ABSTRACT
Candida tropicalis is the leading cause of non-albicans candidemia in tropical/sub-tropical areas. We detected a predominant genotype of azole-resistant C. tropicalis clinical strains in Taiwan from 2014 to 2018. To investigate the potential fomite transmission of drug-resistant C. tropicalis, we characterized yeasts recovered from 17 samples sourced from 6 different kinds of fruits from a supermarket in northern Taiwan in the present study. Fruits were gently washed with buffer, and the solution was then collected for centrifugation. The cell suspension was plated onto CHROMagar Candida medium. Representative yeast species was identified by rDNA sequencing. Drug susceptibilities of C. tropicalis were determined by the broth microdilution method. We found that different types of fruit surfaces had different distributions of yeast species. Washing fruit can significantly eliminate the number of yeasts from the surface. Of 123 identified isolates, C. tropicalis was the most frequently found species, followed by Meyerozyma caribbica and Candida krusei. Among 10 collected C. tropicalis, all 3 fluconazole-resistant ones were non-susceptible to voriconazole. Furthermore, the genotype of all azole-resistant C. tropicalis belonged to the same predominant genotype of azole-resistant C. tropicalis causing candidemia in patients in Taiwan. Hence, fruit can serve as a vehicle of azole-resistant C. tropicalis and other species, especially C. krusei, which is intrinsically resistant to fluconazole.
IMPORTANCE
Of 123 identified isolates from the fruit surface, C. tropicalis was the most frequently found species, followed by Meyerozyma caribbica and Candida krusei. All three fluconazole-resistant C. tropicalis were non-susceptible to voriconazole and belonged to the same predominant genotype of azole-resistant C. tropicalis causing candidemia in patients in Taiwan. Our findings provide evidence that fruit should be washed before eaten not only to remove chemicals but also potential drug-resistant pathogenic microbes, especially for immunocompromised individuals. To keep precious treatment options in patients, we not only continuously implement antimicrobial stewardship in hospitals but also reducing/stopping the use of agricultural fungicide classes used in human medicine.
KEYWORDS: Candida tropicalis, fruit, environment, drug-resistance
INTRODUCTION
The prevalence of fungal infections has increased significantly in recent decades due to the increase of at-risk populations. Fungal infections contribute to approximately 1·5 million deaths annually (1, 2). Additionally, nosocomial infections also result in extra costs, not only from prolongation of hospital stays but also from other medical costs (3). Candida species are the most common pathogens causing severe infections in immunocompromised patients. Candida tropicalis has been reported as the most common non-albicans Candida species causing invasive candidiasis in tropical/subtropical Asia and Latin America (4 – 10).
Previously, we found that approximately 17% of patients were infected by fluconazole-non-susceptible C. tropicalis causing candidemia with cross resistance to itraconazole, voriconazole, and posaconazole, while 55·2% of patients were azole naive (4). Importantly, fluconazole-non-susceptible isolates were genetically closely related, but we did not see time- or place-clustering (4). Furthermore, we also detected that approximately 91% (51/56) of fluconazole-resistant C. tropicalis collected from a national survey, the Taiwan Surveillance of Antimicrobial Resistance of Yeasts (TSARY), in 2014 and 2018 belonged to the clade 4 (11), the same genotype causing candidemia detected in our previous non-TSARY hospital study (4). To evaluate the discriminatory power of multilocus sequence typing (MLST), we compared the mitochondrial genome sequence of selected 34 clinical isolates and found that all the clade 4 tested isolates were clustered together (12). Thus, the MLST method is still a convenient and cost-effective tool for studying the genetic relatedness/diversity of C. tropicalis isolates. In addition to Taiwan, an increasing prevalence of fluconazole resistance among clinical C. tropicalis isolates was reported recently from different areas, including Algeria, China, India, Japan, and Turkey (13 – 19).
In our previous study in 2017, even though one fluconazole-non-susceptible C. tropicalis isolate belonging to the clade 4 genotype on a banana surface among 60 fruit samples from supermarkets was detected, no fluconazole-resistant C. tropicalis was detected (20). Thus, we conducted a prospective follow-up survey of pathogenic yeasts on the surfaces of fruits to identify potential sources of the predominant genotype of azole-resistant C. tropicalis causing infections in humans.
RESULTS
Distribution of yeast species
A total of 123 yeast isolates (Table 1 and Table S2) were recovered and identified to species. There were 31, 24, 21, 21, 14, and 12 isolates from pear, mango, lemon, melon, orange, and guava, respectively (see Fig. S1 for representative images of the fruits). A total of 68 pathogenic yeasts composed of 20 species were detected. The four most frequently isolated species that cause infections in humans were C. tropicalis (10 instances), Meyerozyma caribbica (9 instances), Candida krusei (8 instances), and Moesziomyces aphidis (6 instances).
TABLE 1.
