ABSTRACT
Due to the increasing incidence of antibiotic-resistant bacterial pathogens, the need for the development of alternative compounds is of utmost importance. Bacteriocins are considered a promising alternative to antibiotics. In this study, we determined the in vitro activities of 65 bacteriocins from the PARAGEN collection along with microbisporicin (NAI-107) against 14 WHO reference isolates of Neisseria gonorrhoeae, one isolate of Neisseria meningitidis, and four commensal Neisseria spp. The initial screening of the bacteriocins was carried out using a spot-on-lawn assay wherein 12 bacteriocins (12/66) showed a zone of inhibition against the WHO-P N. gonorrhoeae isolate. Four bacteriocins (4/12), Garvicin KS (GarKS) (ABC), lacticin (lcn) Q, lcn Z, and NAI-107, demonstrated higher activity, and minimum inhibitory concentrations (MICs) using agar dilutions were determined for 19 Neisseria spp. GarKS (ABC), lcn Q, and lcn Z had an MIC range of 4–16 µg/mL, whereas NAI-107 had an MIC range of <0.25 to 2 µg/mL. Using an in vivo Galleria mellonella model, we determined the efficacy of GarKS (ABC), lcn Z, and NAI-107. GarKS (ABC) and lcn Z displayed no in vivo toxicity but showed no in vivo activity against WHO-P N. gonorrhoeae. In contrast, NAI-107 was as effective as ceftriaxone and significantly increased the survival rate of WHO-P N. gonorrhoeae-infected G. mellonella larvae. In conclusion, NAI-107 exhibited potent in vitro and in vivo activities against N. gonorrhoeae. To our knowledge, this is the first study to demonstrate that NAI-107 possesses anti-gonococcal activity in vivo.
IMPORTANCE
We screened 66 bacteriocins to see if they exhibited anti-gonococcal activity. We found 12 bacteriocins with anti-gonococcal effects, and 4 bacteriocins showed higher anti-gonococcal activity. Three bacteriocins, lacticin Z, lacticin Q, and Garvicin KS (ABC), showed in vitro anti-gonococcal activity but no in vivo inhibitory effects against the Neisseria gonorrhoeae (WHO-P) isolate. On the other hand, NAI-107 showed in vivo anti-gonococcal activity. The findings suggest that NAI-107 is a promising alternative to treat gonorrhea infections.
KEYWORDS: Neisseria gonorrhoeae, lantibiotics, NAI-107, microbisporicin, Galleria mellonella
INTRODUCTION
The emergence, spread, and persistence of multidrug-resistant (MDR) bacteria pose a global threat of growing concern to human, animal, and environmental health. The scarcity of new antibacterial drugs in the development pipeline exacerbates the serious threat to public health posed by the spread of antibiotic resistance, which is forecast to reach dramatic levels by 2050 (1 – 4). The majority of antibiotics used in clinical practice today are microbial metabolites or their derivatives, and alternative approaches to antibiotic discovery have not been as successful as microbial product screening (5, 6). The increasing incidence of antibiotic-resistant bacterial pathogens highlights the urgent need for new antimicrobial agents, especially those with new modes of action (2).
Gonorrhea caused by the pathogen Neisseria gonorrhoeae is the second most common bacterial sexually transmitted infection (STI). In 2020, the World Health Organization (WHO) estimated that 82.4 million new gonorrhea cases occurred in adults globally (7). Complications of gonorrhea include pelvic inflammatory disease, infertility, and ectopic pregnancy in women, epididymal orchitis in men, neonatal ophthalmia, and an elevated risk for HIV acquisition and transmission (8 – 11). The increased emergence of antimicrobial resistance (AMR) in N. gonorrhoeae has heightened the need for new first-line antimicrobials for the treatment of gonorrhea (11).
Bacteriocins are antimicrobial peptides produced by bacteria with either a broad or narrow spectrum of antibacterial activity (12 – 14). According to the Cotter et al. scheme, bacteriocins are classified into lanthionine (class I) and non-lanthionine-containing (class II) bacteriocins (15). Lantibiotics (class I) are ribosomally synthesized, post-translationally modified peptides (RiPPs) (16). They are small (<5 kDa) peptides and are synthesized as a precursor peptide, lanthionine (LanA), with an N-terminal leader peptide (NP) and a C-terminal core peptide (CP) (17). They undergo post-translational modifications (PTMs) with the formation of 3-methyllanthionine (MeLan), dehydroalanine (Dha), and/or dehydrobutyrine (Dhb) residues that occur via the dehydration of serine and threonine residues, respectively, which are then crosslinked via a thioether linkage with cysteine residues and a number of dehydrated amino acids that gives lantibiotics their characteristic conformation and stability (15 – 22). Examples include microbisporicin (NAI-107) produced by the actinomycete Microbispora corallina ATCC-PTA-5024 (23 – 28) and nisin Z from Lactococcus lactis IO-1 (29). Class II bacteriocins are the most frequent naturally occurring bacteriocins. They are small (<10 kDa), are heat-stable, and, unlike lantibiotics, do not undergo extensive post-translational modification. Examples include lacticin Z (lcn Z) and lacticin Q (lcn Q) produced by L. lactis QU 14 and QU 5, respectively (30, 31), and Garvicin KS (GarKS) produced by Lactococcus garvieae KS1546 (32).
Bacteriocins have gained considerable attention as potential alternatives to traditional antibiotics due to their narrow-spectrum activity and a lower likelihood of inducing resistance (12 – 14, 33). Most leaderless bacteriocins, such as lacticin Q (53 aa), lacticin Z (53 aa), and Garvicin KS [composed of three peptides (GakA, GakB, and GakC, 30–34 aa)], have been shown to have inhibitory effects against a range of genera, such as Bacillus, Listeria, Staphylococcus, and Enterococcus (32, 34). NAI-107 has been found to be active against a wide range of MDR Gram-positive bacterial pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), glycopeptide-intermediate-resistant S. aureus (GISA), vancomycin-resistant enterococci (VRE), penicillin-resistant Streptococcus pneumoniae, and a selected range of Gram-negative bacteria—N. gonorrhoeae and Moraxella spp. (24, 33, 35). .
