Abstract
Background:
Infants in neonatal units are susceptible to numerous potential iatrogenic risks. One key concern is central line-associated blood stream infection (CLABSI). To ensure patient safety and reduce the incidence of CLABSI toward zero, numerous evidence-based clinical interventions and product innovations have been implemented. Nevertheless, sustaining zero CLABSI for sustained periods remains challenging.
Aim:
The purpose of this study was to evaluate the impact on CLABSI rates of introducing a preassembled closed intravenous (IV) administration set in a neonatal intensive care unit (NICU).
Methods:
This was a retrospective observational analysis of routinely collected anonymized IV therapy infection data in a NICU. The study period was from January 2019 through June 2020.
Results:
Nine-hundred eighty five patients with a Epicutaneo-Caval Catheter (ECC) were included (456 legacy IV set, 529 closed IV set). Patient demographics were comparable between the two groups. ECC dwell time was the only IV characteristic associated (p = 0.04) with CLABSI. Mann-Whitney U-test demonstrated significant differences between the two sets for CLABSI complication events (p = 0.031). Prior to using the closed IV administration sets (January 2019–September 2019) the mean monthly CLABSI rate was 2.87 (/1000 device days). This figure declined to 0.22 (/1000 device days) afterwards (October 2019–June 2020). Zero CLABSIs were observed during January to June 2020.
Conclusions:
Utilization of a pre-assembled closed IV administration set was associated with a reduction in CLABSI rates. The study results suggest that using a pre-assembled closed IV set concurrently with evidence-based central line infection control interventions can help attain extended periods of zero CLABSI.
Keywords: Neonate, NICU, Epicutaneo-Caval Catheter (ECC), Central line-associated blood stream infection, CLABSI, Needle-free connector, Luer activated valve
What is known about the subject?
A common reason for unplanned ECC removal amongst neonates is suspected blood stream infection.
There is broad evidence that practice standardization of protocols, procedures and team membership/training can reduce the incidence of catheter related blood stream infections.
What this study adds
When month-on-month CLABSI rates are low, achieving long-term zero incidence, “zero CLABSI,” requires additional creative efforts beyond standardization of practice.
Intravenous (IV) administration set design innovation can support efforts to achieve zero CLABSI when all other evidence-based measures are already sustainable and consistently implemented.
Implementation of a new IV administration set was associated with a significantly reduced CLABSI rate in a NICU.
Introduction
Central venous catheters (CVC), typically in the manner of peripherally inserted central catheters (Epicutaneo-Caval Catheter, ECC), are widely used in neonatal intensive care settings to provide intravenous (IV) fluids, parenteral nutrition, and medications.1,2 CVCs have several benefits over peripheral IV catheters (PIVC) including: long-term securement of difficult IV access, fewer line exchanges, and the ability to infuse, acidic (<pH 5) or alkaline (pH > 9) solutions and hyperosmolar fluids (Osmolarity > 600 mOsm/L).1–4 When compared to PIVCs, CVCs are generally associated with lower rates of complications, though these figures vary.1,5,6 Importantly, CVCs are not risk-free and their use is associated with several clinically minor and serious complications including CLABSI.1,2,4–6 Exact figures for complication rates due to all causes are debated due to a lack of universal definitions and surveillance practices.
The CLABSI rate is commonly presented as the number of confirmed infections per 1000 catheter days. 7 Actual figures for the incidence of CLABSI vary internationally, between different healthcare settings, and among different patient groups. The observed rate variability reflects infection surveillance methods, and underlying patient risk. However, inconsistencies in CLABSI diagnosis and confirmation (e.g., setting the level of clinical suspicion, interpretation of a positive blood culture, routine or non-routine ECC tip culture, and how robustly other infections are excluded).1,4,5,7–13
Newborns, particularly those born preterm, are considered a high-risk patient group and a focus of considerable effort to reduce infection risk. Measures that have been shown to reduce the incidence of CVC related complications, including CLABSI in the NICU include; standardizing practices and workflows related to insertion, maintenance, use, and removal of CVCs, including ECCs.1,3,5,10–13
There is emerging favorable evidence suggesting alternative insertion procedures, such as the modified Seldinger technique, and modified workflows have a favorable impact on CLABSI rates.14–16 There is some evidence about the benefits and additional risks of using bedside assembled “add on” devices such as extension lines to enhance functionality of the infusion system. 5 However, there is little evidence regarding the impact of consumables, such as advanced closed system IV administration sets, preassembled at the point of manufacture towards reducing CLABSI rates.
