Abstract
The two very rare neurodegenerative diseases historically known as the “neuroacanthocytosis syndromes” are due to mutations of either VPS13A or XK. These are phenotypically similar disorders that affect primarily the basal ganglia and hence result in involuntary abnormal movements as well as neuropsychiatric and cognitive alterations. There are other shared features such as abnormalities of red cell membranes which result in acanthocytes, whose relationship to neurodegeneration is not yet known. Recent insights into the functions of these two proteins suggest dysfunction of lipid processing and trafficking at the subcellular level and may provide a mechanism for neuronal dysfunction and death, and potentially a target for therapeutic interventions.
Keywords: VPS13A, XK, acanthocytosis, chorea, lipid, BLTP
Introduction
VPS13A disease (chorea-acanthocytosis) and XK disease (McLeod syndrome) are the currently recognized “core neuroacanthocytosis syndromes” (Jung et al., 2021; Peikert et al., 2023). These two genetically distinct disorders share remarkable phenotypic similarities, resulting in significant diagnostic confusion, even after the identification of their respective causative genetic mutations (Walker and Danek, 2021). These two disorders were initially thought to constitute a single disease entity (“neuroacanthocytosis” or “Levine-Critchley syndrome”); intriguingly it now appears likely that the involved proteins are functionally linked, suggesting that a common mechanism at the subcellular level may be the cause of the remarkable phenotypic resemblance (Park and Neiman, 2020; Peikert et al., 2022a; Peikert and Danek, 2023).
The descriptive name for these disorders refers to the thorny red blood cells, known as acanthocytes, whose appearance is a diagnostic clue, however, these are neither specific nor obligatory for diagnosis (Peikert et al., 2022b). While acanthocytes can be seen in other conditions, including in disorders of lipid absorption (such as abetalipoproteinemia), their presence in combination with the specific neurologic symptoms seen here is a useful, although not invariable, clue to diagnosis.
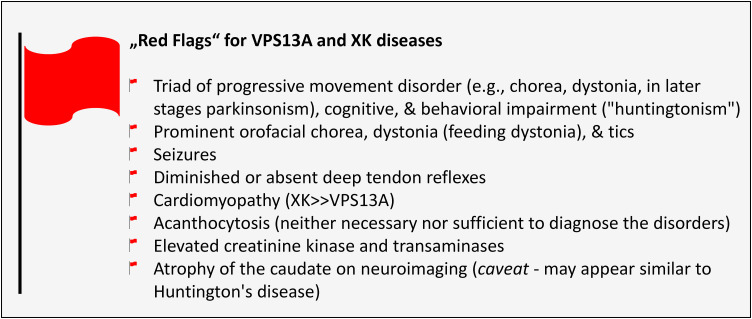
Increased awareness of these disorders over the past two decades has resulted in improvements in diagnosis and management, with a growing appreciation for their protean manifestations, both neurological and nonneurological (see “Red Flags Findings,” Figure 1). Patients with these disorders might present initially to psychiatrists, hematologists, cardiologists, transfusion medicine specialists, gastroenterologists, or others, in addition to neurologists from a variety of subspecialties, including neuromuscular, epilepsy, behavior, sleep, or movement disorders.
Figure 1.
“Red Flags findings” for VPS13A
and XK diseases.
Nomenclature
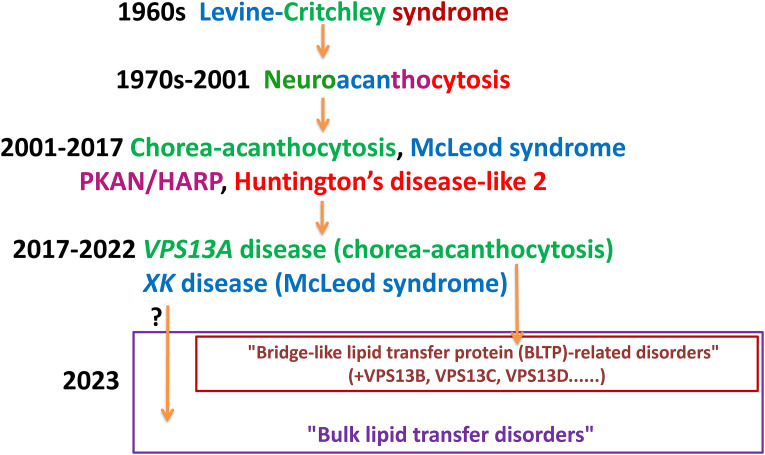
The history of the taxonomy of “neuroacanthocytosis syndromes” might be said to reflect the evolution of identification, diagnosis, and nomenclature of genetic disorders in the 20th and 21st (Walker and Danek, 2021) (Figure 2). In the 1960s, Irvine Levine (Estes et al., 1967; Levine et al., 1968) and Edmund Critchley (Critchley et al., 1967, 1968), each identified families with a novel syndrome with features of erythrocyte acanthocytosis and basal ganglia neurodegeneration resulting in a spectrum of movement disorders, which was termed “neuroacanthocytosis” (NA). This syndrome was well-recognized in Japan (later identified as VPS13A disease due to a founder mutation; Ueno et al., 2001), and at the time was believed to be a single disease, and was given the eponym “Levine-Critchley syndrome.” This term was used interchangeably with the term “neuroacanthocytosis,” most notably in the seminal work published by Hardie in 1991 (Hardie et al., 1991). However, molecular investigation of the subjects in this series determined that they carried mutations causative of several disorders, namely VPS13A, XK, and pantothenate kinase-associated neurodegeneration (PKAN; Gandhi et al., 2008).
Figure 2.
The evolution of nomenclature of neuroacanthocytosis syndromes. VPS13A disease can now be included in the group of disorders due to mutations of genes encoding for other VPS13 family members (bridge-like lipid transfer protein [BLTP]-related disorders), and possibly other related proteins. Further research will determine whether disruption of bulk lipid transfer indeed underlies the pathophysiology of these and other disorders, including XK disease, and whether “bulk lipid transfer disorders” is indeed an appropriate term.
Note. HARP = hypoprebetalipoproteinemia, acanthocytosis, retinitis pigmentosa, and pallidal degeneration; PKAN = pantothenate kinase-associated neurodegeneration.
