Abstract
The ordered assembly of the Caulobacter crescentus flagellum is accomplished in part through the organization of the flagellar structural genes in a regulatory hierarachy of four classes. Class II genes are the earliest to be expressed and are activated at a specific time in the cell cycle by the CtrA response regulator. In order to identify gene products required for early events in flagellar assembly, we used the known phenotypes of class II mutants to identify new class II flagellar genes. In this report we describe the isolation and characterization of a flagellar gene, fliX. A fliX null mutant is nonmotile, lacks a flagellum, and exhibits a marked cell division defect. Epistasis experiments placed fliX within class II of the flagellar regulatory hierarchy, suggesting that FliX functions at an early stage in flagellar assembly. The fliX gene encodes a 15-kDa protein with a putative N-terminal signal sequence. Expression of fliX is under cell cycle control, with transcription beginning relatively early in the cell cycle and peaking in Caulobacter predivisional cells. Full expression of fliX was found to be dependent on ctrA, and DNase I footprinting analysis demonstrated a direct interaction between CtrA and the fliX promoter. The fliX gene is located upstream and is divergently transcribed from the class III flagellar gene flgI, which encodes the basal body P-ring monomer. Analysis of the fliX-flgI intergenic region revealed an arrangement of cis-acting elements similar to that of another set of Caulobacter class II and class III flagellar genes, fliL-flgF, that is also divergently transcribed. In parallel with the FliL protein, FliX copurifies with the membrane fraction, and although its expression is cell cycle controlled, the protein is present throughout the cell cycle.
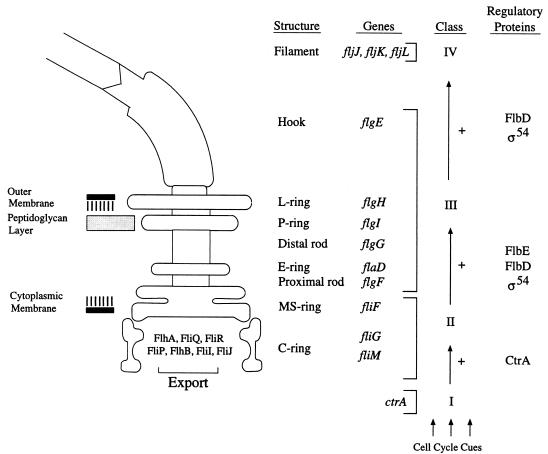
Midway through the Caulobacter cell cycle, the transcription of a cascade of flagellar genes is initiated, culminating in the construction of a single flagellum at one pole of a predivisional cell. The flagellum is comprised of three subassemblies (Fig. 1). The basal body, the most complex subassembly, spans the cell envelope and consists of (i) a compound ring in the inner membrane that is part of the flagellar motor, (ii) a rod that spans the cell wall, and (iii) stabilizing rings. The other subassemblies are a cell surface-associated hook and a long extracellular filament. Assembly of the substructures occurs in a cell-proximal–to–cell-distal order, accomplished, in part, by the organization of the flagellar structural genes in a regulatory hierarachy of four classes (6, 8, 34, 36, 55). The temporal expression of these classes of genes reflects the order in which the gene products are assembled into the growing structure (10, 21, 45).
FIG. 1.
Diagram of the C. crescentus flagellum. The name of each structure is accompanied by its gene designation(s). The structure of the C-ring complex is adapted from that proposed for the Salmonella typhimurium basal body (18). The genes encoding structural proteins are grouped into one of the three known flagellar gene classes (II, III, and IV), which together comprise the flagellar regulatory hierarchy (6, 8, 34, 55). Arrows indicate positive regulation (+) in which the transcription of genes within a class requires the expression of the gene products of the preceding class. The regulatory cascade is initiated by the class I gene product, CtrA, in response to as yet unidentified cell cycle cues (39). Class II gene products include proteins comprising early structural components of the flagellum (FliF), proteins which function in the flagellum-specific export pathway, and the transcription factors FlbD and RpoN (ς54). FlbE is the cognate histidine kinase for FlbD (50).
Class II genes (Fig. 1) are the earliest flagellar genes to be expressed (54). Mutations in these genes result in the cessation of class III and IV flagellar gene expression and a concomitant increase in the expression of other class II genes (34, 55). Class II genes encode (i) early structural components of the flagellum, including FliF, the protein monomer of the MS-ring (22, 36); (ii) components of the flagellum-specific export pathway required for the export of rod, hook, and filament proteins (19, 28, 41, 47, 58); and (iii) transcription factors such as RpoN (ς54) and the response regulator FlbD, which are required for the expression of class III and IV flagellar genes (2, 4, 5, 40, 52, 53). Class II flagellar genes have conserved promoter elements and are activated at a defined time in the Caulobacter cell cycle. With at least three class II gene promoters, PfliL, PfliQ, and PfliF, the activation of transcription is controlled by the CtrA response regulator (13, 39). CtrA is a global regulatory protein which also mediates the control of the initiation of chromosome replication and chromosomal DNA methylation at specific time points in the cell cycle (13, 39).
Strains with mutations in class II flagellar genes, in addition to being nonmotile, exhibit aberrant cell division, resulting in the formation of abnormally long filamentous cells (5, 12, 19, 57, 58). This cell division defect suggests that early events in flagellar biogenesis may function as a morphological checkpoint for cell cycle progression. In order to understand how flagellar biogenesis is coupled to the cell cycle, as well as to identify additional gene products required for early events in flagellar assembly, we used the known phenotypes of class II mutants to identify new flagellar genes. Here we describe the isolation and characterization of a gene, fliX, encoding a membrane protein required for flagellar assembly and normal cell division. Epistasis experiments indicate that fliX is a class II flagellar gene, suggesting that FliX functions at an early stage in flagellar biogenesis. We show that transcription of fliX is under cell cycle control, being expressed prior to the activation of class III flagellar genes, that full expression is dependent on ctrA (as is the case with other class II genes), and that CtrA interacts directly with the fliX promoter. The fliX gene is located upstream and is divergently transcribed from the class III flagellar gene flgI, which encodes the basal body P-ring monomer. This is the second example of a divergent promoter arrangement involving class II and class III flagellar operons. The conserved architecture of cis-acting elements within these intergenic regions may play a role in controlling the timing of flagellar gene expression during the cell cycle.
MATERIALS AND METHODS
Materials.
Oligonucleotides were obtained from either Operon Technologies (Alameda, Calif.) or the Beckman Center protein and nucleic acid facility at Stanford University. DNA-modifying enzymes, including restriction endonucleases, S1 nuclease, DNase I, and T4 polynucleotide kinase, were obtained from Boehringer Mannheim and New England Biolabs. [35S]Trans-Label (l-methionine, l-cysteine) was obtained from ICN Biomedicals, and [γ-32P]ATP was obtained from Amersham. Sequencing was performed with a Thermosequenase cycle sequencing kit from Amersham Life Science. Horseradish peroxidase conjugated to goat anti-rabbit immunoglobulin G was purchased from Boehringer Mannheim. Immobilon-P membranes were purchased from Millipore, and a Renaissance chemiluminescence kit was purchased from DuPont NEN. Formalin-fixed staphylococcus A cells (Immunoprecipitin) were purchased from Life Technologies (BRL). Other reagents were purchased from Sigma Chemical Co.