Galleria mellonella larvae have been previously used as a model host for studying various pathogens and the efficacy of various antimicrobial agents (36 – 43). This system provides an alternative in vivo model to mammalian systems, and its immune system involves both cellular (phagocytosis, nodulization, and encapsulation) and humoral defenses (melanization, hemolymph clotting, and production of antimicrobial peptides) and shares a high degree of structural and functional similarity with that of mammals (36).
The in vitro antimicrobial activity of bacteriocins against relevant pathogenic bacteria (including MDR pathogens) has been well documented (44 – 47). However, the use of in vivo studies is an important step forward for the development of new therapeutic agents (48). The present study investigated the in vitro antibacterial activity of lantibiotics (n = 66) and the in vivo antibacterial activity of three bacteriocins against WHO-P Neisseria gonorrhoeae using the wax moth larva G. mellonella. The results highlight the potential of bacteriocins as alternative therapeutic agents for treating N. gonorrhoeae infections.
MATERIALS AND METHODS
Bacterial isolates
The following isolates were used in the present study: N. gonorrhoeae WHO reference isolates (n = 14), N. meningitidis (n = 1, ITM 3354), Neisseria mucosa (n = 1, ATCC 25999), Neisseria subflava (ITM 3367), Neisseria cinerea (ATCC 14685), Neisseria lactamica (n = 1, ATCC 23970), and L. lactis subsp. lactis IL1403 (n = 1) (Table 2). In Belgium, resistance to gonococcal azithromycin has increased from 0.2% to 18.6% over the past 8 years, despite the use of dual therapy, i.e., ceftriaxone and azithromycin (49). Therefore, in this study, the WHO-P N. gonorrhoeae isolate was used in the initial in vitro screening and in vivo experiments due to its susceptibility to ceftriaxone (0.004 µg/mL) and resistance to azithromycin (4 µg/mL), as determined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria (50).
In vitro studies
Source of bacteriocins and determination of the anti-gonococcal activity of bacteriocins
Sixty-five bacteriocins from the PARAGEN synthetic DNA library collection from the Belgian start-up Syngulon (51) and NAI-107 (AdipoGen Life Sciences) were used in the study. The stock of each bacteriocin from PARAGEN was prepared in molecular biology-grade water (AccuGENE). NAI-107 was dissolved in 15% dimethyl sulfoxide (DMSO). The bacteriocins were stored at −20°C until further use. The antibacterial activities of the bacteriocins against the azithromycin-resistant WHO-P N. gonorrhoeae isolate were tested by spot-on-lawn assay. Briefly, the direct colony suspension method was used to grow the WHO-P isolate, wherein the inoculum was prepared using phosphate-buffered saline (PBS) from isolated colonies grown overnight on a gonococcal (GC) [containing 15 g/L Bacto protease peptone, 1 g/L soluble starch, 4 g/L K2HPO4 (174.18 g/mol), 1 g/L KH2PO4 (136.08 g/mol), and 5 g/L NaCl (58.44 g/mol), supplemented with 1% BD BBL IsoVitaleX] agar plate. The turbidity of the suspension was adjusted to a 0.5 McFarland standard. Specifically, 100 µL of the inoculum suspension was added to 5 mL of GC soft agar, mixed, and overlaid on the surface of the GC agar plate and allowed to solidify. Two microliters of the bacteriocin was spotted on the surface. Plates were incubated in a 5% CO2 incubator at 37 o C for 24 hours. The antimicrobial activity was evaluated based on the zone of inhibition (ZOI). Based on the size of the ZOI, a select number of antimicrobial bacteriocins were used to determine the minimum inhibitory concentration (MIC).
Minimum inhibitory concentrations of lacticin Z, lacticin Q, GarKS ABC, and NAI-107 against Neisseria species
The MICs were determined using the agar dilution method based on the Clinical and Laboratory Standards Institute (CLSI) for the four bacteriocins (lacticin Z, lacticin Q, GarKS ABC, and NAI-107) against 19 Neisseria spp. (52). L. lactis IL1403, for which the PARAGEN collection bacteriocins showed antibacterial activity by spot assays, was used as a positive control (53).
In brief, for the agar dilution, a series of GC agar plates with 1% IsoVitaleX containing the bacteriocin to be tested in increasing concentrations in doubling dilutions (i.e., 2, 4, 8, and 16 µg mL−1 for lacticin Z, lacticin Q, and GarKS ABC and 0.25, 0.5, 1, 2, and 4 µg mL−1 for NAI-107) were prepared. The strains were suspended in Mueller-Hinton (MH) broth to equal the turbidity of a 0.5 McFarland standard [∼1 × 108 colony forming units (CFU) mL−1], and 200 µL of one-tenth dilution of the suspension was placed on the inoculum plate. The inoculum was transferred using an AQS A400 Multipoint Inoculator (the final inoculum is ∼103 CFU/spot). Blood agar and GC agar plates with no bacteriocin added served as the growth control plates. The agar plates were incubated at 37°C for 18–24 hours in 5% CO2. After incubation, the MIC was considered the lowest concentration of the tested material that inhibited the visible growth of the bacteria.
Serial passage experiments and evaluation of the potential for in vitro resistance development to NAI-107
N. gonorrhoeae WHO-P was propagated by serial passages with increasing concentrations of NAI-107 (0.25, 0.5, 1, 2, and 4 µg/mL) in quadruplicate. The selection of WHO-P was performed on GC agar plates supplemented with various concentration gradients of NAI-107 with an initial low sub-MIC concentration (one-fourth of MIC) of 0.25 µg/mL and incubated at 37°C in 5% CO2. After visible growth was attained, colonies were subcultured onto GC agar plates with twofold serial bacteriocin concentrations. The serial passage was repeated until no visible growth was seen on the GC agar plate.