Distribution of yeast species isolated from surfaces of fruits a
| Species | Pear | Mango | Lemon | Melon | Orange | Guava | Total |
|---|---|---|---|---|---|---|---|
| Candida tropicalis* | 2 | 2 | 1 | 3 | 0 | 2 | 10 |
| Meyerozyma caribbica* | 2 | 1 | 3 | 2 | 0 | 1 | 9 |
| Candida krusei* | 3 | 1 | 0 | 3 | 0 | 1 | 8 |
| Rhodotorula taiwanensis | 1 | 2 | 3 | 0 | 1 | 1 | 8 |
| Moesziomyces aphidis* | 1 | 1 | 3 | 0 | 0 | 1 | 6 |
| Sporidiobolus pararoseus | 2 | 3 | 0 | 1 | 0 | 0 | 6 |
| Rhodosporidium paludigenum | 0 | 2 | 1 | 2 | 0 | 0 | 5 |
| Kodamaea ohmeri* | 0 | 1 | 0 | 2 | 1 | 0 | 4 |
| Kurtzmaniella quercitrusa* | 3 | 0 | 0 | 0 | 1 | 0 | 4 |
| Rhodotorula mucilaginosa* | 0 | 0 | 2 | 0 | 2 | 0 | 4 |
| Moesziomyces antarcticus | 0 | 1 | 0 | 3 | 0 | 0 | 4 |
| Papiliotrema ruineniae | 0 | 2 | 0 | 0 | 0 | 2 | 4 |
| Hanseniaspora opuntiae* | 1 | 0 | 0 | 1 | 0 | 1 | 3 |
| Hyphopichia burtonii | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Aureobasidium melanogenum* | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Candida famata var. famata* | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| Candida metapsilosis* | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| Metschnikowia pulcherrima* | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Meyerozyma guilliermondii* | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Trichosporon asahii* | 0 | 0 | 1 | 1 | 0 | 0 | 2 |
| Wickerhamomyces anomalus* | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Yarrowia lipolytica* | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Hanseniaspora guilliermondii | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Hanseniaspora pseudoguilliermondii | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Pichia kluyveri | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Sarocladium strictum | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| Yarrowia deformans | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Others | 6 | 5 | 4 | 2 | 1 | 1 | 19 |
| Total | 31 | 24 | 21 | 21 | 14 | 12 | 123 |
*, species has been reported to cause infections in human. One each of Candida sorboxylosa, *Papiliotrema flavescens, Pichia manshurica, Pichia mexicana, Pseudozyma tsukubaensis, and Starmerella bacillaris from pear; one each of Cyberlindnera xylosilytica, *Lodderomyces elongisporus, Pichia occidentalis, Rhodosporidiobolus ruineniae, and Rhodotorula toruloides from mango; one each of *Dirkmeia churashimaensis, *Diutina catenulata, Hannaella siamensis, and Papiliotrema terrestris from lemon; one of each Starmerella apicola and Torulaspora delbrueckii from melon; Hanseniaspora uvarum from orange and Wickerhamomyces rabaulensis from guava.
We found that different fruits carried different combinations of microbes (Fig. 1). The surface of pear had the highest number of yeasts, followed by guava and lemon. Mango and orange surfaces had a relatively low number of yeasts (Fig. 2). After the third washing, most microbes had been washed off from the fruit surface (Table S3). Removal of the top and bottom ends of lemon, mango, melon, and orange reduced the number of yeast colonies. In contrast, the number of yeast colonies did not decline after the removal of both ends of pear (Fig. 2; Table S3).
Fig 1.
Detection of microbes after treatment. Each sample was washed six times. The colonies on the CHROMagar Candida media of the cell suspensions of the first (1st, 1 and 5), second (2nd, 2 and 6), third (3rd, 3 and 7), and sixth (6th, 4 and 8) washings from whole fruits (1–4) or from fruits of which both ends were removed (treated, 5–8) were photographed after incubation.
Fig 2.
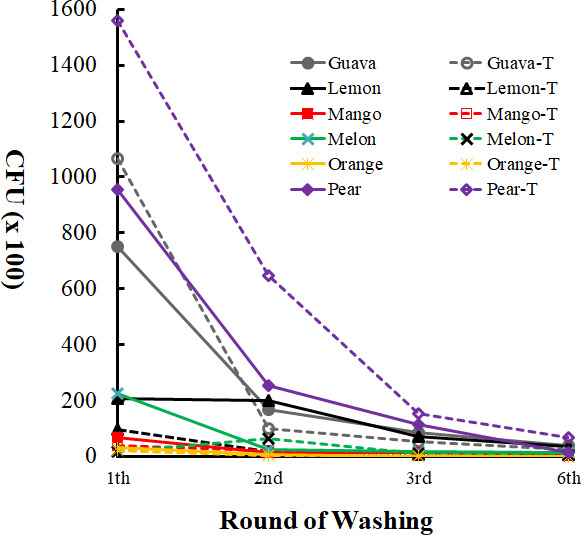
Microbes on the surface of different types of fruit. The effects of washing and removal of both ends were determined. Colony-forming units (CFUs) of 1st, 2nd, 3rd, and 6th washing of whole fruits (solid lines) or from fruits of which both ends were removed (dashed lines) were detected.
Characteristics of 10 collected Candida tropicalis isolates
Candida tropicalis was detected on the surface of all fruits other than oranges. We found three isolates on melon; two each on guava, mango, and pear; and one on lemon (Table 2). Three C. tropicalis isolates were resistant to fluconazole. Two of those were from the melons, belonging to DST506. The third fluconazole-resistant isolate was from pear and belonged to DST1107. Both DST506 and DST1107 belonged to the clade 4, the predominant genotype of the azole-resistant C. tropicalis causing infections in Taiwan (11). Interestingly, of the remaining seven fluconazole-susceptible ones, four belonged to clade 3, and one each to clades 1, 4, and 8 (Table 2). All three fluconazole-resistant isolates were non-susceptible to voriconazole, while YF17294 was resistant to voriconazole, and the remaining two were intermediate. In general, all three fluconazole-resistant isolates were less susceptible to azole used in agriculture (Table 2).