In 2011, descendants of Critchley's original family from eastern Kentucky were found to carry mutations in VPS13A (Velayos-Baeza et al., 2011), while more recently descendants of Levine's family have been found to carry mutations in XK (Walker, Danek, Westhoff, and Vege, personal observations). This pleasing symmetry, and the association of the two genetically distinct disorders under a single term, appears to be further validated by the apparent functional relationship of the affected proteins, VPS13A and XK, in membranes (Park and Neiman, 2020; Guillén-Samander et al., 2022; Park et al., 2022; Ryoden et al., 2022; Sakuragi and Nagata, 2023).
Given the presence of acanthocytosis in PKAN, and the presence of an individual with this disorder in Hardie's series, we initially included PKAN under the umbrella of “neuroacanthocytosis syndromes,” however, it is now clear that the cellular pathophysiology is divergent from that of the other disorders. For a number of years now PKAN has been more appropriately described as the prototypical “neurodegeneration with brain iron accumulation” disorder. In Hardie's series, the individual carried the diagnosis of hypoprebetalipoproteinemia, acanthocytosis, retinitis pigmentosa, and pallidal degeneration (HARP), which has transpired to be allelic with PKAN (Ching et al., 2002).
Huntington's disease-like 2 was also included in this grouping, however, this disease is also now excluded from the group in light of recent reports showing the absence of acanthocytosis in 12 of 12 investigated HDL2 patients (Anderson et al., 2017).
Current usage of NA is understood to encompass the diseases caused by mutations in the genes VPS13A and XK. Biallelic mutations in VPS13A cause what has been termed “chorea-acanthocytosis” or “choreoacanthocytosis” or sometimes just “neuroacanthocytosis”. Mutations in XK cause McLeod syndrome, named after the initial propositus, a Harvard dental student who was found incidentally to carry the rare blood group caused by this mutation (Allen et al., 1961).
In this review, we address these two distinct genetic disorders, which have remarkable phenotypic overlap, in addition to some distinguishing features.
It has been noted that acanthocytes can be absent or appear late in either disorder (Malandrini et al., 1993; Sorrentino et al., 1999; Klempír et al., 2008; Bayreuther et al., 2010) and that the hyperkinetic movement disorder known as “chorea” can be absent in VPS13A disease (Peluso et al., 2017), thus in line with current trends in the nomenclature of mono-genetic disorders from hereon we identify these disorders based upon their genetic cause.
The Relationship Between VPS13A and XK Proteins and Related Diseases
The locations and functions of the hitherto little-known VPS13 protein family (A–D) have only been recently unraveled. The development of Alphafold provided critical insights into protein structure, and hence potential functions. Located at membrane contact sites (MCS), the VPS13 protein family members act as conduits for nonvesicular bulk lipid transfer (Bean et al., 2018; Kumar et al., 2018; Yeshaw et al., 2019; Dziurdzik and Conibear, 2021; Leonzino et al., 2021) and belong to the newly recognized superfamily of “bridge-like lipid transfer proteins (BTLPs)” (Braschi et al., 2022; Levine, 2022; Neuman et al., 2022). The corresponding human genes are of considerable interest as mutations in each lead to a hereditary neurological disease: VPS13A disease (Rampoldi et al., 2001; Ueno et al., 2001), VPS13B disease (Cohen syndrome; Kolehmainen et al., 2003), VPS13C disease (familial parkinsonism with Lewy bodies; Lesage et al., 2016; Smolders et al., 2021) or VPS13D disease (various ataxic-spastic conditions, formerly classified as SCAR4/SCA24/SCASI; Gauthier et al., 2018; Seong et al., 2018). To date, acanthocytosis has not been identified in these clinically distinct syndromes apart from VPS13A disease. Further insights into pathophysiology in diseases due to mutations of these other members of the VPS13 protein family, other BTLPs, and proteins related by a common function, such as XK, may indicate that these are all part of a group of disorders with a common mechanism of impaired bulk lipid transport (Figure 2).
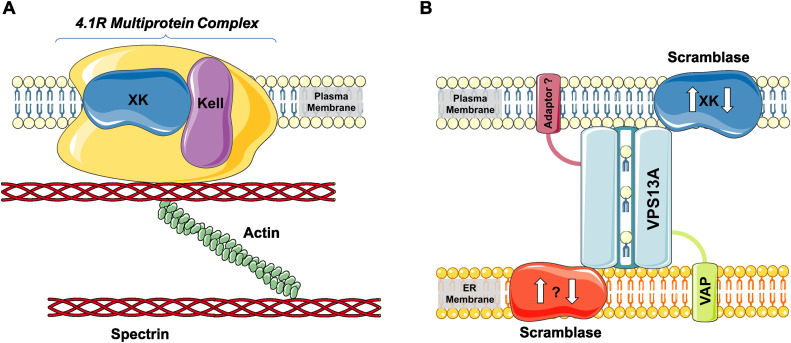
The VPS13A protein resides at MCS between the endoplasmic reticulum (ER) and various organelles; mitochondria, lipid droplets, endosomes, or plasma membrane. At these sites, the protein is arranged with its N-terminus in contact with the ER and the C-terminus near the second organelle (Kumar et al., 2018; Muñoz-Braceras et al., 2019; Yeshaw et al., 2019). Together with adaptor proteins, such as VAP, VPS13A has been described to form a “bridge” or “tunnel” with a hydrophobic groove enabling bulk phospholipid transfer between the respective organelle membranes (Figure 3; Leonzino et al., 2021; Dall'Armellina et al., 2023). While selectivity, direction, and functional consequences of this transfer need to be further investigated, this process seems to be important for expansion (e.g., autophagosome formation) or modification of lipid composition of membranes (Leonzino et al., 2021; Dabrowski et al., 2023). At the plasma membrane, VPS13A interacts with the scramblase XK (Guillén-Samander et al., 2022; Park et al., 2022; Ryoden et al., 2022), while a putative ER-sided scramblase is as yet undefined (Figure 3). This complex may be involved in the transport of lipids between the ER and plasma membrane and lipid scrambling between the two leaflets of the plasma membrane (Guillén-Samander et al., 2022) as has been shown in CD25+ CD4+ T-cells. Here, VPS13A-XK plays a role in ATP-induced phosphatidylserine exposure (by an unidentified P2X7-dependent signal) and subsequent necrotic cell death (Ryoden et al., 2022). The exact role of this process in neurons anf other cell types involved in “neuroacanthocytosis” is the subject of further research. Nevertheless, the XK–VPS13A interaction most likely represents the mechanistic basis for the phenotypic similarities between the respective diseases (Peikert and Danek, 2023).
