Abstract
Bacillus subtilis grown at 37°C synthesizes saturated fatty acids with only traces of unsaturated fatty acids (UFAs). However, when cultures growing at 37°C are transferred to 20°C, UFA synthesis is induced. We report the identification and characterization of the gene encoding the fatty acid desaturase of B. subtilis. This gene, called des, was isolated by complementation of Escherichia coli strains with mutations in either of two different genes of UFA synthesis. The des gene encodes a polypeptide of 352 amino acid residues containing the three conserved histidine cluster motifs and two putative membrane-spanning domains characteristic of the membrane-bound desaturases of plants and cyanobacteria. Expression of the des gene in E. coli resulted in desaturation of palmitic acid moieties of the membrane phospholipids to give the novel mono-UFA cis-5-hexadecenoic acid, indicating that the B. subtilis des gene product is a Δ5 acyl-lipid desaturase. The des gene was disrupted, and the resulting null mutant strains were unable to synthesize UFAs upon a shift to low growth temperatures. The des null mutant strain grew as well as its congenic parent at 20 or 37°C but showed severely reduced survival during stationary phase. Analysis of operon fusions in which the des promoter directed the synthesis of a lacZ reporter gene showed that des expression is repressed at 37°C, but a shift of cultures from 37 to 20°C resulted in a 10- to 15-fold increase in transcription. This is the first report of a membrane phospholipid desaturase in a nonphotosynthetic organism and the first direct evidence for cold induction of a desaturase.
Bacterial adaptation to various environmental stresses has been extensively investigated (11, 12). Interestingly, it has been demonstrated that bacteria respond to high growth temperatures by induction of a group of heat shock proteins but also to low temperatures by induction of a group of cold shock proteins (10, 13, 14). In contrast to heat shock proteins, which include chaperones required for protein folding and peptidases, cold-induced proteins appear to be involved in cellular functions such as general metabolism, transcription, translation, and recombination (10, 13, 14). Recently, major attention has focused on a group of small, acidic proteins found in several microorganisms that are very highly induced by a temperature downshift (10, 13). Although a large body of information concerning these cold-induced proteins has been gathered (10, 14), no defined biochemical role or mode of action has been reported for any of these proteins. This lack of knowledge about the role of the proteins induced at low growth temperatures has probably limited the design of strategies to discover the key regulatory factor(s) responsible for sensing low temperatures and/or governing induction by shifts to decreased temperatures.
A universally conserved adaptation response observed among bacteria and most (if not all) poikilothermic organisms is the adjustment of membrane lipid composition at low temperatures (3, 21). As the growth temperature is lowered, the proportion of unsaturated fatty acids (UFAs) in the membrane lipids increases. This regulatory mechanism, called thermal control of fatty acid synthesis, is thought to be designed to ameliorate the effects of temperature changes on the physical state of the lipid bilayer (3, 21). It is well documented that the proportion of fluid (disordered) lipid to ordered lipid in cell membranes plays a major role in membrane function (3). Increased incorporation of UFAs decreases the melting temperature of the membrane phospholipids, whereas increased incorporation of saturated fatty acids (SFAs) has the opposite effect (3, 21). Thus, the membrane lipid composition can be altered to give optimal membrane function at a new growth temperature.
How the fatty acid composition of membrane lipids is altered in response to the growth temperature appears to depend on the mechanism of UFA synthesis utilized. In bacteria, both anaerobic and aerobic mechanisms are responsible for the synthesis of UFA (for a review, see reference 5). The anaerobic pathway, elucidated in detail for Escherichia coli, produces cis-UFA by a specific 2,3-dehydrase acting at the C-10 level (for a recent review, see reference 3). In certain bacteria, and in eukaryotes, the introduction of double bonds into the fatty acids employs a different mechanism. The reaction is catalyzed by oxygen-dependent desaturation of the full-length fatty acid chain either as an acyl-thioester or as a phospholipid fatty acid moiety and requires a specific electron transport chain (21, 25).
The molecular mechanism of thermal control of UFA biosynthesis has been extensively studied in E. coli (3). In this organism, the UFA synthesized in greater quantity at low temperatures is cis-vaccenic acid. This regulatory response is due to the properties of a specific fatty acid synthetic protein, β-ketoacyl-acyl carrier protein (ACP) synthase II, that converts palmitoleic to cis-vaccenic acid. The enzyme is present at all temperatures but is more active at lower growth temperatures.
