ABSTRACT
The emergence of antibiotic resistance in Mycobacterium tuberculosis has been the culprit behind the inefficient treatment of tuberculosis (TB). Mycobacteriophages being rapid and specific in their activity against mycobacteria including M. tuberculosis are an emerging alternative solution to the antibiotic resistance problem. D29 mycobacteriophage is known as an efficient lytic phage. Its lytic cassette encodes for two lytic proteins, Lysin A (LysA) and Lysin B (LysB), and a holin protein responsible for the translocation of the former two. LysB is a mycolyl-arabinogalactan esterase, which has shown a promising lytic effect on M. tuberculosis. In this study, we explored the lytic efficiency of D29 mycobacteriophage LysB protein on drug-resistant strains of M. tuberculosis. We demonstrate that LysB effectively lyses multi-drug-resistant M. tuberculosis both alone and along with anti-TB drugs. We also report that the surfactant Tween-80, although not vital, potentiates LysB activity. We are able to demonstrate that LysB can eliminate M. tuberculosis residing in mouse macrophages. Thus, by combining our in vitro and ex vivo data, we are able to claim that LysB is a potential phage-derived therapeutic which should undergo pre-clinical and clinical trials.
IMPORTANCE
To combat the rapidly emerging drug-resistant M. tuberculosis, it is now essential to look for alternative therapeutics. Mycobacteriophages can be considered as efficient therapeutics due to their natural ability to infect and kill mycobacteria including M. tuberculosis. Here, we have exploited the mycolyl-arabinogalactan esterase property of LysB encoded from mycobacteriophage D29. This study is novel in terms of targeting a multi-drug-resistant pathogenic strain of M. tuberculosis with LysB and also examining the combination of anti-TB drugs and LysB. All the experiments include external administration of LysB. Therefore, the remarkable lytic activity of LysB overcomes the difficulty to enter the complex cell envelope of mycobacteria. Targeting the intracellularly located M. tuberculosis by LysB and non-toxicity to macrophages take the process of the development of LysB as a drug one step ahead, and also, the interaction studies with rifampicin and isoniazid will help to form a new treatment regimen against tuberculosis.
KEYWORDS: tuberculosis, antibiotic resistance, D29 mycobacteriophage, mycolyl-arabinogalactan esterase, phage therapy, additive effect
INTRODUCTION
Tuberculosis (TB), despite being one of the oldest diseases, ranks second among the leading infectious killers after COVID-19 and is overall positioned at 13th place among the leading causes of death (1). More precisely, one-fourth of the world’s population is estimated to harbor Mycobacterium tuberculosis infection (2). Despite being declared as a global emergency by the World Health Organization in 1993, the disease is far away from elimination (3). Havoc has been created by the emergence of multi-drug-resistant (MDR) and extensively drug resistant (XDR) strains, which are resistant to most of the available drugs. A significant part of health-care activity and research is dedicated to the prevention of the spread of M. tuberculosis besides identification and development of novel therapeutics against it. The standard antibiotic regimen for the treatment of tuberculosis consists of daily administration of an anti-TB drug regimen consisting of four drugs, i.e., isoniazid, rifampicin, ethambutol, and pyrazinamide for 2 months followed by isoniazid and rifampicin thrice weekly for 4 months (4). Despite its proven clinical efficacy, longer treatment duration and hepatotoxicity associated with prolonged administration result in treatment dropout and has raised the probability of the emergence of drug-resistant strains (5).
Concerns about bacterial resistance to antibiotics have prompted researchers to develop new ways or to modify old ways to combat the problem, resulting in the exploration of mycobacteriophages (viruses or phages that infect and kill mycobacterial species) and their related enzymes as new therapeutics to fight against TB. Bacteriophages have been proposed for decades for the treatment of common bacterial infections (6). Phages can combat undesirable bacterial growth by infecting and replicating within their bacterial hosts. The ability of phages to increase in number within the host cell, along with their co-functioning with antibiotics, has led to consideration of phage therapy as a possible therapeutic alternative for the treatment of infections caused by MDR M. tuberculosis strains (7 – 9). Endolysins or lysins are the phage-encoded lytic enzymes that are capable of degrading cell wall peptidoglycan of host cells to release progeny phages and are the potential anti-bacterial regardless of the drug sensitivity profile of host cells. Phage lysins have demonstrated the efficacy in diminishing growth of antibiotic-resistant microorganisms and have exhibited low likelihood of developing bacterial resistance (10). A study showed that Lysin B (LysB) of mycobacteriophage Ms6 has lipolytic activity and can hydrolyse mycolic acids from the mycolylarabinogalactan–peptidoglycan complex and trehalose-6,6′-dimycolate (TDM), a trehalose diester of two mycolic acid molecules, from the outer membrane of the mycobacterial cell wall (11). Recently, a few studies have addressed the anti-mycobacterial effect of LysB against Mycobacterium smegmatis and M. tuberculosis. However, previous studies used either D29 mycobacteriophage or LysB from mycobacteriophages other than D29 mycobacteriophage, which has proven its efficacy repeatedly against M. tuberculosis (12 – 15). Furthermore, these studies utilized either M. smegmatis, a rather distant relative of M. tuberculosis, as the surrogate strain which has a different cell wall composition as compared to M. tuberculosis or auxotrophic strain of M. tuberculosis mc27902, having a different growth profile inside macrophages and lack of lethality in immunocompromised and immunocompetent mice, for the evaluation of anti-mycobacterial efficacy (16 – 19). Moreover, previous studies were limited by use of Tween-80 in the drug susceptibility assays, which is known to alter mycobacterial drug susceptibility and potentiate LysB activity (11, 14, 17). Interestingly, D29 mycobacteriophage-derived LysB can also act externally, suggesting its promising anti-microbial effect (20). However, these studies have not looked into the efficacy of D29 LysB activity against MDR isolates or evaluated its efficacy against mycobacteria residing inside the macrophages.
In this study, we examined the ability of D29 LysB to kill M. tuberculosis so as to use it as a therapeutic alternative to antibiotics. Our data show that D29 LysB targets both drug-sensitive and drug-resistant M. tuberculosis isolates, irrespective of its resistance to isoniazid and rifampicin and, importantly, is able to inhibit the growth of intracellular mycobacteria. The anti-bacterial activity of the enzyme was measured alone as well as in combination with anti-TB drug rifampicin against the M. tuberculosis MDR isolate. Our results, demonstrating the activity of LysB against M. tuberculosis, open a new avenue to explore it as a novel tool to eradicate mycobacteria cells and treat TB.
RESULTS
Purified LysB of D29 mycobacteriophage is able to kill M. tuberculosis in vitro
D29 mycobacteriophage encodes three proteins from its lytic cassette, viz., Lysin A (LysA) (coded by the gene gp10), holin (coded by the gene gp11), and LysB (coded by the gene gp12) (21). Here, we attempted to develop LysB, an esterase involved in disrupting the mycolic acid layer, as a potential “enzybiotic” that is able to kill M. tuberculosis.
