Abstract
We cloned the pS1K1 plasmid in the process of apparently “complementing” a circadian clock mutant of cyanobacterium Synechococcus sp. strain PCC 7942, SP22, which has a 22-h period (T. Kondo, N. F. Tsinoremas, S. S. Golden, C. H. Johnson, S. Kutsuna, and M. Ishiura, Science 266:1233–1236, 1994). Sequence analysis revealed that SP22 did not have a mutation in the genomic DNA segment carried on pS1K1, and the sp22 mutation was later found in a recently cloned new clock gene, kaiC. Therefore, the period-extender gene pex that was carried on pS1K1 was a suppressor gene for the sp22 mutation. The pex gene encoded a protein of 148 amino acid residues. No meaningful homologs were found in DNA or protein databases including the Synechocystis genome database. The pex gene was transcribed from 129 and 164 bp upstream of the translation initiation codon as 0.6-kb transcripts. The Pex protein was detected as a fusion protein with a molecular mass of 15 kDa by the epitope tag fusion method using a c-Myc epitope tag. Disruption of the pex gene in wild-type cells shortened the period of the rhythms by 1 h, although it did not affect other properties of the rhythms, whereas its overexpression extended the period by 3 h with a concomitant reduction in the amplitude of the rhythms. In various clock mutants examined, overexpression caused arrhythmicity. Thus, Pex is likely to function as a modifier of the circadian clock in Synechococcus.
Circadian rhythms, biological oscillations with a period of about 24 h, are found ubiquitously in organisms from cyanobacteria to humans and are thought to be an adaptation to daily changes in environmental conditions on Earth, such as light and temperature (2). Circadian rhythms persist even in constant conditions. Thus, an endogenous mechanism called a circadian clock that generates the rhythms has been postulated, although the mechanism of this clock remains to be solved. To analyze the molecular mechanism for the circadian clock, clock genes and clock-related genes have been cloned from various organisms: the mouse Clock gene (13), the fruit fly Drosophila period (11, 24) and timeless genes (7, 21), the fungus Neurospora frequency (18), white collar-1, and white collar-2 genes (5), and the cyanobacterium Synechococcus kaiA, kaiB, and kaiC genes (9).
To monitor the circadian clock by using a bacterial luciferase luxAB gene set as a reporter, we introduced psbAI reporter construct PpsbAI::luxAB, which is a Synechococcus psbAI promoter segment fused to a promoterless segment of the luxAB gene set, into the cyanobacterium Synechococcus sp. strain PCC 7942. The transgenic strain displays circadian rhythms of bioluminescence both in liquid medium and on agar medium (14, 15). By utilizing this efficient Synechococcus system, we have isolated more than 100 clock mutants that show various abnormalities in their bioluminescence rhythms (16). Recently, the associated mutations have been localized to circadian clock gene cluster kaiABC by complementation of the mutants with a plasmid library for genomic DNA prepared from wild-type cells (9).
Previously, the pS1K1 plasmid had been isolated as a gene that appeared to complement the sp22 mutation (16). We show here that pS1K1 did not carry a true complementing gene but instead carried a suppressor gene for the sp22 mutation, pex, which extended the period of circadian rhythms.
MATERIALS AND METHODS
Bacterial strains, media, cultures, enzymes, and manipulation of DNA.
We used wild-type Synechococcus sp. strain PCC 7942 and psbAI reporter strains of Synechococcus, AMC149 (14) and CR1 (Table 1). The latter two transgenic strains carried psbAI reporter construct PpsbAI::luxAB at a specific targeting site (a unique XhoI site of Synechococcus genomic DNA sequence NSI [3]; TS1) in the genome and showed bioluminescence rhythms. Clock mutants SP22, P30, P331, and LP40, which exhibited altered periods, were isolated from AMC149 (16). These Synechococcus cells were grown in BG-11 liquid medium or on solid medium that contained BG-11 (25) and 1.5% Bacto Agar (Difco Laboratories, Detroit, Mich.) under continuous illumination (LL) of 46 μmol m−2 s−1 from white fluorescent lamps at 30°C (we define this as standard conditions). Synechococcus cells were transformed with plasmid DNA by natural transformation (22). Kanamycin-resistant and spectinomycin-resistant transformant clones were selected with 33 μg of kanamycin sulfate per ml and 40 μg of spectinomycin sulfate per ml, respectively. For RNA or protein analysis, Synechococcus cells were grown in 100 ml of BG-11 liquid medium at 30°C under LL with aeration until the mid-exponential phase (cell density corresponding to an optical density at 730 nm of 0.3). Escherichia coli cells were maintained in Luria-Bertani broth (LB) or on LB agar that contained 1.2% agar (Shouei Kanten Co., Tokyo, Japan) in LB. Plasmids were propagated in E. coli HB101, DH5α, or DH10B. Plasmids were introduced into E. coli by electroporation with an electric pulse generator (Cellject Basic; EquiBio s.a., Angleur, Belgium). Enzymes were obtained from New England Biolabs, Inc. (Beverly, Mass.), Boehringer GmbH (Mannheim, Germany), Fermentas MBI (Vilnius, Lithuania), and Takara Shuzo Co. (Kyoto, Japan). Plasmid DNA was prepared by the boiling lysis method (28). DNA fragments were purified from agarose gels by the glass powder method (28), with an AGC-001K DNA PREP (Diayatron, Tokyo, Japan). The handling of E. coli and the manipulation of DNA for molecular cloning were carried out as described previously (28).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristicsa | Source and/or reference(s) |
|---|---|---|
| Synechococcus sp. strain PCC 7942 | ||
| Wild-type strain | Wild type | S. S. Golden |
| AMC149 | Wild-type strain carrying a PpsbAI::luxAB reporter; Spr | 14 |
| CR1 | Wild-type strain carrying a PpsbAI::luxAB reporter; Cmr | M. Ishiura |
| PREII | Wild-type strain carrying a Ppex::luxAB reporter; Spr | This study |
| Synechococcus circadian clock mutants isolated from AMC149 | ||
| SP22 | sp22 mutation in the kaiC gene; period of 22 h | 9, 16 |
| P30 | p30 mutation in the kaiA gene; period of 30 h | 9, 16 |
| P331 | p331 mutation in the kaiA gene; period of 30 h | 9 |
| LP40 | lp40 mutation in the kaiC gene; period of 40 h | 9, 16 |
| Plasmids | ||
| pS1K1 | Plasmid carrying the pex gene in a 2,095-bp Sau3AI-KpnI Synechococcus genomic DNA segment; Kmr | 16 |