Bacilli have a different mechanism to regulate UFA synthesis in response to low growth temperatures, as first demonstrated 30 years ago by Fulco (7). This adaptive response was extensively characterized in vivo in Bacillus megaterium, which desaturates palmitate to Δ5-hexadecenoate only at low growth temperatures (6). It was proposed that the inability of B. megaterium grown at 35°C to desaturate fatty acids was due to the absence of the mRNA encoding the desaturase (6). Upon transfer of a culture from a high to a low temperature, desaturase synthesis transcription initiation at the new temperature was required (6). However, no direct experimental evidence supported this proposed “on or off” transcriptional regulatory model of desaturase synthesis. Therefore, we studied cold induction of UFA synthesis in Bacillus subtilis. Like B. megaterium, B. subtilis growing at 37°C almost exclusively synthesizes SFA. However, when a culture grown at 37°C is transferred to 20°C, the synthesis of a C-16 mono-UFA is induced (8). We have isolated and studied a cold-induced gene encoding the B. subtilis desaturase to begin to understand how a change in growth temperature regulates the expression of genes required for oxygen-dependent desaturation of fatty acids.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and DNA methods.
The B. subtilis strains used in this study are trpC2 phe-1 derivatives of JH642 and were grown in Luria-Bertani broth (24) or Spizizen minimal salts medium (26) supplemented with glucose (0.5%), vitamin-free casein hydrolysate (0.1%), and tryptophan (40 μg/ml). E. coli K-12 strains CY451 and DM86 are temperature-sensitive fabA and fabB derivatives of strain LE392 (hsdR supE44 supF metB1), respectively. Strain AK7 (fabB5 fadE zfa::Tn10) has been previously described (16), and strain HC71 is a fadE derivative of LE392. The RB medium used to grow the E. coli strains has been previously described (16). Anaerobic growth on RB agar plates was performed in an anaerobic jar with a gas-generating system (Becton Dickinson, Cockeysville, Md.).
Plasmid pHP13 is a B. subtilis-E. coli shuttle vector conferring resistance to chloramphenicol and erythromycin (2). A library made of B. subtilis JH642 DNA cloned into pHP13 was the generous gift of A. Grossman. The fragments were derived by partial digestion with Sau3A and ligated into the BamHI site of the vector. The general techniques for handling DNA followed standard methods (24). DNA sequencing was done automatically with Taq DNA polymerase and fluorescent-dye-labeled terminators.
β-Galactosidase was assayed as described previously (19), and specific activity was expressed in Miller units.
Plasmid and strain constructions.
The chromosomal copy of des was disrupted by first cloning an internal 665-bp fragment of the des gene between the EcoRI and HindIII sites of integrational vector pJM103 (22). The fragment was obtained by PCR amplification from JH642 chromosomal DNA using the oligonucleotides 5′-ACACGAATTCTTATCATCTTCCATGACTGCTTGC-3′ and 5′-TCATTCAAGCTTATAGTTAGGCACCTTTGGACTC-3′ (restriction sites are underlined). The resulting plasmid, named pAK667, was integrated into the chromosome of strain JH642 via single crossover by transformation and selection for resistance to chloramphenicol (5 μg/ml). To construct a transcriptional fusion between des and lacZ, chromosomal DNA of strain JH642 was used in a PCR using oligonucleotides 5′-GTTTGGAATTCACCCCTCAAGTGAGTGGAGC-3′ and 5′-TAGTTAGGATCCTCTCATTGTGTGTCTCGGTTC-3′ to amplify a DNA fragment containing the 660 bp located upstream of the putative translational start of des. The fragment obtained was digested with EcoRI and BamHI and cloned into integrational vector pJM116 (4), generating plasmid pAR10. This plasmid was linearized with ScaI and introduced by a double-crossover event at the amyE locus of the JH642 chromosome, yielding strain AKP2.
Fatty acid analyses.
For measurement of fatty acid desaturation, E. coli cells were grown to exponential phase and 2-ml samples of these cultures were labeled with 2 μCi of [1-14C]palmitate (see Fig. 3A). After incubation, the lipids were extracted from whole cells as previously described (8). The fatty acids of the glycerolipids were converted to their methyl esters with sodium methoxide and separated into unsaturated and saturated fractions by chromatography on 20% silver nitrate-impregnated silica gel thin-layer plates (8). The plates were developed at −17°C and autoradiographed, and the appropriate areas of the silica gel were scraped into vials containing scintillation solutions to determine their radioactivity content (8).
FIG. 3.
