Abstract
Aim:
To describe barriers to implementation of diabetic retinopathy (DR) teleretinal screening programs and artificial intelligence (AI) integration at the University of California (UC).
Methods:
Institutional representatives from UC Los Angeles, San Diego, San Francisco, Irvine, and Davis were surveyed for the year of their program's initiation, active status at the time of survey (December 2021), number of primary care clinics involved, screening image quality, types of eye providers, image interpretation turnaround time, and billing codes used. Representatives were asked to rate perceptions toward barriers to teleretinal DR screening and AI implementation using a 5-point Likert scale.
Results:
Four UC campuses had active DR teleretinal screening programs at the time of survey and screened between 246 and 2,123 patients at 1–6 clinics per campus. Sites reported variation between poor-quality photos (<5% to 15%) and average image interpretation time (1–5 days). Patient education, resource availability, and infrastructural support were identified as barriers to DR teleretinal screening. Cost and integration into existing technology infrastructures were identified as barriers to AI integration in DR screening.
Conclusions:
Despite the potential to increase access to care, there remain several barriers to widespread implementation of DR teleretinal screening. More research is needed to develop best practices to overcome these barriers.
Keywords: artificial intelligence, diabetic retinopathy, telemedicine, informatics, diabetes screening
Introduction
Diabetes is a global epidemic,1 with prevalence estimated to increase from 537 to 783 million adults worldwide between 2021 and 2045.2 Diabetic retinopathy (DR), a common complication of diabetes, is a leading cause of preventable blindness among adults, and its prevalence is expected to increase to 160 million by 2045.3 To address this growing burden, the American Academy of Ophthalmology and American Diabetes Association have recommended diabetes patients undergo annual eye examinations for DR screening.4,5
Despite incorporating DR screening recommendations into quality metrics within the federally supported Merit-based Incentive Payment System (MIPS),6 real-world screening adherence has remained low, ranging from 20% to 60%.7,8 Reasons for these gaps include limited health care access, transportation, cost, education on screening importance, and anxiety about diabetic complications.9 Furthermore, minority status, older age, complex medical comorbidities, feasibility of DR screening,10 diagnostic accuracy,11 and local disease prevalence12 have been shown to complicate access to care. To address these barriers, DR teleretinal screening programs have been previously implemented across several single-center institutions and have demonstrated real-world efficacy in increasing access to care.7,10,11,13
However, a recent study by Thomas et al. identified that despite the early success of several telemedicine programs, addressing issues such as infrastructural support and technology implementation were necessary to ensure long-term sustainability, especially in light of the recent COVID-19 pandemic.14 Within DR telemedicine literature, there is a paucity of studies describing implementation considerations of teleretinal systems for DR.13,15–17 Recent approvals of autonomous artificial intelligence (AI) systems for DR diagnosis such as IDx-DR18,19 and EyeNuk19 have gained U.S. Food and Drug Administration (FDA) approval and represent further opportunities to improve cost-effectiveness and increase patient impact of telemedicine systems.20 However, literature addressing successful integration of autonomous AI systems in DR teleretinal screening has been largely lacking.21,22
We aimed to address these gaps by (1) analyzing variations in implementation of teleretinal DR screening programs across a large multipayer and multi-institutional system at the University of California (UC), and (2) assessing barriers and institutional perceptions of AI integration for DR screening.
Subjects, Materials, and Methods
This study adhered to the Declaration of Helsinki and was approved by the UC San Diego (UCSD) institutional review board; informed consent was waived. We surveyed 1–2 institutional representatives regarding DR screening program implementation from the following campuses: UCSD, UC San Francisco (UCSF), UC Los Angeles (UCLA), UC Irvine (UCI), and UC Davis (UCD). Representatives consisted of primary care physicians (PCPs), endocrinologists, or ophthalmologists leading teleretinal DR screening programs.