In vivo studies
Co-culturing of N. gonorrhoeae with the hemolymph from G. mellonella
The N. gonorrhoeae inoculum for infection in G. mellonella was prepared as follows: the WHO-P N. gonorrhoeae isolate was cultured from frozen stocks onto BD Columbian Blood Agar with 5% sheep blood for 24 hours at 37°C with 5% (v/v) CO2. Single colonies were plated onto fresh BD chocolate blood agar plates and incubated. A loopful of the culture from the agar plates was suspended in PBS containing the hemolymph from G. mellonella larvae. The mixture was incubated at 37°C with 5% (v/v) CO2 for an hour and plated on fresh BD BBL GC-lect selective agar to enrich the growth and recovery of N. gonorrhoeae specifically. This inoculum was used to infect G. mellonella subsequently.
Determining the maximum nonlethal dose of N. gonorrhoeae
G. mellonella larvae were treated with a range of inoculating doses of N. gonorrhoeae WHO-P (1.5 × 108–1.2 × 109 CFU/mL) and observed for death alongside PBS controls. The maximum nonlethal dose was determined and used as the inoculating dose for infection. The nonlethal dose with 80% mortality was observed at concentrations as high as 9.0 × 108 CFU/mL and was used in the G. mellonella model.
Galleria mellonella infection model
Injection of G. mellonella was carried out as described in reference (43). The last larval stage of G. mellonella (Terramania, Arnhem, Netherlands) was used for the experiments. Only macroscopically healthy, non-discolored larvae were selected. The larvae were inoculated in the last left proleg using 0.3-mL U-100 insulin syringes (BD Micro-Fine). Two test groups were evaluated: (i) to assess the efficacy, test group 1 consisted of 20 larvae per concentration of each bacteriocin or ceftriaxone (20 mg/kg). About 30 µL of the 3.0-McFarland (2.7 × 107 CFU/mL) N. gonorrhoeae inoculum in PBS was used to infect G. mellonella larvae. The larvae were treated with 1× MIC, 2× MIC, 4× MIC, and 8× MIC of GarKS (ABC) and lacticin Z and 4× MIC, 8× MIC, and 16× MIC of NAI-107; (ii) to assess the toxicity, test group 2 with five larvae per concentration of bacteriocin was used. The efficacy test group 1 first received the N. gonorrhoeae inoculum, followed by the bacteriocin or antimicrobial, whereas the toxicity test group 2 received only the bacteriocin. One positive and two negative control groups were used. The positive control group received only the N. gonorrhoeae inoculum in PBS. Out of the two negative control groups, one group underwent no manipulation, while the other group was injected with PBS only. Each group was incubated in sterile Petri dishes at 37°C with a 5% (v/v) CO2 atmosphere for the duration of the experiments. The larvae were observed for 120 hours for any indications of illness, necrosis, or paralysis, which enabled an evaluation of the bacteriocin’s toxicity. Larvae were scored dead if they did not respond to touch stimuli by blunt sterile forceps and scored for five consecutive days (120 hours).
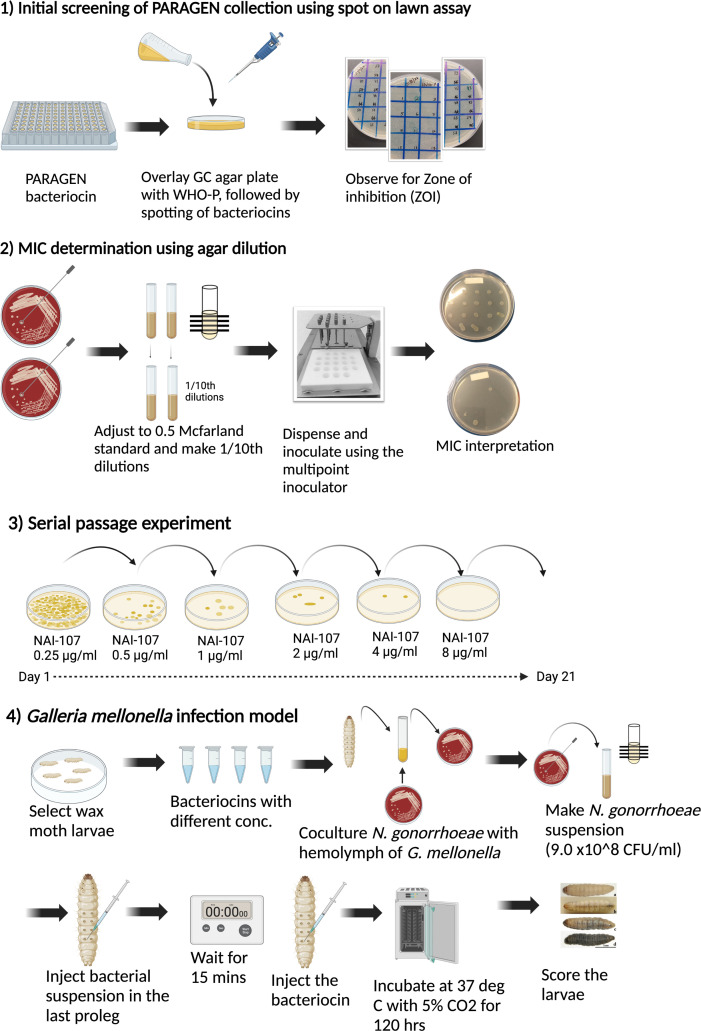
An overview of the study is provided in Fig. 1.
Fig 1.
Overview of the study. The figure was created using BioRender.
Statistical analysis
Data were analyzed using GraphPad Prism v9. Survival plots were created using Kaplan-Meier survival curves. Statistical analysis was carried out using the Mantel-Cox test to compare survival curves between the PBS controls and each treatment arm. A P value of <0.05 was considered statistically significant.
Pearson’s correlation coefficient was determined for NAI-107 vs ceftriaxone and NAI-107 vs azithromycin. A Pearson’s correlation coefficient (r) of 1 indicated a perfect direct relationship, an r value of −1 indicated a perfect inverse relationship, and an r value of 0 indicated no relationship between the two variables. Statistical analysis was performed using RStudio (2022.07.1).