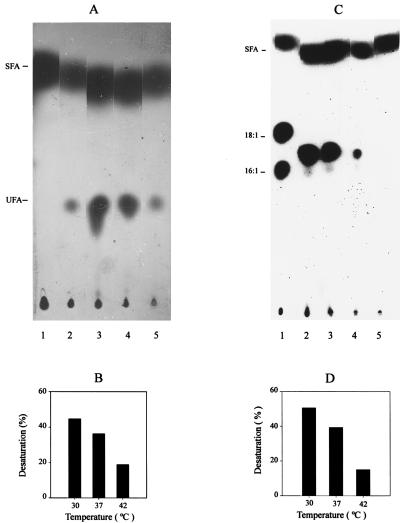
Effects of the expression of the B. subtilis des gene in E. coli. (A) Autoradiogram of the products of [1-14C]palmitate labeling of E. coli strains harboring either vector plasmid pHP13 or des plasmid pDM10. Lane 1 contains a culture of strain AK7/pHP13 that was grown to exponential phase at 30°C. Cells were washed twice with RB medium to remove the oleate, and a 2-ml sample was exposed to 2 μCi of radioactive palmitate for 90 min at the same temperature. Lane 2 contains fatty acids synthesized by B. subtilis JH642 labeled with [1-14C]acetate after a shift from 37 to 20°C (see Materials and Methods). Lanes 3 to 5 contain strain AK7/pDM10 grown to exponential phase at 30°C. A 2-ml sample of each culture was exposed to 2 μCi of radioactive palmitate and then incubated for 90 min at 30°C (lane 3), 37°C (lane 4), or 42°C (lane 5). The lipids were analyzed as described in Materials and Methods. The UFA and SFA migration positions are indicated on the left. (B) Desaturation of [1-14C]palmitic acid at different growth temperatures. The radioactive unsaturated methyl esters shown in lanes 3 to 5 of panel A were quantified by scintillation counting, and the results are expressed as percentages of the total methyl esters recovered at the indicated growth temperatures. (C) Autoradiogram of the products of [1-14C]acetate labeling of E. coli strains harboring vector plasmid pHP13 or des plasmid pDM10. Cultures of strains HC71/pHP13 (lane 1), AK7/pDM10 (lanes 2 to 4), and AK7/pHP13 (lane 5) were grown at 30°C to exponential phase. A 2-ml sample of each culture was exposed to 10 μCi of [1-14C]acetate and then incubated for 90 min at 30°C (lanes 1, 2, and 5), 37°C (lane 3), or 42°C (lane 4). After incubation, the lipids were extracted and chromatographed as in Fig. 2A. The UFAs synthesized by strain HC71/pHP13 are palmitoleic acid (16:1) and cis-vaccenic acid (18:1). Neither UFA is synthesized by AK7/pDM10 or AK7/pHP13 due to the fabB null mutation of the host strain. The final UFAs and SFA positions are indicated on the left. (D) Percentages of UFA synthesis at different growth temperatures. The total radioactive methyl esters were separated into unsaturated and saturated fractions as shown in panel C. The radioactivity contents of lanes 2 to 4 were quantitated as described in Materials and Methods. Results are expressed as percentages of the total methyl esters recovered at the indicated growth temperatures.
For measurement of fatty acid synthesis, E. coli cells were grown to exponential phase and then 2-ml samples were labeled with 10 μCi of sodium [1-14C]acetate (see the legend to Fig. 3C), lipid extracted; and separated into unsaturated and saturated fractions as described above. To label strain AK7/pHP13 (see Fig. 3A and C), the oleate supplement was removed by centrifugation and resuspension of the cells in 2 ml of fresh RB medium. UFA synthesis in B. subtilis was assayed by growing cultures at 37°C to exponential phase with aeration, and then 2-ml samples were shifted to 20°C and exposed to 10 μCi of sodium [1-14C]acetate for 12 h. Following incubation, the labeled UFAs were extracted, chromatographed, and visualized as described above. The double bond position of the mono-UFA synthesized by E. coli AK7/pDM10 was determined by gas chromatography-mass spectrometry (GC-MS) of dimethyl disulfide adducts of these derivatives (28).
Nucleotide sequence accession number.
The sequence reported here has been entered in GenBank under accession no. AF037430.
RESULTS
Rationale of des gene isolation.
Our approach to the cloning of the gene coding for the desaturase of B. subtilis was complementation of two classes of E. coli mutants that require UFAs for growth. UFA synthesis in E. coli requires the products of two genes, fabA and fabB (3). FabA is β-hydroxydecanoylthioester dehydrase which introduces the cis double bond, forming cis-3-decenoyl-ACP (1), the first intermediate in UFA synthesis (Fig. 1). The FabB protein β-ketoacyl-ACP-synthase I catalyzes a rate-limiting step in UFA synthesis (3). This reaction is probably the elongation of cis-3-decenoyl-ACP (3; Fig. 1). The fabA and fabB mutants, although blocked in UFA synthesis, synthesize SFA normally (3). Since the introduction of a cis double bond into long-chain SFAs by desaturation is strictly oxygen dependent (21, 24), we assumed that functional expression of the B. subtilis desaturase would complement the UFA auxotrophy of both fabA and fabB mutants under aerobic culture conditions but would not complement it in the absence of oxygen.
FIG. 1.
