Your patient, a 25-year-old man who smokes and has hypertension, tells you of an article he read in the New York Times1 about a “genetic report card” that will tell people their risk factors. He wants to know how soon your office will be offering the genetic test.
Table 1.
| The role of primary care physicians in genetics |
|---|
|
The impending “genetic revolution” has been so overpromoted by members of the scientific, corporate, media, and political communities that it is tempting to dismiss the role of genetics in primary care practice entirely. However, physicians should not be misled by the hype into missing the true value of genetics.
Although the peer-reviewed medical literature seemingly overlooks the present when asking clinicians to contemplate the future role of genetics in primary care,2 primary care practitioners can incorporate genetics into their practices now by recognizing the role of genetics in their practices (see box).
A genetic origin accounts for a proportion of cases of many common diseases. Furthermore, the identification of a genetic cause may contribute to better patient management. In some cases, genetic information can be used to provide tailored preventive health care. Helping to distinguish those at high risk from those at low risk of a disease allows physicians to target special interventions appropriately.
As the role of genetics in primary care evolves, generalists presumably will need to become skilled in case recognition, risk assessment, and the appropriate use of genetic testing, as discussed in this article.
WHY MUST PRIMARY CARE PHYSICIANS UNDERSTAND GENETICS?
Case recognition
By increasing their awareness of the manifestations of common genetic diseases, practitioners can expand the differential diagnoses of some patients' symptoms to include common genetic diseases. Whereas all diseases have both a genetic and an environmental component, in some, the genetic effect predominates, and these are commonly referred to as “genetic diseases.”
Information requests
Practitioners need to be able to respond to patients' questions about the possibility of a genetic disease in the family. A survey conducted by the American Medical Association in March 1998 found that 71% of patients who questioned whether there was a genetic disease in their family would contact their primary care physician first.3
Primary care of a patient with a genetic disease
Practitioners need to know how patients' primary genetic diseases may affect their health, what secondary diseases they are likely to develop, and the unusual ways that common diseases may present in these patients.
Screening
Genetic screening measures historically have focused on reproductive issues, such as preconception screening for those at risk of being carriers of autosomal recessive diseases (eg, Tay-Sachs disease, cystic fibrosis) or prenatal diagnosis (eg, Down syndrome). Newborn screening is generally mandated by state or federal government health policies and occurs outside the physician's purview (eg, neonatal screening for inborn errors of metabolism).
The role of genetics in routine health care maintenance for adults as a means to assess the genetic risk of disease is under debate. Currently its use is limited by 3 factors. First, the prevalence of identifiable persons at genetically high risk in the primary care population is low. Second, population-based data to provide informative estimates of the predictive value of such predispositional testing in this setting are lacking. Third, high-quality evidence of the effectiveness of interventions for those found to be at high genetic risk is absent.
Take, for example, breast cancer. In a primary care practice with a patient panel of 1,000, one case of inherited breast cancer due to the BRCA1 or BRCA2 gene mutations will be diagnosed every 20 years.4 The penetrance of the mutation, or the percentage of people who have the gene and in whom breast cancer will develop, ranges from 30% to 85%, depending on the population in which it is studied.5,6,7,8,9,10 The evidence demonstrating the efficacy of the interventions offered to women who are carriers of the BRCA1 or BRCA2 mutation—early mammography, ovarian cancer screening, and prophylactic surgery—is based largely on expert opinion and estimates from anecdotal experience.11 In other cases, however, genetics can be used to provide targeted prevention, for example, in early-onset screening for colon cancer in a family with hereditary nonpolyposis colon cancer.12
FAMILY HISTORY AS A FORM OF GENETIC TESTING
There are several forms of genetic testing. The one most commonly used is taking a family history. The family history is often the first step in identifying a genetic contribution to disease.
