ABSTRACT
Because of the stubborn resistance to antibiotics, treating clinically resistant bacteria is a tricky business. Although considerable attention has been devoted to preventing and treating infections from drug-resistant bacteria, few studies are available on combining metabolites and antibiotics to combat methicillin-resistant Staphylococcus aureus (MRSA). This study found that exogenous L-glutamine potentiated aminoglycoside (gentamicin)-mediated killing efficacy in a dose- and time-dependent manner in MRSA (USA300 cell line) and other Gram-positive-resistant bacteria including MRSA 252, Listeria monocytogenes, and Corynebacterium diphtheriae. L-glutamine promoted the uptake of gentamicin through increasing the membrane permeability and was correlated with disrupted pH gradient. Furthermore, L-glutamine decreased intracellular reactive oxygen species by glutathione, which also increased USA300 sensitivity to gentamicin. We also demonstrated that combined treatment with gentamicin and L-glutamine enhanced the survival of MRSA-infected mice. In conclusion, we developed a promising therapeutic strategy for treating Gram-positive-resistant bacterial infections.
IMPORTANCE
Methicillin-resistant Staphylococcus aureus (MRSA) infection severely threatens human health due to high morbidity and mortality; it is urgent to develop novel strategies to tackle this problem. Metabolites belong to antibiotic adjuvants which improve the effect of antibiotics. Despite reports of L-glutamine being applied in antibiotic adjuvant for Gram-negative bacteria, how L-glutamine affects antibiotics against Gram-positive-resistant bacteria is still unclear. In this study, L-glutamine increases the antibacterial effect of gentamicin on MRSA, and it links to membrane permeability and pH gradient (ΔpH), resulting in uptake of more gentamicin. Of great interest, reduced reactive oxygen species (ROS) by glutathione was found under L-glutamine treatment; USA300 becomes sensitive again to gentamicin. This study not only offers deep understanding on ΔpH and ROS on bacterial resistance but also provides potential treatment solutions for targeting MRSA infection.
KEYWORDS: methicillin-resistant Staphylococcus aureus , L-glutamine, gentamicin, ΔpH, membrane permeability, ROS
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a multidrug-resistant (MDR) Gram-positive bacteria, which is one of the most common and drug-resistant pathogens in hospital and community. The severity of invasive MRSA infections in the bloodstream, skin, and soft tissue causes high morbidity and mortality (1 – 3). The World Health Organization has labeled MRSA infection as a major global health problem. Therefore, novel approaches for combating bacterial resistance are urgently needed and underlying mechanisms of MDR Gram-positive bacteria must be understood.
Although antibiotic resistance is a major concern, drugs such as lipoglycopeptides, chloramphenicols, aminoglycosides, and rifamycins remain irreplaceable for combating most S. aureus-related diseases (4, 5). In particular, aminoglycosides such as gentamicin is combined with other antibiotics for treatment of MRSA infection (6, 7). The effectiveness of gentamicin against MRSA lies in its ability to disrupt protein biosynthesis and thus inhibit bacterial growth, but resistance to this drug is already increasing (8). Combining multiple antibiotics is a promising approach to combat MRSA and other drug-resistant bacteria (9 – 11), but resistance will eventually develop in response to novel drug combinations (12). Moreover, combination drug therapy has the potential side effect of increased toxicity for patients (12, 13). Therefore, new therapeutic approaches are needed that do not rely exclusively on antibiotics.
Promising evidence suggests that altering the metabolic state of bacteria is effective against drug resistance (14, 15). Our and other studies found that exogenous metabolites could reprogram bacterial metabolomes from resistant to sensitive phenotypes, restoring antibiotic susceptibility (16 – 24). Thus, the combination of an antibiotic and a non-antibacterial agent has been considered as an alternative therapeutic approach. In recent years, the metabolite L-glutamine could synergize with rifampicin against mycobacterial persisters via promoting energy metabolism (25); Zhao et al. found that it also promoted nucleoside biosynthesis in Escherichia coli and these nucleosides interacted with bacterial two-component system CpxA/CpxR and upregulated OmpF to exert metabolism-altering effects (21). Besides, L-glutamine increased the antibacterial effect of gentamicin to resistant E. coli (23). Still, it is unclear whether L-glutamine could synergistically interact with antibiotics against Gram-positive resistant bacteria, and the important mechanism is poorly understood.
In this study, we aimed to investigate synergistic antibiotic effects of L-glutamine and gentamicin on MRSA in vitro and in vivo, as well as explore the underlying mechanisms. The combination therapy described here may be a promising strategy for combating MRSA infections.
RESULTS
Exogenous L-glutamine promotes aminoglycosides to kill MRSA
When in the present doses of exogenous L-glutamine, gentamicin exerted a dose-dependent inhibitory effect on the viability of USA300, a Gram-positive MRSA strain (Fig. 1A). Alone, 200 µg/mL gentamicin did not exhibit bactericidal activity against USA300 cells, but in combination with L-glutamine, the potentiated efficacy was over 500-fold. Additionally, the cell survival rate was decreased with an increasing dose of gentamicin plus 5 mM L-glutamine (Fig. 1B). Fluorescence microscopic imaging showed that the combination of L-glutamine and gentamicin resulted in enhanced red and orange fluorescence, whereas the monotreatment resulted in green fluorescence only (Fig. 1C). The killing effect was also time dependent, with the highest bactericidal activity effect at 6–8 h (Fig. 1D). To determine whether the bacteria developed resistance to L-glutamine and gentamicin-enabled potentiation, USA300 was propagated in M9 medium containing increasing concentrations of gentamicin with or without glutamine for 20 generations. Up to the 5th generation, the minimum inhibitory concentration (MIC) value remained unchanged for both gentamicin and L-glutamine plus gentamicin. From the 6th generation, the MIC value doubled but no longer increased with increasing generations (Fig. 1E). This suggests that the synergistic use of gentamicin and L-glutamine had a negligible effect on the reemergence of MRSA resistance. Highly synergistic effects of L-glutamine and other aminoglycosides (e.g., kanamycin, tobramycin, streptomycin, and neomycin) against USA300 were also observed (Fig. 1F). These data show that L-glutamine potentiates the bactericidal characteristics of aminoglycosides against Gram-positive-resistant bacteria.