Biosynthesis of UFAs in E. coli. β-Hydroxydecanoyl-ACP dehydrase (HDD) catalyzes the key step in UFA production, whereas β-ketoacyl-ACP synthase I (KAS I) is required for the elongation of these unsaturated acyl-ACP intermediates. β-Ketoacyl-ACP synthase II (KAS II) is capable of participating in SFA synthesis and in the elongation of 16:1 Δ9 palmitoleoyl-ACP but is unable to replace KAS I in the elongation of cis-3-decenoyl-ACP.
A genomic library of B. subtilis chromosome fragments constructed in plasmid pHP13 was screened for clones capable of complementing strains CY457 and DM86, which have temperature-sensitive mutations in the fabA and fabB genes, respectively. A number of complementing clones were found that eliminated the growth requirement (at 42°C) for UFA of both the fabA and fabB strains. The plasmids carried by these strains had a 2.4-kb DNA insert with a common restriction map, and the insert of one of these plasmids, pDM10, was sequenced. This plasmid failed to complement the E. coli mutants when the cultures were grown anaerobically, showing that oxygen-dependent introduction of the double bonds into the membrane fatty acids was responsible for growth of the E. coli mutants. These data strongly suggested that we had isolated a gene encoding a B. subtilis fatty acid desaturase.
Sequence of the des gene.
The 2.4-kb DNA insert of plasmid pDM10 was sequenced and found to contain an open reading frame (ORF) of 352 amino acid residues. This ORF was subsequently found to be identical to ORF yocE of the B. subtilis Genomic Sequence Project upon publication of the genomic sequence (17). The protein product of this ORF had a relatively low sequence identity (typically, about 23%) to the membrane desaturases from cyanobacteria and plants (data not shown). However, the B. subtilis ORF contains all three of the histidine clusters found in the known membrane desaturases (25) and these clusters have the appropriate spacing (Fig. 2). Moreover, the hydropathy profile of the deduced amino acid sequence of the 1,056 nucleotide ORF is similar to those of the desaturases from cyanobacteria and plants and the spacing between each His-containing region and the end of the previous hydrophobic domain is also conserved (Fig. 3). It is believed that the conserved His residues are essential for catalytic activity, likely acting as ligands for the iron atom(s) contained in these enzymes (21, 25). Thus, based on the characteristics of this ORF and the ability of the B. subtilis gene to relieve the UFA requirement of E. coli fabA and fabB auxotrophs, we have named this gene des for desaturase. Southern analysis of several restriction enzyme digests of B. subtilis JH642 DNA with a specific des probe gave only a single hybridization signal, indicating the presence of a single copy of the gene (data not shown).
FIG. 2.
Hydropathy profiles and conserved histidine clusters of the putative desaturase of B. subtilis. The sequence we obtained has been reported to GenBank (accession no. AF037430), and the identical sequence is found as ORF yocE in the genomic sequence of B. subtilis (17; Genbank accession no. Z99114), which was reported since the completion of our work. Hydropathy indices for the B. subtilis, Synechocystis sp. (GenBank accession no. 488509), Anabaena variabilis (GeneBank accession no. 628916), Arabidopsis thaliana (GenBank accession no. 1169601), and Glycine max (GenBank accession no. 1345979) desaturases were calculated by the algorithm of Kyte and Doolittle (18) with a window size of 11 residues. Histidine residues in conserved clusters are indicated by H’s in shaded circles and by the black boxes at the bottom. Shaded boxes indicate hydrophobic domains containing greater than 40 amino acid residues (capable of spanning the membrane twice). Note that the hydropathy profiles are aligned by the histidine clusters, and thus, the N termini of the desaturases are not coincident.
Fatty acid desaturase activity in E. coli.
E. coli cannot introduce double bonds into completed long-chain SFAs (3); thus, a test showing that the B. subtilis des gene product has desaturase activity was done by labeling E. coli cells bearing pDM10 with radioactive palmitate and assaying the conversion of this fatty acid to UFA. To avoid β-oxidation of palmitate by the E. coli host, we transformed plasmid pDM10 into strain AK7, which is deficient in fatty acid degradation (fadE) and carries a fabB null mutation that blocks UFA synthesis by the anaerobic pathway at all growth temperatures. Introduction of plasmid pDM10 into AK7 readily allowed growth in the absence of added UFA. This strain converted [14C]palmitate to a UFA that migrated together with the UFA synthesized by B. subtilis cells shifted from 37 to 20°C (Fig. 3A). These experiments clearly demonstrated that the des gene product possesses desaturase activity and that E. coli cells contain sufficient amounts of the cofactors necessary for aerobic desaturation. The levels of desaturation of exogenously added palmitate by strain AK7/pDM10 were highest in cells grown at 30°C (Fig. 3B).