RESULTS
In vitro anti-gonococcal activity of bacteriocins and MICs
Using the spot-on-lawn assay, 66 bacteriocins were screened, and 13 bacteriocins (PARAGEN collection, n = 12), including NAI-107, showed anti-gonococcal activity against the WHO-P strain. The sequences and the activity of the bacteriocins are provided in Table 1.
TABLE 1.
List of bacteriocins with anti-gonococcal activity detected in this study, their producer organisms, amino acid sequences, and in vitro activity against N. gonorrhoeae WHO-P a
| Bacteriocin | Abbreviation | Producer | Length (aa) | Amino acid sequence | Spot assay (zone of inhibition) |
|---|---|---|---|---|---|
| Garvicin KS - A | GarKS-A+B+C | Lactococcus garvieae | 34 | MGAIIKAGAKIVGKGVLGGGASWLGWNVGEKIWK | +++ |
| Garvicin KS - C | 32 | MGAIIKAGAKIVGKGALTGGGVWLAEKLFGGK | |||
| Garvicin KS - B | 34 | MGAIIKAGAKIIGKGLLGGAAGGATYGGLKKIFG | |||
| Lacticin Z | LcnZ | L. lactis | 53 | MAGFLKVVQILAKYGSKAVQWAWANKGKILDWINAGQAIDWVVEKIKQILGIK | +++ |
| Lacticin Q | LcnQ | L. lactis | 53 | MAGFLKVVQLLAKYGSKAVQWAWANKGKILDWLNAGQAIDWVVSKIKQILGIK | +++ |
| Acidocin LF221B | Acd221B | Lactobacillus gasseri | 48 | NKWGNAVIGAATGATRGVSWCRGFGPWGMTACALGGAAIGGYLGYKSN | ++ |
| Weissellicin M | WeisM | Weissella hellenica | 43 | MVSAAKVALKVGWGLVKKYYTKVMQFIGEGWSVDQIADWLKRH | ++ |
| Lacticin Z_C* | LcnZ_C* | L. lactis | 48 | MLAFLKLVAKLGPKAAKWAWANKSRVLGWIRDGMAIEWIINKINDIVN | + |
| Aureocin A53 | AurA53 | S. aureus | 51 | MSWLNFLKYIAKYGKKAVSAAWKYKGKVLEWLNVGPTLEWVWQKLKKIAGL | + |
| Enterocin 7A | Ent7A+B | Enterococcus faecium | 44 | MGAIAKLVAKFGWPIVKKYYKQIMQFIGEGWAINKIIDWIKKHI | + |
| Enterocin 7B | 43 | MGAIAKLVAKFGWPFIKKFYKQIMQFIGQGWTIDQIEKWLKRH | |||
| Enterocin L50A | EntL50A+B | E. faecium | 44 | MGAIAKLVAKFGWPIVKKYYKQIMQFIGEGWAINKIIEWIKKHI | + |
| Enterocin L50B | 43 | MGAIAKLVTKFGWPLIKKFYKQIMQFIGQGWTIDQIEKWLKRH | |||
| Mutacin BHTB | MutBHTB | Streptococcus rattus | 44 | MWGRILAFVAKYGTKAVQWAWKNKWFLLSLGEAVFDYIRSIWGG | + |
| Microbisporicin (prepropeptide) | NAI-107 | M. corallina | 57 | MPADILETRTSETEDLLDLDLSIGVEEITAGPAVTSWSLCTPGCTSPGGGSNCSFCC | +++ |
Low (+), moderate (++), and high (+++) anti-gonococcal activities of bacteriocins are shown. Bacteriocins with +++ activity were further chosen for MIC determination.
Four bacteriocins, GarKS (ABC), lacticin Q, lacticin Z, and NAI-107, showed higher activity against WHO-P, and their MICs were determined for 19 Neisseria isolates. The MIC of the four bacteriocins against these 19 isolates is provided in Table 2. NAI-107 showed an extended antimicrobial spectrum and was highly active against all N. gonorrhoeae isolates. Against all 14 tested N. gonorrhoeae isolates, the MICs ranged from 0.25 to 2 µg/mL, with no correlation with the isolate’s ceftriaxone or azithromycin susceptibility profiles. The MIC of N. meningiditis was 4 µg/mL, and the MIC range of other Neisseria spp. was from 1 to >4 µg/mL (Table 2). In contrast, the other bacteriocins, GarKS (ABC) (MIC range 4 to >16 µg/mL), lacticin Q (MIC range 16 to >16 µg/mL), and lacticin Z (MIC range 8 to >16 µg/mL), were substantially less active than NAI-107 against Neisseria species. GarKS (ABC), lacticin Q, and lacticin Z showed some activity against N. gonorrhoeae isolates but were inactive against N. gonorrhoeae WHO-N, N. meningiditis, and Neisseria commensals at the highest concentration tested (16 µg/mL) (Table 2).
TABLE 2.