TABLE 2.
Characteristics of 10 Candida tropicalis isolates
| Isolate | Fruit collected date | Clade | DST | Fluconazole | Voiconazole | Difenoconazole | Miconazole | Tebuconazole | Triadimenol | Erg11 ORF |
ERG11
mRNA |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 132 | 154 | |||||||||||
| YF17026 | Mango July 04 | 8 | 1106 | ≤0.125 | ≤0.0156 | ≤1 | 0.25 | ≤1 | ≤2 | YY | SS | 1.5 |
| YF17034 | Melon July 04 | 3 | 766 | 0.25 | 0.0313 | ≤1 | 0.5 | ≤1 | ≤2 | YY | SS | 1.5 |
| YF17091 | Pear July 04 | 4 | 1107 | 32 | 0.5 | 2 | 4 | 4 | >64 | YF | SF | 8.4 |
| YF17195 | Guava Aug 09 | 3 | 1108 | ≤0.125 | ≤0.0156 | ≤1 | 0.25 | ≤1 | ≤2 | YY | SS | 0.4 |
| YF17229 | Melon Aug 08 | 4 | 506 | 8 | 0.25 | ≤1 | 2 | ≤1 | 32 | YF | SF | 1.6 |
| YF17294 | Melon Aug 16 | 4 | 506 | 32 | 1 | 4 | 4 | 4 | >64 | YF | SF | 7.6 |
| YF17304 | Guava Aug 16 | 1 | 765 | ≤0.125 | ≤0.0156 | ≤1 | 0.25 | ≤1 | ≤2 | YY | SS | 0.8 |
| YF17310 | Mango Aug 17 | 3 | 1303 | ≤0.125 | ≤0.0156 | ≤1 | ≤0.125 | ≤1 | ≤2 | YY | SS | 1.0 |
| YF17358 | Pear Aug 17 | 4 | 1110 | 0.25 | ≤0.0156 | ≤1 | 0.25 | ≤1 | ≤2 | YY | SS | 0.7 |
| YF17390 | Lemon Aug 21 | 3 | 767 | 0.25 | ≤0.0156 | ≤1 | 0.25 | ≤1 | ≤2 | YY | SS | 1.5 |
Mechanism contributing to azole resistance of Candida tropicalis
Interestingly, among the three fluconazole-resistant isolates, YF17091 and YF17294 were more resistant to azoles than YF17229 (Table 2). Previously, we detected that a combination of mutation and overexpression of Erg11p, the azole target, was the major mechanism contributing to resistance of clinical isolates (11). To investigate the mechanisms contributing to drug resistance and different levels of azole resistance in the three isolates collected in the present study, we analyzed the Erg11p sequence and ERG11 mRNA expression of the 10 isolates. All three azole-resistant isolates in the present study had both Y132F and S154F mutations on the Erg11p, consistent with that of isolates from patients (11). Furthermore, the relative levels of ERG11 mRNA in YF17091 (8.4) and YF17294 (7.6) were significantly higher than YF17229 (1.6) and the remaining eight (ranging from 0.4 to 1.5) fluconazole susceptible ones.
DISCUSSION
Our findings point to a challenge of azole-resistant C. tropicalis in clinical settings. More importantly, the phenomenon of azole resistance in C. tropicalis has been observed beyond just Taiwan (21 – 23). Approximately 80% of fluconazole-resistant C. tropicalis in China also belonged to the clade 4 (24). The burden of antifungal-resistant C. tropicalis in animals or environments is less investigated than it is in humans. Four percent to seventy-five percent of C. tropicalis recovered from animals in Brazil, India, and Japan were resistant to fluconazole (17, 25 – 28). Even though limited information was available regarding the rate of azole resistance of C. tropicalis recovered from environments, 21% and 25% of isolates from China and Brazil, respectively, were resistant to fluconazole (29, 30). In the present study, we demonstrate that fruit may be one of the sources of fluconazole-resistant C. tropicalis, providing the basis for further understanding of the dissemination of drug-resistant pathogenic yeasts.
Selection pressure from the use of clinical azole drugs and/or from patients acquiring azole-resistant C. tropicalis from the environment are two major routes for patients with azole-resistant C. tropicalis. In our previous studies (4, 11). we found no evidence of patient-to-patient transmission. Nevertheless, there is a possibility that unknown human source is involved, such as that has been reported for C. parapsilosis (31 – 34) and multidrug-resistant Candida auris (35). Hence, azole-resistant C. tropicalis may colonize humans through direct contact or dietary intake of food and water.
The fact that microbes were significantly washed off strongly suggests that for individuals at risk, all kinds of fruits should be carefully washed in running water before being eaten. Also, the fact that fluconazole-resistant C. tropicalis and C. krusei were found, which are intrinsically resistant to fluconazole (36), on fruit surfaces is a reminder of the importance of caution when providing fruits to immunocompromised patients, because fruit surfaces can carry not only pathogenic microbes but also drug-resistant ones.
