ABSTRACT
The use of valganciclovir as secondary prophylaxis for cytomegalovirus (CMV) infection in solid organ transplantation (SOT) is the most prevalent therapeutic choice, but it has been dismissed by the presence of resistance and toxicity. Letermovir (LMV) is indicated as primary prophylaxis in hematopoietic stem cell transplantation, but there is scarce clinical data on its use as secondary prophylaxis in SOT. We present two cases of SOT recipients (lung, liver) who underwent LMV secondary prophylaxis. One patient developed the L595S (UL97)-resistant mutation to ganciclovir/valganciclovir and experienced several CMV relapses under LMV therapy, but no LMV resistance mutations were detected. The second patient developed the C325F (UL56)-resistant mutation to LMV under secondary prophylaxis which was rescued by foscarnet. This observation reviews previously published clinical data on LMV secondary prophylaxis and recommends a cautious use of LMV in the clinical practice due to the early development of UL56 resistance mutations.
IMPORTANCE
This observation provides comprehensive data on the clinical correlates of both cytomegalovirus (CMV) genotypic follow-up and clinical monitoring and outcomes for two different solid organ transplantation recipients that received letermovir as secondary prophylaxis. Our study emphasizes that monitoring of CMV disease in the patient and early genotypic detection of resistance mutations are essential when using new antiviral drugs for off-label indication in patients experiencing CMV relapses or not responding to standard antiviral therapy. These cases and the bibliography reviewed can be helpful for other researchers and clinicians working in the field to optimize the use of new treatments for transplant recipients since drug-resistant CMV infection is an important emerging problem even with new developments in antiviral treatment.
KEYWORDS: cytomegalovirus, letermovir, secondary prophylaxis, transplant recipients, antiviral, resistance mutation
OBSERVATION
Clinical trials have demonstrated the efficacy and safety of letermovir (LMV) for primary prophylaxis of cytomegalovirus (CMV) infection in hematopoietic stem cell transplant (HSCT) patients (1) and in high-risk kidney transplant recipients (2). However, no trials have been conducted on the use of LMV as secondary prophylaxis for CMV disease. The drug of choice for this indication is valganciclovir (3, 4), which can cause resistance or neutropenia that may require discontinuation (3, 4). Foscarnet has many limitations that complicate its use, such as being nephrotoxic and needing to be administered intravenously (3, 4). The efficacy of maribavir has not been demonstrated in dose/response studies (5, 6). Therefore, LMV could be a potential therapeutic choice for this indication (1, 2).
Case reports
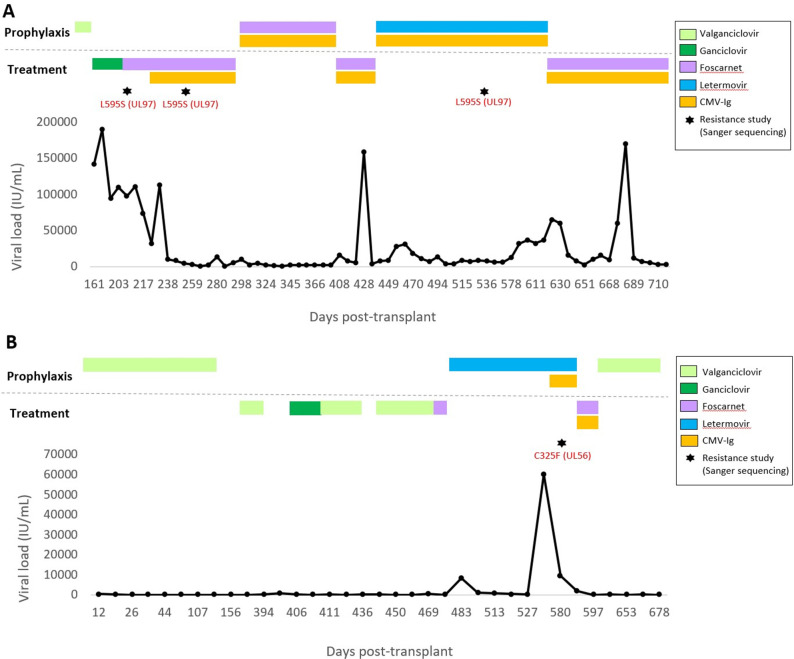
A 56-year-old CMV-seronegative female with idiopathic pulmonary fibrosis received a double lung transplant from a CMV-seronegative donor (D−/R−) in August 2018. Prophylaxis was initiated with valganciclovir, and 6 months after transplantation, the patient developed a breakthrough CMV primary infection (pneumonitis and plasma viral load of 142,000 IU/mL) (Fig. 1A). Subsequent treatment with ganciclovir failed, and a genotypic resistance study performed by Sanger sequencing showed the L595S mutation in the UL97 gene (IC50 × 8.5–9.2; which confers a 8.5- to 9.2-fold decreased susceptibility to ganciclovir/valganciclovir) (7). Treatment with foscarnet combined with CMV-specific immunoglobulin and everolimus controlled the patient’s symptoms. After CMV infection was resolved, the patient started secondary prophylaxis with foscarnet and specific immunoglobulin weekly, which was maintained for approximately 5 months until nephrotoxicity forced the discontinuation of foscarnet. As a result, secondary prophylaxis with LMV (480 mg/24 h) was initiated. CMV load at the time of LMV secondary prophylaxis was 7,740 IU/mL. Prophylaxis was maintained for 6 months, and the patient remained asymptomatic although breakthrough replication was observed. Three