Figure 3.
VPS13A, XK, and their interaction partners. The XK and VPS13A proteins have various interacting proteins. (A) The scramblase XK binds to Kell in the 4.1R multiprotein complex of red cell membranes (drawn simplistically) and (B) interacts with VPS13A at membrane contact sites between the endoplasmic reticulum (ER) and plasma membranes. VPS13A is a bridge-like lipid transfer protein residing at various membrane contact sites where it forms a “tunnel” enabling bulk lipid transfer and where it interacts with adaptor proteins, such as VAP as the ER-sided adaptor (the opposite adaptor is as yet undefined as is a speculative scramblase at the ER side). Reproduced with permission (Creative Commons CC-BY-NC license) from Sage Journals 2023 from Peikert and Danek (2023). (This figure contains modified images from Servier Medical Art [https://smart.servier.com] licensed by a Creative Commons Attribution 3.0 Unported License).
VPS13A Disease
This progressive neurodegenerative disorder is caused by biallelic mutations and typically appears in early adulthood (Peikert et al., 2023). Prevalence is estimated at 1:1,000,000, but underrecognition is likely. Signs and symptoms are consistent with the involvement of the basal ganglia, in particular, the head of the caudate nucleus (Walterfang et al., 2011; Liu et al., 2018). Psychiatric features such as obsessive-compulsive behaviors, tics, or psychosis can be early and confounding features. In addition to the sometimes bizarre appearance of involuntary movements, these can lead to a diagnosis of a functional neurologic disorder (Peikert et al., 2023).
The movement disorders that can develop include dystonia, tics, chorea, and—typically more prevalent in the later disease stages—parkinsonism. There is a predilection for involvement of the lower face, mouth, and oropharynx, thus speech and swallowing are often affected relatively early in the disease course, in comparison to Huntington's disease (HD), which otherwise has similar features (Peikert et al., 2023). Feeding dystonia with involuntary tongue protrusion is an occasional characteristic phenomenon associated with VPS13A disease (Bader et al., 2010).
Other features that distinguish VPS13A disease from disorders that solely affect the basal ganglia, such as HD, include seizures, which can be a presenting feature, and peripheral neuropathy and myopathy. Involvement of skeletal, and possibly cardiac, muscle appears generally to be milder in VPS13A disease than in XK disease, although at times it can be clinically significant. Additionally, the liver and spleen can often be enlarged, with elevation of liver enzymes, and there can be cardiac involvement, albeit typically mild (Quick et al., 2021).
The course of VPS13A disease is one of slow progression over several decades, although there can be sudden death, likely related to seizures or autonomic dysfunction (Walker et al., 2019).
Laboratory features such as elevation of creatine kinase (CK) and liver enzymes can lead to presymptomatic diagnosis by whole exome sequencing (Walker, personal observations). Other potential biomarkers include a reduced erythrocyte sedimentation rate (Darras et al., 2021) and elevated serum neurofilament light chain (Peikert et al., 2020), although the latter finding is a common sign of central and peripheral nervous system damage. In addition to genetic testing, diagnosis can be made by Western blot of peripheral red blood cells to look for the absence of VPS13A (chorein; Dobson-Stone et al., 2004). There appear to be rare cases, likely due to missense mutations, in which VPS13A is present on Western blot but dysfunctional.
When acanthocytosis is present in a significant percentage of red blood cells on peripheral blood smear (ideally performed as per Storch et al., 2005), this is supportive of the diagnosis, however, this is not a consistent finding and can be absent (Malandrini et al., 1993; Sorrentino et al., 1999; Bayreuther et al., 2010; Peikert et al., 2022b). The relationship between this observation and neurodegeneration remains unclear, and there is not a clear correlation between disease severity or progression and this laboratory finding.
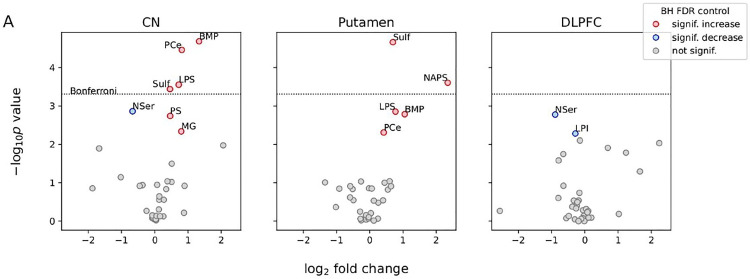
Neuropathological reports have been strikingly uninformative to date, revealing primarily marked neuronal loss and astrogliosis primarily affecting the basal ganglia, most prominently the head of the caudate nucleus (Liu et al., 2018). The latter is in contrast to HD, where neuropathologic changes are more obvious in the caudal region of the neostriatum (Vonsattel et al., 1985). However, recent lipidomic analysis of postmortem human brain tissue is supportive of dysfunctional lipid metabolism (Miltenberger-Miltenyi et al., 2023; Figure 4), as are our observations of apparent lipid accumulation in neurons (Figure 5; Ditzel et al., 2023). Similar lipidomic changes have been reported in Parkinson's disease and various VPS13 model systems, and are discussed in detail elsewhere (Miltenberger-Miltenyi et al., 2023).
Figure 4.
Lipid-level alterations in the brain extracts of ChAc, compared with non-ChAc, patients. (A) The x-axis of the volcano plots shows the log-transformed fold change in the expression of lipid groups in ChAc relative to non-ChAc patients. The shared y-axis of the volcano plots shows the minus log-transformed P values for the null hypothesis of no lipid-level change in patients with ChAc relative to control patients in a given brain region. P values were obtained from linear mixed models controlling for brain region, age at death, and subject-to-subject variability. Labeled red/blue symbols mark lipid groups with significantly increased/ decreased levels in ChAc, respectively, after 5% FDR control with the BH procedure (BH FDR, in the legend). The horizontal dotted line shows the threshold for the more conservative Bonferroni procedure. See reference for methodological details. In summary, there was increased BMP, sulfatide, LPS, and PCE in the caudate nucleus and putamen, but not in the DLPFC; increased PS and MG in the caudate nucleus and increased NAPS in the putamen; decreased NSer in the caudate nucleus and DLPFC and decreased LPI in the DLPFC; of note, there was no single outlier case driving significance. Reproduced with permission © Wiley 2023 from Miltenberger-Miltenyi et al. (2023).