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this work are described in Table 1. The fliX null strain was made recombination deficient as previously described (32). Caulobacter crescentus NA1000 and mutant strains were grown at 30°C in either peptone-yeast extract (PYE) medium or M2 minimal glucose medium (14). C. crescentus cultures containing plasmids were supplemented with 1 μg of tetracycline per ml. PYE agar (1.5% agar) was supplemented with nalidixic acid (20 μg/ml), tetracycline (2 μg/ml), or kanamycin (20 μg/ml) as necessary. PYE swarm plates contained 0.25% agar. Escherichia coli TG-1 and S17-1 were grown at 37°C in Luria-Bertani broth supplemented with ampicillin (50 μg/ml), tetracycline (10 μg/ml), or gentamicin (20 μg/ml). Plasmids complementing the fliX null strain (LS2821) were obtained by subcloning fragments from cosmid pCM1 into the broad-host-range plasmid pMR4. Complementing plasmid subclones and plasmid-borne transcriptional fusions were introduced into C. crescentus cells by mating with E. coli donor strain S17-1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| C. crescentus | ||
| NA1000 | syn-1000 | 16 |
| LS107 | syn-1000 bla-6 | Dickon Alley |
| LS800 | rec-526 (pKM3001) | 37 |
| LS1218 | syn-1000 ΔfliF | 22 |
| LS1917 | syn1000 flgI::Tn5 | 32 |
| LS2195 | syn-1000 ctrA401 | 39 |
| LS2821 | syn-1000 fliX::Tn5 | This work |
| LS2996 | rec526 fliX::Tn5 | This work |
| SC508 | flaS153 (ΔfliQR) | 24 |
| SC1117 | flbN194::Tn5 str-152 | 10 |
| SC1131 | fliLM196::Tn5 | 57 |
| E. coli | ||
| TG1 | F′ lacIqproA+B+ lacZΔM15 supE44 | 7 |
| S17-1 | Integrated RP4-2, Tc::Mu, Km::Tn7 | 46 |
| Plasmids | ||
| pCM1 | pLAFR5-derived cosmid containing flgI | 32 |
| pCM2 | 5.3-kb SacI fragment containing flgI subcloned from pCM1 into pSKII+ | 32 |
| pCM9 | 800-bp PstI fragment subcloned from pCM2 into pRKlac290 | This work |
| pCM10 | 800-bp PstI fragment subcloned from pCM2 into pRKlac290 | This work |
| pCM11 | 5.3-kb SacI fragment containing flgI and fliX subcloned from pCM2 into pMR4 | This work |
| pCM12 | 1.3-kb SacI/XhoI fragment containing fliX subcloned from pCM2 into pMR4 | This work |
| pCM13 | 600-bp NlaIII fragment containing fliX subcloned from pCM2 into pMR4 | This work |
| pCM14 | 400-bp PstI/NlaIII fragment containing truncated fliX subcloned from pCM2 into pMR4 | This work |
| pCM15 | 613-bp PstI/XhoI fragment containing the fliX-flgI intergenic region subcloned from pCM2 into pSKII(+) | This work |
| pCM16 | 613-bp PstI/XhoI fragment containing the fliX-flgI intergenic region subcloned from pCM2 into pSKII(−) | This work |
| pCM17 | fliX cloned as a 420-bp EcoRI/HindIII fragment (generated by PCR) into pET21b | This work |
| pCM18 | 1.1-kb PstI fragment containing dksA subcloned from pCM1 into pSKII(+) | This work |
| pRKlac290 | lacZ transcriptional fusion vector, Tetr IncP1 replicon, mob+ | 20 |
| pMR4 | Broad-host-range vector, Tetr | C. Mohr and R. Roberts |
| pSKII(+) | Cloning and single-stranded phagemid | Stratagene |
| pSKII(−) | Cloning and single-stranded phagemid | Stratagene |
| pET21b | Ampr vector with T7 promoter for protein overexpression and generation of carboxyl-terminal polyhistidine tag | Novagen |
| pKM3001 | Pseudomonas aeruginosa recA-containing fragment in the broad-host-range vector pCP13 (Tetr) | 27 |
Generation of an fliX null strain.
The fliX null strain was generated by random transposon mutagenesis. The suicide plasmid pSUP202 carrying transposon Tn5 was introduced into C. crescentus LS107 by conjugation as previously described (15). Cultures of mutagenized cells were plated directly into swarm agar plates containing kanamycin to select for transposon-mediated antibiotic resistance. C. crescentus strains with mutations in class II flagellar genes, in addition to being nonmotile, exhibit defects in cell division, often giving rise to long filamentous cells. Cultures of cells which failed to form swarms on swarm agar were examined for cell division defects by light microscopy. Nonmotile cells exhibiting the filamentous phenotype were then tested for restoration of motility with cosmids containing known C. crescentus flagellar genes. One of the strains with nonmotile cells was complemented to motility with cosmid pCM1, which carries the flagellar flgI locus (26, 32). The Tn5 insertion in this strain was transduced into strain NA1000 (selecting for Kmr) to generate strain LS2821 (fliX::Tn5).
Electron microscopy.
Bacterial cultures were grown in PYE medium at 30°C to an optical density at 600 nm (OD600) of 0.6, transferred to a sterile 1.5-ml microcentrifuge tube, and concentrated by gentle centrifugation. The supernatant was removed, and the pellets were resuspended in the residual liquid. The concentrated cell suspension was transferred to a Formvar-coated grid and stained with uranyl acetate. Grids were examined in a Phillips model EM300 electron microscope.
DNA sequencing.
DNA sequencing was carried out by the dideoxynucleotide chain termination method (44), with single- or double-stranded DNA as the template. The sequencing ladder used to determine the transcriptional start site of fliX was generated with plasmid pCM4 as the template and the synthetic oligonucleotide FliX2 (5′ TCGACCGATCCAACGCCGGT 3′) corresponding to nucleotides +203 to +184 relative to the fliX +1A transcriptional start site. The sequencing reaction used to determine the transcriptional start site of flgI was generated with plasmid pCM5 as the template and the synthetic oligonucleotide FlgI1 (5′ TCGAGGCTCTGCTTGGTCAT 3′), which corresponds to nucleotides +241 to +222 relative to the flgI +1A transcriptional start site. Sequence compilation was carried out with the Genetics Computer Group package of the University of Wisconsin (9); for database searching, we employed the BLAST algorithm.
Promoter expression.
DNA fragments were inserted into the multiple-cloning site of the pRKlac290 vector to generate transcriptional fusions to lacZ. β-Galactosidase activity was assayed as described by Miller (30), with cells being grown in PYE medium plus tetracycline. Assays were done in triplicate on a minimum of two independent cultures. In order to examine the pattern of expression of the fliX and flgI promoters during the cell cycle, strains harboring plasmid-borne fliX and flgI transcriptional fusions to lacZ were synchronized by gradient centrifugation as previously described (16). At various time points during the cell cycle, 1-ml aliquots of cells were incubated for 5 min with 15 μCi of [35S]Trans-Label, centrifuged, and frozen on dry ice. Cells were lysed and immunoprecipitated as described previously (32). The immunoprecipitated proteins were separated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels and visualized by autoradiography. Quantification was done with a Molecular Dynamics PhosphorImager with ImageQuant software.
S1 nuclease protection analysis.
RNA was isolated from C. crescentus NA1000 via standard protocols (43). Double-stranded end-labeled probes for S1 nuclease assays were generated as described previously (43). Labeled probes (105 cpm) were mixed with RNA (40 or 80 μg), ethanol precipitated, and resuspended in S1 hybridization solution {60% formamide, 40 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] (pH 6.4), 400 mM NaCl, 1 mM EDTA (pH 8)}. The nucleic acids were denatured at 85°C for 10 min and then allowed to hybridize overnight at 50°C. S1 nuclease digestion and product preparation were carried out as described previously (43). The protected fragments were run alongside sequencing ladders generated with plasmids pCM4 and pCM5 as templates and synthetic oligonucleotide primers whose 5′ ends corresponded to the 5′ ends of the labeled probes, thus permitting direct comparison.
DNase I footprinting analysis.
