Abstract
Background:
Biologic medications are emerging as options for treating chronic rhinosinusitis with nasal polyps (CRSwNP). Several questions remain regarding patient selection, indications, clinical efficacy, and cost effectiveness.
Methods:
In November 2019, a group of physicians and scientists gathered to consider strategies for future studies regarding biologics. During the discussion, gaps in knowledge highlighted a need for a consensus on the present day use of biologics in polyp patients.
Results:
The goal of this guideline is to propose recommendations for the current use of biologics in CRSwNP as new evidence continues to emerge and inform practice.
Conclusion:
We suggest that physicians evaluate patients on an individual basis and closely monitor for improvement due to the high cost and unknown long-term effects of biologics.
Keywords: chronic rhinosinusitis, medical therapy of chronic rhinosinusitis, endoscopic sinus surgery, topical therapy for chronic rhinosinusitis, guideline
It is estimated that approximately 20% to 25% of patients with chronic rhinosinusitis (CRS) have nasal polyps.1,2 Current treatment options include medical therapy, such as topical nasal steroids and saline irrigations, as well as oral steroids and antibiotics for symptom flares. Some patients require long-term antibiotics for their anti-inflammatory effects.3 When medical therapy fails, functional endoscopic sinus surgery (FESS) is used to widely open affected sinuses and remove obstructing polyps.4 Continued medical therapy is typically needed postoperatively to control symptoms and avoid polyp regrowth.4 Recently, options for treating patients with CRS with nasal polyps (CRSwNP) have expanded with the introduction of biologic medications. In June 2019, the U.S. Food and Drug Administration (FDA) approved the first biologic, dupilumab, to treat CRSwNP based in part on 2 large multinational randomized controlled trials, which reported significantly improved nasal polyp scores and symptoms with dupilumab treatment as compared to placebo.5 Other biologics are currently under investigation in several trials and will likely become available for nasal polyposis in the near future. As the therapeutic decision algorithm for physicians and patients has become more complicated, the best practices for biologics use in CRSwNP have yet to be determined. Greater clarity of indications for biologics in CRSwNP is needed given the significant concern for inappropriate utilization of costly treatment options with unclear long-term adverse effects.
Thus, in November of 2019, a group of physicians and scientists gathered at the National Institutes of Health (NIH) to discuss current and future research needs for evaluating the use of biologics in patients with CRSwNP.6 Attendees included experts in rhinology, biostatistics, and allergy/immunology, as well as pharmaceutical representatives for relevant emerging biologic and topical steroid medications. The goal of this workshop was to consider future clinical trials addressing gaps in knowledge associated with biologics for CRSwNP. However, the results of such trials may not become available for 5 to 10 years. In the meantime, the panel recognized that literature-based guidance for practical incorporation of biologics was lacking and represents a pressing need. Our discussions highlighted a remarkable heterogeneity in use of biologics by panel members and the community in treatment of CRSwNP.
The goal of this article is to propose recommendations for the current use of biologics for CRSwNP. Using existing data regarding the use of biologics for asthma, our discussions at the NIH, and our understanding of the pathophysiology of CRSwNP, we aim to provide guidance on the contemporary use of biologics for polyp patients.