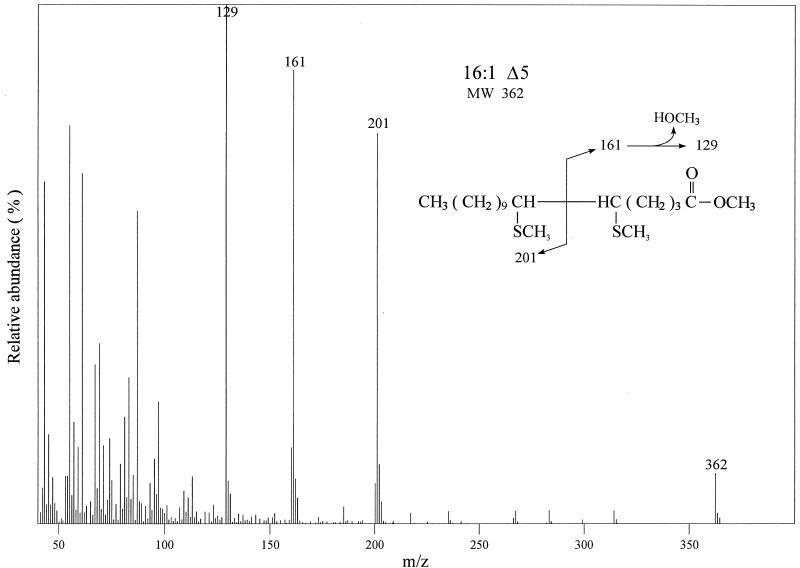
The fatty acid profile of strain AK7 carrying des plasmid pDM10 was compared with that of the same strain carrying vector plasmid pHP13. The fatty acids were labeled by growth of the strains in [14C]acetate, followed by argentation chromatography of the radioactive fatty acids. The strain expressing the des gene accumulated only a single unsaturated fatty acid, whereas the control strain synthesized only SFA (Fig. 3C). As observed for the desaturation of exogenous palmitate (Fig. 3B), higher growth temperatures decreased the UFA synthesis of the E. coli strain that carried the des plasmid (Fig. 3D). The UFA synthesized by strain AK7/pDM10 was identified by GC-MS analysis of the dimethyl disulfide adduct. The mass spectrum of the adduct shows a weak ion at m/z 362 corresponding to the theoretical mass of the molecular ion of the dimethyl disulfide adduct of a 16-carbon monounsaturated fatty acid (Fig. 4). The strong peaks at m/z 161 and m/z 201 indicate the position of the double bond at Δ5 (Fig. 4; the peak observed at m/z 129 was due to loss of methanol from the m/z 161 ion, a characteristic of dimethyl disulfide adducts prepared from fatty acids with unsaturation near the carboxyl group [28]). These results are consistent with the known in vivo activity of the desaturase from bacilli (including B. subtilis) which desaturates palmitate to cis-Δ5-hexadeceonate (7).
FIG. 4.
Analysis of the double bond position of dimethyl disulfide adducts of 16:1 fatty acid methyl esters of strain AK7/pDM10. Strain AK7/pDM10 was grown at 30°C to exponential phase. Lipids were extracted, and the dimethyl disulfide adducts of the fatty acid methyl esters were prepared as described in Materials and Methods. GC-MS analysis of the adducts was carried out on an SPB-1 capillary column (60 m by 0.25 mm) in a Shimadzu QP-5000 gas chromatograph-mass spectrometer.
Disruption of the des gene.
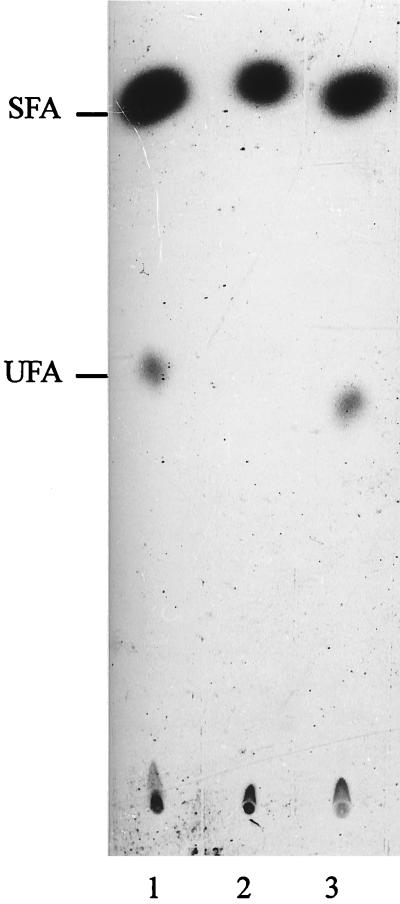
To test if the Δ5 desaturase is a function essential for B. subtilis viability, we disrupted the des gene (see Materials and Methods). Transformation of competent B. subtilis JH642 cells with plasmid pAR10 containing a 665-bp des gene internal fragment resulted in the integration of the chromosomal fragment by a single-crossover (Campbell-type) recombination event. The integration of this plasmid by homologous recombination was confirmed by Southern hybridization (data not shown), and the resultant mutant strain was designated des null. The des null strain formed no detectable UFA after a temperature downshift from 37 to 20°C, although UFAs were formed if the des null strain had been transformed with pDM10 (Fig. 5). These results confirm that the disrupted gene corresponded to that encoding the Δ5 desaturase and that B. subtilis encodes a single desaturase.