The His-CtrA fusion protein used for DNase I footprinting analysis was purified as described previously (39). The DNA probes used for DNase I footprinting analysis were generated by digestion with BspEI (−92 relative to the fliX +1A transcriptional start site), dephosphorylation with alkaline phosphatase, and phosphorylation with [γ-32P]ATP (50 μci) catalyzed by T4 polynucleotide kinase. Following digestion with a second restriction endonuclease, the end-labeled fragments were gel purified. Probes (approximately 5 × 104 cpm) were incubated with different amounts of His-CtrA in a 200-μl reaction mixture containing 20 mM Tris-HCl [pH 7.4], 100 mM KCl, 5 mM MgCl2, 1 mM CaCl2, 2 mM dithiothreitol, 5% glycerol, 50 μg of bovine serum albumin per ml, and 4 μg of calf thymus DNA per ml. The reaction mixtures were incubated at 23°C for 10 min to allow complex formation, followed by a 3-min digestion of the DNA-protein complex with 60 ng of DNase I. The digestion was stopped by the addition of 10 μl of 0.5 M EDTA, and the labeled DNA fragments were isolated with a QIAquick PCR purification kit (Qiagen) according to the manufacturer’s instructions. The products of DNase I digestion were separated on sequencing gels in parallel with a sequencing ladder generated with the 22-mer oligonucleotide FliX1 (5′ CAGTCGCCGAGACACCCCCCGT 3′) extending from +82 to +61 relative to the fliX +1A transcriptional start site.
Production of a His-tagged FliX protein and generation of polyclonal antisera.
The fliX-coding region was amplified by PCR with the following primers: FliX4-EcoRI (5′ TCGGATGAAGAATTCCAGCACG 3′) and FliX5-HindIII (5′ CCCTGGCCTGAAGCTTGGCCA 3′). The resulting 420-bp fragment was digested with EcoRI and HindIII and ligated into pET-21b (Novagen), creating plasmid pCM17. The resulting plasmid generates an in-frame fusion between an N-terminal T7 epitope tag, the cloned fliX fragment, and a C-terminal His tag. Expression of FliX-His from the T7 promoter was induced in E. coli BL21 (DE3) by addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). A 250-ml culture was grown at 37°C in Luria-Bertani broth to an OD600 of 0.6, and cells were harvested by centrifugation 3 h after induction. The cell pellet was dissolved in ice-cold binding buffer (20 mM Tris-HCl [pH 7.9], 5 mM imidazole, 0.5 M NaCl) and lysed by sonication. Following centrifugation (20,000 × g for 15 min), FliX-His was purified from the supernatant fraction by chromatography on His Bind resin (Novagen) according to the manufacturer’s instructions. Column fractions containing FliX-His were pooled, concentrated with Centricon 10 Microconcentrator tubes (Amicon), and used directly to immunize rabbits. Immunization and sampling of the serum were performed by the Berkeley Antibody Company.
Western blot analysis.
Western blots on SDS-polyacrylamide gel electrophoresis (PAGE)-separated gels were performed as previously described (23). Blots were probed with primary antiserum at a dilution of 1:5,000 and then with the secondary antibody (goat anti-rabbit immunoglobulin G; Boehringer Mannheim) at a 1:10,000 dilution. Generation and use of antisera to the C. crescentus CcrM, flagellin, and FlgH proteins have been described previously (23, 48). Western blots were developed with a Renaissance chemiluminescence kit (DuPont NEN) according to the manufacturer’s instructions. Protein standards were prestained SDS-PAGE low-range standards (Bio-Rad).
Analysis of cellular localization of FliX.
C. crescentus cultures were harvested by centrifugation at an OD600 of 0.6 to 0.8. Cell lysis and separation of membrane fractions from soluble proteins by differential centrifugation were performed as described previously (23). Following centrifugation, the supernatant fraction was concentrated in Centricon 10 Microconcentrator tubes (Amicon). To prepare the extracellular protein fraction, a 100-ml sample of culture (OD600 = 0.6) was centrifuged at 7,000 × g for 20 min. The culture supernatant was then passed through a 0.45-μm-pore-size filter. Proteins were precipitated from the supernatant by addition of ammonium sulfate to 50% saturation at 4°C. Precipitated material was collected by centrifugation at 8,000 × g for 20 min. The pellet was washed with 70% ethanol, dried under vacuum, and resuspended in 1 ml of 10 mM Tris (pH 7.5)–1 mM EDTA.
Nucleotide sequence accession numbers.
The nucleotide sequence of the fliX-flgI locus has been previously described (26) and assigned GenBank accession no. M91448. The dksA sequence has been deposited in the GenBank database under accession no. AFO34413.
RESULTS
Isolation and complementation of the fliX null mutant.
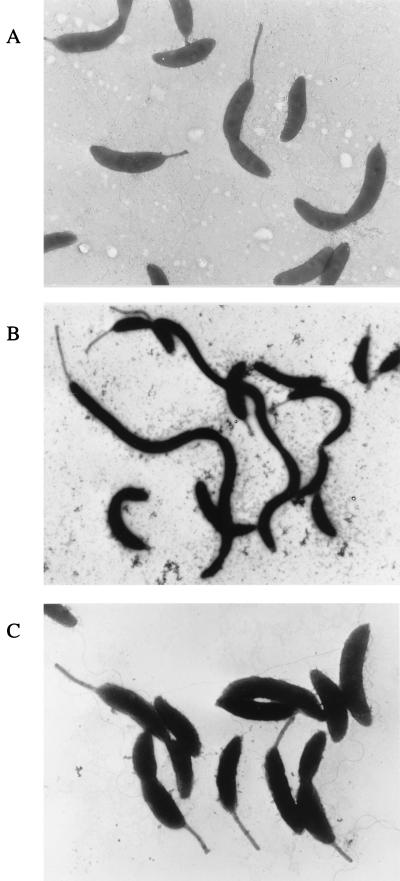
In order to screen for new class II flagellar genes, nonmotile mutants were generated by Tn5 mutagenesis and then examined for defects in cell division (see Materials and Methods). A total of 13 nonmotile mutants exhibiting the filamentous phenotype were generated and examined further. To determine if the mutations were in known flagellar loci, we first attempted to complement the motility defect using cosmids containing known flagellar genes. Three of the mutants were not complemented, and several new class II flagellar genes were identified (31, 47). One of the strains, LS2821, whose cells were nonmotile, had no visible flagella, and exhibited a marked cell division defect (Fig. 2), was complemented by cosmid pCM1, previously shown to contain flgI, a class III flagellar gene encoding the structural protein of the basal body P-ring (26, 32).
FIG. 2.
Electron micrographs of C. crescentus NA1000 and the fliX null strain LS2821. (A) Strain NA1000 showing normal swarmer, stalked, and predivisional cells. (B) LS2821 (fliX::Tn5) lacking flagella and forming filamentous cells. (C). LS2821 complemented with plasmid pCM13.
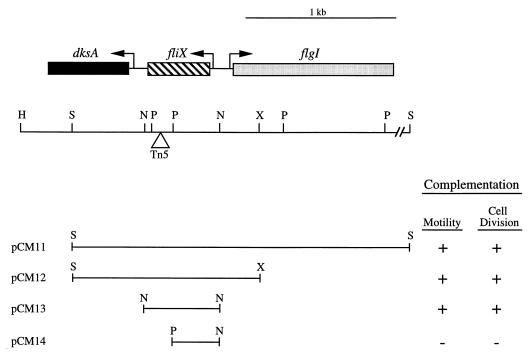
Southern blot hybridization analysis (data not shown) defined the site of Tn5 insertion in LS2821 to within a 240-bp PstI fragment located upstream of flgI (Fig. 3). Based on the previously published sequence of the flgI region (26), we identified two open reading frames (ORFs) upstream and oriented in the direction opposite to that of flgI (Fig. 3). The site of Tn5 insertion in LS2821 is within the first ORF, which we designated fliX, based on its requirement for flagellar assembly and on epistasis experiments that placed it within the flagellar hierarchy (see below). Plasmid pCM13, containing a 620-bp NlaIII fragment encoding only fliX, complemented both the motility defect and the cell division phenotype of LS2821 (Fig. 2 and 3). To confirm that an intact fliX gene was required for complementation, plasmid pCM14, bearing a deletion which lacked 260 bp of the 3′ end of fliX, was constructed. This plasmid failed to complement either the motility or the cell division phenotype of LS2821 (Fig. 3). To rule out possible polar effects, as well as the possibility that complementation by pCM13 containing the intact gene was due to chromosomal integration of the fliX-complementing DNA, complementation experiments were carried out with both LS2821 and LS2996, a fliX rec double mutant background. No observable difference in complementation results were observed with the two strains.