Biologics for asthma
The rhinology/allergy community can benefit and learn from the extensive research and guideline preparation that has been completed regarding biologic use in the asthma population. For example, the Global Initiative for Asthma (GINA) guidelines considers biologics for patients with continued symptoms despite high-dose inhaled steroids with diagnostic testing consistent with eosinophilia or allergies. Anti–immunoglobulin E (anti-IgE) biologics are recommended for patients with skin-prick sensitivities and elevated total serum IgE; anti–interleukin 5 (anti-IL-5) biologies are reserved for patients with blood eosinophils ≥300/μL; and anti-IL-4 biologies are used in patients with eosinophils ≥150/μL and fractional exhaled nitric oxide (FeNO) ≥25 parts per billion (ppb). All patients are assessed at 4 months for initial response.7 Similarly, in the United Kingdom, the National Institute for Health and Care Excellence (NICE) has created an integrated care pathway for biologics use for patients specifically with severe asthma.8 Severe asthma is defined as persistence of symptoms and/or exacerbations while on appropriate medical care, including high-dose inhaled corticosteroids. In line with GINA guidelines, omalizumab is recommended only for patients with confirmed allergic asthma and elevated IgE with positive allergy skin testing. Mepolizumab and reslizumab are both reserved for patients with elevated blood eosinophil levels (≥300/μL and ≥400/μL, respectively). To initiate some biologics, recommendations require specific defined previous use of oral steroids prior to offering biologics. Clear therapy cessation guidance is included within the guidelines because of the expense of these therapeutics. Multiple studies support an early cessation rule for omalizumab at 4 months if symptoms of severity of asthma have not improved. There is less information regarding recommendations for cessation of treatment of mepolizumab and reslizumab, but the NICE guidelines suggest evaluation of patient symptoms at 12 months to determine if the medication should be continued.8
In the United States, it is accepted that biologics be reserved for patients who meet criteria for severe asthma.9 The American Academy of Allergy Asthma and Immunology (AAAAI) is in agreement with this, but with less stringent guidelines than the NICE integrated care pathway. A recent review of the cost-effectiveness of biologics for asthma patients concluded that with current pricing schemes, biologics are not cost-effective and should be reserved for severe asthmatics and those who are shown to be responders to the biologic treatment.10,11
Still, the literature is lacking in head-to-head clinical trials comparing biologics to one another for severe asthma, specifically studies to differentiate between anti-IL-5–monoclonal antibody (mAb) options. These are areas of research in which we expect to see growth as more biologics are coming to the market with similar, but slightly modified, mechanisms of action. Following the analogy of the work done in asthma patients, biologics should be considered for CRSwNP patients if symptoms are not well controlled with the current treatment algorithm of medical and surgical therapy. Similarly, biologics may be considered in recurrent/recalcitrant polyp cases.
Recalcitrant nasal polyps
Although current treatment options work well for most patients with nasal polyps, there is a subset of patients that are difficult to treat. The current treatment algorithm includes saline irrigations, topical nasal steroids, and oral steroids or antibiotics as indicated.12 FESS is reserved as an option for patients who fail medical management. Postoperatively, endoscopy may detect mild polyps and edema, but in most cases, patients are asymptomatic or symptoms are controlled.13 In a study evaluating >9000 CRSwNP patients over 20 years, 70% of patients did not require a revision surgery.14 A larger study that included nearly 30,000 patients who had undergone FESS for CRS reported 75% of CRSwNP patients required only 1 surgery in a mean follow-up of 10 years.14 Oral steroids and antibiotics are typically reserved for severe polyposis and acute exacerbations, and prospectively followed patients have shown improvement in long-term quality-of-life measures.15,16 However, despite these efforts, there is a subset of patients who fail this algorithm, requiring revision surgery or possibly multiple revision surgeries.17
Several studies, through large database analyses and systematic reviews, have evaluated revision rates among patients with CRSwNP with reported frequencies between 4% and 28%.14,18,19 Retrospective studies have identified factors associated with recurrence of polyps and need for revision surgery, including comorbid asthma, aspirin-exacerbated respiratory disease (AERD), and allergic fungal rhinosinusitis (AFRS).18 Loftus et al.18 also identified that patients who were noted to have undergone previous “polypectomy” as compared to “endoscopic sinus surgery” were more likely to undergo a revision surgery in the future, supporting the benefit of a complete sinus surgery addressing all surgical goals. A cluster analysis of CRSwNP patients with histologic assessment of polyps identified subsets of patients with increased risk for need for revision surgery. Patients with eosinophilic polyps as well as young patients with non-eosinophilic polyps but with comorbid asthma had increased risk of requiring revision surgery as compared to older, non-eosinophilic polyp patients.20
Patients with recurrent or recalcitrant polyps may be good candidates for biologics. CRSwNP patients requiring multiple revision surgeries should be carefully considered for these medications.