Background
Beginning in 2010, the NICU of the Women’s Wellness and Research Centre, Hamad Medical Corporation (Doha, Qatar) standardized evidence-based clinical workflows for the insertion and maintenance of CVCs to reduce CLABSIs and other complications in newborn care. These measures included evidence-based care bundles, establishing a dedicated multi-professional vascular access team, disinfecting caps, IV filters, anti-microbial ECC lines, and development of a vascular access decision procedure to facilitate selection of an appropriate vascular access device for the patient and therapy. Finally, a culture of infection prevention was encouraged through a “Speak Up” campaign providing caregivers a mechanism to report infection control concerns, along with staff professional development education and vascular access competency validation.
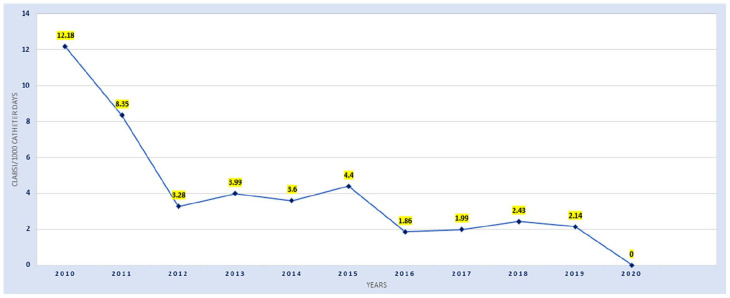
Over time annual CLABSI rates have declined from 12.18/1000 catheter days in 2010 to 2.14 CLABSI/1000 catheter days in 2019 (Figure 1). However, this reduction in CLABSI rate was not linear and included periods of no improvement and deterioration. These improvements in CLABSI rate correlated with times when vascular access practice was subjected to focused quality improvement initiatives. After several years of progress, in April 2019 the NICU team set a quality improvement goal to aim for “zero-CLABSI” and implemented processes, equipment, consumables, and practice elements from current clinical evidence in pursuit of their goal.
Figure 1.
10-year annual trend of CLABSI rates in the NICU 2010-2020.
Aim
The purpose of this retrospective observational study was to evaluate the impact of an innovative closed IV administration set on CLABSI rates in one neonatal intensive care unit.
Methods
The closed IV administration set (011-MC330423, ICU Medical Inc, San Clemente, California, USA, Figure 2) was fully assembled by the manufacturer prior to sterilization and end user packaging. In this paper, the term “closed” was chosen to indicate that the administration set was fully assembled when sterilized/packaged and that the set utilizes self-sealing needle-free connectors for fluid pathway access rather than stopcocks. In contrast, the previously used, “legacy” IV administration set was assembled at the bedside from several components sourced from multiple manufacturers.
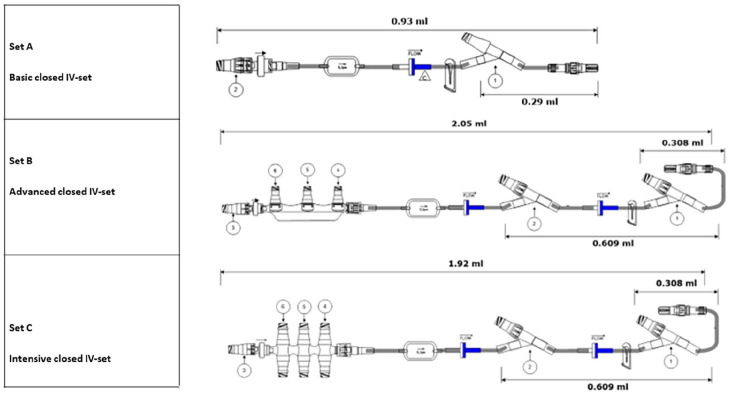
Figure 2.