months after starting treatment with LMV, a genotypic resistance study was performed and showed no mutations in the UL56 gene (the plasma viral load was 7,800 IU/mL at this point). Only the L595S (UL97) mutation was detected over the clinical course of this patient. No mutations were found in UL54 or UL56 antiviral target genes. The patient developed chronic rejection that required increased immunosuppression. Six months after starting LMV, she developed CMV pneumonitis and plasma CMV viral load increased to 170,000 IU/mL; however, no genotypic resistance study was performed at this time. The patient died despite treatment with foscarnet combined with CMV-specific immunoglobulin.
Fig 1.
Description of the virologic course of the two clinical cases presented (panel A, lung transplant; panel B, liver transplant). CMV viral loads (IU/mL) were monitored over the time course after transplantation. Antiviral therapies are indicated according to prophylaxis or CMV treatment regimens. Resistance mutations detected by genotypic resistance studies are pointed with a star.
A 39-year-old CMV-seronegative male with alcoholic liver cirrhosis received a liver transplant from a CMV-seropositive (D+/R−) donor in February 2021. The patient was administered primary prophylaxis with valganciclovir for 4.5 months, which was discontinued due to severe neutropenia (Fig. 1B). One year after transplantation, he developed gastrointestinal CMV disease (detected in biopsy) which was treated with valganciclovir until being discontinued due to neutropenia. The patient then developed CMV retinitis that required treatment with ganciclovir/valganciclovir. Fifteen months after transplant, he developed SARS-COV-2 pneumonia complicated by a pulmonary superinfection with Pneumocystis jirovecii and CMV. Valganciclovir was administered until being replaced with foscarnet due to neutropenia. After clinical and virological response (35 IU/mL), secondary prophylaxis with LMV (480 mg/24 h) was initiated. After 5 weeks of LMV administration, asymptomatic low-grade viral replication (229–1,230 IU/mL) was detected, but LMV was maintained for 4 months. Plasma CMV replication gradually increased until viral load reached 60,000 IU/mL. A genotypic resistance mutation study by Sanger sequencing showed the development of the C325F mutation (3,000-fold decrease susceptibility to LMV) in the UL56 LMV target gene (8), and no resistance mutation was detected in either UL97 or UL54. Although the patient was asymptomatic, LMV was discontinued and treatment with foscarnet combined with CMV-specific immunoglobulin was initiated. The patient is currently continuing secondary prophylaxis with valganciclovir.
Clinical assessment
CMV treatment and monitoring were performed according to the clinical protocol of the center (Reina Sofia University Hospital, Cordoba).
Viral load quantification
Quantification of CMV loads was performed by real-time polymerase chain reaction (qPCR) using the Cobas CMV kit on the Cobas 6800 System (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
Genotypic resistance study
Antiviral resistance studies were performed to detect resistant mutations in the antiviral target genes (UL97, UL54, UL56). This study was requested based on the virological response of the patient to the specific antiviral treatment received. Genotypic resistance studies were performed at the Reference Centre for antiviral resistance, Hospital Clinic of Barcelona, between April 2019 and September 2022. Nucleic acid extraction from plasma samples was performed using a MagNAPure Compact (Roche, Switzerland). Genotypic testing was done by Sanger sequencing based on PCR amplification of HCMV UL97 (residues 270–670), UL54 (300–1,000), and UL56 (180–395) regions, followed by BigDye Terminator v3.1. (Applied Biosystems) dideoxy chain-termination sequencing using previously described primers and procedures (9 – 11). Sequences were analyzed and aligned using the MEGA v.7. software and were compared with the HCMV TB40 strain (GenBank: MF871618.1) using the MRA-Mutation Resistance Analyzer tool provided by the University of Ulm (12).