FIG. 3.
Complementation analysis of LS2821. (A) Genetic and restriction map of the C. crescentus chromosomal region upstream of flgI (26) and plasmids complementing (+) or not complementing (−) the motility and cell division phenotypes of LS2821. The triangle represents the site of Tn5 insertion in LS2821 as determined by Southern blot analysis (data not shown). Abbreviations: H, HindIII; S, SacI; N, NlaIII; P, PstI; X, XhoI.
Identification of the fliX and dksA ORFs.
The fliX ORF begins at nucleotide 874 and ends at nucleotide 440 of the published sequence of the flgI region (26). A potential ribosome binding site (GGAG) is located 7 bp upstream of the putative ATG start codon. The nucleotide sequence of fliX encodes a putative protein of 144 amino acids (Fig. 4A) with a predicted molecular mass of 14.5 kDa. The fliX gene product has no significant similarity to proteins in current databases. The N-terminal region of FliX has characteristics of signal-peptide sequences (38), including a charged amino terminus and a potential signal peptidase cleavage site (Gly-X-Ser ↑). The most probable cleavage site for the putative signal peptide is between amino acids 24 and 25. Hydrophobicity analysis showed that FliX has two hydrophobic domains (Fig. 4B). The first region, from residues 31 to 52, constitutes a typical alpha-helical transmembrane domain, suggesting that FliX may be an integral membrane protein.
FIG. 4.
(A) Predicted amino acid sequence of FliX. The possible signal peptide sequence is underlined, and the cleavage site is indicated by a vertical arrow. Numbers indicate amino acids. (B) Hydrophobicity plot of the FliX protein. The graph was generated by the Hydrophobicity Plot program in the DNA Strider package. Negative values indicate hydrophilic regions, and positive values indicate hydrophobic regions. (C) Alignment of C. crescentus (Cc) DksA with the homologs from E. coli (Ec) (25) and H. influenzae (Hi) (17). Asterisks indicate identical amino acids.
A second ORF, located adjacent to fliX, was designated dksA based on the similarity of its predicted protein product to DksA homologs from other organisms (Fig. 4C). Homology to the N termini of known dksA gene products was detected in database searches with the published sequence of the flgI region (26). This homology extended to the SacI site located at position 1 of the published sequence, suggesting that the remainder of the dksA gene extended downstream of the SacI site. A 1.1-kb PstI fragment with the SacI site located approximately midway in the fragment was subcloned from cosmid pCM1, and the complete nucleotide sequence of dksA was determined on both strands. The putative dksA initiation codon is located 208 bp downstream of the fliX termination codon. The dksA gene appears to be transcribed from its own promoter based on the activity of reporter fusions with the dksA-fliX intervening region (31). The C. crescentus DksA protein is 45% identical to the Haemophilus influenzae and E. coli DksA proteins (Fig. 4C). The dksA gene was originally identified in E. coli as a multicopy suppressor of the temperature-sensitive growth of a dnaK null strain (25). More recently, dksA has been identified as a multicopy suppressor of mutations in the chromosome-partitioning gene mukB (56), in the periplasmic protease tsp (3), and in the origin of replication of plasmid pCS101 (35). In E. coli the dksA gene has been shown to be nonessential (25). Insertional inactivation of the C. crescentus dksA gene has no observable affect on motility, growth, or cell division under normal growth conditions (31).
Placement of the fliX gene in class II of the flagellar regulatory hierarchy.
Epistasis experiments were performed in order to determine the position of fliX within the flagellar regulatory hierarchy. The nonmotile and aberrant cell division phenotypes of the fliX null strain are consistent with a mutation in a class II flagellar gene. Mutations in class II flagellar genes typically result in an increased expression of other class II flagellar genes and a dramatic reduction in the expression of class III and IV flagellar genes (34, 55). In order to analyze the effect of the fliX mutant on flagellar gene expression, transcriptional fusions of representative class II, III, and IV flagellar gene promoters to lacZ were introduced into LS2821 (fliX::Tn5) on low-copy-number plasmids and β-galactosidase activity was measured and compared to that of NA1000. The expression of class II promoters was elevated in LS2821, while that of class III and IV promoters was dramatically reduced (Table 2), consistent with the phenotype expected of a class II flagellar mutant.
TABLE 2.
Flagellar gene expression in the fliX null strain LS2821
| Class | Promoter | β-Galactosidase activity (U)
|
|
|---|---|---|---|
| NA1000 | LS2821 | ||
| II | fliF | 1,169 | 3,274 |
| fliLM | 968 | 2,738 | |
| fliQ | 1,194 | 3,429 | |
| III | flgH | 675 | 160 |
| flgI | 243 | 55 | |
| IV | fljL | 1,941 | 218 |
Conversely, we examined the effect of known flagellar mutants on fliX expression. An 800-bp PstI fragment extending 200 bp into the fliX coding region and 400 bp into the flgI coding region was cloned into pRKlac290, yielding recombinant plasmids pCM9 and pCM10, with the two possible orientations of the PstI insertion. The pCM9 construct places lacZ under the control of the fliX promoter, while the pCM10 construct places lacZ under the control of the promoter for the class III flagellar gene flgI. The pCM9 and pCM10 constructs introduced into strain NA1000 produced approximately 1,700 and 700 U of β-galactosidase, respectively. As shown in Table 3, the fliX::lacZ fusion (pCM9) showed elevated expression in class II flagellar mutants and was relatively unaffected by mutations in class III flagellar genes, as expected for the expression of a class II flagellar gene. In contrast, expression of the flgI::lacZ fusion (pCM10) was dramatically reduced in class II flagellar mutants and increased roughly twofold in class III flagellar mutants, the expected expression pattern for a class III flagellar gene.
TABLE 3.
Effects of flagellar gene mutations on fliX and flgI transcriptiona
| Strain (temp, °C) | Mutant gene | Class | β-Galactosidase activity (U) with:
|
|
|---|---|---|---|---|
| pCM9 (fliX::lacZ) | pCM10 (flgI::lacZ) | |||
| NA1000 | 1,683 | 683 | ||
| LS1917 | flgI | III | 1,577 | 1,400 |
| SC1117 | flgH | III | 1,474 | 1,211 |
| LS1218 | fliF | II | 3,870 | 26 |
| SC1131 | fliM | II | 3,727 | 30 |
| SC508 | fliQR | II | 4,834 | 31 |
| LS2821 | fliX | II | 4,051 | 68 |
| LS2195 (28) | ctrA401 | I | 1,065 | 745 |
| LS2195 (37) | ctrA401 | I | 300 | 157 |
Plasmids pCM9 (fliX::lacZ) and pCM10 (flgI::lacZ) were mated into strain NA1000, flagellar class II and III mutants, and strain LS2195 containing a temperature-sensitive mutation in ctrA (39). β-Galactosidase activity was measured as described in Materials and Methods and compared to that of NA1000.
A number of class II flagellar genes have been shown to be under transcriptional regulation by the CtrA response regulator (39). We therefore tested the activity of the fliX::lacZ fusion in strain LS2195, which contains a temperature-sensitive mutation in ctrA (39). Two hours after a shift to the nonpermissive temperature, β-galactosidase activity was reduced by over 50% (Table 3). A similar reduction in activity of the class III flgI-lacZ fusion was most likely indirect, due to the block in flagellar class II gene expression.