| pSS | Deletion derivative of pS1K1; Kmr | This study |
| pSEII | Deletion derivative of pS1K1; Kmr | This study |
| pSBI | Deletion derivative of pS1K1; Kmr | This study |
| pNS | Deletion derivative of pS1K1; Kmr | This study |
| pBS | Deletion derivative of pS1K1; Kmr | This study |
| pRV | Deletion derivative of pS1K1; Kmr | This study |
| pTS2KC | pTS2K carrying the chloramphenicol resistance gene in a 1.1-kb Tth111I-NheI fragment from pACYC184 (4, 26) in the NdeI site; Kmr Cmr | M. Ishiura |
| pPEX-474 | pTS2KC carrying the 1.0-kb BamHI segment of pS1K1; Kmr | This study |
| pPEX-363 | pTS2KC carrying nucleotides −363 to +536 (a BamHI site located downstream) of the pex gene; Kmr | This study |
| pPEX-225 | pTS2KC carrying nucleotides −225 to +536 of the pex gene; Kmr | This study |
| pPEX-113 | pTS2KC carrying nucleotides −113 to +536 of the pex gene; Kmr | This study |
| pTS2/C | Targeting vector which targets its insert to TS2 in the Synechococcus genome; Cmr | This study |
| pTS2Slux | pTS2/C carrying a 2.7-kb HindIII-PvuII promoterless segment of the luxAB gene set from V. harveyi (1) and a 2.2-kb Ω fragment (23) with a multicloning site; the luxAB segment carries the Ω fragment in the SalI site; Spr Cmr | This study |
| pTS2Ppex::lux | An 872-bp BamHI-Ecl136II segment was inserted upstream of the luxAB gene set in pTS2Slux; Spr | This study |
| p322Ptrc | pBR322 carrying lacIq and Ptrc segments in replace of the 0.97-kb EcoRI-NruI segment; Apr | This study |
| pTS2KCPtrc | pTS2KC carrying the lacIq-Ptrc construct in a 2.2-kb BglII fragment from p322Ptrc in the BamHI site; Kmr Cmr | This study |
| pTS2Cpex | pTS2KCPtrc carrying nucleotides −377 to +447 (stop codon) of the pex gene; Kmr Cmr | This study |
| pTS2Cpex-myc | pTS2KCPtrc carrying a pex–c-myc-tag fusion gene; Kmr Cmr | This study |
| pACYC177 | E. coli vector; Apr Kmr | 4, 27; New England Biolabs |
| pPEX-E | pACYC177 carrying a modified pex gene in the 1.0-kb BamHI segment of pS1K1 in which the pex ORF is interrupted by insertion of an EcoRI site | This study |
| pIpex | pPEX-E carrying the Ω fragment from pBR322ΩE (23) cloned into the EcoRI site; Spr | This study |
| pDpex | pGEM-T carrying a pex gene in which most of the pex ORF is replaced with the Ω fragment from pBR322ΩE; Spr | This study |
| pGEMpex | pGEM-T carrying nucleotides −499 to +705 of the pex gene; Kmr | This study |
| pTS2CPtrc::pex | pTS2KCPtrc carrying nucleotides +1 to +447 (stop codon) of the pex ORF; Kmr Cmr | This study |
PpsbAI::luxAB, gene fusion of the psbAI promoter and a bacterial luciferase luxAB gene set; kaiA, the kaiA gene in the circadian clock gene cluster kaiABC; kaiC, the kaiC gene in the gene cluster kaiABC; Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Spr, spectinomycin resistance; Ptrc, the trc promoter; the first nucleotide (A) of the translation initiation codon ATG of the pex gene is numbered +1.
DNA sequencing and sequence analysis.
DNA sequencing was carried out with a Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Inc., Foster City, Calif.) and a model 373A DNA sequencing system (Applied Biosystems, Inc.). DNA sequences were analyzed by the DNASIS (version 3.5; Hitachi Software Engineering, Yokohama, Japan) and CodonUse programs (version 3.0d12; C. Halling, Department of Molecular Genetics and Cell Biology, University of Chicago).
Sequencing of the pex gene from wild-type cells and SP22 mutant cells.
We directly determined the sequence of the pex gene from wild-type cells and SP22 mutant cells after in vitro amplification of the pex gene segment by PCR. Genomic DNA was prepared from Synechococcus cells by the method of Porter (22). A 987-bp BamHI-SalI segment which carried the pex gene was amplified by PCR, with genomic DNA from wild-type cells and SP22 cells as templates and the following four pairs of primers: AU (5′-GAAGTGGAAGACCTTGGTAAT-3′; nucleotides −498 to −478 of the pex gene; the first nucleotide, A, of the translation initiation codon of the pex gene is numbered +1) and AL (5′-CCTAAGTTGATCCCTCACACC-3′; −32 to −52), BU (5′-AATCCCCGCAGAGAATAAAAA-3′; −149 to −129) and BL (5′-CTCAGTGCCGTAGGAGTCTTC-3′; +186 to +166), CU (5′-CTGCTATGTGTTGGCGGTGC-3′; +138 to +157) and CL (5′-CATTCTCCAGACTCTGCAGG-3′; +554 to +535), and DU (5′-GAAGCACGCTGAAAATCTGAC-3′; +436 to +456) and DL (5′-TAGGTAAACCTGTGGTCCAAC-3′; +705 to +685). The four kinds of PCR products amplified from the genomic DNAs prepared from wild-type cells and SP22 cells were cloned into the pGEM-T vector (Promega Co., Madison, Wis.), and their sequences were determined with the T7 and SP6 primers (Promega Co.).
Deletion mapping of the pex gene carried on pS1K1.
We constructed a series of deletion derivatives of pS1K1 (16). pS1K1 carries a 2,095-bp Sau3AI-KpnI genomic DNA insert carrying the pex gene cloned into the BamHI site of a derivative of pBR322 (30), which carried the 1.4-kb kanamycin resistance gene from pACYC177 (4, 27) at the PstI site. pSS, which lacked the 627-bp SalI segment of pS1K1 carrying a 221-bp SalI-KpnI genomic DNA segment, was constructed by digestion of pS1K1 with SalI followed by circularization with T4 DNA ligase (Fig. 1). pSEII, which lacked the 115-bp Ecl136II-SalI segment of pSS, was constructed by digestion of pSS with Ecl136II and SalI, followed by the filling-in reaction with Klenow enzyme and circularization with T4 DNA ligase. pSBI, which lacked the 1,857-bp BsaBI segment of pSS carrying an 834-bp BsaBI-SalI genomic DNA segment, was constructed by digestion of pSS with BsaBI followed by circularization with T4 DNA ligase. pNS, which lacked the 837-bp ClaI-NcoI segment of pSS carrying a 485-bp ClaI-NcoI genomic DNA segment, was constructed by digestion of pSS with ClaI and NcoI, followed by the filling-in reaction with Klenow enzyme and circularization with T4 DNA ligase. pBS, which lacked the 410-bp NcoI-BamHI segment, was constructed by digestion of pSS with NcoI and BamHI, followed by the filling-in reaction with Klenow enzyme and circularization with T4 DNA ligase. pRV, which lacked the 1,944-bp EcoRV segment carrying a 1,754-bp genomic DNA segment, was constructed by digestion of pS1K1 with EcoRV, followed by circularization with T4 DNA ligase. We transformed SP22 mutant cells with pS1K1 and these deletion derivatives of pS1K1 and used the resulting kanamycin-resistant transformant clones that grew on BG-11 agar plates for an assay of bioluminescence rhythms.
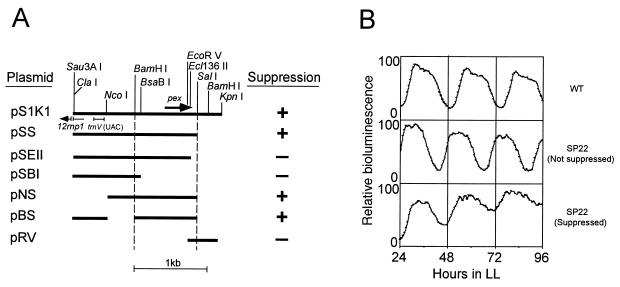
FIG. 1.