We employed a mixed methods approach to gather quantitative and qualitative responses. Each representative provided data regarding operational details of their screening programs with numeric (e.g., number of patients seen) and categorical responses (e.g., billing codes). We asked for the year of program initiation, active status at the time of survey (December 2021), number of primary care clinics involved in each program, screening image quality, types of eye care providers, turnaround time for image interpretation, billing codes used, and financial sustainability of the screening program.
Five-point Likert items (1 = strongly disagree, 2 = somewhat disagree, 3 = neutral, 4 = somewhat agree, and 5 = strongly agree) were used to assess perceived barriers and facilitators to screening program implementation and perceptions of AI integration. Proportions of Likert scale responses were expressed as percentages, and mean Likert scale scores were reported. We also solicited free-text responses regarding conducive or prohibitive factors to implementing teleretinal screening programs, and the impact of the COVID-19 pandemic.
Additional qualitative information regarding DR screening program implementation was obtained from monthly meetings hosted by the UC Office of the President called “UC Diabetes Initiative: Medical Home subgroup.” These were standing monthly meetings of representatives from each of the participating UC sites to provide updates and share lessons learned during implementation. Qualitative comments from these meetings provided additional context.
STATISTICAL ANALYSES
Data were collected from December 2021 to February 2022 and were analyzed during March and April 2022. Descriptive analyses were performed for program characteristics. Formal hypothesis testing was not performed, as this was a descriptive study of variation in implementation among few sites. All data analyses were performed in R 4.0.5 (R Foundation, Vienna, Austria).
Results
TELERETINAL DR SCREENING PROGRAMS ACROSS THE UC
Table 1 describes teleretinal DR screening programs at each UC campus. The UCD program began in 2018 with a single fundus camera at a primary care clinic with electronic health records (EHRs)-based ordering and reporting. The program has since grown to six clinics with ∼1,000 patients screened (Table 1). The UCI program was initiated in 2010 by PCPs and employed a fundus camera at a primary care clinic. Imaging interpretation was performed by a neuro-ophthalmologist. However, due to poor reimbursements, the screening program was not financially sustainable and was terminated in 2015. The UCLA program was recently initiated in 2020 and had expanded its use of retinal cameras to both endocrinology and primary care clinics (six cameras in total).
Table 1.
Summary Information Regarding Teleretinal Diabetic Retinopathy Screening Programs Across Five University of California Sites According to Institutional Representative Responses (December 2021–February 2022)
| UCD | UCI | UCLA | UCSD | UCSF | |
|---|---|---|---|---|---|
| Year initiated | 2018 | 2010 (note: program ended in 2015) | 2020 | 2020 | 2012 |
| Current active status | Yes | No | Yes | Yes | Yes |
| Approximate number of patients screened to date | 1,000 | 700–1,000 | 1,178 | 500 | 2,123 |
| Number of eye care providers for image interpretation | 2–5 | 1 | 2 | 2 | 4 |
| Type of eye care providers | Optometrists and retina specialists | Other ophthalmic subspecialists | General ophthalmologists | Optometrists and general ophthalmologists | Optometrists and retina specialists |
| Number of primary care clinics with cameras | 6 | 1 | 3 | 5 | 5 |
| Percentage of poor-quality photos | <5% | 6–9% | 10–15% | 6–9% | <5% |
| Average turnaround time (days): imaging acquisition to interpretation | 2–3 | >5 | 4–5 | 1 | >5 |
UCD, University of California Davis; UCI, University of California Irvine; UCLA, University of California Los Angeles; UCSD, University of California San Diego; UCSF, University of California San Francisco.
The UCSD program began in 2020 and developed a new teleretinal screening program through information technology (IT) tools for automated billing, health maintenance completion, and image interpretation.23 Imaging acquisition to interpretation time was only 1 day compared with 2 or longer for other programs. The UCSF program was developed with the Zuckerberg San Francisco General Hospital to screen patients under the San Francisco Health Plan and also employed a mobile screening van with a mounted camera.12 The program had been operational for >10 years and had screened >2,000 patients. At the time of the study, a new teleretinal DR screening program with UCSF primary care clinics was being developed, but had not yet been implemented.