Fig 1.
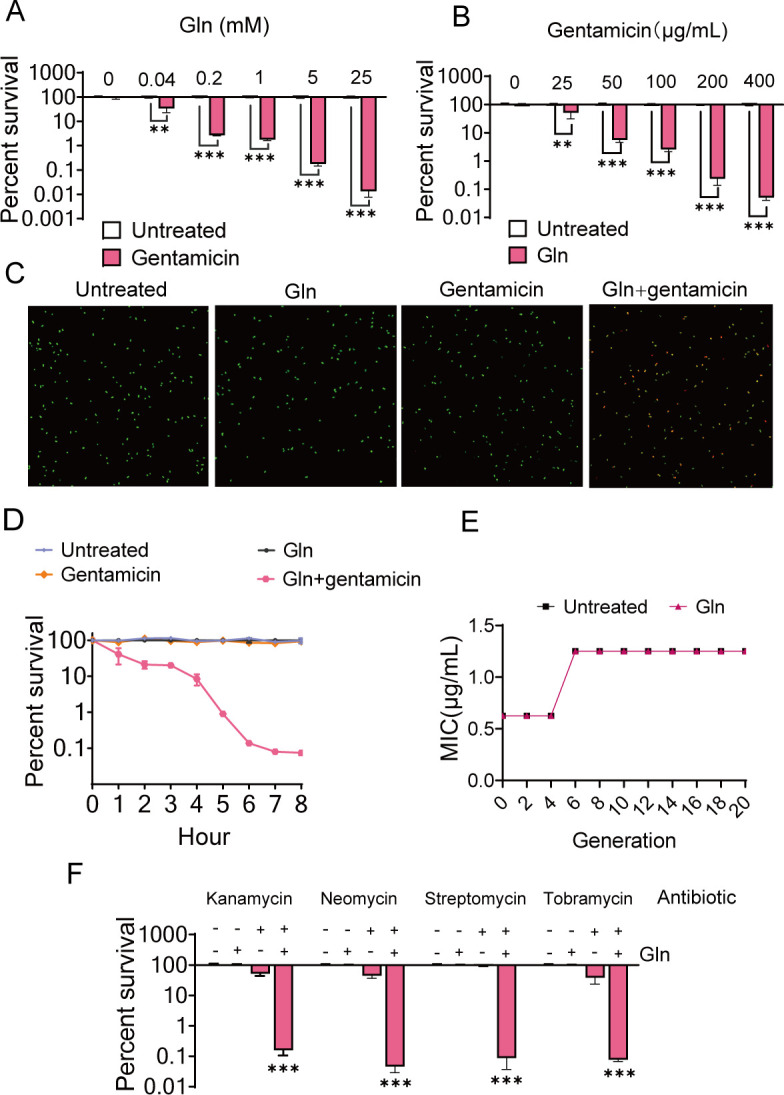
Effect of exogenous L-glutamine on the susceptibility of Gram-positive bacteria USA300 to aminoglycosides. (A) Percent survival of USA300 treated with different concentrations of L-glutamine (Gln) and/or gentamicin (200 µg/mL). (B) Percent survival of USA300 treated with different concentrations of gentamicin (200 µg/mL) and/or L-glutamine (5 mM). (C) Confocal micrographs of USA300 with different concentrations of PBS, L-glutamine, gentamicin, and their combination for 6 h stained by the Live & Dead Bacterial Staining Kit. Viable bacteria were stained green with DMAO, while dead bacteria were stained red with EthD-III. (D) The time effect of bactericidal efficacy with L-glutamine (5 mM) and/or gentamicin (200 µg/mL) treatment. (E) MIC of USA300, which was propagated in M9 medium with or without glutamine (5 mM) for twenty generations, and the concentration of gentamicin was 200 µg/mL. (F) Percent survival of USA300 treated with L-glutamine and other aminoglycosides, kanamycin (400 µg/mL), tobramycin (400 µg/mL), streptomycin (800 µg/mL), and neomycin (1.0 mg/mL). All data are displayed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, determined by one-way ANOVA.
L-glutamine increases gentamicin uptake through disrupting ΔpH and increasing membrane permeability
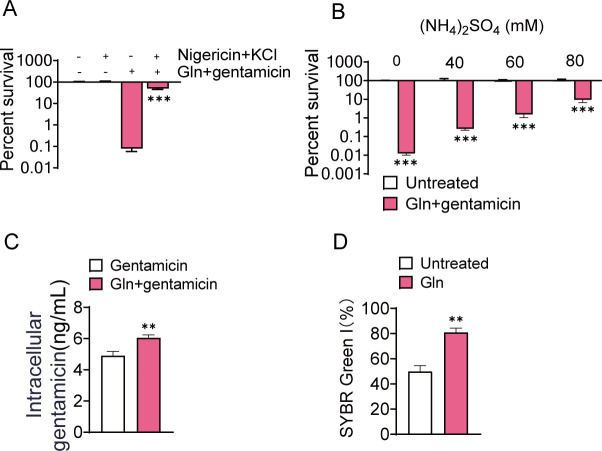
It has been reported that exogenous metabolites could improve antibiotic activity through altering pH gradient (ΔpH) (26, 27). For example, nigericin/excess K+ decreased the intracellular pH, which was responsible for the loss of potentiating activity for sodium acetate in the combination ceftazidime against Pseudomonas aeruginosa biofilms (27). We found that nigericin/excess K+ abolished the synergistic effect of L-glutamine and gentamicin against USA300, similarly (Fig. 2A). Additionally, (NH4)2SO4 eliminated the potentiating bactericidal effect of L-glutamine and gentamicin (Fig. 2B), possibly through triggering decreases of intracellular pH (26). The results suggest that the disappearance of the synergistic effect resulted from the disrupted ΔpH by nigericin/excess K+ and (NH4)2SO4. Besides, we also observed increased gentamicin uptake by USA300 cells after L-glutamine treatment (Fig. 2C). The research showed that an alteration in ΔpH elevates gentamicin uptake (26, 28, 29), which was also demonstrated in our study. Since antibiotics absorption was attributable to alteration of the membrane permeability, we measured permeability in USA300 by SYBR Green I and demonstrated a clear increase after treatment with L-glutamine (Fig. 2D and Fig. S1). These results indicate that L-glutamine promotes gentamicin uptake via disrupting ΔpH and increasing membrane permeability.