Deletion mapping of the suppressor activity for the sp22 mutation on pS1K1. A series of deletion derivatives of pS1K1 which carried 2,095-bp Sau3AI-KpnI genomic DNA segments from cells of wild-type Synechococcus were constructed and introduced into SP22 mutant cells, and the resulting kanamycin-resistant transformant clones were grown on BG-11 agar plates under LL (light intensity was 46 μmol m−2 s−1 from white fluorescent lamps) at 30°C. After synchronization of the circadian clocks by exposure of the cells to a 12-h dark period at 30°C, the bioluminescence rhythms of the transformant clones were monitored under LL at 30°C. (A) Restriction map of pS1K1 and a series of deletion derivatives of pS1K1 and their suppressor activities. The bars indicate the genomic DNA segments carried on the plasmids. + and −, presence and absence of suppressor activity, respectively. (B) Representative bioluminescence rhythms of wild-type cells and SP22 mutant cells which were suppressed or not suppressed by transformation of pS1K1 derivatives. The vertical axis indicates relative bioluminescence; the highest value of bioluminescence in each rhythm is defined as 100. WT, wild-type cells; SP22 (Not suppressed), SP22 transformant cells which were not suppressed; SP22 (Suppressed), SP22 transformant cells which were suppressed.
Northern blotting analysis.
Cells of wild-type Synechococcus sp. strain PCC 7942 were grown in BG-11 medium and harvested at the mid-logarithmic growth phase by centrifugation at 1,000 × g for 10 min, and then the pellets were immediately frozen in liquid nitrogen. RNA was extracted from each frozen sample as described by Mohamed and Jansson (19). RNA was subjected to electrophoresis on 1.0% agarose gels containing 1.0% formaldehyde (5 μg of total RNA was loaded per lane), blotted onto positively charged nylon membranes (Boehringer GmbH), and hybridized with a 32P-labeled or digoxigenin (DIG)-labeled pex probe. The entire open reading frame (ORF) of the pex gene was amplified by PCR with two primers used for the construction of an overexpression construct for the pex gene described below (5′-CCACATGTCGAGCGGGGTAGC-3′ and 5′-TCGGATCCTCAGCGTGCTTCGACAG-3′) and with pS1K1 as a template, labeled with [α-32P]dCTP (3,000 Ci/mmol; Amersham International plc, Little Chalfont, Buckinghamshire, England) by using a Random Primer DNA Labeling Kit (version 2; Takara Shuzo Co.), and used as a 32P-labeled DNA probe. Hybridization was carried out under standard stringent conditions (28). To make a DIG-labeled RNA probe, the pex ORF amplified by PCR was subcloned into pGEM-T and then labeled with DIG by a DIG RNA labeling kit (Boehringer GmbH). We used a 600-ng aliquot of the DIG-labeled probe in 2 ml of hybridization buffer for hybridization at 65°C and then detected pex transcripts by chemiluminescence, by using a DIG nucleic acid detection kit (Boehringer GmbH).
Determination of the transcriptional initiation site of the pex gene by the primer extension method.
We carried out primer extension experiments by standard procedures (28) with SuperScript II RNaseH− reverse transcriptase (Gibco BRL, Gaithersburg, Md.) and with oligonucleotide 5′-AGCAGCTCAGCCTGTGTCGTCGCAGCATC-3′ as a primer. This primer corresponded to nucleotides −59 to −87 of the pex gene. The 5′ end of the primer was labeled with [γ-32P]ATP (>5,000 Ci/mmol; Amersham International plc) by the T4 polynucleotide kinase reaction (T4 polynucleotide kinase; Promega Co.). Thirty micrograms of Synechococcus total RNA and 4 ng of 32P-labeled primer (∼105 cpm) in 30 μl of hybridization buffer were denatured by boiling and then incubated overnight without heating to allow annealing. RNA annealed with primer was recovered by ethanol precipitation and dried. The RNA and 5 μl (1,000 U) of reverse transcriptase (Gibco BRL) in 50 μl of reaction mixture for reverse transcriptase were incubated at 37°C for 2 h for the primer extension reaction. The reaction product was recovered by ethanol precipitation and applied to sequencing gels. To make sequencing ladders by which the position of the reverse transcription product in the gel was determined, we also carried out a sequencing reaction with an fmol DNA sequencing kit (Promega Co.) by using pS1K1 and the 32P-labeled primer as the template and primer, respectively. The sequencing gels were dried and then analyzed by a Bio-Image Analyzer BAS2000 (Fuji Film Co., Kanagawa, Japan).
Deletion mapping analysis of the promoter region of the pex gene.
From a 1.0-kb BamHI segment which carried the pex gene, we constructed a series of deletion derivatives carrying upstream regions of various lengths (Fig. 3A and Table 1). To construct the deletion derivatives, appropriate segments were amplified by PCR with the following oligonucleotides: upper primers, 5′-GGCAAAGGGGATCCCGGTGG-3′ (nucleotides −363 to −358 of the pex gene; a BamHI restriction site harbored by the oligonucleotide is underlined), 5′-GTGTTCGGATCCTGTGTG-3′ (−225 to −217), and 5′-CTGGATCCGGTAATCCCTGTCTGTAACC-3′ (−113 to −94); lower primer, 5′-TAGGTAAACCTGTGGTCCAAC-3′ (+685 to +705), which corresponded to a sequence between the BamHI and KpnI sites located in the downstream region of the pex gene (Fig. 1). The PCR products were digested with BamHI and cloned into the unique BamHI site of pTS2KC by which the insert portions could be targeted to a specific targeting site (a unique BstEII site of the Synechococcus genomic DNA sequence NSII [8] [accession no. in the GenBank/EMBL/DDBJ database, U44761]; TS2; insertion of a DNA segment into TS2 does not affect the growth of Synechococcus cells) in the genome. The original BamHI fragment was also cloned into pTS2KC as a control (pPEX-474). We transformed SP22 mutant cells with these plasmids to target the insert DNAs to TS2 in the genome. In more than 90% of the transformants obtained with our TS2-targeting vectors, targeting of genomic DNA segments as large as 6 kb to TS2 occurs as expected (10a). We confirmed by this Southern blotting analysis and PCR. A DNA segment flanked with NSII was amplified by PCR from the genomic DNA prepared from each transformant and plasmid DNA used for transformation to obtain the transformant by using TS2 upper (5′-CTCGATGGCATTCAGCA-3′; nucleotides 2462 to 2478 of NSII) and lower (5′-CTGCTTGGAACTGCACA-3′; nucleotides 2304 to 2320 of NSII) primers and was subjected to electrophoresis on 0.8% agarose gels. The genomic DNA from SP22 cells was analyzed similarly. Bands of the same expected sizes (the sizes of the bands in the transformants obtained by transformation with pPEX-474, pPEX-363, pPEX-225, and pPEX-113 were 3.3, 3.2, 3.1, and 3.0 kb, respectively) were found in each pair of samples, and a 0.18-kb band that resulted from the original TS2 and that was observed in SP22 cells was not detected in the transformants (data not shown). These results suggested that targeting occurred as expected and eliminated the possibility that integration at the pex region via a single homologous recombination might have occurred in the transformants. The genomic and plasmid DNAs were digested with HindIII, subjected to electrophoresis on 0.8% agarose gels, and examined by Southern blotting analysis using a DIG-labeled 1.6-kb DraI-BsaBI ori segment of pBR322 as a hybridization probe. The vector portions (the pBR322 ori segment), which should not be targeted to TS2 or other sites in the genome, were not detected in the transformant genome, whereas expected bands (the sizes of the bands in transformants obtained by transformation with pPEX-474, pPEX-363, pPEX-225, and pPEX-113 were 6.5, 6.4, 6.2, and 6.1 kb, respectively) were detected in the plasmids, also eliminating the possibility of integration of the plasmids into the genome (data not shown). Transformant clones were grown on BG-11 agar plates and used for an assay of bioluminescence rhythms.