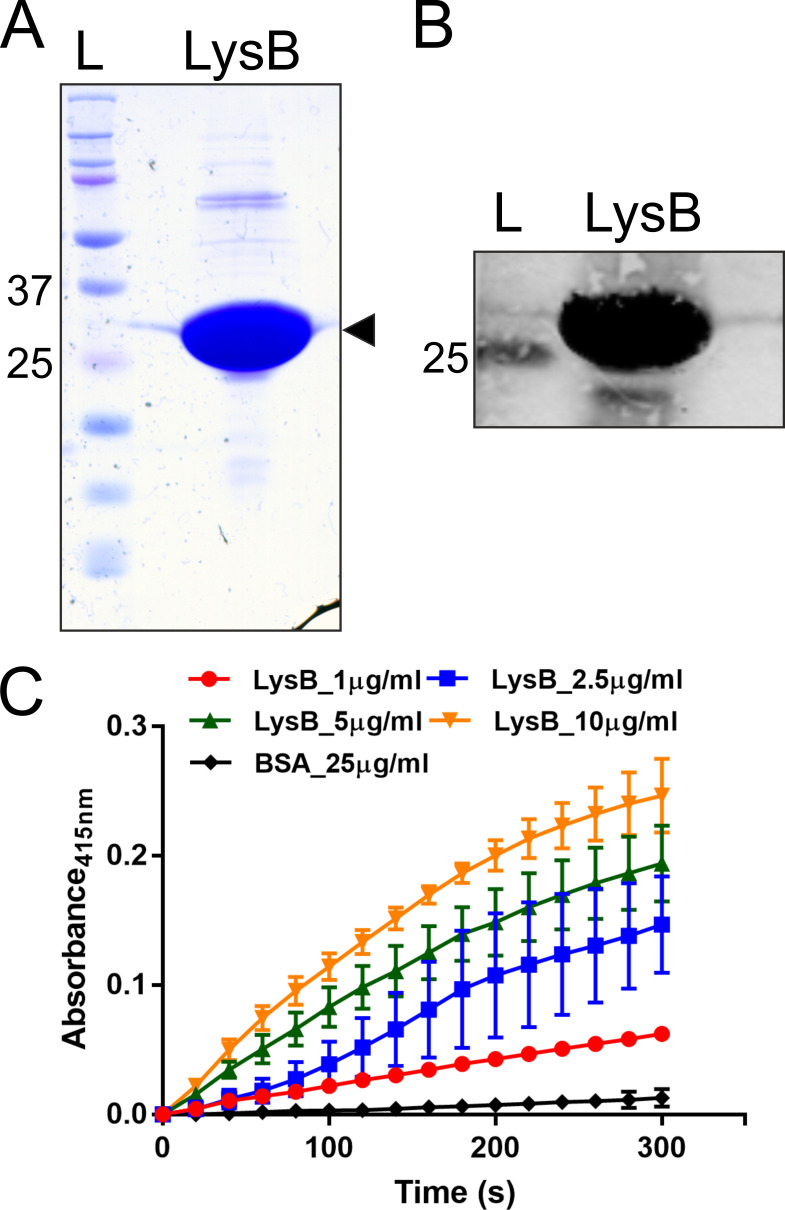
We cloned, expressed, and purified D29 LysB from Escherichia coli. The purified protein was examined on a Coomassie blue-stained polyacrylamide gel (Fig. 1A) and was assessed by Western blotting using the anti-LysB antibodies (Fig. 1B). Next, the protein’s esterase activity was examined in vitro using 4-nitrophenyl butyrate as the substrate. Our LysB enzyme assay clearly shows an increase in the absorbance at 415 nm with time, which is due to the release of p-nitrophenol from the substrate demonstrating the action of LysB on it (Fig. 1C). This profile was monitored with increasing concentration of the LysB, along with bovine serum albumin (BSA) acting as a negative control. The data thus confirm that the purified enzyme is active in vitro.
FIG 1.
Purified LysB of D29 is an active esterase. Panel A shows a Coomassie Brilliant Blue-stained SDS-polyacrylamide gel with purified D29 LysB (marked with an arrowhead). Panel B shows the Western blot of this protein using anti-LysB antibodies. In both panels, ‘L’ is the protein ladder with molecular weights marked in kilodalton. Panel C shows the activity assay of LysB. The assay was carried out by incubating the substrate nitrophenyl butyrate with purified LysB or BSA, and the reaction was monitored by measuring the absorbance of released 4-nitrophenol at 415 nm with time. Different concentrations of LysB (1.0-, 2.5-, 5.0-, and 10.0-µg/mL reaction volumes as indicated) were taken for the assay. BSA was used as negative control. In this panel, the data represent an average of at least three experiments with error bars representing standard deviation with 99% significant score. BSA, bovine serum albumin.
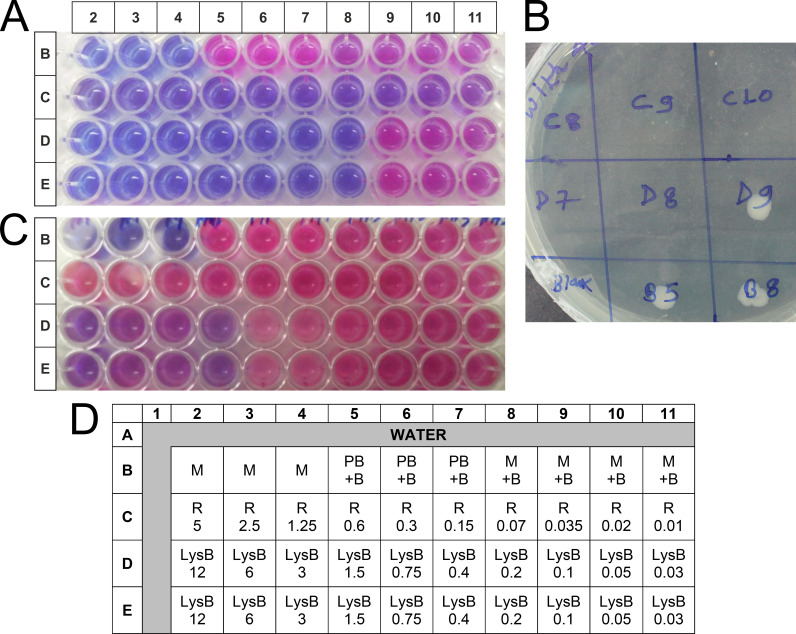
We next measured minimum inhibitory concentration (MIC) of LysB for M. tuberculosis using the microtiter plate-based colorimetric assay. Some studies have assessed the inoculum’s size effect and noted the increase in MIC of an antibiotic with increase in inoculum size, which may increase the probability of emergence of drug resistance (22). We, therefore, investigated the effect of different inoculum sizes (1 × 105 CFU/mL and 2 × 106 CFU/mL) on the susceptibility of M. tuberculosis H37Rv to LysB and rifampicin. The MIC of LysB for M. tuberculosis following the standard protocol (22) using 105 CFU/mL of mycobacteria was found to be 0.20 µg/mL (Fig. 2A; Table 1). The rifampicin MIC was found to be 0.02 µg/mL and was similar to the MIC reported elsewhere (22). A few microliters of the different samples were tested on the Middlebrook (MB) agar plate for the bacterial growth (Fig. 2B), which further confirmed the killing of M. tuberculosis.
FIG 2.
The in vitro anti-mycobacterial activity of D29 mycobacteriophage-derived LysB against M. tuberculosis was measured using the REMA assay. Mycobacteria were incubated with different concentrations (0.03–12.0 µg/mL) of the LysB against M. tuberculosis (A and C, respectively). (A) The 1 × 104 CFU/well of M. tuberculosis in 100 µL of medium was treated with 100 µL of different concentration of LysB for 6 days. The viability of mycobacteria was determined by a change in color of the resazurin dye; the blue color shows the anti-M. tuberculosis activity/effect, and the pink color indicates bacterial growth. (B) Culture (3 µL) from selected wells from panel A was spotted on MB 7H11 agar plate to confirm the REMA results. (C) M. tuberculosis (2 × 105 CFU/well) in 100 µL of medium was treated with 100 µL of different concentrations of LysB for 48 h. The viability of mycobacteria was determined by a change in color of the resazurin dye added on the third day. (D) Plate layout plan for the experiment. B, M. tuberculosis H37Rv bacteria; LysB, Lysin B; M, 7H9 medium; M + B, M. tuberculosis H37Rv growth controls (without any treatment); PB, buffer used for suspending lyophilized protein; R, rifampicin; REMA, resazurin microtiter assay plate.
TABLE 1.