Secondary prophylaxis with letermovir: review of the literature
Table 1 summarizes the published cases of secondary prophylaxis with LMV (13 – 17), as well as the two cases presented in this observation. A total of 13 transplant recipients have been reported (8 lung, 3 kidney, 1 heart, and 1 liver). All recipients were high-risk seronegative patients who received seropositive organs (D+/R−), except one who received a seronegative organ (D−/R−) that had a primary infection. All patients had previously received valganciclovir in addition to other antiviral therapies (foscarnet, cidofovir, leflunomide, CMV-specific immunoglobulin). Toxicity, resistance, or failure of other therapies leads to LMV administration as secondary prophylaxis. Seven patients developed CMV infection with confirmed viremia while on secondary prophylaxis with LMV. Aryal et al. (13) reported three cases with breakthrough viremia, but no resistance study was performed. In the rest of the reports, four patients developed confirmed resistance to LMV with UL56 mutations.
TABLE 1.
Review of use of letermovir as secondary prophylaxis for cytomegalovirus infection after solid organ transplantation (only references with disaggregated data) b
| Reference | SOT | D/R serology | Previous treatment/prophylaxis | Reason for letermovir prophylaxis | Previous CMV infection | CMV infection on letermovir prophylaxis | Letermovir resistance | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| (13) | Lung | D+/R− | Valganciclovir | Leukopenia | Viremia | No | No | Not applicable | Alive |
| (13) | Lung | D+/R− | Valganciclovir | Leukopenia | Viremia | No | No | Not applicable | Alive |
| (13) | Lung | D+/R− | Valganciclovir | Leukopenia | Viremia | No | No | Not applicable | Alive |
| (13) | Lung | D+/R− | Valganciclovir | Leukopenia | Viremia | Viremia (1,910 IU/mL) a | Not performed | Not available | Alive (failure therapy) |
| (13) | Lung | D+/R− | Valganciclovir | Leukopenia | Viremia | Viremia (33,392 IU/mL) a | Not performed | Not available | Alive (failure therapy) |
| (13) | Lung | D+/R− | Valganciclovir | Resistance (UL97 mutation) | Viremia | Viremia (2,499 IU/mL) a | Not performed | Not available | Death (for CMV infection) |
| (14) | Kidney | D+/R− | Valganciclovir Foscarnet |
Resistance (UL97 mutations, L595F, H520Q, and M460V and UL54 mutation, A987G | CMV syndrome CMV colitis? |
Intermittent viremia up to 200 copies/mL a | UL56 mutation, C325Y; persistence of the UL97 mutation M460V; and a new UL54 mutation P522S | Foscarnet, CMV immunoglobulin, everolimus | Alive (with functional graft) |
| (14) | Kidney | D+/R− | Valganciclovir Foscarnet CMV immune globulin |
Resistance (UL97 mutation, C607F) | Disseminated disease | Viremia (13,200 copies/mL) a | UL56 mutation, C325Y | Foscarnet | Death (PTLD) |
| (15) | Heart | D+/R− | Valganciclovir* Cidofovir, foscarnet** Leflunomide*** |
*Resistance (UL 97 mutation, C603W) **Intolerance ***Failure |
CMV syndrome with viremia | No | No | Not applicable | Alive |
| (16) | Kidney | D+/R− | Valganciclovir* Cidofovir** |
*Resistance (UL 97 mutation, C603W) **Intolerance |
Gastrointestinal CMV disease with viremia | Low-grade asymptomatic viremia | No | Cidofovir | Alive (graft failure) |
| (17) | Lung | D+/R− | Valganciclovir CMV immune globulin Leflunomide |
Resistance (UL 97 mutation, M460V and UL54 mutation, L516P) | CMV syndrome with viremia | Viremia (4.1 log10 copies/mL) a | UL56 mutation C325Y (IC50 x >8,000) UL 97 mutation, M460V |
Foscarnet | Alive (with functional graft) |
| Case 1 | Lung | D−/R− | Valganciclovir* Foscarnet** CMV immune globulin |
*Resistance (UL97 mutation, L595S). **Intolerance (nephrotoxicity) |
Pneumonitis | Pneumonitis | No (3 months of treatment) Unknown at failure (6 months of treatment) |
Foscarnet + CMV-specific immunoglobulins | Death (chronic rejection + CMV disease) |
| Case 2 | Liver | D+/R− | Valganciclovir* Foscarnet |
*Intolerance (leukopenia) | CMV colitis | Viremia (60,000 IU/mL) | UL56 mutation, C325F (IC50 x >3.000) | Foscarnet + CMV-specific immunoglobulins | Alive |
Viral loads are expressed in IU/mL or copies/mL according to the data reported in the cited studies.