Since we have found that overexpression of mutated ERG11, instead of CDR1 or MDR1, is a major mechanism contributing to fluconazole resistance in clade 4 C. tropicalis from patients (11), we investigated the potential factors affecting the drug susceptibility for those four clade 4 strains collected in the present study. The YF17358 has wild-type Erg11p and does not overexpress ERG11 and is susceptible to fluconazole. We found that strains YF17091 and YF17294, which overexpress mutant Erg11p, have a higher level of fluconazole resistance than the strain YF17229, which contains a mutant and does not overexpress Erg11p. This observation is consistent with the stepwise evolution of drug-resistance. Furthermore, whether alternation of ERG11 gene copy and/or gain-of-function in Upc2p, a regulator of ERG11, contributes to the upregulation of ERG11 needs further investigation. The observation that strains YF17091 and YF17294, which overexpress mutant Erg11p, have a higher level of fluconazole resistance than the strain YF17229, which contains a mutant and does not overexpress Erg11p, is consistent with the stepwise evolution of drug-resistance (37). Furthermore, whether alternation of ERG11 gene copy and/or gain-of-function in Upc2, a regulator of ERG11, contributes to the upregulation of ERG11 needs further investigation.
Previously, we detected pathogenic yeasts on the fruit surface (20). In addition to patients, we have identified the clade 4 predominant genotype azole-resistant C. tropicalis from fruits in the present study. There are limitations in the present study. First, we can’t distinguish whether the clade 4 predominant genotype azole-resistant C. tropicalis originated from patients and/or the environment; Second, we cannot distinguish whether the azole-resistant C. tropicalis originated from the environment, or farmers of orchards, or was contaminated by customs or supermarket staff. Nevertheless, our findings clearly indicate that fruit can be a vehicle of azole-resistant C. tropicalis. In the present study, 17 samples taken from 6 different kinds of available and popular fruits from a supermarket in 3 time periods were analyzed. Increase the number of fruits from various sources and regions or fruits directly from orchards would be our next follow-up investigation to identify a potential hotspot for fluconazole-resistant C. tropicalis, but it is beyond the current scope of the study. Third, we did not confirm the genetic relatedness by whole genome sequencing. Depending on different principles, a wide variety of genotyping methods from the traditional pulsed-field gel electrophoresis to the whole genome sequencing are available. Both microsatellite typing and MLST are rapid, specific, and relative highly discriminatory and frequently used to type Candida species (38 – 40). In the present study, we used MLST and the data were consistent with those in mitochondrial DNA sequencing (12). One report shows that results from both MLST and microsatellite typing are comparable for C. tropicalis (41). However, for some Candida species, such as C. albicans, C. parapsilosis, and Diutina (Candida) catenulata, other approaches, such as microsatellite typing, may be more suitable (38 – 40).
How the original clade 4 azole-resistant C. tropicalis was selected, either from patients taking clinical azole drugs and/or originating from environments with agricultural fungicides, needs to be investigated. Based on the concept of “one health” (42), antifungal stewardship efforts in hospitals are expected to contribute to a reduction in selection for resistant organisms, but if no parallel efforts are made in agriculture to reduce/stop the use of fungicide classes used in medicine, vulnerable patients would continue to be infected with highly resistant organisms with limited treatment options. Hence, how to implement proper use of azole fungicide in agriculture is equally critical and urgently needed. Furthermore, it is also important to closely monitor the drug susceptibilities of C. tropicalis, including clade 4 and non-clade 4, from both patients and environmental sources.
MATERIALS AND METHODS
Yeast isolation
To avoid contamination by researchers, we collected fruits carefully to avoid touching them by hand. Then, those fruits were washed in a sterilized bag. Yeasts recovered from 17 samples taken from 6 different kinds of available and popular fruits in 3 different time periods from a supermarket in northern Taiwan in 2017 were characterized. Together, whole fruits from the same sampling were gently washed with 200 mL buffer (1% peptone, 0.5% NaCl) in a 10 L sterilized bag six times. The solution was then collected for centrifugation. To avoid residue from the previous cleaning, the fruits were transferred into a new bag for each round of washing. The pellet was re-suspended in 0.5 mL yeast extract peptone dextrose (YPD) broth. An inoculation loop was used to transfer the cell suspension for plating onto CHROMagar Candida medium (BBL, Becton Dickinson Cockeysville, MD, USA) containing 0.5 g/L chloramphenicol to eliminate the growth of bacteria. After three days of incubation at 25°C, three (if there were) representative colonies of each morphotype were picked for subsequent workup. One isolate per species per sample was analyzed. A total of 139 yeast isolates were analyzed.
Yeast identification
For identification, all isolates were subjected to ribosomal DNA (rDNA) sequencing. The internal transcribed spacer (ITS) region was amplified by the primers ITS1 5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4 5′-TCCTCCGCTTATTGATATGC-3′, and/or the D1/D2 region of rDNA was amplified by the primers NL1 5′-GCATATCAATAAGCGGAGGAAAAG-3′ and NL4 5′-GGTCCGTGTTTCAAGACGG-3′ (43). Among 139 analyzed isolates, 123 were identified to species. Thus, we reported the characteristics of those 123 isolates.