MIC for D29 mycobacteriophage-derived LysB and anti-TB drug rifampicin against M. tuberculosis at different inoculum concentrations
| Organism | Inoculum size (CFU/mL) | D29LysB (µg/mL) | Rifampicin (µg/mL) |
|---|---|---|---|
| M. tuberculosis H37Rv | 105 | 0.20 | 0.02 |
| 106 | 12.0 | >5.0 | |
| Fold change in MIC | 60.0 | >250.0 |
We noticed that an increase in inoculum size affected the activity of both LysB and rifampicin. The increase in MIC value was more prominent for rifampicin (from 0.02 to >5.0 µg/mL), while for LysB, the change was moderate (from 0.2 to 12.0 μg/mL, Fig. 2C; Table 1). The plausible mechanism for change in MIC may be attributed to the increase in bacterial density resulting in reduced ratio of available drug molecules per target rendering them less effective in killing mycobacteria (23).
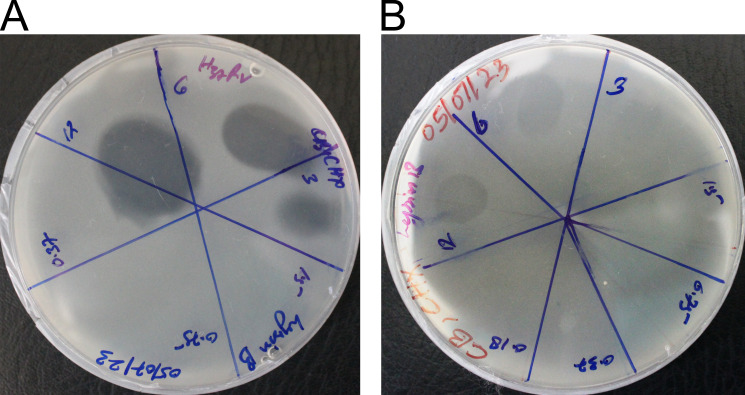
We also tested the anti-microbial activity of LysB against M. tuberculosis in a plate lysis assay in the presence and absence of Tween-80. The presence of Tween-80 potentiated the LysB activity, and M. tuberculosis was susceptible to the lowest protein concentration used (3 µg/mL, Fig. 3A). In the absence of Tween-80, the lowest concentration of LysB resulting in a clear spot zone indicating cell lysis shifted to 6 µg/mL (Fig. 3B). These results demonstrate that LysB is a potent molecule against M. tuberculosis, which can function at a very low concentration. D29 LysB enzyme in this study exhibits significant anti-bacterial activity in both presence and the absence of Tween-80. However, the enhanced anti-mycobacterial activity observed in the presence of Tween-80 could be attributed to the action of oleic acid released by hydrolysis of Tween-80 by LysB.
FIG 3.
The anti-mycobacterial activity of LysB was determined by plate lysis assay on MB 7H11 medium plate in the (A) presence or (B) absence of Tween-80. Anti-mycobacterial activity is indicated by the formation of a lytic zone. In the presence of Tween-80, the clear spot zone was observed at 3 µg/mL. In the absence of Tween-80, the concentration up to which a clear spot zone was observed increased and was visible at 6 µg/mL.
LysB is an effective anti-bacterial against MDR TB isolate in vitro and shows additive effect with anti-TB drug rifampicin
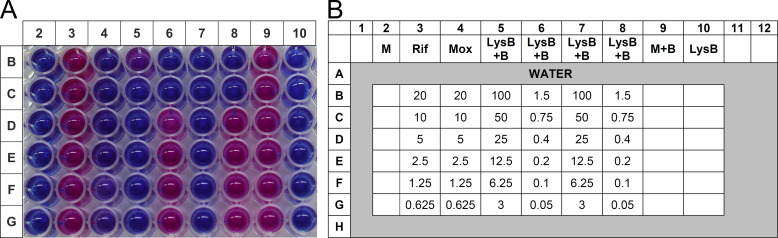
The in vitro anti-mycobacterial activity of LysB was also determined against the previously characterized MDR TB clinical isolate (JAL-19187) procured from the repository of National JALMA Institute for Leprosy and Other Mycobacterial Diseases [Indian Council of Medical Research (ICMR)], Agra, India. Resazurin microtiter assay plate (REMA) was used to determine the MIC values of LysB against the MDR isolates (Fig. 4). LysB was found to be active against the M. tuberculosis MDR isolate, with an MIC value of 0.75 µg/mL (Table 2). The clinical isolate of M. tuberculosis was resistant to the first-line anti-TB drug rifampicin and was susceptible to the second-line anti-TB drug moxifloxacin (24).
FIG 4.
Effect of LysB against M. tuberculosis MDR isolate using the REMA assay. (A) Mycobacteria were incubated with different concentrations (0.0625–20.0 µg/mL) of the anti-TB drugs rifampicin or moxifloxacin or LysB (concentration ranging from 0.05 to 20.0 µg/mL) for 6 days. The viability of mycobacteria was determined by a change in color of the REMA; the blue color shows the anti-M. tuberculosis activity/effect, and the pink color indicates bacterial growth. (B) Plate layout of the experiment shown in panel A. B, M. tuberculosis bacteria; LysB, well containing only Lysin B protein; M, blank media control without inoculation; M + B, M. tuberculosis growth control (without any treatment); Mox, mycobacteria treated with moxifloxacin; Rif, mycobacteria treated with rifampicin.
TABLE 2.
Drug sensitivity profile of M. tuberculosis MDR isolate against standard drugs (MIC in μg/mL)
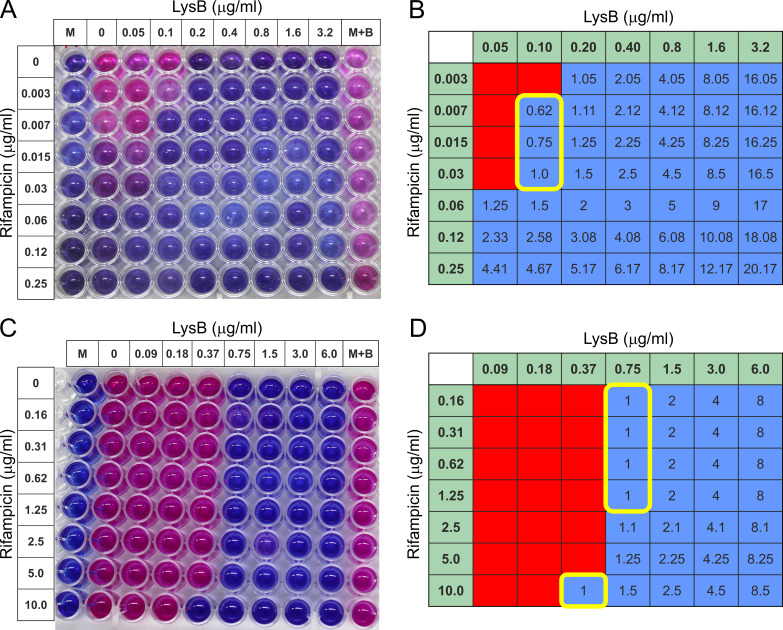
Next, we evaluated the impact of LysB in combination with anti-TB drug rifampicin on M. tuberculosis H37Rv (drug-sensitive strain) and MDR isolate, respectively, using the checkerboard assay. The fractional inhibitory concentration (FIC) for each combination was calculated and is presented in Table 3 and in Fig. 5A through D. In checkerboard titrations, LysB displayed additive activity against drug-sensitive M. tuberculosis H37Rv strain with MICs of LysB and rifampicin lowered from 0.20 and 0.06 µg/mL alone to 0.10 and 0.007 µg/mL in combination, respectively, with fractional inhibitory concentration index (FICI) of ≤0.62 (Fig. 5A and B). Similar results were obtained for drug-resistant M. tuberculosis MDR isolate with MICs of LysB and rifampicin lowered from 0.75 and >20 µg/mL alone to 0.37 and 10 µg/mL in combination, respectively, with FICI of ≤1.0 (Fig. 5C and D). Checkerboard experiments were repeated twice to confirm the results, and results were consistent.
TABLE 3.