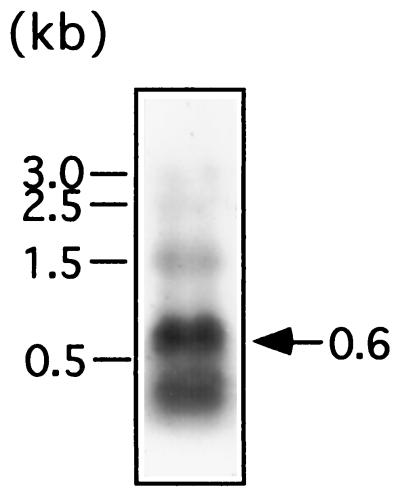
FIG. 3.
Identification of pex transcripts by Northern blotting analysis. RNA was prepared from cells of wild-type Synechococcus, subjected to electrophoresis on 1.0% agarose gels containing 1.0% formaldehyde, blotted onto positively charged nylon membranes, and hybridized with a DIG-labeled pex probe. Five micrograms of total RNA was loaded per lane. The arrow indicates pex transcripts. The 1.5-kb band may be a degradation product of rRNA. The bars on the left indicate the positions of ribosomal RNAs and their degradation products serving as size markers.
Monitoring of the expression of the pex gene by a luciferase reporter as bioluminescence.
To monitor the expression of the pex gene by using luciferase bioluminescence as a reporter, we constructed pex reporter construct Ppex::luxAB. An 872-bp BamHI-Ecl136II segment carrying the upstream region and 5′ coding region (nucleotides −474 to +398) of the pex gene was inserted upstream of the coding region of the luciferase luxAB gene set from Vibrio harveyi (1) in targeting vector pTS2Slux to obtain pTS2Ppex::lux. pTS2Slux carried Synechococcus genomic DNA segment NS2 containing TS2 into which the Ω fragment carrying a spectinomycin resistance gene (23), a multicloning site, and the luxAB gene set were inserted. We transformed wild-type cells of Synechococcus sp. strain PCC 7942 with pTS2Ppex::lux to target the reporter construct to TS2 in the genome. Transformant clones (PREII strain) were selected with spectinomycin and grown under LL on BG-11 agar plates until the transformant clones developed colonies 2 mm in diameter. Then, the cells were subjected to a 12-h dark period to synchronize their circadian clocks and were used for the assay of bioluminescence.
Detection of pex gene product by the epitope tag fusion method by using a c-Myc epitope tag.
To detect the pex gene product by the epitope tag fusion method using a c-Myc epitope tag (20), we overexpressed a pex–c-myc tag fusion gene in Synechococcus cells by using the Ptrc-IPTG (isopropyl-β-d-thiogalactopyranoside) induction system for Synechococcus cells (6). We first amplified by PCR a DNA segment which carried a genomic DNA segment comprising nucleotides −363 to +444 of the pex gene with upper primer 5′-GGCAAAGGGGATCCCGGTGG-3′ (−363 to −358; a BamHI site is underlined) and lower primer 5′-TCGGATCCTCAGTTCAGATCTTCTTCGCTGATCAGCTTTTGTTCGCGTGCTTCGACAGGGATC-3′ (+444 to +426; a BamHI site is underlined and the nucleotide sequence for the human c-Myc peptide tag, EQKLISEEDLN, is in boldface). The PCR product, which contained the upstream region and ORF of the pex gene, was digested with BamHI and inserted into the unique BamHI site downstream of the trc promoter of targeting vector pTS2KCPtrc to obtain pTS2Cpex/myc. We transformed SP22 cells with the resulting plasmid to target the pex–c-myc tag fusion gene together with the lacIq gene to the TS2 in the genome. We also constructed a control plasmid without a c-Myc epitope tag, pTS2Cpex. A DNA segment carrying the pex gene was amplified by PCR with primers 5′-GGCAAAGGGGATCCCGGTGG-3′ (−363 to −358; a BamHI site is underlined) and 5′-TCGGATCCTCAGCGTGCTTCGACAG-3′ (+447 to +431), digested with BamHI, and inserted into the unique BamHI site of pTS2KCPtrc. Then, we transformed SP22 cells with control plasmid pTS2Cpex. Kanamycin-resistant transformant cells were grown in BG-11 liquid medium in the presence of 1 mM IPTG to induce the expression of the pex–c-myc tag fusion gene. Cells were collected from 100 ml of culture (optical density at 730 nm, 0.5) by centrifugation, and about 100 μl of the pellet was resuspended in 250 μl of 2× sodium dodecyl sulfate (SDS) gel loading buffer without bromophenol blue (100 mM Tris-Cl [pH 6.8], 200 mM dithiothreitol, 4% SDS, 20% glycerol) (28), and the cell suspension was mixed with 750 μl of zirconium beads 0.1 mm in diameter (Biospec Products, Bartlesville, Okla.) and vortexed for 2 to 10 s. The mixture was frozen in liquid nitrogen and warmed at 37°C for 2 min to allow a partial melting. To disrupt the cells, the mixture was agitated for 30 s at the maximum speed by a Mini Bead-Beater (Biospec Products). After the mixture was refrozen in liquid nitrogen, this agitation was repeated. Supernatant (cell extracts) was obtained by centrifugation at 2,500 × g for 10 min at 4°C. Ten-microliter aliquots of cell extracts containing 10 μg of total protein were mixed with equal volumes of 0.4% bromophenol blue, boiled for 10 min, and then subjected to electrophoresis on 15% polyacrylamide gels containing 0.1% SDS. The fractionated proteins were transferred onto polyvinylidine difluoride membranes (Hybond-P; Amersham International plc). c-Myc epitope-tagged fusion proteins were detected by an ECL Western blotting detection kit (Amersham International plc) by using anti-c-Myc antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) and anti-mouse immunoglobulin G antibody labeled with horseradish peroxidase (Santa Cruz Biotechnology).
Disruption of the pex gene.
We disrupted the pex gene by insertion of the Ω fragment (23) carrying a spectinomycin resistance gene, by using plasmid pIpex. pIpex carried a 3.0-kb BamHI fragment carrying the pex gene which had been inactivated by the insertion of the Ω fragment. A 1.0-kb BamHI fragment of pS1K1 carrying the whole pex gene was circularized with T4 DNA ligase (New England Biolabs, Inc.) and used as a template for PCR with mutagenic primers 5′-GGAATTCTACGGCACTGAGCTGATCC-3′ (+175 to +193; an EcoRI site is underlined) and 5′-GGAATTCGGAGTCTTCGTGCCGAAGC-3′ (+174 to +156) to obtain a 1.0-kb PCR product carrying EcoRI sites at both ends. This product was digested with EcoRI, circularized with T4 DNA ligase, and then digested with BamHI to obtain a modified 1.0-kb BamHI fragment carrying an EcoRI site between the 58th and 59th codons of the pex gene. The 1.0-kb modified BamHI fragment was cloned into the unique BamHI site of pACYC177 (4, 27) to obtain pPEX-E. A 2.1-kb EcoRI Ω fragment of pBRR322ΩE (22) carrying a spectinomycin resistance gene was inserted into the unique EcoRI site of pPEX-E to obtain pIpex.
