2005a) | |
| Domestic cats | 2.8% (5/181) | Italy | Paoletti et al. (2011) | |
| Domestic cats | 6.2% (9/146) | Italy | Mancianti et al. (2015) | |
| Shelter cats | 1.5% (1/65) | Italy | Gil et al. (2017) | |
| Giardia-positive cat | 100% (1/1) | Japan | Itagaki et al. (2005) | |
| Domestic cats | 1.8% (1/55) | Japan | Yoshiuchi et al. (2010) | |
| Cats from cat café’s and petshops | 6.2% (20/321) | Japan | Suzuki et al. (2011) | |
| Domestic cats | 12.1% (4/33) | Poland | Piekarska et al. (2016) | |
| Shelter cats | 3.8% (6/158) | South Korea | Kwak and Seo (2020) | |
| Domestic cats | 5.9% (2/34) | Spain | de Lucio et al. (2017) | |
| Giardia-positive cats | 66.6% (12/18) | Sweden | Lebbad et al. (2010) | |
| Domestic cats | 31.1% (38/122) | UK | Krumrie et al. (2022) | |
| Cat colony cats | 44.4% (8/18) | USA | Fayer et al. (2006) | |
| Domestic cats | 4.4% (11/250) | USA | Vasilopulos et al. (2007) | |
| Giardia-positive cat | 100% (1/1) | USA | Miska et al. (2009) | |
| Giardia-positive domestic cats | 56.7% (21/37) | USA | Saleh et al. (2019) | |
| Mixed F and A | Giardia-positive domestic cats | 30.8% (4/13) | Canada | McDowall et al. (2011) |
| Domestic & shelter cats | 1.5% (4/264) | Greece | Kostopoulou et al. (2017) | |
| Giardia-positive domestic cats | 10.8% (4/37) | USA | Saleh et al. (2019) | |
| Other mixtures | Stray cats | A + B (n = 2) | Italy | Guadano Procesi et al. (2022) |
4 samples typed as assemblage A at 18S locus.
Table 2.
Zoonotic Cryptosporidium and Giardia species and assemblages identified in dogs globally.
| Species/Assemblage | Hosts | Prevalence | Country | Reference |
|---|---|---|---|---|
| Cryptosporidium | ||||
| C. canis | Shelter & domestic dogs | 0.3% (4/1400) | Australia | Palmer et al. (2008a,b) |
| Wild dogs | 20.5% (9/44) | Australia | Ng et al. (2011) | |
| Wild dogs | 3.1% (1/32) | Australia | Nolan et al. (2013) | |
| Wild dogs | 100% (2/2) | Australia | Zahedi et al. (2018) | |
| Cryptosporidium-positive dogs | 100% (5/5) | Australia, USA | Morgan et al. (2000b) | |
| Cryptosporidium-positive domestic dogs | 100% (9/9) | Brazil | Thomaz et al. (2007) | |
| Domestic dogs | 3.1% (4/128) | Brazil | Alves et al. (2018) | |
| Domestic dogs | 4.7% (3/64) | Brazil | de Oliveira et al. (2021) | |
| Domestic, shelter & pet shop dogs | 1.9% (4/209) | Canada | Uehlinger et al. (2013) | |
| Various | 1.7% (5/294) | Canada | Julien et al. (2019) | |
| Domestic dogs | 0.3% (3/860) | Canada | Smith et al. (2020) | |
| Various | 3.8% (29/770) | China | Jian et al. (2014) | |
| Domestic dogs | 1.6% (5/315) | China | Gu et al. (2015) | |
| Domestic dogs | 8.0% (39/485) | China | Xu et al. (2016) | |
| Dogs with diarrhoea | 4.1% (20/485) | China | Yu et al. (2018) | |
| Various | 2.9% (19/651) | China | Liao et al. (2020) | |
| Domestic dogs | 4.6% (12/262) | China | Wang et al. (2021) | |
| Domestic dogs | 1.6% (10/604) | China | Cao et al. (2022) | |
| Domestic dogs | 1.8% (4/218) | Egypt | Elmahallawy et al. (2023) | |
| Stray & domestic dogs | 5.0% (15/300) | Egypt | Khalifa et al. (2023) | |
| Domestic dogs | 2.0% (3/150) | Finland | Rimhanen-Finne et al. (2007) | |
| Domestic dogs | 2.6% (3/116) | France | Osman et al. (2015) | |
| Domestic dogs | 9.4% (33/349) | Germany | Murnik et al. (2022) | |
| Domestic & shelter dogs | 0.2% (2/807) | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 0.6% (2/315) | Iran | Homayouni et al. (2019) | |
| Kennel & domestic dogs | 0.4% (1/240) | Italy | Giangaspero et al. (2006) | |
| Kennel dogs | 1.0% (3/285) | Italy | Simonato et al. (2015) | |
| Domestic dogs | 0.1% (1/705) | Italy | Simonato et al. (2017) | |
| Domestic dogs | 9.3% (13/140) | Japan | Abe et al. (2002) | |
| Domestic dogs | 0.1% (1/906) | Japan | Yamamoto et al. (2009) | |
| Domestic dogs | 3.9% (3/77) | Japan | Yoshiuchi et al. (2010) | |
| Kennel dogs | 21.1% (66/314) | Japan | Itoh et al. (2019) | |
| Domestic dog | 100% (1/1) | Nigeria | Ayinmode et al. (2018a) | |
| Domestic dogs | 1.1% (3/264) | Poland | Piekara-Stępińska et al. (2021) | |
| Domestic dogs | 3.6% (2/55) | Spain | de Lucio et al. (2017) | |
| Shelter dogs | 2.6% (5/194) | Spain | Gil et al. (2017) | |
| Domestic dogs | 1.6% (4/252) | Spain | Mateo et al. (2023) | |
| Temple dogs | 2.1% (2/95) | Thailand | Koompapong et al. (2014) | |
| Domestic dogs | 13.8% (15/109) | Thailand | Tangtrongsup et al. (2017) | |
| Domestic & shelter dogs | 2.6% (8/301) | Thailand | Tangtrongsup et al. (2020) | |
| Monastery dogs | 0.7% (4/540) | Thailand | Khine et al. (2021) | |
| Shelter dogs | 4.2% (28/676) | UK | Rosanowski et al. (2018) | |
| Domestic dogs | 0.8% (1/129) | USA | Wang et al. (2012) | |
| Domestic dogs | 4.8% (4/84) | USA | Scorza and Lappin (2017) | |
| Domestic dogs | 33.3% (1/3) | Vietnam | Iwashita et al. (2021) | |
| C. hominis | Wild dogs | 2.3% (1/44) | Australia | Ng et al. (2011) |
| Shelter dogs | 0.5% (1/194) | Italy | Gil et al. (2017) | |
| C. parvum | Domestic dogs | 1.6% (2/128) | Brazil | Alves et al. (2018) |
| Domestic dogs | 3.1% (2/64) | Brazil | de Oliveira et al. (2021) | |
| Various | 0.3% (2/651) | China | Liao et al. (2020) | |
| Domestic dogs | 1.7% (1/58) | Costa Rica | Scorza et al. (2011) | |
| Domestic dogs | 50.0% (1/2) | Czech Republic | Hajdušek et al. (2004) | |
| Stray dogs | 10.0% (2/20) | Egypt | El-Madawy et al. (2010) | |
| Domestic dogs | 10.0% (5/50) | Egypt | Gharieb et al. (2018) | |
| Domestic dogs | 1.2% (1/81) | Germany | Sotiriadou et al. (2013) | |
| Domestic dogs | 0.6% (2/349) | Germany | Murnik et al. (2022) | |
| Kennel & privately-owned dogs | 2.9% (7/240) | Italy | Giangaspero et al. (2006) | |
| Domestic dogs | 1.4% (10/705) | Italy | Simonato et al. (2017) | |
| Street dogs | 1.5% (3/203) | Nigeria | Ayinmode et al. (2018b) | |
| Domestic dogs | 0.8% (2/264) | Poland | Piekara-Stępińska et al. (2021) | |
| Domestic dogs | 0.4% (1/252) | Spain | Mateo et al. (2023) | |
| Domestic dogs | 6.4% (7/109) | Thailand | Tangtrongsup et al. (2017) | |
| Domestic & shelter dogs | 0.3% (1/301) | Thailand | Tangtrongsup et al. (2020) | |
| Farm dog | 100% (1/1) | UK | Smith et al. (2010) | |
| Shelter dogs | 0.3% (2/676) | UK | Rosanowski et al. (2018) | |
| C. muris | Wide range | 0.2% (1/601) | China | Li et al. (2019b) |
| Street dogs | 1.0% (2/203) | Nigeria | Ayinmode et al. (2018b) | |
| Kennel dogs | 8.6% (6/70) | USA | Lupo et al. (2008) | |
| C. scrofarum | Domestic & shelter dogs | 0.1% (1/807) | Greece | Kostopoulou et al. (2017) |
| C. meleagridis | Domestic dogs | 50.0% (1/2) | Czech Republic | Hajdušek et al. (2004) |
|
C. ubiquitum |
Wide range |
0.4% (1/267) |
China |
Li et al. (2015) |
| Giardia | ||||
| Assemblage A | Domestic dogs | 13.8% (5/36) | Argentina | Kuthyar et al. (2021) |
| Shelter & domestic dogs | 0.07% (1/1400) | Australia | Palmer et al. (2008a, b) | |
| Wild dogs | 6.8% (3/44) | Australia | Ng et al. (2011) | |
| Wild dogs | 3.1% (1/32) | Australia | Nolan et al. (2013) | |
| Various | 3.5% (40/1159) | Belgium | Claerebout et al. (2009) | |
| Stray dogs | 36.8% (7/19) | Brazil | Volotão et al. (2007) | |
| Giardia-positive stray dogs | 100% (60/60) | Brazil | Fantinatti et al. (2018) | |
| Dogs from a remote indigenous community | 8.4% (13/155) | Canada | Himsworth et al. (2010) | |
| Dogs in indigenous communities | 13.8% (32/231) | Canada | Schurer et al. (2012) | |
| Domestic, shelter & pet shop dogs | 1.4% (3/209) | Canada | Uehlinger et al. (2013) | |
| Domestic dogs | 2.4% (5/209) | China | Li et al. (2012) | |
| Police & farm dogs | 12.2% (25/205) | China | Li et al. (2013) | |
| Domestic dogs | 3.2% (7/216) | China | Zheng et al. (2014) | |
| Giardia-positive domestic dogs | 40.0% (8/20) | China | Tan et al. (2016) | |
| Domestic dogs | 4.7% (23/485) | China | Xu et al. (2016) | |
| Stray dogs | 4.9% (26/527) | China | Pan et al. (2018) | |
| Domestic dogs | 0.2% (1/604) | China | Cao et al. (2022) | |
| Domestic dogs | 18.2% (2/11)b | Côte dʼIvoire | Berrilli et al. (2012) | |
| Giardia-positive domestic & kennel dogs | 2.7% (2/96) | Croatia | Beck et al. (2012) | |
| Domestic dogs | 10.2% (10/98) | Cuba | Puebla et al. (2017) | |
| Stray & domestic dogs | 0.6% (2/300) | Egypt | Khalifa et al. (2023) | |
| Giardia-positive dog samples | 22.8% (137/600) | Europe | Sprong et al. (2009) | |
| Giardia-positive domestic dogs | 60.0% (33/55) | Germany | Leonhard et al. (2007) | |
| Domestic dogs | 6.2% (5/81) | Germany | Sotiriadou et al. (2013) | |
| Giardia-positive domestic dogs | 19.5% (24/123) | Germany | Pallant et al. (2015) | |
| Domestic dogs | 0.5% (2/376) | Germany | Sommer et al. (2018) | |
| Domestic dogs | 6.5% (2/31) | Germany | Rehbein et al. (2019) | |
| Domestic dogs | 2.3% (9/386) | Germany | Murnik et al. (2023) | |
| Domestic & shelter dogs | 0.9% (7/807)a | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 4.9% (5/101) | India | Traub et al. (2004) | |
| Domestic dogs | 0.5% (1/212) | India | Utaaker et al. (2018) | |
| Domestic dogs | 0.3% (1/315) | Iran | Homayouni et al. (2019) | |
| Giardia-positive domestic dogs | 11.8% (2/17) | Italy | Berrilli et al. (2004) | |
| Domestic & kennel dogs | 0.8% (2/240) | Italy | Paoletti et al. (2008) | |
| Domestic dogs | 6.3% (8/127) | Italy | Scaramozzino et al. (2009) | |
| Stray dogs | 64.3% (9/14) | Italy | Marangi et al. (2010) | |
| Domestic dogs | 0.3% (2/655) | Italy | Pipia et al. (2014) | |
| Sheltered dogs | 3.6% (7/194) | Italy | Gil et al. (2017) | |
| Shelter dogs | 9.5% (16/168) | Italy | Agresti et al. (2021) | |
| Domestic dogs | 19.5% (44/225) | Jamaica | Lee et al. (2017) | |
| Giardia-positive domestic dogs | 66.6% (14/21) | Japan | Itagaki et al. (2005) | |
| Shelter dogs | 1.0% (2/202) | Korea | Shin et al. (2015) | |
| Giardia-positive domestic dogs | 100% (2/2) | Mexico | Ponce-Macotela et al. (2002) | |
| Giardia-positive domestic dogs | 100% (11/11) | Mexico | Eligio-Garcia et al. (2005) | |
| Puppies | 100% (5/5) | Mexico | Lalle et al. (2005b) | |
| Various | 1.1% (2/189) | Netherlands | Uiterwijk et al. (2020) | |
| Domestic dogs | 3.5% (2/58) | Nicaragua | Roegner et al. (2019) | |
| Domestic dogs | 1.4% (5/348) | Spain | Adell-Aledon et al. (2018) | |
| Domestic dogs | 0.4% (1/252) | Spain | Mateo et al. (2023) | |
| Giardia-positive dog samples | 3.6% (1/28) | Sweden | Lebbad et al. (2010) | |
| Temple dogs | 2.2% (5/229) | Thailand | Inpankaew et al. (2007) | |
| Temple dogs | 14.4% (33/229) | Thailand | Traub et al. (2009) | |
| Monastery dogs | 0.4% (2/540) | Thailand | Khine et al. (2021) | |
| Domestic dogs | 0.7% (1/152) | The Netherlands | Overgaauw et al. (2009) | |
| Giardia-positive shelter dogs | 2.4% (1/41) | UK | Upjohn et al. (2010) | |
| Domestic dogs | 1.9% (1/52) | UK | Krumrie et al. (2022) | |
| Giardia-positive domestic dogs | 11.1% (1/9) | Unknown | Read et al. (2004) | |
| Giardia-positive domestic dogs | 28.0% (36/128) | USA | Covacin et al. (2011) | |
| Domestic dogs | 13.3% (16/120) | USA | Munoz and Mayer (2016) | |
| Assemblage B | Domestic dog | 100% (1/1) | Argentina | Minvielle et al. (2008) |
| Various | 0.3% (4/1159) | Belgium | Claerebout et al. (2009) | |
| Domestic dogs | 3.1% (1/32) | Brazil | Colli et al. (2015) | |
| Domestic dogs | 2.2% (2/94) | Cambodia | Inpankaew et al. (2014) | |
| Domestic dogs | 1.9% (6/315) | China | Gu et al. (2015) | |
| Domestic dogs | 0.2% (1/485) | China | Xu et al. (2016) | |
| Giardia-positive domestic & kennel dogs | 23.3% (17/96) | Croatia | Beck et al. (2012) | |
| Giardia-positive dog samples | 8.8% (53/600) | Europe | Sprong et al. (2009) | |
| Giardia-positive domestic dogs | 6.5% (8/123) | Germany | Pallant et al. (2015) | |
| Domestic & shelter dogs | 5.7% (46/807) | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 2.0% (2/101) | India | Traub et al. (2004) | |
| Domestic dogs | 0.5% (1/212) | India | Utaaker et al. (2018) | |
| Kennel dogs | 0.3% (1/318) | Italy | Simonato et al. (2015) | |
| Sheltered dogs | 4.1% (8/194) | Italy | Gil et al. (2017) | |
| Domestic dogs | 0.1% (1/705) | Italy | Simonato et al. (2017) | |
| Shelter dogs | 3.6% (6/168) | Italy | Agresti et al. (2021) | |
| Domestic dogs | 8.6% (5/58) | Nicaragua | Roegner et al. (2019) | |
| Domestic dogs | 0.8% (1/128) | Poland | Piekarska et al. (2016) | |
| Household & kennel dogs | 1.6% (2/126) | Portugal | Ferreira et al. (2011) | |
| Domestic dogs | 2.3% (8/348) | Spain | Adell-Aledon et al. (2018) | |
| Temple dogs | 3.9% (9/229) | Thailand | Traub et al. (2009) | |
| Domestic dogs | 10.8% (51/473) | Turkey | Gultekin et al. (2017) | |
| Giardia-positive domestic dogs | 22.2% (2/9) | Unknown | Read et al. (2004) | |
| Giardia-positive domestic dogs | 40.6% (52/128) | USA | Covacin et al. (2011) | |
| Assemblage C | Giardia-positive domestic dogs | 90.9% (10/11) | Australia | Monis et al. (1998, 2003) |
| Shelter & domestic dogs | 2.9% (41/1400) | Australia | Palmer et al. (2008a, b) | |
| Wild dogs | 11.4% (5/44) | Australia | Ng et al. (2011) | |
| Shelter dogs | 1.4% (1/70) | Austria | Joachim et al. (2023) | |
| Various | 2.2% (26/1159) | Belgium | Claerebout et al. (2009) | |
| Giardia-positive domestic dogs | 29.9% (7/27) | Brazil | Souza et al. (2007) | |
| Domestic dogs | 3.1% (1/32) | Brazil | Colli et al. (2015) | |
| Domestic dogs | 1.4% (2/147) | Brazil | Paim Arruda Trevisan et al. (2020) | |
| Giardia-positive domestic dogs | 16.6% (2/12) | Brazil | Silva et al. (2022) | |
| Domestic dogs | 4.3% (4/94) | Cambodia | Inpankaew et al. (2014) | |
| Giardia-positive domestic dogs | 30.6% (23/75) | Canada | McDowall et al. (2011) | |
| Domestic, shelter & pet shop dogs | 4.8% (10/209) | Canada | Uehlinger et al. (2013) | |
| Various | 0.7% (2/294) | Canada | Julien et al. (2019) | |
| Domestic dogs | 1.6% (14/860) | Canada | Smith et al. (2020) | |
| Police & farm dogs | 1.0% (2/205) | China | Li et al. (2013) | |
| Domestic dogs | 0.5% (1/216) | China | Zheng et al. (2014) | |
| Wide range | 2.6% (7/267) | China | Li et al. (2015) | |
| Giardia-positive domestic dogs | 25.0% (5/20) | China | Tan et al. (2016) | |
| Domestic dogs | 5.4% (26/485) | China | Xu et al. (2016) | |
| Domestic dogs | 15.8% (3/19) | China | Liu et al. (2017) | |
| Stray dogs | 5.7% (9/159) | China | Zhang et al. (2017) | |
| Stray dogs | 3.4% (18/527) | China | Pan et al. (2018) | |
| Wide range | 4.5% (27/601) | China | Li et al. (2019b) | |
| Various | 0.8% (5/651) | China | Liao et al. (2020) | |
| Domestic dogs | 1.5% (4/262) | China | Wang et al. (2021) | |
| Domestic dogs | 2.3% (14/604) | China | Cao et al. (2022) | |
| Domestic dogs | 2.9% (13/448) | China | Sui et al. (2022) | |
| Domestic dogs | 3.8% (4/105) | China | Wu et al. (2022) | |
| Domestic dogs | 3.0% (3/100) | Colombia | Hernandez et al. (2021) | |
| Domestic dogs | 9.1% (1/11) | Côte dʼIvoire | Berrilli et al. (2012) | |
| Giardia-positive domestic & kennel dogs | 36.9% (27/73) | Croatia | Beck et al. (2012) | |
| Giardia-positive domestic dogs | 38.8% (21/54) | Czech Republic | Lecová et al. (2020) | |
| Domestic & shelter dogs | 3.0% (50/1645) | Eastern European countries | Sommer et al. (2015) | |
| Domestic dogs | 0.5% (1/218) | Egypt | Elmahallawy et al. (2023) | |