Checkerboard assay of LysB in combination with rifampicin against drug-sensitive M. tuberculosis H37Rv strain and MDR M. tuberculosis isolate, respectively a
| Drug combination | MIC (µg/mL) | FIC | FICI | |
|---|---|---|---|---|
| Alone | Combination | |||
| M. tuberculosis H37Rv strain | ||||
| LysB | 0.20 | 0.10 | 0.5 | 0.62 |
| Rifampicin | 0.06 | 0.007 | 0.12 | |
| MDR M. tuberculosis isolate | ||||
| LysB | 0.75 | 0.37 | 0.5 | 1.0 |
| Rifampicin | >20 | 10 | 0.5 | |
Synergy is defined as fractional inhibitory concentration index (FICI) FICI of ≤0.5, 0.5 < FICI ≤ 1.0 as additive, 1.0 < FICI ≤ 4.0 as indifferent, and FICI >4.0 as antagonism.
FIG 5.
Checkerboard microtiter plate assay testing the combination of rifampicin and LysB against drug-sensitive M. tuberculosis H37Rv and multi-drug-resistant M. tuberculosis isolate. (A) M. tuberculosis H37Rv strain: twofold serial dilutions were performed ranging from 0.05 to 3.2 µg/mL for LysB and from 0.003 to 0.25 µg/mL for rifampicin. The addition of resazurin dye revealed the viability of the bacteria. (C) M. tuberculosis MDR isolate: twofold serial dilutions were performed ranging from 0.1 to 6.0 µg/mL for LysB and from 0.16 to 10.0 µg/mL for rifampicin. The addition of resazurin dye revealed the viability of the bacteria. (B and D) FICs: wells corresponding to no growth by color development (blue color) during REMA assay were shaded blue, while wells corresponding to growth by visual inspection of color (pink color) developed during REMA assay were shaded red. Values for LysB and rifampicin along x- and y-axes, respectively, represent the concentration of the drug in that column or row alone. The value in each cell is the FICI, or the sum of the FICs, i.e., the ratio of the concentration of the drug in that column or row to the minimum inhibitory concentration of that drug alone of the two drugs in that well. The yellow line-bordered box encloses wells with a FICI of ≤1. The combination is considered additive because the minimum FICI is 0.62 and 1.0 for M. tuberculosis drug-susceptible and MDR isolates, respectively. FIC, fractional inhibitory concentration.
Intracellular killing activity of LysB
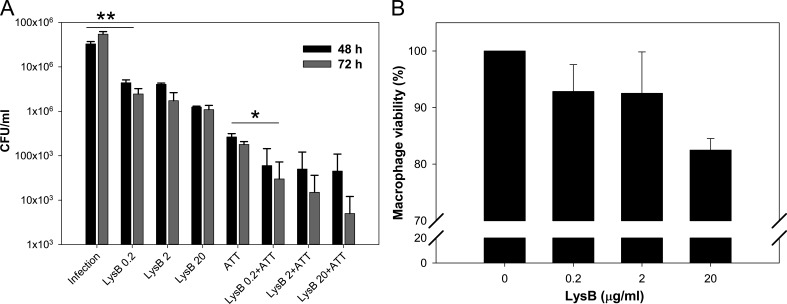
M. tuberculosis is an intracellular pathogen which resides inside macrophages, and the anti-TB drugs are able to target these bacilli. However, in order to use LysB as an effective anti-TB therapeutic, it is important to examine if it can target M. tuberculosis residing inside macrophages. Thus, to check the ability of LysB to kill intracellular M. tuberculosis, the RAW 264.7 macrophages were infected with M. tuberculosis H37Rv. Following infection for 3 h, to investigate the effect of time and increasing concentration of LysB, infected cell lines were treated with 0.2, 2.0, or 20.0 µg/mL of LysB for 48 or 72 h. Our data clearly show the dose and time-dependent mycobacterial killing by LysB (Fig. 6A). A significant difference was observed between the infected and non-treated and infected and treated groups. The observed activity was comparable to that elicited by ATT (isoniazid and rifampicin). To evaluate whether combination therapy of LysB and ATT would enhance the efficacy, we treated macrophages with ATT along with different concentrations of LysB. Interestingly, benefits of combined LysB–antibiotic therapy for the control of mycobacteria under in vitro condition were also visualized against intracellular M. tuberculosis. The combination of ATT and LysB (concentration: 3 µg/mL each of isoniazid (INH) and rifampicin (RIF) and 0.2 µg/mL, respectively) significantly reduced the mycobacterial survival compared to the individual treatment (P < 0.1) (Fig. 6A). These results suggest that LysB can synergize with anti-TB drugs and potentiate its action against intracellular M. tuberculosis. The results also suggest the compatibility of LysB with two main anti-TB drugs, i.e., isoniazid and rifampicin.
FIG 6.
Killing of intracellular M. tuberculosis by D29 LysB. (A) RAW 264.7 macrophages infected with M. tuberculosis (multiplicity of infection [MOI], 10) for 3 h were treated with 0.2, 2.0, or 20.0 µg/mL of LysB either alone or in combination with ATTs, i.e., isoniazid (3 µg/mL) and rifampicin (3 µg/mL) for 48 or 72 h, respectively. The differences in the intracellular survival of M. tuberculosis between different treatments were analyzed by the Student’s t-test. Each bar represents the average value of three independent experiments, and the error bars represent the standard deviations. Significant differences are apparent between groups in pairwise comparisons using a two-tailed t-test at a confidence interval of P < 0.1 (*) or P < 0.05 (**). (B) In vitro cytotoxicity study of RAW 264.7 macrophage cells after 24 h of exposure to LysB. Assays were carried out in triplicate; error bars represent standard deviations. ATT, anti-TB drug.
We also evaluated the cyto-compatibility of LysB with macrophages using (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT) assay. RAW 264.7 mouse macrophages were incubated with various concentrations of LysB for 24 h, and cells were assessed based on the activity of the live cells to convert tetrazolium MTT to purple formazan. Treatment with 1% solution of Triton X-100 served as positive control and resulted in complete cell lysis. Incubation with LysB at all the tested concentrations showed no significant change in macrophage viability, thus demonstrating no signs of cytotoxicity (Fig. 5B). These results indicate that LysB is cyto-compatible with macrophages.
DISCUSSION
Tuberculosis mostly occurs as a chronic infection and is one of the major global health threats. The emergence, evolution, and spread of drug-resistant strains have put pressure to update the chemotherapy regimen for faster and efficient treatment. The presence of unique cell wall has made it difficult for the drug to go inside and act on the pathogen. Thus, there is an urgent need to search for new sources or agents having either anti-mycobacterial activities or an ability to potentiate the existing regimen.
Mycobacteriophage is a bacterial virus that has the ability to infect and kill mycobacteria. Mycobacteriophage D29 is able to infect and kill M. tuberculosis, and hence, it has been considered as potential next-generation therapeutics (15, 27). D29 phage encodes for LysA, LysB, and holin to target peptidoglycan cell wall, mycolic acid layer, and the cell membrane, respectively, in order to release phage progeny at the end of the lytic cycle (21). D29 LysB protein possesses esterase activity that can break down mycolylarabinogalactan present in the mycobacterial cell wall (17). One study evaluated activity of D29 mycobacteriophage-derived endolysins against mycolylarabinogalactan–peptidoglycan isolated from M. smegmatis, while another study evaluated the effect of MS6 LysB activity against extractable lipids from M. smegmatis, Mycobacterium bovis BCG, and M. tuberculosis H37Ra (11, 17). However, its activity against viable planktonic and intracellular M. tuberculosis and against M. tuberculosis MDR isolates has not yet been reported. Further, no studies have looked into the effect of inoculum size on LysB activity. We therefore, investigated the activity of D29-derived LysB against TB-causing bacteria.
