Abstract
The inner centromere is a region on the mitotic chromosome that serves as a platform for mitotic signaling and possesses unique biophysical properties that enable it to withstand relatively large pulling-forces that are generated by kinetochores during chromosome segregation. The chromosomal passenger complex (CPC) localizes to and is the key regulator of inner centromere organization and function during mitosis. Recently, we demonstrated that in addition to its kinase and histone code-reading activities, the CPC also can undergo liquid-liquid phase separation (LLPS) and proposed that the inner centromere is a membraneless organelle scaffolded by the CPC. In this perspective, we explore mechanisms that can allow the formation and dissolution of this membraneless body. The cell cycle regulated spatially defined assembly and disassembly of the CPC condensate at the inner centromere can reveal general principles about how histone modifications control chromatin-bound membraneless organelles. We further explore how the ability of the CPC to undergo LLPS may contribute to the organization and function of the inner centromere during mitosis.
Liquid-liquid phase separation a potential organizing principle in mitosis:
During mitosis, the complex task of chromosome segregation is accomplished with remarkable fidelity. Although the parts list for macromolecules that carry out mitotic processes is almost complete, the design principles that underlie these processes are only beginning to be uncovered. During mitosis many biochemical reactions need to be compartmentalized at key regulatory centers in mitotic cytoplasm with high spatiotemporal precision. Such compartmentalization of biochemical reactions in mitotic cytoplasm is not trivial given that mitotic regulatory regions, such as centrosomes, centromeres, kinetochores, etc., are not membrane-bound and most membrane-bound organelles are either disassembled or fragmented during mitosis in vertebrates.
In combination with classical membrane-bound compartmentalization, liquid-liquid phase separation (LLPS) is fast becoming a key organizational principle driving subcellular compartmentalization at the mesoscale [1]. LLPS is an emergent biophysical property of multivalent proteins or nucleic acids that collectively behave as liquids under specific conditions and lead to the formation of aqueous two- or multi-phase system [1]. The cytoplasmic environment was theorized to be conducive for phase separation as early as 1995 [2] and LLPS is indeed increasingly understood to be a ubiquitous phenomenon in both the nucleus and cytoplasm [1, 3]. LLPS allows the local concentration of biomolecules in the cytoplasm and enables the formation of conditions that can trigger unique biochemical reactions that otherwise would not be readily possible in a diffused cytoplasm [1, 3, 4]. Due to the membrane-independent nature of compartmentalization driven by LLPS, in theory, LLPS can play an especially important role in organizing mitotic cytoplasm as well as nucleoplasm. Since the role of LLPS in organizing/regulating the mitotic spindle was recently explored [5], here we will concentrate on the role it may play in organizing mitotic chromosomes.
The inner centromere is a specialized chromatin region between sister centromeres that acts as a key regulatory center on every mitotic chromosome [6]. Overexpression of inner centromere components is tightly correlated with high amounts of aneuploidy in breast tumors, underscoring their key role in maintaining genomic stability [7]. The inner centromere has at least three biochemical and biophysical properties that are distinct from the rest of the mitotic chromosome [6]. It is the key signaling center during mitosis and is also the region where cohesion between sister chromatids is maintained until anaphase. The chromatin at the inner centromere is also uniquely organized allowing it to withstand microtubule pulling forces that act upon this region during mitosis [8, 9]. To enable these functions the inner centromere has a specific set of proteins that are confined to this chromatin region during mitosis.
The CPC is a central regulator of inner centromere organization and function during mitosis [6, 10–12]. The CPC is a four-protein complex composed of the kinase Aurora B, the scaffold protein INCENP and two targeting proteins Borealin and Survivin [13–19]. The CPC is concentrated at inner centromeres during mitosis [20, 21]. Recently, we demonstrated that the CPC undergoes LLPS in vivo and in vitro [22]. We hypothesized that CPC phase separation imparting unique biochemical and biophysical properties to the inner centromere during mitosis. In this review, we will integrate previous results with this new finding and explore this hypothesis further. Using the example of the CPC we will also highlight an emerging principle, where the combination of histone code reading and phase separation activity on the same protein or protein complex can allow specification of the site at which membraneless organelle will form. Since, the CPC condensates are assembled and disassembled at the defined chromatin locus in a cell cycle regulated manner understanding how CPC condensation is regulated during mitosis will uncover general principles applicable to many chromatin-bound membraneless organelles.
Mechanism of Assembly and Disassembly of the Inner Centromere Organelle
Since phase separation can organize cellular events at the mesoscale, the generation and dissolution of membraneless organelles in cells becomes a critical form of regulation for a number of cellular processes [23]. CPC condensates assemble at the inner centromere during early prophase and dissolve at the beginning of anaphase [13, 22]. The rapid and periodic assembly and disassembly of the CPC condensates at a single location on each chromosome pair every mitosis makes it an outstanding system to study the principles behind assembly and disassembly of membraneless organelles.
Why does the CPC phase separate at inner centromeres but not in the cytoplasm? This is explained by our observation that although inner centromere targeting regions of the CPC can spontaneously phase separate in vitro, the concentration range at which this occurs is above the total cellular concentration of the CPC but below its concentration at the inner centromere [22]. Thus, mechanisms must exist that dictate where and when the CPC phase separation occurs in the cell. Multiple mechanisms can regulate the formation of CPC condensate at the inner centromere during mitosis. First, the local enrichment of CPC on inner centromere chromatin can “nucleate” the formation of the CPC condensate during mitosis (Figure 1A). This seeding can be achieved by the initial enrichment of the CPC to the inner centromere by two orthogonal histone marks that were first shown to drive localization of the CPC to inner centromeres [24]. The haspin kinase phosphorylates inner centromeric histone H3 on Thr3 and this mark is directly read by the Survivin subunit of the CPC [24–28]. A second landing mark comes from kinetochores localized kinase Bub1 that phosphorylates histone H2a on Thr120 [29–31]. Current models suggest that this mark is directly read by the Sgo1 protein, which then interacts with the CPC [29, 31]. These histone marks are enriched at the inner centromere, thus leading to initial recruitment of the CPC to this region [24]. This initial recruitment of the CPC could then initiate condensate formation after a critical concentration of CPC is recruited to a specific chromatin region. Interestingly, while the strength of the interaction between CPC and both H3 pThr3 and Sgo1 are weak in the diffused state (dissociation constants in the μM range), these interactions occur readily in the CPC condensate [26, 28, 30]. The high local concentrations of binding sites in condensates will push the equilibrium of the binding reaction towards the bound form and can thus allow such weak interactions to take place even at physiological protein concentrations, allowing them to exert observable effect. Allowing weak interactions to become physiologically relevant may be an emergent generally applicable outcome of condensate biology. Therefore, the histone marks can drive local enrichment of the CPC to allow initiation of condensate formation and the condensate state in turn further enhances the CPC-histone interaction to robustly and stably recruit more CPC.