AERD
AERD is an interesting phenotype of CRSwNP defined as a subgroup of patients with nasal polyps, asthma, and a sensitivity to drugs that inhibit the cyclooxygenase enzyme, including aspirin and all nonspecific nonsteroidal anti-inflammatory drugs (NSAIDs).21 The surgical revision rate has been noted to be higher in AERD patients than other CRSwNP subgroups, with 1 retrospective review reporting an average of 2.6 surgeries per lifetime for AERD patients.22 Other work has identified that within 5 years of their initial surgery, over 35% of AERD patients will require a second surgical intervention.23 Aspirin desensitization and daily aspirin maintenance therapy has been shown to improve symptoms, endoscopy, and quality of life, and revision FESS rates as low as 9% have been reported.23–26 Current expert panel guidelines state that aspirin desensitization with daily aspirin maintenance therapy is indicated for recurrent polyps after surgery, severe asthma, and sinus symptoms despite standard therapy, or a need for aspirin therapy due to cardiac disease or stroke.27 However many patients are not offered this treatment option or opt out of aspirin desensitization.28 Likely there is a lack of accessibility and/or adoption of this treatment in some practices,28 or patients and physicians decide against maintenance therapy due to risks of gastrointestinal complications or challenges with convenience of this daily treatment. Additionally, most studies regarding aspirin maintenance have had relatively short follow-up and some patients do not respond to aspirin desensitization.24,29
Given the high rates of revision sinus surgery and significant group of AERD patients with poor compliance or who cannot tolerate, decline, or do not respond to aspirin therapy, biologics could be considered in this population.
Cost of biologics
Currently, there is limited data regarding the cost utility of biologics for CRSwNP.30 For initial treatment of CRSwNP, FESS appears to be more cost effective than biologics.30,31 Although not well studied, biologics were designed as a long-term treatment for asthma.32 Based on current data demonstrating relapse of polyps after discontinuation of biologic therapy, long-term use will likely be needed for CRSwNP as well. The wholesale acquisition cost of dupilumab is approximately $37,000/year, with similar costs for other available biologics.10 Recently, the Emory group presented data regarding the cost of surgery for CRSwNP patients as compared to biologic treatment. Using a large insurance claims repository called MarketScan, it was estimated that the mean cost of surgery over a 6-year-time period was approximately $7000/per patient (panel discussion at the Annual ARS Meeting, September 2019, New Orleans, LA). Although this analysis did not account for indirect costs or medication usage, costs of surgery and topical steroid delivery appear to be dramatically lower than the estimated cost of biologic therapy over the same time period. More research on this front will be critical in the years to come.
Proposed treatment algorithm
As we work to study available and emerging biologics to determine the most appropriate treatments for our patients, we need to make decisions regarding management with the currently available data. At the present time, our treatment paradigm of medical therapy includes the off-label use of high-volume saline irrigations, topical nasal steroid delivery, and oral steroids and antibiotics as indicated.12 In the workup of CRSwNP, it is important to evaluate for comorbidities and clinical features that suggest a specific CRSwNP endotype such as AERD or AFRS.33 Management of comorbid asthma is critical during the medical treatment of CRS. Should medical therapy fail, FESS is beneficial and sufficient for over 70% of CRSwNP patients along with ongoing and sufficient topical steroid therapy.14 A history of aspirin sensitivity or diagnosis of AERD should elicit consideration for aspirin sensitivity testing and desensitization, which is coordinated within weeks after sinus surgery.34 Presence of elevated serum and sinus tissue eosinophils are risk factors for recalcitrant disease and may signal a need for strong compliance with medical therapy.35,36 AFRS should be evaluated by allergy testing to fungi and histologic evaluation of surgical specimen. Consideration of these factors may aid in avoidance of polyp recurrence.
In the event that this management algorithm fails, we believe that the following should be offered to the patient: consideration of additional surgery, specifically if prior surgeries did not address all surgical goals based on endoscopic and computed tomography (CT) findings and/or if appropriate postoperative treatment was not started following the initial surgery. Appropriate treatment may include leukotriene modifiers,37 allergy immunotherapy for documented sensitivities,38 saline irrigations, topical steroids in various forms,39 or aspirin desensitization in interested patients. If patients were not compliant with medical therapy following their initial surgery, oral steroids followed by topical steroids, which are available via various delivery mechanisms,39,40 should be considered prior to a revision surgery. Additional surgical goals such as improved access to sinus cavities involving partial middle turbinectomy or an endoscopic modified Lothrop procedure are options for recurrent polyp patients and should be considered. Some authors argue that comorbid asthma and aspirin intolerance are correlated with more aggressive disease and polyp recurrence and that an endoscopic modified Lothrop procedure should be carefully considered in AERD patients.41 Regardless, we acknowledge that complete FESS, which typically involves maximal openings of the frontal, maxillary and sphenoid sinuses with thorough removal of all ethmoid partitions, and surgical goals, may evolve and are subject to interpretation. Consequently, the extent of surgery will range among otolaryngologists.