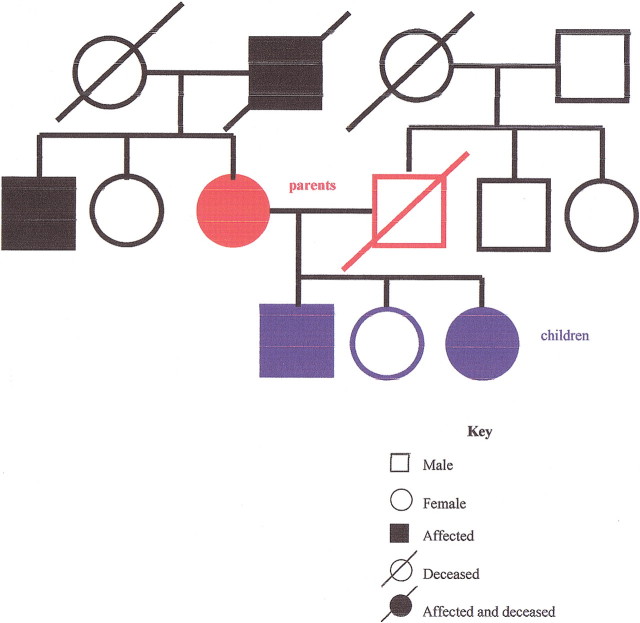
A family history may reveal patterns consistent with a strong genetic influence, and it may suggest the mode of inheritance. In an autosomal dominant disorder (figure 1), the history reveals multiple generations of affected relatives with the unusually early onset of a common disease, or multiple generations of family members with a rare disease. In an autosomal recessive disorder (figure 2), siblings are more likely to be affected than parents. In families with an X-linked recessive disorder (figure 3), the mother is a carrier, and in the next generation, only the sons will be affected. If the affected sons have children, unless the mother is also a carrier, none of the sons will be affected, but the daughters will be carriers. A positive family history may lead to a specific genetic diagnosis or to the identification of an increased risk, leading to opportunities for improved prevention.
Figure 1.
Pedigree of autosomal dominant inheritance
Figure 2.
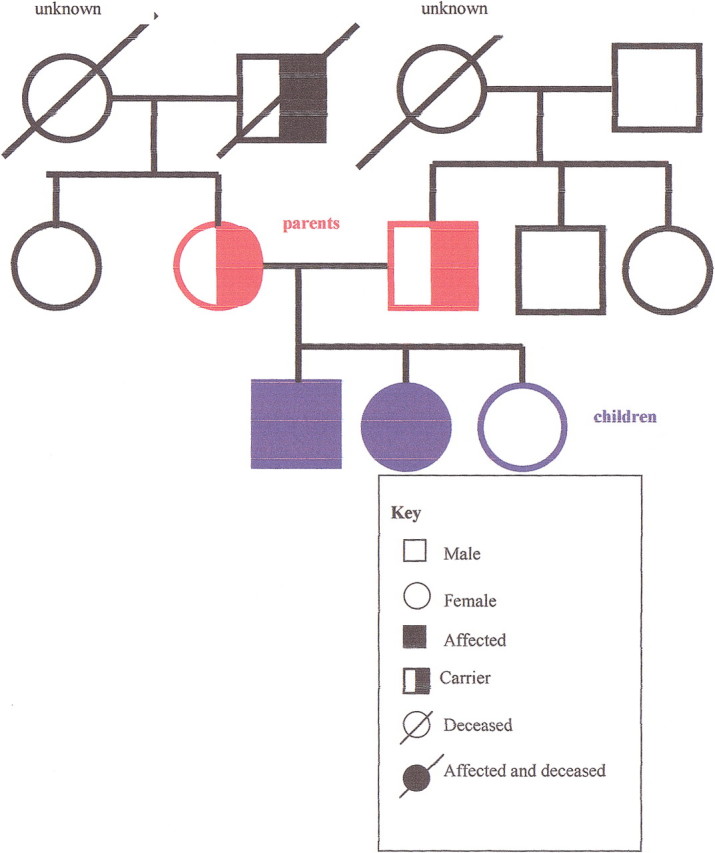
Pedigree of autosomal recessive inheritance
Figure 3.