Figure 1: Model for inner centromere body formation.
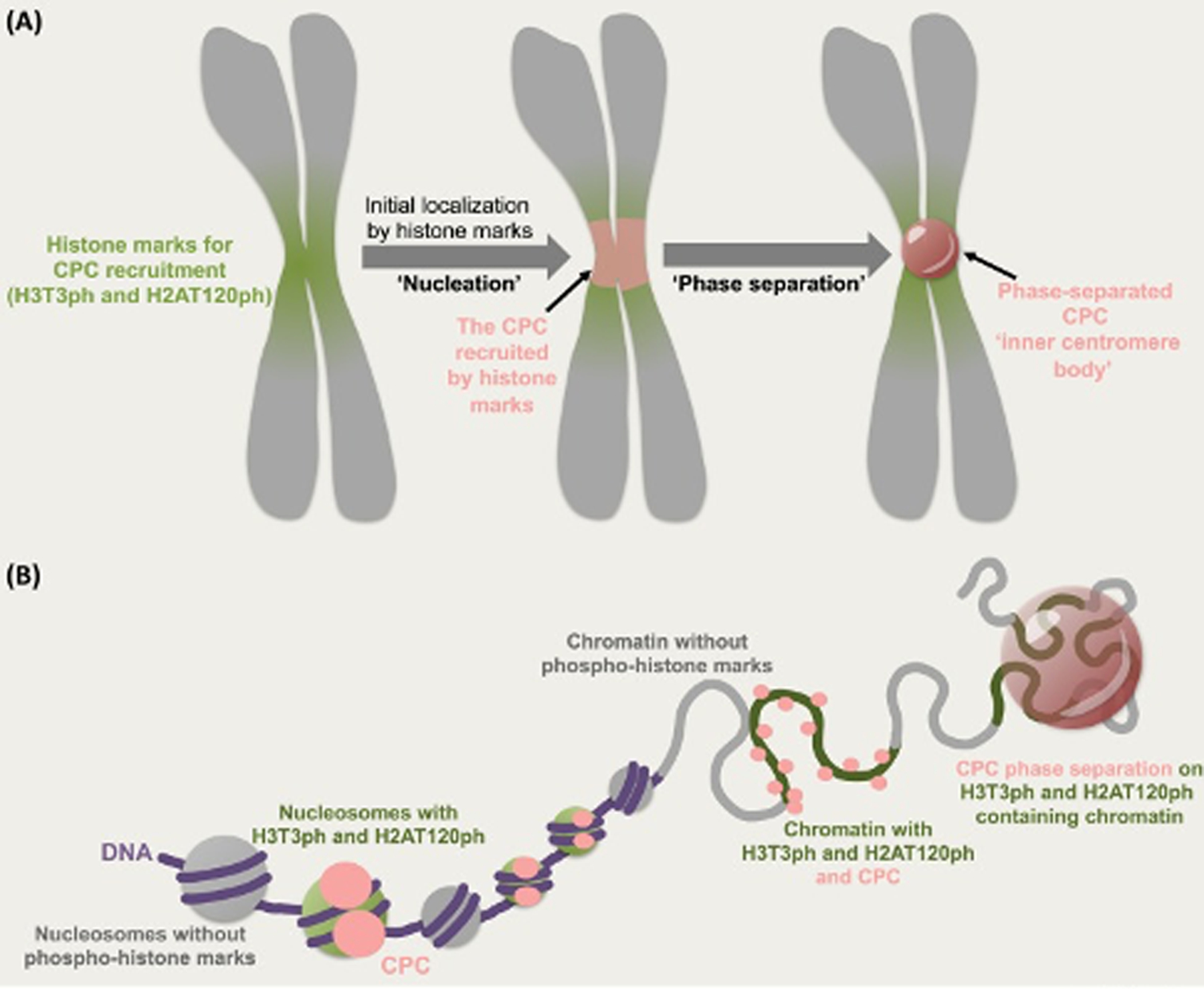
(A) The histone post-translational modifications (H3 pThr3 and H2A pThr120) initially recruit the CPC to the inner centromere during early mitosis. The initial enrichment of the CPC to the inner centromere leads to the “nucleation” of the CPC condensate. The local concentration of the CPC by histone marks and the presence of inner centromeric components allow the CPC phase separation to occur at the inner centromere. (B) This process can play a role in the organization of the inner centromeric chromatin since the nucleosomes/chromatin containing the phospho-histone marks (green circle /green line) will be preferentially incorporated in the CPC condensate (pink droplet). Grey represents chromatin, green represents chromatin containing H3 pThr3 and H2A pThr120 modifications, the CPC is shown as pink circle, and DNA is shown in purple.