VARIATIONS IN BILLING
Current Procedural Terminology (CPT) codes utilized by PCPs and eye providers were reported. Because CPT 92227 and 92228 definitions changed on 10/2021 before this survey was released, the definitions for CPT codes were not collected. Two sites (UCD and UCSF) utilized 92227: “Imaging of retina for detection of disease; with remote clinical staff review and report,” originally intended for screening patients without DR. In 2021, the 92227 definition changed to “Imaging of retina for detection or monitoring of disease; with remote clinical staff review and report.”
Three sites (UCD, UCSD, and UCSF) utilized 92228: “Imaging of retina for detection or monitoring of disease; with remote physician or other qualified healthcare professional interpretation and report,” intended for detection or monitoring of diseases in patients with existing retinopathy, and requires an eye care provider to provide an interpretation/summary report with recommendations. In 10/2021, the 92228 definition was modified to be similar to 92227 with the exception that interpretation occurred by: “…remote physician or other qualified health care [professionals].”
Two sites (UCLA and UCSD) utilized CPT 92250: “Fundus photography with interpretation and report.” Although not listed under Medicare's telehealth list, 92250 has been appropriate under several commercial insurers. UCSD initially used 92250 in 2020 but switched to 92228 in 2021 when updated definitions for 92250 required that image collection by a physician or ophthalmology technician. This made primary care image collection, typically performed by a medical assistant or nurse, no longer compatible with billing for 92250. Despite changes in CPT definitions, perceptions of financial sustainability were mixed: half of representatives were unsure about financial sustainability, two (40%) felt their programs were financially sustainable, and one (10%) program reported their program was unsustainable.
FACILITATORS AND BARRIERS TO DR SCREENING IMPLEMENTATION
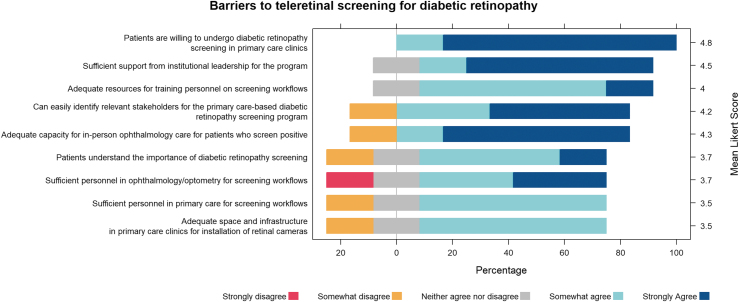
Institutional representatives felt optimistic about teleretinal screening implementation (All mean Likert scores >3; Fig. 1). They agreed (Likert score ≥4) that patients were willing to undergo DR screening in primary care clinics, they had adequate resources for training personnel on screening workflows, they had sufficient support from institutional leadership, could identify relevant stakeholders, and there was adequate capacity for in-person screening. Factors perceived as neutral to slightly positive (Likert score 3–4) included patients' understanding of the importance of screening, sufficient personnel for screening workflows, and adequate space in the clinic for fundus cameras. Subanalysis by ophthalmologists versus nonophthalmologists did not show remarkable differences in sentiment (Supplementary Fig. S1).
Fig. 1.
Barriers to screening for diabetic retinopathy. Institutional representatives of the University of California system and their perception of barriers to AI implementation. AI, artificial intelligence.
Qualitative free-text responses from representatives conveyed cost, limited staffing, reimbursement, and insurance variability as common barriers and having physician champions, support from leadership, physician financial incentives, and population health funding facilitating their teleretinal programs. The COVID-19 pandemic was generally felt to be disruptive, brought integration delays and staffing challenges, and furthered the backlog. However, one institution felt their teleretinal screening program accelerated due to the need to reduce in-person visits.