Closed IV administration sets with NanoClave™ needle-free connectors (set A, B, C) and manifolds (Set B, C).
The closed IV administration set includes several features and components which may contribute to a reduction in infectious complications. The set incorporates a 0.2-micron filter which serves to support the elimination particulate matter and is air-eliminating and bacterial retentive. The set includes a NanoClave™ needle-free connector manifold with integral back check valve. High residual volumes of fluid within IV administration sets are areas of potential flow stasis and provide areas where bacterial replication may occur. The residual volume of the advanced closed administration set ranges from 0.93 ml to 2.05 ml depending on set configuration which allows for lower priming and flush volumes decreasing the risk of fluid or medication overload compared to the legacy model. In comparison, the residual volume of the legacy set ranged from 1.26 ml to 2.32 ml depending on configuration.
Additional clinical benefits of the closed IV administration set include an option for a manifold that allows for the connection, visualization, and monitoring of multiple infusions. The manifold may also facilitate line spacing and organization, reducing “spaghetti syndrome,” the tangling of multiple tubing sets. 17 Finally, the back check valve in the closed IV administration set reduces reflux and subsequently mixing of fluids into the tubing at low infusion rates and reduces the influence of gravity on flow rates for dose critical medications like inotropes. 18
The clinical team choose to implement the closed IV administration set configurations based on those previously designed for the NICU of Antwerp University Hospital, Belgium. To meet heterogeneous clinical needs for infusion therapy, 19 three closed set configurations were developed: (a) Basic closed IV-set, (b) Advanced closed IV-set and (c) the Intensive closed IV-set with NanoClave™ manifold (Figure 2). The primary difference between the sets is the number of needle-free connectors available for the simultaneous infusion of multiple compatible medications and fluids. The basic set (a) is designated to be used for patients with limited IV therapy requirements. The advanced set (b) is used for increased IV therapy requirements and the Intensive set (c) with NanoClave™ manifold is for patients with complex IV therapy requirements.
Importantly, the infusion of multiple fluids and medications through a common fluid pathway requires confirmation of compatibility and enables the use of fewer intravenous access points, whether they be peripheral or central catheters. The components used in the IV administration sets require clinician awareness of the impact on the fluid path including the requirements for air removal, priming volume, and residual volume.
Design and setting
This retrospective observational study analyzed routinely collected anonymized IV device use data from January 2019 to June 2020. The study outcome was the incidence of CLABSI in the patient population before and after introduction of the closed IV administration sets. The study was carried out at the 112 bed NICU of the Women’s Wellness and Research Centre (WWRC) of Hamad Medical Corporation (HMC), in Doha, Qatar. The study protocol (MRC-01-20-851) was approved by the local institutional review board (IRB). As the data was anonymized, the local IRB deemed that participant consent was not required and classified the study as a “chart review.”
Patient and public involvement statement
Study participants and parents were not involved in the design, conduct or reporting of this study.
Participants and sample size
Infants who were admitted to the NICU and who required intravenous therapy using peripherally inserted central catheters (ECC) were included in this study. Overall ECC insertion success was 83.5% (985/1179 attempts) Participants were excluded from the sample if insertion was not successful, data was incomplete, or non-ECC vascular access devices alone were utilized for infusion therapy such as peripheral IV catheters, extended-dwell or midline catheters, and other central catheters (e.g., umbilical catheters). The legacy set period prior to the implementation of the advanced administration sets included 4113 central line (CL) days with 456 patients. While the period after implementation included 5255 CL days with 529 patients. In total 9368 CL days and 985 patients were included in the sample.