Note. BH = Benjamini–Hochberg; BMP = bis(monoacylglycerol)phosphate; ChAc = chorea-acanthocytosis; DLPFC = dorsolateral prefrontal cortex; FDR = false discovery rate; LPI = lysophosphatidylinositol; LPS = lysophosphatidylserine; MG = monoacylglycerol; NAPS = N-acyl phosphatidylserine; NSer = N-acyl serine; PCE = phosphatidylcholine ether; PS = phosphatidylserine..
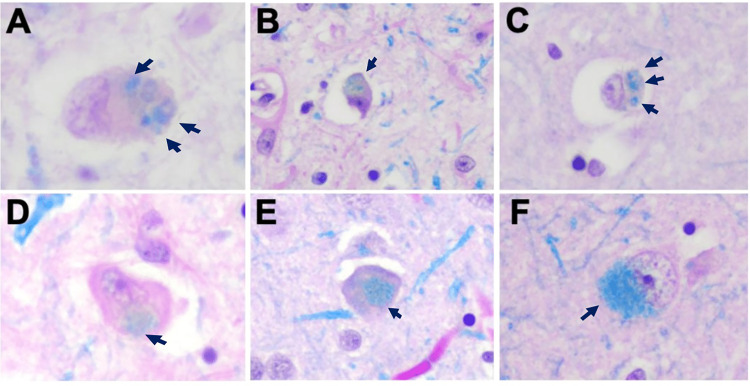
Figure 5.
Lipid inclusions. Examples of lipid accumulation morphologies in VPS13A disease (chorea-acanthocytosis). Sections stained with Luxol fast blue (LFB) showed variable patterns of lipid accumulation (arrows) with (A, B, C) puncta, (D, E) single cytoplasmic, or (F) diffuse patterns. 20× magnification for all images. Reproduced with permission © Wiley 2023 from Ditzel et al. (2023).
XK Disease
Symptoms of X-chromosomal XK disease typically appear in middle-aged men, although similar to VPS13A disease, identification of elevated CK and transaminases, the latter sometimes misinterpreted as of hepatic origin, can lead to diagnosis in earlier life (Tian et al., 2019). Affected subjects may also be identified if they undergo blood-typing, as they have an atypical pattern of erythrocyte antigen expression, namely absent Kx antigen and weakened Kell antigens, known as the “McLeod blood group phenotype” (Redman and Marsh, 1993; Redman et al., 1999). The protein product of the XK gene, XK, is expressed on the surface of erythrocytes and presents an antigen, known as Kx (Redman and Marsh, 1993; Russo et al., 1998; Redman et al., 1999). Most mutations of XK result in absent or dysfunctional (and hence mislocated) XK and hence Kx; additionally XK is linked to another protein, Kell, in the erythrocyte membrane within the 4.1R multiprotein complex, thus when XK is absent, expression of Kell antigens is markedly reduced (Peikert and Danek, 2023; Figure 3).
As with VPS13A disease, acanthocytes can be an informative feature when present, but can be absent (Klempír et al., 2008).
XK disease appears to be even rarer than VPS13A disease, with an estimated prevalence of 1:10,000,000, although again, underdiagnosis is likely a factor. Symptoms of XK disease are generally very similar to those of VPS13A disease, although there can be much more variation in presentation, severity, and rate of disease progression. In addition, symptom onset is usually later and some patients may manifest as late as their 60s (Jung et al., 2021; Peikert et al., 2022a). Milder symptoms appear to be related to mutations that result only in dysfunctional, rather than absent, XK (Walker et al., 2007). Severe involvement of speech and swallowing is a less consistent feature. The other most striking feature is the involvement of cardiac muscle, which can result in significant morbidity and mortality (Oechslin et al., 2009; Walker et al., 2019; Quick et al., 2021). Cardiac involvement can be the presenting feature, and can even require cardiac transplantation (Laurencin et al., 2018). Myopathy of skeletal muscle and peripheral neuropathy can also be significant and a cause of morbidity (Jung and Brandner, 2002; Hewer et al., 2007; Vaisfeld et al., 2021). Sleep disturbances, such as sleep apnea and periodic limb movements, have recently been recognized and may compound other issues (Lim et al., 2022).
Similar to VPS13A disease, neuropathological evaluation demonstrates neuronal loss and gliosis in the basal ganglia (Deutschländer et al., 2022).
Management
Management of these two disorders, in common with other neurodegenerative disorders at the time of writing, is directed at controlling symptoms (Walker, 2015) and preventing future complications, for example, by annual monitoring for cardiac complications (Jung et al., 2021; Peikert et al., 2023). Neurologic symptoms are managed as in other diseases with similar manifestations; this principle extends to deep brain stimulation which has been employed in a number of patients, primarily with VPS13A disease, with at least short-term benefits. Weight loss can be significant, and placement of a feeding tube can be a valuable strategy (unlike in Alzheimer's disease, where it is not found to improve life expectancy or quality).
Similar to other neurodegenerative diseases affecting the basal ganglia, such as HD, in both of these disorders behavioral issues can be very challenging to manage and can be a major determinant of functioning and quality of life. The involvement of a psychiatrist can be valuable.
In XK disease, affected patients are encouraged to bank their blood in case of future need, to avoid the possibility of a transfusion reaction. Regular screening for potential heart involvement regardless of the presence of specific symptoms is recommended.
Ongoing multidisciplinary care involving therapists of all modalities, including physiotherapy, speech, occupational, and psychological, is recommended.
Potential Disease-Modifying Strategies
Based on preclinical evidence of Lyn kinase hyperactivity with subsequent perturbation of autophagy, impaired protein phosphorylation of red cell membrane proteins and neuronal hyperexcitability in VPS13A disease (De Franceschi et al., 2011; Lupo et al., 2016; Stanslowsky et al., 2016), three subjects were given the protein kinase inhibitor dasatinib (Peikert et al., 2021b). Although target engagement in red blood cells was achieved, clinical parameters remained essentially unchanged. Follow-up studies suggested failure of central nervous system penetrance by orally administered dasatinib (Peikert et al., 2021a), which indicated that, based on current knowledge, this approach cannot be generally recommended to treat VPS13A disease patients.