After complete FESS and appropriate postoperative medical management, or in the event that a patient is not interested in or is not stable for an initial or revision surgery, we believe that biologics should be considered, likely with continuation of topical nasal steroids.5 In addition, patients requiring repeated rounds of oral steroids despite intermittent improvement may also be considered for revision surgery or biologics, similar to the treatment of asthma. At this time, in patients without asthma, the only biologic approved specifically for CRSwNP is dupilumab. However, once other biologics become approved for CRSwNP, omalizumab may be considered for patients with IgE-mediated disease. Similar to published recommendations for biologics in asthma patients, mepolizumab and possibly other anti-IL-5 agents may be appropriate for those with elevated serum eosinophils or sinus tissue notable for elevated eosinophils. Dupilumab may also be useful in patients with eosinophilia and IgE-mediated disease. Further work on biomarkers of response will likely yield better tests for differentiating between biologics for appropriate patient-centered care. This work should focus on the various identified endotypes of CRSwNP that may differ in their response to specific biologics. Additional investigation into the role of other comorbid Type 2 mediated diseases such as asthma, eczema, and eosinophilic esophagitis may also impact future recommendations for biologics use.
An evaluation to consider response to biologics is very important. In line with the asthma literature, we support an evaluation at 4 months to consider an early stopping point if treatment response is lacking, due to the high cost of these medications.7,8 This evaluation should include assessment of improvement of sinusitis symptoms, endoscopic evaluation, prescription utilization including oral steroids, and respiratory symptoms, if applicable. Of note, an expert panel representing the European Forum for Research and Education in Allergy and Airway Diseases has published recommendations for biologics use in CRSwNP patients.42 We agree with their recommendations for assessment of response at 16 weeks, but have included slightly more stringent and deliberate indications for beginning biologic treatment in CRSwNP patients (see Fig. 1).42
FIGURE 1.
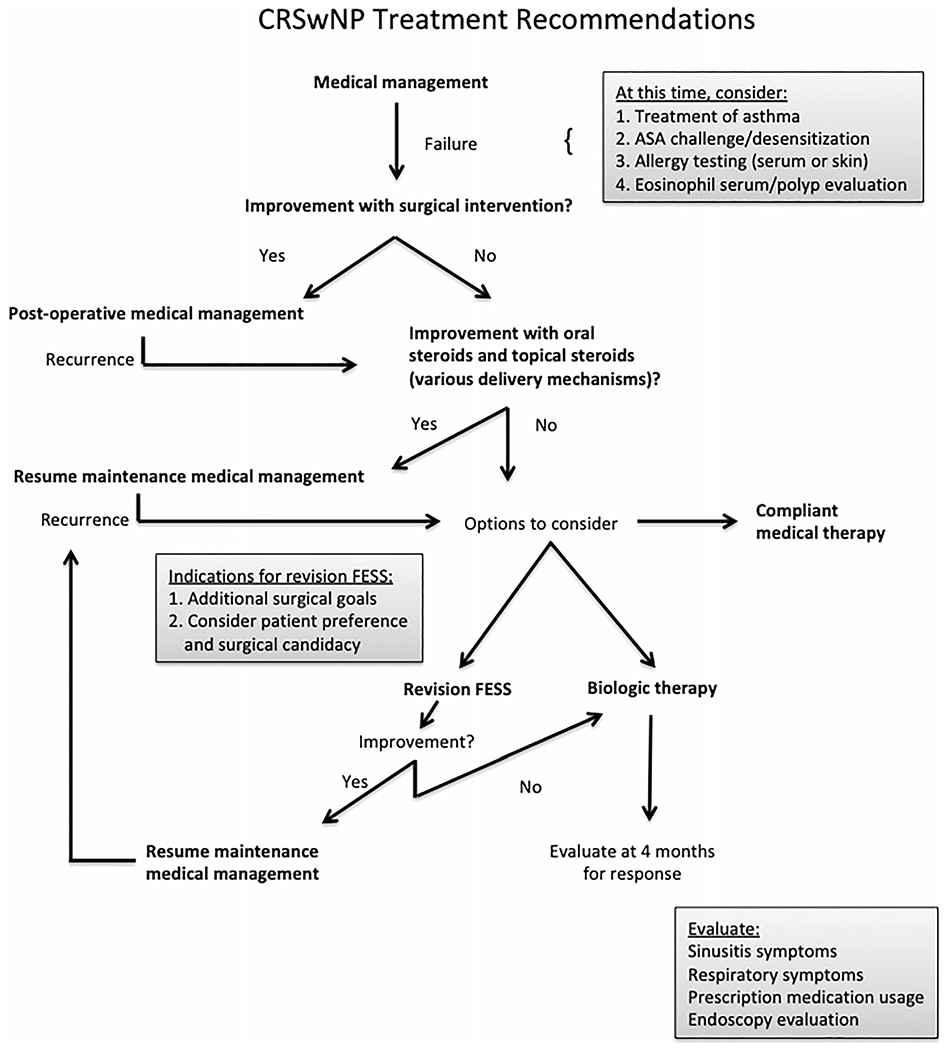
CRSwNP treatment recommendations. CRSwNP = chronic rhinosinusitis with nasal polyps.