| Giardia-positive dog samples | 31.8% (191/600) | Europe | Sprong et al. (2009) | |
| Domestic dogs | 2.0% (3/150) | Finland | Rimhanen-Finne et al. (2007) | |
| Giardia-positive dogs | 76.9% (30/39) | France | Kaufmann et al. (2022) | |
| Giardia-positive domestic dogs | 35.9% (33/92) | Germany | Barutzki et al. (2007) | |
| Giardia-positive domestic dogs | 36.4% (20/55)b | Germany | Leonhard et al. (2007) | |
| Giardia-positive domestic dogs | 42.3% (52/123) | Germany | Pallant et al. (2015) | |
| Domestic dogs | 10.1% (38/376) | Germany | Sommer et al. (2018) | |
| Domestic dogs | 3.2% (1/31) | Germany | Rehbein et al. (2019) | |
| Domestic dogs | 11.2% (43/386) | Germany | Murnik et al. (2023) | |
| Domestic & shelter dogs | 5.7% (46/807) | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 2.2% (5/229) | Hungary | Szénási et al. (2007) | |
| Domestic dogs | 4.2% (9/212) | India | Utaaker et al. (2018) | |
| Domestic dogs | 0.9% (3/315) | Iran | Homayouni et al. (2019) | |
| Giardia-positive domestic dogs | 64.7% (11/17) | Italy | Berrilli et al. (2004) | |
| Domestic & kennel dogs | 1.2% (3/240) | Italy | Paoletti et al. (2008) | |
| Domestic dogs | 11.0% (14/127) | Italy | Scaramozzino et al. (2009) | |
| Domestic dogs | 2.6% (17/655) | Italy | Pipia et al. (2014) | |
| Domestic dogs | 2.4% (6/253) | Italy | Zanzani et al. (2014) | |
| Domestic dogs | 3.0% (15/502) | Italy | Paoletti et al. (2015) | |
| Kennel dogs | 17.2% (49/285) | Italy | Simonato et al. (2015) | |
| Domestic dogs | 1.3% (9/705) | Italy | Simonato et al. (2017) | |
| Stray dogs | 2.3% (6/262) | Italy | Liberato et al. (2018) | |
| Dogs with primary chronic enteropathy | 2.2% (1/47) | Italy | Perrucci et al. (2020) | |
| Shelter dogs | 1.2% (2/168) | Italy | Agresti et al. (2021) | |
| Giardia-positive domestic dogs | 4.8% (1/21) | Japan | Itagaki et al. (2005) | |
| Pet shop puppies | 0.5% (9/1794) | Japan | Itoh et al. (2011) | |
| Shelter dogs | 0.5% (1/202) | Korea | Shin et al. (2015) | |
| Domestic dogs | 1.4% (9/640) | Korea | Kim et al. (2019) | |
| Various | 9.0% (17/189) | Netherlands | Uiterwijk et al. (2020) | |
| Domestic dogs | 2.0% (2/100) | Nicaragua | Lebbad et al. (2008) | |
| Domestic dogs | 5.2% (3/58) | Nicaragua | Roegner et al. (2019) | |
| Domestic dogs | 1.5% (9/605) | Peru | Cooper et al. (2010) | |
| Domestic & shelter dogs | 0.7% (1/148) | Poland | Solarczyk and Majewska (2010) | |
| Sled dogs | 8.0% (2/25) | Poland | Bajer et al. (2011) | |
| Domestic dogs | 14.0% (18/128) | Poland | Piekarska et al. (2016) | |
| Household & kennel dogs | 11.9% (15/126) | Portugal | Ferreira et al. (2011) | |
| Domestic dogs | 3.7% (3/80) | Portugal | Pereira et al. (2021) | |
| Domestic dogs | 20.5% (8/39) | Romania | Adriana et al. (2016) | |
| Domestic dogs | 5.4% (3/55) | Spain | de Lucio et al. (2017) | |
| Domestic dogs | 0.3% (2/348) | Spain | Adell-Aledon et al. (2018) | |
| Domestic dogs | 1.6% (4/252) | Spain | Mateo et al. (2023) | |
| Giardia-positive dog samples | 28.6% (8/28) | Sweden | Lebbad et al. (2010) | |
| Domestic dogs | 2.4% (1/42) | Taiwan | Liang et al. (2012) | |
| Stray dogs | 5.9% (7/118) | Taiwan | Tseng et al. (2014) | |
| Temple dogs | 0.4% (1/229) | Thailand | Inpankaew et al. (2007) | |
| Temple dogs | 2.2% (5/229) | Thailand | Traub et al. (2009) | |
| Domestic dogs | 7.3% (8/109) | Thailand | Tangtrongsup et al. (2017) | |
| Domestic & shelter dogs | 0.3% (1/301) | Thailand | Tangtrongsup et al. (2020) | |
| Monastery dogs | 1.3% (7/540) | Thailand | Khine et al. (2021) | |
| Stray dogs | 3.8% (4/104) | Trinidad and Tobago | Mark-Carew et al. (2013) | |
| Giardia-positive shelter dogs | 24.4% (10/41) | UK | Upjohn et al. (2010) | |
| Domestic dogs | 19.2% (10/52) | UK | Krumrie et al. (2022) | |
| Giardia-positive domestic dogs | 44.4% (4/9) | Unknown | Read et al. (2004) | |
| Giardia-positive domestic dogs | 100% (15/15) | USA | Sulaiman et al. (2003) | |
| Giardia-positive dog samples | 33.3% (1/3) | USA | Miska et al. (2009) | |
| Giardia-positive domestic dogs | 14.8% (19/128) | USA | Covacin et al. (2011) | |
| Domestic dogs | 0.8% (1/129) | USA | Wang et al. (2012) | |
| Shelter dogs | 0.1% (1/672)b | USA | Johansen et al. (2014) | |
| Domestic dogs | 4.8% (10/209) | Vietnam | Nguyen et al. (2018) | |
| Assemblage D | Giardia-positive domestic dogs | 9.1% (10/11) | Australia | Monis et al. (1998, 2003) |
| Shelter & domestic dogs | 3.1% (44/1400) | Australia | Palmer et al. (2008a, b) | |
| Various | 4.2% (49/1159) | Belgium | Claerebout et al. (2009) | |
| Giardia-positive domestic dogs | 74.1% (20/27) | Brazil | Souza et al. (2007) | |
| Kennel dogs | 16.5% (16/97) | Brazil | Fava et al. (2016) | |
| Domestic dogs | 4.3% (4/94) | Cambodia | Inpankaew et al. (2014) | |
| Giardia-positive domestic dogs | 68.0% (51/75) | Canada | McDowall et al. (2011) | |
| Domestic, shelter & pet shop dogs | 9.1% (19/209) | Canada | Uehlinger et al. (2013) | |
| Various | 1.0% (3/294) | Canada | Julien et al. (2019) | |
| Domestic dogs | 1.5% (13/860) | Canada | Smith et al. (2020) | |
| Domestic dogs | 8.6% (18/209) | China | Li et al. (2012) | |
| Domestic dogs | 0.5% (1/216) | China | Zheng et al. (2014) | |
| Domestic dogs | 1.3% (4/315) | China | Gu et al. (2015) | |
| Giardia-positive domestic dogs | 35.0% (7/20) | China | Tan et al. (2016) | |
| Domestic dogs | 11.9% (58/485) | China | Xu et al. (2016) | |
| Domestic dogs | 15.8% (3/19) | China | Liu et al. (2017) | |
| Stray dogs | 5.7% (9/159) | China | Zhang et al. (2017) | |
| Stray dogs | 2.5% (13/527) | China | Pan et al. (2018) | |
| Dogs with diarrhoea | 3.1% (15/485) | China | Yu et al. (2018) | |
| Wide range | 4.3% (26/601) | China | Li et al. (2019b) | |
| Various | 2.1% (14/651) | China | Liao et al. (2020) | |
| Domestic dogs | 12.2% (32/262) | China | Wang et al. (2021) | |
| Domestic dogs | 1.2% (7/604) | China | Cao et al. (2022) | |
| Domestic dogs | 3.1% (14/448) | China | Sui et al. (2022) | |
| Domestic dogs | 1.0% (1/105) | China | Wu et al. (2022) | |
| Domestic dogs | 3.0% (3/100) | Columbia | Hernandez et al. (2021) | |
| Domestic dogs | 3.4% (2/58) | Costa Rica | Scorza et al. (2011) | |
| Domestic dogs | 18.2% (2/11) | Côte dʼIvoire | Berrilli et al. (2012) | |
| Giardia-positive domestic & kennel dogs | 36.9% (27/73) | Croatia | Beck et al. (2012) | |
| Giardia-positive domestic dogs | 59.2% (32/54) | Czech Republic | Lecová et al. (2020) | |
| Domestic & shelter dogs | 4.1% (68/1645) | Eastern European countries | Sommer et al. (2015) | |
| Domestic dogs | 0.4% (4/986) | Egypt | Abdelaziz and Sorour (2021) | |
| Domestic dogs | 1.4% (3/218) | Egypt | Elmahallawy et al. (2023) | |
| Stray & domestic dogs | 1.6% (5/300) | Egypt | Khalifa et al. (2023) | |
| Giardia-positive dog samples | 35.8% (215/600) | Europe | Sprong et al. (2009) | |
| Domestic dogs | 2.6% (4/150) | Finland | Rimhanen-Finne et al. (2007) | |
| Giardia-positive dogs | 23.1% (9/39) | France | Kaufmann et al. (2022) | |
| Giardia-positive domestic dogs | 54.3% (50/92) | Germany | Barutzki et al. (2007) | |
| Giardia-positive domestic dogs | 3.6% (2/55) | Germany | Leonhard et al. (2007) | |
| Giardia-positive domestic dogs | 56.1% (69/123) | Germany | Pallant et al. (2015) | |
| Domestic dogs | 12.5% (47/376) | Germany | Sommer et al. (2018) | |
| Domestic dogs | 6.5% (2/31) | Germany | Rehbein et al. (2019) | |
| Domestic dogs | 10.4% (40/386) | Germany | Murnik et al. (2023) | |
| Domestic & shelter dogs | 2.7% (22/807) | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 6.5% (15/229) | Hungary | Szénási et al. (2007) | |
| Domestic dogs | 3.3% (7/212) | India | Utaaker et al. (2018) | |
| Domestic dogs | 0.6% (2/315) | Iran | Homayouni et al. (2019) | |
| Giardia-positive domestic dogs | 5.8% (1/17) | Italy | Berrilli et al. (2004) | |
| Domestic & kennel dogs | 10.4% (25/240) | Italy | Paoletti et al. (2008) | |
| Domestic dogs | 3.2% (4/127) | Italy | Scaramozzino et al. (2009) | |
| Domestic dogs | 3.5% (23/655) | Italy | Pipia et al. (2014) | |
| Domestic dogs | 2.0% (5/253) | Italy | Zanzani et al. (2014) | |
| Domestic dogs | 0.4% (2/502) | Italy | Paoletti et al. (2015) | |
| Domestic dogs | 10.2% (29/285) | Italy | Simonato et al. (2015) | |
| Domestic dogs | 1.6% (11/705) | Italy | Simonato et al. (2017) | |
| Stray dogs | 7.2% (19/262) | Italy | Liberato et al. (2018) | |
| Dogs with primary chronic enteropathy | 10.6% (5/47) | Italy | Perrucci et al. (2020) | |
| Giardia-positive domestic dogs | 100% (6/6) | Italy | Ciuca et al. (2021) | |
| Giardia-positive domestic dogs | 100% (4/4) | Japan | Abe et al. (2003) | |
| Giardia-positive domestic dogs | 28.6% (6/21) | Japan | Itagaki et al. (2005) | |
| Domestic dogs | 2.6% (2/77) | Japan | Yoshiuchi et al. (2010) | |
| Pet shop puppies | 1.1% (20/1794) | Japan | Itoh et al. (2011) | |
| Domestic dogs | 1.4% (9/640) | Korea | Kim et al. (2019) | |
| Various | 25.9% (49/189) | Netherlands | Uiterwijk et al. (2020) | |
| Domestic dogs | 5.0% (5/100) | Nicaragua | Lebbad et al. (2008) | |
| Domestic dogs | 5.2% (3/58) | Nicaragua | Roegner et al. (2019) | |
| Domestic dogs | 5.3% (32/605) | Peru | Cooper et al. (2010) | |
| Domestic & shelter dogs | 0.7% (1/148) | Poland | Solarczyk and Majewska (2010) | |
| Domestic dogs | 1.6% (2/128) | Poland | Piekarska et al. (2016) | |
| Household & kennel dogs | 9.5% (12/126) | Portugal | Ferreira et al. (2011) | |
| Domestic dogs | 13.7% (11/80) | Portugal | Pereira et al. (2021) | |
| Domestic dogs | 27.6% (29/39) | Romania | Adriana et al. (2016) | |
| Domestic dogs | 3.7% (13/348) | Spain | Adell-Aledon et al. (2018) | |
| Domestic dogs | 1.6% (4/252) | Spain | Mateo et al. (2023) | |
| Giardia-positive dog samples | 50.0% (14/28) | Sweden | Lebbad et al. (2010) | |
| Domestic dogs | 7.1% (3/42) | Taiwan | Liang et al. (2012) | |
| Stray dogs | 3.4% (4/118) | Taiwan | Tseng et al. (2014) | |
| Temple dogs | 1.3% (3/229) | Thailand | Inpankaew et al. (2007) | |
| Temple dogs | 5.7% (13/229) | Thailand | Traub et al. (2009) | |
| Domestic dogs | 11.0% (12/109) | Thailand | Tangtrongsup et al. (2017) | |
| Domestic & shelter dogs | 6.0% (18/301) | Thailand | Tangtrongsup et al. (2020) | |
| Monastery dogs | 1.3% (7/540) | Thailand | Khine et al. (2021) | |
| Stray dogs | 20.2% (21/104) | Trinidad and Tobago | Mark-Carew et al. (2013) | |
| Giardia-positive shelter dogs | 70.7% (29/41) | UK | Upjohn et al. (2010) | |
| Giardia-positive domestic dogs | 22.2% (2/9) | Unknown | Read et al. (2004) | |
| Giardia-positive dog samples | 66.6% (2/3) | USA | Miska et al. (2009) | |
| Giardia-positive domestic dogs | 16.4% (21/128) | USA | Covacin et al. (2011) | |
| Domestic dogs | 1.5% (2/129) | USA | Wang et al. (2012) | |
| Domestic dogs | 0.8% (1/120) | USA | Munoz and Mayer (2016) | |
| Domestic dogs | 4.8% (10/209) | Vietnam | Nguyen et al. (2018) | |
| Mixed C& D | Domestic dogs | 2.7% (1/36) | Argentina | Kuthyar et al. (2021) |
| Shelter & domestic dogs | 0.1% (2/1400) | Australia | Palmer et al. (2008a, b) | |
| Domestic, shelter & pet shop dogs | 0.5% (1/209) | Canada | Uehlinger et al. (2013) | |
| Domestic & stray dogs | 8.5% (80/940) | China | Qi et al. (2016) | |
| Wide range | 0.8% (5/601) | China | Li et al. (2019b) | |
| Domestic dogs | 1.1% (5/448) | China | Sui et al. (2022) | |
| Giardia-positive domestic dogs | 1.8% (1/54) | Czech Republic | Lecová et al. (2020) | |
| Giardia-positive dogs | 2.6% (1/39) | France | Kaufmann et al. (2022) | |
| Giardia-positive domestic dogs | 8.7% (8/92) | Germany | Barutzki et al. (2007) | |
| Domestic dogs | 3.1% (12/386)b | Germany | Murnik et al. (2023) | |
| Domestic & shelter dogs | 1.9% (15/807) | Greece | Kostopoulou et al. (2017) | |
| Domestic dogs | 0.4% (1/229) | Hungary | Szénási et al. (2007) | |
| Giardia-positive domestic dogs | 5.8% (1/17) | Italy | Berrilli et al. (2004) | |
| Domestic dogs | 0.6% (3/502) | Italy | Paoletti et al. (2015) | |
| Various | 7.4% (14/189) | Netherlands | Uiterwijk et al. (2020) | |
| Domestic dogs | 1.0% (1/100) | Nicaragua | Lebbad et al. (2008) | |
| Domestic dogs | 4.3% (26/605) | Peru | Cooper et al. (2010) | |
| Domestic dogs | 0.8% (1/128) | Poland | Piekarska et al. (2016) | |
| Domestic dogs | 5.0% (4/80) | Portugal | Pereira et al. (2021) | |
| Domestic dogs | 2.6% (1/39) | Romania | Adriana et al. (2016) | |
| Domestic dogs | 0.4% (1/252) | Spain | Mateo et al. (2023) | |
| Giardia-positive dog samples | 17.8% (5/28) | Sweden | Lebbad et al. (2010) | |
| Domestic dogs | 0.9% (1/109) | Thailand | Tangtrongsup et al. (2017) | |
| Giardia-positive shelter dogs | 2.4% (1/41) | UK | Upjohn et al. (2010) | |
| Domestic dogs | 0.8% (1/129) | USA | Wang et al. (2012) | |
| Shelter dogs | 24.4% (164/672) | USA | Johansen et al. (2014) | |
| Domestic dogs | 2.3% (7/300) | USA | Hascall et al. (2016) | |
| Domestic dogs | 10.7% (9/84) | USA | Scorza and Lappin (2017) | |
| Assemblage E | Wild dogs | 4.5% (2/44) | Australia | Ng et al. (2011) |
| Domestic dogs | 1.4% (2/143) | Brazil | Harvey et al. (2023) | |
| Domestic, shelter & pet shop dogs | 0.5% (1/209) | Canada | Uehlinger et al. (2013) | |
| Various | 0.3% (1/294) | Canada | Julien et al. (2019) | |
| Wide range | 1.9% (5/267) | China | Li et al. (2015) | |
| Giardia-positive dog samples | 0.8% (5/600) | Europe | Sprong et al. (2009) | |
| Domestic dogs | 0.6% (1/150) | Finland | Rimhanen-Finne et al. (2007) | |
| Domestic dogs | 0.5% (2/376) | Germany | Sommer et al. (2018) | |
| Domestic dogs | 0.5% (1/212) | India | Utaaker et al. (2018) | |
| Domestic dogs | 2.6% (1/39) | Romania | Adriana et al. (2016) | |
| Stray dogs | 1.0% (1/104) | Trinidad and Tobago | Mark-Carew et al. (2013) | |
| Assemblage F | Giardia-positive domestic dogs | 16.6% (2/12) | Brazil | Silva et al. (2022) |
| Dogs with diarrhoea | 0.2% (1/485) | China | Yu et al. (2018) | |
| Various | 0.1% (1/651) | China | Liao et al. (2020) | |
| Giardia-positive domestic dogs | 4.9% (6/123) | Germany | Pallant et al. (2015) | |
| Domestic dogs | 0.5% (2/376) | Germany | Sommer et al. (2018) | |
| Domestic dogs | 15.4% (8/52) | UK | Krumrie et al. (2022) | |
| Other mixtures | Domestic dogs | C + A (1/36) | Argentina | Kuthyar et al. (2021) |
| Wild dogs | A + E (3/44) | Australia | Ng et al. (2011) | |
| Domestic dogs | A + C (1/147) | Brazil | Paim Arruda Trevisan et al. (2020) | |
| Domestic, shelter & pet shop dogs | A + C (1/209) | Canada | Uehlinger et al. (2013) | |
| B + D (1/209) | ||||
| D + E (1/209) | ||||
| Domestic dogs | 5.1% (11/216)b | China | Zheng et al. (2014) | |
| Domestic dogs | A + B (4/105) | China | Wu et al. (2022) | |
| A + D (1/105) | ||||
| A + B + D (2/105) | ||||
| Giardia-positive domestic dogs | A + D (1/92) | Germany | Barutzki et al. (2007) | |
| Domestic dogs | A + B (1/31) | Germany | Rehbein et al. (2019) | |
| Giardia-positive domestic dogs | A + C (2/17) | Italy | Berrilli et al. (2004) | |
| Temple dogs | A + B (3/229) A + D (1/229) | Thailand | Inpankaew et al. (2007) | |
| Domestic dogs | A + B (38/473) | Turkey | Gultekin et al. (2017) | |
Also contained mixtures with C and D.
Various mixtures.