Fig 2.
ΔpH and membrane permeability are involved in the synergistic bactericidal effect of L-glutamine and gentamicin against Gram-positive bacteria USA300. (A) Percent survival of USA300 treated with nigericin/excess K+ and/or the combined treatment with gentamicin (200 µg/mL) and L-glutamine (5 mM). (B) Percent survival of USA300 treated with different concentrations of (NH4)2SO4 and/or the combined treatment with gentamicin (200 µg/mL) and L-glutamine (5 mM). (C) Change in the intracellular gentamicin content under gentamicin treatment with or without L-glutamine. (D) Changes in the membrane permeability induced by glutamine treatment. Membrane permeability was detected by 1 × SYBR Green I. All data are displayed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, determined by one-way ANOVA.
L-glutamine-induced decreases in ROS levels are involved in the bactericidal effect
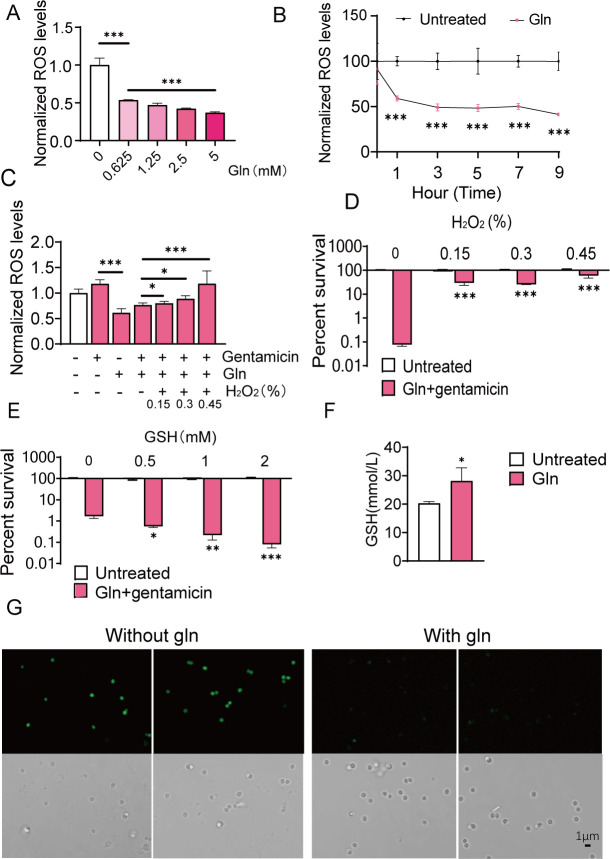
It has been proven that antibiotic effectiveness is associated with reactive oxygen species (ROS) production and exogenous metabolites have been found to influence ROS levels (22). Here, we observed that L-glutamine treatment could decrease ROS in a dose- dependent manner (Fig. 3A) and generate a lower level of ROS in the 9th hour (Fig. 3B) and the ROS fluorescence signal was significantly weakened after L-glutamine treatment by fluorescence detection (Fig. 3G). We next examined the effect of H2O2 (contributor to ROS) on the bactericidal effect of L-glutamine plus gentamicin. Addition of gentamicin alone could increase ROS levels, and H2O2 restored the ROS content with the increase of concentration when combining L-glutamine with gentamicin (Fig. 3C). Furthermore, adding a dose of H2O2 abolished the bactericidal effects of L-glutamine and gentamicin (Fig. 3D). Conversely, GSH (eliminator to ROS) could improve gentamicin and L-glutamine to kill UAS300 (Fig. 3E). In addition, more GSH was produced under L-glutamine treatment (Fig. 3F). Thus, L-glutamine-induced decreases by GSH in intracellular ROS levels contribute to gentamicin sensitivity of USA300 cells.
Fig 3.
ROS is involved in the synergistic bactericidal effect of L-glutamine and gentamicin. (A) Changes in ROS levels induced by different concentrations of L-glutamine. (B) ROS levels induced by L-glutamine for time-gradient incubation. (C) Gentamicin and/or L-glutamine (5 mM) treatments induced changes in ROS levels. (D) The role of H2O2 in the synergistic bactericidal effect of L-glutamine (5 mM) and gentamicin (200 µg/mL). (E) The role of GSH in the synergistic bactericidal effect of L-glutamine (1.25 mM) and gentamicin (200 µg/mL). (F) The level of GSH under L-glutamine (5 mM) treatments. (G) Bright-field and corresponding fluorescence images of USA300 under L-glutamine (5 mM) treatment stained by DCFH-DA. All data are displayed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, determined by one-way ANOVA.