FIG. 5.
Fatty acids synthesized by des mutant cultures. Cultures of the B. subtilis JH642 wild type (lane 1), the JH642 des null mutant (lane 2), and the JH642 des null mutant carrying pDM10 (lane 3) were grown to exponential phase at 37°C. A 2-ml sample of each culture was shifted to 20°C and exposed to 10 μCi of [1-14C]acetate for 12 h. The lipids were then extracted and transesterified, and the resulting methyl esters were chromatographed as described in the legend to Fig. 3. The sample in lane 1 contained 10,000 and 1,400 cpm of radioactivity in the SFA and UFA fractions, respectively. The sample in lane 2 contained 8,000 cpm of radioactivity in the SFA fraction, while the UFA fraction contained only background levels of radioactivity. The sample in lane 3 contained 10,000 and 1,750 cpm of radioactivity in the SFA and UFA fractions, respectively. des plasmid pDM10 was introduced into the des null mutant by transformation of competent cells, followed by selection for resistance to erythromycin (10 μg/ml).
To investigate whether the growth rate or viability of the des null strain was affected, the mutant and parent strains were cultured at different growth temperatures. Although no change in cell morphology and growth was visible, loss of Des affects cell viability during prolonged growth in rich medium at 20 or 37°C, where B. subtilis in unable to sporulate (<1% sporulation). Fourteen days after the mutant reached the stationary phase of growth at 20°C, its viability was about 50-fold lower than that of the wild type (Table 1). However, when the des null mutant was transformed with pDM10, cell viability returned to values similar to those reached by the wild-type strain (Table 1). Similar results were obtained during growth at 37°C, except that the viability of the mutant strain was reduced about 10-fold within 72 h after it reached the stationary phase (Table 1).
TABLE 1.
Viability of B. subtilis cultures at 20 and 37°Ca
| Strainb | 20°C
|
37°C
|
||
|---|---|---|---|---|
| No. of cells/ml in stationary phasec | No. of cells/ml in late stationary phased | No. of cells/ml in stationary phasee | No. of cells/ml in late stationary phasef | |
| JH642 | 1.0 × 108 | 5 × 107 | 1.0 × 109 | 1.4 × 107 |
| AK671 | 1.0 × 108 | 1 × 106 | 9.5 × 108 | 2.0 × 106 |
| AK671/pDM10 | 1.0 × 108 | 5.6 × 107 | 1.0 × 109 | 1.5 × 107 |
The data are means of at least three experiments with a mean cell concentration error of less than 10%. Numbers of viable cells were determined at various times by dilution and plating on LB plates.
Strain AK671 is a des null mutant constructed as described in Materials and Methods.
Cells were grown in LB medium at 37°C to an optical density at 600 nm of 0.45 and then shifted to 20°C. The cell concentration was determined after 16 h of growth at 20°C, when the cells had reached an optical density at 600 nm of 2.8.
Numbers of viable cells were determined 14 days after the cells reached stationary phase by dilution and plating on LB plates.
Cells were grown in LB medium at 37°C to an optical density at 600 nm of 2.8.
Numbers of viable cells were determined 72 h after the cells reached stationary phase.
Temperature control of des gene expression.