Minimum inhibitory concentrations (MICs) of GarKS (ABC), lacticin Q, lacticin Z, and NAI-107 determined using the agar dilution method against 19 Neisseria spp., including L. lactis as a positive control a
| S. no. | Bacterial species | Strains | GarKS (ABC) (µg/mL) | Lacticin Q (µg/mL) | Lacticin Z (µg/mL) | NAI-107 (µg/mL) | Ceftriaxone (µg/mL) | Azithromycin (µg/mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | N. gonorrhoeae | WHO G | 8 | 16 | 8 | 1 | 0.008 | 0.25 |
| 2 | WHO K | 8 | 16 | 8 | <0.25 | 0.064 | 0.25 | |
| 3 | WHO M | 8 | 16 | 8 | 0.5 | 0.016 | 0.25 | |
| 4 | WHO L | 16 | 16 | 16 | 1 | 0.25 | 0.5 | |
| 5 | WHO N | >16 | >16 | >16 | 2 | 0.004 | 0.25 | |
| 6 | WHO O | 8 | 16 | 8 | 2 | 0.032 | 0.25 | |
| 7 | WHO P | 8 | 16 | 8 | 1 | 0.004 | 4 | |
| 8 | WHO U | 4 | 16 | 8 | 0.5 | 0.002 | 4 | |
| 9 | WHO V | 16 | 16 | 8 | 2 | 0.064 | >256 | |
| 10 | WHO W | 8 | 16 | 8 | 1 | 0.064 | 0.5 | |
| 11 | WHO F | 8 | 16 | 8 | 2 | <0.002 | 0.125 | |
| 12 | WHO X | 8 | 16 | 8 | 1 | 2 | 0.5 | |
| 13 | WHO Y | >16 | >16 | 8 | 0.5 | 1 | 1 | |
| 14 | WHO Z | 4 | 16 | 8 | 1 | 0.5 | 1 | |
| 15 | N. meningitidis | ITM-3354 | >16 | >16 | >16 | 4 | nd | nd |
| 16 | Neisseria commensals | N. lactamica | >16 | 16 | 16 | 1 | nd | nd |
| 17 | N. mucosa | >16 | >16 | 16 | >4 | nd | nd | |
| 18 | N. subflava | >16 | >16 | >16 | 4 | nd | nd | |
| 19 | N. cinerea | >16 | >16 | 16 | 2 | nd | nd | |
| 20 | L. lactis | <2 | 16 | 4 | <0.25 | nd | nd |
Ceftriaxone and azithromycin MICs have been adapted from reference (50). nd, not determined. For NAI-107 vs ceftriaxone, Pearson’s correlation (r) = −0.207, P value = 0.477, and for NAI-107 vs azithromycin, r = 0.396, P value = 0.159.
In vivo toxicity of bacteriocins in G. mellonella
GarKS (ABC), lacticin Z, and NAI-107, which were active against the N. gonorrhoeae WHO-P isolate, were chosen for the in vivo experiments. GarKS (ABC), lacticin Z, and NAI-107 administered at doses up to 64 and 16 µg/mL, respectively, did not result in the death of the larvae (Fig. 2; Fig. S1a and b).
Fig 2.
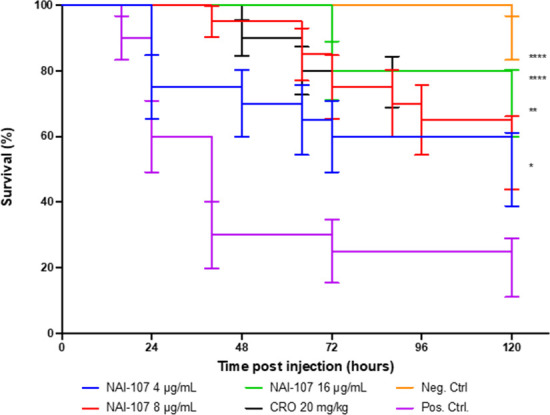
In vivo efficacy (survival) of NAI-107 and ceftriaxone (20 mg/kg) against N. gonorrhoeae, the WHO-P reference strain in G. mellonella larvae. The test groups were injected with 2.7 × 107 CFU/mL of N. gonorrhoeae, the WHO-P strain in PBS, followed by NAI-107 (doses 4, 8, and 16 µg/mL) or ceftriaxone (20 mg/kg). The negative control group was injected with 30 µL of PBS, and the positive control group was injected with 2.7 × 107 CFU/mL of N. gonorrhoeae in PBS. Both the test and control groups consisted of 20 larvae and were incubated for 120 hours at 37°C. Error bars represent the standard errors. Asterisks represent the significance levels based on the P values comparing the survival curves between the PBS-positive controls and each treatment arm (Mantel-Cox test). A single asterisk (*) denotes a P value between 0.01 and 0.09. Two (**), three (***), and four (****) asterisks represent a P value in the range of 0.001–0.009, 0.0001–0.0009, and <0.0001, respectively.
NAI-107 can protect G. mellonella larvae from infection with WHO-P N. gonorrhoeae
The in vivo efficacy of GarKS (ABC), lacticin Z, and NAI-107 was determined against N. gonorrhoeae WHO-P in comparison to ceftriaxone. Despite displaying in vitro activity against N. gonorrhoeae, the two bacteriocins, GarKS (ABC) and lacticin Z, did not demonstrate any detectable efficacy in vivo (Fig. S1a and b). NAI-107 was effective in vivo at all doses tested (P values 0.01 to <0.0001). A small dose-response effect may have been present, with mortality being lowest at the highest dose tested (16 µg/mL) and increasing stepwise at decreasing doses. At the two highest doses tested (8 and 16 µg/mL), the survival of G. mellonella was similar to that of the ceftriaxone group (Fig. 2).
Lack of in vitro resistance in N. gonorrhoeae
After subjecting N. gonorrhoeae WHO-P to four consecutive subcultures for 21 days in the presence of doubling concentrations of NAI-107, it resulted in a modest increase in the NAI-107 MIC, specifically a twofold increase (4 µg/mL).