L-glutamine improves gentamicin efficacy in vitro and in vivo
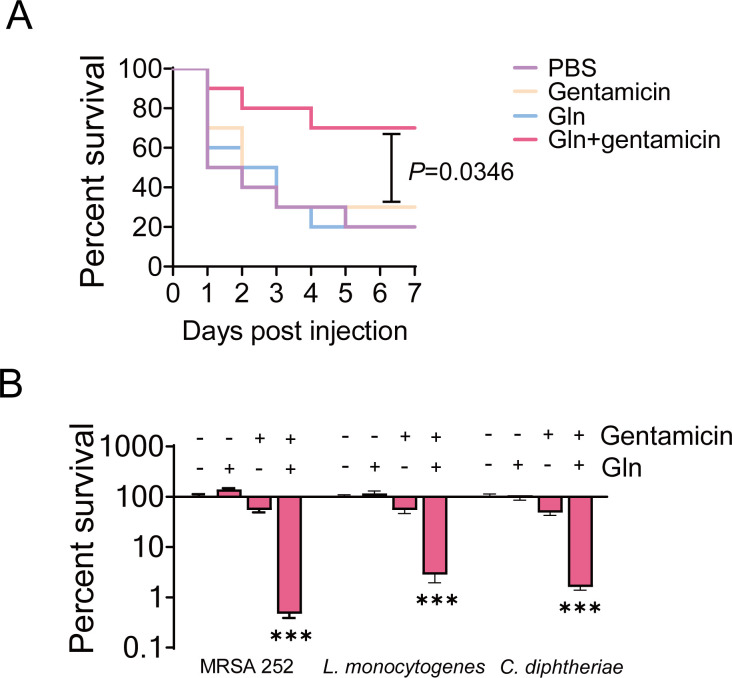
Previous studies have demonstrated the synergistic bactericidal effects of L-glutamine with antibiotics against Gram-negative bacteria in vitro. Here, we demonstrated the potentiated efficacy against other Gram-positive drug-resistant bacteria, such as MRSA 252, Corynebacterium diphtheria, and Listeria monocytogenes (Fig. 4A). Subsequently, we evaluated the synergistic effects of L-glutamine and gentamicin in vivo. First, BALB/c mice were infected with USA300 (1 × 109 CFU) via tail vein injection and then treated with a single dose of gentamicin and/or L-glutamine via intraperitoneal injection. Percent survival of MRSA-infected mice was not significantly improved after a single treatment with gentamicin or glutamine. However, combining gentamicin and L-glutamine caused mouse survival to reach 70% (Fig. 4B), which had similar good effect to the positive treatment with vancomycin (100 mg kg−1) alone (Fig. S2).These results suggest that combined treatment enhances antimicrobial activity against Gram-positive bacteria, thus improving survival rates in MRSA-infected mice.
Fig 4.
L-Glutamine improves the antimicrobial efficacy of gentamicin in vitro and in vivo. (A) The synergistic bactericidal effects of combined treatment with L-glutamine (5 mM) and gentamicin against MRSA252, L. monocytogenes, and C. diphtheriae, with gentamicin concentrations of 400 µg/ mL, 800 µg/mL, and 4 µg/mL, respectively. (B) Percent survival of MRSA-infected mouse in the presence of L-glutamine and/or gentamicin. All data are displayed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, determined by one-way ANOVA.
DISCUSSION
The synergistic bactericidal activity of metabolites and antibiotics has been widely studied (30 – 32). Metabolites, such as amino acids, are typical adjuvants for antibiotics. For example, combined alanine and gentamicin treatment elevated bactericidal efficacy against antibiotic-resistant Vibrio alginolyticus (33). Moreover, glycine, serine, and threonine potentiated kanamycin-mediated killing of Edwardsiella piscicida (17). Recently, glutamine promoted ampicillin to eliminate multidrug-resistant uropathogenic E. coli (21). L-glutamine is a special amino acid with two NH4 +. Current researches of L-glutamine mainly focus on its relation with cancer, given that glutamine is the metabolic fuel supporting enhanced tumor proliferation, invasion, and bioenergetics (34, 35). However, few studies are available on the role of L-glutamine in restoring antibiotic susceptibility of Gram-positive resistant bacteria.
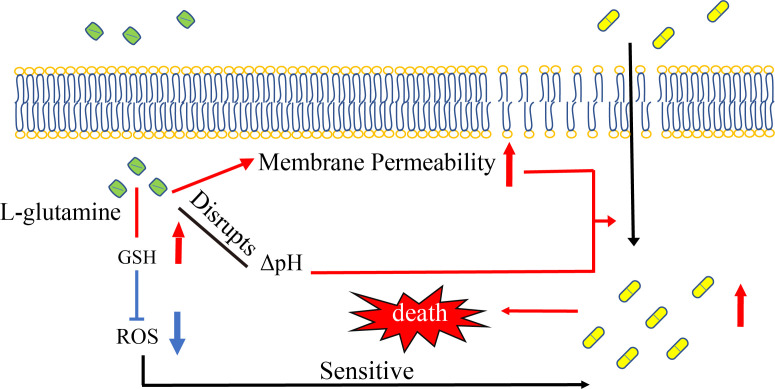
In this study, we found strong synergistic bactericidal effects of gentamicin and L-glutamine against MRSA. The combined treatment altered ΔpH, cell membrane permeability, and ROS levels (Fig. 5). Multiple studies have demonstrated that pH plays an important role in bacterial resistance. For instance, ΔpH mediated aminoglycoside uptake (36), and alkaline conditions increased antibiotic efficacy (37). Thus, pH imbalance may contribute to antibiotic resistance via inducing changes in the antibiotic efflux systems and proton motive force (28, 38, 39). Nigericin/excess K+ or (NH4)2SO4 was used to disrupt ΔpH. Besides, our findings also revealed that exogenous L-glutamine increased USA300 cell membrane permeability. Similarly, previous studies have reported that L-lysine improves membrane permeability of bacterial species such as Acinetobacter baumannii, E. coli, and Klebsiella pneumoniae (26). The results showed ΔpH and membrane permeability did influence the synergistic effect of L-glutamine and gentamicin. Therefore, L-glutamine induced a change of ΔpH and an increase of cell membrane permeability elevated gentamicin uptake, resulting in greater bactericidal effect.
Fig 5.