There have been several reports of C. parvum in cats involving mostly single reports in individual cats from various countries (Table 1). There have also been a few reports of C. muris and rat genotypes III and IV in cats, which may be due to mechanical carriage from ingesting rodents or an accidental infection (Table 1). Cryptosporidium muris oocysts from a cat have been shown to be infective to laboratory mice (Pavlásek and Ryan, 2007), indicating that they may retain viability following passage through a cat. Another study indicated that C. muris was infectve to cats, as DNA sequencing of paraffin-embedded sections of intestinal and stomach biopsies from a cat with persistant diarrhoea detected C. muris in the stomach and C. felis in the intestine (FitzGerald et al., 2011). A follow-up study 12 months later on the same cat, detected C. muris-like oocysts from endoscopic biopsies but C. felis oocysts were not identified (FitzGerald et al., 2011). The identification of C. muris and other rodent genotypes in cats is likely due to frequent contact between cats and rodents, with the study by FitzGerald et al. (2011) showing C. muris can infect the gastric mucosa of cats. A previous cross-transmission study also reported that cats were “highly susceptible” to C. muris, whereas dogs had low susceptibility (Iseki et al., 1989). There has been one report of C. canis in one cat, which is likely due to mechanical carriage (Table 1).
There have only been two reports of C. hominis in dogs; one in a wild dog in Sydney catchments in Australia (Ng et al., 2011) and the other in a shelter dog faecal sample in Spain (Gil et al., 2017). The parasite was identified by PCR and sequencing at the 18S locus, but in both cases, amplification at the gp60 locus was not successful (Ng et al., 2011; Gil et al., 2017), suggesting that oocyst numbers were low and may have been present due to coprophagy of human faeces. There have been several reports of C. parvum in dogs, with the highest prevalence (10%; 5/50) reported in household dogs in Egypt (Gharieb et al., 2018), and in two puppies with diarrhoea in Egypt (10%; 2/20) (El-Madawy et al., 2010). Another study in Thailand reported C. parvum in 6.4% (7/109) of dogs (31.8% of the 22 typed samples) (Tangtrongsup et al., 2017) (Table 2). In the majority of other studies, the prevalence of C. parvum has been low (0.3–2.9%) (Table 2).
Only a few studies have subtyped the C. parvum detected in dogs. Among them, subtypes IIaA15G2R1 and IIaA17G2R1 have been detected in dogs in the UK (Smith et al., 2010; Rosanowski et al., 2018), and subtype IIaA15G2R1 in dogs in Germany (Murnik et al., 2022). These are common subtypes in cattle and humans (Feng and Xiao, 2017; Holzhausen et al., 2019; Santín, 2020; Guo et al., 2021, 2022; Yang et al., 2021). In the study by Smith et al. (2010), IIaA17G2R1 was detected in the single farm dog sampled, and was the dominant subtype detected in cattle on the farm. Epidemiological analysis indicates that dogs are at a higher risk of being infected with C. parvum from cattle when living in areas on or near cattle farms (Moreira et al., 2018). In the study of shelter dogs in the UK (Rosanowski et al., 2018), the authors reported that “some of the dogs arriving at the shelter, may have been roaming as strays prior to submission, with the potential that the environment was more contaminated than for owned dogs”. The study in Germany, which identified IIaA15G2R1 in two dogs (Murnik et al., 2022), was conducted in Saxony, and IIaA15G2R1 was the most prevalent subtype in calves in the same area in a previous study (Holzhausen et al., 2019). In another study, C. parvum subtype IIcA5G3b was identified in two street dogs in Nigeria (Ayinmode et al., 2018b). The C. parvum IIc subtype family almost exclusively infects humans (Yang et al., 2021), suggesting that its presence in dogs may have been due to coprophagy of human faeces by the dogs.
There have been two reports of C. muris in dogs (Table 2). Oocysts of C. muris directly isolated from the stomach of infected mice have previously been orally inoculated in dogs with very few oocysts (7–12) detected between 7 and 12 days post-infection, and a few C. muris life-cycle stages were identified in the gastric mucosa of one dog (Aydin and Ozkul, 1996). These findings suggest that dogs may not be a natural host of C. muris, which is consistent with a study by Iseki et al. (1989). In one study in Texas, Cryptosporidium antigens were detected in 70% (49/70) of kennel dogs (Lupo et al., 2008), with six of the positives being amplified at the 18S locus and identified as C. muris and “C. muris-like”. Oocysts were also identified in these six samples (Lupo et al., 2008). The actual prevalence of C. muris in the study remains unknown, but the reported 70% prevalence is likely an over-estimate, as there have been numerous reports of false positives by Cryptosporidium enzyme antigen tests, especially considering the fact that most coproantigen kits are not expected to detect C. muris (CDC, 1999; Doing et al., 1999; Katanik et al., 2001).
There has also been a single report of C. scrofarum in one shelter dog in Greece (Kostopoulou et al., 2017), C. meleagridis in a domestic dog in the Czech Republic, and C. ubiquitum and rat genotype IV in individual dogs in China (Li et al., 2019a). These are all likely due to coprophagy.
Cryptosporidium oocyst shedding from cats and dogs appears to be low (Asahi et al., 1991; Cox et al., 2005; Hamnes et al., 2007; Labana et al., 2018; Smith et al., 2020). For example, oocyst concentrations ranging from 0 to 17 and 0–5000 oocysts per gram of faeces (OPG) have been reported in cats and dogs, respectively, using direct immunofluorescence microscopy (DFA) (Cox et al., 2005). A more recent larger study of 869 dogs reported median Cryptosporidium oocyst excretion of 3.8 × 10 OPG using DFA (Smith et al., 2020). In comparison, livestock, particularly cattle, shed much higher numbers of oocysts, with calves shedding > 5.5 × 106 oocysts per day and > 3 × 1010 oocysts over a 6-day period (Nydam et al., 2001). Infected lambs can shed up to 3.7 × 109 OPG (Yang et al., 2014), while humans can shed up to 108 oocysts in a single bowel movement (Goodgame et al., 1993).
3. Giardia in cats and dogs
The prevalence of Giardia in cats (∼1.3–44.4%) and dogs (∼1–57.9%) varies widely but is generally < 10% (Feng and Xiao, 2011; Bouzid et al., 2015; López-Arias et al., 2019; Nagamori et al., 2020; Cai et al., 2021; Sui et al., 2022) (Table 1, Table 2). In one meta-analysis, the global prevalence of Giardia was calculated at 2.61% (112,513/4,309,451) in dogs and 2.33% (5807/248,195) in cats (Bouzid et al., 2015). In China, the overall prevalence of Giardia in dogs has been estimated at 12.3% by molecular testing (Zhao et al., 2022). Factors that influence the prevalence of Giardia include age, presence of diarrhoea, socioeconomic situation, and origin of the samples (e.g. household pets versus kennel/shelter dogs/cats) and the number of doses of anthelmintic administered each year (Bugg et al., 1999; Bouzid et al., 2015; Cai et al., 2021; French et al., 2023; Murnik et al., 2023).
The highest prevalence of Giardia in dogs and cats has been reported from young animals (< 6 months) (Bouzid et al., 2015), in dogs from low- to middle-income areas, and from kennel/shelter dogs, where the potential for transmission is greater due to high densities of animals, continuous environmental contamination and frequent introduction of new animals. For example, the overall prevalence of Giardia in dogs was 44.4% (16/36) (Kuthyar et al., 2021) in Argentina, and 41.1% (69/168) in shelter dogs in Italy (Agresti et al., 2021). In another study from Spain, the highest prevalence was detected in breeding (45.8%; 11/24) and shelter dogs (40.4%; 88/218) (Adell-Aledon et al., 2018). In China, a recent study reported a prevalence of 40.3% (60/149) in shelter dogs compared to only 3.0% (6/199) in household dogs (Liao et al., 2020). Similarly in Portugal, 47.0% (23/49) of kennel dogs were Giardia-positive, but household dogs (n = 97) were negative (Ferreira et al., 2011). In cats, in one study in Italy, an overall prevalence of 37% (10/27) was reported for stray and domestic cats and all were assemblage A (Papini et al., 2007), and in the USA, 44.4% of colony cats were positive for Giardia (Fayer et al., 2006). However, another study reported similar prevalences of Giardia in shelter/breeding cats and household cats (Sommer et al., 2018).
Microscopy-based screening studies generally under-report the prevalence of Giardia due to sporadic shedding of cysts and the poor sensitivity of flotation assays and miscoscopy (Thompson, 2004). Many of the studies conducted the initials screens for Giardia by microscopy and then genotyped the positives samples, which can also underestimate the true prevalence. For example, one study detected Giardia in 57.9% (165/285) of kennel dogs by qPCR analysis of the 18S rRNA gene, compared to only 14.6% (39/268) detected by microscopy (Simonato et al., 2015). However, of the qPCR-positive samples, only 64% (106/165) were confirmed by nested PCR, and of these, only 74.5% (79/106) produced sequences (Simonato et al., 2015), emphasising the importance of sequencing to confirm positives.
Assemblages C and D are the most commonly reported Giardia in dogs, while assemblages F and A are most common in cats (Table 1, Table 2) (Ramírez-Ocampo et al., 2017; Cai et al., 2021; Zhao et al., 2022). In addition to assemblages C and D, assemblages A, B, E and F have also been reported in dogs. Similarly, assemblages B, C, D and occasionally E have also been reported in cats (Table 1, Table 2). Although assemblages C and D dominate in dogs, assemblages A and B (which infect humans) have also been detected. The detection of assemblages A and B in dogs and cats is thought to be mainly due to coprophagy and/or spill-back from human contact (Feng and Xiao, 2011; Cai et al., 2021), but this remains to be conclusively determined.
Despite the presence of host-adapted assemblages, assemblages A and B are common in cats and dogs (Table 1, Table 2). Between them, studies conducted in China revealed that assemblage A was the dominant assemblage in stray cats and dogs (Pan et al., 2018), and in police and farm dogs (92.6% of typed isolates; 25/27) (Li et al., 2013). Assemblage A was also the dominant assemblage in dogs in Japan (66.6%; 14/21 typed isolates) (Itagaki et al., 2005) and in Germany (60%; 33/55 typed isolates) (Leonhard et al., 2007). Assemblage A was the only assemblage identified in slum and stray dogs in Brazil (Volotão et al., 2007; Fantinatti et al., 2018), domestic dogs in Jamaica (Lee et al., 2017), and dogs in indigenous communities in Saskatchewan, Canada (32/32 typed isolates) (Schurer et al., 2012). Subtyping of assemblage A in cats and dogs has identified mostly sub-assemblage AI, which is mainly confined to animal hosts (Lalle et al., 2005b; Souza et al., 2007; Volotão et al., 2007; Marangi et al., 2010; Zheng et al., 2015; Saleh et al., 2019; Mateo et al., 2023). However, some studies have identified sub-assemblage AII in cats and dogs (e.g. Claerebout et al., 2009; Traub et al., 2004; Gil et al., 2017; Ito et al., 2017; Agresti et al., 2021; Guadano Procesi et al., 2022), which is mainly human-associated and even sub-assemblage AIII (Claerebout et al., 2009; Guadano Procesi et al., 2022), which is wild ruminant-associated (Klotz et al., 2022). Differences in sub-assemblage A assignment may be related to the typing locus used, as a meta-analysis revealed that subtyping assemblage A at multiple loci produced divergent results, with sub-assemblage AI dominating amongst assemblage A identified in dogs, when the tpi locus was used, and sub-assemblage AII dominating at the bg locus (Cai et al., 2021). Therefore, the identity of A sub-assemblages in cats and dogs is unclear.
Assemblage B has been reported less frequently in cats and dogs than assemblage A (Feng and Xiao, 2011; Cai et al., 2021) (Table 1, Table 2). However, assemblage B was identified as the dominant assemblage in these animals in some studies. For example, in Spain, assemblage B was detected in 42.1% (8/19) of typed Giardia-positive dogs, followed by assemblage A (7/19), with assemblages C and D only detected in 4 samples (Gil et al., 2017). A study in the USA, also reported assemblage B as the dominant assemblage (41% of typed samples) in household dogs, followed by assemblage A (28% of typed samples) (Covacin et al., 2011). In Turkey, assemblage B was reported in all 89 typed Giardia-positive dogs, but 42.7% (38/89) of these were mixed infections with assemblage A (Gultekin et al., 2017). Moreover, also in Turkey, assemblage B was the only assemblage detected in 30 typed Giardia-positive symptomatic cats (Sursal et al., 2020). Interestingly, in a study conducted in Romania, assemblage D was the only assemblage detected in 80% (8/10) of cats screened (Adriana et al., 2016). In that study, seven of the cats positive for assemblage D resided in the same house, and although dogs were not present, all cats had outdoor access (Adriana et al., 2016).
Many of these studies, particularly earlier studies, have relied on one locus for identification of Giardia assemblages in cats and dogs. Due to discordance in genotyping results between loci, however, it is possible for Giardia isolates from cats and dogs to be typed as ‘zoonotic’ at one locus but ‘host-specific’ at another (Cacciò and Ryan, 2008), which makes interpretation difficult. In many studies, where multiple loci were used, there was significant discordance between loci (e.g. Covacin et al., 2011; Beck et al., 2012; Johansen et al., 2014; Pallant et al., 2015; Kostopoulou et al., 2017), with one study reporting that the 18S locus preferentially amplified assemblages B, D and C, whereas the bg locus preferentially amplified assemblages A and B (Covacin et al., 2011).
Giardia cyst excretion is highly variable and sporadic and relatively few studies have examined cyst excretion in dogs and cats. In DFA analysis, cyst concentrations ranging from 0 to 7143 and 0–6061 (median 835) cysts per gram of faeces (CPG) have been reported in cats and dogs, respectively (Cox et al., 2005). Another study of Giardia cyst shedding in 104 dogs and 21 cats from Germany, reported that 92.3% (96/104) of dogs and 76.2% (16/21) of cats, shed low numbers of cysts (< 50 cysts per slide) (Sommer et al., 2018). In Canada, a study of 869 dog faecal samples from urban parks reported median Giardia cyst excretion rates of 1.0 × 104 CPG (Smith et al., 2020). Similarly, studies of Giardia-positive dogs in Italy (Ciuca et al., 2021) and France (Kaufmann et al., 2022) reported a mean of 2 × 104 CPG (2.7 × 103–4.8 × 104) and a geometric mean excretion of 2.1 × 105 CPG, respectively. A more recent study of Giardia in dogs and cats in Egypt using qPCR reported that 56% (47/84) of the Giardia positives from dogs and 90.7% (39/43) of the Giardia positives from cats had high qPCR CT values indicating “light infections” (Elmahallawy et al., 2023). In comparison, cattle can shed > 1 × 106 Giardia cysts per day (Nydam et al., 2001), while infected humans can shed 108–109 cysts per day (Danciger and Lopez, 1975; Rendtorff, 1979).
4. Zoonotic risk of cryptosporidiosis and giardiasis from cats and dogs
The dominant Cryptosporidium species and Giardia assemblages infecting cats are C. felis and assemblages F and A, compared with C. canis and assemblages C and D in dogs (Table 1, Table 2). Although C. felis, C. canis, and assemblages C, D and F are considered to be of limited zoonotic potential, this risk is not negligible as there have been numerous reports of C. felis and C. canis in humans, as well as a few reports of assemblages C, D and F in humans (Table 3). The prevalence of C. felis and C. canis in humans is usually low (1–3% of cryptosporidiosis cases); however, a few studies have reported higher prevalences; 5–40% for C. canis and 7.1–37.5% for C. felis cryptosporidiosis cases, mostly in children or HIV-positive individuals (Moore et al., 2016; Dacal et al., 2018; Sannella et al., 2019; Uran-Velasquez et al., 2022; Wang et al., 2022b; Khalifa et al., 2023).
Table 3.
Reports of Cryptosporidium felis, C. canis and Giardia assemblages C, D and F in humans globally.
| Species/Assemblage | No. of cases | Comment | Country | Reference |
|---|---|---|---|---|
| Cryptosporidium | ||||
| C. felis | 2 | HIV+ patients | Algeria | Semmani et al. (2023) |
| 1 | 21-year-old female with diarrhoea | Australia | Ebner et al. (2015) | |
| 5 | HIV+ patients | Brazil | Lucca et al. (2009) | |
| 4 | School children | Brazil | Pacheco et al. (2022) | |
| 2 | Sporadic cases | Canada | Guy et al. (2021) | |
| 2 | Hospitalised children (median 36 months-old) | China | Feng et al. (2012) | |
| 8 | 1 HIV+, 4 with diarrhoea, 2 with non-gastrointestinal illnesses | China | Liu et al. (2020) | |
| 3 | Kindergarten children aged 2–6 years | China | Wang et al. (2022b) | |
| 1 | Average age 5 years | Colombia | Higuera et al. (2020) | |
| 2 | 1 child and 1 HIV+ patient | Colombia | Uran-Velasquez et al. (2022) | |
| 1 | C. felis identified in owner’s cat | Colombia | Potes-Morales and Crespo-Ortiz (2023) | |
| 5 | HIV+/AIDS patients | Ethiopia | Adamu et al. (2014) | |
| 6 | HIV+ patients | France | Guyot et al. (2001) | |
| 15 | 10 HIV+ patients, 3 transplant patients | France | ANOFEL Cryptosporidium National Network (2010) | |
| 10 | Immunodeficient individuals | France | Costa et al. (2018) | |
| 6 | 40% immunodeficient patients | France | Costa et al. (2020) | |
| 2 | AIDS patients | Hatai | RaccurtBrasseur et al. (2006) | |
| 1 | HIV+ patients | India | Muthusamy et al. (2006) | |
| 3 | Patients with diarrhoea; many had underlying clinical conditions | India | Khalil et al. (2017) | |
| 1 | HIV+ patients | Jamaica | Gatei et al. (2008) | |
| 2 | Children aged < 5 years | Kenya | Gatei et al. (2006) | |
| 1 | HIV+/AIDS patients | Malaysia | Lim et al. (2011) | |
| 4 | Children aged 3–14 years | Mozambique | Muadica et al. (2021) | |
| 1 | HIV+/AIDS patients | Nigeria | Ukwah et al. (2017) | |
| 1 | All children; not associated with diarrhoea | Peru | Xiao et al. (2001) | |
| 24 | 3.3% of typed positives from HIV+/AIDS patients | Peru | Cama et al. (2003) | |
| 9 | HIV+ patients | Peru | Cama et al. (2006) | |
| 6 | HIV+ patients | Peru | Cama et al. (2007) | |
| 4 | Children aged < 3 years | Peru | Cama et al. (2008) | |
| 1 | HIV+ patients | Portugal | Alves et al. (2001) | |
| 4 | HIV+ patients | Portugal | Matos et al. (2004) | |
| 1 | HIV+ patient | Spain | Cieloszyk et al. (2012) | |
| 1 | – | Spain | Abal-Fabeiro et al. (2014) | |
| 1 | 37-year-old immunocompetent female with a range of underlying health issues and used inhaled steroids, pet cat also had C. felis | Sweden | Beser et al. (2015) | |
| 4 | Sporadic cases - one had travelled to Asia | Sweden | Lebbad et al. (2021) | |
| 3 | HIV+ patients | Switzerland | Morgan et al. (2000a) | |
| 3 | HIV+ patients | Thailand | Gatei et al. (2002) | |
| 1 | HIV+ patients | Thailand | Tiangtip and Jongwutiwes (2002) | |
| 1 | HIV+/AIDS patients | Thailand | Srisuphanunt et al. (2011) | |
| 7 | HIV+ patients | Thailand | Sannella et al. (2019) | |
| 1 | Primary school children | Thailand | Sutthikornchai et al. (2021) | |
| 4 | One HIV+ patient, one had a severe underlying condition, one aged 1 year | UK | Pedraza-Días et al. (2001) | |
| 6 | 2 were known to be immunocompromised | UK | Leoni et al. (2006) | |
| 4 | 0.01% (4/8000) of typed cases | UK | Chalmers et al. (2009b) | |
| 38 | 0.3% (38/14,469) of typed cases, 6/38 were immunocompromised | UK | Elwin et al. (2012b) | |
| 1 | HIV+ patients | USA | Pieniazek et al. (1999) | |
| 3 | Rural Nebraska; age range 7 months to 79 years | USA | Loeck et al. (2020) | |
| 10 | New York City residents. 1.8% (10/547) of typed samples | USA | Alderisio et al. (2023) | |
| 1 | Patients with diarrhoea | Zambia | Mulunda et al. (2020) | |
| 2 | One patient infected while on holiday in India and second patient had “contact with a kitten with diarrhoea 3 weeks before disease onset” | Sweden | Insulander et al. (2013) | |
| C. canis | 1 | School children (10% prevalence) | Angola | Dacal et al. (2018) |
| 1 | HIV+ patients | Brazil | Lucca et al. (2009) | |
| 5 | Symptomatic children attending hospital | Cambodia | Moore et al. (2016) | |
| 1 | Hospitalised children (median 36 months-old) | China | Feng et al. (2012) | |
| 2 | Both with non-gastrointestinal illnesses | China | Liu et al. (2020) | |
| 18 | 40% (12/30) of dog owners and 24% of (6/25) dog shelter workers | Egypt | Khalifa et al. (2023) | |
| 2 | HIV+/AIDS patients | Ethiopia | Adamu et al. (2014) | |
| 4 | 3 HIV+ patients | France | ANOFEL Cryptosporidium National Network (2010) | |
| 3 | 40% immunodeficient patients | France | Costa et al. (2020) | |
| 1 | HIV+ patients | Jamaica | Gatei et al. (2008) | |
| 1 | Critically ill child in hospital with additional co-infections | Jordan | Hijjawi et al. (2010) | |
| 3 | Children aged < 5 years | Kenya | Gatei et al. (2006) | |
| 2 | Children (24- and 14 months old) | New Zealand | Learmonth et al. (2004) | |
| 1 | Children aged 6 months to 6 years | Nigeria | Molloy et al. (2010) | |
| 2 | All children; not associated with diarrhoea | Peru | Xiao et al. (2001) | |
| 36 | 4% of typed samples from HIV+/AIDS patients | Peru | Cama et al. (2003) | |
| 12 | HIV+ patients | Peru | Cama et al. (2006) | |
| 6 | HIV+ patients | Peru | Cama et al. (2007) | |
| 2 | 32-month-old female, 6.5-year-old brother (and household dog) | Peru | Xiao et al. (2007) | |
| 4 | Children aged < 3 years | Peru | Cama et al. (2008) | |
| 2 | HIV+ patients | Thailand | Gatei et al. (2002) | |
| 1 | HIV+/AIDS patients | Thailand | Srisuphanunt et al. (2011) | |
| 12 | HIV+ patients | Thailand | Sannella et al. (2019) | |
| 1 | Child aged 2 years | UK | Pedraza-Días et al. (2001) | |
| 1 | Child aged 1 year; had returned from Africa | UK | Leoni et al. (2006) | |
| 1 | 0.01% (1/8000) of typed cases | UK | Chalmers et al. (2009a) | |
| 1 | 0.007% (1/14,469) of typed cases | UK | Elwin et al. (2012b) | |
| 1 | HIV+ patients | USA | Pieniazek et al. (1999) | |
| 1 | Rural Nebraska; age range 7 months to 79 years | USA | Loeck et al. (2020) | |
| 3 | New York City residents. 0.5% (3/547) of typed samples | USA | Alderisio et al. (2023) | |
| 1 | HIV+ patients | Venezuela | Certad et al. (2006) | |
| 2 | 1 human diarrheal and 1 non-diarrheal sample | Vietnam | Iwashita et al. (2021) | |
| Giardia | ||||
| Assemblage C | 5 | Children attending a day-care centre | Brazil | Durigan et al. (2014) |
| 6 | Immunocompromised patients | Brazil | Jeske et al. (2022) | |
| 16 | Typing only conducted at 1 locus | China | Liu et al. (2014) | |
| 3 | HIV+/AIDS patients | China | Jiang et al. (2023) | |
| 1 | Adult immunocompromised male with cancer and diarrhoea | Egypt | Soliman et al. (2011) | |
| 1 | 44-year-old female with chronic diarrhoea; was not a pet owner | Slovakia | Štrkolcová et al. (2015) | |
| Assemblage D | 2 | Travel returnees from Southeastern Asia | Germany | Broglia et al. (2013) |
| 1 | Rural village, lack of water and sanitation. Only typed at 1 locus | Argentina | Candela et al. (2021) | |
| 1 | Immunocompromised patients | Brazil | Jeske et al. (2022) | |
| Assemblage F | 1 | 10-month-old child | Brazil | Silva et al. (2022) |
| 3 | Children. Mixed A and F | Ethiopia | Gelanew et al. (2007) | |
| 3 | Children living with poor sanitation | Slovakia | Pipiková et al. (2022) | |
| 1 | Primary school children | Thailand | Sutthikornchai et al. (2021) | |
Cryptosporidium canis and C. felis are however, largely host-specific, as the prevalence of C. felis and C. canis in humans is usually low, despite the close contact between humans and companion animals. For example, in the USA, ∼14% of households own a cat and ∼22.2% of households own a dog, equating to 111.6 million households (APPA, 2022). Across Europe, 26% of households own one or more cats and 25% own one or more dogs, equating to ∼127.2 million cats and 104.4 million dogs (FEDIAF, 2023). In Australia, 48% of Australian households have dogs and 33% of households have cats across both urban and rural areas (Animal Medicines Australia). The majority of reports of C. felis, C. canis and assemblages C, D and F in humans have been in immunologically immature children or immunocompromised adults and predominantly in low-income countries (Table 3). Therefore, the zoonotic risk from C. felis, C. canis and assemblages C, D and F in immunocompetent humans is probably low.