We also constructed pDpex, which could replace most of the pex ORF in the genome with the Ω fragment by transformation of cells. A DNA segment from −499 to +705 of the pex gene was amplified by PCR with the pS1K1 template and primers 5′-GAAGTGGAAGACCTTGGTAAT-3′ (−499 and −479) and 5′-TAGGTAAACCTGTGGTCCAAC-3′ (+705 to +685) and cloned into pGEM-T to obtain pGEMpex. A DNA segment from −499 to −1 of the pex gene was also amplified, with primers 5′-GAAGTGGAAGACCTTGGTAAT-3′ (−499 and −479) and 5′-TAGGCCTTTCGCCAACGCATGAG-3′ (−1 to −16; a StuI site is underlined). The 506-bp PCR product was digested with NheI and StuI. The resulting 302-bp NheI-StuI fragment and a 2.0-kb SmaI Ω fragment of pBR322ΩE carrying a spectinomycin resistance gene were inserted into pGEMpex digested with NheI and EcoRV to replace a 690-bp NheI-EcoRV fragment encoding the pex gene carried on pGEMpex; thus was obtained pDpex.
We transformed CR1 cells, a psbAI reporter strain of Synechococcus carrying the chloramphenicol resistance gene from pACYC184 (4, 26) as a selective marker gene, with pIpex and pDpex and isolated spectinomycin-resistant transformant clones. We performed Southern blotting analysis of the genomic DNA prepared from the transformant cells with a DIG-labeled pex probe and confirmed that the pex genes of the transformant cells had been inactivated by the insertion of the Ω fragment or replaced with the Ω fragment as expected (data not shown). The transformants were grown on BG-11 agar plates and used for the assay of bioluminescence rhythms.
Overexpression of the pex gene.
We first constructed an overexpression construct for the pex gene. A modified pex gene was synthesized by PCR with upper primer 5′-CCACATGTCGAGCGGGGTAGC-3′ (+1 to +17; an AflIII site is underlined, and the translation initiation codon ATG of the pex gene is in boldface) and lower primer 5′-TCGGATCCTCAGCGTGCTTCGACAG-3′ (+447 to +431; a BamHI site is underlined). After digestion with AflIII and BamHI, the PCR product was ligated with p322Ptrc digested with NcoI and BamHI to obtain p322Ptrc::pex. A 2.6-kb BglII fragment carrying a lacIq-Ptrc::pex segment was cloned into the unique BamHI site of the pTS2KC targeting vector to obtain pTS2CPtrc::pex. We transformed AMC149 and clock mutants SP22, P30, P331, and LP40 with pTS2CPtrc::pex to target the lacIq-Ptrc::pex segment to TS2 in the genome. We also transformed AMC149 and SP22 with a control vector without a pex insert (pTS2KCPtrc) to construct control strains. The resulting transformants were named AMC149/Ptrc::pex, SP22/Ptrc::pex, P30/Ptrc::pex, P331/Ptrc::pex, and LP40/Ptrc::pex, whereas control strains were named AMC149/Ptrc and SP22/Ptrc. The transformants were inoculated on BG-11 agar plates with and without IPTG, were grown under standard conditions for 2 days, and were then used for an assay of bioluminescence rhythms.
Assay of bioluminescence rhythms.
Cells of transgenic bioluminescent strains were spotted on BG-11 agar plates and then grown to dots 2 mm in diameter under standard conditions (LL, 46 μmol m−2 s−1 from white fluorescent lamps, at 30°C). The cells were further cultured under standard light conditions for 2 or 3 days and then maintained for 12 h in the dark at 30°C to synchronize their circadian clocks. A small dish (8 mm in diameter) that contained 0.3 ml of n-decanal (Sigma Chemical Co., St. Louis, Mo.) dissolved in vacuum pump oil at a concentration of 3% (vol/vol) was placed within each plate. We monitored bioluminescence rhythms of cells on agar plates under standard conditions (under LL at 30°C) with a bioluminescence-monitoring charge-coupled device camera apparatus as described previously (15).
Nucleotide sequence accession number.
The nucleotide sequence of the pex gene has been deposited in the GenBank/EMBL/DDBJ database and has been given accession no. AB009574.
RESULTS AND DISCUSSION
Identification of the pex gene as a suppressor gene for circadian clock mutation sp22.
Previously, we isolated the pS1K1 plasmid as carrying a candidate gene for complementing the sp22 mutation (16). pS1K1 came from a plasmid library for genomic DNA of wild-type cells of Synechococcus, and it carried a 2,095-bp DNA segment. To find the sp22-complementing gene which was carried on pS1K1, we constructed a series of deletion derivatives of pS1K1, introduced the derivatives into SP22 mutant cells, and assayed their complementing activity by monitoring the bioluminescence rhythms of the transformant clones.
Figure 1 shows the effects of deletions on the apparent complementing activity of pS1K1. pS1K1 extended the period of SP22 mutant cells from 22 to 24 h (Fig. 1B). Deletion derivatives pSS, pNS, and pBS carried the activity, while derivatives pSEII, pSBI, and pRV lost the activity. This indicates that the 987-bp BamHI-SalI segment carried the activity. Thus, we expected that this segment would carry the sp22 mutation.
We determined the nucleotide sequence of the genomic DNA insert carried on pS1K1 (Fig. 2). To find the putative sp22 mutation in the 987-bp BamHI-SalI segment in the SP22 genome, we also directly determined the nucleotide sequence of this segment from both wild-type cells and SP22 cells after amplification of the segment by PCR. However, we could not find any differences in nucleotide sequence between wild-type cells and SP22 cells. This result indicates that pS1K1 did not carry a true complementing gene for the sp22 mutation but instead carried a suppressor gene for the sp22 mutation that affects the circadian period by a gene dosage effect. Consistent with this hypothesis, we recently cloned circadian clock gene cluster kaiABC from Synechococcus sp. strain PCC 7942; this cluster is essential for the clock functions. This goal was accomplished by complementation of different clock mutants, and we identified the sp22 mutation as a missense mutation on the kaiC gene (an amino acid change of Ala-87 to Val resulted from a nucleotide change of C-260 to T; the first A nucleotide of translation initiation codon ATG of the kaiC gene is numbered +1) (9). We also found that a plasmid carrying clock gene cluster kaiABC complemented mutant SP22 (9). Gene cluster kaiABC and pS1K1 do not overlap, so these data indicated that pS1K1 did not carry a true complementing gene but instead carried a suppressor gene for the sp22 mutation.
FIG. 2.
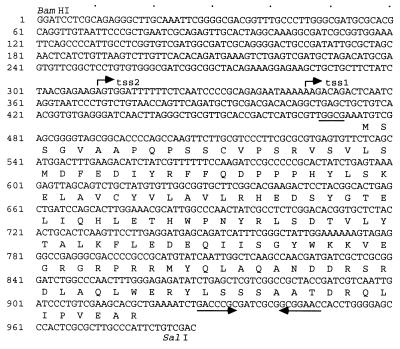
Nucleotide sequence of the pex gene and the predicted amino acid sequence of the pex gene product. A putative ribosome-binding sequence is underlined. An inverted repeat sequence is shown by the opposing arrows. The two transcriptional start sites determined by the primer extension method (Fig. 4) are shown as tss1 and tss2. The dots above the nucleotide sequence indicate the positions of each 10 nucleotides.
To find the protein coding region of the sp22 suppressor gene, we analyzed the sequence of the genomic DNA insert carried on pS1K1 with the CodonUse program and found a 447-bp ORF whose codons were frequently used in Synechococcus (Fig. 2). This ORF is located upstream of the 12rnp1 and trnV (UAC) genes (29). We named this ORF a period- extender (pex) gene. pex encoded a 148-amino-acid protein with a molecular mass of 17 kDa. We could not find any functional motifs in the Pex protein and also could not find significant homologs by a search of DNA and protein databases including a search of the whole-genome sequence of the cyanobacterium Synechocystis sp. strain PCC 6803 (12). Southern blotting analysis of the genomic DNA with a 32P-labeled pex ORF probe demonstrated that the Synechococcus cells are likely to have a single copy of the pex gene (data not shown).