Proposed mechanism involved in the synergistic bactericidal effects of L-glutamine and gentamicin against MRSA. The treatment of L-glutamine disrupted the ΔpH and increased cell membrane permeability, which boosted the uptake of gentamicin and is resultant of bacterial death. Meanwhile, L-glutamine treatment decreased ROS levels by GSH, which promoted the sensitivity of MRSA to gentamicin.
Surprisingly, the presence of L-glutamine eliminated a portion of gentamicin-induced ROS. L-glutamine is an important biosynthetic component of some antioxidants, such as glutathione (40). Our results showed glutathione was increased which metabolized from L-glutamine. In fact, the role of ROS in antibiotic tolerance and resistance is complex, and there are some controversies and challenges (41, 42), being deleterious or beneficial depending on the conditions (43 – 45). For example, ROS induced by non-lethal antibiotics could contribute to the development of antibiotic resistance (46 – 48). Moreover, an increase in ROS levels lowered MRSA sensitivity to Amomum villosum Lour essential oil (49). Another study found that N-acetylcysteine lowered wild-type mutagenesis through decreasing intracellular ROS levels, in turn hampering the development of antibiotic resistance (50). These studies suggest that limiting intracellular ROS levels helps suppress antibiotic resistance in pathogenic bacteria. Our study found that L-glutamine treatment decreased ROS levels implying that the metabolite contributes to the synergistic bactericidal activity of L-glutamine and gentamicin.
In conclusion, L-glutamine increased gentamicin efficacy against Gram-positive bacteria and improved the survival rate of mice infected with MRSA. Further mechanistic studies indicated that L-glutamine enhanced aminoglycoside uptake through disrupting ΔpH, increasing cell membrane permeability and decreasing ROS. However, the influence of L-glutamine on ROS requires further research. Our findings suggest that L-glutamine had antioxidant characteristics that benefited the synergistic bactericidal activity of L-glutamine and gentamicin.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemical agents
Methicillin-resistant S. aureus USA300_FPR3757 was kindly provided by Dr. Hua Zhou, from Zhejiang University. MRSA 252, Listeria monocytogenes, and Corynebacterium diphtheriae were kindly provided by Dr. Haixin Chen, from Shenzhen Bay Lab. These strains were cultured in 30 mL Luria-Bertani (LB) broth (HuanKai Microbiology Technology Co. Ltd., Guangdong, China) at 37°C and 220 rpm for 12 h. L-Glutamine and gentamicin (Aladdin, China), other antibiotics (Macklin, China), Live & Dead Bacterial Staining Kit (YESEN, China), 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Sigma, USA), nigericin (APExBIO), NH4Cl and (NH4)2SO4 (Macklin, China), SYBR Green I (Biosharp, China), and glutathione (GSH) (Biosharp, China) were used in this study.
Bactericidal study
Overnight bacterial cultures were collected and adjusted to an optical density of 0.2 at 600 nm (OD600) with M9 medium (containing 10 mM acetate, 1 mM MgSO4, and 100 µM CaCl2) and incubated in a constant-temperature incubator at 37°C and 220 rpm for 6 h. Bacteria were treated with L-glutamine, antibiotics, and/or other chemical agents in the M9 medium. Finally, 100 µL of the culture was serially diluted. An aliquot (10 µL) of each dilution was plated onto LB agar to determine the bacterial count and calculated CFU/mL (14).
Assessment of drug resistance development
Based on the method reported by Zhao et al. (21), bacteria were cultured in LB medium overnight, collected by centrifugation at 8,000 rpm for 5 min, and washed three times with sterile saline. The bacteria were resuspended in M9 medium and diluted to OD600 = 1.0. Next, 100 µL of the bacterial suspension (5 × 107 CFU) was added to each well of a 96-well microplate. Additionally, 100 µL of a series of twofold dilutions of gentamicin or gentamicin plus 5 mM glutamine diluted in M9 medium was added to each well. The mixture was then incubated at 37°C. The minimum inhibitory concentration was determined after 16 h based on the lowest concentration of antimicrobial agent that visually inhibited the growth of microorganisms. The surviving bacteria on LB agar were recultured, and the MIC was determined repeatedly for 20 generations. Three biological replicates were used for each experiment.
Measurement of ROS
The intracellular ROS in the USA300 strain was measured using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma, USA) (51). Overnight bacterial cultures were collected and adjusted to OD600 = 0.6 with M9 medium and incubated in a constant-temperature incubator at 37°C and 220 rpm for 6 h with gentamicin and/or L-glutamine. A mixture containing 194 µL of bacterial cultures and 4 µL of 2′,7′-dichlorodihydrofluorescein diacetate (200 µL, final concentration 20 µM) was incubated in microplates at 37°C for 1 h in the dark. Fluorescence was immediately measured at an excitation wavelength of 485 nm and emission wavelength of 515 nm using a plate reader (CLARIO Star Plus, Germany).
Laser scanning confocal microscopy was used to determine the fluorescence of DCFH-DA. Overnight bacterial cultures were collected and adjusted to OD600 = 0.2 with M9 medium and incubated in a constant-temperature incubator at 37°C and 220 rpm for 6 h with or without L-glutamine. A mixture including 98 µL of bacterial cultures and 2 µL of 2′,7′-dichlorodihydrofluorescein diacetate (100 µL, final concentration 20 µM) was incubated in microplates at 37°C for 1 h in the dark and observed using 35-mm laser scanning confocal microscopy at the wavelength of 488 nm (OLYMPUS, Japan).
Measurement of GSH
Overnight bacterial cultures were collected and adjusted to OD600 = 1.0 with M9 medium and incubated in a constant-temperature incubator at 37°C and 220 rpm for 6 h with or without L-glutamine. A total of 10 mL of the cultures was collected to assess the concentration of GSH using a GSH test kit (Nanjing Jiancheng Bioengineering Institute, China). All tests were performed in duplicate.