PERCEPTIONS OF AI INTEGRATION
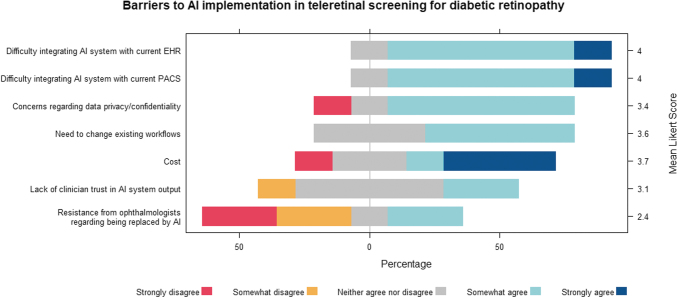
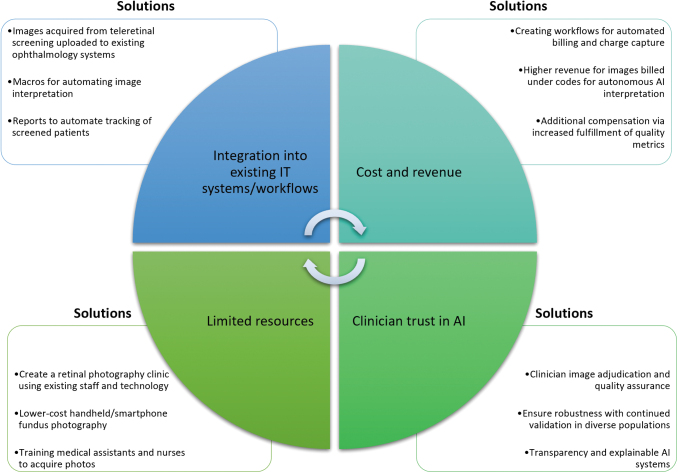
Eighty percent of representatives showed strong interest in AI integration with DR teleretinal screening (mean Likert score = 4). Despite interest, no UC sites had yet implemented any AI systems for DR screening. Representatives generally agreed (mean Likert scores = 3.4–4) that AI integration into EHR and current DR telemedicine systems such as eye Picture Archiving and Communication System (PACS), data privacy, changes existing workflows, and cost were barriers (Fig. 2). They were neutral regarding clinician trust in AI (mean Likert score = 3.1). Ophthalmologists felt more strongly that they would not be replaced by AI compared with non-ophthalmologists (mean Likert score = 1.3 vs. 3.5, respectively; Supplementary Fig. S2). Possible solutions addressing barriers to teleretinal screening implementation and AI integration are shown in Figure 3.
Fig. 2.
Interest in and barriers to AI in teleretinal screening for diabetic retinopathy. Institutional representatives of the University of California system overall expressed interest in integrating AI into teleretinal screening for diabetic retinopathy but expressed concerns regarding barriers to implementation. EHR, electronic health record; PACS, Picture Archiving and Communication System.
Fig. 3.
Barriers and proposed solutions for increasing access to teleretinal screening and AI implementation for DR. These solutions have been previously implemented among sites included in this analysis or proposed in the literature and may be useful for other institutions interested in creating a cost-effective and efficient teleretinal DR screening system. DR, diabetic retinopathy; IT, information technology.
Discussion
In this study, we described implementations of DR screening programs within the UC system and described barriers to DR screening and AI integration. This study has three key findings: (1) there was substantial variation in implementations of teleretinal DR screening systems, even among institutions within the same parent organization; (2) cost considerations and lack of infrastructure support were barriers to DR teleretinal implementation; and (3) despite strong interest, there remain several barriers to implementing AI systems for DR screening.
There was significant variation in DR teleretinal program implementation, even among institutions within the same parent organization. UCD demonstrated a 14% increase in DR screening and saved $42.53 per patient over 2018–2019 when accounting for incentive payments and downstream revenue from ophthalmology referrals.24 With a sustainable program in place, their screening rates accelerated during the COVID-19 pandemic due to increased demand for virtual visits, although the use of teleophthalmology nationally did not show a sustained increase.25
Conversely, UCI found their program to be cost-prohibitive and unsustainable. These results are consistent with a recent study demonstrating that telehealth perception and program efficacy across specialties is variable and dependent on several factors, including existing telemedicine infrastructural workflows.26 Our results highlighted differences in proportion of poor-quality images, which may be partly due to hardware differences, lack of standardized protocols, and staff turnover and training. Prior studies have expressed similar concerns, including workflow and image quality variability among urban safety net clinics.27,28 It is unclear what percentage of unreadable images were due to pathology causing media opacity, small pupils, or patient cooperation. Unreadable images may prompt referrals to ophthalmologists for an in-person examination and require undoing of testing status and billing. More studies in this area are needed to minimize operational error and to maximize utilization.