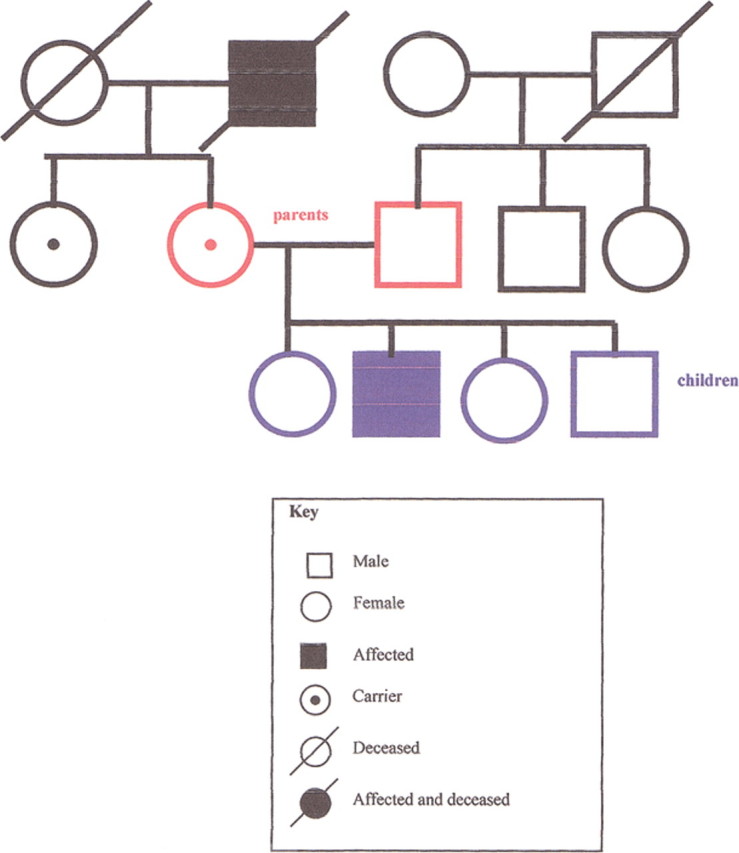
Pedigree of X-linked recessive inheritance
Although geneticists often speak of taking a 3-generation family history, this is unlikely to be feasible in a primary care setting. For example, a recent study of family practitioners found that a family history of cancer was sought in only half of new patient visits.13 In primary care, unlike in a genetic consultation, family history information must be assessed efficiently within the comprehensive care of the patient and the time constraints of practice, with attention to those measures that affect management. Using a triage approach to family history may militate against the time demands involved in taking a family history because most people will not have a significant family history. This approach involves starting with general questions and looking for red flags that signal the provider to delve deeper.
For example, if a patient says that his father developed dementia at age 40, it may be worth taking a detailed family history of 3 generations, if the information is available, to pursue the diagnosis of early-onset Alzheimer disease, a condition estimated by 1 study to occur in 41.2 per 100,000 people at risk (ages 40-59) (GeneClinics web site: www.geneclinics.org).14 It may not make sense to spend the time acquiring this detailed family history on every patient seen in the practice.
An example of a technique to obtain and interpret a family history that suggests an increased risk of cancer is shown in the box.15 The longitudinal relationship in primary care also allows obtaining family history information suggestive of increased risk over time.
Table 2.
| FAMILY Mnemonic for obtaining and interpreting family history* |
|---|
|
Adapted from Pinsky et al,15 with permission.