Identification of pex transcripts by Northern blotting analysis.
We analyzed pex transcripts by Northern blotting analysis using an RNA probe specific for the pex ORF and detected a 0.6-kb transcript (Fig. 3). This result suggests that the 447-bp pex ORF was transcribed as a 0.6-kb RNA. A 0.3-kb minor transcript was also detected. This might be a degradation product of the 0.6-kb transcript or another transcript which was initiated from inside the pex ORF. A 1.5-kb band is likely to be a degradation product of rRNA which hybridized nonspecifically with the RNA probe because the band apparently corresponded to the degradation product and because such a band was often observed with various RNA probes specific to other genes. With a DNA probe, the 0.6- and 0.3-kb bands were also detected, but the 1.5-kb band was not detected (data not shown).
Analysis of the promoter region of the pex gene by primer extension and deletion mapping.
We determined the transcriptional start site of the pex gene by the primer extension method (28) and found two transcriptional start sites at positions 164 (tss2) and 129 bp (tss1) upstream from the translation initiation codon (the first A nucleotide of the translation initiation codon of the pex gene is numbered +1) (Fig. 4A). Because an inverted repeat sequence (nucleotides +454 to +474 of the pex gene) that might function as a possible transcription terminator was found downstream of the pex ORF (Fig. 2), the pex gene appears to be transcribed from −164 and −129 to around the inverted repeat sequence as 0.6-kb transcripts.
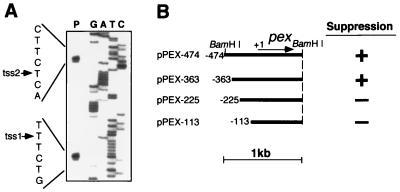
FIG. 4.
Analysis of the promoter region of the pex gene by the primer extension and deletion mapping methods. (A) Determination of the transcription start site by the primer extension method. Total RNA was hybridized to a primer whose 5′ end was labeled with 32P and reverse transcribed with reverse transcriptase, and the product was analyzed on a sequencing gel (lane P). A sequencing ladder (lanes G, A, T, and C) was obtained by a sequencing reaction in which the same primer was used as a sequencing primer. The arrows indicate the transcription start sites detected (the sites are also shown on the nucleotide sequence of the pex gene [Fig. 2]). (B) Deletion mapping of the promoter region. A series of deletion fragments carrying the upstream region of various lengths from the 1.0-kb BamHI segment of pS1K1 were amplified by PCR and cloned into targeting vector pTS2KC to target the insert DNAs to the TS2 of SP22 mutant cells by transformation of the cells with the resulting plasmid. Transformant clones were grown on BG-11 agar plates and used for the assay of bioluminescence rhythms. Other conditions were the same as those described in the legend for Fig. 1. The plasmids carry the genomic DNA segments indicated by the bars. The first nucleotide of translation initiation codon ATG of the pex gene is numbered +1. The arrow shows the pex ORF. + and −, presence and absence of suppressor activity for the sp22 mutation, respectively.
To analyze the promoter region of the pex gene by deletion mapping, we first determined whether the 1.0-kb BamHI segment carrying the pex gene could suppress the sp22 mutation even if this segment was targeted to a specific genomic targeting site (TS2) of SP22 mutant cells. The BamHI segment targeted to TS2 by pPEX-474 also suppressed the sp22 mutation (Fig. 4B). This indicates that the 1.0-kb BamHI segment carried a functional pex gene.
To determine the promoter region of the pex gene, we constructed a series of deletion derivatives of pPEX-474, which carried the upstream region of various lengths of the pex gene, targeted the derivatives to the TS2 of SP22 cells (Fig. 4B), and determined the suppression activity of the derivatives. Deletion derivative pPEX-363 carried the suppression activity, while derivatives pPEX-225 and pPEX-113 lost the activity. This indicates that nucleotides −363 to −129 (tss1) of the pex gene carry the pex promoters. No typical −10 or −35 bacterial promoter sequences were found.
Expression of the pex gene monitored by a luciferase reporter as bioluminescence.
We monitored the expression of the pex gene by using a bacterial luciferase luxAB gene set as a reporter gene. We fused an 872-bp BamHI-Ecl136II segment which carried the upstream region of the pex gene and the 5′ region of the pex ORF (nucleotides −474 to +398) with a promoterless segment of the luxAB gene set derived from V. harveyi (1), targeted the resulting Ppex::luxAB construct to TS2 of wild-type cells, and monitored the bioluminescence of the transformed cells. As shown in Fig. 5, the expression of the pex gene monitored as bioluminescence showed rhythms with a period of 24 h. Thus, the pex gene also showed circadian expression, as do many genes in Synechococcus (17). The phase of the rhythms observed in the pex reporter strain was similar to that of the rhythms observed in the psbAI reporter strains. The level of bioluminescence of the pex reporter strain was approximately one-fifth to one-third that of the psbAI reporter strains.
FIG. 5.
Bioluminescence rhythms of pex gene expression monitored by a luciferase reporter. Cells of pex reporter strain PREII were grown on BG-11 agar plates as colonies under LL, were subjected to a 12-h dark period to synchronize the circadian clocks, and then had their bioluminescence monitored under LL. Other conditions were the same as those described in the legend for Fig. 1.
Identification of pex gene product.
We examined the pex gene product by the epitope tag fusion method with a c-Myc epitope tag. To produce a Pex protein with a c-Myc epitope tag in Synechococcus, we constructed a pex–c-myc tag fusion gene which encoded a Pex protein fusion with a c-Myc epitope tag at the C terminus and which was inducible with IPTG. This construct was targeted to the TS2 of SP22 mutant cells. We also targeted a control construct without a c-Myc tag (lacIq-Ptrc::pex) to TS2. We induced the overexpression of the pex–c-myc tag fusion gene by the addition of 1 mM IPTG, prepared cell extracts from the transformant cells, and detected Pex protein in the extracts by Western blotting analysis with an antibody against the c-Myc epitope tag.
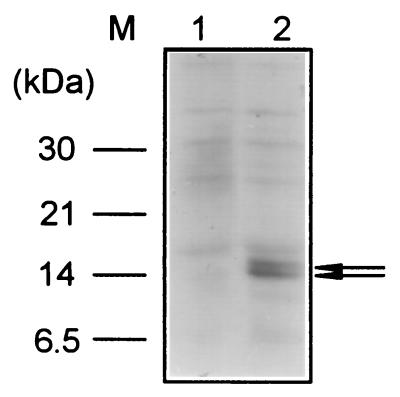
We detected two bands of c-Myc epitope-tagged products around a 14-kDa marker (Fig. 6, lane 2), while no bands were detected when cell extracts were prepared from cells carrying the control construct without a c-Myc epitope tag (lane 1). The smaller 14-kDa protein might be a degraded or processed product of the 15-kDa protein. The size of the tagged protein detected (15 kDa) was slightly smaller than its expected size (18 kDa). This discrepancy could be due to anomalous migration of this protein on SDS-polyacrylamide gels. Another possibility is that the N terminus of the Pex protein might be processed after its translation. It is also possible that the translation of pex mRNA might start from potential translation initiation codons (ATG or GTG) found within the pex ORF. Whichever is the explanation for the slight discrepancy between predicted and observed molecular masses, we conclude that the pex transcript was translated into the Pex protein.