Finally, we reported a wide range of turnaround time between sites. Delays in interpretation may be in part due to integration differences between EHRs and EyePACS, availability of eye care providers, and other administrative barriers. Workflow standardization should be employed to improve sustainability and scalability.16 For example, UCSD used informatics-based approaches to standardize a workflow for image interpretation using an EHR-based list of patients undergoing screening compatible with existing ophthalmology and primary care IT systems (Fig. 3).23 Future research should focus on how teleretinal DR systems implementation could be optimized across individual and multi-institutional organizations.
Cost and infrastructure support were key barriers to DR teleretinal implementation. Financial feasibility of a teleretinal program is crucial for long-term feasibility and provider buy-in. For example, UCI no longer offers DR teleretinal screening due to poor compensation. This may be due to significant variation in the billing codes approved by insurance companies and institutions; for example, 92227 and 92228 are billed for $15.79 and $30.23 on average nationally, whereas the 92250 allowable is $45.83.29 With prior definitions, there was also no professional fee payment with 92227, with reimbursement only covering image acquisition, whereas 92228 and 92250 provided payment for image capture and image interpretation. The lack of a separate fee for image interpretation presented additional solvency challenges for primary care-based screening, where images were captured in one department and interpreted by clinicians in another, but only one service was reimbursed.
In addition, prior definitions required that screening be billed under 92227 but any finding of retinopathy to be billed under 92228, which required modifying the CPT code during the completion of the order based on findings. Modification of a CPT code for a procedure depending on interpretation required significantly modified software builds and changes in workflow. Moreover, recent definition changes of 92227 and 92228 from distinguishing between screening for pre-retinopathy versus existing retinopathy to staff versus eye provider image interpretation will require future studies to evaluate the impact of these changes on screening reimbursements. Some institutions in this study also utilized CPT 92250, which may allow for remote use depending on the insurer, but allows for physician interpretation, and can be used both to monitor or screen disease.
Overall, the complexity of the billing environment and frequent regulatory changes has adversely affected the financial sustainability of teleretinal screening,30 thus other avenues of compensation should be explored to increase the financial incentives (Fig. 3). Teleretinal programs may increase the percentage of patients meeting quality metrics in MIPS such as Centers for Medicare and Medicaid Services (CMS) Measure 117, which evaluates eligible diabetic patients who underwent a DR eye examination. These quality-based payments and downstream revenue associated with screening may financially sustain teleretinal programs beyond direct billing24 and ultimately reduce system costs.31
Infrastructural support and resource limitations were identified as key barriers to DR teleretinal screening and are crucial in ensuring operational success. For example, many representatives expressed concern regarding inadequate capacity for in-person ophthalmic evaluations, an operational challenge previously expressed by private practice DR screening programs.17 This may also relate to lack of standardized workflows and infrastructural challenges that stem from inconsistent billing definitions. Although current implementation articles focus largely on population demographics and disease prevalence,16,32 there is a paucity of literature describing the implementation of health IT processes,33 which can be highly variable as seen among the universities within our study.23
In light of the recent COVID-19 pandemic, several studies have shown that having readily available infrastructure is key to a rapid data-driven response and evaluation of informatics tools33 and necessary to sustain existing telemedicine programs postpandemic.14 In our experience, recurring regional network meetings within the UC system have been important for disseminating lessons learned throughout each institution's implementation process, many of which are ongoing. These discussions also facilitate ideas for ongoing quality improvement. Other solutions previously implemented include the creation of retinal photography clinics in the LA county system and training medical assistants for fundus photography using existing personnel and resources (Fig. 3).16
Finally, institutions expressed interest in AI integration, although several barriers to implementation remain. Although autonomous AI for DR screening were the first in clinical medicine to gain FDA approval,19 there is a paucity of studies demonstrating successful AI implementation beyond the trials used to gain approval.34,35 These autonomous AI models have demonstrated real-world potential in increasing access to care and patient outcomes, especially in low-resource settings.36 In our study, barriers to AI implementation included difficulty integrating AI into EHR or EyePACS and cost.