Procedure
In the patient assessment stage for vascular access requirements, the team follows a locally developed mnemonic, the “5Rights for Vascular Access” [after, 20 ] that is, the Right device, for the Right vein, with the Right therapy, for the Right duration, for the Right patient. The design and decision to purchase the preassembled closed IV administration set was based upon application of current evidence-based infusion standards1,5 and local contexts such as, product compatibility with existing infusion devices, hospital purchasing decisions, and practitioner consensus. The insertion, use, and maintenance care of vascular access devices and IV administration sets is performed according to hospital policy based on international guidelines.1,5,10
In September 2019, all nursing staff were educated and trained on the use of the closed IV administration sets. An internally developed training video for the closed sets was shared with all staff and accessible 24 h a day at the bedside. The competency checks for bedside nurses were completed by IV team nurses. After 1 month of training all bedside nurses were deemed competent to use, work and troubleshoot the closed administration IV sets. In October 2019, the closed sets were introduced into everyday use.
Standard clinical practice throughout the study periods incorporated infection prevention education of nursing staff with courses on Aseptic Non-Touch Technique (ANTT) and certification earned through successful demonstrations. Reminders for the proper aseptic technique to nursing staff were provided periodically during the “safety huddle rounds” at the beginning of each shift.
Measurements and data collection
This study utilizes a convenience sample limited to ECC lines to facilitate evaluation of CLABSI rates associated with legacy-versus the closed administration sets. PIVCs, extended-dwell catheters, umbilical lines, and surgically inserted catheters are included in the database but are excluded from this initial analysis.
Analyzed data includes patient, procedural, and vascular access device characteristics. Patients’ demographics and baseline data included sex, gestational age at birth in weeks and days, birth weight, and current body weight in grams. Data regarding the ECC line insertion procedure were the date and time of cannulation, as well as the number of attempts needed to successful cannulation, cannulation side (left or right), vein of cannulation, used type and size of device (PremiCath®, PremiStar®, NutriLine®), the use of catheter securement glue, the indication for intravenous treatment (long-term intravenous therapy, fluid characteristics, patient characteristics), the date and time of removal of the ECC, total dwell time of the ECC in days (calculated as the removal date minus the insertion date), and the reason for removal of the ECC (therapy completed and elective removal, catheter related complications, maintenance related complications, suspected sepsis and administrative censoring at death or patient transfer).
Statistical analyses
Statistical analyses were completed using software SPSS version 22.0. Frequency, percentage, mean, and standard deviation were used to describe the patient demographics, annual trends of CLABSI, occurrence of CLABSI with the legacy and closed IV sets. The Mann-Whitney U-test was used to assess for significant differences between the legacy- and closed IV sets and CLABSI events. The CLABSI rate is calculated per 1000 central line-days by dividing the number of CLABSIs by the number of central line-days and multiplying the result by 1000. 7 Regression analysis incorporating variables known to be important in CLABSI1,2,5–14,21–23 was used to identify predictors or factors associated with the CLABSI. Significant differences were determined to be associated with a Mann-Whitney U-test p-value of equal to or less than 0.05.
Results
A total of 456 patients with a ECC were in the legacy set group and 529 patients with a ECC were in the closed set group. Analysis of patient demographics (birth weight, current weight, gestational age, sex) demonstrated no statistical differences between the two groups, though males were overrepresented in both groups. In the legacy set group, 61.7% of the patients were male while in the closed set group included 58.4% were male. The patient ages ranged from 23 weeks to more than 37 weeks and weights ranged from ⩽999 g to ⩾2500 g. Neonates’ demographic characteristics such as sex, gestational age at birth, current gestational age, gestational age at birth in days, current gestational age in days, age from birth, birth weight and current weight, were not associated with differences in CLABSI rate (p > 0.05).