There are additional activities that promote or inhibit the CPC phase separation at the inner centromere. We identified both protein and nucleic acid components at inner centromeres and mitotic post-translational modifications can drive condensate formation in vitro [22]. Specifically, the inner centromere components such as DNA or chromatin, phosphorylated HP1α, and Cdk1 phosphorylation on the CPC, all lower the concentration at which the CPC phase separates in vitro [22]. Of these inner centromere components phosphorylated HP1α is enriched on the inner centromeres and also has the biggest effect on enhancing the CPC phase separation in vitro [22]. The presence of multiple activities that promote CPC phase separation at the inner centromere makes it an ideal location for CPC phase separation during mitosis (Figure 1A, B). Since phase separation is driven by weak multivalent interactions between components [1, 4, 23, 32], DNA/chromatin and pHP1α are likely to participate as one of the multivalent interactors that drive LLPS. Recently, the Borealin subunit was shown to bind chromatin through the region that is close to the one that drives phase separation [33]. Also, a DNA binding motif was identified on the N-terminal region of INCENP [34]. Further evaluation of the impact that interactions between the CPC and other inner centromere components, and modified chromatin has on the phase behavior of the CPC will be important to understand mechanisms of inner centromere assembly.
Many interphase membraneless organelles are dissolved in mitosis and the dissolution of liquid demixed bodies is at least in part driven by the DYRK3 kinase that can phosphorylate S/TP sites in TY motifs that drive phase separation of the intrinsically disordered regions of many of the interphase scaffolded proteins [35]. Thus, the principles that generate the CPC condensate at the inner centromere in mitosis may have different rules than most of the phase-separated organelles. For example, TY motifs were not found in a region of Borealin required for phase separation in vitro and in vivo. Moreover, CDK1, which phosphorylates a similar motif as DYRK3, enhances (rather than inhibits) phase separation of the CPC in vitro [22].
Growing evidence suggests that the mediators of pericentric LLPS switch during the transition from interphase to mitosis. The heterochromatin regulator HP1α mediates LLPS in interphase heterochromatin [35, 36]. In interphase, HP1 directly binds chromatin through its interaction with K9me2/3 on histone H3. In G2/prophase, the CPC is recruited to heterochromatin region where it is activated [39, 40] [43] and phosphorylates histone H3 on Ser10, which hides the mark recruiting HP1. However, in mitosis direct interaction of the CPC with HP1 allows HP1 to accumulate inner centromeres [41, 42], where it promotes CPC condensate formation at the pericentric region. These series of events would thus lead to a switch of key LLPS mediators at pericentric chromatin from HP1 in interphase to the CPC in mitosis.
How inner centromere condensates are rapidly disassembled at the end of mitosis is an important area of future research. There is strong precedent for the rapid disassembly of LLPS organelles at the entry of mitosis so the same principles, such as phosphoregulation[35], may drive the disassembly of the inner centromere. Much of the CPC is removed from centromeres after chromosomes align in non-transformed cells and the remaining CPC is removed from the inner centromere at the metaphase to anaphase transition [13, 36]. The dephosphorylation of the histone marks that recruit the CPC is the simplest model, but there are inconsistencies between the dynamics of reduction in the histone marks and removal of the CPC from the inner centromere. In fact, the level of CPC at the inner centromere is reduced before the H3 pThr3 marks are removed [37] and variable data exist for the dynamics of H2a pThr120 and Sgo1 at the centromere during anaphase [37, 38]. Thus, the CPC may be actively removed from the inner centromere even when the marks for its localization are present. ATP dependent processes are required to remove the CPC from chromatin at the metaphase to anaphase transition so it may take energy to rapidly disassemble membraneless organelles. The kinesin motor MKLP2 competes with a DNA binding site on INCENP to displace the CPC from chromatin and the ATP dependent motor activity then move it to a central region of midzone microtubules [34, 39]. Apart from Mklp2, the ubiquitin ligase Cul3-KLHL21 [40], KLHL9 [41], and KLHL13 [41] are also involved in extraction of the CPC from the inner centromere the exact mechanism for how these proteins work in extraction of the CPC is unclear. Interestingly, Ubiquitin-binding proteins UBASH3B interacts with ubiquitinated CPC during metaphase and targets the CPC for extraction by allowing its interaction with Mklp2. The VCP/Cdc48/p97 AAA-ATPase, which usually plays a role in extracting ubiquitinated proteins from chromatin, has also been proposed to extract CPC from the chromatin during mitosis [42, 43]. However, it is unclear if VCP extracts the CPC at the beginning of anaphase or if its function is restricted to earlier stages of mitosis. Interestingly, VCP has been previously implicated in disassembly of stress granules [44, 45], another phase-separated structure. It will be important to determine if the cell needs to employ these ATPases to physically break apart liquid demixed condensates at the end of mitosis. It will also be important to identify the relative contributions of ATP motors and post-translational modifications of the LLPS driving motifs. For example, CPC phase separation is promoted by Cdk1 activity [22], suggesting the reduction in Cdk1 activity during the anaphase onset would contribute to condensate dissolution. Cdk1 activity also negatively controls MKLP2-INCENP[46] interaction and thus changes to phosphorylation may not only dissolve CPC droplets but also allow its extraction and transport to midzones. Finally, we point out that it is also possible that the CPC condensates are not completely disassembled at the beginning of anaphase, but instead transported as smaller chunks/droplets of condensates onto midzone microtubules by the microtubule motor activity of MKLP2.
Control of Inner Centromere Activities
The inner centromere is the site for at least three biochemical reactions that are essential during mitosis - Aurora-B kinase activation, kinetochore-microtubule error correction, and cohesion protection. The general principles of LLPS biology such as the ability of the condensates to act as reaction crucibles by concentrating enzymes and substrates [47] or allowing weak protein-protein interactions to become physiologically relevant is likely to have significant impact on this inner centromere biology. Below, we will explore how CPC phase separation suggests new models for these key functions of the inner centromeres.
Aurora B Kinase Activation
Cells often initiate signaling events by concentrating kinases that can phosphorylate their own T-loops and other activating sites in trans. Aurora kinases are such autoactivating kinases[48, 49]. It is likely that the phase separation property of the CPC enhances its autoactivation, since it highly concentrates the CPC at the inner centromere during mitosis, and at the midzone during anaphase and thus could facilitate the autoactivation reaction [22]. Moreover, phase separation is a mechanism to buffer the concentration of proteins, which can normalize the amount of signal that would be generated from a coacervate [50]. Spatial information may even emerge from these concentrated signaling centers as the CPC forms activity gradients emanating from inner centromere during mitosis and from the midzone during anaphase [51, 52]. CPC activity gradients could translate into different levels of substrate phosphorylation at distinct location along the gradient, thus providing spatial information for mitotic processes such as error correction and cytokinesis. The co-occurrence of autoactivating kinases and phase separating properties in a protein complexes is emerging as a common mechanism to initiate intracellular signaling events as it may also be used by Plk4 and Aurora-A at centrosomes and spindle poles [53, 54].