Measurement of membrane permeability
Overnight bacterial cultures were collected and adjusted to OD600 = 0.2 with M9 medium and incubated in a constant-temperature incubator at 37°C and 220 rpm for 6 h with or without L-glutamine. A total of 100 µL of the culture was obtained and diluted 10-fold and then stained with 1 × SYBR Green I for 15 min. Green (FTIC: absorption wavelength/emission wavelength = 488 nm/520 nm) fluorescence intensity was determined using a flow cytometer (the total number of cells was up to 10,000), and the threshold of FSC and SSC was 8,000 (Beckman Coulter, Brea, CA, USA) (52). All tests were performed in duplicate, and the positivity rate of the stained bacteria was evaluated.
Determination of intracellular gentamicin concentration
Overnight bacterial cultures were collected and adjusted to OD600 = 1.0 with M9 medium and incubated in a constant-temperature incubator at 37°C and 220 rpm for 6 h with or without L-glutamine. Bacterial cultures were harvested, washed three times with PBS, and then lysed by sonication (200 W total power with 60% output, 2 s pulse, and 3 s pause) for 40 min on ice. The supernatant was collected by centrifugation at 12,000 rpm for 10 min at 4°C. Finally, gentamicin concentration was assessed using a gentamicin ELISA rapid test kit (Cusabio, Hubei, China) (53).
In vivo synergy in the mouse infection model
To evaluate the efficacy of the drugs against MRSA strains in vivo, BALB/c mice were used. Overnight bacterial cultures were washed, collected by centrifugation at 8,000 rpm for 5 min, and adjusted to a final density of 1 × 109 CFU/mL with PBS. After 1 week of adaptation, BALB/c mice (8–10 weeks) were inoculated with a total of 100 µL 1 × 109 CFU/mL MRSA (USA300) bacterial suspension in PBS (20 g/mouse) by tail vein injection. After 6 h, mice were randomly divided into five groups (n = 10 per group) and intraperitoneally injected with 200 µL PBS (200 µL), gentamicin (5 mg kg−1), glutamine (100 mg kg−1), gentamicin (5 mg kg−1) + glutamine (100 mg kg−1), or vancomycin (100 mg kg−1) twice a day for a week. The mice were observed, and the observations were recorded daily. The log-rank test was used to analyze the survival of mice.
ACKNOWLEDGMENTS
This research was funded by the National Natural Science Foundation of China projects (42076095), Natural Science Foundation of Xiamen, China (3502Z20227233), and Natural Science Foundation of Guangdong grant (2022A1515010408).
Y.B.S. and G.Z.Y. conceptualized and designed the project. L.Y.F., Y.B.S., and G.Z.Y. wrote the manuscript. Y.B.S., G.Z.Y., and L.Y.F. interpreted the data. L.Y.F., Z.Y.P., Y.L.Z., J.G., X.L., R.P., and Q.Q.X. performed the experiments and the data analysis.
The authors declare no competing interests.
Contributor Information
Guozhu Ye, Email: gzye@iue.ac.cn.
Yubin Su, Email: suyb7@jnu.edu.cn.
Ayush Kumar, University of Manitoba, Winnipeg, Manitoba, Canada .
DATA AVAILABILITY
Data will be made available upon request.
ETHICS APPROVAL
Animal infection experiments were approved by Institutional Animal Care and Use Committee at the Guangdong Institute of Microbiology (Animal Welfare Assurance Number GT-IACUC201907031).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01619-23.
Legends to Fig. S1 and S2.
Fig. S1 (Changes in the membrane permeability induced by L-glutamine treatment).
Fig. S2 (Percent survival of MRSA-infected mouse in the presence of vancomycin [100 mg/kg]).
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Boucher H, Miller LG, Razonable RR. 2010. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 51 Suppl 2:S183–97. doi: 10.1086/653519 [DOI] [PubMed] [Google Scholar]
- 2. Gherardi G. 2023. Staphylococcus aureusinfection: pathogenesis and antimicrobial resistance. Int J Mol Sci 24:8182. doi: 10.3390/ijms24098182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, Harbarth S. 2018. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers 4:18033. doi: 10.1038/nrdp.2018.33 [DOI] [PubMed] [Google Scholar]
- 4. Vestergaard M, Frees D, Ingmer H. 2019. Antibiotic resistance and the MRSA problem. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maraolo AE, Giaccone A, Gentile I, Saracino A, Bavaro DF. 2021. Daptomycin versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bloodstream infection with or without endocarditis: a systematic review and meta-analysis. Antibiotics (Basel) 10:1014. doi: 10.3390/antibiotics10081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boot W, Schmid T, D’Este M, Guillaume O, Foster A, Decosterd L, Richards RG, Eglin D, Zeiter S, Moriarty TF. 2021. A hyaluronic acid hydrogel loaded with gentamicin and vancomycin successfully eradicates chronic methicillin-resistant Staphylococcus aureus orthopedic infection in a sheep model. Antimicrob Agents Chemother 65:e01840-20. doi: 10.1128/AAC.01840-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, Holland TL, Fowler VG. 2019. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 17:203–218. doi: 10.1038/s41579-018-0147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson J, Lo J, Hsu E, Burt HM, Shademani A, Lange D. 2021. The combined use of gentamicin and silver nitrate in bone cement for a synergistic and extended antibiotic action against gram-positive and gram-negative bacteria. Materials 14:3413. doi: 10.3390/ma14123413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright GD. 2016. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol 24:862–871. doi: 10.1016/j.tim.