In this study, we used two different lytic assay procedures to quantify the activity of D29 LysB against M. tuberculosis. Mycobacterial survival quantified by REMA assay suggested that the addition of LysB even at a very low concentration inhibited M. tuberculosis growth. The MIC of LysB against MDR clinical isolate was found to be 0.75 µg/mL. Our observations were similar to the study by Fraga et al., who reported the MIC of LysB for M. tuberculosis and Mycobacterium ulcerans (20). The observed MIC was strain dependent and was found to be 0.20 and 0.079 µg/mL, respectively, for M. tuberculosis and M. ulcerans. It has been suggested that the observed strain-dependent variation in MIC can be attributed to an exceptionally complex cell wall based on sugars and lipids, which differs between pathogenic and non-pathogenic mycobacteria and also accounts for difference in pathogenesis (28). The ultra-structural study by Singh et al. has also revealed that there is thickening of the cell envelope in drug-resistant isolates and has been assumed to be one of the primary reasons for observed resistance toward rifampicin and isoniazid (29).
To further characterize the activity levels, plate lysis assays were performed with LysB. Plate lysis assay used a high-level inoculum compared with the REMA assay. The results in the plate lysis assay with M. tuberculosis were generally similar to the REMA assay, though there was a shift in the lowest concentration of LysB at which activity was observed (0.20 µg/mL to 3 µg/mL). In order to confirm whether the observed shift was due to inoculum size (105 CFU/mL in REMA assay to 107 CFU/mL in plate lysis assay), we further investigated the effect of inoculum size on drug activity against M. tuberculosis by broth micro-dilution method. In our study, the increase in inoculum size from 105 to 106 CFU/mL in REMA assay resulted in shift in rifampicin MIC from 0.02 to >5.0 µg/mL. Several studies have shown that the efficacy of anti-microbials is influenced by inoculum size. Taneja and Tyagi found MIC higher than reported MICs for anti-TB drugs at higher inoculums (22). MICs were similar to the reported values with the inoculum size of 5 × 104 CFU/well as was used in the present study. A study by Banfi et al. noted a significant change in MIC of streptomycin, i.e., from 0.25 to 1.0 µg/mL against M. tuberculosis following a 10-fold increase in inoculum concentration. The same study reported a twofold increase in rifampicin MIC following a 10-fold shift in mycobacterial concentration (30). A study by Jung et al. also confirmed that inoculum size affects drug susceptibility test against mycobacteria, leading to reduced drug activity from the increased bacterial cell density (30).
Tween-80 is commonly used during mycobacterial culture in order to reduce the aggregates due to bacterial surface hydrophobicity. It has been suggested that Tween-80 may promote the anti-mycobacterial activity of LysB due to the action of the oleic acid released by hydrolysis of the surfactant by LysB or due to the interaction of Tween-80 with mycobacterial cell wall component inducing membrane permeabilization (11, 31). In our study, LysB exhibited significant anti-bacterial activity in both the presence and the absence of Tween-80. The observed results are supported by the additive effect with rifampicin, an anti-mycobacterial drug, which probably resulted from increased cell wall permeability in the presence of LysB. A separate study showed the activity of D29 LysB against M. tuberculosis mc27902_Lux, and the observed MIC in the presence or absence of Tween-80 was 0.08 or ≥0.4 µM, respectively (19). A study showed that D29 LysB demonstrated equal activity against both Tween-80 and Tween-20 (12). As MIC of D29 LysB was lower in the presence of Tween-80, keeping in view of future translation into development of product for clinical use where Tween-80 has been successfully included in inhaled therapeutics, we tested LysB activity in the presence or absence of Tween-80.
The therapeutic efficacy of LysB treatment depends on the presence of biologically active LysB in vivo. Studies have shown a rapid decrease in endolysin levels after administration, leading to loss in activity over time (32, 33). Based on these observations, we decided to investigate the effect of LysB on survival of intracellular mycobacteria in ex vivo macrophage infection model. In our study, LysB administration effectively decreased the bacterial load in the infected macrophages by almost 50% at the MIC dose of 0.20 µg/mL. Our observations are similar to the recent work by various laboratories who reported the activity of endolysins against intracellular bacteria. Lai et al. reported that the treatment of M. smegmatis infected RAW 264.7 macrophages with BTCU-1-derived LysB (5 µg/mL) for 12 h reduced the survival of bacteria by 80% following treatment (13); it may be noted here that LysB of BTCU-1 shows 63% sequence similarity with that from D29 LysB. Another study by Shen et al. showed that bacteriophage-encoded PlyC endolysin can cross epithelial cell membranes and clear intracellular Streptococcus pyogenes in a dose-dependent manner (34). The present study is limited by lack of mechanistic study facilitating internalization of LysB within the intracellular environment. Moreover, before using LysB as a potential therapeutic for TB treatment, a number of important factors such as drug toxicity, immunogenicity, efficacy, resistance, and synergy are to be investigated. A number of pre-clinical studies suggest that lysin administration by topical, systemic, or intravenous route has no harmful, abnormal, or irritant side effects (35 – 37). Several studies have shown that though lysins can elicit an immune response, this does not neutralize their activity or prevent their use as anti-bacterial in the treatment of systemic infections (38, 39). Nevertheless, data on the pharmacokinetics and pharmacodynamics of endolysins as potential drugs are limited. A study showed no signs of toxicity in the rodent single- or repeated-dose tests for central nervous system function of respiratory function. Although multiple dosing led to some adverse effect, it was noted to be mild and self-limiting (36). Although the results of safety evaluations and immunogenicity studies to date largely support the idea of endolysins as a potential therapeutic, a greater volume of research in this area needs to be carried out to ensure that different classes of lysins do not pose a significant risk to the host. Further, the occurrence of lysin-resistant bacteria is unlikely since phages have naturally evolved with their bacterial hosts over millions of years to produce these enzymes that are essential for the release of their progeny. Nevertheless, studies with different clinical isolates of both drug-sensitive and drug-resistant M. tuberculosis are required to look into the effect of variability of TDM on LysB efficacy.
Nonetheless, our data indicate that the D29 LysB can be internalized within macrophages and that the protein retains bacteriolytic efficacy against M. tuberculosis. For therapeutic use, an anti-microbial agent should not affect mammalian cells and only target the pathogen. In our study, the treatment of RAW 264.7 macrophages with LysB even at the highest tested concentration, i.e., 20 µg/mL, did not result in a significant decrease in viability, and the observed effect was due to LysB activity within the intracellular environment. The findings were similar to those of Shen et al., where treatment of eukaryotic cells with PlyC lysin did not affect the membrane integrity (34). The study by van Schie et al. also discussed that lung delivery of therapeutic enzyme up to 0.13 mg/mL is possible without adverse effect (19). The observed MIC in our study is much lower than estimated doses, and it may be decreased further in the presence of Tween-80, which has been successfully included in inhalation therapy. The observed synergy between anti-TB drugs and LysB against drug-susceptible and MDR M. tuberculosis isolates in the present study suggests that D29 LysB when combined with anti-TB drugs can improve the therapeutic efficacy of anti-TB drugs even against drug-resistant tuberculosis and may even shorten the duration of treatment.
Conclusions
The present report is the first of its kind demonstrating the effect of LysB against M. tuberculosis drug-susceptible and MDR isolates both in intracellular and extracellular environments. The study also showed that although Tween-80 potentiated the activity of LysB, it is not essential for its activity. Importantly, LysB could be used as an adjuvant to improve the current antibiotic regimen, given its in vitro additive effect when it is provided in combination with isoniazid and rifampicin. Although the present study did not evaluate the efficacy of LysB in the animal model, nevertheless, we present a proof of concept of the anti-microbial activity of LysB against M. tuberculosis. Systematic research is required to explore the activities of D29 LysB both in pre-clinical and clinical trials before they may be used in adjunctive therapy with standard anti-TB drugs for better management of the disease. Special attention should be given to the studies on safety parameters before incorporation in the present treatment regimen to improve the existing therapeutic practices. Nonetheless, our study lays the foundation for an alternative treatment regimen by providing proof of concept of the anti-microbial activity of LysB against M. tuberculosis infection.