Future Directions
Further studies are required to expand our findings in VPS13A disease and to extend our observations by performing lipidomics and neuropathology in XK disease. In addition, further studies are needed to demonstrate that disordered lipid transfer at MCS due to loss of protein function and subsequent neuronal dysfunction is the proximate cause of neurodegeneration, prior to this being the focus for the development of potential therapy targets.
The cause of the abnormal red cell membrane structure that results in acanthocytosis is not known, although there is evidence of abnormal protein phosphorylation in red cell membranes (De Franceschi et al., 2011) and of depolymerized cortical actin (Foller et al., 2012). These cells do not contain nuclei or subcellular organelles once they are in the peripheral circulation, however, it is possible that abnormal lipid metabolism and organellar transfer during maturation (erythropoiesis) affects their membrane composition.
In order to achieve “clinical trial readiness” once a disease-modifying strategy/compound has been identified, it is essential to identify potential study subjects (at the international level, given the rarity of these diseases), and to further characterize disease natural history and biomarkers.
Conclusions
Recent insights into the group of proteins involved in bridge-like lipid transfer have elucidated mechanisms of subcellular lipid processing. Mutations of VPS13A result in rather localized neurodegeneration and dysfunction of lipid metabolism, thus providing a tantalizing indication that this dysfunction might be a target for therapeutic intervention. Mutations resulting in loss or dysfunction of the scramblase XK, which interacts with VPS13A, produce a remarkably similar disease, potentially suggesting a common mechanism, and hence a shared pathway to intervention.
Acknowledgments
We are grateful to Glenn (†) and Ginger Irvine as the founders of the Advocacy for Neuroacanthocytosis Patients (www.naadvocacy.org) and to Susan Wagner and Joy Willard-Williford as representatives of the NA Advocacy USA (www.naadvocacyusa.org). We thank the Advocacies for their support and research funding.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose receipt of the following financial support for the research and/or authorship of this article: Ruth H. Walker receives research support from the Department of Veterans Affairs, USA, and Neuroacanthocytosis Advocacy USA. Kevin Peikert is supported by the Rostock Academy of Science (RAS). Andreas Hermann is supported by the “Hermann und Lilly Schilling-Stiftung für medizinische Forschung im Stifterverband.”
ORCID iDs: Ruth H. Walker https://orcid.org/0000-0002-8366-072X
Kevin Peikert https://orcid.org/0000-0002-0845-2453
References
- Allen FH, Krabbbe SM, Corcoran PA. (1961). A new phenotype (McLeod) in the Kell blood-group system. Vox Sang 6, 555–560. doi: 10.1111/j.1423-0410.1961.tb03203.x [DOI] [PubMed] [Google Scholar]
- Anderson DG, Carmona S, Naidoo K, Coetzer TL, Carr J, Rudnicki DD, Walker RH, Margolis RL, Krause A. (2017). Absence of acanthocytosis in Huntington's disease-like 2: A prospective comparison with Huntington's disease. Tremor Other Hyperkinet Mov (N Y) 7, 512. doi: 10.7916/D81J9PDX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader B, Walker RH, Vogel M, Prosiegel M, McIntosh J, Danek A. (2010). Tongue protrusion and feeding dystonia: A hallmark of chorea-acanthocytosis. Mov Disord 25, 127–129. doi: 10.1002/mds.22863 [DOI] [PubMed] [Google Scholar]
- Bayreuther C, Borg M, Ferrero-Vacher C, Chaussenot A, Lebrun C. (2010). Chorea-acanthocytosis without acanthocytes. Rev Neurol (Paris) 166, 100–103. doi: 10.1016/j.neurol.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Bean BDM, Dziurdzik SK, Kolehmainen KL, Fowler CMS, Kwong WK, Grad LI, Davey M, Schluter C, Conibear E. (2018). Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites. J Cell Biol 217, 3593–3607. doi: 10.1083/jcb.201804111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi B, Bruford EA, Cavanagh AT, Neuman SD, Bashirullah A. (2022). The bridge-like lipid transfer protein (BLTP) gene group: Introducing new nomenclature based on structural homology indicating shared function. Hum Genomics 16, 66. doi: 10.1186/s40246-022-00439-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching KH, Westaway SK, Gitschier J, Higgins JJ, Hayflick SJ. (2002). HARP syndrome is allelic with pantothenate kinase-associated neurodegeneration. Neurology 58, 1673–1674. doi: 10.1212/wnl.58.11.1673 [DOI] [PubMed] [Google Scholar]
- Critchley EM, Clark DB, Wikler A. (1967). An adult form of acanthocytosis. Trans Am Neurol Assoc 92, 132–137. [PubMed] [Google Scholar]
- Critchley EM, Clark DB, Wikler A. (1968). Acanthocytosis and neurological disorder without betalipoproteinemia. Arch Neurol 18, 134–140. doi: 10.1001/archneur.1968.00470320036004 [DOI] [PubMed] [Google Scholar]
- Dabrowski R, Tulli S, Graef M. (2023). Parallel phospholipid transfer by Vps13 and Atg2 determines autophagosome biogenesis dynamics. J Cell Biol 222, e202211039. doi: 10.1083/jcb.202211039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Armellina F, Stagi M, Swan LE. (2023). In silico modeling human VPS13 proteins associated with donor and target membranes suggests lipid transfer mechanisms. Proteins 91, 439–455. doi: 10.1002/prot.26446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras A, Peikert K, Rabe A, Yaya F, Simionato G, John T, Dasanna AK, Buvalyy S, Geisel J, Hermann A, et al. (2021). Acanthocyte sedimentation rate as a diagnostic biomarker for neuroacanthocytosis syndromes: Experimental evidence and physical justification. Cells 10, 788. doi: 10.3390/cells10040788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L, Tomelleri C, Matte A, Brunati AM, Bovee-Geurts PH, Bertoldi M, Lasonder E, Tibaldi E, Danek A, Walker RH, et al. (2011). Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood 118, 5652–5663. doi: 10.1182/blood-2011-05-355339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschländer AB, Dickson DW, Wszolek ZK. (2022). Neuropathology of McLeod syndrome. Mov Disord 37, 644–646. doi: 10.1002/mds.28882 [DOI] [PubMed] [Google Scholar]