FIG. 6.
Detection of Pex protein by the epitope tag fusion method with a c-Myc epitope tag. To express a Pex–c-Myc epitope tag fusion protein in Synechococcus cells, an overexpression construct carrying a pex–c-myc tag fusion gene together with the lacIq gene was constructed and targeted to the TS2 of SP22 mutant cells. Transformant cells were grown in BG-11 liquid medium in the presence of 1 mM IPTG to induce the overexpression of the pex–c-myc tag fusion gene. Cell extracts were prepared, subjected to electrophoresis on SDS-polyacrylamide gels, transferred to membranes, and analyzed by Western blotting experiments using anti-c-Myc antibody and anti-mouse immunoglobulin G antibody labeled with horseradish peroxidase. About 10 μg of total protein was applied to each lane. The presence of Pex–c-Myc epitope tag fusion protein expressed in Synechococcus cells is indicated by the arrow. Lane 1, SP22 cells carrying a control construct without a c-Myc epitope tag; lane 2, SP22 cells carrying the pex–c-myc tag fusion gene.
Effects of the disruption of the pex gene on bioluminescence rhythms.
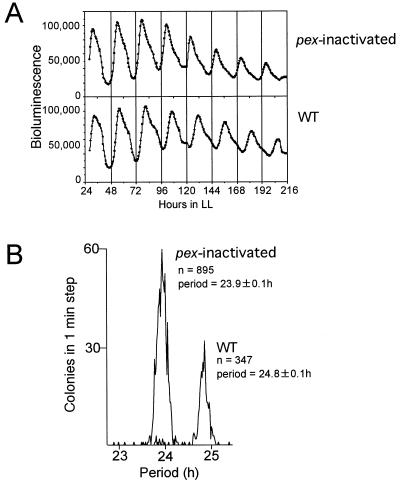
To examine the function of the pex gene in the circadian clock, we inactivated the pex gene by insertion of the Ω fragment (23) carrying a spectinomycin resistance gene into the pex ORF and monitored the bioluminescence rhythms of the pex-inactivated cells (Fig. 7A). The pex-inactivated cells grew as well as did wild-type cells and developed normal colonies, suggesting that the Pex protein is not likely to be fundamental to the metabolism of Synechococcus. The period of pex-inactivated cells was slightly shorter than that of wild-type cells: the means of the periods of pex-inactivated and wild-type cells ± standard deviations were 23.9 ± 0.1 (n = 895) and 24.8 ± 0.1 (n = 347) h, respectively (Fig. 7B). On the other hand, the amplitudes and waveforms of the rhythms were not much affected by the inactivation of the pex gene (Fig. 7A).
FIG. 7.
Effects of the inactivation of the pex gene on bioluminescence rhythms. The pex gene of CR1 cells was inactivated by insertion of the Ω fragment. Other conditions were the same as those described in the legend for Fig. 1. pex-inactivated, pex-inactivated cells; WT, wild-type cells (CR1 strain). (A) Bioluminescence rhythms of pex-inactivated cells and wild-type cells. (B) Histograms of the periods of bioluminescence rhythms. The vertical and horizontal axes indicate the number of colonies in a 1-min step and the period of the rhythm, respectively. n, number of colonies examined; period, means of periods with standard deviations. pex-inactivated and wild-type cells were able to be separately grouped.
To exclude the possibility that a partially active truncated protein might be synthesized in the pex-inactivated cells, we also replaced the whole pex ORF with the Ω fragment and carried out similar experiments. We obtained essentially the same results. Thus, disruption of the pex gene shortened the period of rhythms by about 0.9 h.
Disruption of the pex gene in pPEX-474 by insertion of the Ω fragment eliminated its sp22 suppressor activity (data not shown).
Effects of overexpression of the pex gene on the rhythms.
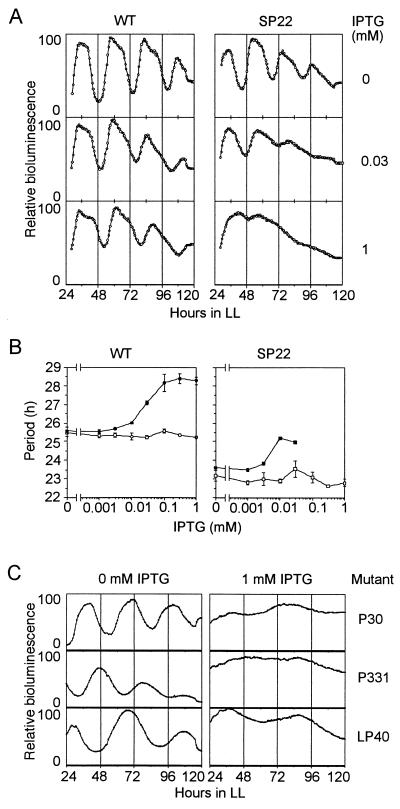
To confirm the period extension by the Pex protein, we overexpressed the pex gene in wild-type cells and SP22 mutant cells by induction with IPTG and monitored the bioluminescence rhythms.
We found that the periods of the rhythms were extended with IPTG in both wild-type cells and SP22 cells, while IPTG did not affect the rhythms in either type of control cells which carried a control vector without a pex insert (Fig. 8A). The period was extended gradually by increasing the concentration of IPTG. In wild-type cells, the means ± standard deviations of periods were 25.6 ± 0.2, 26.0 ± 0.05, 28.2 ± 0.5, and 28.3 ± 0.2 h (n = 4 in each case) at IPTG concentrations of 0, 0.01, 0.1, and 1 mM, respectively. Corresponding values for SP22 cells were 23.6 ± 0.2, 23.5 ± 0.3, and 25.0 ± 0.6 h (n = 4 in each case) at IPTG concentrations of 0, 0.001, and 0.01 mM, respectively (Fig. 8B). Higher doses of IPTG (0.03 to 1 mM) decreased the amplitude of rhythms. In SP22 cells, 1 mM IPTG caused arrhythmicity.
FIG. 8.
Effects of the overexpression of the pex gene on bioluminescence rhythms in wild-type cells and various clock mutant cells. To overexpress the pex gene, a lacIq-Ptrc::pex construct was constructed and targeted to TS2 in the genomes of AMC149, SP22, P30, P331, and LP40 cells by transformation of the cells with the resulting plasmids. A control construct without a pex insert was also targeted to the TS2 of both AMC149 and SP22 cells to construct control strains. The transformant clones were inoculated on BG-11 agar plates with or without IPTG and were grown under LL for 2 days. Other conditions were the same as those described in the legend for Fig. 1. WT, wild-type cells (AMC149); SP22, SP22 mutant cells. (A) Typical rhythms altered by overexpression of the pex gene. (B) Extension of the periods of rhythms in wild-type cells (AMC149) and SP22 mutant cells by overexpression of the pex gene induced with various concentrations of IPTG. The vertical axis indicates the mean of the period (± the standard deviation) calculated from four cultures. Open circles, AMC149 cells carrying the control construct without a pex insert (AMC149/Ptrc); solid circles, AMC149 cells carrying the pex overexpression construct (AMC149/Ptrc::pex); open squares, SP22 cells carrying the control construct (SP22/Ptrc); solid squares, SP22 cells carrying the pex overexpression construct (SP22/Ptrc::pex). (C) Decrease in the amplitude of rhythms in clock mutants caused by overexpression of the pex gene.