MATERIALS AND METHODS
Bacteria and culture conditions
M. tuberculosis H37Rv and characterized M. tuberculosis MDR isolate resistant to rifampicin and isoniazid (JAL-19187 obtained from the repository of National JALMA ICMR, Agra, India) were used for the study. M. tuberculosis strains were grown in Middlebrook 7H9 broth (Difco, USA) supplemented with ADC (Himedia, India), at 37°C with shaking, or on Middlebrook 7H11 agar (Difco) supplemented with 0.5% glycerol and 10% OADC (BD Biosciences, USA) for plate lysis assay.
Cloning, protein expression, and lyophilization of LysB
The gp12 gene coding for LysB was PCR amplified using a set of primers (gp12_for: 5′ CCTGGAACCGGCTAGCAAGCCCTGGCTGTTCACC and gp12_rev: 5′ CACCCCGCCTCGCGGCCGCGATCTGTCGTAGGAACTCG) and D29 genomic DNA as template, and cloning was carried out in pET21b between NheI and NotI sites to yield a hexa-histidine tag at the C-terminus of the expressed protein. LysB was purified on Ni-NTA affinity chromatography as described elsewhere with some modifications (21). Briefly, E. coli BL21(DE3) carrying the desired plasmid was grown at 37°C with constant shaking at 200 rpm until the OD600 reached 0.6. The culture was then induced by addition of Isopropyl-β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM and was further grown for 12 h at 22°C with constant shaking at 150 rpm. The cells were harvested by centrifugation and lysed by sonication after resuspending in lysis buffer (40 mM Tris–Cl, pH 8.0, 400 mM NaCl, 5 mM imidazole, and 5 mM 2-mercaptoethanol). The lysate was clarified by centrifugation, and the supernatant was incubated with Ni-NTA (Qiagen) beads pre-equilibrated with lysis buffer for 2 h. Beads were washed by 25 column volumes of washing buffer (40 mM Tris–Cl, pH 8.0, 400 mM NaCl, 25 mM imidazole, and 5 mM 2-mercaptoethanol). The protein was then eluted in elution buffer (40 mM Tris–Cl, pH 8.0, 400 mM NaCl, 300 mM imidazole, and 5 mM 2-mercaptoethanol). The eluted protein was examined on SDS-PAGE. Fractions containing the highest amount of protein were dialyzed twice against the storage buffer (40 mM Tris–Cl, pH 8.0, 200 mM NaCl, 1 mM dithiothreitol (DTT)). This was followed by dialysis against water to remove salts. After three to four successive changes, the insoluble protein was separated by centrifugation, and the clarified solution was dialyzed against deionized water and lyophilized. The lyophilized protein was stored at –20°C or room temperature as needed. Before use, 5 mg of lyophilized protein was suspended in phosphate-buffered saline (PBS) and quantified for LysB protein using BCA kit (Sigma Aldrich, USA) following manufacturer’s protocol.
Activity against p-nitrophenyl esters
In vitro esterase activity of D29 LysB was determined by treating 10 mM of p-nitrophenyl ester (Sigma) in 25 mM Tris–Cl buffer (pH 8.0) with different amounts of LysB (1.0, 2.5, 5.0, and 10.0 µg/mL); BSA (25 µg/mL) was used as negative control. The reaction was monitored by measuring the absorbance at 415 nm for 5 min at 37°C.
Determination of M. tuberculosis susceptibility to D29 mycobacteriophage LysB
The MIC determination of D29 LysB against M. tuberculosis and M. tuberculosis MDR isolate was performed using the REMA method (40). Briefly, D29 LysB (0.03–12.0 µg/mL for drug-sensitive M. tuberculosis H37Rv strain and 0.05–100.0 µg/mL for M. tuberculosis MDR isolate) suspended in protein buffer (40 mM Tris–Cl, pH 8.0, 200 mM NaCl, and 1 mM DTT) was diluted serially twofold in Middlebrook 7H9 containing ADC (10%), and 100-µL of it was placed in a 96-well microtiter plate. The medium used during the experiments was supplemented with Tween-80 (0.05%). M. tuberculosis grown in Middlebrook 7H9 medium was harvested at 2,500 × g, and bacterial concentration was adjusted to 1.0 McFarland standard. To determine the effect of inoculum size, the phage lysin activity was determined at two different bacterial concentrations, i.e., 0.05 McFarland unit (equivalent to ~1 × 105 CFU/mL) (recommended for determination of antibiotic susceptibility by REMA method) (22) or 1 McFarland standard (equivalent to ~2 × 106 CFU/mL) (41). Rifampicin was used as the positive control. For the M. tuberculosis MDR isolate, LysB MIC determination was done at bacterial concentration of 1 × 105 CFU/mL. Sterile distilled water (200 µL) was distributed in the perimeter wells of the microtiter plate to avoid dehydration of medium during incubation (Himedia; 96 well plate, flat bottoms).
The plate seeded with 1 McFarland unit CFU was incubated at 37°C for 48 h, while the plate seeded with 0.05 McFarland unit CFU was incubated for 6 days. After incubation for the respective time period at 37°C, resazurin dye (0.02%) (Sigma Aldrich, Germany) was added to all the wells and incubated overnight before visualization of change in color from blue to pink. Visual MIC was defined as the lowest concentration of drug that prevented a color change. The assays were performed in entirety with two replicates on at least two separate occasions. To confirm the results visualized by REMA assay, each well was mixed using a pipette and 3 µL aliquots were spotted onto Middlebrook 7H11 agar plates supplemented with OADC and incubated for 3–4 weeks at 37°C (42).
Screening of D29 LysB against M. tuberculosis by plate lysis method
To test the activity of LysB against M. tuberculosis, purified proteins (final concentration 1.5–400.0 μg/mL) were spotted onto a freshly spread lawn of log-phase M. tuberculosis. The double agar overlay technique was used to identify the anti-mycobacterial activity of lysin. Briefly, soft agar was prepared by adding agar (0.8%) to 7H9 media along with the required supplements. The suspension was autoclaved and allowed to cool down to 42°C. Late log phase bacterial culture (0.5 mL) having OD600 ≤1 (~1 × 107 CFU/mL) was added to 4.5 mL of the soft agar and was poured onto an MB 7H11 agar plate supplemented with 10% OADC (oleic acid, albumin, dextrose, and catalase). In a parallel experiment, the soft agar was supplemented with Tween-80 (1%) to evaluate the effect of Tween-80 on LysB activity. The soft agar was allowed to cool down and solidify for 45 min-1 h. The 10 µL solution of purified LysB were spotted onto bacterial lawns and was air dried for 30 min. Protein buffer was spotted as a negative control. Plates were incubated at 37°C for approximately 2–3 weeks until visible lawns were obtained. Anti-microbial activity of LysB was indicated by a clear lysis zone within the lawn wherever M. tuberculosis growth was inhibited.
Determination of D29 LysB interactions with anti-TB drugs using a REMA checkerboard assay
To determine whether LysB interact synergistically, additively or antagonistically, checkerboard assay experiments were performed in a 96-well plate against drug-sensitive M. tuberculosis H37Rv and multi-drug-resistant M. tuberculosis MDR isolate, respectively (Fig. 5A through D). For M. tuberculosis MDR isolate, in the first step, seven concentrations of D29 LysB (ranging from 0.09 to 6.0 µg/mL) were double diluted column-wise, while six concentrations of rifampicin (ranging from 0.16 to 10.0 µg/mL) were double diluted row-wise. For drug-sensitive M. tuberculosis H37Rv strain, D29 LysB concentration ranged from 0.05 to 3.2 µg/mL while rifampicin concentration ranged from 0.003 to 0.25 µg/mL, respectively. Mycobacterial culture (100 µL) containing 1 × 105 CFU/mL was added to each well and incubated for six days. Resazurin dye was added, and results were recorded after overnight incubation. The results were interpreted as the FICI calculated as FIC of D29 LysB + FIC of drug. FIC was calculated as MIC of LysB or drug in combination/MIC of LysB or drug alone. FICI values of ≤0.5, >0.5–1.0, >1.0–4.0, and >4.0 were interpreted as indicating “synergy,” “additive,” “indifference,” and “antagonism,” respectively (43).