EVALUATING THE USE OF GENETIC INFORMATION TO IMPROVE PATIENT MANAGEMENT
Genetic information is predicted to serve as the basis for a new, more effective approach to preventive care, in which individual risk can be predicted more accurately and strategies to reduce risk and improve health outcome are tailored to individual susceptibilities.1
The presence of a gene does not translate directly into the presence of disease. For example, the risk for hemochromatosis, a genetic disorder of excess iron accumulation, can be detected genotypically through DNA testing and phenotypically through serum iron levels that are elevated well before symptoms occur. However, not everyone with the implicated genotypes develops phenotypic changes, and not everyone with elevated serum iron levels develops disease, even in the absence of treatment.16,17
Such discrepancies are the rule rather than the exception in genetics, even in classically severe genetic diseases. For example, homozygosity for the deltaF508 mutation in CFTR, the cystic fibrosis gene, may result in clinical manifestations ranging from mild to severe illnesses.18,19 In common disorders such as heart disease, cancer, or Alzheimer disease, most mutations result in an increased risk, rather than a certainty, of disease.20
Although the provision of information of risk has historically been viewed as an effective prompt for change, this belief is not supported by current evidence. Giving people information about genetic risks to their health does not actually increase their motivation to change behavior beyond that achieved with nongenetic information.21
In thinking about genetic testing, it is helpful to think of the benefit of a test in its predictive value and the efficacy of available treatment. Primary care physicians may play an important role in the evaluation of this potential from an evidence-based perspective, by addressing several questions:
How accurately can genetic risk assessment identify a subgroup with increased risk?
What is the prevalence of the genetically susceptible subgroup?
What is the positive predictive value (penetrance) of identifying a subgroup through genotypic or early phenotypic changes?
What effective risk reduction strategies are available?
Are these risk strategies already effectively employed without genetic testing?
How much morbidity and mortality can be prevented by tailored prevention in the genetically susceptible subgroup?
What are the costs and risks of the genetic assessment process?
Although generalists do not want to miss early diagnosis in high-risk patients, they equally hope not to raise concerns in most of their patients for whom genetic testing will bring no health gain.22 The rarity of high-risk individuals in a primary care setting necessitates population-based estimates of predictive value before genetic risk assessment can be applied to a primary care population.
ETHICAL, LEGAL, AND SOCIAL ISSUES IN GENETICS
Genetic information carries possible risks of stigmatization; personal, job, or insurance discrimination; and the disruption of families.23 A process of informed decision making and consent should be done, informing the person of the risks and benefits of this approach to the patient and the family (see “Genetic testing,” the second article in this series, in wjm May 2001).24
The identification of genetic risk in a patient usually indicates a possible genetic risk in family members as well. Some case law and some expert opinion suggest that primary care physicians may be obligated to disclose such risk information to other family members. We must ask ourselves, “If obligations exist, how can they be discharged?” Primary care providers must grapple with how we can efficiently communicate medical information to genetically related members of the family while maintaining patient confidentiality.
CONCLUSION
Primary care physicians will play a crucial role in the integration of genetics into clinical practice. In a study of genetic referral in the United Kingdom, most referrals were found to be low-risk persons who could have received reassurance from their primary care physicians.25 By increasing our skills in case recognition, management, and patient education in genetics, we will improve the care of our patients.
The patient has substantial risk factors for coronary artery disease that do not require genetic testing to ascertain or to treat. Although initial pharmocogenetic studies have suggested that some people respond better to thiazide diuretics than others, the test does not differentiate enough to change the basic approach of starting with this medication for the treatment of hypertension. In studies of smokers at increased genetic risk for lung cancer, those who had genetic testing showed positive changes in perceptions of risk and beliefs about quitting, but they were no more likely to stop smoking. Similarly, people who theoretically could be found to have a greater risk for nicotine addiction may use this information to change, or they may become fatalistic and reduce their efforts at smoking cessation.21 In these instances, genetic information will not affect patient outcome.
However, the patient's care may be affected by learning of a family history of premature coronary artery disease. In this case, the American College of Physicians and the US Preventive Services Task Force suggest doing cholesterol testing in a patient of this age, in deviation from their usual care guidelines. This may lead to early initiation of cholesterol-lowering medication, along with advice about smoking cessation and lifestyle modification or medication for the treatment of hypertension, all to lower his risk of coronary disease.
Summary points
While avoiding hype about the future of genetics, primary care physicians can use genetic assessment to improve patient care now
Generalists need to become skilled in case recognition, risk assessment, and the appropriate use of genetic testing
Family history is an important tool for genetic assessment in primary care
Primary care physicians must view genetics from an evidence-based perspective, judging the value of genetic information to health outcomes
Providing people information about genetic risks to their health does not increase motivation to change behavior beyond that achieved with nongenetic information
Competing interests: None declared
References
- 1.Jones M. The genetic report card. New York Times Magazine, June 11, 2000, p 80.