There have been several reports suggesting possible zoonotic transmission between owners and pets using genotyping analysis of human and animal samples from the same household or workplace (Xiao et al., 2007; Beser et al., 2015). For example, C. felis was characterised in a 37-year-old immunocompetent woman and her cat in Sweden (Beser et al., 2015) and C. canis was identified in a 2.5 year-old child, her 6.5 year-old brother and their dog in Peru (Xiao et al., 2007). In a recent study in Egypt, C. canis was identified in 12 domestic dog owners and 6 dog shelter workers (Khalifa et al., 2023).
The recent development of gp60 subtyping tools for C. felis and C. canis has facilitated studies of zoonotic transmission of these two pathogens (Rojas-Lopez et al., 2020; Jiang et al., 2021). Results of these studies have identified both zoonotic and human-adapted subtypes in C. felis. Therefore, subtypes XIXc, XIXd and XIXe have been reported only in humans, while the remaining two subtypes (XIXa and XIXb) have been reported in both humans and cats (Jiang et al., 2020; Rojas-Lopez et al., 2020; Guy et al., 2021; Li et al., 2021b; Joachim et al., 2023). In total, nine C. canis subtype families (XXa to XXi) have been identified (Jiang et al., 2021; Murnik et al., 2022; Wang et al., 2022a; Elmahallawy et al., 2023; Mateo et al., 2023). The limited typing to date in C. canis has supported potential zoonotic transmission (Jiang et al., 2021), including re-analysis of samples from a suspected case of zoonotic transmission of C. canis in a household (Xiao et al., 2007), with C. canis subtype XXa4 identified from the 2.5 year-old female index case, her 6.5-year-old brother and their dog (Jiang et al., 2021). Similarly, subtyping has confirmed zoonotic transmission of C. felis between two human cases and their cats in Sweden (Rojas-Lopez et al., 2020).
Cats and particularly dogs can also be infected with C. parvum, which is a more significant public health risk. However, in most studies where C. parvum was detected, with a few exceptions, the occurrence rate was low and it is unknown if dogs and cats are mechanically carrying C. parvum due to coprophagy of human or cattle faeces or actually infected. It is possible that cats and particularly dogs from rural locations close to ruminants, may have a higher prevalence of C. parvum. Zoonotic transmission of C. parvum from cats and dogs has been suggested (Neira et al., 2010; Gharieb et al., 2018). For example, C. parvum was detected in children and their pet dogs in Egypt (Gharieb et al., 2018) and in a pregnant immunocompetent woman and her cat (Neira et al., 2010). The direction of transmission, i.e. spillover from pets to humans or spillback from humans to pets, in these studies is unknown. In contrast, a study in Spain reported no evidence supporting zoonotic transmission between owners and their pet cats and dogs (de Lucio et al., 2017). Similarly, in households in Peru, only Giardia assemblages A and B were identified in humans, while assemblages C and D were identified in their dogs, with the exception of one dog with a mixed assemblage B/D infection (Cooper et al., 2010). There have only been two reports of C. hominis in dogs and in both cases, PCR analysis of a second locus was unsuccessful, indicating very low oocyst numbers (Ng et al., 2011; Gil et al., 2017).
As with Cryptosporidium, several studies have suggested possible transmission of Giardia from pets to owners (Traub et al., 2004; Palmer et al., 2008b; Marangi et al., 2010; Quadros et al., 2016; Rehbein et al., 2019; Kuthyar et al., 2021; Harvey et al., 2023; Khalifa et al., 2023), particularly in socially-deprived areas. For example, in a low socioeconomic community in southern Italy, sub-assemblage AI was identified in nine of 14 dogs and six of 14 children (Marangi et al., 2010). In Argentina, assemblage A was detected in 12 humans and five pet dogs (Kuthyar et al., 2021). In one study in Brazil, assemblage E was the only assemblage detected in dogs, and assemblages A, B and E were recovered from children from the same rural community (Harvey et al., 2023). Another study of Giardia-positive samples from humans, dogs and cats in Brazil, identified assemblage F in one human and assemblages C, D and F in dogs and F in one cat (Silva et al., 2022). These studies are suggestive of zoonotic transmission but have not been confirmed by finding identical multilocus subtypes in both humans and their companion animals. Furthermore, it would also be necessary to demonstrate that the animals acquired the infection prior to human infection (Cai et al., 2021). Other studies have found no evidence of transmission between companion animals and their owners (Hopkins et al., 1997; Inpankaew et al., 2014; Wu et al., 2022). For example, one study examined Giardia assemblages in dogs and humans in rural Cambodia and found the occurrence of different assemblages between the two host species, leading to the conclusion that the risk of zoonotic transmission was low (Inpankaew et al., 2014). Another recent study in Egypt detected assemblages A and D in domestic and shelter dogs but assemblage A only in their owners and dog shelter workers (Khalifa et al., 2023).
Many of the typing studies conducted to date on Giardia in cats and dogs are problematic due to (i) high levels of mixed infections with multiple Giardia assemblages, (ii) the use of only one locus or lack of agreement between multiple loci, and (iii) the high levels of allelic sequence hetrerozygosity (ASH) in assemblages C and D, which make assemblage assignment unreliable (Morrison et al., 2007; Franzén et al., 2009; Kooyman et al., 2019; Veldhuis et al., 2022; Klotz et al., 2023). Therefore, it is difficult to draw firm conclusions on the zoonotic potential of Giardia infections in companion animals. It has been suggested that in dogs, two main transmission cycles exist, with assemblage C and D predominating, particularly in kennel/shelter dogs, and assemblage A and occasionally assemblage B transmission between dogs and humans in households (Cai et al., 2021). In agreement with this, a case-control study in the UK, reported a significant correlation between dog ownership and assemblage A infection in humans (Minetti et al., 2015). Others argue that overall, humans and pets are preferentially infected with sub-assemblages AII and AI, respectively (Feng and Xiao, 2011).
5. Conclusions
Our understanding of the zoonotic transmission of C. felis and C. canis from cats and dogs to humans will be improved by the recent development of molecular typing tools, with preliminary evidence supporting some level of zoonotic transmission (Rojas-Lopez et al., 2020; Jiang et al., 2020, 2021; Guy et al., 2021; Li et al., 2021a). Most molecular-based household transmission studies to date, however, have focussed on middle to high-income countries, where the prevalence of C. felis and C. canis in humans is low. Relatively few studies have been conducted in dogs and cats in low-income countries, where the prevalence of C. felis and C. canis is much higher in humans. Overall, the risk of zoonotic transmission from cats and dogs to humans is uncertain but likely to be low due to the relatively low zoonotic risk from C. canis, C. felis and assemblages C, D and F, the overall low prevalence of C. parvum (and C. hominis) in cats and dogs, and low oocyst shedding (Lucio-Forster et al., 2010; de Lucio et al., 2016; Rehbein et al., 2019; Li et al., 2021a; Cai et al., 2021). This would be even more the case for G. duodenalis in cats and dogs due to differences in host preferences of this parasite at the assemblage and sub-assemblage levels. Future epidemiological studies should concentrate on applying the most recently developed Cryptosporidium and Giardia multi-locus sub-typing tools, as well as whole genome sequencing, to longitudinal Giardia-positive samples from cats and dogs and their owners, before a clearer understanding of the extent of spillover and/or spillback of Cryptosporidium and Giardia between pets and their owners can be determined.
Funding
This work was funded by Water Research Australia (WaterRA) project number 1156 and the 111 Project of China (D20008).
Ethical approval
Not applicable.
CRediT authorship contribution statement
Amanda D. Barbosa: Writing – original draft, Writing – review & editing. Siobhon Egan: Writing – review & editing. Yaoyu Feng: Writing – review & editing, Funding acquisition. Lihua Xiao: Writing – review & editing, Funding acquisition. Una Ryan: Writing – original draft, Writing – review & editing, Project administration, Funding acquisition, All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Given their role as Guest Editor, Amanda D. Barbosa had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dr Frank Katzer (Co-Editor) and Professor Aneta Kostadinova (Editor-in-Chief).
Data availability
The data supporting the conclusions of this article are included within the article.
References
- Abal-Fabeiro J.L., Maside X., Llovo J., Bello X., Torres M., Treviño M., et al. High-throughput genotyping assay for the large-scale genetic characterization of Cryptosporidium parasites from human and bovine samples. Parasitology. 2014;141:491–500. doi: 10.1017/S0031182013001807. [DOI] [PubMed] [Google Scholar]
- Abdelaziz A.R., Sorour S.S.G. Prevalence and molecular characterization of Giardia duodenalis assemblage D of dogs in Egypt, and its zoonotic implication. Microbes Infect. Chemother. 2021;1:e1268. doi: 10.54034/mic.e1268. [DOI] [Google Scholar]
- Abe N., Sawano Y., Yamada K., Kimata I., Iseki M. Cryptosporidium infection in dogs in Osaka, Japan. Vet. Parasitol. 2002;108:185–193. doi: 10.1016/s0304-4017(02)00204-2. [DOI] [PubMed] [Google Scholar]
- Abe N., Kimata I., Iseki M. Identification of genotypes of Giardia intestinalis isolates from dogs in Japan by direct sequencing of the PCR amplified glutamate dehydrogenase gene. J. Vet. Med. Sci. 2003;65:29–33. doi: 10.1292/jvms.65.29. [DOI] [PubMed] [Google Scholar]
- Adamu H., Petros B., Zhang G., Kassa H., Amer S., Ye J., et al. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell-Aledon M., Köster P.C., de Lucio A., Puente P., Hernández-de-Mingo M., Sánchez-Thevenet P., Dea-Ayuela M.A., Carmena D. Occurrence and molecular epidemiology of Giardia duodenalis infection in dog populations in eastern Spain. BMC Vet. Res. 2018;14:26. doi: 10.1186/s12917-018-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriana G., Zsuzsa K., Mirabela Oana D., Mircea G.C., Viorica M. Giardia duodenalis genotypes in domestic and wild animals from Romania identified by PCR-RFLP targeting the gdh gene. Vet. Parasitol. 2016;217:71–75. doi: 10.1016/j.vetpar.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Agresti A., Berrilli F., Maestrini M., Guadano Procesi I., Loretti E., Vonci N., Perrucci S. Prevalence, risk factors and genotypes of Giardia duodenalis in sheltered dogs in Tuscany (Central Italy) Pathogens. 2021;11:12. doi: 10.3390/pathogens11010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderisio K.A., Mergen K., Moessner H., Madison-Antenucci S. Identification and evaluation of Cryptosporidium species from New York City cases of cryptosporidiosis (2015 to 2018): A watershed perspective. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.03921-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M., Matos O., Pereira, Da Fonseca I., Delgado E., Lourenco A.M., Antunes F. Multilocus genotyping of Cryptosporidium isolates from human HIV-infected and animal hosts. J. Eukaryot. Microbiol. 2001;2001:17S–18S. doi: 10.1111/j.1550-7408.2001.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Alves M.E.M., Martins F.D.C., Bräunig P., Pivoto F.L., Sangioni L.A., Vogel F.S.F. Molecular detection of Cryptosporidium spp. and the occurrence of intestinal parasites in fecal samples of naturally infected dogs and cats. Parasitol. Res. 2018;117:3033–3038. doi: 10.1007/s00436-018-5986-4. [DOI] [PubMed] [Google Scholar]
- Animal Medicines Australia Pets in Australia: A national survey of pets and people. 2022. https://animalmedicinesaustralia.org.au/report/pets-in-australia-a-national-survey-of-pets-and-people-2/
- ANOFEL Cryptosporidium National Network Laboratory-based surveillance for Cryptosporidium in France, 2006–2009. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- APPA . 2022. 2021–2022 APPA National Pet Owners Survey. American Pet Products Manufacturers Association (APPA)http://www.americanpetproducts.org/press_industrytrends.asp [Google Scholar]
- Asahi H., Koyama T., Arai H., Funakoshi Y., Yamaura H., Shirasaka R., Okutomi K. Biological nature of Cryptosporidium sp. isolated from a cat. Parasitol. Res. 1991;77:237–240. doi: 10.1007/BF00930864. [DOI] [PubMed] [Google Scholar]
- Aydin Y., Ozkul I.A. Infectivity of Cryptosporidium muris directly isolated from the murine stomach for various laboratory animals. Vet. Parasitol. 1996;66:257–262. doi: 10.1016/s0304-4017(96)01007-2. [DOI] [PubMed] [Google Scholar]
- Ayinmode A.B., Oliveira B.C.M., Obebe O.O., Dada-Adgebola H.O., Ayede A.I., Widmer G. Genotypic characterization of Cryptosporidium species in humans and peri-domestic animals in Ekiti and Oyo States, Nigeria. J. Parasitol. 2018;104:639–644. doi: 10.1645/17-74. [DOI] [PubMed] [Google Scholar]
- Ayinmode A.B., Obebe O.O., Falohun O.O. Molecular detection of Cryptosporidium species in street-sampled dog faeces in Ibadan, Nigeria. Vet. Parasitol. Reg. Stud. Reports. 2018;14:54–58. doi: 10.1016/j.vprsr.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Bajer A., Bednarska M., Rodo A. Risk factors and control of intestinal parasite infections in sled dogs in Poland. Vet. Parasitol. 2011;175:343–350. doi: 10.1016/j.vetpar.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Ballweber L.R., Panuska C., Huston C.L., Vasilopulos R., Pharr G.T., Mackin A. Prevalence of and risk factors associated with shedding of Cryptosporidium felis in domestic cats of Mississippi and Alabama. Vet. Parasitol. 2009;160:306–310. doi: 10.1016/j.vetpar.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Barutzki D., Thompson R.C., Wielinga C., Parka U., Schaper R. Observations on Giardia infection in dogs from veterinary clinics in Germany. Parasitol. Res. 2007;101(Suppl. 1):153–156. [Google Scholar]
- Beck R., Sprong H., Pozio E., Cacciò S.M. Genotyping Giardia duodenalis isolates from dogs: Lessons from a multilocus sequence typing study. Vector Borne Zoonotic Dis. 2012;12:206–213. doi: 10.1089/vbz.2011.0751. [DOI] [PubMed] [Google Scholar]
- Berrilli F., Di Cave D., De Liberato C., Franco A., Scaramozzino P., Orecchia P. Genotype characterisation of Giardia duodenalis isolates from domestic and farm animals by SSU-rRNA gene sequencing. Vet. Parasitol. 2004;122:193–199. doi: 10.1016/j.vetpar.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Berrilli F., DʼAlfonso R., Giangaspero A., Marangi M., Brandonisio O., Kaboré Y., et al. Giardia duodenalis genotypes and Cryptosporidium species in humans and domestic animals in Côte dʼIvoire: Occurrence and evidence for environmental contamination. Trans. R. Soc. Trop. Med. Hyg. 2012;106:191–195. doi: 10.1016/j.trstmh.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Beser J., Toresson L., Eitrem R., Troell K., Winiecka-Krusnell J., Lebbad M. Possible zoonotic transmission of Cryptosporidium felis in a household. Infect. Ecol. Epidemiol. 2015;5 doi: 10.3402/iee.v5.28463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M., Halai K., Jeffreys D., Hunter P.R. The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples. Vet. Parasitol. 2015;207:181–202. doi: 10.1016/j.vetpar.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Broglia A., Weitzel T., Harms G., Cacció S.M., Nöckler K. Molecular typing of Giardia duodenalis isolates from German travellers. Parasitol. Res. 2013;112:3449–3456. doi: 10.1007/s00436-013-3524-y. [DOI] [PubMed] [Google Scholar]
- Bugg R.J., Robertson I.D., Elliot A.D., Thompson R.C. Gastrointestinal parasites of urban dogs in Perth, Western Australia. Vet. J. 1999;157:295–301. doi: 10.1053/tvjl.1998.0327. [DOI] [PubMed] [Google Scholar]
- Cacciò S.M., Ryan U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008;160:75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Cacciò S.M., Beck R., Lalle M., Marinculic A., Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int. J. Parasitol. 2008;38:1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Cai W., Ryan U., Xiao L., Feng Y. Zoonotic giardiasis: An update. Parasitol. Res. 2021;120:4199–4218. doi: 10.1007/s00436-021-07325-2. [DOI] [PubMed] [Google Scholar]
- Cama V.A., Bern C., Sulaiman I.M., Gilman R.H., Ticona E., Vivar A., et al. Cryptosporidium species and genotypes in HIV-positive patients in Lima, Peru. J. Eukaryot. Microbiol. 2003;50(Suppl. l.):531–533. doi: 10.1111/j.1550-7408.2003.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Cama V., Gilman R.H., Vivar A., Ticona E., Ortega Y., Bern C., Xiao L. Mixed Cryptosporidium infections and HIV. Emerg. Infect. Dis. 2006;12:1025–1028. doi: 10.3201/eid1206.060015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama V.A., Ross J.M., Crawford S., Kawai V., Chavez-Valdez R., Vargas D., et al. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J. Infect. Dis. 2007;196:684–691. doi: 10.1086/519842. [DOI] [PubMed] [Google Scholar]
- Cama V.A., Bern C., Roberts J., Cabrera L., Sterling C.R., Ortega Y., et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg. Infect. Dis. 2008;14:1567–1574. doi: 10.3201/eid1410.071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela E., Goizueta C., Periago M.V., Muñoz-Antoli C. Prevalence of intestinal parasites and molecular characterization of Giardia intestinalis, Blastocystis spp. and Entamoeba histolytica in the village of Fortín Mbororé (Puerto Iguazú, Misiones, Argentina) Parasites Vectors. 2021;14:510. doi: 10.1186/s13071-021-04968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Fang C., Deng J., Yu F., Ma D., Chuai L., Wang T., Qi M., Li J. Molecular characterization of Cryptosporidium spp. and Giardia duodenalis in pet dogs in Xinjiang, China. Parasitol. Res. 2022;121:1429–1435. doi: 10.1007/s00436-022-07468-w. [DOI] [PubMed] [Google Scholar]
- CDC Centers for Disease Control and Prevention: False-positive laboratory tests for Cryptosporidium involving an enzyme-linked immunosorbent assay - United States. MMWR Morb. Mortal. Wkly. Rep. 1999;48:4–8. [PubMed] [Google Scholar]
- Certad G., Ngouanesavanh T., Hernan A., Rojas E., Contreras R., Pocaterra L., et al. First molecular data on cryptosporidiosis in Venezuela. J. Eukaryot. Microbiol. 2006;53:S30–S32. doi: 10.1111/j.1550-7408.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- Chalmers R.M., Robinson G., Elwin K., Hadfield S.J., Xiao L., Ryan U., et al. Cryptosporidium sp. rabbit genotype, a newly identified human pathogen. Emerg. Infect. Dis. 2009;15:829–830. doi: 10.3201/eid1505.081419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers R.M., Elwin K., Thomas A.L., Guy E.C., Mason B. Long-term Cryptosporidium typing reveals the aetiology and species-specific epidemiology of human cryptosporidiosis in England and Wales, 2000 to 2003. Euro Surveill. 2009;14 doi: 10.2807/ese.14.02.19086-en. [DOI] [PubMed] [Google Scholar]