Intracellular bactericidal assay
Mouse peritoneal macrophage cell line RAW 264.7 (procured from National Centre for Cell Sciences, Pune, India) was used to study the treatment of intracellular mycobacterial infection as previously standardized in our laboratory (44). RAW 264.7 macrophages were cultured in Dulbecco’s Modified Eagle Medium (Himedia) supplemented with 10% heat in-activated fetal bovine serum (Himedia) and L-glutamine (2 mM). RAW 264.7 macrophages (2 × 105 cells/well) were seeded into a 96-well tissue culture plate (Himedia) and incubated overnight at 37°C with an atmosphere of 5% CO2. M. tuberculosis H37Rv was used to infect RAW 264.7 macrophages in the ratio of 10:1. Following infection with M. tuberculosis H37Rv for 3 h, the supernatant was removed, and wells were washed thrice with 1× PBS. The infected macrophage monolayers were subsequently treated for 48 or 72 h with either 1×, 10×, or 100× MIC of LysB. The control group included macrophages treated with either anti-TB drugs, i.e., isoniazid and rifampicin (3 µg/mL each), or cells without treatment (negative control) (45). Following incubations at respective time points, cell monolayers were washed three times and lysed with 140 µL of 0.01% Triton X-100. The cell lysates were plated on Middlebrook 7H11 agar to quantify viable intracellular M. tuberculosis. The test was repeated thrice and the number of CFUs recovered per well (mean number ±S.D.) was determined.
Cell viability assay
The cell viability of RAW 264.7 macrophages following treatment with D29 LysB was evaluated by MTT assay as described previously (44). Briefly, RAW 264.7 macrophages (2 × 105 cells/well) were seeded as described before. The adhered cells were treated with LysB at concentrations of 0.2, 2.0, and 20.0 µg/mL (equivalent to 1×, 10×, or 100× MIC of LysB) for 24 h. Blank well containing medium only and untreated wells were included as controls. At specified time points, MTT reagent (Himedia) to an amount equal to 10% of the total volume of wells was added and incubated for 3–4 h in the dark. Formazan crystals formed were dissolved in 100 µL of dimethyl sulfoxide and mixed by pipetting up and down, and the resultant absorbance was measured at 570 nm using a microplate spectrophotometer (BioTek Epoch, USA).
Statistical analysis
The data are expressed as the mean ± standard error of the mean. CFUs were enumerated, and the data were analyzed between groups in pair-wise comparisons using a two-tailed t-test. P value of <0.05 was considered statistically significant. OriginLab software (version 6.0; Origin Lab Corp., Northampton, MA, USA) was used for all the calculations.
ACKNOWLEDGMENTS
We thank Amol Pohane for cloning of LysB in pET21b vector. R.G. thanks IISER Bhopal for the fellowship. We are grateful for the support from staff of ABSL-3 Lab, Indian Council of Medical Research-NJIL & OMD, Agra, especially Nilesh Pal and Amit Kumar for their helpful technical assistance.
The work was supported by a research grant (number 5/8/5/38/2019/ECD-I) from Indian Council of Medical Research, New Delhi, to both A.K.S. and V.J., and in part by the funds from Science and Engineering Research Board, Government of India, to V.J. (#CRG/2020/004231).
Conceptualization and funding acquisition: A.K.S., S.P., and V.J.; methodology: A.K.S. and R.G.; investigation: A.K.S., R.G., H.S.T., S.K.R., and V.J.; writing: A.K.S., R.G., and V.J.; supervision: A.K.S. and V.J.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Amit Kumar Singh, Email: amits.hq@icmr.gov.in.
Vikas Jain, Email: vikas@iiserb.ac.in.
Prabagaran Narayanasamy, University of Nebraska Medical Center, Omaha, Nebraska, USA .
REFERENCES
- 1. WHO . 2021. Fact sheet details tuberculosis.
- 2. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, Kapata N, Mfinanga S, Hasnain SE, Katoto P, Bulabula ANH, Sam-Agudu NA, Nachega JB, Tiberi S, McHugh TD, Abubakar I, Zumla A. 2021. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis 113:S7–S12. doi: 10.1016/j.ijid.2021.02.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grange JM, Zumla A. 2002. The global emergency of tuberculosis: what is the cause J R Soc Promot Health 122:78–81. doi: 10.1177/146642400212200206 [DOI] [PubMed] [Google Scholar]
- 4. (WHO) WHO. 2017. Guidelines for treatment of drug-susceptible tuberculosis and patient care. Available from: https://appswhoint/iris/bitstream/handle/10665/255052/9789241550000-engpdf
- 5. Steele MA, Burk RF, DesPrez RM. 1991. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest 99:465–471. doi: 10.1378/chest.99.2.465 [DOI] [PubMed] [Google Scholar]
- 6. Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. 2011. Phage treatment of human infections. Bacteriophage 1:66–85. doi: 10.4161/bact.1.2.15845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordillo Altamirano FL, Barr JJ. 2019. Phage therapy in the postantibiotic era. Clin Microbiol Rev 32:e00066-18. doi: 10.1128/CMR.00066-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatfull GF. 2022. Mycobacteriophages: from Petri dish to patient. PLoS Pathog 18:e1010602. doi: 10.1371/journal.ppat.1010602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diacon AH, Guerrero-Bustamante CA, Rosenkranz B, Rubio Pomar FJ, Vanker N, Hatfull GF. 2022. Mycobacteriophages to treat tuberculosis: dream or delusion? Respiration 101:1–15. doi: 10.1159/000519870 [DOI] [PubMed] [Google Scholar]
- 10. Taati Moghadam M, Amirmozafari N, Shariati A, Hallajzadeh M, Mirkalantari S, Khoshbayan A, Masjedian Jazi F. 2020. How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect Drug Resist 13:45–61. doi: 10.2147/IDR.S234353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gil F, Grzegorzewicz AE, Catalão MJ, Vital J, McNeil MR, Pimentel M. 2010. Mycobacteriophage Ms6 LysB specifically targets the outer membrane of Mycobacterium smegmatis. Microbiology (Reading) 156:1497–1504. doi: 10.1099/mic.0.032821-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abouhmad A, Korany AH, Grey C, Dishisha T, Hatti-Kaul R. 2020. Exploring the enzymatic and antibacterial activities of novel mycobacteriophage Lysin B enzymes. Int J Mol Sci 21:3176. doi: 10.3390/ijms21093176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai MJ, Liu CC, Jiang SJ, Soo PC, Tu MH, Lee JJ, Chen YH, Chang KC. 2015. Antimycobacterial activities of endolysins derived from a mycobacteriophage, BTCU-1. Molecules 20:19277–19290. doi: 10.3390/molecules201019277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gigante AM, Hampton CM, Dillard RS, Gil F, Catalão MJ, Moniz-Pereira J, Wright ER, Pimentel M. 2017. The Ms6 mycolyl-arabinogalactan esterase LysB is essential for an efficient mycobacteriophage-induced lysis. Viruses 9:343. doi: 10.3390/v9110343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lapenkova MB, Smirnova NS, Rutkevich PN, Vladimirsky MA. 2018. Evaluation of the efficiency of lytic mycobacteriophage D29 on the model of M. tuberculosis-infected macrophage RAW 264 cell line. Bull Exp Biol Med 164:344–346. doi: 10.1007/s10517-018-3986-0 [DOI] [PubMed] [Google Scholar]