CMV, cytomegalovirus; IC50, 50% inhibitory concentration; PTLD, post-transplant lymphoproliferative disorder.
It is difficult to review the literature because the descriptions include cases of both prolonged treatment and secondary prophylaxis. This is what occurs with the 27 cases with SOT reported by Linder et al. (18) and in the 16 cases described by Saullo et al. (19). In the report of Linder et al. (18), 30% of 27 recipients patients were treated for graft rejection in the 3 months prior to initiation of LMV, and the most frequent indications for initiating LMV were intolerance to other agents (77%) and detection of proven antiviral resistance to others antiviral (32%). This study included 9 patients with SOT who initiated LMV with a viral load >1,000 IU/mL, of whom 5 had a reduction in viral load of at least 1 log 2–4 weeks after initiating LMV and 3/9 patients had a viral load <1,000 IU/mL at the time of stopping LMV treatment. One of the patients who had a CMV viral load rebound at week 9–12 post-LMV treatment had the C325Y mutation in the UL56. Two other patients died. In the cases with SOT published by Saullo et al. (19), valganciclovir was discontinued in 10 cases due to myelosuppression, mainly leukopenia, and in 6 cases due to resistance mutations in both UL97 and UL54 genes. One patient developed breakthrough viremia that responded to foscarnet, and a resistance study for the UL56 gene was not performed. Ten patients developed low-grade viremia that did not require treatment (no resistance studies were performed).
In conclusion, focusing on the 27 cases using LMV as secondary prophylaxis from the articles reviewed (13 – 17, 19), LMV was successful in seven cases, it failed in eight cases that displayed increasing CMV viremia, and LMV prophylaxis was discontinued in three other cases for other reasons (lack of insurance coverage, transition to palliative care, or loss to follow-up due to transfer to another medical center); therefore, control of viral replication was achieved in 46.7% (7/15) of the cases. The remaining nine cases were still on prophylaxis at the time of study completion.
In 12 of the 27 cases, resistance mutations were detected in UL97 and UL54, so it was decided that LMV would be used as a prophylactic option. Of the eight cases in which viremia could not be controlled with LMV, resistance in UL56 was assessed in four patients, and mutations were detected in three of them (75%).
Discussion
The main reasons of valganciclovir failure as prophylaxis in SOT are adverse effects and the development of resistant mutations (20, 21). In our observation, we described two cases of LMV secondary prophylaxis. In the first case, the patient developed a valganciclovir/ganciclovir resistant mutation (L595S) that compelled a switch to LMV; in the second case, neutropenia caused by valganciclovir/ganciclovir precipitated the use of LMV.
These two cases together with the reviewed bibliography suggest that alternatives to valganciclovir are needed. Even though LMV seems to be a potential drug for prophylaxis regimens, it should be used with caution because of the early development of resistance. This was exemplified in the second case of our study, in which LMV administration caused C325F-resistant mutation to emerge in UL56. It has been suggested that the UL56 LMV target gene has a low genetic barrier to mutate under antiviral selection pressure (22) but not without (10); therefore, exposure to low doses of LMV may lead to the development of UL56 mutants (23). This study together with previous trials showed that LMV resistance is frequent when used as secondary prophylaxis, which could be caused by the continuation of LMV therapy even under CMV relapses. Thus, it has been highly recommended to initiate LMV secondary prophylaxis when CMV loads are undetectable in three or more consecutive tests to avoid UL56 resistance development. Clinical experience showed that primary prophylaxis with LMV does neither prevent CMV blips, which should not imply a risk of resistance if dosage is correct and there is not treatment interruption (1, 2).