- 2.Collins FS. Shattuck lecture—medical and societal consequences of the Human Genome Project. N Engl J Med 1999;341: 28-37. [DOI] [PubMed] [Google Scholar]
- 3.American Medical Association. Genetic testing: a study of consumer attitudes, March 1998: AMA survey results. Available at: www.amaassn.org/ama/pub/article/2304-2937.html. Accessed May 8, 2001.
- 4.Rosenthal TC, Puck SM. Screening for genetic risk of breast cancer. Am Fam Physician 1999;59: 99-104, 106. [PubMed] [Google Scholar]
- 5.Burke W, Press N, Pinsky L. BRCA1 and BRCA2: a small part of the puzzle [editorial]. J Natl Cancer Inst 1999;91: 904-905. [DOI] [PubMed] [Google Scholar]
- 6.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families: The Breast Cancer Linkage Consortium. Am J Hum Genet 1998;62: 676-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorlacius S, Struewing JP, Hartge P, et al. Population-based study of risk of breast cancer in carriers of BRCA2 mutation. Lancet 1998;352: 1337-1339. [DOI] [PubMed] [Google Scholar]
- 8.Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 1999;91: 943-949. [DOI] [PubMed] [Google Scholar]
- 9.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997;336: 1401-1408. [DOI] [PubMed] [Google Scholar]
- 10.Fodor FH, Weston A, Bleiweiss IJ, et al. Frequency and carrier risk associated with common BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer patients. Am J Hum Genet 1998;63: 45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer, II: BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA 1997;277: 997-1003. [PubMed] [Google Scholar]
- 12.Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer, I: hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997;277: 915-919. [PubMed] [Google Scholar]
- 13.Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC. Family history-taking in community family practice: implications for genetic screening. Genet Med 2000;2: 180-185. [DOI] [PubMed] [Google Scholar]
- 14.Campion D, Dumanchin C, Hannequin D, et al Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet 1999;65: 664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinsky L, Berg AO, Press N, Burke W. Assessing Genetic Risk for Breast Cancer: Primary Care Panel Recommendations. White Paper. National Institutes of Health grant R01 HG01085.
- 16.Cogswell ME, McDonnell SM, Khoury MJ, Franks AL, Burke W, Brittenham G. Iron overload, public health, and genetics: evaluating the evidence for hemochromatosis screening. Ann Intern Med 1998;129: 971-979. [DOI] [PubMed] [Google Scholar]
- 17.Beutler E, Felitti V, Gelbart T, Ho N. The effect of HFE genotypes on measurements of iron overload in patients attending a health appraisal clinic. Ann Intern Med 2000;133: 329-337. [DOI] [PubMed] [Google Scholar]
- 18.Burke W, Aitken ML, Chen SH, Scott CR. Variable severity of pulmonary disease in adults with identical cystic fibrosis mutations. Chest 1992;102: 506-509. [DOI] [PubMed] [Google Scholar]
- 19.The Cystic Fibrosis Genotype-Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 1993;329: 1308-1313. [DOI] [PubMed] [Google Scholar]
- 20.Welch HG, Burke W. Uncertainties in genetic testing for chronic disease. JAMA 1998;280: 1525-1527. [DOI] [PubMed] [Google Scholar]
- 21.Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ 2001;322: 1056-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bock GH, Vliet Vlieland TP, Hageman GC, Oosterwijk JC, Springer MP, Kievit J. The assessment of genetic risk of breast cancer: a set of GP guidelines. Fam Pract 1999;16: 71-77. [DOI] [PubMed] [Google Scholar]
- 23.Marteau TM, Croyle RT. The new genetics: psychological responses to genetic testing. BMJ 1998;316: 693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagon RA, Hanson NB, Neufeld-Kaiser W, Covington ML. Genetic testing. West J Med 2001;174: 344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris R, Harris HJ. Primary care for patients at genetic risk [editorial]. BMJ 1995;311: 579-580. [DOI] [PMC free article] [PubMed] [Google Scholar]