- Charmaraman L., Cobas S., Weed J., Gu Q., Kiel E., Chin H., et al. From regulating emotions to less lonely screen time: Parents’ qualitative perspectives of the benefits and challenges of adolescent pet companionship. Behav. Sci. 2022;12:143. doi: 10.3390/bs12050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieloszyk J., Goñi P., García A., Remacha M.A., Sánchez E., Clavel A. Two cases of zoonotic cryptosporidiosis in Spain by the unusual species Cryptosporidium ubiquitum and Cryptosporidium felis. Enferm. Infecc. Microbiol. Clín. 2012;30:549–551. doi: 10.1016/j.eimc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Cirak V.Y., Bauer C. Comparison of conventional coproscopical methods and commercial coproantigen ELISA kits for the detection of Giardia and Cryptosporidium infections in dogs and cats. Berl. Münchener Tierärztliche Wochenschr. 2004;117:410–413. [PubMed] [Google Scholar]
- Ciuca L., Pepe P., Bosco A., Caccio S.M., Maurelli M.P., Sannella A.R., et al. Effectiveness of fenbendazole and metronidazole against Giardia infection in dogs monitored for 50-days in home-conditions. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.626424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claerebout E., Casaert S., Dalemans A.C., De Wilde N., Levecke B., Vercruysse J., et al. Giardia and other intestinal parasites in different dog populations in Northern Belgium. Vet. Parasitol. 2009;161:41–46. doi: 10.1016/j.vetpar.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Colli C.M., Bezagio R.C., Nishi L., Bignotto T.S., Ferreira É.C., Falavigna-Guilherme A.L., et al. Identical assemblage of Giardia duodenalis in humans, animals and vegetables in an urban area in southern Brazil indicates a relationship among them. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M.A., Sterling C.R., Gilman R.H., Cama V., Ortega Y., Adam R.D. Molecular analysis of household transmission of Giardia lamblia in a region of high endemicity in Peru. J. Infect. Dis. 2010;202:1713–1721. doi: 10.1086/657142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D., Razakandrainibe R., Sautour M., Valot S., Basmaciyan L., Gargala G., et al. Human cryptosporidiosis in immunodeficient patients in France (2015–2017) Exp. Parasitol. 2018;192:108–112. doi: 10.1016/j.exppara.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Costa D., Razakandrainibe R., Valot S., Vannier M., Sautour M., Basmaciyan L., et al. Epidemiology of cryptosporidiosis in France from 2017 to 2019. Microorganisms. 2020;8:1358. doi: 10.3390/microorganisms8091358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacin C., Aucoin D.P., Elliot A., Thompson R.C.A. Genotypic characterisation of Giardia from domestic dogs in the USA. Vet. Parasitol. 2011;177:28–32. doi: 10.1016/j.vetpar.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Cox P., Griffith M., Angles M., Deere D., Ferguson C. Concentrations of pathogens and indicators in animal feces in the Sydney watershed. Appl. Environ. Microbiol. 2005;71:5929–5934. doi: 10.1128/AEM.71.10.5929-5934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacal E., Saugar J.M., de Lucio A., Hernandez-de-Mingo M., Robinson E., Koster P.C., et al. Prevalence and molecular characterization of Strongyloides stercoralis, Giardia duodenalis, Cryptosporidium spp., and Blastocystis spp. isolates in school children in Cubal, Western Angola. Parasites Vectors. 2018;11:67. doi: 10.1186/s13071-018-2640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danciger M., Lopez M. Numbers of Giardia in the feces of infected children. Am. J. Trop. Med. Hyg. 1975;24:237–242. doi: 10.4269/ajtmh.1975.24.237. [DOI] [PubMed] [Google Scholar]
- Davies R.H., Lawes J.R., Wales A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019;60:329–339. doi: 10.1111/jsap.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucio A., Amor-Aramendía A., Bailo B., Saugar J.M., Anegagrie M., Arroyo A., et al. Prevalence and genetic diversity of Giardia duodenalis and Cryptosporidium spp. among school children in a rural area of the Amhara region, north-west Ethiopia. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucio A., Bailo B., Aguilera M., Cardona G.A., Fernández-Crespo J.C., Carmena D. No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Álava, northern Spain. Acta Trop. 2017;170:48–56. doi: 10.1016/j.actatropica.2017.02.024. [DOI] [PubMed] [Google Scholar]
- de Oliveira A.G.L., Sudré A.P., Bergamo do Bomfim T.C., Santos H.L.C. PLoS One, 16. 2021. Molecular characterization of Cryptosporidium spp. in dogs and cats in the city of Rio de Janeiro, Brazil, reveals potentially zoonotic species and genotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doing K.M., Hamm J.L., Jellison J.A., Marquis J.A., Kingsbury C. False-positive results obtained with the Alexon ProSpecT Cryptosporidium enzyme immunoassay. J. Clin. Microbiol. 1999;37:1582–1583. doi: 10.1128/jcm.37.5.1582-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durigan M., Abreu A.G., Zucchi M.I., Franco R.M., de Souza A.P. Genetic diversity of Giardia duodenalis: multilocus genotyping reveals zoonotic potential between clinical and environmental sources in a metropolitan region of Brazil. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner J., Koehler A.V., Robertson G., Bradbury R.S., Jex A.R., Haydon S.R., et al. Genetic analysis of Giardia and Cryptosporidium from people in Northern Australia using PCR-based tools. Infect. Genet. Evol. 2015;36:389–395. doi: 10.1016/j.meegid.2015.08.034. [DOI] [PubMed] [Google Scholar]
- El-Madawy R.S., Khalifa N.O., Khater H.F. Detection of cryptosporidial infection among Egyptian stray dogs by using Cryptosporidium parvum outer wall protein gene. Bulg. J. Vet. Med. 2010;13:104–110. [Google Scholar]
- Eligio-Garcia L., Cortes-Campos A., Jimenez-Cardoso E. Genotype of Giardia intestinalis isolates from children and dogs and its relationship to host origin. Parasitol. Res. 2005;97:1–6. doi: 10.1007/s00436-005-1368-9. [DOI] [PubMed] [Google Scholar]
- Elmahallawy E.K., Gareh A., Abu-Okail A., Köster P.C., Dashti A., Asseri J., et al. Molecular characteristics and zoonotic potential of enteric protists in domestic dogs and cats in Egypt. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1229151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwin K., Hadfield S.J., Robinson G., Chalmers R.M. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000–2008. Epidemiol. Infect. 2012;140:673–683. doi: 10.1017/S0950268811000860. [DOI] [PubMed] [Google Scholar]
- Enemark H.L., Starostka T.P., Larsen B., Takeuchi-Storm N., Thamsborg S.M. Giardia and Cryptosporidium infections in Danish cats: Risk factors and zoonotic potential. Parasitol. Res. 2020;119:2275–2286. doi: 10.1007/s00436-020-06715-2. [DOI] [PubMed] [Google Scholar]
- ESCCAP . ESCCAP Guideline 06, 2nd edition. European Specialist Counsel Companion Animal Parasites. 2018. Control of intestinal protozoa in dogs and cats.https://www.esccap.org/guidelines/gl6 [Google Scholar]
- Fantinatti M., Caseca A.C., Bello A.R., Fernandes O., Da-Cruz A.M. The presence of Giardia lamblia assemblage A in dogs suggests an anthropozoonotic cycle of the parasite in Rio de Janeiro, Brazil. Infect. Genet. Evol. 2018;65:265–269. doi: 10.1016/j.meegid.2018.07.025. [DOI] [PubMed] [Google Scholar]
- Fava N.M., Soares R.M., Scalia L.A., Cunha M.J., Faria E.S., Cury M.C. Molecular typing of canine Giardia duodenalis isolates from Minas Gerais, Brazil. Exp. Parasitol. 2016;161:1–5. doi: 10.1016/j.exppara.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Fayer R., Santín M., Trout J.M., Dubey J.P. Detection of Cryptosporidium felis and Giardia duodenalis Assemblage F in a cat colony. Vet. Parasitol. 2006;140:44–53. doi: 10.1016/j.vetpar.2006.03.005. [DOI] [PubMed] [Google Scholar]
- FEDIAF . 2023. European Pet Food Industry Federation: Annual Report 2023.https://europeanpetfood.org/wp-content/uploads/2023/06/FEDIAF_Annual-Report_2023_Facts-Figures.pdf [Google Scholar]
- Feng Y., Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Xiao L. Molecular epidemiology of cryptosporidiosis in China. Front. Microbiol. 2017;8:1701. doi: 10.3389/fmicb.2017.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Wang L., Duan L., Gomez-Puerta L.A., Zhang L., Zhao X., et al. Extended outbreak of cryptosporidiosis in a pediatric hospital, China. Emerg. Infect. Dis. 2012;18:312–314. doi: 10.3201/eid1802.110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F.S., Pereira-Baltasar P., Parreira R., Padre L., Vilhena M., Távora Tavira L., et al. Intestinal parasites in dogs and cats from the district of Évora, Portugal. Vet. Parasitol. 2011;179:242–245. doi: 10.1016/j.vetpar.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Alho A.M., Otero D., Gomes L., Nijsse R., Overgaauw P.A.M., et al. Urban dog parks as sources of canine parasites: contamination rates and pet owner behaviours in Lisbon, Portugal. J. Environ. Public Health. 2017;2017 doi: 10.1155/2017/5984086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald L., Bennett M., Ng J., Nicholls P., James F., Elliot A., et al. Morphological and molecular characterisation of a mixed Cryptosporidium muris/Cryptosporidium felis infection in a cat. Vet. Parasitol. 2011;17:160–164. doi: 10.1016/j.vetpar.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Franzén O., Jerlström-Hultqvist J., Castro E., Sherwood E., Ankarklev J., Reiner D.S., et al. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: Is human giardiasis caused by two different species? PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S.K., Kotwa J.D., Singh B., Greer T., Pearl D.L., Elsemore D.A., et al. Factors associated with Giardia infection in dogs in southern Ontario, Canada. Vet. Parasitol. Reg. Stud. Reports. 2023;41 doi: 10.1016/j.vprsr.2023.100870. [DOI] [PubMed] [Google Scholar]
- Gatei W., Suputtamongkol Y., Waywa D., Ashford R.W., Bailey J.W., Greensill J., et al. Zoonotic species of Cryptosporidium are as prevalent as the anthroponotic in HIV-infected patients in Thailand. Ann. Trop. Med. Parasitol. 2002;96:797–802. doi: 10.1179/000349802125002202. [DOI] [PubMed] [Google Scholar]
- Gatei W., Kamwati S.K., Mbae C., Waruru A., Hart C.A., Wamae C.N., et al. Cryptosporidiosis: Prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am. J. Trop. Med. Hyg. 2006;75:78–82. [PubMed] [Google Scholar]
- Gatei W., Barrett D., Lindo J.F., Eldemire-Shearer D., Cama V., Xiao L. Unique Cryptosporidium population in HIV-infected persons, Jamaica. Emerg. Infect. Dis. 2008;14:841–843. doi: 10.3201/eid1405.071277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelanew T., Lalle M., Hailu A., Pozio E., Caccio S.M. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gharieb R.M.A., Merwad A.M.A., Saleh A.A., Abd El-Ghany A.M. Molecular screening and genotyping of Cryptosporidium species in household dogs and in contact children in Egypt: Risk factor analysis and zoonotic importance. Vector Borne Zoonotic Dis. 2018;18:424–432. doi: 10.1089/vbz.2017.2254. [DOI] [PubMed] [Google Scholar]
- Giangaspero A., Iorio R., Paoletti B., Traversa D., Capelli G. Molecular evidence for Cryptosporidium infection in dogs in Central Italy. Parasitol. Res. 2006;99:297–299. doi: 10.1007/s00436-006-0169-0. [DOI] [PubMed] [Google Scholar]
- Gil H., Cano L., de Lucio A., Bailo B., de Mingo M.H., Cardona G.A., et al. Detection and molecular diversity of Giardia duodenalis and Cryptosporidium spp. in sheltered dogs and cats in northern Spain. Infect. Genet. Evol. 2017;50:62–69. doi: 10.1016/j.meegid.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Goodgame R.W., Genta R.M., White A.C., Chappell C.L. Intensity of infection in AIDS-associated cryptosporidiosis. J. Infect. Dis. 1993;167:704–709. doi: 10.1093/infdis/167.3.704. [DOI] [PubMed] [Google Scholar]
- Gu Y.F., Wang K., Liu D.Y., Mei N., Chen C., Chen T., et al. Molecular detection of Giardia lamblia and Cryptosporidium species in pet dogs. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2015;33:362–367. [PubMed] [Google Scholar]
- Guadano Procesi I., Carnio A., Berrilli F., Montalbano, Di Filippo M., Scarito A., et al. Giardia duodenalis in colony stray cats from Italy. Zoonoses Public Health. 2022;69:46–54. doi: 10.1111/zph.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultekin M., Ural K., Aysul N., Ayan A., Balikci C., Akyildiz G. Prevalence and molecular characterization of Giardia duodenalis in dogs in Aydin, Turkey. Int. J. Environ. Health Res. 2017;27:161–168. doi: 10.1080/09603123.2017.1310187. [DOI] [PubMed] [Google Scholar]
- Guo Y., Li N., Ryan U., Feng Y., Xiao L. Small ruminants and zoonotic cryptosporidiosis. Parasitol. Res. 2021;120:4189–4198. doi: 10.1007/s00436-021-07116-9. [DOI] [PubMed] [Google Scholar]
- Guo Y., Ryan U., Feng Y., Xiao L. Emergence of zoonotic Cryptosporidium parvum in China. Trends Parasitol. 2022;38:335–343. doi: 10.1016/j.pt.2021.12.002. [DOI] [PubMed] [Google Scholar]
- Guy R.A., Yanta C.A., Muchaal P.K., Rankin M.A., Thivierge K., Lau R., et al. Molecular characterization of Cryptosporidium isolates from humans in Ontario, Canada. Parasites Vectors. 2021;14:69. doi: 10.1186/s13071-020-04546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot K., Follet-Dumoulin A., Lelievre E., Sarfati C., Rabodonirina M., Nevez G., et al. Molecular characterization of Cryptosporidium isolates obtained from humans in France. J. Clin. Microbiol. 2001;39:3472–3480. doi: 10.1128/JCM.39.10.3472-3480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdušek O., Ditrich O., Šlapeta J. Molecular identification of Cryptosporidium spp. in animal and human hosts from the Czech Republic. Vet. Parasitol. 2004;122:183–192. doi: 10.1016/j.vetpar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Hamnes I.S., Gjerde B.K., Robertson L.J. A longitudinal study on the occurrence of Cryptosporidium and Giardia in dogs during their first year of life. Acta Vet. Scand. 2007;49:22. doi: 10.1186/1751-0147-49-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey T.V., Carvalho J.P.D.S., Aquino M.C.C., Oliveira B.C.M., Barros L.D., Fehlberg H.F., et al. Giardiasis in children and dogs, and the first report of assemblage E in dogs from northeastern Brazil. Rev. Bras. Parasitol. Vet. 2023;32 doi: 10.1590/S1984-29612023010. [DOI] [PubMed] [Google Scholar]
- Hascall K.L., Kass P.H., Saksen J., Ahlmann A., Scorza A.V., Lappin M.R., Marks S.L. Prevalence of enteropathogens in dogs attending 3 regional dog parks in Northern California. J. Vet. Intern. Med. 2016;30:1838–1845. doi: 10.1111/jvim.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P.C., Morales de la Pava L., Chaparro-Olaya J., Lopez-Osorio S., Lopez-Arias A., Chaparro-Gutierrez J.J. Multilocus genotyping of Giardia intestinalis in pet dogs of Medellin, Colombia. Vet. Parasitol. Reg. Stud. Reports. 2021;23 doi: 10.1016/j.vprsr.2020.100520. [DOI] [PubMed] [Google Scholar]
- Higuera A., Villamizar X., Herrera G., Giraldo J.C., Vasquez-A L.R., Urbano P., et al. Molecular detection and genotyping of intestinal protozoa from different biogeographical regions of Colombia. PeerJ. 2020;8 doi: 10.7717/peerj.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijjawi N., Ng J., Yang R., Atoum M.F., Ryan U. Identification of rare and novel Cryptosporidium gp60 subtypes in human isolates from Jordan. Exp. Parasitol. 2010;125:161–164. doi: 10.1016/j.exppara.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Himsworth C.G., Skinner S., Chaban B., Jenkins E., Wagner B.A., Harms N.J., et al. Multiple zoonotic pathogens identified in canine feces collected from a remote Canadian indigenous community. Am. J. Trop. Med. Hyg. 2010;83:338–341. doi: 10.4269/ajtmh.2010.10-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney B., Ederer C., Stengl C., Wilding K., Štrkolcová G., Harl J., et al. Enteric protozoa of cats and their zoonotic potential - a field study from Austria. Parasitol. Res. 2015;114:2003–2006. doi: 10.1007/s00436-015-4408-0. [DOI] [PubMed] [Google Scholar]
- Holzhausen I., Lendner M., Göhring F., Steinhöfel I., Daugschies A. Distribution of Cryptosporidium parvum gp60 subtypes in calf herds of Saxony, Germany. Parasitol. Res. 2019;118:1549–1558. doi: 10.1007/s00436-019-06266-1. [DOI] [PubMed] [Google Scholar]
- Homayouni M.M., Razavi S.M., Shaddel M., Asadpour M. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia intestinalis in household dogs and cats from Shiraz, southwestern Iran. Vet. Ital. 2019;55:311–318. doi: 10.12834/VetIt.1710.9049.3. [DOI] [PubMed] [Google Scholar]
- Hoopes J., Hill J.E., Polley L., Fernando C., Wagner B., Schurer J., et al. Enteric parasites of free-roaming, owned, and rural cats in prairie regions of Canada. Can. Vet. J. 2015;56:495–501. [PMC free article] [PubMed] [Google Scholar]
- Hopkins R.M., Meloni B.P., Groth D.M., Wetherall J.D., Reynoldson J.A., Thompson R.C.A. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J. Parasitol. 1997;83:44–51. [PubMed] [Google Scholar]
- Inpankaew T., Traub R., Thompson R.C., Sukthana Y. Canine parasitic zoonoses in Bangkok temples. Southeast Asian J. Trop. Med. Publ. Health. 2007;38:247–255. [PubMed] [Google Scholar]
- Inpankaew T., Schär F., Odermatt P., Dalsgaard A., Chimnoi W., Khieu V., et al. Low risk for transmission of zoonotic Giardia duodenalis from dogs to humans in rural Cambodia. Parasites Vectors. 2014;7:412. doi: 10.1186/1756-3305-7-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insulander M., Silverlas C., Lebbad M., Karlsson L., Mattsson J.G., Svenungsson B. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol. Infect. 2013;141:1009–1020. doi: 10.1017/S0950268812001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki M., Maekawa T., Moriya K., Uni S., Takada S. Infectivity of Cryptosporidium muris (strain RN 66) in various laboratory animals. Parasitol. Res. 1989;75:218–222. doi: 10.1007/BF00931279. [DOI] [PubMed] [Google Scholar]
- Itagaki T., Kinoshita S., Aoki M., Itoh N., Saeki H., Sato N., et al. Genotyping of Giardia intestinalis from domestic and wild animals in Japan using glutamate dehydrogenase gene sequencing. Vet. Parasitol. 2005;133:283–287. doi: 10.1016/j.vetpar.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Ito Y., Itoh N., Iijima Y., Kimura Y. Molecular prevalence of Cryptosporidium species among household cats and pet shop kittens in Japan. JFMS Open Rep. 2017;3 doi: 10.1177/2055116917730719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Itagaki T., Kawabata T., Konaka T., Muraoka N., Saeki H., et al. Prevalence of intestinal parasites and genotyping of Giardia intestinalis in pet shop puppies in east Japan. Vet. Parasitol. 2011;176:74–78. doi: 10.1016/j.vetpar.2010.10.048. [DOI] [PubMed] [Google Scholar]