Most of the literature about LMV secondary prophylaxis refers to clinical cases in which LMV was associated with nonresponse or the development of antiviral resistance, which bias the clinical use of LMV for the mentioned indication. Therefore, larger clinical trials and well-designed observational cohort studies are needed, which can only be achieved through collaborations or multicenter groups.
Contributor Information
María-Ángeles Marcos, Email: mmarcos@clinic.cat.
Maria Antonia De Francesco, University of Brescia, Brescia, Italy .
REFERENCES
- 1. Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA, Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y, Kartsonis NA, Leavitt RY, Badshah C. 2017. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 377:2433–2444. doi: 10.1056/NEJMoa1706640 [DOI] [PubMed] [Google Scholar]
- 2. Limaye AP, Budde K, Humar A, Garcia-Diaz J, Carroll RP, Murata Y, Teal VL, Gilbert CL, Haber BA. 2022. Lb2307. safety and efficacy of letermovir (LET) versus valganciclovir (VGCV) for prevention of cytomegalovirus (CMV) disease in kidney transplant recipients (KTRs): a phase 3 randomized study. Open Forum Infect Dis 9. doi: 10.1093/ofid/ofac492.1897 [DOI] [Google Scholar]
- 3. Torre-Cisneros J, Aguado JM, Caston JJ, Almenar L, Alonso A, Cantisán S, Carratalá J, Cervera C, Cordero E, Fariñas MC, Fernández-Ruiz M, Fortún J, Frauca E, Gavaldá J, Hernández D, Herrero I, Len O, Lopez-Medrano F, Manito N, Marcos MA, Martín-Dávila P, Monforte V, Montejo M, Moreno A, Muñoz P, Navarro D, Pérez-Romero P, Rodriguez-Bernot A, Rumbao J, San Juan R, Vaquero JM, Vidal E. 2016. Management of cytomegalovirus infection in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplantation Reviews 30:119–143. doi: 10.1016/j.trre.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 4. Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A, The Transplantation Society International CMV Consensus Group . 2018. The third International consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 102:900–931. doi: 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 5. Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia-Diaz JB, Goss JA, Clough L, Avery R, Limaye AP, Ericzon BG, Navasa M, Troisi RI, Chen H, Villano SA, Uknis ME, 1263-301 Clinical Study Group . 2012. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant 12:3021–3030. doi: 10.1111/j.1600-6143.2012.04231.x [DOI] [PubMed] [Google Scholar]
- 6. Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young J-AH, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M, Maribavir 1263-300 Clinical Study Group . 2011. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 11:284–292. doi: 10.1016/S1473-3099(11)70024-X [DOI] [PubMed] [Google Scholar]
- 7. Lurain NS, Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 23:689–712. doi: 10.1128/CMR.00009-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou S. 2015. Rapid in vitro evolution of human cytomegalovirus Ul56 mutations that confer letermovir resistance. Antimicrob Agents Chemother 59:6588–6593. doi: 10.1128/AAC.01623-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santos Bravo M, Plault N, Sánchez-Palomino S, Rodríguez C, Navarro Gabriel M, Mosquera MM, Fernández Avilés F, Suarez-Lledó M, Rovira M, Bodro M, Moreno A, Linares L, Cofan F, Berengua C, Esteva C, Cordero E, Martin-Davila P, Aranzamendi M, Pérez Jiménez AB, Vidal E, Fernández Sabé N, Len O, Hantz S, Alain S, Marcos MÁ, Spanish network for research in infectious diseases (REIPI) and the group for the study of infection in transplantation (GESITRA) . 2022. Genotypic and phenotypic study of antiviral resistance mutations in refractory cytomegalovirus infection. J Infect Dis 226:1528–1536. doi: 10.1093/infdis/jiac349 [DOI] [PubMed] [Google Scholar]