AI integration may be challenging because current algorithms often lack interoperability and straightforward integration with existing infrastructure.37 A previous study has shown that clinician trust in AI remains a significant barrier,38 although in our study, institutional representatives were largely neutral. Although autonomous AI DR screening may hypothetically be cost-effective,39 these studies do not account for the capital costs of acquiring and configuring an AI system, including integration into an organization's IT systems and the cost of new hardware.
Recently, the CMS approved formal billing codes for autonomous AI (92229), which are tentatively billed at $45.69 on average, higher than current billing for physicians ($30.23).29 This represents an important step toward increasing the financial sustainability of autonomous AI teleretinal programs. Interestingly, ophthalmologists were much less concerned regarding the risk of AI replacing ophthalmologists compared with non-ophthalmologist representatives, consistent with previously published qualitative assessments among physicians.40 Our results are also consistent with previously published perceptions of AI among clinicians, which has historically been highly variable due to ethical considerations, impact on physician workflows, and liability.41 More research within AI transparency and generalizability as well as multidisciplinary collaboration among physicians, informaticians, and administrators will be essential to increase stakeholder buy-in (Fig. 3).
LIMITATIONS
Our study has additional limitations. First, our data were provided by institutional leaders, and larger studies will be needed to better evaluate the diversity of the medical community's perceptions of the benefits and barriers to telemedicine and AI integration within it. Second, our study was retrospective. Prospective studies will be needed to evaluate the impact of telemedicine on patient outcomes, satisfaction of quality metrics, and clinic revenues. Third, we did not collect data on visual outcomes of patients screened at each institution as part of this study. More study is needed to understand if and how variations in DR screening implementation across various institutions may affect patient outcomes.
Conclusions
Increasing adoption of telemedicine holds promise for expanding access to care. For diabetes patients, performing DR screening in primary care settings may help expand adherence to national guidelines and facilitate early diagnosis and treatment. Our study describes variations in implementation among several academic medical centers and highlights some challenges associated with teleretinal DR screening program implementation and AI integration. We also highlight possible solutions to address these barriers. Ongoing study is needed to better understand these barriers and develop best practices to overcome them. This will be critical to improving access to care and promoting health equity for this vision-threatening disease.
Supplementary Material
Acknowledgment
In addition, the authors thank the University of California Health Diabetes Initiative Workgroup for valuable insights in the process of conducting this study.
Disclosure Statement
S.L.B. has none specifically related to this study; other financial disclosures: equipment from Optomed and consulting fees from voxelcloud.io. G.Y. is consultant for Abbvie, Adverum, Alimera, Anlong, Bausch & Lomb, Cholgene, Clearside, Endogena, Genentech, Gyroscope, Intergalactic, Iridex, NGM Bio, Regeneron, Thea, Topcon, and Zeiss. J.S. has none related to this study; other: Merck. M.A.H. has none. M.F. received research grant Novo Nordisk.
Funding Information
Sally Baxter is supported by NIH Grant DP5OD29610 (Bethesda, MD, USA) and an unrestricted departmental grant from Research to Prevent Blindness (New York, NY, USA). Glenn Yiu is supported by NIH Grants R01 EY032238, R34 EY031719, and BrightFocus Foundation. M.F. is supported by NIH Grants NIH 1R56DE030469-01, 1RO1NR017190-01A1, and 1RO1DK116719-01A1.