The phase separation of the CPC can also add other forms of previously unappreciated complex regulation to the CPC activation reaction. For example, the CPC phase can enrich or exclude proteins that allosterically activate or repress CPC activity. So far, recruitment of only activators such as chromatin and tubulin/MTs to the CPC condensates have been observed [22]. An added layer of complexity can come from the material property of the CPC condensate, which exist in a gel-like state in vitro [22], although posttranslational modifications such as phosphorylation may alter the material state of the CPC condensate and make it more liquid-like or solid-like. The change in material property of the CPC condensate can in theory lead to altered activation kinetics due to changes in diffusion characteristics of the constituent proteins within the condensate or due to other allosteric effects. Thus, reconstitution of the CPC activation reaction that occurs at the inner centromere may now be possible in the CPC condensates and will allow systematic understanding of this complex process.
Kinetochore-Microtubule Error Correction
Improper kinetochore-microtubule (KT-MT) attachments are often a source of aneuploidy and may underlie chromosomal instability in cancer [55]. KT-MT attachment stability is a function of Aurora B and phosphatase activity at the kinetochores. Aurora B destabilizes improper KT-MT attachments [56], mainly by phosphorylating the key microtubule-binding protein, Ndc80, at the kinetochore and reducing its affinity for microtubules [57–59]. Apart from Ndc80 phosphorylation, KT-MT attachment error correction may also involve other events under the regulation of Aurora B activity such as localization of microtubule depolymerizing kinesin MCAK to the centromeres [60, 61]. As mentioned previously, the maximum amount of the CPC is localized to the inner centromere; however, the key substrates for error correction are localized ~100s of nanometers away at the kinetochore. How the CPC phosphorylates the kinetochore substrates is thus an important area of research.
It has been suggested that the CPC at the inner centromere directly phosphorylates kinetochore substrates to drive error correction; however, mounting evidence from multiple model systems suggests that the CPC that is not present at the inner centromere directly phosphorylates the kinetochore substrates. Two mutually non-exclusive models have been proposed for how this might occur (Figure 2). In the first model, three independent pools of CPC are suggested to exist at the inner centromere, at the inner kinetochore, and at the kinetochore [62, 63] (Figure 2, Model 1). The CPC pool at kinetochores is suggested to directly bind an unknown kinetochore protein, becoming activated there to phosphorylate kinetochore substrates [64]. In yeast, the CCAN complex is proposed to recruit the CPC to the inner-kinetochore [65, 66]; whether CCAN is also the receptor for the CPC in mammals is unclear. How such a mechanism allows destabilization specifically of the improperly attached kinetochore is also unclear. In a second model, the kinase becomes activated in the inner centromere, but then diffuse to kinetochores and phosphorylates substrates [49, 51]. It was suggested that soluble phosphatase and the activated CPC form a bi-stable system that limits the extent to which the diffusing CPC can remain active [49]. The kinetochore substrates are pulled out of the area of phosphorylation by the forces generated by properly attached microtubules. Microtubules are also suggested to play a role in allowing CPC to phosphorylate kinetochore substrates [67, 68]. Recently, we proposed a variation on the second model [69] based on the fact that the CPC has at least two microtubule-binding activities that are required for the error-correction process and specifically for kinetochore phosphorylation during prometaphase [67, 69, 70]. In this model, the inner centromere proximal microtubules (present during lateral kinetochore attachment and at merotelic attachments but absent at properly attached kinetochores) provide conduits that effectively direct the diffusion of the CPC along microtubules to one-dimension and allowing the activated CPC to reach kinetochores before it is inactivated by phosphatases.
Figure 2: Current models for kinetochore substrate phosphorylation by the CPC.
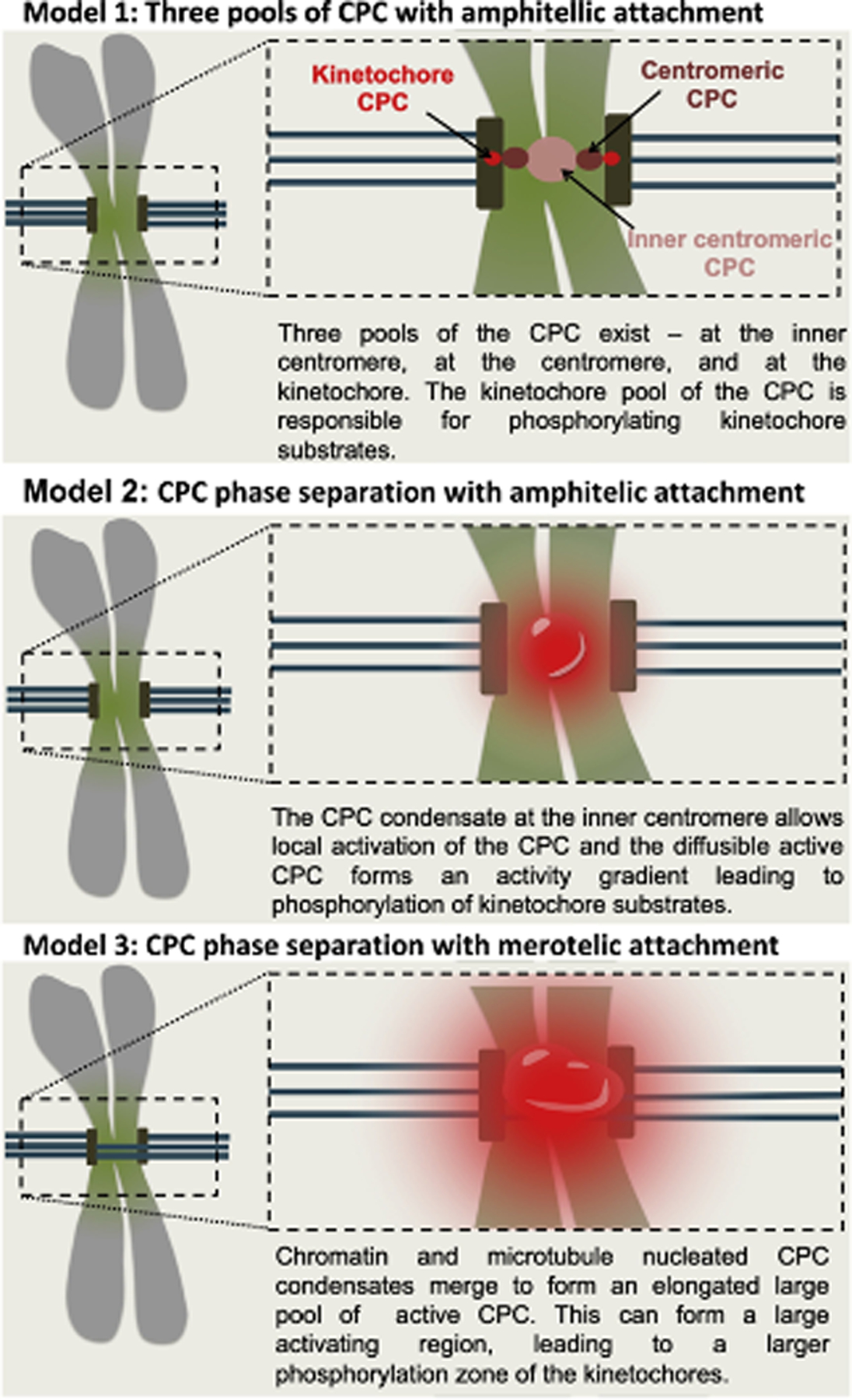