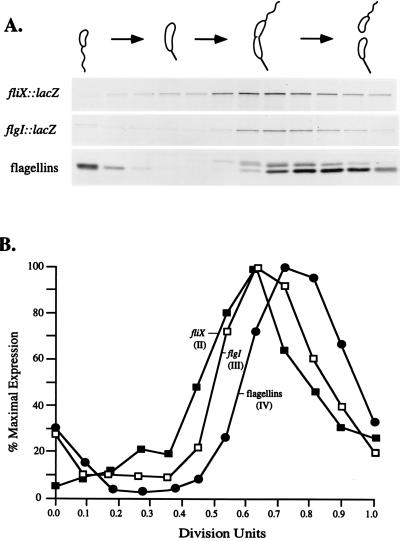
The transcription of class II flagellar genes is activated midway through the cell cycle, prior to the activation of class III and then class IV flagellar genes. To test the relative time of fliX transcriptional activation, swarmer cells were isolated from cultures of NA1000 containing either pCM9 (fliX::lacZ) or pCM10 (flgI::lacZ) and allowed to progress synchronously through the cell cycle. At 15-min intervals, samples were pulse-labeled with [35S]Trans-Label for 5 min and cell extracts were immunoprecipitated with anti-β-galactosidase antibody. As an internal control, samples of the same cell extracts were also immunoprecipitated with antibodies to the class IV flagellar filament proteins. Both fliX::lacZ and flgI::lacZ exhibited temporal regulation, with fliX transcription initiating somewhat before flgI relative to the flagellin internal control (Fig. 5). fliX promoter expression began between 0.3 and 0.4 division units and peaked in the predivisional cell, similar to that reported for other class II flagellar genes (36, 41, 49, 58). The expression of flgI began between 0.4 and 0.5 division units, slightly later than that of fliX, consistent with the time of activation of other class III flagellar promoters (10, 22, 32).
FIG. 5.
Patterns of fliX and flgI transcription during the cell cycle. Swarmer cells from cultures of NA1000 containing either pCM9 (fliX::lacZ) or pCM10 (flgI::lacZ) were isolated and allowed to progress synchronously through the cell cycle. At 15-min intervals, a portion of the culture was removed and proteins were pulse-labeled with [35S]Trans-Label. (A) Results of immunoprecipitation of 35S-labeled β-galactosidase from strains carrying either pCM9 (fliX::lacZ) or pCM10 (flgI::lacZ) and of 35S-labeled flagellar filament proteins, followed by electrophoresis on an SDS–10% polyacrylamide gel and autoradiography. The flagellin proteins were monitored as an internal control and indicator for the quality of cell synchrony. Shown are flagellins recovered from cells carrying the flgI::lacZ fusion. Flagellin proteins recovered from the cells carrying the fliX::lacZ fusion gave nearly identical immunoprecipitation and quantification profiles. The cell types present at each time point, monitored microscopically, are represented schematically above the graphs and the autoradiograms. (B) Quantification of the data in panel A with a Molecular Dynamics PhosphorImager, reported as the percentage of maximal expression for each protein. The duration of the cell cycle was approximately 150 min. Filled boxes represent expression of the fliX::lacZ fusion, open boxes represent expression of the flgI::lacZ fusion, and filled circles represent expression of flagellin. The Roman numerals in parentheses indicate the flagellar class for each gene (fliX, flgI) or protein (flagellins) examined.
Thus, the phenotype of the fliX mutant, the results of the epistasis experiments, and the time of fliX transcriptional activation relative to those of other flagellar genes leads to the conclusion that fliX is a class II flagellar gene and that the fliX gene product is likely to be required at an early stage in flagellar assembly.
Mapping the transcriptional start sites for fliX and flgI.
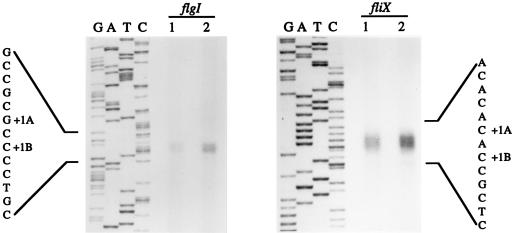
S1 nuclease protection assays were used to determine the fliX and flgI transcriptional start sites (Fig. 6). The 568-bp probe used for mapping the fliX transcriptional start site was end labeled at the SalI site located 158 bp into the fliX coding sequence. The 600-bp probe used for mapping the transcriptional start site of flgI was end labeled at the XhoI site located 212 bp into the flgI coding region. To define precisely the ends of the protected transcripts, DNA sequencing reactions were performed with primers whose 5′ ends matched the 5′ ends of the labeled probes. Two products of equal intensities, corresponding to two transcriptional start points, were consistently seen in S1 nuclease experiments with either the fliX or the flgI promoter probe (Fig. 6). The length of the protected fragments corresponding to the fliX transcript indicated that the transcriptional start sites (+1A and +1B) (Fig. 6) are located 45 and 43 bp, respectively, upstream of the fliX initiation codon. The lengths of the protected fragments corresponding to the flgI transcript indicated that the transcriptional start sites +1A and +1B (Fig. 6) are located 29 and 27 bp, respectively, upstream of the flgI initiation codon. The distance between the fliX and flgI transcriptional start sites (+1A) is 158 bp (Fig. 7B).
FIG. 6.
S1 nuclease mapping of the flgI and fliX transcriptional start sites. The lanes labeled G, A, T, and C show the products of the sequencing reactions that used oligonucleotide primers with 5′ sequences matching the labeled ends of the S1 nuclease probes. Lanes labeled 1 and 2 contained labeled probe hybridized to 40 and 80 μg of C. crescentus total RNA, respectively. The identified transcriptional start sites (+1A and +1B) are indicated next to the sequence.
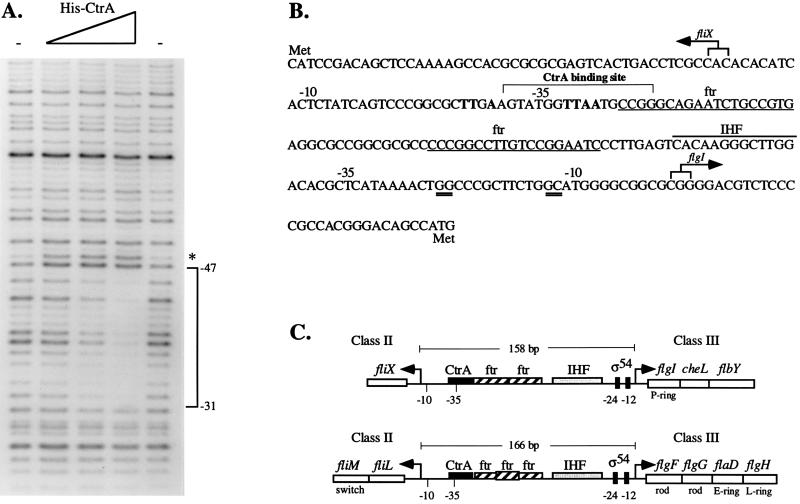
FIG. 7.
(A) Footprinting analysis of His-CtrA at the fliX promoter. An end-labeled BspEI-PstI fragment containing sequences from −92 to +220 (relative to the +1A transcriptional start site) of the fliX promoter was incubated in the presence or absence of CtrA and then digested with DNase I as described in Materials and Methods. The triangle indicates increasing concentrations of His-CtrA (10, 20, and 40 μg). The minus signs indicate that no protein was added, and the asterisk indicates a hypersensitive site. (B) Nucleotide sequence of the fliX-flgI intergenic region showing −10 and −35 regions for fliX and flgI as well as the CtrA binding site and potential cis-acting regulatory elements. Numbers are given relative to the +1A transcriptional start sites. The nucleotide sequence is shown from 5′ to 3′ in the direction of transcription of flgI. Boldface nucleotides denote the consensus CtrA binding site, and ftr denotes the potential binding sites for the transcriptional activator FlbD. Double-underlined sequences at −24 and −12 of the flgI promoter conform to the consensus sequence for ς54-dependent promoters. Overlined is the conserved binding site for integration host factor (IHF). Arrows indicate the transcriptional start sites determined by S1 nuclease analysis. (C) Comparison of the organization of cis-acting elements in the fliX-flgI (26) and fliL-flgF (29, 53) intergenic regions. Arrows denote transcriptional start sites.