- Itoh N., Tanaka H., Iijima Y., Kameshima S., Kimura Y. Molecular prevalence of Cryptosporidium spp. in breeding kennel dogs. Kor. J. Parasitol. 2019;57:197–200. doi: 10.3347/kjp.2019.57.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita H., Takemura T., Tokizawa A., Sugamoto T., Thiem V.D., Nguyen T.H., et al. Molecular epidemiology of Cryptosporidium spp. in an agricultural area of northern Vietnam: A community survey. Parasitol. Int. 2021;83 doi: 10.1016/j.parint.2021.102341. [DOI] [PubMed] [Google Scholar]
- Jeske S.T., Macedo M.R.P., Bianchi T., Leon Í.F., Pinheiro N.B., Borsuk S., Villela M. Molecular characterization of Giardia lamblia and risk factors for giardiasis among immunocompromised patients in southern Brazil. Braz. J. Biol. 2022;82 doi: 10.1590/1519-6984.265055. [DOI] [PubMed] [Google Scholar]
- Jian F., Qi M., He X., Wang R., Zhang S., Dong H., et al. Occurrence and molecular characterization of Cryptosporidium in dogs in Henan province, China. BMC Vet. Res. 2014;10:26. doi: 10.1186/1746-6148-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Roellig D.M., Lebbad M., Beser J., Troell K., Guo Y., et al. Subtype distribution of zoonotic pathogen Cryptosporidium felis in humans and animals in several countries. Emerg. Microb. Infect. 2020;9:2446–2454. doi: 10.1080/22221751.2020.1840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Roellig D.M., Guo Y., Li N., Feng Y., Xiao L. Development of a subtyping tool for zoonotic pathogen Cryptosporidium canis. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.02474-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Liu L., Yuan Z., Liu A., Cao J., Shen Y. Molecular identification and genetic characteristics of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in human immunodeficiency virus/acquired immunodeficiency syndrome patients in Shanghai, China. Parasites Vectors. 2023;16:53. doi: 10.1186/s13071-023-05666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Osman M., Green B.A., Yang Y., Ahuja A., Lu Z., et al. Evidence for the transmission of antimicrobial resistant bacteria between humans and companion animals: A scoping review. One Health. 2023;17 doi: 10.1016/j.onehlt.2023.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim A., Auersperg V., Drüe J., Wiedermann S., Hinney B., Spergser J. Parasites and zoonotic bacteria in the feces of cats and dogs from animal shelters in Carinthia, Austria. Res. Vet. Sci. 2023;164 doi: 10.1016/j.rvsc.2023.105022. [DOI] [PubMed] [Google Scholar]
- Johansen K.M., Castro N.S., Lancaster K.E., Madrid E., Havas A., Simms J., et al. Characterization of Giardia lamblia genotypes in dogs from Tucson, Arizona using SSU-rRNA and ß-giardin sequences. Parasitol. Res. 2014;113:387–390. doi: 10.1007/s00436-013-3666-y. [DOI] [PubMed] [Google Scholar]
- Julien D.A., Sargeant J.M., Guy R.A., Shapiro K., Imai R.K., Bunce A., et al. Prevalence and genetic characterization of Giardia spp. and Cryptosporidium spp. in dogs in Iqaluit, Nunavut, Canada. Zoonoses Public Health. 2019;66:813–825. doi: 10.1111/zph.12628. [DOI] [PubMed] [Google Scholar]
- Karimi P., Shafaghi-Sisi S., Meamar A.R., Razmjou E. Molecular identification of Cryptosporidium, Giardia, and Blastocystis from stray and household cats and cat owners in Tehran, Iran. Sci. Rep. 2023;13:1554. doi: 10.1038/s41598-023-28768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanik M.T., Schneider S.K., Rosenblatt J.E., Hall G.S., Procop G.W. Evaluation of ColorPAC Giardia/Cryptosporidium rapid assay and ProSpecT Giardia/Cryptosporidium microplate assay for detection of Giardia and Cryptosporidium in fecal specimens. J. Clin. Microbiol. 2001;39:4523–4525. doi: 10.1128/JCM.39.12.4523-4525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H., Zenner L., Benabed S., Poirel M.T., Bourgoin G. Lack of efficacy of fenbendazole against Giardia duodenalis in a naturally infected population of dogs in France. Parasite. 2022;29:49. doi: 10.1051/parasite/2022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa M.M., Fouad E.A., Kamel N.O., Auda H.M., El-Bahy M.M., Ramadan R.M. Dogs as a source for the spreading of enteric parasites including zoonotic ones in Giza Province, Egypt. Res. Vet. Sci. 2023;161:122–131. doi: 10.1016/j.rvsc.2023.06.015. [DOI] [PubMed] [Google Scholar]
- Khalil S., Mirdha B.R., Panda A., Singh Y., Makharia G., Paul J. Cryptosporidium species subtypes and associated clinical manifestations in Indian patients. Gastroenterol. Hepatol. Bed. Bench. 2017;10:311–318. [PMC free article] [PubMed] [Google Scholar]
- Khine N.O., Chimnoi W., Kamyingkird K., Kengradomkij C., Saetiew N., Simking P., et al. Molecular detection of Giardia duodenalis and Cryptosporidium spp. from stray dogs residing in monasteries in Bangkok, Thailand. Parasitol. Int. 2021;83 doi: 10.1016/j.parint.2021.102337. [DOI] [PubMed] [Google Scholar]
- Kim H.Y., Lee H., Lee S.H., Seo M.G., Yi S., Kim J.W., et al. Multilocus genotyping and risk factor analysis of Giardia duodenalis in dogs in Korea. Acta Trop. 2019;199 doi: 10.1016/j.actatropica.2019.105113. [DOI] [PubMed] [Google Scholar]
- Klotz C., Sannella A.R., Weisz F., Chaudhry U., Sroka J., Tůmová P., et al. Extensive testing of a multi-locus sequence typing scheme for Giardia duodenalis assemblage A confirms its good discriminatory power. Parasites Vectors. 2022;15:489. doi: 10.1186/s13071-022-05615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz C., Schmid M.W., Winter K., Ignatius R., Weisz F., Saghaug C.S., et al. Highly contiguous genomes of human clinical isolates of Giardia duodenalis reveal assemblage- and sub-assemblage-specific presence-absence variation in protein-coding genes. Microb. Genom. 2023;9 doi: 10.1099/mgen.0.000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koompapong K., Mori H., Thammasonthijarern N., Prasertbun R., Pintong A.R., Popruk S., et al. Molecular identification of Cryptosporidium spp. in seagulls, pigeons, dogs, and cats in Thailand. Parasite. 2014;21:52. doi: 10.1051/parasite/2014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman F.N.J., Wagenaar J.A., Zomer A. Whole-genome sequencing of dog-specific assemblages C and D of Giardia duodenalis from single and pooled cysts indicates host-associated genes. Microb. Genom. 2019;5 doi: 10.1099/mgen.0.000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köseoğlu A.E., Can H., Karakavuk M., Güvendi M., Değirmenci Döşkaya A., Manyatsi P.B., et al. Molecular prevalence and subtyping of Cryptosporidium spp. in fecal samples collected from stray cats in İzmir, Turkey. BMC Vet. Res. 2022;18:89. doi: 10.1186/s12917-022-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulou D., Claerebout E., Arvanitis D., Ligda P., Voutzourakis N., Casaert S., et al. Abundance, zoonotic potential and risk factors of intestinal parasitism amongst dog and cat populations: The scenario of Crete, Greece. Parasites Vectors. 2017;10:43. doi: 10.1186/s13071-017-1989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu H., Ogasawara S., Kikusui T., Nagasawa M. Ownership of dogs and cats leads to higher levels of well-being and general trust through family involvement in late adolescence. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumrie S., Capewell P., McDonald M., Dunbar D., Panarese R., Katzer F., et al. Molecular characterisation of Giardia duodenalis from human and companion animal sources in the United Kingdom using an improved triosephosphate isomerase molecular marker. Curr. Res. Parasitol. Vector Borne Dis. 2022;2 doi: 10.1016/j.crpvbd.2022.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuthyar S., Kowalewski M.M., Seabolt M., Roellig D.M., Gillespie T.R. Molecular characterization of Giardia duodenalis and evidence for cross-species transmission in Northern Argentina. Transbound. Emerg. Dis. 2021;69:2209–2218. doi: 10.1111/tbed.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kváč M., Hofmannová L., Ortega Y., Holubová N., Horčičková M., Kicia M., et al. Stray cats are more frequently infected with zoonotic protists than pet cats. Folia Parasitol. 2017;64:2017.034. doi: 10.14411/fp.2017.034. [DOI] [PubMed] [Google Scholar]
- Kwak D., Seo M.G. Genetic analysis of zoonotic gastrointestinal protozoa and microsporidia in shelter cats in South Korea. Pathogens. 2020;9:894. doi: 10.3390/pathogens9110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labana R.V., Dungca J.Z., Nissapatorn V. Community-based surveillance of Cryptosporidium in the indigenous community of Boliwong, Philippines, from April to December 2017. Epidemiol. Health. 2018;40 doi: 10.4178/epih.e2018047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M., Pozio E., Capelli G., Bruschi F., Crotti D., Cacciò S.M. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 2005;35:207–213. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Lalle M., Jimenez-Cardosa E., Caccio S.M., Pozio E. Genotyping of Giardia duodenalis from humans and dogs from Mexico using a beta-giardin nested polymerase chain reaction assay. J. Parasitol. 2005;91:203–205. doi: 10.1645/GE-293R. [DOI] [PubMed] [Google Scholar]
- Learmonth J.J., Ionas G., Ebbett K.A., Kwan E.S. Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Appl. Environ. Microbiol. 2004;70:3973–3978. doi: 10.1128/AEM.70.7.3973-3978.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbad M., Ankarklev J., Tellez A., Leiva B., Andersson J.O., Svärd S. Dominance of Giardia assemblage B in Leon, Nicaragua. Acta Trop. 2008;106:44–53. doi: 10.1016/j.actatropica.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lebbad M., Mattsson J.G., Christensson B., Ljungström B., Backhans A., Andersson J.O., et al. From mouse to moose: Multilocus genotyping of Giardia isolates from various animal species. Vet. Parasitol. 2010;168:231–239. doi: 10.1016/j.vetpar.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lebbad M., Winiecka-Krusnell J., Stensvold C.R., Beser J. High diversity of Cryptosporidium species and subtypes identified in cryptosporidiosis acquired in Sweden and abroad. Pathogens. 2021;10:523. doi: 10.3390/pathogens10050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecová L., Hammerbauerová I., Tůmová P., Nohýnková E. Companion animals as a potential source of Giardia intestinalis infection in humans in the Czech Republic - a pilot study. Vet. Parasitol. Reg. Stud. Reports. 2020;21 doi: 10.1016/j.vprsr.2020.100431. [DOI] [PubMed] [Google Scholar]
- Lee M.F., Cadogan P., Eytle S., Copeland S., Walochnik J., Lindo J.F. Molecular epidemiology and multilocus sequence analysis of potentially zoonotic Giardia spp. from humans and dogs in Jamaica. Parasitol. Res. 2017;116:409–414. doi: 10.1007/s00436-016-5304-y. [DOI] [PubMed] [Google Scholar]
- Leonhard S., Pfister K., Beelitz P., Wielinga C., Thompson R.C.A. The molecular characterisation of Giardia from dogs in southern Germany. Vet. Parasitol. 2007;150:33–38. doi: 10.1016/j.vetpar.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Leoni F., Amar C., Nichols G., Pedraza Díaz S., McLauchlin J. Genetic analysis of Cryptosporidium from 2414 humans with diarrhoea in England between 1985 and 2000. J. Med. Microbiol. 2006;55:703–707. doi: 10.1099/jmm.0.46251-0. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang P., Wang P., Alsarakibi M., Zhu H., Liu Y., et al. Genotype identification and prevalence of Giardia duodenalis in pet dogs of Guangzhou, southern China. Vet. Parasitol. 2012;188:368–371. doi: 10.1016/j.vetpar.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Li W., Liu C., Yu Y., Li J., Gong P., Song M., et al. Molecular characterization of Giardia duodenalis isolates from police and farm dogs in China. Exp. Parasitol. 2013;135:223–226. doi: 10.1016/j.exppara.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Li W., Li Y., Song M., Lu Y., Yang J., Tao W., et al. Prevalence and genetic characteristics of Cryptosporidium, Enterocytozoon bieneusi and Giardia duodenalis in cats and dogs in Heilongjiang Province, China. Vet. Parasitol. 2015;208:125–134. doi: 10.1016/j.vetpar.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Li J., Dan X., Zhu K., Li N., Guo Y., Zheng Z., et al. Genetic characterization of Cryptosporidium spp. and Giardia duodenalis in dogs and cats in Guangdong, China. Parasites Vectors. 2019;12:571. doi: 10.1186/s13071-019-3822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Liu X., Gu Y., Liu J., Luo J. Prevalence of Cryptosporidium, Giardia, Blastocystis, and trichomonads in domestic cats in east China. J. Vet. Med. Sci. 2019;81:890–896. doi: 10.1292/jvms.19-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ryan U., Guo Y., Feng Y., Xiao L. Advances in molecular epidemiology of cryptosporidiosis in dogs and cats. Int. J. Parasitol. 2021;51:787–795. doi: 10.1016/j.ijpara.2021.03.002. [DOI] [PubMed] [Google Scholar]
- Li J., Yang F., Liang R., Guo S., Guo Y., Li N., Feng Y., et al. Subtype Characterization and zoonotic potential of Cryptosporidium felis in cats in Guangdong and Shanghai, China. Pathogens. 2021;10:89. doi: 10.3390/pathogens10020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Tsaihong J.C., Cheng Y., Peng S. Occurrence and genotype of Giardia cysts isolated from faecal samples of children and dogs and from drinking water samples in an aboriginal area of central Taiwan. Exp. Parasitol. 2012;131:204–209. doi: 10.1016/j.exppara.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Liao S., Lin X., Sun Y., Qi N., Lv M., Wu C., et al. Occurrence and genotypes of Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. in household, shelter, breeding, and pet market dogs in Guangzhou, southern China. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-74299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberato C., Berrilli F., Odorizi L., Scarcella R., Barni M., Amoruso C., et al. Parasites in stray dogs from Italy: Prevalence, risk factors and management concerns. Acta Parasitol. 2018;63:27–32. doi: 10.1515/ap-2018-0003. [DOI] [PubMed] [Google Scholar]
- Lim Y.A., Iqbal A., Surin J., Sim B.L., Jex A.R., Nolan M.J., et al. First genetic classification of Cryptosporidium and Giardia from HIV/AIDS patients in Malaysia. Infect. Genet. Evol. 2011;11:968–974. doi: 10.1016/j.meegid.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Liu H., Shen Y., Yin J., Yuan Z., Jiang Y., Xu Y., et al. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrhoeal outpatients in China. BMC Infect. Dis. 2014;14:25. doi: 10.1186/1471-2334-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Shen Y., Liu A., Yin J., Yuan Z., Jiang Y., et al. Occurrence and multilocus genotyping of Giardia duodenalis in pets and zoo animals in Shanghai, China. J. Infect. Dev. Ctries. 2017;11:479–486. doi: 10.3855/jidc.8421. [DOI] [PubMed] [Google Scholar]
- Liu A., Gong B., Liu X., Shen Y., Wu Y., Zhang W., et al. A retrospective epidemiological analysis of human Cryptosporidium infection in China during the past three decades (1987–2018) PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeck B.K., Pedati C., Iwen P.C., McCutchen E., Roellig D.M., Hlavsa M.C., et al. Genotyping and subtyping Cryptosporidium to identify risk factors and transmission patterns - Nebraska, 2015–2017. MMWR Morb. Mortal. Wkly. Rep. 2020;69:335–338. doi: 10.15585/mmwr.mm6912a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arias Á., Villar D., López-Osorio S., Calle-Vélez D., Chaparro-Gutiérrez J.J. Giardia is the most prevalent parasitic infection in dogs and cats with diarrhea in the city of Medellín, Colombia. Vet. Parasitol. Reg. Stud. Reports. 2019;18 doi: 10.1016/j.vprsr.2019.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucca P., De Gaspari E.N., Bozzoli L.M., Funada M.R., Silva S.O., Iuliano W., Soares R.M. Molecular characterization of Cryptosporidium spp. from HIV-infected patients from an urban area of Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:341–343. doi: 10.1590/s0036-46652009000600006. [DOI] [PubMed] [Google Scholar]
- Lucio-Forster A., Griffiths J.K., Cama V.A., Xiao L., Bowman D.D. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010;26:174–179. doi: 10.1016/j.pt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Lupo P.J., Langer-Curry R.C., Robinson M., Okhuysen P.C., Chappell C.L. Cryptosporidium muris in a Texas canine population. Am. J. Trop. Med. Hyg. 2008;78:917–921. [PubMed] [Google Scholar]
- Mancianti F., Nardoni S., Mugnaini L., Zambernardi L., Guerrini A., Gazzola V., et al. A retrospective molecular study of select intestinal protozoa in healthy pet cats from Italy. J. Feline Med. Surg. 2015;17:163–167. doi: 10.1177/1098612X14533549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangi M., Berrilli F., Otranto D., Giangaspero A. Genotyping of Giardia duodenalis among children and dogs in a closed socially deprived community from Italy. Zoonoses Public Health. 2010;57:e54–e58. doi: 10.1111/j.1863-2378.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- Mark-Carew M.P., Adesiyun A.A., Basu A., Georges K.A., Pierre T., Tilitz S., et al. Characterization of Giardia duodenalis infections in dogs in Trinidad and Tobago. Vet. Parasitol. 2013;196:199–202. doi: 10.1016/j.vetpar.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Martins C.F., Soares J.P., Cortinhas A., Silva L., Cardoso L., Pires M.A., Mota M.P. Petʼs influence on humansʼ daily physical activity and mental health: A meta-analysis. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.1196199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo M., Montoya A., Bailo B., Köster P.C., Dashti A., Hernández-Castro C., et al. Prevalence and public health relevance of enteric parasites in domestic dogs and cats in the region of Madrid (Spain) with an emphasis on Giardia duodenalis and Cryptosporidium sp. Vet. Med. Sci. 2023;9:2542–2558. doi: 10.1002/vms3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos O., Alves M., Xiao L., Cama V., Antunes F. Cryptosporidium felis and C. meleagridis in persons with HIV, Portugal. Emerg. Infect. Dis. 2004;10:2256–2257. doi: 10.3201/eid1012.031068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall R.M., Peregrine A.S., Leonard E.K., Lacombe C., Lake M., Rebelo A.R., Cai H.Y. Evaluation of the zoonotic potential of Giardia duodenalis in fecal samples from dogs and cats in Ontario. Can. Vet. J. 2011;52:1329–1333. [PMC free article] [PubMed] [Google Scholar]
- Meng X.Z., Li M.Y., Lyu C., Qin Y.F., Zhao Z.Y., Yang X.B., et al. The global prevalence and risk factors of Cryptosporidium infection among cats during 1988–2021: A systematic review and meta-analysis. Microb. Pathog. 2021;158 doi: 10.1016/j.micpath.2021.105096. [DOI] [PubMed] [Google Scholar]