DISCUSSION
Previously, microbisporicin (NAI-107) was reported to have in vitro efficacy against N. gonorrhoeae (33). Using a spot-on-lawn assay, we screened 65 bacteriocins from the PARAGEN collection along with NAI-107 against WHO-P N. gonorrhoeae (33, 51). Out of the 65 PARAGEN bacteriocins, a zone of inhibition was observed for 12 bacteriocins, and 3 bacteriocins showed the highest in vitro activity (Table 2). The N. gonorrhoeae MIC values of three PARAGEN bacteriocins, GarKS (ABC) (MIC range 4 to >16 µg/mL), lacticin Q (MIC range 16 to >16 µg/mL), and lacticin Z (MIC range 8 to >16 µg/mL), were higher than those for NAI-107 (MIC range <0.25 to 2 µg/mL). This MIC range was similar to that reported by the study of Brunati et al. (33), where the MIC range of N. gonorrhoeae (n = 18) isolates with intermediate or high resistance to penicillin had MICs that ranged from 0.015 to 2 µg/mL. Interestingly, there was no apparent correlation between the MIC of NAI-107 and N. gonorrhoeae’s ceftriaxone and azithromycin susceptibility profiles. In particular, the isolates with high ceftriaxone MICs had low NAI-107 MICs. This, in conjunction with its similar half-life to ceftriaxone, makes it an attractive option for combination therapy to prevent the further emergence of ceftriaxone resistance (33). Our in vivo tests in G. mellonella confirmed the therapeutic efficacy and lack of toxicity of NAI-107. Finally, the in vitro passage experiments showed only a modest increase in the NAI-107 MIC; specifically, a twofold increase in MICs was observed. The same was observed in Gram-positive organisms, such as S. aureus, and interestingly, no spontaneous resistant mutants were observed (33, 54)
In contrast to NAI-107, the two PARAGEN bacteriocins [GarKS (ABC) and lacticin Z] did not demonstrate in vivo efficacy against N. gonorrhoeae; however, they showed no in vivo toxicity. The observed disparity between the in vitro and in vivo activities of the PARAGEN bacteriocins suggests the presence of additional factors influencing their efficacy in vivo. Several possibilities warrant consideration. Little is known about the pharmacodynamics and pharmacokinetics of these bacteriocins in the G. mellonella infection model (55 – 57). It is possible, for example, that NAI-107 is the only bacteriocin assessed that is not metabolized by the host. Various modes of action have been postulated for leaderless bacteriocins. Generally, leaderless bacteriocins have been shown not to involve a receptor/docking molecule to exhibit their antimicrobial action (46).
For instance, lacticin Q forms a huge toroidal pore (HTP) in the bacterial cell membrane, is translocated beneath the cell membrane, does not require a docking molecule, and is thus highly dependent on the physiological features of the cell membrane. This is thought to partly explain its limited spectrum of antibacterial activity and selective antimicrobial action (58, 59). The leaderless aureocin A53 produced by S. aureus A53 shows a stronger interaction with neutral membranes than negatively charged lipids and permeates the bacterial membranes without forming pores (60). In another instance, the leaderless bacteriocin LsbB, isolated from L. lactis subsp. lactis BGMN1–5, requires a zinc-dependent membrane metallopeptidase, YvjB, as its receptor molecule, which is contrary to the general understanding that leaderless bacteriocins do not require a receptor molecule for their mode of action (46, 61). The lantibiotic NAI-107, unlike nisin, does not form stable pores but causes depolarization of the membrane, resulting in bacterial cell death (28). It is thus plausible that the absence of a signal peptide and, in general, various modes of action of the leaderless peptides affects their processing, localization, or recognition by immune cells, leading to reduced efficacy in vivo (46). Further research is warranted to elucidate the underlying mechanisms responsible for the reduced in vivo activity of leaderless peptides, such as PARAGEN bacteriocins. Investigating these peptides’ stability, secretion pathways, and host interactions could provide valuable insights into their efficacy and pave the way for their optimization as therapeutic agents.
The contrasting results observed for NAI-107 and the PARAGEN bacteriocins raise other interesting questions. While in vitro studies provide valuable initial insights, they may not fully reflect the complexity of the in vivo environment.
The study’s caveats include a lack of testing in a mammalian model of N. gonorrhoeae. Notably, NAI-107 has been shown to be effective against methicillin-resistant S. aureus in a rat model (62). While no evidence of toxicity was detected in this study, further studies are required to assess toxicity in other animal models before NAI-107 can be tested in humans. Further testing should assess if there is evidence of synergy or antagonism between NAI-107 and ceftriaxone and other potential combination therapies.
Despite the above shortcomings, the results of the current work indicate that NAI-107 represents a promising option for addressing the severe threat of antibiotic resistance in N. gonorrhoeae and possibly in N. meningitidis.
ACKNOWLEDGMENTS
Conceptualization: S.S.M.-B. and C.K.; Methodology: N.H, Z.G., S.A., S.S.M.-B. and C.K; Formal analysis: N.H., S.S.M.-B. and C.K.; Investigation: N.H., S.S.M.-B, and C.K.; Data curation: N.G., S.S.M.-B. and C.K.; writing—original draft preparation: S.S.M.-B. and C.K.; writing—review and editing: Z.G, A.M, S.S.M.-B., and C.K. All authors have read and agreed to the published version of the manuscript.
Contributor Information
Sheeba Santhini Manoharan-Basil, Email: sbasil@itg.be.
John M. Atack, Griffith University - Gold Coast Campus, Southport, Gold Coast, Queensland, Australia
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02825-23.