DNase I footprinting analysis of the CtrA binding site in the fliX promoter.
Class II flagellar genes are driven by a unique class of promoters which contain a conserved DNA motif recognized by the CtrA response regulator (39). Examination of the fliX promoter region revealed a sequence, centered around −35, which matched seven of nine bases of the consensus CtrA binding site (TTAA-N7-TTAAC) (Fig. 7B). The CtrA binding motif is typically located in the −35 regions of other class II flagellar promoters (39). To determine if CtrA recognizes this site and binds directly to the fliX promoter, DNase I footprinting studies were performed with a purified His-CtrA fusion protein. The His-CtrA fusion protein has previously been shown to bind the class II fliQ promoter, at a region overlapping the CtrA binding motif (39). In the presence of CtrA, a single protected region of 17 bp, partially overlapping the binding motif, was observed. This region extended from positions −31 to −47 relative to the fliX +1A transcriptional start site (Fig. 7A and B). These results, and the finding that transcription of fliX is decreased in a strain bearing a ctrA401 allele, suggest that CtrA plays a direct role in the regulation of fliX transcription.
Analysis of the fliX-flgI intergenic region revealed a number of other potential cis-acting regulatory elements (Fig. 7B and C). Overlapping the CtrA binding site within the fliX promoter is the first of two ftr (flagellar transcriptional regulation) elements. The ftr elements are binding sites for the transcriptional activator FlbD and are typically present in pairs approximately 100 bp upstream of the transcriptional start sites for class III flagellar genes (4, 33, 51, 53). Sequences at −24 and −12 of the flgI promoter conform to the consensus sequence for ς54-dependent promoters typically found in other class III flagellar genes (53). Between this sequence and the ftr elements is a consensus binding site for integration host factor. The presence and arrangement of regulatory elements within the fliX-flgI intergenic region is similar to those of the fliL-flgF intergenic region (29), which controls the class II fliLM operon and the class III flgFGDH flagellar operon (Fig. 7C).
FliX is a membrane protein that is present throughout the cell cycle.
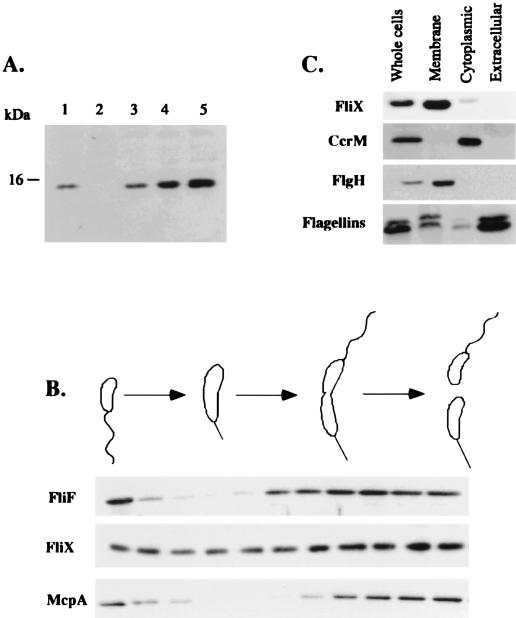
A polyhistidine-tagged FliX protein was overexpressed, purified, and used to generate antibodies. The FliX antibody recognized a protein of 15 kDa (Fig. 8A) which is similar in size to the 14.5-kDa protein predicted from the fliX nucleotide sequence. The fliX null strain LS2821 completely lacks the 15-kDa protein, which was restored when the fliX gene was provided in trans on the complementing plasmid pCM13 (Fig. 8A, lanes 2 and 3). Elevated levels of the 15-kDa protein were observed in protein extracts from the class II fliF and fliQR flagellar mutants (Fig. 8A, lanes 4 and 5), as was expected from the expression analysis of the fliX promoter (Table 3).
FIG. 8.
Western blot characterization of FliX. (A) Western blot analysis of the FliX protein in NA1000 and flagellar mutants. Equal amounts of protein from whole-cell extracts were separated by SDS–12% PAGE and immunoblotted with anti-FliX antibody. Lanes: 1, NA1000; 2, LS2821 (fliX::Tn5); 3, LS2821 complemented with plasmid pCM13; 4, LS1218 (fliF mutant); 5, SC508 (fliQR mutant). (B) FliX protein levels throughout the cell cycle. Swarmer cells isolated from a culture of NA1000 were suspended in fresh medium and allowed to progress through the cell cycle. At 15-min intervals, 1-ml aliquots were removed. Equal amounts of total protein from each time point were separated by SDS-PAGE and immunoblotted with antisera to FliX, FliF, and McpA proteins. Shown schematically above the autoradiograms are the cell types present at each time point as determined by light microscopy. (C) Subcellular localization of the FliX protein. Cell extracts from strain NA1000 were fractionated into membrane, cytoplasmic, and extracellular fractions as described in Materials and Methods. Equal amounts of protein from each fraction and a whole-cell control were separated by SDS-PAGE and blotted onto polyvinylidene difluoride membranes. Membranes were probed separately with polyclonal antisera to FliX, the CcrM DNA methyltransferase, the FlgH flagellar ring protein, and C. crescentus flagellins.
Cell-type-specific proteolysis has been shown to play an important role in maintaining asymmetry in the predivisional cell. The McpA chemoreceptor (1) and FliF flagellar motor protein (22), for example, are specifically degraded during the transition from swarmer to stalked cell. In contrast, the flagellar FlgH ring protein and the FliL protein are present throughout the cell cycle (23, 32). In order to examine FliX protein levels during the cell cycle, cell extracts were prepared from samples of synchronous cultures at several times during the cell cycle and assayed by Western blot analysis with anti-FliX antibodies. As controls, the extracts were also assayed with anti-FliF or anti-McpA antibodies. As previously shown, the FliF and McpA proteins are turned over during the transition from swarmer to stalked cells (Fig. 8B). In contrast, the steady-state level of the FliX protein remains constant (Fig. 8B), suggesting that FliX is not turned over during the cell cycle, even though its synthesis is under temporal control (Fig. 5).
The N-terminal region of FliX contains a putative cleavable signal sequence, followed by a hydrophobic transmembrane domain, suggesting that FliX might function extracytoplasmically. To determine the cellular location of the FliX protein, cell extracts from strain NA1000 were separated into cytoplasmic, membrane, and extracellular fractions and Western blot analysis with anti-FliX antibody was used to detect the proteins in these fractions. As a control for the purity of the fractions, each sample was also probed with antisera to the cytoplasmic CcrM DNA methyltransferase (48), the outer membrane L-ring protein, FlgH (23), and the predominantly extracellular flagellins. FliX was found almost exclusively in the membrane fraction, although a small but detectable amount was also seen in the cytoplasmic fraction (Fig. 8C).