In Model 1, three independent pools of the CPC exist at the centromere and kinetochore region. Kinetochore substrate phosphorylation is mediated by the kinetochore bound CPC pool. In Model 2, the CPC in the inner centromere acts as an activation pool that activates the diffusible CPC molecules. This creates a gradient of active CPC molecules (red haze) emanating from the inner centromere. The diffusible active CPC molecules eventually phosphorylate the kinetochore substrates. In Model 3, at the merotelic attachment, the CPC condensates nucleated at the inner centromere and the chromatin proximal microtubules fuse. This leads to an expansion of the activating pool of the CPC, leading to a larger volume of active CPC, which destabilizes kinetochore-microtubule attachments through phosphorylation of kinetochore substrates. Mitotic chromosome is in grey, chromatin with histone H3 pThr3 and H2A pThr120 is in light green, microtubules are depicted as dark green bars, kinetochores are shown as brown vertical rectangle.
Cells expressing a mutant of the CPC that is deficient in phase separation generate lagging chromosomes suggesting they are deficient at kinetochore-microtubule error correction [22]. We speculate that the CPC phase separation can aid in the error correction process in multiple ways. First, the CPC phase separation at the inner centromere provides a mechanism to concentrate large amounts of CPC, thus allowing a robust activating pool to exist at each inner centromere (Figure 2, Model 2). This mechanism will affect models of error correction that rely on centromeric CPC pool for error correction (Figure 2, Models 2 and 3). Second, since both chromatin and microtubules promote nucleation of the CPC condensates in vitro. It is possible that under situations such as merotelic attachments, where microtubule and the inner centromeric chromatin are near each other, the CPC condensates nucleated at the inner centromere and on microtubules fuse leading to a larger condensate in the inner centromeric region. This larger activating pool of inner centromere proximal pool of the CPC at the merotelic kinetochores would lead to hyperphosphorylation and destabilization of the merotelic KT-MT attachments (Figure 2, Model 3). Such enrichment of the CPC at the inner-centromeres of the merotelically attached kinetochores has been observed in Xenopus cells (69). Third, the microtubules of a merotelically attached kinetochores lie across the inner centromeres and thus physically contact the organelle. We suggest that these microtubules might act as tracks that connect the inner centromere and enable rapid diffusion of CPC to the improperly attached regions of the kinetochore. Thus, a CPC condensate could essentially “flow” on the microtubule tracks from its activating region directly to the improperly attached kinetochore as a mechanism to distinguish improper from correct attachments. Since the CPC condensates undergo gelation very quickly on chromatin, the material property of the CPC must be altered to a more liquid like state locally on microtubules to allow “flow”. Interestingly, apart from forming droplets on microtubules the CPC can also coat or “wet” microtubules under conditions of high relative CPC concentration indicating that microtubules drive CPC condensates from gels to liquids (22,77).
Cohesion Protection
To sort chromatids during segregation, it is critical to protect cohesion of sister chromatids until all of the chromosomes are bipolarly attached to the spindle microtubules. This is accomplished by the cohesin complex, which forms rings that topologically entrap the chromatin of two sister chromatids [71]. Cohesins also play an important function in organizing chromatin domains during interphase [72–74] and most of the cohesins are removed early in mitosis when mitotic phosphorylation drives ring opening to release cohesin molecules [75–77]. A small pool of cohesins remains on the chromatids and most of this pool is in the inner centromere, which underlies the classic X-shaped chromosomes visualized in karyotypes. Cohesion protection is mediated by the Sgo1 protein, which recruits PP2A phosphatase to counter phosphorylation-dependent opening of the cohesin ring [38, 78–81]. The CPC plays a central role in cohesion protection at the inner centromere; however, the mechanism behind this is poorly understood [6, 82, 83]. The CPC has two roles in cohesion protection. First, Aurora B kinase activity is required for cohesion protection [84, 85]. Second, the CPC has a kinase-independent role in cohesion protection through its inner centromere localization region that also drives phase separation [82]. This role in cohesion protection is uncovered when microtubules pulling forces on kinetochores are present. We can envision three mechanisms by which phase separation could mediate the CPC’s protection of cohesion. First, the CPC phase separation could enable robust Sgo1 recruitment to the inner centromere. Second, phase separation could enable increased Aurora B kinase activity to phosphorylate an unknown substrate required for cohesion protection. Third, the CPC phase separation could act in parallel with cohesins to provide mechanical stability to chromatin, enabling it to counter the pulling forces of microtubules on kinetochores.