Supplemental Figure S1a and b, showing two Kaplan-Meier plots.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. O’Neill J. 2016. Tackling drug-resistant infections globally: final report and recommendations. Available from: https://doi.org/https://doi.org/APO-63983
- 2. Miethke M, Pieroni M, Weber T, Brönstrup M, Hammann P, Halby L, Arimondo PB, Glaser P, Aigle B, Bode HB, Moreira R, Li Y, Luzhetskyy A, Medema MH, Pernodet J-L, Stadler M, Tormo JR, Genilloud O, Truman AW, Weissman KJ, Takano E, Sabatini S, Stegmann E, Brötz-Oesterhelt H, Wohlleben W, Seemann M, Empting M, Hirsch AKH, Loretz B, Lehr C-M, Titz A, Herrmann J, Jaeger T, Alt S, Hesterkamp T, Winterhalter M, Schiefer A, Pfarr K, Hoerauf A, Graz H, Graz M, Lindvall M, Ramurthy S, Karlén A, van Dongen M, Petkovic H, Keller A, Peyrane F, Donadio S, Fraisse L, Piddock LJV, Gilbert IH, Moser HE, Müller R. 2021. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 5:726–749. doi: 10.1038/s41570-021-00313-1 [DOI] [PubMed] [Google Scholar]
- 3. AMR Industry alliance . 2021. 2021 progress report (AMR industry alliance, 2021)
- 4. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. 2022. Lancet:629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093. doi: 10.1126/science.1176667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newman DJ, Cragg GM. 2007. Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477. doi: 10.1021/np068054v [DOI] [PubMed] [Google Scholar]
- 7. WHO . 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections
- 8. Quillin SJ, Seifert HS. 2018. Neisseria Gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 16:226–240. doi: 10.1038/nrmicro.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger RE, Alexander ER, Harnisch JP, Paulsen CA, Monda GD, Ansell J, Holmes KK. 1979. Etiology, manifestations and therapy of acute epididymitis: prospective study of 50 cases. J Urol 121:750–754. doi: 10.1016/s0022-5347(17)56978-5 [DOI] [PubMed] [Google Scholar]
- 10. Reekie J, Donovan B, Guy R, Hocking JS, Kaldor JM, Mak D, Preen D, Ward J, Liu B, Chlamydia and Reproductive Health Outcome Investigators . 2019. Risk of ectopic pregnancy and tubal infertility following gonorrhea and chlamydia infections. Clin Infect Dis 69:1621–1623. doi: 10.1093/cid/ciz145 [DOI] [PubMed] [Google Scholar]
- 11. Riou J, Althaus CL, Allen H, Cole MJ, Grad YH, Heijne JCM, Unemo M, Low N. 2023. Projecting the development of antimicrobial resistance in neisseria gonorrhoeae from antimicrobial surveillance data: a mathematical modelling study. BMC Infect Dis 23:252. doi: 10.1186/s12879-023-08200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F. 2012. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113:723–736. doi: 10.1111/j.1365-2672.2012.05338.x [DOI] [PubMed] [Google Scholar]
- 13. Ghodhbane H, Elaidi S, Sabatier J-M, Achour S, Benhmida J, Regaya I. 2015. Bacteriocins active against multi-resistant gram negative bacteria implicated in nosocomial infections. Infect Disord Drug Targets 15:2–12. doi: 10.2174/1871526514666140522113337 [DOI] [PubMed] [Google Scholar]
- 14. Marshall SH, Arenas G. 2003. Antimicrobial peptides: a natural alternative to chemical antibiotics and a potential for applied biotechnology. Electron. J. Biotechnol 6:271–284. doi: 10.2225/vol6-issue3-fulltext-1 [DOI] [Google Scholar]
- 15. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- 16. Entian KD, Klein C. 1993. Lantibiotics, a class of ribosomally synthesized peptide antibiotics. Naturwissenschaften 80:454–460. doi: 10.1007/BF01136035 [DOI] [PubMed] [Google Scholar]
- 17. Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian K-D, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rebuffat S, Ross RP, Sahl H-G, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RD, Tagg JR, Tang G-L, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep 30:108–160. doi: 10.1039/C2NP20085F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McAuliffe O, Ross RP, Hill C. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev 25:285–308. doi: 10.1111/j.1574-6976.2001.tb00579.x [DOI] [PubMed] [Google Scholar]
- 19. Guder A, Wiedemann I, Sahl HG. 2000. Posttranslationally modified bacteriocins--the Lantibiotics. Biopolymers 55:62–73. doi: [DOI] [PubMed] [Google Scholar]
- 20. van Kraaij C, de Vos WM, Siezen RJ, Kuipers OP. 1999. Lantibiotics: biosynthesis, mode of action and applications. Nat Prod Rep 16:575–587. doi: 10.1039/a804531c [DOI] [PubMed] [Google Scholar]
- 21. Asaduzzaman SM, Sonomoto K. 2009. Lantibiotics: diverse activities and unique modes of action. J Biosci Bioeng 107:475–487. doi: 10.1016/j.jbiosc.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 22. Repka LM, Chekan JR, Nair SK, van der Donk WA. 2017. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem Rev 117:5457–5520. doi: 10.1021/acs.chemrev.6b00591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foulston LC, Bibb MJ. 2010. Microbisporicin gene cluster reveals unusual features of lantibiotic biosynthesis in Actinomycetes. Proc Natl Acad Sci U S A 107:13461–13466. doi: 10.1073/pnas.1008285107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castiglione F, Lazzarini A, Carrano L, Corti E, Ciciliato I, Gastaldo L, Candiani P, Losi D, Marinelli F, Selva E, Parenti F. 2008. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem Biol 15:22–31. doi: 10.1016/j.chembiol.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 25. Sosio M, Gallo G, Pozzi R, Serina S, Monciardini P, Bera A, Stegmann E, Weber T. 2014. Draft genome sequence of the microbispora SP, . In Strain ATCC-PTA-5024, producing the Lantibiotic NAI-107. doi: 10.1128/genomeA.01198-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maffioli SI, Iorio M, Sosio M, Monciardini P, Gaspari E, Donadio S. 2014. Characterization of the congeners in the lantibiotic NAI-107 complex. J Nat Prod 77:79–84. doi: 10.1021/np400702t [DOI] [PubMed] [Google Scholar]
- 27. Foulston L, Bibb M. 2011. Feed-forward regulation of microbisporicin biosynthesis in microbispora corallina. J Bacteriol 193:3064–3071. doi: 10.1128/JB.00250-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Münch D, Müller A, Schneider T, Kohl B, Wenzel M, Bandow JE, Maffioli S, Sosio M, Donadio S, Wimmer R, Sahl H-G. 2014. The lantibiotic NAI-107 binds to bactoprenol-bound cell wall precursors and impairs membrane functions. J Biol Chem 289:12063–12076. doi: 10.1074/jbc.M113.537449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsusaki H, Endo N, Sonomoto K, Ishizaki A. 1996. Lantibiotic nisin Z fermentative production by lactococcus lactis iO-1: relationship between production of the lantibiotic and lactate and cell growth. Appl Microbiol Biotechnol 45:36–40. doi: 10.1007/s002530050645 [DOI] [PubMed] [Google Scholar]