Drug susceptibility testing
The drug susceptibilities of C. tropicalis were determined by the in vitro antifungal susceptibility testing procedure established in our laboratory (10), modified from the guidelines of the Clinical and Laboratory Standards Institute (CLSI) document M27-A3 (44). The growth of each isolate was measured by Multiskan FC Microplate Photometer (Thermo Fisher Scientific, USA) after incubation at 35°C for 24 h.
Standard powders of two clinical antifungal agents, fluconazole and voriconazole, were kindly provided by Pfizer; and azoles used in agriculture—difenoconazol (36531, Merck), miconazole nitrate salt (M3512, Merck), tebuconazole (N-12006, Chem Service), and triadimenol (N-11129, Chem Service)—were dissolved in dimethyl sulfoxide. The final concentrations of difenoconazol and tebuconazole ranged from 1 mg/L to 32 mg/L, 0.125 mg/L to 64 mg/L for fluconazole, 0.125 mg/L to 4 mg/L for miconazole, 2 mg/L to 64 mg/L for triadimenol, and 0.0156 mg/L to 8 mg/L for voriconazole. RPMI medium 1640 (31800-022, Gibco BRL) was used for the dilution and growth of the yeast culture. Strains from American Type Culture Collection (ATCC)—including Candida albicans (ATCC 90028), Candida krusei (ATCC 6258), and Candida parapsilosis (ATCC 22019)—were used as the standard controls.
Minimum inhibitory concentrations (MICs) were defined as the concentration of drugs capable of reducing the turbidity of cells to more than 50%. The newly defined species-specific breakpoints for common Candida species were applied in the present study (45). The CLSI newly defined species-specific breakpoints were also applied (46). For fluconazole, the clinical breakpoints were MICs ≤2 mg/L, susceptible; 4 mg/L, susceptible-dose dependent; ≥8 mg/L, resistant. For voriconazole, the clinical breakpoints were MICs ≤0.125 mg/L, susceptible; 0.25 mg/L–0.5 mg/L, intermediate; and ≥1 mg/L, resistant.
Qualitative analysis of the transcript level of ERG11 involved in drug resistance by real-time PCR
We further determined the expression levels of ERG11. The cells were harvested at OD600 0.7 to 0.9 after growing in YPD liquid medium at 30°C for 6 h. Total RNAs were isolated using the RNeasy Mini kit (QIAGEN), followed by treatment with RQ1 RNase-Free DNase (Promega) to digest the possible contaminating DNA. The purified RNAs were converted to first-strand cDNA using a GoScript Reverse Transcription System kit (Promega). ACT1 was used as an internal control. The reaction system comprised 10 µL SensiFAST SUBR Lo-ROX kit (meridian), 1 µM forward and reverse primers and cDNAs, in a final reaction volume of 20 µL. Negative controls (DNA-free water) were included in each run. All the real-time PCRs (RT-PCRs) were performed on the QuantStudio 6 Flex (Applied Biosystems). Fold changes in gene expression were determined using the ΔCT method, and data were normalized against the expression of ACT1. Then the mRNA level of a fluconazole-susceptible isolate, YFA120877, was used as the denominator for normalization. The primers used in the present study are listed in Table S1.
Multilocus sequence typing of Candida tropicalis
Multilocus sequence typing was conducted as described in our previous report (11, 47). Phylogenetic analysis was performed using the unweighted pair group method with arithmetic average (UPGMA), which was created by MEGA X software. A cutoff P distance of 0.01 was chosen because it separated clades that contained known examples of isolates. We constructed a diploid sequence type (DST)-based phylogenetic tree global C. tropicalis composed of 1368 DSTs as listed in the C. tropicalis MLST database, and clades containing more than 10 genetically closely related DSTs were labeled. The primers used are listed in Table S1.
ACKNOWLEDGMENTS
We would like to thank Pfizer for supplying anidulafungin, fluconazole, and voriconazole. We thank Prof. Shau-Ku Huang and Mr. Mark Swofford for editing the manuscript.
This work was supported in part by grants from the National Health Research Institutes (IV-111-PP-10 and IV-111-SP-01), the Taiwan Centers for Disease Control (MOHW111-CDC-C-114-124301), and the National Science and Technology Council (108-2314-B-400-010 and 110-2314-B-400-040). The funding sources played no role in the study design or conduct, the data collection, the analysis or interpretation, the writing of the manuscript, or the decision to submit it for publication.
Contributor Information
Chung-Yu Lan, Email: cylan@life.nthu.edu.tw.
Hsiu-Jung Lo, Email: hjlo@nhri.org.tw.
Paschalis Vergidis, Mayo Foundation for Medical Education and Research, Rochester, Minnesota, USA .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01471-23.
Photos of the fruits.
Primers used in the present study.
Sources of the 123 isolates from the fruit surfaces.