Supplementary Material
REFERENCES
- 1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14(2):88–98. [DOI] [PubMed] [Google Scholar]
- 2. Ogurtsova K, Guariguata L, Barengo NC, et al. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pr 2022;183:109118. [DOI] [PubMed] [Google Scholar]
- 3. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021;128(11):1580–1591. [DOI] [PubMed] [Google Scholar]
- 4. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020;127(1):P66–P145. [DOI] [PubMed] [Google Scholar]
- 5. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017;40(3):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quality ID#117 (NQF0055): Diabetes: Eye Exam. Available from: https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2020_Measure_117_MIPSCQM.pdf [Last accessed: April 15, 2022].
- 7. Bresnick G, Cuadros JA, Khan M, et al. Adherence to ophthalmology referral, treatment and follow-up after diabetic retinopathy screening in the primary care setting. BMJ Open Diabetes Res Care 2020;8(1):e001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuo J, Liu JC, Gibson E, et al. Factors associated with adherence to screening guidelines for diabetic retinopathy among low-income metropolitan patients. Mo Med 2020;117(3):258–264. [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Zupan NJ, Shiyanbola OO, et al. Factors influencing patient adherence with diabetic eye screening in rural communities: A qualitative study. PLoS One 2018;13(11):e0206742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Misra A, Bachmann MO, Greenwood RH, et al. Trends in yield and effects of screening intervals during 17 years of a large UK community-based diabetic retinopathy screening programme. Diabet Med 2009;26(10):1040–1047. [DOI] [PubMed] [Google Scholar]
- 11. Walton OB IV, Garoon RB, Weng CY, et al. Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol 2016;134(2):204–209. [DOI] [PubMed] [Google Scholar]
- 12. Afshar AR, Oldenburg CE, Stewart JM. A novel hybrid fixed and mobile ultra-widefield imaging program for diabetic teleretinopathy screening. Ophthalmol Retina 2019;3(7):576–579. [DOI] [PubMed] [Google Scholar]
- 13. Khou V, Khan MA, Jiang IW, et al. Evaluation of the initial implementation of a nationwide diabetic retinopathy screening programme in primary care: A multimethod study. BMJ Open 2021;11(8):e044805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas EE, Haydon HM, Mehrotra A, et al. Building on the momentum: Sustaining telehealth beyond COVID-19. J Telemed Telecare 2022;28(4):301–308. [DOI] [PubMed] [Google Scholar]
- 15. Conlin PR, Fisch BM, Orcutt JC, et al. Framework for a national teleretinal imaging program to screen for diabetic retinopathy in Veterans Health Administration patients. J Rehabil Res Dev 2006;43(6):741–748. [DOI] [PubMed] [Google Scholar]
- 16. Daskivich LP, Vasquez C, Martinez C Jr, et al. Implementation and Evaluation of a Large-Scale Teleretinal Diabetic Retinopathy Screening Program in the Los Angeles County Department of Health Services. JAMA Intern Med 2017;177(5):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mamillapalli CK, Prentice JR, Garg AK, et al. Implementation and challenges unique to teleretinal diabetic retinal screening (TDRS) in a private practice setting in the United States. J Clin Transl Endocrinol 2020;19:100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Autonomous diabetic retinopathy screening system gains FDA approval. 2020. Available from: https://www.aao.org/headline/autonomous-diabetic-retinopathy-screening-system-g [Last accessed: April 15, 2022].
- 19. Office of the Commissioner. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. 2018. Available from: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye [Last accessed: April 15, 2022].