- 10. Santos Bravo M, Tilloy V, Plault N, Palomino SS, Mosquera MM, Navarro Gabriel M, Fernández Avilés F, Suárez Lledó M, Rovira M, Moreno A, Linares L, Bodro M, Hantz S, Alain S, Marcos MÁ. 2022. Assessment of Ul56 mutations before letermovir therapy in refractory cytomegalovirus transplant recipients. Microbiol Spectr 10:e0019122. doi: 10.1128/spectrum.00191-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. López-Aladid R, Guiu A, Sanclemente G, López-Medrano F, Cofán F, Mosquera MM, Torre-Cisneros J, Vidal E, Moreno A, Aguado JM, Cordero E, Martin-Gandul C, Pérez-Romero P, Carratalá J, Sabé N, Niubó J, Cervera C, Cervilla A, Bodro M, Muñoz P, Fariñas C, Codina MG, Aranzamendi M, Montejo M, Len O, Marcos MA, Group for Study of Infection in Transplantation of the Spanish Society of Infectious Diseases Clinical Microbiology GESITRA-SEIMC Spanish Network for Research in Infectious . 2017. Detection of cytomegalovirus drug resistance mutations in solid organ transplant recipients with suspected resistance. J Clin Virol 90:57–63. doi: 10.1016/j.jcv.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 12. Chevillotte M, von Einem J, Meier BM, Lin F-M, Kestler HA, Mertens T. 2010. A new tool linking human cytomegalovirus drug resistance mutations to resistance phenotypes. Antiviral Res 85:318–327. doi: 10.1016/j.antiviral.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 13. Aryal S, Katugaha SB, Cochrane A, Brown AW, Nathan SD, Shlobin OA, Ahmad K, Marinak L, Chun J, Fregoso M, Desai S, King C. 2019. Single‐center experience with use of letermovir for CMV prophylaxis or treatment in thoracic organ transplant recipients. Transpl Infect Dis 21:e13166. doi: 10.1111/tid.13166 [DOI] [PubMed] [Google Scholar]
- 14. Hofmann E, Sidler D, Dahdal S, Bittel P, Suter-Riniker F, Manuel O, Walti LN, Hirzel C. 2021. Emergence of letermovir resistance in solid organ transplant recipients with ganciclovir resistant cytomegalovirus infection: a case series and review of the literature. Transpl Infect Dis 23:e13515. doi: 10.1111/tid.13515 [DOI] [PubMed] [Google Scholar]
- 15. Chong PP, Teiber D, Prokesch BC, Arasaratnam RJ, Peltz M, Drazner MH, Garg S. 2018. Letermovir successfully used for secondary prophylaxis in a heart transplant recipient with ganciclovir-resistant cytomegalovirus syndrome (Ul97 Mutation). Transpl Infect Dis 20:e12965. doi: 10.1111/tid.12965 [DOI] [PubMed] [Google Scholar]
- 16. Koepf US, Klehr HU, Eis-Huebinger AM, Aldabbagh S, Strassburg CP, Boes D, Lutz P. 2020. Suppression of CMV infection with letermovir in a kidney transplant patient. Eur J Case Rep Intern Med 7:001622. doi: 10.12890/2020_001622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cherrier L, Nasar A, Goodlet KJ, Nailor MD, Tokman S, Chou S. 2018. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant 18:3060–3064. doi: 10.1111/ajt.15135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linder KA, Kovacs C, Mullane KM, Wolfe C, Clark NM, La Hoz RM, Smith J, Kotton CN, Limaye AP, Malinis M, Hakki M, Mishkin A, Gonzalez AA, Prono MD, Ostrander D, Avery R, Kaul DR. 2021. Letermovir treatment of cytomegalovirus infection or disease in solid organ and hematopoietic cell transplant recipients. Transpl Infect Dis 23:e13687. doi: 10.1111/tid.13687 [DOI] [PubMed] [Google Scholar]
- 19. Saullo JL, Baker AW, Snyder LD, Reynolds JM, Zaffiri L, Eichenberger EM, Ferrari A, Steinbrink JM, Maziarz EK, Bacchus M, Berry H, Kakoullis SA, Wolfe CR. 2022. Cytomegalovirus prevention in thoracic organ transplantation: a single-center evaluation of letermovir prophylaxis. J Heart Lung Transplant 41:508–515. doi: 10.1016/j.healun.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou S. 2020. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antiviral Res 176:104711. doi: 10.1016/j.antiviral.2020.104711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleiboeker S, Nutt J, Schindel B, Dannehl J, Hester J. 2014. Cytomegalovirus antiviral resistance: characterization of results from clinical specimens. Transplant Infectious Dis 16:561–567. doi: 10.1111/tid.12241 [DOI] [PubMed] [Google Scholar]
- 22. Chou S. 2015. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr Opin Infect Dis 28:293–299. doi: 10.1097/QCO.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lischka P, Michel D, Zimmermann H. 2016. Characterization of cytomegalovirus breakthrough events in a phase 2 prophylaxis trial of letermovir (AIC246, MK 8228). J Infect Dis 213:23–30. doi: 10.1093/infdis/jiv352 [DOI] [PubMed] [Google Scholar]