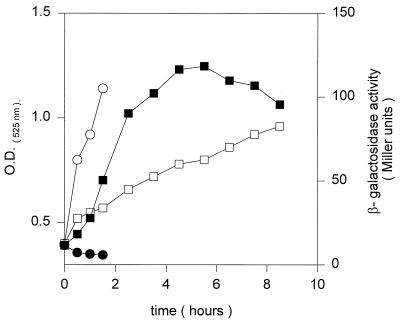
Fujii and Fulco (6) proposed that initiation of UFA synthesis by bacilli after a downshift from 37 to 20°C requires transcriptional induction of the desaturase. This proposal was based exclusively on the finding that desaturation of fatty acids at low temperatures is prevented by the addition of antibiotics that inhibit either transcription or translation (6). However, given the indirect nature of those experiments, it remained possible that the Δ5 desaturase is synthesized constitutively and that shifts to low temperatures induce another cofactor required in aerobic desaturation, such as the electron donor or a component of the electron transport chain. To test the induction of desaturase expression upon a temperature shift, we assayed the β-galactosidase activity of a des promoter-lacZ fusion construction (pPAR10; see Materials and Methods) integrated ectopically into the B. subtilis chromosomal amyE locus. When B. subtilis AKP2 carrying the promoter-lacZ fusion construct was grown at 37°C, the levels of β-galactosidase were very low (Fig. 6). However, when cells containing this fusion growing at 37°C were shifted to 20°C, β-galactosidase synthesis began about 1 h after the temperature downshift and continued for 4 h, reaching induction levels 10- to 15-fold higher than the levels at 37°C (Fig. 6). After about six h at 20°C, the β-galactosidase levels began to decrease. It is interesting that when pPAR10 was introduced into E. coli, the β-galactosidase activity of the des-lacZ fusion was constitutively expressed at 37°C (data not shown). Thus, we concluded that transcription of des in B. subtilis is specifically controlled by the growth temperature.
FIG. 6.
Pattern of des-lacZ expression after a temperature shift. B. subtilis AKP2 cells harboring a des-lacZ transcriptional fusion located in the amyE locus were grown at 37°C to an optical density (O.D.) of 0.35 (at 525 nm) and then divided into two samples. One sample was transferred to 20°C (□, ▪), and the second was kept at 37°C (○, •). Optical densities (□, ○) and β-galactosidase specific activities (▪, •) were determined at the indicated time intervals.
DISCUSSION
The synthesis of UFAs in bacilli is an attractive system to study thermal gene regulation. In contrast to the well-studied constitutive mechanism used by E. coli to increase the amount of UFAs in its phospholipids at low temperatures, expression of the Bacillus desaturation system is a cold-inducible process (6, 8). However, mechanistic study of this environmental response has been hampered by the failure to identify the gene coding for the desaturase. In this report, we describe the isolation and characterization of the des gene encoding the B. subtilis desaturase. The primary sequence of the B. subtilis desaturase displays the characteristic features of membrane-bound desaturases, and two different in vivo assays, desaturation of palmitic acid by E. coli and the double-bond position of the UFA product, indicate that the B. subtilis desaturase inserts a double bond at the Δ5 position of a 16:0 fatty acid. Thus, this is the first report describing the sequence and functional analysis of a bacterial desaturase other than that of cyanobacteria. The failure of E. coli cells to elongate 16:1Δ5 to 18:1Δ7 supports our previous results suggesting that the B. subtilis desaturase introduces a double bond into the acyl chain of membrane phospholipids rather than into the acyl-thioester intermediate of lipid synthesis (8). The E. coli elongation enzymes catalyze the addition of malonyl-ACP to fatty acyl thioester intermediates but are unable to utilize the acyl chains of membrane phospholipids (3). Thus, the failure of E. coli to elongate the 16:1Δ5 made by the B. subtilis desaturase indicates that des encodes an acyl-lipid desaturase similar to those of cyanobacteria and plants (21). It should be noted that other data suggest a relatively close phylogenetic relationship between gram-positive bacteria and cyanobacteria (27). However, while B. subtilis possesses a single desaturase, cyanobacterial cells contain four distinct desaturases (21, 23). Disruption of the desaturase gene resulted in a survival defect during stationary phase at 20 or 37°C but failed to cause any major change in the growth rate of B. subtilis cells at these temperatures. In contrast, UFAs are essential for cyanobacteria, in which desaturation of lipids has been correlated with the tolerance of photosynthetic activity to low growth temperatures (21). The ability of B. subtilis to grow without UFA can be attributed to the nature of the SFA of this organism. In place of the normal straight-chain SFA synthesized by most organisms, bacilli synthesize SFAs that have a large fraction of iso branches and anteiso-methyl branches (5, 15). Branched-chain fatty acids share with double bonds the ability to disrupt the close packing of phospholipid acyl chains and lower the temperature of the phase transition essential for normal membrane function (15, 20). Therefore, although branched-chain fatty acids maintain the proper membrane fluidity necessary for growth, they are not sufficient for survival. Thus, UFAs are necessary to optimize membrane function for viability during the stationary phase. The finding that the des gene product seems to be important for survival at 37°C raises the question of why cells grown to stationary phase at this temperature do not synthesize more UFAs than do cells growing in exponential phase. In fact, in either the exponential or stationary phase of growth, B. subtilis synthesizes only traces of UFAs at 37°C (8, 9, and data not shown).