- 20. Gunasekeran DV, Ting DSW, Tan GSW, Wong TY. Artificial intelligence for diabetic retinopathy screening, prediction and management. Curr Opin Ophthalmol 2020;31(5):357–365. [DOI] [PubMed] [Google Scholar]
- 21. Grzybowski A, Brona P. Analysis and comparison of two artificial intelligence diabetic retinopathy screening algorithms in a pilot study: IDx-DR and Retinalyze. J Clin Med Res 2021;10(11):2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Heijden AA, Abramoff MD, Verbraak F, et al. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol 2018;96(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baxter S, Quackenbush Q, Cerda J, et al. Implementing clinical informatics tools for primary care-based diabetic retinopathy screening. Am J Manag Care 2022;28(10):e355–e362. [DOI] [PubMed] [Google Scholar]
- 24. Ellis MP, Bacorn C, Luu KY, et al. Cost analysis of teleophthalmology screening for diabetic retinopathy using teleophthalmology billing codes. Ophthalmic Surg Lasers Imaging Retina 2020;51(5):S26–S34. [DOI] [PubMed] [Google Scholar]
- 25. Lee SC, Alber S, Lieng MK, et al. Teleophthalmology using remote retinal imaging during the COVID-19 pandemic. Telemed J E Health 2023;29(1):81–86; doi: 10.1089/tmj.2022.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Connolly SL, Miller CJ, Gifford AL, Charness ME. Perceptions and use of telehealth among mental health, primary, and specialty care clinicians during the COVID-19 pandemic. JAMA Netw Open 2022;5(6):e2216401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fish A, George S, Terrien E, et al. Workflow concerns and workarounds of readers in an urban safety net teleretinal screening study. AMIA Annu Symp Proc 2011;2011:417–426. [PMC free article] [PubMed] [Google Scholar]
- 28. Ogunyemi O, George S, Patty L, et al. Teleretinal screening for diabetic retinopathy in six Los Angeles urban safety-net clinics: Final study results. AMIA Annu Symp Proc 2013;2013:1082–1088. [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Medicare and Medicaid Services. Medicare Program; CY 2022 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Provider Enrollment Regulation Updates; and Provider and Supplier Prepayment and Post-Payment Medical Review Requirements. Fed Regist 2021;86:64996–66031. [Google Scholar]
- 30. Lee SC, Lieng MK, Alber S, et al. Trends in remote retinal imaging utilization and payments in the United States. Ophthalmology 2022;129(3):354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Snoswell CL, Taylor ML, Comans TA, et al. Determining if telehealth can reduce health system costs: Scoping review. J Med Internet Res 2020;22(10):e17298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bastos de Carvalho A, Ware SL, Lei F, et al. Implementation and sustainment of a statewide telemedicine diabetic retinopathy screening network for federally designated safety-net clinics. PLoS One 2020;15(11):e0241767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Payne PRO, Wilcox AB, Embi PJ, Longhurst CA. Better together: Integrating biomedical informatics and healthcare IT operations to create a learning health system during the COVID-19 pandemic. Learn Health Syst 2022;6(2):e10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heydon P, Egan C, Bolter L, et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30 000 patients. Br J Ophthalmol 2021;105(5):723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ipp E, Liljenquist D, Bode B, et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open 2021;4(11):e2134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathenge W, Whitestone N, Nkurikiye J, et al. Impact of artificial intelligence assessment of diabetic retinopathy on referral service uptake in a low resource setting: The RAIDERS randomized trial. Ophthalmol Sci 2022;2(4):100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lehne M, Sass J, Essenwanger A, et al. Why digital medicine depends on interoperability. NPJ Digit Med 2019;2:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evans NG, Wenner DM, Cohen IG, et al. Emerging ethical considerations for the use of artificial intelligence in ophthalmology. Ophthalmol Sci 2022;2(2):100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolf RM, Channa R, Abramoff MD, Lehmann HP. Cost-effectiveness of autonomous point-of-care diabetic retinopathy screening for pediatric patients with diabetes. JAMA Ophthalmol 2020;138(10):1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al-Khaled T, Valikodath N, Cole E, et al. Evaluation of physician perspectives of artificial intelligence in ophthalmology: A pilot study. Invest Ophthalmol Vis Sci 2020;61(7):2023. [Google Scholar]
- 41. Laï MC, Brian M, Mamzer MF. Perceptions of artificial intelligence in healthcare: Findings from a qualitative survey study among actors in France. J Transl Med 2020;18(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.