- Minetti C., Lamden K., Durband C., Cheesbrough J., Platt K., Charlett A., et al. Case-control study of risk factors for sporadic giardiasis and parasite assemblages in North-West England. J. Clin. Microbiol. 2015;53:3133–3140. doi: 10.1128/JCM.00715-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minvielle M.C., Molina N.B., Polverino D., Basualdo J.A. First genotyping of Giardia lamblia from human and animal feces in Argentina, South America. Mem. Inst. Oswaldo Cruz. 2008;103:98–103. doi: 10.1590/s0074-02762008000100015. [DOI] [PubMed] [Google Scholar]
- Miska K.B., Jenkins M.C., Trout J.M., Santín M., Fayer R. Detection and comparison of Giardia virus (GLV) from different assemblages of Giardia duodenalis. J. Parasitol. 2009;95:1197–1200. doi: 10.1645/GE-1876.1. [DOI] [PubMed] [Google Scholar]
- Molloy S.F., Kirwan P., Asaolu S.O., Holland C.V., Nichols R.A.B., Connelly L., Smith H.V. Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am. J. Trop. Med. Hyg. 2010;82:608–613. doi: 10.4269/ajtmh.2010.09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis P.T., Andrews R.H., Mayrhofer G., Mackrill J., Kulda J., Isaac-Renton J.L., Ey P.L. Novel lineages of Giardia intestinalis identified by genetic analysis of organisms isolated from dogs in Australia. Parasitology. 1998;116:7–19. doi: 10.1017/s0031182097002011. [DOI] [PubMed] [Google Scholar]
- Monis P.T., Andrews R.H., Mayrhofer G., Ey P.L. Genetic diversity within the morphological species Giardia intestinalis and its relationship to host origin. Infect. Genet. Evol. 2003;3:29–38. doi: 10.1016/s1567-1348(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Moore C.E., Elwin K., Seng C., Mao S., Suy K., Kumar V., et al. Molecular characterization of Cryptosporidium species and Giardia duodenalis from symptomatic Cambodian children. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A.S., Baptista C.T., Brasil C.L., Valente J.S.S., Bruhn F.R.P., Pereira D.I.B. Risk factors and infection due to Cryptosporidium spp. in dogs and cats in southern Rio Grande do Sul. Rev. Bras. Parasitol. Vet. 2018;27:112–117. doi: 10.1590/S1984-296120180012. [DOI] [PubMed] [Google Scholar]
- Morgan U.M., Sargent K.D., Elliot A., Thompson R.C. Cryptosporidium in cats - additional evidence for C. felis. Vet. J. 1998;156:159–161. doi: 10.1016/s1090-0233(05)80047-4. [DOI] [PubMed] [Google Scholar]
- Morgan U., Weber R., Xiao L., Sulaiman I., Thompson R.C., Ndiritu W., et al. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J. Clin. Microbiol. 2000;38:1180–1183. doi: 10.1128/jcm.38.3.1180-1183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan U.M., Xiao L., Monis P., Fall A., Irwin P.J., Fayer R., et al. Cryptosporidium spp. in domestic dogs: the “dog” genotype. Appl. Environ. Microbiol. 2000;66:2220–2223. doi: 10.1128/aem.66.5.2220-2223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H.G., McArthur A.G., Gillin F.D., Aley S.B., Adam R.D., Olsen G.J., et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- Muadica A.S., Koster P.C., Dashti A., Bailo B., Hernández-de-Mingo M., Balasegaram S., Carmena D. Molecular diversity of Giardia duodenalis, Cryptosporidium spp. and Blastocystis sp. in symptomatic and asymptomatic school children in Zambezia province (Mozambique) Pathogens. 2021;10:255. doi: 10.3390/pathogens10030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulunda N.R., Hayashida K., Yamagishi J., Sianongo S., Munsaka G., Sugimoto C., Mutengo M.M. Molecular characterization of Cryptosporidium spp. from patients with diarrhoea in Lusaka, Zambia. Parasite. 2020;27:53. doi: 10.1051/parasite/2020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J., Mayer D.C. Toxoplasma gondii and Giardia duodenalis infections in domestic dogs in New York City public parks. Vet. J. 2016;211:97–99. doi: 10.1016/j.tvjl.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Murnik L.C., Daugschies A., Delling C. Cryptosporidium infection in young dogs from Germany. Parasitol. Res. 2022;121:2985–2993. doi: 10.1007/s00436-022-07632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnik L.C., Daugschies A., Delling C. Gastrointestinal parasites in young dogs and risk factors associated with infection. Parasitol. Res. 2023;122:585–596. doi: 10.1007/s00436-022-07760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy D., Rao S.S., Ramani S., Monica B., Banerjee I., Abraham O.C., et al. Multilocus genotyping of Cryptosporidium sp. isolates from human immunodeficiency virus-infected individuals in South India. J. Clin. Microbiol. 2006;44:632–634. doi: 10.1128/JCM.44.2.632-634.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori Y., Payton M.E., Looper E., Apple H., Johnson E.M. Retrospective survey of parasitism identified in feces of client-owned cats in North America from 2007 through 2018. Vet. Parasitol. 2020;277 doi: 10.1016/j.vetpar.2019.109008. [DOI] [PubMed] [Google Scholar]
- Neira P., Munoz N., Rosales J. Cryptosporidium parvum infection in a pregnant immunocompetent woman with occupational risk. Rev. Chilena Infectol. 2010;27:345–349. doi: 10.4067/s0716-10182010000500011. [DOI] [PubMed] [Google Scholar]
- Ng J., Yang R., Whiffin V., Cox P., Ryan U. Identification of zoonotic Cryptosporidium and Giardia genotypes infecting animals in Sydneyʼs water catchments. Exp. Parasitol. 2011;128:138–144. doi: 10.1016/j.exppara.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Nguyen S.T., Fukuda Y., Nguyen D.T., Dao H.T., Le D.Q., Bui K.L., et al. Prevalence, genotyping and risk factors of Giardia duodenalis from dogs in Vietnam. J. Vet. Med. Sci. 2018;80:92–97. doi: 10.1292/jvms.17-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M.J., Jex A.R., Koehler A.V., Haydon S.R., Stevens M.A., Gasser R.B. Molecular-based investigation of Cryptosporidium and Giardia from animals in water catchments in southeastern Australia. Water Res. 2013;47:1726–1740. doi: 10.1016/j.watres.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Nydam D.V., Wade S.E., Schaaf S.L., Mohammed H.O. Number of Cryptosporidium parvum oocysts or Giardia spp. cysts shed by dairy calves after natural infection. Am. J. Vet. Res. 2001;62:1612–1615. doi: 10.2460/ajvr.2001.62.1612. [DOI] [PubMed] [Google Scholar]
- Önder Z., Yetişmiş G., Pekmezci D., Delibaşı Kökçü N., Pekmezci G.Z., Çiloğlu A., et al. Investigation of zoonotic Cryptosporidium and Giardia intestinalis species and genotypes in cats (Felis catus) Turk. Parazitoloji Derg. 2021;45:252–256. doi: 10.4274/tpd.galenos.2021.46320. [DOI] [PubMed] [Google Scholar]
- Osman M., Bories J., El Safadi D., Poirel M.T., Gantois N., Benamrouz-Vanneste S., et al. Prevalence and genetic diversity of the intestinal parasites Blastocystis sp. and Cryptosporidium spp. in household dogs in France and evaluation of zoonotic transmission risk. Vet. Parasitol. 2015;214:167–170. doi: 10.1016/j.vetpar.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Overgaauw P.A., van Zutphen L., Hoek D., Yaya F.O., Roelfsema J., Pinelli E., et al. Zoonotic parasites in fecal samples and fur from dogs and cats in the Netherlands. Vet. Parasitol. 2009;163:115–122. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Overgaauw P.A.M., Vinke C.M., Hagen M.A.E.V., Lipman L.J.A. A One Health perspective on the human-companion animal relationship with emphasis on zoonotic aspects. Int. J. Environ. Res. Publ. Health. 2020;17:3789. doi: 10.3390/ijerph17113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco F.T.F., Freitas H.F., Silva R.K.N.R., Carvalho S.S., Martins A.S., Menezes J.F., et al. Cryptosporidium diagnosis in different groups of children and characterization of parasite species. Rev. Soc. Bras. Med. Trop. 2022;55 doi: 10.1590/0037-8682-0041-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paim Arruda Trevisan Y., Do Bom Parto Ferreira de Almeida A., Nakazato L., Dos Anjos Pacheco T., Iglesias de Souza J., Henrique Canei D., et al. Frequency of Giardia duodenalis infection and its genetic variability in dogs in Cuiabá, Midwest Brazil. J. Infect. Dev. Ctries. 2020;14:1431–1436. doi: 10.3855/jidc.13095. [DOI] [PubMed] [Google Scholar]
- Pallant L., Barutzki D., Schaper R., Thompson R.C.A. The epidemiology of infections with Giardia species and genotypes in well cared for dogs and cats in Germany. Parasites Vectors. 2015;8:2. doi: 10.1186/s13071-014-0615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C.S., Thompson R.C., Traub R.J., Rees R., Robertson I.D. National study of the gastrointestinal parasites of dogs and cats in Australia. Vet. Parasitol. 2008;151:181–190. doi: 10.1016/j.vetpar.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Palmer C.S., Traub R.J., Robertson I.D., Devlin G., Rees R., Thompson R.C. Determining the zoonotic significance of Giardia and Cryptosporidium in Australian dogs and cats. Vet. Parasitol. 2008;154:142–147. doi: 10.1016/j.vetpar.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Pan W., Wang M., Abdullahi A.Y., Fu Y., Yan X., Yang F., et al. Prevalence and genotypes of Giardia lamblia from stray dogs and cats in Guangdong, China. Vet. Parasitol. Reg. Stud. Reports. 2018;13:30–34. doi: 10.1016/j.vprsr.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Paoletti B., Iorio R., Capelli G., Sparagano O.A., Giangaspero A. Epidemiological scenario of giardiosis in dogs from central Italy. Ann. N. Y. Acad. Sci. 2008;1149:371–374. doi: 10.1196/annals.1428.005. [DOI] [PubMed] [Google Scholar]
- Paoletti B., Otranto D., Weigl S., Giangaspero A., Di Cesare A., Traversa D. Prevalence and genetic characterization of Giardia and Cryptosporidium in cats from Italy. Res. Vet. Sci. 2011;91:397–399. doi: 10.1016/j.rvsc.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Paoletti B., Traversa D., Iorio R., De Berardinis A., Bartolini R., Salini R., Di Cesare A. Zoonotic parasites in feces and fur of stray and private dogs from Italy. Parasitol. Res. 2015;114:2135–2141. doi: 10.1007/s00436-015-4402-6. [DOI] [PubMed] [Google Scholar]
- Papini R., Cardini G., Paoletti B., Giangaspero A. Detection of Giardia assemblage A in cats in Florence, Italy. Parasitol. Res. 2007;100:653–656. doi: 10.1007/s00436-006-0290-0. [DOI] [PubMed] [Google Scholar]
- Patterson J. Toxocarosis in humans: How much of a problem is it in the UK? Drug Therapeut. Bull. 2023;61:7–11. doi: 10.1136/dtb.2022.000052. [DOI] [PubMed] [Google Scholar]
- Pavlásek I., Ryan U. The first finding of a natural infection of Cryptosporidium muris in a cat. Vet. Parasitol. 2007;144:349–352. doi: 10.1016/j.vetpar.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Pedraza-Días S., Amar C., Iversen A.M., Stanley P.J., McLauchlin J. Unusual Cryptosporidium species recovered from human faeces: First description of Cryptosporidium felis and Cryptosporidium “dog type” from patients in England. J. Med. Microbiol. 2001;50:293–296. doi: 10.1099/0022-1317-50-3-293. [DOI] [PubMed] [Google Scholar]
- Pereira A., Teixeira J., Sousa S., Parreira R., Campino L., Meireles J., Maia C. Giardia duodenalis infection in dogs from the metropolitan area of Lisbon, Portugal: Prevalence, genotyping and associated risk factors. J. Parasit. Dis. 2021;45:372–379. doi: 10.1007/s12639-020-01307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrucci S., Berrilli F., Procopio C., Di Filippo M.M., Pierini A., Marchetti V. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Vet. J. 2020;10:74–79. doi: 10.4314/ovj.v10i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekara-Stępińska A., Piekarska J., Gorczykowski M. Cryptosporidium spp. in dogs and cats in Poland. Ann. Agric. Environ. Med. 2021;28:345–347. doi: 10.26444/aaem/120467. [DOI] [PubMed] [Google Scholar]
- Piekarska J., Bajzert J., Gorczykowski M., Kantyka M., Podkowik M. Molecular identification of Giardia duodenalis isolates from domestic dogs and cats in Wroclaw, Poland. Ann. Agric. Environ. Med. 2016;23:410–415. doi: 10.5604/12321966.1219178. [DOI] [PubMed] [Google Scholar]
- Pieniazek N.J., Bornay-Llinares F.J., Slemenda S.B., da Silva A.J., Moura I.N., Arrowood M.J., et al. New Cryptosporidium genotypes in HIV-infected persons. Emerg. Infect. Dis. 1999;5:444–449. doi: 10.3201/eid0503.990318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipia A.P., Varcasia A., Tamponi C., Sanna G., Soda M., Paoletti B., et al. Canine giardiosis in Sardinia Island, Italy: Prevalence, molecular characterization, and risk factors. J. Infect. Dev. Ctries. 2014;8:655–660. doi: 10.3855/jidc.4255. [DOI] [PubMed] [Google Scholar]
- Pipiková J., Papajová I., Majláthová V., Šoltys J., Bystrianska J., Schusterová I., Vargová V. First report on Giardia duodenalis assemblage F in Slovakian children living in poor environmental conditions. J. Microbiol. Immunol. Infect. 2022;53:148–156. doi: 10.1016/j.jmii.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Ponce-Macotela M., Martínez-Gordillo M.N., Bermúdez-Cruz R.M., Salazar-Schettino P.M., Ortega-Pierres G., Ey P.L. Unusual prevalence of the Giardia intestinalis A-II subtype amongst isolates from humans and domestic animals in Mexico. Int. J. Parasitol. 2002;32:1201–1202. doi: 10.1016/s0020-7519(02)00086-3. [DOI] [PubMed] [Google Scholar]
- Potes-Morales C., Crespo-Ortiz M.D.P. Molecular diagnosis of intestinal protozoa in young adults and their pets in Colombia, South America. PLoS One. 2023;18 doi: 10.1371/journal.pone.0283824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puebla L.E.J., Nunez F.A., Rivero L.R., Hernandez Y.R., Millan I.A., Muller N. Prevalence of intestinal parasites and molecular characterization of Giardia duodenalis from dogs in La Habana, Cuba. Vet. Parasitol. Reg. Stud. Reports. 2017;8:107–112. doi: 10.1016/j.vprsr.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Qi M., Dong H., Wang R., Li J., Zhao J., Zhang L., Luo J. Infection rate and genetic diversity of Giardia duodenalis in pet and stray dogs in Henan Province, China. Parasitol. Int. 2016;65:159–162. doi: 10.1016/j.parint.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Quadros R.M., Weiss P.H., Marques S.M., Miletti L.C. Potential cross-contamination of similar Giardia duodenalis assemblage in children and pet dogs in southern Brazil, as determined by PCR-RFLP. Rev. Inst. Med. Trop. Sao Paulo. 2016;58:66. doi: 10.1590/S1678-9946201658066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccurt C.P., Brasseur P., Verdier R.I., Li X., Eyma E., Stockman C.P., et al. Human cryptosporidiosis and Cryptosporidium spp. in Haiti. Trop. Med. Int. Health. 2006;11:929–934. doi: 10.1111/j.1365-3156.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- Ramírez-Ocampo S., Cotte-Alzate J.D., Escobedo Á.A., Rodríguez-Morales A.J. Prevalence of zoonotic and non-zoonotic genotypes of Giardia intestinalis in cats: A systematic review and meta-analysis. Infez. Med. 2017;25:326–338. [PubMed] [Google Scholar]
- Ranjbar R., Mirhendi H., Izadi M., Behrouz B., Mohammadi Manesh R. Molecular identification of Cryptosporidium spp. in Iranian dogs using seminested PCR: A first report. Vector Borne Zoonotic Dis. 2018;18:96–100. doi: 10.1089/vbz.2017.2136. [DOI] [PubMed] [Google Scholar]
- Read C.M., Monis P.T., Thompson R.C. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 2004;4:125–130. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Rehbein S., Klotz C., Ignatius R., Müller E., Aebischer A., Kohn B. Giardia duodenalis in small animals and their owners in Germany: A pilot study. Zoonoses Public Health. 2019;66:117–124. doi: 10.1111/zph.12541. [DOI] [PubMed] [Google Scholar]
- Rendtorff R.C. Waterborne Transmission of Giardiasis. Proceeding of a Symposium; Cincinnati, USA: 1979. The experimental transmission of Giardia lamblia among volunteer subjects. [Google Scholar]
- Rimhanen-Finne R., Enemark H.L., Kolehmainen J., Toropainen P., Hanninen M.L. Evaluation of immunofluorescence microscopy and enzyme-linked immunosorbent assay in detection of Cryptosporidium and Giardia infections in asymptomatic dogs. Vet. Parasitol. 2007;145:345–348. doi: 10.1016/j.vetpar.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Roegner A.F., Daniels M.E., Smith W.A., Gottdenker N., Schwartz L.M., Liu J., et al. Giardia infection and Trypanosoma cruzi exposure in dogs in the Bosawas biosphere reserve, Nicaragua. EcoHealth. 2019;16:512–522. doi: 10.1007/s10393-019-01434-2. [DOI] [PubMed] [Google Scholar]
- Rojas-Lopez L., Elwin K., Chalmers R.M., Enemark H.L., Beser J., Troell K. Development of a gp60-subtyping method for Cryptosporidium felis. Parasites Vectors. 2020;13:39. doi: 10.1186/s13071-020-3906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanowski S.M., Banica M., Ellis E., Farrow E., Harwood C., Jordan B., et al. The molecular characterisation of Cryptosporidium species in relinquished dogs in Great Britain: A novel zoonotic risk? Parasitol. Res. 2018;117:1663–1667. doi: 10.1007/s00436-018-5857-z. [DOI] [PubMed] [Google Scholar]
- Ryan U., Feng Y., Fayer R., Xiao L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia - a 50-year perspective (1971–2021) Int. J. Parasitol. 2021;51:1099–1119. doi: 10.1016/j.ijpara.2021.08.007. [DOI] [PubMed] [Google Scholar]
- Ryan U., Zahedi A., Feng Y., Xiao L. An update on zoonotic Cryptosporidium species and genotypes in humans. Animals. 2021;11:3307. doi: 10.3390/ani11113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M.N., Lindsay D.S., Leib M.S., Zajac A.M. Giardia duodenalis assemblages in cats from Virginia, USA. Vet. Parasitol. Reg. Stud. Reports. 2019;15 doi: 10.1016/j.vprsr.2018.100257. [DOI] [PubMed] [Google Scholar]
- Saleh M.N., Allen K.E., Lineberry M.W., Little S.E., Reichard M.V. Ticks infesting dogs and cats in North America: Biology, geographic distribution, and pathogen transmission. Vet. Parasitol. 2021;294 doi: 10.1016/j.vetpar.2021.109392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannella A.R., Suputtamongkol Y., Wongsawat E., Cacciò S.M. A retrospective molecular study of Cryptosporidium species and genotypes in HIV-infected patients from Thailand. Parasites Vectors. 2019;12:91. doi: 10.1186/s13071-019-3348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santín M. Cryptosporidium and Giardia in ruminants. Vet. Clin. North. Am. Food Anim. Pract. 2020;36:223–238. doi: 10.1016/j.cvfa.2019.11.005. [DOI] [PubMed] [Google Scholar]