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 10. Kussmann M, Obermueller M, Karer M, Kriz R, Chen RY, Hohl L, Schneider L, Burgmann H, Traby L, Vossen MG. 2021. Synergistic effect of cefazolin plus fosfomycin against Staphylococcus aureusin vitro and in vivo in an experimental Galleria mellonella model. Front. Pharmacol 12. doi: 10.3389/fphar.2021.685807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samura M, Kitahiro Y, Tashiro S, Moriyama H, Hamamura Y, Takahata I, Kawabe R, Enoki Y, Taguchi K, Takesue Y, Matsumoto K. 2022. Efficacy and safety of daptomycin versus vancomycin for bacteremia caused by methicillin-resistant Staphylococcus aureus with vancomycin minimum inhibitory concentration > 1 mg/mL: a systematic review and meta-analysis. Pharmaceutics 14:714. doi: 10.3390/pharmaceutics14040714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown NM, Goodman AL, Horner C, Jenkins A, Brown EM. 2021. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): updated guidelines from the UK. JAC Antimicrob Resist 3:dlaa114. doi: 10.1093/jacamr/dlaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pritchard JR, Lauffenburger DA, Hemann MT. 2012. Understanding resistance to combination chemotherapy. Drug Resist Updat 15:249–257. doi: 10.1016/j.drup.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng B, Su YB, Li H, Han Y, Guo C, Tian YM, Peng XX. 2015. Alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab 21:249–262. doi: 10.1016/j.cmet.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 15. Stokes JM, Lopatkin AJ, Lobritz MA, Collins JJ. 2019. Bacterial metabolism and antibiotic efficacy. Cell Metab 30:251–259. doi: 10.1016/j.cmet.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su YB, Peng B, Han Y, Li H, Peng X. 2015. Fructose restores susceptibility of multidrug-resistant Edwardsiella tarda to kanamycin. J Proteome Res 14:1612–1620. doi: 10.1021/pr501285f [DOI] [PubMed] [Google Scholar]
- 17. Ye JZ, Lin XM, Cheng ZX, Su YB, Li WX, Ali FM, Zheng J, Peng B. 2018. Identification and efficacy of glycine serine and threonine metabolism in potentiating kanamycin-mediated killing of Edwardsiella piscicida. J Proteomics 183:34–44. doi: 10.1016/j.jprot.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 18. Su YB, Peng B, Li H, Cheng ZX, Zhang TT, Zhu JX, Li D, Li MY, Ye JZ, Du CC, Zhang S, Zhao XL, Yang MJ, Peng XX. 2018. Pyruvate cycle increases aminoglycoside efficacy and provides respiratory energy in bacteria. Proc Natl Acad Sci U S A 115:E1578–E1587. doi: 10.1073/pnas.1714645115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan LY, Pan ZY, Liao X, Zhong YL, Guo J, Pang R, Chen XH, Ye GZ, Su YB. 2023. Uracil restores susceptibility of methicillin-resistant Staphylococcus aureus to aminoglycosides through metabolic reprogramming. Front Pharmacol 14:1133685. doi: 10.3389/fphar.2023.1133685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ming J, Su Y, Ye J, Li H, Kuang S, Wu J, Li S, Peng X, Peng B. 2023. Ampicillin-controlled glucose metabolism manipulates the transition from tolerance to resistance in bacteria. Sci Adv 9. doi: 10.1126/sciadv.ade8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao XL, Chen ZG, Yang TC, Jiang M, Wang J, Cheng ZX, Yang MJ, Zhu JX, Zhang TT, Li H, Peng B, Peng XX. 2021. Glutamine promotes antibiotic uptake to kill multidrug-resistant uropathogenic bacteria. Sci Transl Med 13:eabj0716. doi: 10.1126/scitranslmed.abj0716 [DOI] [PubMed] [Google Scholar]
- 22. Ye JZ, Su YB, Lin XM, Lai SS, Li WX, Ali F, Zheng J, Peng B. 2018. Alanine enhances aminoglycosides-induced ROS production as revealed by Proteomic analysis. Front Microbiol 9:29. doi: 10.3389/fmicb.2018.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen YT, Ma YM, Peng XX, Li H. 2022. Glutamine potentiates gentamicin to kill lab-evolved gentamicin-resistant and clinically isolated multidrug-resistant Escherichia coli. Front. Microbiol 13. doi: 10.3389/fmicb.2022.1071278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen XW, Wu JH, Liu YL, Munang’andu HM, Peng B. 2023. Fructose promotes ampicillin killing of antibiotic-resistant Streptococcus agalactiae. Virulence 14:2180938. doi: 10.1080/21505594.2023.2180938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang X, Duan XK, Li J, Niu JJ, Yuan SQ, Wang XY, Lambert N, Li X, Xu J, Gong Z, Yan S, Xie L, Xie J. 2018. The synergistic effect of exogenous glutamine and Rifampicin against Mycobacterium Persisters. Front Microbiol 9:1625. doi: 10.3389/fmicb.2018.01625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng WY, Fu TW, Zhang Z, Jiang X, Xie JP, Sun H, Hu P, Ren H, Zhou P, Liu Q, Long QX. 2020. L-lysine potentiates aminoglycosides against Acinetobacter baumannii via regulation of proton motive force and antibiotics uptake . Emerging Microbes & Infections 9:639–650. doi: 10.1080/22221751.2020.1740611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bao X, Bové M, Coenye T. 2022. Organic acids and their salts potentiate the activity of selected antibiotics against Pseudomonas aeruginosa biofilms grown in a synthetic cystic fibrosis sputum medium. Antimicrob Agents Chemother 66. doi: 10.1128/AAC.01875-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lebeaux D, Chauhan A, Létoffé S, Fischer F, de Reuse H, Beloin C, Ghigo J-M. 2014. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J Infect Dis 210:1357–1366. doi: 10.1093/infdis/jiu286 [DOI] [PubMed] [Google Scholar]
- 29. Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meylan S, Andrews IW, Collins JJ. 2018. Targeting antibiotic tolerance, pathogen by pathogen. Cell 172:1228–1238. doi: 10.1016/j.cell.2018.01.037 [DOI] [PubMed] [Google Scholar]