DISCUSSION
We have isolated and characterized fliX, a gene required for flagellar assembly and normal cell division in C. crescentus. Epistasis experiments place fliX in class II of the flagellar regulatory hierarchy, indicating that the fliX gene product functions at an early stage in flagellar biogenesis. The observations that FliX copurifies with the membrane fraction and that the FliX predicted amino acid sequence has a potential N-terminal signal sequence and at least one transmembrane domain indicate that FliX functions either in or in association with the membrane. In Caulobacter, the only flagellar proteins other than FliX that possess N-terminal signal sequences are the protein monomers for the P- and L-rings (11, 26). The P- and L-ring proteins are presumed to be exported across the cytoplasmic membrane to their respective destinations in the cell envelope via the general secA-dependent pathway. This export is in contrast to that of the axial rod, hook, and filament subunit proteins, which do not have cleaveable signal sequences and are exported by a flagellum-specific export apparatus (28). The presence of an N-terminal signal sequence suggests that FliX may use the same pathway as the P- and L-ring proteins for translocation to the membrane. The function of FliX in the cell envelope has not been determined. Although the genes encoding the known substructures of the flagellum have been identified (Fig. 1), it is possible that FliX functions as a transient component of the flagellum that is required for the assembly process. FliX may contribute to the targeting or assembly of the P- and L-ring protein monomers at the cell pole. Immunolocalization and coimmunoprecipitation studies with the anti-FliX antibodies are under way to further elucidate the role of FliX.
A hallmark of the flagellar regulatory hierarchy in Caulobacter is the sequential activation of the genes required to assemble the flagellum. It has recently been shown that the cues which initiate flagellar biogenesis at a specific time in the cell cycle are mediated through the signal transduction response regulator CtrA, by regulating the transcriptional activity of class II flagellar genes (39). We have shown that fliX expression is activated at the same time in the cell cycle as other class II genes and that its full expression is dependent on CtrA, which interacts directly with the fliX promoter. We propose that in vivo, the temporal control of fliX expression during the Caulobacter cell cycle is mediated directly through binding and transcriptional activation by CtrA.
While the CtrA protein regulates the transcription of class II flagellar genes, the FlbD response regulator mediates the transition from early to late flagellar gene expression by activating class III and IV flagellar genes (4, 40, 52, 53). We have shown that the class II fliX gene and the class III flgI gene are divergently transcribed and that their transcriptional start sites are separated by a 158-bp intergenic region. We have demonstrated that the intergenic region mediates the cell cycle control of fliX and flgI expression. Analysis of this region revealed not only the presence of a CtrA binding motif but also two FlbD binding sites, termed ftr (Fig. 7B). This arrangement of cis-acting elements is similar to that of another set of class II and class III flagellar genes, fliL-flgF, that is also divergently transcribed (see Fig. 7C). We have recently demonstrated that CtrA binds to the class II fliL promoter in vitro (42). A direct interaction between FlbD and the flgI and flgF promoters has not yet been demonstrated. However, Wu et al. (53) have shown that flgI and flgF promoter fragments, with the ftr elements intact, can be transcriptionally activated by FlbD, indicating that FlbD binds to the ftr elements to control flgI and flgF expression. The fliX-flgI and fliL-flgF intergenic regions, therefore, appear to be important control sites for both the initiation of flagellar biogenesis by CtrA and the transition from early to late flagellar gene expression mediated by FlbD.
Does the regulatory control exerted on the fliX-flgI and fliL-flgF intergenic regions reflect a functional relationship between the products of these divergent transcription units? The class II fliLM operon encodes FliL, a membrane protein of unknown function, and FliM, a flagellar switch protein. The class III flgF operon encodes the basal body rod proteins and FlgH, the L-ring monomer. We have previously shown that the FlgH protein is expressed, but unstable, in a flgI null strain (32), suggesting that the assembly of FlgI into the P-ring and FlgH into the L-ring is coordinately controlled. In an attempt to establish a similar relationship between fliX and the products of the fliLM operon, we examined FliL and FliM protein levels in LS2821, the fliX null strain. We found that FliL and FliM were present in the fliX null strain, indicating that the absence of FliX does not affect the stability of these proteins (31). Alternatively, the regulatory control exerted on the fliX-flgI intergenic region may reflect a functional relationship between FliX and FlgI. For example, if FliX has a role in the assembly of the periplasmic FlgI P-ring monomers, then the regulatory control of the fliX-flgI intergenic region would ensure that FliX synthesis occurs prior to that of FlgI, its target substrate.
ACKNOWLEDGMENTS
We thank members of the Shapiro lab for critical readings of the manuscript.
This work was supported by National Institutes of Health grant GM 32506/5120MZ.
REFERENCES
- 1.Alley M R, Maddock J R, Shapiro L. Requirement of the carboxyl terminus of a bacterial chemoreceptor for its targeted proteolysis. Science. 1993;259:1754–1757. doi: 10.1126/science.8456303. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D K, Ohta N, Wu J, Newton A. Regulation of the Caulobacter crescentus rpoN gene and function of the purified sigma 54 in flagellar gene transcription. Mol Gen Genet. 1995;246:697–706. doi: 10.1007/BF00290715. [DOI] [PubMed] [Google Scholar]
- 3.Bass S, Gu Q, Christen A. Multicopy suppressors of Prc mutant Escherichia coli include two HtrA (DegP) protease homologs (HhoAB), DksA, and truncated RlpA. J Bacteriol. 1996;178:1154–1161. doi: 10.1128/jb.178.4.1154-1161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson A K, Ramakrishnan G, Ohta N, Feng J, Ninfa A J, Newton A. The Caulobacter crescentus FlbD protein acts at ftr sequence elements both to activate and to repress transcription of cell cycle-regulated flagellar genes. Proc Natl Acad Sci USA. 1994;91:4989–4993. doi: 10.1073/pnas.91.11.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun Y V, Shapiro L. A temporally controlled sigma-factor is required for polar morphogenesis and normal cell division in Caulobacter. Genes Dev. 1992;6:2395–2408. doi: 10.1101/gad.6.12a.2395. [DOI] [PubMed] [Google Scholar]
- 6.Bryan R, Glaser D, Shapiro L. A genetic regulatory hierarchy in Caulobacter development. Adv Genet. 1990;27:1–31. doi: 10.1016/s0065-2660(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 7.Carter P, Bedouelle H, Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985;13:4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champer R, Dingwall A, Shapiro L. Cascade regulation of Caulobacter flagellar and chemotaxis genes. J Mol Biol. 1987;194:71–80. doi: 10.1016/0022-2836(87)90716-9. [DOI] [PubMed] [Google Scholar]
- 9.Devereux D, Hableri P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwall A, Garman J D, Shapiro L. Organization and ordered expression of Caulobacter genes encoding flagellar basal body rod and ring proteins. J Mol Biol. 1992;228:1147–1162. doi: 10.1016/0022-2836(92)90322-b. [DOI] [PubMed] [Google Scholar]
- 11.Dingwall A, Gober J W, Shapiro L. Identification of a Caulobacter basal body structural gene and a cis-acting site required for activation of transcription. J Bacteriol. 1990;172:6066–6076. doi: 10.1128/jb.172.10.6066-6076.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingwall A, Zhuang W Y, Quon K, Shapiro L. Expression of an early gene in the flagellar regulatory hierarchy is sensitive to an interruption in DNA replication. J Bacteriol. 1992;174:1760–1768. doi: 10.1128/jb.174.6.1760-1768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domian I J, Quon K C, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 14.Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 1991;204:372–384. doi: 10.1016/0076-6879(91)04019-k. [DOI] [PubMed] [Google Scholar]
- 15.Ely B, Croft R H. Transposon mutagenesis in Caulobacter crescentus. J Bacteriol. 1982;149:620–625. doi: 10.1128/jb.149.2.620-625.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J, Dougherty B A, Merrick J M, McKenney K, Sutton G, Fitzhugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L, Glodek A, Kelley J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 18.Francis N R, Sosinsky G E, Thomas D, DeRosier D J. Isolation, characterization, and structure of bacterial flagellar motors containing the switch complex. J Mol Biol. 1994;235:1261–1270. doi: 10.1006/jmbi.1994.1079. [DOI] [PubMed] [Google Scholar]
- 19.Gober J W, Boyd C H, Jarvis M, Mangan E K, Rizzo M F, Wingrove J A. Temporal and spatial regulation of fliP, an early flagellar gene of Caulobacter crescentus that is required for motility and normal cell division. J Bacteriol. 1995;177:3656–3667. doi: 10.1128/jb.177.13.3656-3667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gober J W, Shapiro L. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol Biol Cell. 1992;3:913–926. doi: 10.1091/mbc.3.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahnenberger K M, Shapiro L. Identification of a gene cluster involved in flagellar basal body biogenesis in Caulobacter crescentus. J Mol Biol. 1987;194:91–103. doi: 10.1016/0022-2836(87)90718-2. [DOI] [PubMed] [Google Scholar]
- 22.Jenal U, Shapiro L. Cell cycle-controlled proteolysis of a flagellar motor protein that is asymmetrically distributed in the Caulobacter predivisional cell. EMBO J. 1996;15:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 23.Jenal U, White J, Shapiro L. Caulobacter flagellar function, but not assembly, requires FliL, a non-polarly localized membrane protein present in all cell types. J Mol Biol. 1994;243:227–244. doi: 10.1006/jmbi.1994.1650. [DOI] [PubMed] [Google Scholar]
- 24.Johnson R C, Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979;137:627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang P J, Craig E A. Identification and characterization of a new Escherichia coli gene that is a dosage-dependent suppressor of a dnaK deletion mutation. J Bacteriol. 1990;172:2055–2064. doi: 10.1128/jb.172.4.2055-2064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khambaty F M, Ely B. Molecular genetics of the flgI region and its role in flagellum biosynthesis in Caulobacter crescentus. J Bacteriol. 1992;174:4101–4109. doi: 10.1128/jb.174.12.4101-4109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokjohn T A, Miller R. Characterization of the Pseudomonas aeruginosa recA analog and its protein product: rec-102 is a mutant allele of the P. aeruginosa PAO recA gene. J Bacteriol. 1987;169:1499–1508. doi: 10.1128/jb.169.4.1499-1508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macnab R. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 29.Marques M V, Gober J. Activation of a temporally regulated Caulobacter promoter by upstream and downstream sequence elements. Mol Microbiol. 1995;16:279–289. doi: 10.1111/j.1365-2958.1995.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 31.Mohr, C. Unpublished data.