A total 985 ECCs were inserted. All ECCs were inserted by members of a dedicated team of clinicians (nurse and doctor). First attempt insertion success was 74.8% (737/985) with a maximum of two attempts per clinician, which did not exceed the number allowed for by hospital policy. The indwelling time varied from 1 day to 65 days, with a mean dwell time of 9 days for the legacy set and 10 for the closed set per inserted ECC. In 73.5% of patients’ (n = 724) removal of the ECC was an elective decision after completion of the prescribed therapy. However, 141 (14.3%) patients experienced therapy failure and had their ECC removed prior to the completion of therapy for several reasons. These reasons included suspected sepsis 16.3% (n = 23), maintenance-related complications (accidental removal and occlusion) 24.8% (n = 35), and catheter-related complications (leaking due to catheter damage, breakage of the catheter) 58.9% (n = 83). A further 120 patients (12.2%) are lacking outcome data for their ECC. These patients either died or were more usually transferred to other facilities (administrative censoring) with their ECC in situ.
Nine ECC line characteristics, reason for insertion, catheter details and type, insertion technique, number of insertion attempts, blood vessels inserted, side of the body, octyl-butyl-cyanoacrylate glue applied, reason for removal, and dwell time was analyzed to determine which factors were associated with a diagnosis of CLABSI (Table 1). Only one factor, dwell time was associated with the occurrence of CLABSI (p = 0.04). These findings suggest that when consistent, standardized evidence-based practices are robustly adhered to ECC line characteristics, except for dwell time, have no significant independent association with CLABSI.
Table 1.
The association between IV characteristics and having CLABSI.
| Insertion Characteristics | B | Standard Error | Wald Statistic | df | p-value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Reason for Insertion | 0.61 | 0.45 | 1.83 | 1 | 0.18 | 0.76 | 4.46 |
| Catheter Type | −0.00 | 0.09 | 0.00 | 1 | 1.00 | 0.84 | 1.19 |
| Insertion Technique | −0.46 | 0.71 | 0.41 | 1 | 0.52 | 0.16 | 2.56 |
| Number of Insertion Attempts | 0.17 | 0.46 | 0.15 | 1 | 0.70 | 0.49 | 2,92 |
| Blood Vessel Inserted Into | 0.14 | 0.17 | 0.65 | 1 | 0.42 | 0.82 | 1.61 |
| Side of the Body | 0.24 | 0.33 | 0.55 | 1 | 0.46 | 0.67 | 2.42 |
| Cyanoacrylate glue Applied | 1.47 | 1.21 | 1.48 | 1 | 0.22 | 0.41 | 46.92 |
| Reason for Removal | 0.14 | 0.17 | 0.70 | 1 | 0.40 | 0.82 | 1.62 |
| Dwell Time | −0.05 | 0.03 | 4.04 | 1 | 0.04* | 0.90 | 1.00 |
Significant at the level of significance α = 0.05.
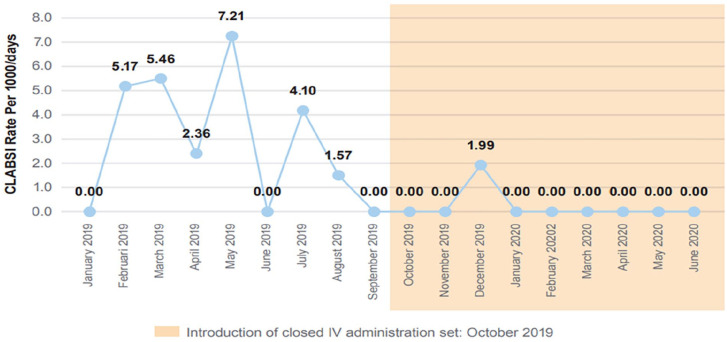
In contrast, the introduction of the closed IV administration set was associated with decreased CLABSI rates (Table 2 and Figure 3). During the 9 months of closed IV administration sets a monthly CLABSI rate of 0.22/1000 catheter days was determined. In comparison, during preceding 9 months (legacy set) the monthly CLABSI rate was 2.87/1000 catheter days. Statistical analysis using the Mann-Whitney U-test demonstrates a significant difference between the legacy set and advanced closed set CLABSI complication rates (Table 2). The CLABSI events (p = 0.031) and the CLABSI rate per 1000/days (p = 0.040) during the use of the closed set are significantly less than the rates observed while the legacy set was in use. The data includes a significantly greater number of advanced closed set CL days than legacy set CL days (p = 0.040).