Current models suggest that Sgo1 is recruited to inner centromeres by three mechanisms, first the Bub1 kinase phosphorylates histone H2a on T120, which is directly bound by the Sgo1 protein [30]. Second, Sgo1 can directly bind cohesion [86]. Third, Aurora B recruits Bub1 to kinetochores [11], and it is possible that the CPC plays only an indirect role in Sgo1 localization [29–31, 87, 88], however Sgo1 may be recruited through a direct binding to the CPC [30]. The latter interaction has been poorly characterized because the binding between the CPC and Sgo1 is very weak and difficult to measure (requiring cross-linkers to detect it in cytoplasm). However, the interaction between the CPC and N-terminus of Sgo1 happens robustly in the CPC condensates as mentioned earlier [22]. The CPC condensation can thus influence all of these mechanisms, since it can potentially enhance the kinase activity as well as allow weak interactions to become robust.
A key function of the inner centromere is that it must act as a spring that can counter the pulling forces of microtubules on kinetochores [8, 89]. The condensin complex, which like cohesin has an ATP dependent chromatin loop extruding activity, plays an important role in this activity, suggesting that assembly of inner centromere chromatin is central to this activity [8, 90–93]. Cohesin is required but it is unclear if this is only a requirement to cohere sisters or if cohesins additionally contribute to the chromatin structure. Computational studies in yeast have suggested exciting mechanisms for condensin and cohesin proteins to fold and intertwine chromatins into structures that can withstand pulling forces [91]. The CPC condensates mature into gels [22], suggesting an additional mechanism that can contribute to the emergent property of the chromatin that can counter the pulling forces (Figure 3). Specifically, the assembly of a gel around centromeric chromatin would add mechanical stability because forces would need to pull chromatin strands through the gel (Figure 3). This model is consistent with the demonstration that the part of the CPC that drives phase separation has a role in cohesion protection [22, 82]. Interestingly, chromosomes had to be under pulling forces to uncover this role, which is consistent with a role for the gel in cohesion protection. If true then the dissolution of the inner centromere condensate might be a requirement for the anaphase segregation of sisters in conjunction with the cleavage of cohesion by separase.
Figure 3: Effect of CPC phase separation on centromere cohesion protection.

In the absence of CPC phase separation, the centromeric and inner centromeric chromatin under pulling forces (blue arrows) from the spindle microtubules (dark green bars) will get stretched. The cohesin molecules will bear most of the pulling forces, leading to weakening of the centromeric cohesion and an increase in the inter-centromere distance (red bar). In the presence of CPC phase separation and gelation at the inner centromere, the CPC condensate will undergo gelation on the chromatin and cohesins, leading to their entrapment in the gel. The CPC gel will provide mechanical stability to the inner centromere and reduce the strain on the cohesin molecules. This will lead to the preservation of centromeric cohesion and reduced distance between sister centromeres.
Concluding Remarks
The number of processes that involve LLPS is rapidly expanding and the next challenge is to understand its role in each process. LLPS maybe especially relevant for organizing events during mitosis when most membrane-bound organelles are disassembled. As discussed in this article, the LLPS property of the CPC generates new models for mitotic events and has a profound impact on our understanding of the inner centromere function. Understanding how the CPC condensates enable various mitotic functions and how the assembly and disassembly of CPC condensates are regulated during mitosis are some of the key avenues for future research. It is also important to identify the principles that drive phase separation in mitosis as they are likely to be different from those that drive LLPS in interphase since these are broken down in mitosis. Spatiotemporally reproducible assembly and disassembly of the CPC condensates in a matter of minutes during mitosis combined with the plethora of structural information available for the CPC makes it an ideal system to uncover principles generally applicable to chromatin-bound condensates.
Outstanding Questions:
What is the effect of phase separation on kinase activity of the CPC?
What are the regulators of the CPC phase behavior during mitosis?
Does CPC phase separation and gelation alter the material property of the inner centromeric chromatin?
Does the CPC form gels around chromatin in vivo and are their mechanisms that enable the CPC condensates to transition between gels and liquids?
What regulates the material property of the CPC during mitosis?
Does CPC phase separation play a role in cohesion protection?
How does CPC phase separation impact the CPC signaling during mitosis?
How is the inner centromeric CPC condensate disassembled at anaphase onset?
Highlights:
The CPC undergoes phase separation in-vitro and in-vivo.
Histone phosphorylation specifies the site for the CPC phase separation during mitosis.
Inner centromeric components and mitotic post translational modifications promote CPC phase separation.
The CPC phase separation ensures faithful mitosis possibly through multiple mechanisms.
Acknowledgements:
P.T and P.T.S were funded by R01GM124042. We thank D. Burke, S. Mattada and E. Niedzialkowska for insightful discussions.
Glossary:
- Centrosomes:
Major microtubule nucleating and organizing organelle in mitosis and interphase.
- Centromeres:
Specialized chromosome locus on which the kinetochore assembles.
- Kinetochores:
Multi-protein structure assembled on centromeres that serve as the site for attachment of spindle microtubules with mitotic chromosomes.
- Microtubule pulling forces:
Force exerted on the kinetochore/centromere by depolymerization of kinetochore attached spindle microtubules.
- Scaffold protein:
A protein that simultaneously binds multiple distinct proteins.
- CPC condensates:
Phase separated assembly made of chromosome passenger complex (CPC).
- Histone marks:
Post-translation modifications on histones.
- Landing marks:
Phosphorylated histone marks (H3 pThr3 and/or H2A pThr120) that specify CPC localization site. Survivin subunit of the CPC directly binds H3 pThr3 and Borealin subunit of the CPC indirectly binds H2A pThr120.