- Ditzel RM, Jr, Walker RH, Nirenberg MJ, Tetlow AM, Farrell K, Lind-Watson KJ, Thorn EL, Dangoor DK, Gordon R, De Sanctis C, et al. (2023). An autopsy series of seven cases of VPS13A disease (chorea-acanthocytosis). Mov Disord. Epub ahead of print. doi: 10.1002/mds.29589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson-Stone C, Velayos-Baeza A, Filippone LA, Westbury S, Storch A, Erdmann T, Wroe SJ, Leenders KL, Lang AE, Dotti MT, et al. (2004). Chorein detection for the diagnosis of chorea-acanthocytosis. Ann Neurol 56, 299–302. doi: 10.1002/ana.20200 [DOI] [PubMed] [Google Scholar]
- Dziurdzik SK, Conibear E. (2021). The Vps13 family of lipid transporters and its role at membrane contact sites. Int J Mol Sci 22, 2905. doi: 10.3390/ijms22062905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JW, Morley TJ, Levine IM, Emerson CP. (1967). A new hereditary acanthocytosis syndrome. Am J Med 42, 868–881. doi: 10.1016/0002-9343(67)90068-x [DOI] [PubMed] [Google Scholar]
- Foller M, Hermann A, Gu S, Alesutan I, Qadri SM, Borst O, Schmidt EM, Schiele F, vom Hagen JM, Saft C, et al. (2012). Chorein-sensitive polymerization of cortical actin and suicidal cell death in chorea-acanthocytosis. FASEB J 26, 1526–1534. doi: 10.1096/fj.11-198317 [DOI] [PubMed] [Google Scholar]
- Gandhi S, Hardie RJ, Lees AJ. (2008). An update on the Hardie neuroacanthocytosis series. In: Neuroacanthocytosis Syndromes II, eds. Walker R.H., Saiki S., Danek A., Berlin, Heidelberg: Germany: Springer, 43–51. doi: 10.1007/978-3-540-71693-8_3 [DOI] [Google Scholar]
- Gauthier J, Meijer IA, Lessel D, Mencacci NE, Krainc D, Hempel M, Tsiakas K, Prokisch H, Rossignol E, Helm MH, et al. (2018). Recessive mutations in VPS13D cause childhood onset movement disorders. Ann Neurol 83, 1089–1095. doi: 10.1002/ana.25204 [DOI] [PubMed] [Google Scholar]
- Guillén-Samander A, Wu Y, Pineda SS, García FJ, Eisen JN, Leonzino M, Ugur B, Kellis M, Heiman M, De Camilli P. (2022). A partnership between the lipid scramblase XK and the lipid transfer protein VPS13A at the plasma membrane. Proc Natl Acad Sci U S A 119, e2205425119. doi: 10.1073/pnas.2205425119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RJ, Pullon HW, Harding AE, Owen JS, Pires M, Daniels GL, Imai Y, Misra VP, King RH, Jacobs JM. (1991). Neuroacanthocytosis. A clinical, haematological and pathological study of 19 cases. Brain 114, 13–49. [PubMed] [Google Scholar]
- Hewer E, Danek A, Schoser BG, Miranda M, Reichard R, Castiglioni C, Oechsner M, Goebel HH, Heppner FL, Jung HH. (2007). McLeod myopathy revisited: more neurogenic and less benign. Brain 130, 3285–3296. doi: 10.1093/brain/awm269 [DOI] [PubMed] [Google Scholar]
- Jung HH, Brandner S. (2002). Malignant McLeod myopathy. Muscle Nerve 26, 424–427. doi: 10.1002/mus.10199 [DOI] [PubMed] [Google Scholar]
- Jung HH, Danek A, Walker RH, Frey BM, Peikert K. (2021). McLeod neuroacanthocytosis syndrome. In: GeneReviews®, eds. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., Seattle, WA: University of Washington, Seattle, 1993–2023. https://www.ncbi.nlm.nih.gov/books/NBK1354/ [Google Scholar]
- Klempír J, Roth J, Zárubová K, Písacka M, Spacková N, Tilley L. (2008). The McLeod syndrome without acanthocytes. Parkinsonism Relat Disord 14, 364–366. doi: 10.1016/j.parkreldis.2007.07.011 [DOI] [PubMed] [Google Scholar]
- Kolehmainen J, Black GC, Saarinen A, Chandler K, Clayton-Smith J, Traskelin AL, Perveen R, Kivitie-Kallio S, Norio R, Warburg M, et al. (2003). Cohen syndrome is caused by mutations in a novel gene, COH1, encoding a transmembrane protein with a presumed role in vesicle-mediated sorting and intracellular protein transport. Am J Hum Genet 72, 1359–1369. doi: 10.1086/375454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Leonzino M, Hancock-Cerutti W, Horenkamp FA, Li P, Lees JA, Wheeler H, Reinisch KM, De Camilli P. (2018). VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol 217, 3625–3639. doi: 10.1083/jcb.201807019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencin C, Sebbag L, Jousserand G, Demontes M, Campean L, Thivolet-Bejui F, Lebre AS, Thobois S. (2018). Novel XK mutation in a McLeod patient diagnosed after heart transplant. Clin Neurol Neurosurg 168, 64–66. doi: 10.1016/j.clineuro.2018.02.039 [DOI] [PubMed] [Google Scholar]
- Leonzino M, Reinisch KM, De Camilli P. (2021). Insights into VPS13 properties and function reveal a new mechanism of eukaryotic lipid transport. Biochim Biophys Acta Mol Cell Biol Lipids 1866, 159003. doi: 10.1016/j.bbalip.2021.159003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Drouet V, Majounie E, Deramecourt V, Jacoupy M, Nicolas A, Cormier-Dequaire F, Hassoun SM, Pujol C, Ciura S, et al. (2016). Loss of VPS13C function in autosomal-recessive Parkinsonism causes mitochondrial dysfunction and increases PINK1/Parkin-dependent mitophagy. Am J Hum Genet 98, 500–513. doi: 10.1016/j.ajhg.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine IM, Estes JW, Looney JM. (1968). Hereditary neurological disease with acanthocytosis. A new syndrome. Arch Neurol 19, 403–409. doi: 10.1001/archneur.1968.00480040069007 [DOI] [PubMed] [Google Scholar]