A des-lacZ transcriptional fusion gives a large increase in des transcription upon a shift to a low growth temperature. Our results are in agreement with the early indirect studies of Fujii and Fulco (6), who suggested that synthesis of the desaturase enzyme in B. megaterium is regulated at the level of transcription. We think it is likely that a transcription regulatory protein (activator or repressor) participates in the thermal regulation of des expression. Another level of control may be at the function of the desaturase enzyme. The enzyme seems poorly functional at high growth temperatures, since although the des-lacZ transcriptional fusion is constitutively expressed in E. coli cells, desaturation is decreased at high growth temperatures (Fig. 3).
ACKNOWLEDGMENTS
We thank Alan Grossman for the gift of the clone bank.
This work was supported by the Commission of the European Communities (grant 937004 AR), the Rockefeller Foundation, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Fundación Antorchas, and the NIH (grant AI15650). P. Aguilar is a fellow of CONICET, and D. de Mendoza is a Career Investigator of the same institution.
REFERENCES
- 1.Bloch K. β-Hydroxydecanoyl thioester dehydrase. In: Boyer P D, editor. The enzymes. New York, N.Y: Academic Press, Inc.; 1971. pp. 441–464. [Google Scholar]
- 2.Bron S. Plamids. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 75–138. [Google Scholar]
- 3.Cronan J E, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 612–636. [Google Scholar]
- 4.Dartois V, Djavakhishvili T, Hoch J A. Identification of a membrane protein involved in activation of the KinB pathway to sporulation in Bacillus subtilis. J Bacteriol. 1996;178:1178–1186. doi: 10.1128/jb.178.4.1178-1186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Mendoza D, Grau R, Cronan J E., Jr . Biosynthesis and function of membrane lipids. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 411–421. [Google Scholar]
- 6.Fujii D K, Fulco A J. Biosynthesis of unsaturated fatty acid in bacilli: hyperinduction and modulation of desaturase synthesis. J Biol Chem. 1977;252:3660–3670. [PubMed] [Google Scholar]
- 7.Fulco A J. The effect of temperature on the formation of delta 5-unsaturated fatty acids by bacilli. Biochim Biophys Acta. 1967;144:701–703. doi: 10.1016/0005-2760(67)90065-3. [DOI] [PubMed] [Google Scholar]
- 8.Grau R, de Mendoza D. Regulation of the synthesis of unsaturated fatty acids in Bacillus subtilis. Mol Microbiol. 1993;8:535–542. doi: 10.1111/j.1365-2958.1993.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 9.Grau R, Gardiol D, Glikin G, de Mendoza D. DNA supercoiling and thermal regulation of unsaturated fatty acid synthesis in Bacillus subtilis. Mol Microbiol. 1994;11:933–941. doi: 10.1111/j.1365-2958.1994.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 10.Graumman P, Marahiel M. Some like it cold: response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300. doi: 10.1007/s002030050386. [DOI] [PubMed] [Google Scholar]
- 11.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 206–216. [Google Scholar]
- 12.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 13.Jones P G, VanBogelen R A, Neihdardt F C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones P G, Inouye M. The cold-shock response—a hot topic. Mol Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klages-Ulrich A, de Mendoza D, Garwin J L, Cronan J E., Jr Genetic and biochemical analysis of Escherichia coli mutants altered in the regulation of membrane lipid composition by temperature. J Bacteriol. 1983;154:221–230. doi: 10.1128/jb.154.1.221-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunst F, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 18.Kyte J, Doolittle R F. A simple method for displaying the hydrophathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 19.Mansilla M C, de Mendoza D. l-Cysteine biosynthesis in Bacillus subtilis: identification, sequencing, and functional characterization of the gene coding for phosphoadenylsulfate sulfotransferase. J Bacteriol. 1997;179:976–981. doi: 10.1128/jb.179.3.976-981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McElhaney R N. Effects of membrane lipids on transport and enzymatic activities. Curr Top Membr Transp. 1982;17:317–380. [Google Scholar]
- 21.Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimation to cold of cyanobacteria. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perego M. Integrational vectors for genetic manipulations in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 23.Sakamoto T, Bryant D A. Temperature-regulated mRNA accumulation and stabilization for fatty acid desaturase genes in the cyanobacterium Synechococcus sp. strain PCC 7002. Mol Microbiol. 1997;23:1281–1292. doi: 10.1046/j.1365-2958.1997.3071676.x. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Shanklin J, Whittle E, Fox B G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry. 1994;33:12787–12794. doi: 10.1021/bi00209a009. [DOI] [PubMed] [Google Scholar]
- 26.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viale A M, Arakaki A K, Soncini F C, Ferreyra R G. Evolutionary relationships among eubacterial groups as inferred from GroEL (chaperonin) sequence comparisons. Int J Syst Bacteriol. 1994;44:527–533. doi: 10.1099/00207713-44-3-527. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Shibahara A, Nakayama T, Kajimoto G. Determination of double-bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulfide adducts. Chem Phys Lipids. 1991;60:39–50. [Google Scholar]