Table 2.
Comparison of CLABSI events, number of CL days, and CLABSI rate per 1000/days between pre- and post-introduction of the closed IV set, monthly rate.
| Comparison | IV System | Minimum | Maximum | Mean | Standard Deviation | Mann-Whitney U-test p-value |
|---|---|---|---|---|---|---|
| CLABSI rate per 1000/days | Legacy | 0.00 | 7.21 | 2.8744 | 2.72 | 0.031* |
| Closed | 0.00 | 1.99 | 0.2211 | 0.66 | ||
| Number of CL days | Legacy | 366.00 | 636.00 | 457.00 | 93.62 | 0.040* |
| Closed | 422.00 | 817.00 | 583.89 | 133.15 | ||
| CLABSI Events | Legacy | 0.00 | 3.00 | 1.22 | 1.09 | 0.031* |
| Closed | 0.00 | 1.00 | 0.11 | 0.33 |
Significant at the level of significance .
Figure 3.
Occurrence of CLABSI during the study period pre- and post-introduction of the closed IV set.
Based on the regression analysis results in Table 3, we reject the null hypothesis that the IV set used, legacy or advanced closed set, has no significant association with CLABSI (p-value = 0.000). These results suggest that the advanced closed set had a statistically significant effect on reducing the incidence of CLABSI.
Table 3.
Regression analysis between the IV system (Legacy vs Closed) and CLABSI.
| Independent Variable | B | Standard Error | Wald Statistic | df | p-value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| IV System | 3.60 | 0.27 | 176.31 | 1 | 0.00** | 21.56 | 62.47 |
Significant at the level of significance .
Significant at the level of significance .
Discussion
Overall 73.5% of patients’ completed their therapy before their ECC was removed; a figure broadly in alignment with those published elsewhere in the neonatal literature.21–23 The results of this study evaluating the effect of the pre-assembled advanced closed IV administration set on infectious complications in a NICU patient population receiving infusion therapy through ECC lines were associated with a significant reduction in the CLABSI rate. Prior to the adoption of the closed set, evidence-based initiatives had reduced, but not eliminated CLABSI. The addition of the closed IV administration set to the evidence based clinical workflows, enabled additional and sustained reductions to be realized. However, it is possible that other factors (clinical, behavioral, methodological) that are known to affect CLABSI risk1,5,6,8,13–16 or study setting characteristics could have influenced this result. Further research using randomized controlled trial methodologies are required to unequivocally determine this relationship.
The closed sets may have reduced CLABSI through several mechanisms and design features. The closed set is fully assembled, packaged, and sterilized by the manufacturer, whereas the legacy set was assembled from IV add on devices at the bedside. This full assembly reduces the need for add on devices and manual assembly tasks which could be associated with touch contamination. 5 Back check valves promote one-way fluid flow into the patient and may reduce the risk of infection by reducing potential backflow of patient blood into the fluid path, reducing opportunities for line occlusion, and resulting in fewer manipulations/handling to resolve this issue.
The NanoClave™ connector surface features a protruding silicone seal, whereas the legacy IV administration set needle-free connector has a recessed, concave surface. It is plausible that the protruding seal design may be more effectively disinfected during swabbing to reduce contamination and or biofilm formation at the IV port point of entry. Additionally, the housing of the closed set needle-free connector is made of clear colorless plastic, which enables clinical staff to confirm flush completion and air elimination. In contrast, the legacy set connectors did not enable visualization within the component fluid path, due to non-transparent construction materials. Assurance of complete flushing may contribute to a reduction of infectious complications as uncleared fluids may provide substrates for bacterial seeding or growth, (glucose, nutrition, blood products, etc.).