As always in our specialty, we believe that treatment should be tailored to the patient’s comfort and wishes and that a shared decision making model is critical. Specifically for aspirin maintenance therapy and biologic medications, both of which require a substantial commitment, the expectations and side effect profiles must be adequately disclosed. This proposed algorithm may be used as a guide for treatment of our patients as we continue to learn more about biologics, their safety profiles, and biomarkers of response.
NIH discussion
Participants of the NIH workshop for the use of biologics in CRSwNP discussed several important aspects of future research. First and foremost, we identified that consistency among grading and evaluation of CRSwNP, a largely subjective disease, is important for interpretation of results among multiple studies. Second, we are currently lacking reliable clinical biomarkers for differentiating among CRSwNP phenotypes and endotypes, which is relevant given the availability of multiple treatment options.
Future directions for CRSwNP research should include determination of appropriate initiation of biologics, cessation recommendations when there is a lack of response, and biomarkers of treatment success. Additionally, the most effective biologic for each patient should be determined for patient-centered care. Therefore, future studies should compare patients with varying levels of medical treatment and surgery prior to the initiation of biologics. Cessation rules should be determined based on cost effectiveness and evaluation of response to treatment. Last, patient-centered care, although challenging, should be the goal of our practice. Due to the heterogeneity of our population, research should also evaluate biomarkers for determination of the appropriate biologic for each patient, and biologics with similar mechanisms of action should be compared in head-to-head blinded trials.
Conclusion
Currently, only dupilumab is FDA-approved for CRSwNP, but other biologics are under investigation. Given lifetime costs, biologics should be reserved for patients who do not respond to other therapies. Future clinical trials are needed to determine recommendations for initiation of biologics, and to compare biologics both to current standard of care treatment, and between biologic medication options. Although this is an ongoing area of research and conversation, the above recommendations have emerged from recent NIH workshop discussions as a possible guideline for the current incorporation of biologics in CRSwNP management.