Yeast colony-forming units of six different types of fruits.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 3:57. doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 3. Sheng WH, Chie WC, Chen YC, Hung CC, Wang JT, Chang SC. 2005. Impact of nosocomial infections on medical costs, hospital stay, and outcome in hospitalized patients. J Formos Med Assoc 104:318–326. [PubMed] [Google Scholar]
- 4. Chen PY, Chuang YC, Wu UI, Sun HY, Wang JT, Sheng WH, Lo HJ, Wang HY, Chen YC, Chang SC. 2019. Clonality of fluconazole-nonsusceptible Candida tropicalis in bloodstream infections Taiwan, 2011-2017. Emerg Infect Dis 25:1660–1667. doi: 10.3201/eid2509.190520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombo AL, Júnior JN de A, Guinea J. 2017. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis 30:528–538. doi: 10.1097/QCO.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 6. Tan BH, Chakrabarti A, Li RY, Patel AK, Watcharananan SP, Liu Z, Chindamporn A, Tan AL, Sun P-L, Wu U-I, Chen Y-C, Asia Fungal Working Group (AFWG) . 2015. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect 21:946–953. doi: 10.1016/j.cmi.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 7. Wu PF, Liu WL, Hsieh MH, Hii IM, Lee YL, Lin YT, Ho MW, Liu CE, Chen YH, Wang FD. 2017. Epidemiology and antifungal susceptibility of candidemia isolates of non-albicans Candida species from cancer patients. Emerg Microbes Infect 6:e87. doi: 10.1038/emi.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang YL, Cheng MF, Wang CW, Wang AH, Cheng WT, Lo HJ, Hospitals T. 2010. The distribution of species and susceptibility of amphotericin B and fluconazole of yeast pathogens isolated from sterile sites in Taiwan. Med Mycol 48:328–334. doi: 10.3109/13693780903154070 [DOI] [PubMed] [Google Scholar]
- 9. Yang YL, Ho YA, Cheng HH, Ho M, Lo HJ. 2004. Susceptibilities of Candida species to amphotericin B and fluconazole: the emergence of fluconazole resistance in Candida tropicalis. Infect Control Hosp Epidemiol 25:60–64. doi: 10.1086/502294 [DOI] [PubMed] [Google Scholar]
- 10. Zhou Z-L, Lin C-C, Chu W-L, Yang Y-L, Lo H-J, TSARY Hospitals . 2016. The distribution and drug susceptibilities of clinical Candida species in TSARY 2014. Diagn Microbiol Infect Dis 86:399–404. doi: 10.1016/j.diagmicrobio.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 11. Zhou ZL, Tseng KY, Chen YZ, Tsai DJ, Wu CJ, Chen YC, Peng HL, Yang YL, Hsieh LY, Chen CH, Hsu CH, Wang LS, Cheng MF, Hsu GJ, Kao CC, Hu BS, Lee YT, Liu JW, Liu KS, Miu WC, Yang HM, Yeh YC, Lo HJ. 2022. Genetic relatedness among azole-resistant Candida tropicalis clinical strains in Taiwan from 2014 to 2018. Int J Antimicrob Agents 59:106592. doi: 10.1016/j.ijantimicag.2022.106592 [DOI] [PubMed] [Google Scholar]
- 12. Tseng KY, Liao YC, Chen FC, Chen FJ, Lo HJ. 2022. A predominant genotype of azole-resistant Candida tropicalis clinical strains. Lancet Microbe 3:e646. doi: 10.1016/S2666-5247(22)00179-3 [DOI] [PubMed] [Google Scholar]
- 13. Arastehfar A, Daneshnia F, Hafez A, Khodavaisy S, Najafzadeh MJ, Charsizadeh A, Zarrinfar H, Salehi M, Shahrabadi ZZ, Sasani E, Zomorodian K, Pan W, Hagen F, Ilkit M, Kostrzewa M, Boekhout T. 2020. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med Mycol 58:766–773. doi: 10.1093/mmy/myz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arastehfar A, Hilmioğlu-Polat S, Daneshnia F, Hafez A, Salehi M, Polat F, Yaşar M, Arslan N, Hoşbul T, Ünal N, Metin DY, Gürcan Ş, Birinci A, Koç AN, Pan W, Ilkit M, Perlin DS, Lass-Flörl C. 2020. Recent increase in the prevalence of fluconazole-non-susceptible Candida tropicalis blood isolates in Turkey: clinical implication of azole-non-susceptible and fluconazole tolerant phenotypes and genotyping. Front Microbiol 11:587278. doi: 10.3389/fmicb.2020.587278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, Chhina D, Rao R, Eshwara VK, Xess I, Kindo AJ, Umabala P, Savio J, Patel A, Ray U, Mohan S, Iyer R, Chander J, Arora A, Sardana R, Roy I, Appalaraju B, Sharma A, Shetty A, Khanna N, Marak R, Biswas S, Das S, Harish BN, Joshi S, Mendiratta D. 2015. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med 41:285–295. doi: 10.1007/s00134-014-3603-2 [DOI] [PubMed] [Google Scholar]
- 16. Favarello LM, Nucci M, Queiroz-Telles F, Guimarães T, Salles MJ, Sukiennik TCT, da Matta DA, Melo ASA, Colombo AL. 2021. Trends towards lower azole susceptibility among 200 Candida tropicalis bloodstream isolates from Brazilian medical centres. J Glob Antimicrob Resist 25:199–201. doi: 10.1016/j.jgar.2021.03.018 [DOI] [PubMed] [Google Scholar]