- 32.Mohr C D, Jenal U, Shapiro L. Flagellar assembly in Caulobacter crescentus: a basal body P-ring null mutation affects stability of the L-ring protein. J Bacteriol. 1996;178:675–682. doi: 10.1128/jb.178.3.675-682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullin D, Minnich S, Chen L S, Newton A. A set of positively regulated flagellar gene promoters in Caulobacter crescentus with sequence homology to the nif gene promoters of Klebsiella pneumoniae. J Mol Biol. 1987;195:939–943. doi: 10.1016/0022-2836(87)90497-9. [DOI] [PubMed] [Google Scholar]
- 34.Newton A, Ohta N, Ramakrishnan G, Mullin D, Raymon G. Genetic switching in the flagellar gene hierarchy of Caulobacter requires negative as well as positive regulation of transcription. Proc Natl Acad Sci USA. 1989;86:6651–6655. doi: 10.1073/pnas.86.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkubo S, Yamaguchi K. A suppressor of mutations in the region adjacent to iterons of pSC101 ori. J Bacteriol. 1997;179:2089–2091. doi: 10.1128/jb.179.6.2089-2091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta N, Chen L S, Mullin D A, Newton A. Timing of flagellar gene expression in the Caulobacter cell cycle is determined by a transcriptional cascade of positive regulatory genes. J Bacteriol. 1991;173:1514–1522. doi: 10.1128/jb.173.4.1514-1522.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Neill E A, Hynes R H, Bender R A. Recombination deficient mutant of Caulobacter crescentus. Mol Gen Genet. 1985;198:275–278. doi: 10.1007/BF00383006. [DOI] [PubMed] [Google Scholar]
- 38.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan G, Newton A. FlbD of Caulobacter crescentus is a homologue of the NtrC (NRI) protein and activates sigma 54-dependent flagellar gene promoters. Proc Natl Acad Sci USA. 1990;87:2369–2373. doi: 10.1073/pnas.87.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramakrishnan G, Zhao J L, Newton A. The cell cycle-regulated flagellar gene flbF of Caulobacter crescentus is homologous to a virulence locus (lcrD) of Yersinia pestis. J Bacteriol. 1991;173:7283–7292. doi: 10.1128/jb.173.22.7283-7292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reisenauer, A. Unpublished data.
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro L. The bacterial flagellum: from genetic network to complex architecture. Cell. 1995;80:525–527. doi: 10.1016/0092-8674(95)90505-7. [DOI] [PubMed] [Google Scholar]
- 46.Simon R, Piefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1993;1:784–790. [Google Scholar]
- 47.Stephens C, Mohr C, Boyd C, Maddock J, Gober J, Shapiro L. Identification of the fliI and fliJ components of the Caulobacter flagellar type III protein secretion system. J Bacteriol. 1997;179:5355–5365. doi: 10.1128/jb.179.17.5355-5365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephens C, Reisenauer A, Wright R, Shapiro L. A cell cycle-regulated bacterial DNA methyltransferase is essential for viability. Proc Natl Acad Sci USA. 1996;93:1210–1214. doi: 10.1073/pnas.93.3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens C M, Shapiro L. An unusual promoter controls cell-cycle regulation and dependence on DNA replication of the Caulobacter fliLM early flagellar operon. Mol Microbiol. 1993;9:1169–1179. doi: 10.1111/j.1365-2958.1993.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 50.Wingrove J A, Gober J. Identification of an asymmetrically localized sensor histidine kinase responsible for temporally and spatially regulated transcription. Science. 1996;274:597–601. doi: 10.1126/science.274.5287.597. [DOI] [PubMed] [Google Scholar]
- 51.Wingrove J A, Gober J W. A sigma 54 transcriptional activator also functions as a pole-specific repressor in Caulobacter. Genes Dev. 1994;8:1839–1852. doi: 10.1101/gad.8.15.1839. [DOI] [PubMed] [Google Scholar]
- 52.Wingrove J A, Mangan E K, Gober J W. Spatial and temporal phosphorylation of a transcriptional activator regulates pole-specific gene expression in Caulobacter. Genes Dev. 1993;7:1979–1992. doi: 10.1101/gad.7.10.1979. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Benson A K, Newton A. Global regulation of a sigma 54-dependent flagellar gene family in Caulobacter crescentus by the transcriptional activator FlbD. J Bacteriol. 1995;177:3241–3250. doi: 10.1128/jb.177.11.3241-3250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J, Newton A. Regulation of the Caulobacter flagellar gene hierarchy; not just for motility. Mol Microbiol. 1997;24:233–239. doi: 10.1046/j.1365-2958.1997.3281691.x. [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Dingwall A, Shapiro L. Negative transcriptional regulation in the Caulobacter flagellar hierarchy. Proc Natl Acad Sci USA. 1989;86:6656–6660. doi: 10.1073/pnas.86.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamanaka K, Mitani T, Ogura T, Niki H, Hiraga S. Cloning, sequencing, and characterization of multicopy suppressors of a mukB mutation in Escherichia coli. Mol Microbiol. 1994;13:301–312. doi: 10.1111/j.1365-2958.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 57.Yu J, Shapiro L. Early Caulobacter crescentus genes fliL and fliM are required for flagellar gene expression and normal cell division. J Bacteriol. 1992;174:3327–3338. doi: 10.1128/jb.174.10.3327-3338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhuang W Y, Shapiro L. Caulobacter FliQ and FliR membrane proteins, required for flagellar biogenesis and cell division, belong to a family of virulence factor export proteins. J Bacteriol. 1995;177:343–356. doi: 10.1128/jb.177.2.343-356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]