- Santín M., Trout J.M., Vecino J.A., Dubey J.P., Fayer R. Cryptosporidium, Giardia and Enterocytozoon bieneusi in cats from Bogota (Colombia) and genotyping of isolates. Vet. Parasitol. 2006;141:334–339. doi: 10.1016/j.vetpar.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Sargent K.D., Morgan U.M., Elliot A., Thompson R.C. Morphological and genetic characterisation of Cryptosporidium oocysts from domestic cats. Vet. Parasitol. 1998;77:221–227. doi: 10.1016/s0304-4017(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Scaramozzino P., Di Cave D., Berrilli F., DʼOrazi C., Spaziani A., Mazzanti S., et al. A study of the prevalence and genotypes of Giardia duodenalis infecting kennelled dogs. Vet. J. 2009;182:231–234. doi: 10.1016/j.tvjl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Schurer J.M., Hill J.E., Fernando C., Jenkins E.J. Sentinel surveillance for zoonotic parasites in companion animals in indigenous communities of Saskatchewan. Am. J. Trop. Med. Hyg. 2012;87:495–498. doi: 10.4269/ajtmh.2012.12-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza A.V., Lappin M.R. Prevalence of selected zoonotic and vector-borne agents in dogs and cats on the pine ridge reservation. Vet. Sci. 2017;4:43. doi: 10.3390/vetsci4030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza A.V., Duncan C., Miles L., Lappin M.R. Prevalence of selected zoonotic and vector-borne agents in dogs and cats in Costa Rica. Vet. Parasitol. 2011;183:178–183. doi: 10.1016/j.vetpar.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Semmani M., Costa D., Achour N., Cherchar M., Ziane H., Mouhajir A., et al. Occurrence and molecular characterization of Cryptosporidium infection in HIV/AIDS patients in Algeria. Viruses. 2023;15:362. doi: 10.3390/v15020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.C., Reyes A.W., Kim S.H., Kim S., Park H.J., Seo K.W., Song K.H. Molecular detection of Giardia intestinalis from stray dogs in animal shelters of Gyeongsangbuk-do (Province) and Daejeon, Korea. Kor. J. Parasitol. 2015;53:477–481. doi: 10.3347/kjp.2015.53.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.C.D.S., Martins F.D.C., Ladeia W.A., Kakimori M.T.A., Lucas J.I., Sasse J.P., et al. First report of Giardia duodenalis assemblage F in humans and dogs in southern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2022;89 doi: 10.1016/j.cimid.2022.101878. [DOI] [PubMed] [Google Scholar]
- Simonato G., Frangipane di Regalbono A., Cassini R., Traversa D., Beraldo P., Tessarin C., Pietrobelli M. Copromicroscopic and molecular investigations on intestinal parasites in kenneled dogs. Parasitol. Res. 2015;114:1963–1970. doi: 10.1007/s00436-015-4385-3. [DOI] [PubMed] [Google Scholar]
- Simonato G., Frangipane di Regalbono A., Cassini R., Traversa D., Tessarin C., DiCesare A., Pietrobelli M. Molecular detection of Giardia duodenalis and Cryptosporidium spp. in canine faecal samples contaminating public areas in northern Italy. Parasitol. Res. 2017;116:3411–3418. doi: 10.1007/s00436-017-5671-z. [DOI] [PubMed] [Google Scholar]
- Smith R.P., Chalmers R.M., Mueller-Doblies D., Clifton-Hadley F.A., Elwin K., et al. Investigation of farms linked to human patients with cryptosporidiosis in England and Wales. Prev. Vet. Med. 2010;94:9–17. doi: 10.1016/j.prevetmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Smith A.F., Neumann N., Banting G., Klein C., Liccioli S., Massolo A. Molecular characterization of Giardia spp. and Cryptosporidium spp. from dogs and coyotes in an urban landscape suggests infrequent occurrence of zoonotic genotypes. Vet. Parasitol. 2020;281 doi: 10.1016/j.vetpar.2020.109115. [DOI] [PubMed] [Google Scholar]
- Solarczyk P., Majewska A.C. A survey of the prevalence and genotypes of Giardia duodenalis infecting household and sheltered dogs. Parasitol. Res. 2010;106:1015–1019. doi: 10.1007/s00436-010-1766-5. [DOI] [PubMed] [Google Scholar]
- Soliman R.H., Fuentes I., Rubio J.M. Identification of a novel assemblage B subgenotype and a zoonotic assemblage C in human isolates of Giardia intestinalis in Egypt. Parasitol. Int. 2011;60:507–511. doi: 10.1016/j.parint.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Sommer M.F., Beck R., Ionita M., Stefanovska J., Vasić A., Zdravković N., Hamel D., et al. Multilocus sequence typing of canine Giardia duodenalis from south-eastern European countries. Parasitol. Res. 2015;114:2165–2174. doi: 10.1007/s00436-015-4405-3. [DOI] [PubMed] [Google Scholar]
- Sommer M.F., Rupp P., Pietsch M., Kaspar A., Beelitz P. Giardia in a selected population of dogs and cats in Germany - diagnostics, coinfections and assemblages. Vet. Parasitol. 2018;249:49–56. doi: 10.1016/j.vetpar.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Sotiriadou I., Pantchev N., Gassmann D., Karanis P. Molecular identification of Giardia and Cryptosporidium from dogs and cats. Parasite. 2013;20:8. doi: 10.1051/parasite/2013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza S.L., Gennari S.M., Richtzenhain L.J., Pena H.F., Funada M.R., Cortez A., et al. Molecular identification of Giardia duodenalis isolates from humans, dogs, cats and cattle from the state of São Paulo, Brazil, by sequence analysis of fragments of glutamate dehydrogenase (gdh) coding gene. Vet. Parasitol. 2007;149:258–264. doi: 10.1016/j.vetpar.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Sprong H., Cacciò S.M., van der Giessen J.W., ZOOPNET network and partners Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009;3:e558. doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisuphanunt M., Saksirisampant W., Karanis P. Prevalence and genotyping of Cryptosporidium isolated from HIV/AIDS patients in urban areas of Thailand. Ann. Trop. Med. Parasitol. 2011;105:463–468. doi: 10.1179/1364859411Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štrkolcová G., Maďar M., Hinney B., Goldová M., Mojžišová J., Halánová M. Dogʼs genotype of Giardia duodenalis in human: First evidence in Europe. Acta Parasitol. 2015;60:796–799. doi: 10.1515/ap-2015-0113. [DOI] [PubMed] [Google Scholar]
- Sui Y., Zhang X., Wang H., Yu F., Zheng L., Guo Y., et al. Prevalence and genetic diversity of Giardia duodenalis in pet dogs from Zhengzhou, central China and the association between gut microbiota and fecal characteristics during infection. One Health. 2022;14 doi: 10.1016/j.onehlt.2022.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman I.M., Fayer R., Bern C., Gilman R.H., Trout J.M., Schantz P.M., et al. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003;9:1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sursal N., Simsek E., Yildiz K. Feline giardiasis in Turkey: Prevalence and genetic and haplotype diversity of Giardia duodenalis based on the β-giardin gene sequence in symptomatic cats. J. Parasitol. 2020;106:699–706. doi: 10.1645/19-183. [DOI] [PubMed] [Google Scholar]
- Sutthikornchai C., Popruk S., Mahittikorn A., Arthan D., Soonthornworasiri N., Paratthakonkun C., et al. Molecular detection of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in school children at the Thai-Myanmar border. Parasitol. Res. 2021;120:2887–2895. doi: 10.1007/s00436-021-07242-4. [DOI] [PubMed] [Google Scholar]
- Suzuki J., Murata R., Kobayashi S., Sadamasu K., Kai A., Takeuchi T. Risk of human infection with Giardia duodenalis from cats in Japan and genotyping of the isolates to assess the route of infection in cats. Parasitology. 2011;138:493–500. doi: 10.1017/S0031182010001459. [DOI] [PubMed] [Google Scholar]
- Szénási Z., Marton S., Kucsera I., Tánczos B., Horváth K., Orosz E., et al. Preliminary investigation of the prevalence and genotype distribution of Giardia intestinalis in dogs in Hungary. Parasitol. Res. 2007;101:145–152. [Google Scholar]
- Taghipour A., Olfatifar M., Bahadory S., Godfrey S.S., Abdoli A., Khatami A., Javanmard E., Shahrivar F. The global prevalence of Cryptosporidium infection in dogs: A systematic review and meta-analysis. Vet. Parasitol. 2020;281 doi: 10.1016/j.vetpar.2020.109093. [DOI] [PubMed] [Google Scholar]
- Tahir D., Davoust B., Parola P. Vector-borne nematode diseases in pets and humans in the Mediterranean Basin: An update. Vet. World. 2019;12:1630–1643. doi: 10.14202/vetworld.2019.1630-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wu S., Abdullahi A.Y., Yu X., Hu W., Song M., et al. PCR-RFLP method to detect zoonotic and host-specific Giardia duodenalis assemblages in dog fecal samples. Parasitol. Res. 2016;115:2045–2050. doi: 10.1007/s00436-016-4948-y. [DOI] [PubMed] [Google Scholar]
- Tangtrongsup S., Scorza A.V., Reif J.S., Ballweber L.R., Lappin M.R., Salman M.D. Prevalence and multilocus genotyping analysis of Cryptosporidium and Giardia isolates from dogs in Chiang Mai, Thailand. Vet. Sci. 2017;4:26. doi: 10.3390/vetsci4020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangtrongsup S., Scorza A.V., Reif J.S., Ballweber L.R., Lappin M.R., Salman M.D. Seasonal distributions and other risk factors for Giardia duodenalis and Cryptosporidium spp. infections in dogs and cats in Chiang Mai, Thailand. Prev. Vet. Med. 2020;174 doi: 10.1016/j.prevetmed.2019.104820. [DOI] [PubMed] [Google Scholar]
- Thomaz A., Meireles M.V., Soares R.M., Pena H.F., Gennari S.M. Molecular identification of Cryptosporidium spp. from fecal samples of felines, canines and bovines in the state of São Paulo, Brazil. Vet. Parasitol. 2007;150:291–296. doi: 10.1016/j.vetpar.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A. Zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tiangtip R., Jongwutiwes S. Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop. Med. Int. Health. 2002;7:357–364. doi: 10.1046/j.1365-3156.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- Traub R.J., Monis P.T., Robertson I., Irwin P., Mencke N., Thompson R.C. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology. 2004;128:253–262. doi: 10.1017/s0031182003004505. [DOI] [PubMed] [Google Scholar]
- Traub R.J., Inpankaew T., Reid S.A., Sutthikornchai C., Sukthana Y., Robertson I.D., Thompson R.C. Transmission cycles of Giardia duodenalis in dogs and humans in Temple communities in Bangkok - a critical evaluation of its prevalence using three diagnostic tests in the field in the absence of a gold standard. Acta Trop. 2009;111:125–132. doi: 10.1016/j.actatropica.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Tseng Y.C., Ho G.D., Chen T.T.W., Huang B.F., Cheng P.C., Chen J.L., Peng S.Y. Prevalence and genotype of Giardia duodenalis from faecal samples of stray dogs in Hualien city of eastern Taiwan. Trop. Biomed. 2014;31:305–311. [PubMed] [Google Scholar]
- Uehlinger F.D., Greenwood S.J., McClure J.T., Conboy G., O'Handley R., Barkema H.W. Zoonotic potential of Giardia duodenalis and Cryptosporidium spp. and prevalence of intestinal parasites in young dogs from different populations on Prince Edward Island, Canada. Vet. Parasitol. 2013;196:509–514. doi: 10.1016/j.vetpar.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Uiterwijk M., Mughini-Gras L., Nijsse R., Wagenaar J.A., Ploeger H.W., Kooyman F.N.J. Giardia duodenalis multi-locus genotypes in dogs with different levels of synanthropism and clinical signs. Parasites Vectors. 2020;13:605. doi: 10.1186/s13071-020-04496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukwah B.N., Ezeonu I.M., Ezeonu C.T., Roellig D., Xiao L. Cryptosporidium species and subtypes in diarrheal children and HIV-infected persons in Ebonyi and Nsukka, Nigeria. J. Infect. Dev. Ctries. 2017;11:173–179. doi: 10.3855/jidc.8034. [DOI] [PubMed] [Google Scholar]
- Upjohn M., Cobb C., Monger J., Geurden T., Claerebout E., Fox M. Prevalence, molecular typing and risk factor analysis for Giardia duodenalis infections in dogs in a central London rescue shelter. Vet. Parasitol. 2010;172:341–346. doi: 10.1016/j.vetpar.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Uran-Velasquez J., Alzate J.F., Farfan-Garcia A.E., Gomez-Duarte O.G., Martinez-Rosado L.L., Dominguez-Hernandez D.D., et al. Multilocus sequence typing helps understand the genetic diversity of Cryptosporidium hominis and Cryptosporidium parvum isolated from Colombian patients. PLoS One. 2022;17 doi: 10.1371/journal.pone.0270995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utaaker K.S., Tysnes K.R., Krosness M.M., Robertson L.J. Not just a walk in the park: Occurrence of intestinal parasites in dogs roaming recreational parks in Chandigarh, Northern India. Vet. Parasitol. Reg. Stud. Reports. 2018;14:176–180. doi: 10.1016/j.vprsr.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Vasilopulos R.J., Rickard L.G., Mackin A.J., Pharr G.T., Huston C.L. Genotypic analysis of Giardia duodenalis in domestic cats. J. Vet. Intern. Med. 2007;21:352–355. doi: 10.1892/0891-6640(2007)21[352:gaogdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Veldhuis F.L., Nijsse R., Wagenaar J.A., Arkesteijn G., Kooyman F.N.J. Variation in haplotypes in single cysts of assemblages C and D, but not of assemblage E of Giardia duodenalis. BMC Microbiol. 2022;22:166. doi: 10.1186/s12866-022-02581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyna-Salazar N.P., Cantó-Alarcón G.J., Olvera-Ramírez A.M., Ruiz-López F.J., Bernal-Reynaga R., Bárcenas-Reyes I., Durán-Aguilar M. Occurrence of Giardia duodenalis in cats from Queretaro and the risk to public health. Animals. 2023;13:1098. doi: 10.3390/ani13061098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volotão A.C., Costa-Macedo L.M., Haddad F.S.M., Brandao A., Peralta J.M., Fernandes O. Genotyping of Giardia duodenalis from human and animal samples from Brazil using beta-giardin gene: A phylogenetic analysis. Acta Trop. 2007;102:10–19. doi: 10.1016/j.actatropica.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Wang A., Ruch-Gallie R., Scorza V., Lin P., Lappin M.R. Prevalence of Giardia and Cryptosporidium species in dog park attending dogs compared to non-dog park attending dogs in one region of Colorado. Vet. Parasitol. 2012;184:335–340. doi: 10.1016/j.vetpar.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Wang Y.G., Zou Y., Yu Z.Z., Chen D., Gui B.Z., Yang J.F., et al. Molecular investigation of zoonotic intestinal protozoa in pet dogs and cats in Yunnan Province, Southwestern China. Pathogens. 2021;10:1107. doi: 10.3390/pathogens10091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wei Y., Cao S., Wu W., Zhao W., Guo Y., et al. Divergent Cryptosporidium species and host-adapted Cryptosporidium canis subtypes in farmed minks, raccoon dogs and foxes in Shandong, China. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.980917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Wei Z., Zhang Y., Zhang Q., Zhang L., Yu F., et al. Molecular detection and genetic characterization of Cryptosporidium in kindergarten children in Southern Xinjiang, China. Infect. Genet. Evol. 2022;103 doi: 10.1016/j.meegid.2022.105339. [DOI] [PubMed] [Google Scholar]
- Wood L., Martin K., Christian H., Nathan A., Lauritsen C., Houghton S., et al. The pet factor-companion animals as a conduit for getting to know people, friendship formation and social support. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Yao L., Chen H., Zhang W., Jiang Y., Yang F., et al. Giardia duodenalis in patients with diarrhea and various animals in northeastern China: Prevalence and multilocus genetic characterization. Parasites Vectors. 2022;15:165. doi: 10.1186/s13071-022-05269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Bern C., Limor J., Sulaiman I., Roberts J., Checkley W., et al. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 2001;183:492–497. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- Xiao L., Cama V.A., Cabrera L., Ortega Y., Pearson J., Gilman R.H. Possible transmission of Cryptosporidium canis among children and a dog in a household. J. Clin. Microbiol. 2007;45:2014–2016. doi: 10.1128/JCM.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Jin Y., Wu W., Li P., Wang L., Li N., Feng Y., Xiao L. Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasites Vectors. 2016;9:121. doi: 10.1186/s13071-016-1409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Kon M., Saito T., Maeno N., Koyama M., Sunaoshi K., et al. Prevalence of intestinal canine and feline parasites in Saitama Prefecture, Japan. Kansenshogaku Zasshi. 2009;83:223–228. doi: 10.11150/kansenshogakuzasshi.83.223. [DOI] [PubMed] [Google Scholar]
- Yang R., Jacobson C., Gardner G., Carmichael I., Campbell A.J., Ng-Hublin J., Ryan U. Longitudinal prevalence, oocyst shedding and molecular characterisation of Cryptosporidium species in sheep across four states in Australia. Vet. Parasitol. 2014;200:50–58. doi: 10.1016/j.vetpar.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Yang R., Ying J.L., Monis P., Ryan U. Molecular characterisation of Cryptosporidium and Giardia in cats (Felis catus) in Western Australia. Exp. Parasitol. 2015;155:13–18. doi: 10.1016/j.exppara.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Yang X., Guo Y., Xiao L., Feng Y. Molecular epidemiology of human cryptosporidiosis in low- and middle-income countries. Clin. Microbiol. Rev. 2021;34 doi: 10.1128/CMR.00087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiuchi R., Matsubayashi M., Kimata I., Furuya M., Tani H., Sasai K. Survey and molecular characterization of Cryptosporidium and Giardia spp. in owned companion animal, dogs and cats, in Japan. Vet. Parasitol. 2010;174:313–316. doi: 10.1016/j.vetpar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Yu Z., Ruan Y., Zhou M., Chen S., Zhang Y., Wang L., et al. Prevalence of intestinal parasites in companion dogs with diarrhea in Beijing, China, and genetic characteristics of Giardia and Cryptosporidium species. Parasitol. Res. 2018;117:35–43. doi: 10.1007/s00436-017-5631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablan K., Melvin G., Hayley A. Older adult companion animal-owner wellbeing during the COVID-19 pandemic: A qualitative exploration. Anthrozoös. 2023;36:237–256. [Google Scholar]
- Zahedi A., Gofton A.W., Greay T., Monis P., Oskam C., Ball A., et al. Profiling the diversity of Cryptosporidium species and genotypes in wastewater treatment plants in Australia using next generation sequencing. Sci. Total Environ. 2018;644:635–648. doi: 10.1016/j.scitotenv.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Zanzani S.A., Gazzonis A.L., Scarpa P., Berrilli F., Manfredi M.T. Intestinal parasites of owned dogs and cats from metropolitan and micropolitan areas: Prevalence, zoonotic risks, and pet owner awareness in northern Italy. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/696508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhong Z., Deng L., Wang M., Li W., Gong C., et al. Detection and multilocus genotyping of Giardia duodenalis in dogs in Sichuan Province, China. Parasite. 2017;24:31. doi: 10.1051/parasite/2017032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.Y., Li M.H., Lyu C., Meng X.Z., Qin Y.F., Yang X.B., et al. Prevalence of Giardia duodenalis among dogs in China from 2001 to 2021: A systematic review and meta-analysis. Foodb. Pathog. Dis. 2022;19:179–191. doi: 10.1089/fpd.2021.0073. [DOI] [PubMed] [Google Scholar]
- Zheng G., Alsarakibi M., Liu Y., Hu W., Luo Q., Tan L., Li G. Genotyping of Giardia duodenalis isolates from dogs in Guangdong, China based on multi-locus sequence. Kor. J. Parasitol. 2014;52:299–304. doi: 10.3347/kjp.2014.52.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Hu W., Liu Y., Luo Q., Tan L., Li G. Occurrence and molecular identification of Giardia duodenalis from stray cats in Guangzhou, southern China. Kor. J. Parasitol. 2015;53:119–124. doi: 10.3347/kjp.2015.53.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article.