- Levine TP. (2022). Sequence analysis and structural predictions of lipid transfer bridges in the repeating beta groove (RBG) superfamily reveal past and present domain variations affecting form, function and interactions of VPS13, ATG2, SHIP164, Hobbit and Tweek. Contact (Thousand Oaks) 5, 251525642211343. doi: 10.1177/25152564221134328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Sarva H, Hiller A, Feitell S, Oates P, Barone D, Walker RH. (2022). Sleep disorders in McLeod syndrome: A case series. Parkinsonism Relat Disord 102, 86–88. doi: 10.1016/j.parkreldis.2022.07.013 [DOI] [PubMed] [Google Scholar]
- Liu J, Heinsen H, Grinberg LT, Alho E, Amaro E, Jr, Pasqualucci CA, Rub U, den Dunnen W, Arzberger T, Schmitz C, et al. (2018). Subcortical neurodegeneration in chorea: Similarities and differences between chorea-acanthocytosis and Huntington's disease. Parkinsonism Relat Disord 49, 54–59. doi: 10.1016/j.parkreldis.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Lupo F, Tibaldi E, Matte A, Sharma AK, Brunati AM, Alper SL, Zancanaro C, Benati D, Siciliano A, Bertoldi M, et al. (2016). A new molecular link between defective autophagy and erythroid abnormalities in chorea-acanthocytosis. Blood 128, 2976–2987. doi: 10.1182/blood-2016-07-727321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandrini A, Fabrizi GM, Palmeri S, Ciacci G, Salvadori C, Berti G, Bucalossi A, Federico A, Guazzi GC. (1993). Choreo-acanthocytosis like phenotype without acanthocytes: clinicopathological case report. A contribution to the knowledge of the functional pathology of the caudate nucleus. Acta Neuropathol 86, 651–658. doi: 10.1007/BF00294306 [DOI] [PubMed] [Google Scholar]
- Miltenberger-Miltenyi G, Jones A, Tetlow AM, Conceição VA, Crary JF, Ditzel RM, Farrell K, Nandakumar R, Barton B, Karp BI, et al. (2023). Sphingolipid and phospholipid levels are altered in human brain in chorea-acanthocytosis. Mov Disord 38, 1535–1541. doi: 10.1002/mds.29445 [DOI] [PubMed] [Google Scholar]
- Muñoz-Braceras S, Tornero-Écija AR, Vincent O, Escalante R. (2019). VPS13A is closely associated with mitochondria and is required for efficient lysosomal degradation. Dis Model Mech 12, dmm036681. doi: 10.1242/dmm.036681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman SD, Levine TP, Bashirullah A. (2022). A novel superfamily of bridge-like lipid transfer proteins. Trends Cell Biol 32, 962–974. doi: 10.1016/j.tcb.2022.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin E, Kaup D, Jenni R, Jung HH. (2009). Cardiac abnormalities in McLeod syndrome. Int J Cardiol 132, 130–132. doi: 10.1016/j.ijcard.2007.07.167 [DOI] [PubMed] [Google Scholar]
- Park JS, Hu Y, Hollingsworth NM, Miltenberger-Miltenyi G, Neiman AM. (2022). Interaction between VPS13A and the XK scramblase is important for VPS13A function in humans. J Cell Sci 135, jcs260227. doi: 10.1242/jcs.260227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Neiman AM. (2020). XK is a partner for VPS13A: a molecular link between chorea-acanthocytosis and McLeod syndrome. Mol Biol Cell 31, 2425–2436. doi: 10.1091/mbc.E19-08-0439-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikert K, Akgün K, Beste C, Ziemssen T, Buhmann C, Danek A, Hermann A. (2020). Neurofilament light chain in serum is significantly increased in chorea-acanthocytosis. Parkinsonism Relat Disord 80, 28–31. doi: 10.1016/j.parkreldis.2020.09.004 [DOI] [PubMed] [Google Scholar]
- Peikert K, Danek A. (2023). VPS13 forum proceedings: XK, XK-related and VPS13 proteins in membrane lipid dynamics. Contact 6, 25152564231156994. doi: 10.1177/25152564231156994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikert K, Dobson-Stone C, Rampoldi L, Miltenberger-Miltenyi G, Neiman A, Camilli PD, Hermann A, Walker R, Monaco A, Danek A. (2023). VPS13A Disease. In: GeneReviews®, eds. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., Seattle, WA: University of Washington, Seattle, 1993–2023. https://www.ncbi.nlm.nih.gov/books/NBK1387/ [Google Scholar]
- Peikert K, Federti E, Matte A, Constantin G, Pietronigro EC, Fabene PF, Defilippi P, Turco E, Del Gallo F, Pucci P, et al. (2021a). Therapeutic targeting of Lyn kinase to treat chorea-acanthocytosis. Acta Neuropathol Commun 9, 81. doi: 10.1186/s40478-021-01181-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikert K, Glaß H, Federti E, Matte A, Pelzl L, Akgün K, Ziemssen T, Ordemann R, Lang F, The Network For Translational Research For Neurocanthocytosis Patients, et al. (2021b). Targeting Lyn kinase in chorea-acanthocytosis: A translational treatment approach in a rare disease. J Pers Med 11, 392. doi: 10.3390/jpm11050392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikert K, Hermann A, Danek A. (2022a). XK-associated McLeod syndrome: Nonhematological manifestations and relation to VPS13A disease. Transfus Med Hemother 49, 4–12. doi: 10.1159/000521417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikert K, Storch A, Hermann A, Landwehrmeyer GB, Walker RH, Simionato G, Kaestner L, Danek A. (2022b). Commentary: Acanthocytes identified in Huntington's disease. Front Neurosci 16, 1049676. doi: 10.3389/fnins.2022.1049676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso S, Bilo L, Esposito M, Antenora A, De Rosa A, Pappatà S, De Michele G. (2017). Chorea-acanthocytosis without chorea: expanding the clinical phenotype. Parkinsonism Relat Disord 41, 124–126. doi: 10.1016/j.parkreldis.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Quick S, Heidrich FM, Winkler MV, Winkler AH, Ibrahim K, Linke A, Speiser U, Grabmaier U, Buhmann C, Marxreiter F, et al. (2021). Cardiac manifestation is evident in chorea-acanthocytosis but different from McLeod syndrome. Parkinsonism Relat Disord 88, 90–95. doi: 10.1016/j.parkreldis.2021.05.015 [DOI] [PubMed] [Google Scholar]