Training and educational efforts coincided with the introduction of the advanced sets and are expected to have had a limited impact on CLABSI. The training was primarily focused on proper use of the new IV set components. Staff procedures such as insertion site disinfection, care bundles for catheter insertion/removal, dressing changes, use of securement glue, etc. were unchanged versus historic practices. Differences in staff practices after the introduction of advanced IV administration sets include adoption of the ivWatch Model 400TM (ivWatch LLC, Hampton, VA) for peripheral site monitoring, the addition of medical grade cyanoacrylate as an option for catheter securement, and COVID-19 pandemic preparations. These differences are not considered to be a primary driver of CLABSI reduction in this study. Additional factors which were consistent with the legacy IV set group and the advanced closed IV set group include ECC insertion practices and nursing personnel.
Limitations
The current study was carried out according to the STROBE statement 24 and used a large dataset to analyze the effects of a practice innovation on CLABSI incidence. There was no randomization and data collection was from a single study site; which creates a potential risk of selection bias. However, participant demographics (Table 1) and CVC insertion numbers remained similar throughout both time periods of the study. The potentially confounding impact of training and education in connection with the introduction of the advanced IV sets is noted. However, our use of standardized education and training packages and limiting vascular access to a dedicated team mitigates this variability. Data on the ECC outcomes that were not available for neonates (death or were transferred out of the facility) were deemed as “administrative censoring.” Although this population was small, patients lost to follow-up may have a differing outcome than those who completed the study. Further studies are required to broaden the limited literature available in this area. Future neonatal vascular access research, in larger multi-center populations should include a consideration of the impacts of product design, such as closed IV administration sets, on infusion therapy complications.
Conclusion
This study suggests that when there is high compliance to infection control central line evidence-base care bundles there may be benefits in terms of CLABSI rates associated with using a pre-assembled closed IV administration set design in the NICU setting. In this evaluation, the period during which the closed design was used was associated with a significantly lower CLABSI rate (0.22/1000 CL days) than the legacy design. This observation may be a result of multiple device characteristics which reduce risk of contamination, facilitate disinfection, and reduce opportunity for bacterial growth. However, other confounding factors might also feature, and further study is warranted.
Acknowledgments
The authors would like to thank the NICU’s vascular access team and all our other colleagues in the NICU of the Women’s Wellness and Research Center.
Footnotes
Contributions: All authors contributed to the conceptualization of the study and analysis and interpretation of the data; MvR, KH, KG, AAF contributed to the design of the initial study and data collection; MvR, KH and AF drafted the initial manuscript; ICC provided infection data and related statistical support; MvR, KH and MMA, AAF provided all editorial content review and all revisions the manuscript; all authors critically reviewed and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ICU Medical sponsored open publication of this article. These sponsors had no role in study design, data collection, data analysis, decision to publish, nor approval of the final manuscript.
ORCID iD: Matheus F.P.T. van Rens  https://orcid.org/0000-0002-9595-0265
https://orcid.org/0000-0002-9595-0265
References
- 1. Mason-Wyckoff M, Sharpe EI. Peripherally inserted central catheters: guideline for practice. 3rd ed. Chicago, IL: National Association of Neonatal Nurses, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Hugill K, van Rens MFPT. Inserting central lines via the peripheral circulation in neonates. Brit J Nurs 2020; 29(19): S12–S18. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence, (NICE). Neonatal parenteral nutrition (NG154). www.nice.org.uk/guidance/ng154 (2020, accessed 15 March 2022). [PubMed]
- 4. Ainsworth SB, McGuire W. Peripherally inserted central catheters vs peripheral cannulas for delivering parenteral nutrition in neonates. JAMA 2016; 315(23): 2612–2613. [DOI] [PubMed] [Google Scholar]
- 5. Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice. J Infus Nurs 2021; 44(1S Suppl 1): S1-S224. [DOI] [PubMed] [Google Scholar]
- 6. Pet GC, Eickhoff JC, McNevin KE, et al. Risk factors for peripherally inserted central catheter complications in neonates. J Perinatol 2020; 40: 581–588. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. The 2019 National and State healthcare-associated infections (HAI) progress report. https://www.cdc.gov/hai/data/portal/progress-report.html (2019, accessed 15 March 2022).