Potential conflicts of interest:
L.T.R.: Consultant for Tissium. T.L.S.: Research support from the NIH. R.J.S.: Consulting and/or speaker arrangements with Stryker, Olympus, Optinose, GSK, Regeneron, and Roche; owns stock/equity in Healthy Humming; receives research support from Gossamer, Stryker, Optinose, GSK, AstraZeneca, Regeneron, Roche, and Healthy Humming. Z.M.S.: Consultant for Olympus, Optinose, Novartis, Regeneron and Healthy Humming. A.T.P.: Consultant for Astra Zeneca, Sanofi Regeneron, and Optinose. T.M.L.: Consultant for GSK and Regeneron. B.S.B.: funding from the NIH/National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS108968-01); Consultant for Olympus, Karl Storz, Medtronic, Sinopsys, Baxter, and 3D Matrix; Equity in Interscope; Royalties from Thieme. V.R.: Consultant for Medtronic, Inc. and Optinose US, which are not affiliated with this investigation. V.R.R.: Supported by NIH/National Institute on Deafness and Other Communication Disorders (NIDCD) (K23 DC014747). E.T.: Consultant for Fiagan; Advisory board of Optinose; has received research support from Sanofi. D.W.K.: Consultant for Medtronic, Fiagon, NeurEnt, GSK; Royalties from Medtronic. A.U.L.: Consultant for Aerin Medical (Sunnyvale, CA), Lyra Therapeutics (Watertown, MA), and Stryker (Kalamazoo, MI); Advisory board for ENTvantage (Austin, TX); Participated in advisory board meetings for Sanofi (Paris, France) and Novartis (Basel, Switzerland). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M, Stevens W, Li N, et al. Clinical characteristics of patients with chronic rhinosinusitis without nasal polyps in an academic setting. J Allergy Clin Immunol Pract. 2019;7:1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oakley GM, Harvey RJ, Lund VJ. The role of macrolides in chronic rhinosinusitis (CRSsNP and CRSwNP). Curr Allergy Asthma Rep. 2017;17:30. [DOI] [PubMed] [Google Scholar]
- 4.Alobid I, Mullol J. Role of medical therapy in the management of nasal polyps. Curr Allergy Asthma Rep. 2012;12:144–153. [DOI] [PubMed] [Google Scholar]
- 5.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. [DOI] [PubMed] [Google Scholar]
- 6.Naclerio R, Baroody F, Bachert C, et al. Clinical research needs for the management of chronic rhinosinusitis with nasal polyps in the new era of biologics. a national institute of allergy and infectious diseases workshop. J Allergy Clin Immunol Pract. 2020;8:1532–1549.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Strategy for Asthma Management and Prevention (GINA). Global Strategy for Asthma Management and Prevention, 2019. Fontana, WI: GINA; 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed June 21, 2020. [Google Scholar]
- 8.Bousquet J, Brusselle G, Buhl R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J. 2017;50:1701782. [DOI] [PubMed] [Google Scholar]
- 9.Darveaux J, Busse WW. Biologics in asthma—the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3:152–160; quiz 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson WC 3rd, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122:367–372. [DOI] [PubMed] [Google Scholar]
- 11.Tice J, Walsh J, Synnott P, et al. Biologic therapies for treatment of asthma associated with type 2 inflammation: effectiveness, value, and value-based price benchmarks. Boston, MA: Institute for Clinical and Economic Review (ICER); 2018:1–132. https://icer-review.org/wp-content/uploads/2018/04/ICER_Asthma_Final_Report_122018-1.pdf. Accessed June 21, 2020. [Google Scholar]
- 12.Grayson JW, Harvey RJ. Topical corticosteroid irrigations in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(S1):S9–S15. [DOI] [PubMed] [Google Scholar]
- 13.DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith KA, Orlandi RR, Oakley G, Meeks H, Curtin K, Alt JA. Long-term revision rates for endoscopic sinus surgery. Int Forum Allergy Rhinol. 2019;9:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith TL, Schlosser RJ, Mace JC, et al. Long-term outcomes of endoscopic sinus surgery in the management of adult chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9:831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudmik L, Mace J, Soler ZM, Smith TL. Long-term utility outcomes in patients undergoing endoscopic sinus surgery. Laryngoscope. 2014;124:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loftus CA, Soler ZM, Desiato VM, et al. Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2020;10:289–302. [DOI] [PubMed] [Google Scholar]
- 18.Loftus CA, Soler ZM, Koochakzadeh S, et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol. 2019;10:199–207. [DOI] [PubMed] [Google Scholar]