- 17. Khalifa HO, Watanabe A, Kamei K. 2022. Azole and echinocandin resistance mechanisms and genotyping of Candida tropicalis in Japan: cross-boundary dissemination and animal-human transmission of C. tropicalis infection. Clin Microbiol Infect 28:302. doi: 10.1016/j.cmi.2021.10.004 [DOI] [PubMed] [Google Scholar]
- 18. Megri Y, Arastehfar A, Boekhout T, Daneshnia F, Hörtnagl C, Sartori B, Hafez A, Pan W, Lass-Flörl C, Hamrioui B. 2020. Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: the urgent need for antifungal stewardship and infection control measures. Antimicrob Resist Infect Control 9:50. doi: 10.1186/s13756-020-00710-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, An N, Yang Y, Yang X, Fan Y, Feng J. 2021. Candida Tropicalis distribution and drug resistance is correlated with ERG11 and UPC2 expression. Antimicrob Resist Infect Control 10:54. doi: 10.1186/s13756-021-00890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lo HJ, Tsai SH, Chu WL, Chen YZ, Zhou ZL, Chen HF, Lee CF, Yang YL. 2017. Fruits as the vehicle of drug resistant pathogenic yeasts. J Infect 75:254–262. doi: 10.1016/j.jinf.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 21. Neves-Junior A, Cartágenes-Pinto AC, Rocha DA, de LF, Junqueira Mde L, Ferreira-Pereira A. 2015. Prevalence and fluconazole susceptibility profile of Candida Spp. Clinical Isolates in a Brazilian Tertiary Hospital in Minas Gerais [DOI] [PubMed] [Google Scholar]
- 22. Trouvé C, Blot S, Hayette M-P, Jonckheere S, Patteet S, Rodriguez-Villalobos H, Symoens F, Van Wijngaerden E, Lagrou K. 2017. Epidemiology and reporting of candidaemia in Belgium: a multi-centre study. Eur J Clin Microbiol Infect Dis 36:649–655. doi: 10.1007/s10096-016-2841-3 [DOI] [PubMed] [Google Scholar]
- 23. Zheng YJ, Xie T, Wu L, Liu XY, Zhu L, Chen Y, Mao EQ, Han LZ, Chen EZ, Yang ZT. 2021. Epidemiology, species distribution, and outcome of nosocomial Candida spp. bloodstream infection in Shanghai: an 11-year retrospective analysis in a tertiary care hospital. Ann Clin Microbiol Antimicrob 20:34. doi: 10.1186/s12941-021-00441-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Shi C, Liu J-Y, Li W-J, Zhao Y, Xiang M-J. 2016. Multilocus sequence typing of Candida tropicalis shows clonal cluster enrichment in azole-resistant isolates from patients in Shanghai, China. Infect Genet Evol 44:418–424. doi: 10.1016/j.meegid.2016.07.026 [DOI] [PubMed] [Google Scholar]
- 25. Brilhante RSN, de Aragão Rodrigues PH, de Alencar LP, Riello GB, Ribeiro JF, de Oliveira JS, Castelo-Branco D de SCM, Bandeira T de JPG, Monteiro AJ, Rocha MFG, Cordeiro R de A, Moreira JLB, Sidrim JJC. 2015. Evidence of fluconazole-resistant Candida species in tortoises and sea turtles. Mycopathologia 180:421–426. doi: 10.1007/s11046-015-9923-0 [DOI] [PubMed] [Google Scholar]
- 26. Cordeiro R de A, de Oliveira JS, Castelo-Branco D de SCM, Teixeira CEC, Marques FJ de F, Bittencourt PV, Carvalho VL, Bandeira T de JPG, Brilhante RSN, Moreira JLB, Pereira-Neto W de A, Sidrim JJC, Rocha MFG. 2015. Candida tropicalis isolates obtained from veterinary sources show resistance to Azoles and produce virulence factors. Med Mycol 53:145–152. doi: 10.1093/mmy/myu081 [DOI] [PubMed] [Google Scholar]
- 27. Subramanya SH, Sharan NK, Baral BP, Hamal D, Nayak N, Prakash PY, Sathian B, Bairy I, Gokhale S. 2017. Diversity, in-vitro virulence traits and antifungal susceptibility pattern of gastrointestinal yeast flora of healthy poultry, Gallus gallus domesticus. BMC Microbiol 17:113. doi: 10.1186/s12866-017-1024-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi H, Ueda K, Itano EN, Yanagisawa M, Murata Y, Murata M, Yaguchi T, Murakami M, Kamei K, Inomata T, Miyahara H, Sano A, Uchida S. 2010. Candida albicans and C tropicalis isolates from the expired breathes of captive dolphins and their environments in an aquarium. Vet Med Int 2010:349364. doi: 10.4061/2010/349364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y, Chen Z, Li J, Zhu Z, Pang S, Xu J, Wu J. 2022. Extensive diversity and prevalent fluconazole resistance among environmental yeasts from tropical China. Genes 13:444. doi: 10.3390/genes13030444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zuza-Alves DL, de Medeiros SSTQ, de Souza LBFC, Silva-Rocha WP, Francisco EC, de Araújo MCB, Lima-Neto RG, Neves RP, Melo AS de A, Chaves GM. 2016. Evaluation of virulence factors in vitro, resistance to osmotic stress and antifungal susceptibility of Candida tropicalis isolated from the coastal environment of Northeast Brazil. Front Microbiol 7:1783. doi: 10.3389/fmicb.2016.01783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arastehfar A, Shaban T, Zarrinfar H, Roudbary M, Ghazanfari M, Hedayati MT, Sedaghat A, Ilkit M, Najafzadeh MJ, Perlin DS. 2021. Candidemia among Iranian patients with severe COVID-19 admitted to Icus. J