- Rampoldi L, Dobson-Stone C, Rubio JP, Danek A, Chalmers RM, Wood NW, Verellen C, Ferrer X, Malandrini A, Fabrizi GM, et al. (2001). A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat Genet 28, 119. doi: 10.1038/88821 [DOI] [PubMed] [Google Scholar]
- Redman CM, Marsh WL. (1993). The Kell blood group system and the McLeod phenotype. Semin Hematol 30, 209–218. [PubMed] [Google Scholar]
- Redman CM, Russo D, Lee S. (1999). Kell, Kx and the McLeod syndrome. Baillieres Best Pract Res Clin Haematol 12, 621–635. doi: 10.1053/beha.1999.0045 [DOI] [PubMed] [Google Scholar]
- Russo D, Redman C, Lee S. (1998). Association of XK and Kell blood group proteins. J Biol Chem 273, 13950–13956. doi: 10.1074/jbc.273.22.13950 [DOI] [PubMed] [Google Scholar]
- Ryoden Y, Segawa K, Nagata S. (2022). Requirement of Xk and Vps13a for the P2X7-mediated phospholipid scrambling and cell lysis in mouse T cells. Proc Natl Acad Sci U S A 119, e2119286119. doi: 10.1073/pnas.2119286119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragi T, Nagata S. (2023). Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases. Nat Rev Mol Cell Biol 24, 576–596. doi: 10.1038/s41580-023-00604-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong E, Insolera R, Dulovic M, Kamsteeg EJ, Trinh J, Bruggemann N, Sandford E, Li S, Ozel AB, Li JZ, et al. (2018). Mutations in VPS13D lead to a new recessive ataxia with spasticity and mitochondrial defects. Ann Neurol 83, 1075–1088. doi: 10.1002/ana.25220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders S, Philtjens S, Crosiers D, Sieben A, Hens E, Heeman B, Van Mossevelde S, Pals P, Asselbergh B, Dos Santos Dias R, et al. (2021). Contribution of rare homozygous and compound heterozygous VPS13C missense mutations to dementia with Lewy bodies and Parkinson's disease. Acta Neuropathol Commun 9, 25. doi: 10.1186/s40478-021-01121-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G, De Renzo A, Miniello S, Nori O, Bonavita V. (1999). Late appearance of acanthocytes during the course of chorea-acanthocytosis. J Neurol Sci 163, 175–178. doi: 10.1016/s0022-510x(99)00005-2 [DOI] [PubMed] [Google Scholar]
- Stanslowsky N, Reinhardt P, Glass H, Kalmbach N, Naujock M, Hensel N, Lubben V, Pal A, Venneri A, Lupo F, et al. (2016). Neuronal dysfunction in iPSC-derived medium spiny neurons from chorea-acanthocytosis patients is reversed by Src kinase inhibition and F-actin stabilization. J Neurosci 36, 12027–12043. doi: 10.1523/jneurosci.0456-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch A, Kornhass M, Schwarz J. (2005). Testing for acanthocytosis A prospective reader-blinded study in movement disorder patients. J Neurol 252, 84–90. doi: 10.1007/s00415-005-0616-3 [DOI] [PubMed] [Google Scholar]
- Tian PC, Wang Y, Chen Z, Shi DD, Wang HL, Luo Q. (2019). The first case report of McLeod syndrome in an infant with a novel mutation (c.89C >��A, p. Ser30X) in XK. Clin Neurol Neurosurg 184, 105421. doi: 10.1016/j.clineuro.2019.105421 [DOI] [PubMed] [Google Scholar]
- Ueno S-I, Maruki Y, Nakamura M, Tomemori Y, Kamae K, Tanabe H, Yamashita Y, Matsuda S, Kaneko S, Sano A. (2001). The gene encoding a newly discovered protein, chorein, is mutated in chorea-acanthocytosis. Nat Genet 28, 121. doi: 10.1038/88825 [DOI] [PubMed] [Google Scholar]
- Vaisfeld A, Bruno G, Petracca M, Bentivoglio AR, Servidei S, Vita MG, Bove F, Straccia G, Dato C, Di Iorio G, et al. (2021). Neuroacanthocytosis syndromes in an Italian cohort: clinical spectrum, high genetic variability and muscle involvement. Genes (Basel) 12, 344. doi: 10.3390/genes12030344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos-Baeza A, Holinski-Feder E, Neitzel B, Bader B, Critchley EM, Monaco AP, Danek A, Walker RH. (2011). Chorea-acanthocytosis genotype in the original critchley kentucky neuroacanthocytosis kindred. Arch Neurol 68, 1330–1333. doi: 10.1001/archneurol.2011.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP. (1985). Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol 44, 559–577. doi: 10.1097/00005072-198511000-00003 [DOI] [PubMed] [Google Scholar]
- Walker RH. (2015). Management of neuroacanthocytosis syndromes. Tremor Other Hyperkinet Mov (N Y) 5, 346. doi: 10.7916/d8w66k48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Danek A. (2021). Neuroacanthocytosis” - overdue for a taxonomic update. Tremor Other Hyperkinet Mov (N Y) 11, 1. doi: 10.5334/tohm.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Danek A, Uttner I, Offner R, Reid M, Lee S. (2007). McLeod phenotype without the McLeod syndrome. Transfusion 47, 299–305. doi: 10.1111/j.1537-2995.2007.01106.x [DOI] [PubMed] [Google Scholar]
- Walker RH, Miranda M, Jung HH, Danek A. (2019). Life expectancy and mortality in chorea-acanthocytosis and McLeod syndrome. Parkinsonism Relat Disord 60, 158–161. doi: 10.1016/j.parkreldis.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Walterfang M, Looi JC, Styner M, Walker RH, Danek A, Niethammer M, Evans A, Kotschet K, Rodrigues GR, Hughes A, Velakoulis D. (2011). Shape alterations in the striatum in chorea-acanthocytosis. Psychiatry Res 192, 29–36. doi: 10.1016/j.pscychresns.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshaw WM, van der Zwaag M, Pinto F, Lahaye LL, Faber AI, Gomez-Sanchez R, Dolga AM, Poland C, Monaco AP, van IJzendoorn Sven CD, et al. (2019). Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. Elife 8, e43561. doi: 10.7554/eLife.43561 [DOI] [PMC free article] [PubMed] [Google Scholar]