- Stress granule:
Protein and RNA assemblies formed in the cytosol under the condition of cellular stress.
- Constitutive Centromere Associated Network (CCAN) complex:
Protein complex assembled on CENP-A nucleosomes and serves as a base on which outer kinetochore is assembled during mitosis.
- Amphitelic attachment:
A configuration of kinetochore-microtubule attachment where each sister kinetochore is attached to microtubules emanating from a single and opposite pole.
- Merotellic attachment:
A configuration of erroneous kinetochore-microtubule attachment where a single sister kinetochore is attached to microtubules emanating from both spindle poles.
References
- 1.Banani SF et al. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18 (5), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter H and Brooks DE (1995) Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett 361 (2–3), 135–9. [DOI] [PubMed] [Google Scholar]
- 3.Stroberg W and Schnell S (2018) Do Cellular Condensates Accelerate Biochemical Reactions? Lessons from Microdroplet Chemistry. Biophys J 115 (1), 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman AA et al. (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- 5.Tiwary AK and Zheng Y (2019) Protein phase separation in mitosis. Curr Opin Cell Biol 60, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi P and Stukenberg PT (2016) A Centromere-Signaling Network Underlies the Coordination among Mitotic Events. Trends Biochem Sci 41 (2), 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister K et al. (2018) Identification of Drivers of Aneuploidy in Breast Tumors. Cell Rep 23 (9), 2758–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom KS (2014) Centromeric heterochromatin: the primordial segregation machine. Annu Rev Genet 48, 457–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaqaman K et al. (2010) Kinetochore alignment within the metaphase plate is regulated by centromere stiffness and microtubule depolymerases. J Cell Biol 188 (5), 665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindriksen S et al. (2017) The Ins and Outs of Aurora B Inner Centromere Localization. Front Cell Dev Biol 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauf S et al. (2003) The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161 (2), 281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallio MJ et al. (2002) Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol 12 (11), 900–5. [DOI] [PubMed] [Google Scholar]
- 13.Cooke CA et al. (1987) The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol 105 (5), 2053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams RR et al. (2000) INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol 10 (17), 1075–8. [DOI] [PubMed] [Google Scholar]
- 15.Kaitna S et al. (2000) Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol 10 (19), 1172–81. [DOI] [PubMed] [Google Scholar]
- 16.Gassmann R et al. (2004) Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol 166 (2), 179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolton MA et al. (2002) Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell 13 (9), 3064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeyaprakash AA et al. (2007) Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell 131 (2), 271–85. [DOI] [PubMed] [Google Scholar]
- 19.Honda R et al. (2003) Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell 14 (8), 3325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahen R et al. (2014) Comparative assessment of fluorescent transgene methods for quantitative imaging in human cells. Mol Biol Cell 25 (22), 3610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wachsmuth M et al. (2015) High-throughput fluorescence correlation spectroscopy enables analysis of proteome dynamics in living cells. Nat Biotechnol 33 (4), 384–9. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi P et al. (2019) The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat Cell Biol 21 (9), 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler RJ and Hyman AA (2018) Controlling compartmentalization by non-membrane-bound organelles. Philos Trans R Soc Lond B Biol Sci 373 (1747):20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamagishi Y et al. (2010) Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330 (6001), 239–43. [DOI] [PubMed] [Google Scholar]
- 25.Wang F et al. (2010) Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330 (6001), 231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedzialkowska E et al. (2012) Molecular basis for phosphospecific recognition of histone H3 tails by Survivin paralogues at inner centromeres. Mol Biol Cell 23 (8), 1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly AE et al. (2010) Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330 (6001), 235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J et al. (2012) Structural basis for recognition of H3T3ph and Smac/DIABLO N-terminal peptides by human Survivin. Structure 20 (1), 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawashima SA et al. (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327 (5962), 172–7. [DOI] [PubMed] [Google Scholar]
- 30.Tsukahara T et al. (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467 (7316), 719–23. [DOI] [PubMed] [Google Scholar]
- 31.Ricke RM et al. (2012) Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol 199 (6), 931–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XH et al. (2018) Function and Regulation of Phase-Separated Biological Condensates. Biochemistry 57 (17), 2452–2461. [DOI] [PubMed] [Google Scholar]
- 33.Abad MA et al. (2019) Borealin-nucleosome interaction secures chromosome association of the chromosomal passenger complex. J Cell Biol 218 (12), 3912–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serena M et al. (2020) Molecular basis of MKLP2-dependent Aurora B transport from chromatin to the anaphase central spindle. J Cell Biol 219 (7), e201910059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rai AK et al. (2018) Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 559 (7713), 211–216. [DOI] [PubMed] [Google Scholar]
- 36.Adams RR et al. (2001) Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol 11 (2), 49–54. [DOI] [PubMed] [Google Scholar]
- 37.van der Horst A et al. (2015) Inter-domain Cooperation in INCENP Promotes Aurora B Relocation from Centromeres to Microtubules. Cell Rep 12 (3), 380–7. [DOI] [PubMed] [Google Scholar]
- 38.Liu H et al. (2013) Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol 15 (1), 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krupina K et al. (2016) Ubiquitin Receptor Protein UBASH3B Drives Aurora B Recruitment to Mitotic Microtubules. Dev Cell 36 (1), 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maerki S et al. (2009) The Cul3-KLHL21 E3 ubiquitin ligase targets aurora B to midzone microtubules in anaphase and is required for cytokinesis. J Cell Biol 187 (6), 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumara I et al. (2007) A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell 12 (6), 887–900. [DOI] [PubMed] [Google Scholar]
- 42.Ramadan K et al. (2007) Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature 450 (7173), 1258–62. [DOI] [PubMed] [Google Scholar]
- 43.Dobrynin G et al. (2011) Cdc48/p97-Ufd1-Npl4 antagonizes Aurora B during chromosome segregation in HeLa cells. J Cell Sci 124 (Pt 9), 1571–80. [DOI] [PubMed] [Google Scholar]
- 44.Buchan JR et al. (2013) Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153 (7), 1461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turakhiya A et al. (2018) ZFAND1 Recruits p97 and the 26S Proteasome to Promote the Clearance of Arsenite-Induced Stress Granules. Mol Cell 70 (5), 906–919 e7. [DOI] [PubMed] [Google Scholar]