- 8. Cherifi S, Mascart G, Dediste A, et al. Variations in catheter-related bloodstream infections rates based on local practices. Antimicrob Resist Infect Control 2013; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heijting IE, Antonius TAJ, Tostmann A, et al. Sustainable neonatal CLABSI surveillance: consensus towards new criteria in the Netherlands. Antimicrob Resist Infect Control 2021; 10(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loveday HP, Wilson JA, Pratt RJ, et al. Epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014; 86(Suppl 1): S1–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savage T, Hodge DE, Pickard K, et al. Sustained reduction and prevention of neonatal and pediatric central line-associated bloodstream infection following a nurse-driven quality improvement initiative in a pediatric facility. JAVA 2018; 23(1): 30–41. [Google Scholar]
- 12. Payne V, Hall M, Prieto J, et al. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonat Ed 2018; 103(5): F422–F429. [DOI] [PubMed] [Google Scholar]
- 13. Ista E, van der Hoven B, Kornelisse RF, et al. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16(6): 724–734. [DOI] [PubMed] [Google Scholar]
- 14. Arnts IJJ, Schrijvers NM, van der Flier M, et al. Central line bloodstream infections can be reduced in newborn infants using the modified Seldinger technique and care bundles of preventative measures. Acta Paediatr 2015; 104(4): e152–e157. [DOI] [PubMed] [Google Scholar]
- 15. Taylor T, Massaro A, Williams L, et al. Effect of a dedicated percutaneously inserted central catheter team on neonatal catheter-related bloodstream infection. Adv Neonat Care 2011; 11(2): 122–128. [DOI] [PubMed] [Google Scholar]
- 16. Bayoumi MAA, Van Rens MFP, Chandra P, et al. Effect of implementing an epicutaneo-caval catheter team in neonatal intensive care unit. JVA 2020; 22(2): 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chopra V, Haynes J, Bowers K, et al. Managing spaghetti syndrome in critical care with a novel device: a nursing perspective. Crit Care Nurse 2015; 35(6): 38–45. [DOI] [PubMed] [Google Scholar]
- 18. van der Eijk AC, van Rens MFPT, Dunkelman MJ, et al. Flow rate variability in neonatal IV-therapy; what do we know about the flow? Paediatr Anesth 2013; 23(1): 9–21. DOI: 10.1111/pan.12039. [DOI] [PubMed] [Google Scholar]
- 19. Plaidy P, Chennell P, Wamen I, et al. Montages de perfusion en néonatologie: quelles pratiques en France? Ann Pharm Fr. Epub ahead of print 4 September 2021. DOI: 10.1016/j.pharma.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 20. Steere L, Ficara C, Davis M, et al. Reaching one peripheral intravenous catheter (PIVC) per patient visit with lean multimodal strategy: the PIV5rights™ bundle. JAVA 2019; 24(3): 31–43. [Google Scholar]
- 21. Prado NCda C, Silva RARda, Costa RHS, et al. Non-elective removal of the peripherally inserted central catheter in the neonatal unit. Rev Eletr Enf 2018; 20: V20a13. DOI: 10.5216/ree.v20.45559. [DOI] [Google Scholar]
- 22. Colacchio K, Deng Y, Northrup V, et al. Complications associated with central and non-central venous catheters in a neonatal intensive care unit. J Perinatol 2012; 32(12): 941–946. [DOI] [PubMed] [Google Scholar]
- 23. Cartwright DW. Central venous lines in neonates: a study of 2186 catheters. Arch Dis Child Fetal Neonat Ed 2004; 89(6): F504–F508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370(9596): 1453–1457. [DOI] [PubMed] [Google Scholar]