- 31. Li ZY, Sun FQ, Fu XM, Chen YJ. 2022. 5-Methylindole kills various bacterial pathogens and potentiates aminoglycoside against methicillin-resistant Staphylococcus aureus. PeerJ 10:e14010. doi: 10.7717/peerj.14010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park SY, Lee JH, Kim YG, Hu LB, Lee J. 2022. Fatty acids as aminoglycoside antibiotic adjuvants against Staphylococcus aureus. Front Microbiol 13:876932. doi: 10.3389/fmicb.2022.876932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuang SF, Chen YT, Chen JJ, Peng XX, Chen ZG, Li H. 2021. Synergy of alanine and gentamicin to reduce nitric oxide for elevating killing efficacy to antibiotic-resistant Vibrio alginolyticus. Virulence 12:1737–1753. doi: 10.1080/21505594.2021.1947447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang L, Moss T, Mangala LS, Marini J, Zhao H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, Roopaimoole R, Rodriguez-Aguayo C, Mercado-Uribe I, Lopez-Berestein G, Liu JS, Tsukamoto T, Sood AK, Ram PT, Nagrath D. 2014. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol 10:728. doi: 10.1002/msb.20134892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cha YJ, Kim ES, Koo JS. 2018. Amino acid transporters and glutamine metabolism in breast cancer. Int J Mol Sci 19:907. doi: 10.3390/ijms19030907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mates SM, Eisenberg ES, Mandel LJ, Patel L, Kaback HR, Miller MH. 1982. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A 79:6693–6697. doi: 10.1073/pnas.79.21.6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sabath LD, Toftegaard I. 1974. Rapid microassays for clindamycin and gentamicin when present together and the effect of pH and of each on the antibacterial activity of the other. Antimicrob Agents Chemother 6:54–59. doi: 10.1128/AAC.6.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hillman T. n.d. Antibiotics, efflux, and pH. Arch Proteom Bioinform 3:15–18. doi: 10.33696/Proteomics.3.013 [DOI] [Google Scholar]
- 39. Le D, Krasnopeeva E, Sinjab F, Pilizota T, Kim M, Cooper VS. 2021. Active efflux leads to heterogeneous dissipation of proton motive force by protonophores in bacteria. mBio 12:e0067621. doi: 10.1128/mBio.00676-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fläring UB, Rooyackers OE, Wernerman J, Hammarqvist F. 2003. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci (Lond) 104:275–282. doi: 10.1042/CS20020198 [DOI] [PubMed] [Google Scholar]
- 41. Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. 2013. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339:1213–1216. doi: 10.1126/science.1232688 [DOI] [PubMed] [Google Scholar]
- 42. Imlay JA. 2015. Diagnosing oxidative stress in bacteria: not as easy as you might think. Curr Opin Microbiol 24:124–131. doi: 10.1016/j.mib.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Y, Vulić M, Keren I, Lewis K. 2012. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56:4922–4926. doi: 10.1128/AAC.00921-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mosel M, Li L, Drlica K, Zhao XL. 2013. Superoxide-mediated protection of Escherichia coli from antimicrobials. Antimicrob Agents Chemother 57:5755–5759. doi: 10.1128/AAC.00754-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li H, Zhou XD, Huang YY, Liao BY, Cheng L, Ren B. 2021. Corrigendum: reactive oxygen species in pathogen clearance: the killing mechanisms, the adaption response, and the side effects. Front Microbiol 12:685133. doi: 10.3389/fmicb.2021.685133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blanchard JL, Wholey W-Y, Conlon EM, Pomposiello PJ. 2007. Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks. PLoS One 2:e1186. doi: 10.1371/journal.pone.0001186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kohanski MA, DePristo MA, Collins JJ. 2010. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell 37:311–320. doi: 10.1016/j.molcel.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takahashi N, Gruber CC, Yang JH, Liu X, Braff D, Yashaswini CN, Bhubhanil S, Furuta Y, Andreescu S, Collins JJ, Walker GC. 2017. Lethality of MalE-LacZ hybrid protein shares mechanistic attributes with oxidative component of antibiotic lethality. Proc Natl Acad Sci U S A 114:9164–9169. doi: 10.1073/pnas.1707466114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang CL, Chen JL, Zhou Y, Ding P, He GZ, Zhang LX, Zhao ZM, Yang DP. 2021. Exploring antimicrobial mechanism of essential oil of Amomum villosum lour through metabolomics based on gas chromatography-mass spectrometry in methicillin-resistant Staphylococcus aureus. Microbiological Research 242:126608. doi: 10.1016/j.micres.2020.126608 [DOI] [PubMed] [Google Scholar]
- 50. Rodríguez-Rosado AI, Valencia EY, Rodríguez-Rojas A, Costas C, Galhardo RS, Rodríguez-Beltrán J, Blázquez J. 2019. N-acetylcysteine blocks SOS induction and mutagenesis produced by fluoroquinolones in Escherichia coli. J Antimicrob Chemother 74:2188–2196. doi: 10.1093/jac/dkz210 [DOI] [PubMed] [Google Scholar]
- 51. Kou TS, Wu JH, Chen XW, Chen ZG, Zheng J, Peng B. 2022. Exogenous glycine promotes oxidation of glutathione and restores sensitivity of bacterial pathogens to serum-induced cell death. Redox Biol 58:102512. doi: 10.1016/j.redox.2022.102512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen JZ, Wang XY, Wang SY, Chen C, Zhang WH, Zhang Y. 2021. Ultra-rapid drug susceptibility testing for Klebsiella pneumoniae clinical isolates in 60 min by SYBR green I/propidium iodide viability assay. Front. Microbiol 12:694522. doi: 10.3389/fmicb.2021.694522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang S, Wang J, Jiang M, Xu D, Peng B, Peng X, Li H. 2019. Reduced redox-dependent mechanism and glucose-mediated reversal in gentamicin-resistant Vibrio alginolyticus. Environmental Microbiology 21:4724–4739. doi: 10.1111/1462-2920.14811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legends to Fig. S1 and S2.
Fig. S1 (Changes in the membrane permeability induced by L-glutamine treatment).
Fig. S2 (Percent survival of MRSA-infected mouse in the presence of vancomycin [100 mg/kg]).
Data Availability Statement
Data will be made available upon request.