We examined the effects of pex overexpression on the rhythms of mutants with various periods (period mutants) and also observed decreases in amplitude in conjunction with lengthening periods due to the overexpression of the pex gene induced with 1 mM IPTG in the period mutants P30, P331, and LP40 (Fig. 8C). Thus, we conclude that Pex protein extended the period of the circadian clock in multiple genetic backgrounds.
In summary, we have identified what initially appeared to be a complementing gene for the sp22 mutation (16) to be a suppressor that acts as a period-extender gene (pex) that lengthens the period of the circadian clock in Synechococcus sp. strain PCC 7942. The apparent complementation of the sp22 mutation by transformation of SP22 mutant cells with the pS1K1 plasmid was caused by the duplication of the pex gene in the transformant genome. The pex gene is not essential for growth because disruption of the pex gene did not affect growth. The pex gene also does not have a crucial function in the generation of the circadian oscillation per se because the amplitude and shape of rhythms were little affected by the disruption of the pex gene (Fig. 7A). At present, we do not know the molecular mechanism for the period extension by the Pex protein because its sequence is not similar to those of any other known proteins. In preliminary experiments, we examined the effects of the disruption of the pex gene on the expression of circadian clock gene cluster kaiABC, which is essential for the clock function (10) and found that the expression of the kaiA gene was greatly enhanced by the pex disruption (16a). This result suggests that the Pex protein represses the expression of the kaiA gene. Pex may be a modifier of the circadian clock, while at the same time its expression is controlled by the circadian clock and therefore by clock gene cluster kaiABC (Fig. 5). We also examined possible interactions among the Pex and Kai proteins (KaiA, KaiB, and KaiC proteins) in vitro by using an in vitro transcription-translation system (16a) but could not discover any significant interactions. No pex homolog was found in the whole genome of the cyanobacterium Synechocystis sp. strain PCC 6803. At present, it is uncertain whether other cyanobacteria except for Synechocystis carry the pex gene.
ACKNOWLEDGMENTS
We are grateful for helpful discussions with S. S. Golden (Texas A&M University), and S. Itoh (National Institute for Basic Biology). We thank K. Furukawa, M. Sugita, M. Mutsuda, and T. Nohara of Nagoya University for helpful advice on Western blotting analysis and K. Nakai (Osaka University) and T. Kaneko and S. Tabata of Kazusa DNA Research Institute for the similarity search. We also thank C. H. Johnson (Vanderbilt University) for valuable comments on the manuscript.
This research was supported by grants from the Japanese Ministry of Education, Science and Culture, the Ishida Foundation (Nagoya, Japan), the Nissan Foundation (Tokyo, Japan), the Yamada Foundation (Osaka, Japan), the Chiba-Geigy Foundation for the Promotion of Science (Takarazuka, Japan), the Kurata Research Grant (Tokyo, Japan), and grants from the Japanese Ministry of Education, Science and Culture (04807006 and 05670079), the JSPS (U.S.-Japan Cooperative Program; BSAR382) and the Shimadzu Foundation (Kyoto, Japan) to T.K. S.K. was supported by the Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Baldwin T O, Berends T, Bunch T A, Holzman T F, Rausch S K, Shamansky L, Treat M L, Ziegler M M. Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry. 1984;23:3663–3667. doi: 10.1021/bi00311a014. [DOI] [PubMed] [Google Scholar]
- 2.Bünning E. The physiological clock. Heidelberg, Germany: Springer-Verlag; 1973. [Google Scholar]
- 3.Bustos S A, Golden S S. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J Bacteriol. 1991;173:7525–7533. doi: 10.1128/jb.173.23.7525-7533.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosthwaite S K, Dunlap J C, Loros J J. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 6.Geerts D, Bovy A, Vrieze G D, Borrias M, Weisbeek P. Inducible expression of heterologous genes targeted to a chromosomal platform in the cyanobacterium Synechococcus sp. PCC 7942. Microbiology. 1995;141:831–841. doi: 10.1099/13500872-141-4-831. [DOI] [PubMed] [Google Scholar]
- 7.Gekakis N, Saez L, Delahaye-Brown A M, Myers M P, Sehgal A, Young M W, Weitz C J. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science. 1995;270:811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- 8.Golden, S. S. Unpublished data.
- 9.Ishiura, M., S. Aoki, S. Kutsuna, C. R. Andersson, H. Iwasaki, S. S. Golden, C. H. Johnson, and T. Kondo. A gene cluster kaiABC that is essential for the circadian clock in cyanobacteria. Submitted for publication.
- 10.Ishiura, M., S. Kutsuna, and T. Kondo. Feedback expression control of the clock gene cluster kaiABC by kaiABC-encoded proteins in cyanobacteria. Submitted for publication.
- 10a.Ishiura, M., and S. Kutsuna. Unpublished data.
- 11.Jackson F R, Bargiello T A, Yun S H, Young M W. Product of per locus of Drosophila shares homology with proteoglycans. Nature. 1986;320:185–188. doi: 10.1038/320185a0. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 13.King D P, Zhao Y, Sangoram A M, Wilsbacher L D, Tanaka M, Antoch M P, Steeves T D, Vitaterna M H, Kornhauser J M, Lowrey P L, Turek F W, Takahashi J S. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo T, Strayer C A, Kulkarni R D, Taylor W, Ishiura M, Golden S S, Johnson C H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci USA. 1993;90:5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo T, Ishiura M. Circadian rhythms of cyanobacteria: monitoring the biological clocks of individual colonies by bioluminescence. J Bacteriol. 1994;176:1881–1886. doi: 10.1128/jb.176.7.1881-1885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo T, Tsinoremas N F, Golden S S, Johnson C H, Kutsuna S, Ishiura M. Circadian clock mutants of cyanobacteria. Science. 1994;266:1233–1236. doi: 10.1126/science.7973706. [DOI] [PubMed] [Google Scholar]
- 16a.Kutsuna, S., et al. Unpublished data.
- 17.Liu Y, Tsinoremas N F, Johnson C H, Lebedeva N V, Golden S S, Ishiura M, Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995;9:1469–1478. doi: 10.1101/gad.9.12.1469. [DOI] [PubMed] [Google Scholar]
- 18.McClung C R, Fox B A, Dunlap J C. The Neurospora clock gene frequency shares a sequence element with the Drosophila clock gene period. Nature (London) 1989;339:558–562. doi: 10.1038/339558a0. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed A, Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989;13:693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- 20.Munro S, Pelham H R B. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 21.Myers M P, Wager-Smith K, Wesley C S, Young M W, Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995;270:805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- 22.Porter R D. DNA transformation. Methods Enzymol. 1988;167:703–712. doi: 10.1016/0076-6879(88)67081-9. [DOI] [PubMed] [Google Scholar]
- 23.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 24.Reddy P, Jacquier A C, Abovich N, Petersen G, Rosbash M. The period clock locus of D. melanogaster codes for a proteoglycan. Cell. 1986;46:53–61. doi: 10.1016/0092-8674(86)90859-7. [DOI] [PubMed] [Google Scholar]
- 25.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 26.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose R E. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Sugita M, Sugiura M. The existence of eukaryotic ribonucleoprotein consensus sequence-type RNA-binding proteins in a prokaryote, Synechococcus 6301. Nucleic Acids Res. 1994;22:25–31. doi: 10.1093/nar/22.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsinoremas N F, Kutach A K, Strayer C A, Golden S S. Efficient gene transfer in Synechococcus sp. strains PCC 7942 and PCC 6301 by interspecies conjugation and chromosomal recombination. J Bacteriol. 1994;176:6764–6768. doi: 10.1128/jb.176.21.6764-6768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]