- 46.Hummer S and Mayer TU (2009) Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr Biol 19 (7), 607–12. [DOI] [PubMed] [Google Scholar]
- 47.Shin Y and Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357 (6357):eaaf4382. [DOI] [PubMed] [Google Scholar]
- 48.Sessa F et al. (2005) Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell 18 (3), 379–91. [DOI] [PubMed] [Google Scholar]
- 49.Zaytsev AV et al. (2016) Bistability of a coupled Aurora B kinase-phosphatase system in cell division. Elife 5, e10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klosin A et al. (2020) Phase separation provides a mechanism to reduce noise in cells. Science 367 (6476), 464–468. [DOI] [PubMed] [Google Scholar]
- 51.Wang E et al. (2011) Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. J Cell Biol 194 (4), 539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuller BG et al. (2008) Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453 (7198), 1132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JE et al. (2019) Phase separation of Polo-like kinase 4 by autoactivation and clustering drives centriole biogenesis. Nat Commun 10 (1), 4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y et al. (2018) Aurora A activation in mitosis promoted by BuGZ. J Cell Biol 217 (1), 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cimini D et al. (2001) Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol 153 (3), 517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cimini D et al. (2006) Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol 16 (17), 1711–8. [DOI] [PubMed] [Google Scholar]
- 57.DeLuca JG et al. (2006) Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127 (5), 969–82. [DOI] [PubMed] [Google Scholar]
- 58.Welburn JP et al. (2010) Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell 38 (3), 383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeLuca KF et al. (2011) Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci 124 (Pt 4), 622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lan W et al. (2004) Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol 14 (4), 273–86. [DOI] [PubMed] [Google Scholar]
- 61.Knowlton AL et al. (2006) Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol 16 (17), 1705–10. [DOI] [PubMed] [Google Scholar]
- 62.Hadders MA et al. (2020) Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis. J Cell Biol 219 (3):e201907087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broad AJ et al. (2020) Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J Cell Biol 219 (3):e201905144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krenn V and Musacchio A (2015) The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front Oncol 5, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischbock-Halwachs J et al. (2019) The COMA complex interacts with Cse4 and positions Sli15/Ipl1 at the budding yeast inner kinetochore. Elife 8:e42879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Rodriguez LJ et al. (2019) Aurora B-INCENP Localization at Centromeres/Inner Kinetochores Is Required for Chromosome Bi-orientation in Budding Yeast. Curr Biol 29 (9), 1536–1544 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheelock MS et al. (2017) Dual recognition of chromatin and microtubules by INCENP is important for mitotic progression. J Cell Biol 216 (4), 925–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banerjee B et al. (2014) EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B. J Cell Biol 204 (6), 947–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trivedi P et al. (2019) The binding of Borealin to microtubules underlies a tension independent kinetochore-microtubule error correction pathway. Nat Commun 10 (1), 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samejima K et al. (2015) The Inner Centromere Protein (INCENP) Coil Is a Single alpha-Helix (SAH) Domain That Binds Directly to Microtubules and Is Important for Chromosome Passenger Complex (CPC) Localization and Function in Mitosis. J Biol Chem 290 (35), 21460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasmyth K and Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43, 525–58. [DOI] [PubMed] [Google Scholar]
- 72.Schwarzer W et al. (2017) Two independent modes of chromatin organization revealed by cohesin removal. Nature 551 (7678), 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davidson IF et al. (2019) DNA loop extrusion by human cohesin. Science 366 (6471), 1338–1345. [DOI] [PubMed] [Google Scholar]
- 74.Tedeschi A et al. (2013) Wapl is an essential regulator of chromatin structure and chromosome segregation. Nature 501 (7468), 564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waizenegger IC et al. (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103 (3), 399–410. [DOI] [PubMed] [Google Scholar]
- 76.Nishiyama T et al. (2013) Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc Natl Acad Sci U S A 110 (33), 13404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hauf S et al. (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol 3 (3), e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Z et al. (2006) PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell 10 (5), 575–85. [DOI] [PubMed] [Google Scholar]
- 79.McGuinness BE et al. (2005) Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol 3 (3), e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitajima TS et al. (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441 (7089), 46–52. [DOI] [PubMed] [Google Scholar]
- 81.Shintomi K and Hirano T (2009) Releasing cohesin from chromosome arms in early mitosis: opposing actions of Wapl-Pds5 and Sgo1. Genes Dev 23 (18), 2224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hengeveld RCC et al. (2017) Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat Commun 8, 15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Resnick TD et al. (2006) INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev Cell 11 (1), 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawashima SA et al. (2007) Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev 21 (4), 420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanno Y et al. (2010) Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev 24 (19), 2169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu H et al. (2015) Mitotic Transcription Installs Sgo1 at Centromeres to Coordinate Chromosome Segregation. Mol Cell 59 (3), 426–36. [DOI] [PubMed] [Google Scholar]
- 87.Saurin AT et al. (2011) Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun 2, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Waal MS et al. (2012) Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep 13 (9), 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harasymiw LA et al. (2019) Centromere mechanical maturation during mammalian cell mitosis. Nat Commun 10 (1), 1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bloom K and Costanzo V (2017) Centromere Structure and Function. Prog Mol Subcell Biol 56, 515–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stephens AD et al. (2011) Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring. J Cell Biol 193 (7), 1167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribeiro SA et al. (2009) Condensin regulates the stiffness of vertebrate centromeres. Mol Biol Cell 20 (9), 2371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yong-Gonzalez V et al. (2007) Condensin function at centromere chromatin facilitates proper kinetochore tension and ensures correct mitotic segregation of sister chromatids. Genes Cells 12 (9), 1075–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