Fungi (Basel) 7:280. doi: 10.3390/jof7040280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fekkar A, Blaize M, Bouglé A, Normand AC, Raoelina A, Kornblum D, Kamus L, Piarroux R, Imbert S. 2021. Hospital outbreak of fluconazole-resistant Candida parapsilosis: arguments for clonal transmission and long-term persistence. Antimicrob Agents Chemother 65. doi: 10.1128/AAC.02036-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magobo RE, Lockhart SR, Govender NP. 2020. Fluconazole-resistant Candida parapsilosis strains with a Y132F substitution in the ERG11 gene causing invasive infections in a neonatal unit, South Africa . Mycoses 63:471–477. doi: 10.1111/myc.13070 [DOI] [PubMed] [Google Scholar]
- 34. Singh A, Singh PK, de Groot T, Kumar A, Mathur P, Tarai B, Sachdeva N, Upadhyaya G, Sarma S, Meis JF, Chowdhary A. 2019. Emergence of clonal fluconazole-resistant Candida parapsilosis clinical isolates in a multicentre laboratory-based surveillance study in India. J Antimicrob Chemother 74:1260–1268. doi: 10.1093/jac/dkz029 [DOI] [PubMed] [Google Scholar]
- 35. Meis JF, Chowdhary A. 2018. Candida auris: a global fungal public health threat. Lancet Infect Dis 18:1298–1299. doi: 10.1016/S1473-3099(18)30609-1 [DOI] [PubMed] [Google Scholar]
- 36. Akova M, Akalin HE, Uzun O, Gür D. 1991. Emergence of Candida krusei infections after therapy of oropharyngeal Candidiasis with fluconazole. Eur J Clin Microbiol Infect Dis 10:598–599. doi: 10.1007/BF01967286 [DOI] [PubMed] [Google Scholar]
- 37. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. 2017. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X [DOI] [PubMed] [Google Scholar]
- 38. Dong D, Li Z, Zhang L, Jiang C, Mao E, Wang X, Peng Y. 2015. Clinical and microbiological investigation of fungemia from four hospitals in China. Mycopathologia 179:407–414. doi: 10.1007/s11046-014-9855-0 [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Hermoso D, Desnos-Ollivier M, Bretagne S. 2016. Typing Candida species using microsatellite length polymorphism and multilocus sequence typing. Methods Mol Biol 1356:199–214. doi: 10.1007/978-1-4939-3052-4_15 [DOI] [PubMed] [Google Scholar]
- 40. Nourrisson C, Moniot M, Lavergne RA, Robert E, Bonnin V, Hagen F, Grenouillet F, Cafarchia C, Butler G, Cassaing S, Sabou M, Le Pape P, Poirier P, Morio F. 2023. Acquired fluconazole resistance and genetic clustering in Diutina (Candida) Catenulata from clinical samples. Clin Microbiol Infect 29:257. doi: 10.1016/j.cmi.2022.09.021 [DOI] [PubMed] [Google Scholar]
- 41. Wu Y, Zhou H, Che J, Li W, Bian F, Yu S, Zhang L, Lu J. 2014. Multilocus Microsatellite markers for molecular typing of Candida tropicalis isolates. BMC Microbiol 14:245. doi: 10.1186/s12866-014-0245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castelo‐Branco D, Lockhart SR, Chen Y, Santos DA, Hagen F, Hawkins NJ, Lavergne R, Meis JF, Le Pape P, Rocha MFG, Sidrim JJC, Arendrup M, Morio F. 2022. Collateral consequences of agricultural fungicides on pathogenic yeasts: a one health perspective to tackle azole resistance. Mycoses 65:303–311. doi: 10.1111/myc.13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leaw SN, Chang HC, Barton R, Bouchara JP, Chang TC. 2007. Identification of medically important Candida and non-Candida yeast species by an oligonucleotide array. J Clin Microbiol 45:2220–2229. doi: 10.1128/JCM.00543-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clinical and Laboratory Standards Institute . 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition. CLSI document M27-A3, Wayne, PA. [Google Scholar]
- 45. Pfaller MA, Espinel-Ingroff A, Canton E, Castanheira M, Cuenca-Estrella M, Diekema DJ, Fothergill A, Fuller J, Ghannoum M, Jones RN, Lockhart SR, Martin-Mazuelos E, Melhem MSC, Ostrosky-Zeichner L, Pappas P, Pelaez T, Peman J, Rex J, Szeszs MW. 2012. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp as determined by CLSI broth microdilution. J Clin Microbiol 50:2040–2046. doi: 10.1128/JCM.00248-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clinical and Laboratory Standards Institute . 2020. Performance standards for Antifungl susceptibility testing of yeasts. 2nd ed. CLSI supplement M60, Wayne, PA. [Google Scholar]
- 47. Chou HH, Lo HJ, Chen KW, Liao MH, Li SY. 2007. Multilocus sequence typing of Candida tropicalis shows clonal cluster enriched in isolates with resistance or trailing growth of fluconazole. Diagn Microbiol Infect Dis 58:427–433. doi: 10.1016/j.diagmicrobio.2007.03.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photos of the fruits.
Primers used in the present study.
Sources of the 123 isolates from the fruit surfaces.
Yeast colony-forming units of six different types of fruits.