- 16. Davies CG, Reilly K, Altermann E, Hendrickson HL. 2021. PLAN-M; mycobacteriophage endolysins fused to biodegradable nanobeads mitigate mycobacterial growth in liquid and on surfaces. Front Microbiol 12:562748. doi: 10.3389/fmicb.2021.562748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Payne K, Sun Q, Sacchettini J, Hatfull GF. 2009. Mycobacteriophage Lysin B is a novel mycolylarabinogalactan esterase. Mol Microbiol 73:367–381. doi: 10.1111/j.1365-2958.2009.06775.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada H CK, Aono A, Igarashi Y, Lina Y. 2018. Can M. Smegmatis be used as a real alternative for M. tuberculosis? Eur Respir J 48:A2789. doi: 10.1111/aos.13172 [DOI] [Google Scholar]
- 19. van Schie L, Borgers K, Michielsen G, Plets E, Vuylsteke M, Tiels P, Festjens N, Callewaert N. 2021. Exploration of synergistic action of cell wall-degrading enzymes against Mycobacterium tuberculosis. Antimicrob Agents Chemother 65:e0065921. doi: 10.1128/AAC.00659-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraga AG, Trigo G, Murthy RK, Akhtar S, Hebbur M, Pacheco AR, Dominguez J, Silva-Gomes R, Gonçalves CM, Oliveira H, Castro AG, Sharma U, Azeredo J, Pedrosa J, Roeltgen K. 2019. Antimicrobial activity of mycobacteriophage D29 Lysin B during mycobacterium ulcerans infection. PLoS Negl Trop Dis 13:e0007113. doi: 10.1371/journal.pntd.0007113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pohane AA, Joshi H, Jain V. 2014. Molecular dissection of phage endolysin: an interdomain interaction confers host specificity in Lysin A of Mycobacterium phage D29. J Biol Chem 289:12085–12095. doi: 10.1074/jbc.M113.529594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taneja NK, Tyagi JS. 2007. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J Antimicrob Chemother 60:288–293. doi: 10.1093/jac/dkm207 [DOI] [PubMed] [Google Scholar]
- 23. Udekwu KI, Parrish N, Ankomah P, Baquero F, Levin BR. 2009. Functional relationship between bacterial cell density and the efficacy of antibiotics. J Antimicrob Chemother 63:745–757. doi: 10.1093/jac/dkn554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta VK, Kaushik A, Chauhan DS, Ahirwar RK, Sharma S, Bisht D. 2018. Anti-Mycobacterial activity of some medicinal plants used traditionally by tribes from Madhya Pradesh, India for treating tuberculosis related symptoms. J Ethnopharmacol 227:113–120. doi: 10.1016/j.jep.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 25. Schönfeld N, Bergmann T, Vesenbeckh S, Mauch H, Bettermann G, Bauer TT, Rüssmann H. 2012. Minimal inhibitory concentrations of first-line drugs of multidrug-resistant tuberculosis isolates. Lung India 29:309–312. doi: 10.4103/0970-2113.102794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koh W-J, Lee SH, Kang YA, Lee C-H, Choi JC, Lee JH, Jang SH, Yoo KH, Jung KH, Kim KU, Choi SB, Ryu YJ, Chan Kim K, Um S, Kwon YS, Kim YH, Choi W-I, Jeon K, Hwang YI, Kim SJ, Lee YS, Heo EY, Lee J, Ki YW, Shim TS, Yim J-J. 2013. Comparison of levofloxacin versus moxifloxacin for multidrug-resistant tuberculosis. Am J Respir Crit Care Med 188:858–864. doi: 10.1164/rccm.201303-0604OC [DOI] [PubMed] [Google Scholar]
- 27. Hassan S, Dusthackeer A, Subramanyam B, Ponnuraja C, Sivaramakrishnan GN, Kumar V. 2010. Lytic efficiency of mycobacteriophages. Open Sys Biol J 3:21–28. doi: 10.2174/1876392801003010021 [DOI] [Google Scholar]
- 28. Alderwick LJ, Harrison J, Lloyd GS, Birch HL. 2015. The mycobacterial cell wall--peptidoglycan and arabinogalactan. Cold Spring Harb Perspect Med 5:a021113. doi: 10.1101/cshperspect.a021113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh R, Varshney I, Chauhan DS, Prasad T. 2022. Cell envelope thickening: a mechanism of drug resistance in Mycobacterium tuberculosis. ECS Trans 107:19671–19680. doi: 10.1149/10701.19671ecst [DOI] [Google Scholar]
- 30. Banfi E, Scialino G, Monti-Bragadin C. 2003. Development of a microdilution method to evaluate Mycobacterium tuberculosis drug susceptibility. J Antimicrob Chemother 52:796–800. doi: 10.1093/jac/dkg439 [DOI] [PubMed] [Google Scholar]
- 31. Stoops JK, Arora R, Armitage L, Wanger A, Song L, Blackburn MR, Krueger GR, Risin SA. 2010. Certain surfactants show promise in the therapy of pulmonary tuberculosis. In Vivo 24:687–694. [PubMed] [Google Scholar]
- 32. Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. 2008. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis 197:1519–1522. doi: 10.1086/587942 [DOI] [PubMed] [Google Scholar]
- 33. Schmelcher M, Donovan DM, Loessner MJ. 2012. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. doi: 10.2217/fmb.12.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen Y, Barros M, Vennemann T, Gallagher DT, Yin Y, Linden SB, Heselpoth RD, Spencer DJ, Donovan DM, Moult J, Fischetti VA, Heinrich F, Lösche M, Nelson DC. 2016. A bacteriophage endolysin that eliminates intracellular streptococci. Elife 5:e13152. doi: 10.7554/eLife.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fenton M, Ross P, McAuliffe O, O’Mahony J, Coffey A. 2010. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. doi: 10.4161/bbug.1.1.9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jun SY, Jung GM, Yoon SJ, Choi Y-J, Koh WS, Moon KS, Kang SH. 2014. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother 58:2084–2088. doi: 10.1128/AAC.02232-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad Lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, Yagyu K-I, Muraoka A, Sugai M, Hiramatsu K, Honke K, Matsuzaki S. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis 196:1237–1247. doi: 10.1086/521305 [DOI] [PubMed] [Google Scholar]
- 39. Harhala M, Nelson DC, Miernikiewicz P, Heselpoth RD, Brzezicka B, Majewska J, Linden SB, Shang X, Szymczak A, Lecion D, Marek-Bukowiec K, Kłak M, Wojciechowicz B, Lahutta K, Konieczny A, Dąbrowska K. 2018. Safety studies of pneumococcal endolysins Cpl-1 and Pal. Viruses 10:638. doi: 10.3390/v10110638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palomino JC, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peñuelas-Urquides K, Villarreal-Treviño L, Silva-Ramírez B, Rivadeneyra-Espinoza L, Said-Fernández S, de León MB. 2013. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz J Microbiol 44:287–289. doi: 10.1590/S1517-83822013000100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guerrero-Bustamante CA, Dedrick RM, Garlena RA, Russell DA, Hatfull GF, Ehrt S. 2021. Toward a phage cocktail for tuberculosis: susceptibility and tuberculocidal action of mycobacteriophages against diverse Mycobacterium tuberculosis strains. mBio 12:e00973-21. doi: 10.1128/mBio.00973-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hong HW, Kim YD, Jang J, Kim MS, Song M, Myung H. 2022. Combination effect of engineered endolysin EC340 with antibiotics. Front Microbiol 13:821936. doi: 10.3389/fmicb.2022.821936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma A, Vaghasiya K, Ray E, Gupta P, Gupta UD, Singh AK, Verma RK. 2020. Targeted pulmonary delivery of the green tea polyphenol epigallocatechin gallate controls the growth of Mycobacterium tuberculosis by enhancing the autophagy and suppressing bacterial burden. ACS Biomater Sci Eng 6:4126–4140. doi: 10.1021/acsbiomaterials.0c00823 [DOI] [PubMed] [Google Scholar]
- 45. Singh AK, Yadav AB, Sharma S, Garg R, Bose M, Misra A. 2013. HLA-Drb1*1501 and VDR Polymorphisms and survival of Mycobacterium tuberculosis in human Macrophages exposed to Inhalable Microparticles. Pharmacogenomics 14:531–540. doi: 10.2217/pgs.13.12 [DOI] [PubMed] [Google Scholar]