- 19.Miglani A, Divekar RD, Azar A, Rank MA, Lal D. Revision endoscopic sinus surgery rates by chronic rhinosinusitis subtype. Int Forum Allergy Rhinol. 2018;8:1047–1051. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Huh G, Rhee CS, et al. Unsupervised cluster analysis of chronic rhinosinusitis with nasal polyp using routinely available clinical markers and its implication in treatment outcomes. Int Forum Allergy Rhinol. 2019;9:79–86. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Jimenez JC, Moreno-Paz FJ, Teran LM, Guani-Guerra E. Aspirin exacerbated respiratory disease: current topics and trends. Respir Med. 2018;135:62–75. [DOI] [PubMed] [Google Scholar]
- 22.Stevens WW, Peters AT, Hirsch AG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2017;5:1061–1070.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy JM, Smith TL. Is aspirin desensitization indicated for the treatment recalcitrant chronic rhinosinusitis with nasal polyposis in aspirin-exacerbated respiratory disease? Laryngoscope. 2017;127:776–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu DK, Lee DJ, Lee KM, Schunemann HJ, Szczeklik W, Lee JM. Benefits and harms of aspirin desensitization for aspirin-exacerbated respiratory disease: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2019;9:1409–1419. [DOI] [PubMed] [Google Scholar]
- 25.Adappa ND, Ranasinghe VJ, Trope M, et al. Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. 2018;8:49–53. [DOI] [PubMed] [Google Scholar]
- 26.Smith TL. Scientific abstracts for RhinoWorld 2019. Int Forum Allergy Rhinol. 2019;9(S2):S49–S124. [DOI] [PubMed] [Google Scholar]
- 27.Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. 2019;74:28–39. [DOI] [PubMed] [Google Scholar]
- 28.Roland LT, Nagy C, Wang H, et al. Treatment practices for aspirin-exacerbated respiratory disease: analysis of a national insurance claims database. Int Forum Allergy Rhinol. 2020;10:190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters KM, Waldram JD, Woessner KM, White AA. Long-term clinical outcomes of aspirin desensitization with continuous daily aspirin therapy in aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2018;32:280–286. [DOI] [PubMed] [Google Scholar]
- 30.Codispoti CD, Mahdavinia M. A call for cost-effectiveness analysis for biologic therapies in chronic rhinosinusitis with nasal polyps. Ann Allergy Asthma Immunol. 2019;123:232–239. [DOI] [PubMed] [Google Scholar]
- 31.Scangas GA, Wu AW, Ting JY, et al. Cost utility analysis of dupilumab versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Laryngoscope. (in press). Epub April 3, 2020. 10.1002/lary.28648. [DOI] [PubMed] [Google Scholar]
- 32.McCracken JL, Tripple JW, Calhoun WJ. Biologic therapy in the management of asthma. Curr Opin Allergy Clin Immunol. 2016;16:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roland LT, Wang H, Mehta CC, et al. Longitudinal progression of aspirin-exacerbated respiratory disease: analysis of a national insurance claims database. Int Forum Allergy Rhinol. 2019;9:1420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larivee N, Chin CJ. Aspirin desensitization therapy in aspirin-exacerbated respiratory disease: a systematic review. Int Forum Allergy Rhinol. 2020;10:450–464. [DOI] [PubMed] [Google Scholar]
- 35.Bassiouni A, Ou J, Rajiv S, Cantero D, Vreugde S, Wormald PJ. Subepithelial inflammatory load and basement membrane thickening in refractory chronic rhinosinusitis with nasal polyposis: a histopathological study. Int Forum Allergy Rhinol. 2016;6:248–255. [DOI] [PubMed] [Google Scholar]
- 36.Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol. 2018;8:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SE, Farquhar DR, Adams KN, et al. Effect of zileuton treatment on sinonasal quality of life in patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2019;33:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8:108–352. [DOI] [PubMed] [Google Scholar]
- 39.Kuan EC, Kovacs AJ, Workman AD, Bosso JV, Adappa ND. Efficacy of fluticasone exhalation delivery system in the management of chronic rhinosinusitis: what is the evidence? Int Forum Allergy Rhinol. 2019;9(S1):S16–S21. [DOI] [PubMed] [Google Scholar]
- 40.Han JK, Kern RC. Topical therapies for management of chronic rhinosinusitis: steroid implants. Int Forum Allergy Rhinol. 2019;9(S1):S22–S26. [DOI] [PubMed] [Google Scholar]
- 41.Bassiouni A, Wormald PJ. Role of frontal sinus surgery in nasal polyp recurrence. Laryngoscope. 2013;123:36–41. [DOI] [PubMed] [Google Scholar]
- 42.Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]