- 30. Fujita K, Ichimasa S, Zendo T, Koga S, Yoneyama F, Nakayama J, Sonomoto K. 2007. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl Environ Microbiol 73:2871–2877. doi: 10.1128/AEM.02286-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwatani S, Zendo T, Yoneyama F, Nakayama J, Sonomoto K. 2007. Characterization and structure analysis of a novel bacteriocin, lacticin Z, produced by lactococcus Lactis QU 14. Biosci Biotechnol Biochem 71:1984–1992. doi: 10.1271/bbb.70169 [DOI] [PubMed] [Google Scholar]
- 32. Ovchinnikov KV, Chi H, Mehmeti I, Holo H, Nes IF, Diep DB. 2016. Novel group of leaderless multipeptide bacteriocins from gram-positive bacteria. Appl Environ Microbiol 82:5216–5224. doi: 10.1128/AEM.01094-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brunati C, Thomsen TT, Gaspari E, Maffioli S, Sosio M, Jabes D, Løbner-Olesen A, Donadio S. 2018. Expanding the potential of NAI-107 for treating serious ESKAPE pathogens: synergistic combinations against gram-negatives and bactericidal activity against non-dividing cells. J Antimicrob Chemother 73:414–424. doi: 10.1093/jac/dkx395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chi H, Holo H. 2018. Synergistic antimicrobial activity between the broad spectrum bacteriocin garvicin KS and nisin, farnesol and polymyxin B against gram-positive and gram-negative bacteria. Curr Microbiol 75:272–277. doi: 10.1007/s00284-017-1375-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maffioli SI, Cruz JCS, Monciardini P, Sosio M, Donadio S. 2016. Advancing cell wall inhibitors towards clinical applications. J Ind Microbiol Biotechnol 43:177–184. doi: 10.1007/s10295-015-1703-9 [DOI] [PubMed] [Google Scholar]
- 36. Hoffmann JA. 1995. Innate immunity of insects. Curr Opin Immunol 7:4–10. doi: 10.1016/0952-7915(95)80022-0 [DOI] [PubMed] [Google Scholar]
- 37. Jander G, Rahme LG, Ausubel FM. 2000. Positive correlation between virulence of pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 182:3843–3845. doi: 10.1128/JB.182.13.3843-3845.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fedhila S, Daou N, Lereclus D, Nielsen-LeRoux C. 2006. Identification of bacillus cereus Internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol Microbiol 62:339–355. doi: 10.1111/j.1365-2958.2006.05362.x [DOI] [PubMed] [Google Scholar]
- 39. Evans BA, Rozen DE. 2012. A streptococcus pneumoniae infection model in larvae of the wax moth galleria mellonella. Eur J Clin Microbiol Infect Dis 31:2653–2660. doi: 10.1007/s10096-012-1609-7 [DOI] [PubMed] [Google Scholar]
- 40. Desbois AP, Coote PJ. 2011. Wax moth larva (Galleria Mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J Antimicrob Chemother 66:1785–1790. doi: 10.1093/jac/dkr198 [DOI] [PubMed] [Google Scholar]
- 41. Gibreel TM, Upton M. 2013. Synthetic epidermicin NI01 can protect galleria mellonella larvae from infection with Staphylococcus aureus. J Antimicrob Chemother 68:2269–2273. doi: 10.1093/jac/dkt195 [DOI] [PubMed] [Google Scholar]
- 42. Dijokaite A, Humbert MV, Borkowski E, La Ragione RM, Christodoulides M. 2021. Establishing an invertebrate galleria mellonella greater wax moth larval model of neisseria gonorrhoeae infection. Virulence 12:1900–1920. doi: 10.1080/21505594.2021.1950269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dijokaite A, Humbert V-M, Christodoulides M. 2020. Galleria mellonella – a novel infection model for the study of neisseria gonorrhoeae virulence and pathogenicity. Access Microbiology 2. doi: 10.1099/acmi.ac2020.po1042 [DOI] [Google Scholar]
- 44. Cui Y, Zhang C, Wang Y, Shi J, Zhang L, Ding Z, Qu X, Cui H. 2012. Class IIa bacteriocins: diversity and new developments. Int J Mol Sci 13:16668–16707. doi: 10.3390/ijms131216668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gabrielsen C, Brede DA, Nes IF, Diep DB. 2014. Circular bacteriocins: biosynthesis and mode of action. Appl Environ Microbiol 80:6854–6862. doi: 10.1128/AEM.02284-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perez RH, Zendo T, Sonomoto K. 2018. Circular and leaderless bacteriocins: biosynthesis, mode of action, applications, and prospects. Front Microbiol 9:2085. doi: 10.3389/fmicb.2018.02085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Newstead LL, Varjonen K, Nuttall T, Paterson GK. 2020. Staphylococcal-produced bacteriocins and antimicrobial peptides: their potential as alternative treatments for staphylococcus aureus infections. Antibiotics (Basel) 9:40. doi: 10.3390/antibiotics9020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benítez-Chao DF, León-Buitimea A, Lerma-Escalera JA, Morones-Ramírez JR. 2021. Bacteriocins: an overview of antimicrobial, toxicity, and biosafety assessment by in vivo models. Front. Microbiol 12:630695. doi: 10.3389/fmicb.2021.630695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsoumanis A, Van Dijck C, Hens N, Kenyon C. 2023. Rethinking screening intensity in terms of reducing prevalence or increasing selection pressure for the emergence of resistant gonorrhea: a modeling study of men who have sex with men in belgium. Open Forum Infect Dis 10:fad165. doi: 10.1093/ofid/ofad165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, Ohnishi M, Lahra MM, Limnios A, Sikora AE, Wi T, Harris SR. 2016. The novel 2016 WHO neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 71:3096–3108. doi: 10.1093/jac/dkw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gabant P, Borrero J. 2019. PARAGEN 1.0: a standardized synthetic gene library for fast cell-free bacteriocin synthesis. Front Bioeng Biotechnol 7:213. doi: 10.3389/fbioe.2019.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. CLSI . 2023. Performance standards for antimicrobial disk susceptibility tests. In CLSI, performance standards for antimicrobial disk susceptibility tests, 33rd ed [Google Scholar]
- 53. Jaumaux F, Petit K, Martin A, Rodriguez-Villalobos H, Vermeersch M, Perez-Morga D, Gabant P. 2023. Selective bacteriocins: a promising treatment for staphylococcus aureus skin infections reveals insights into resistant mutants. Antibiotics 12:947. doi: 10.3390/antibiotics12060947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Foulston L. 2010. Cloning and analysis of the microbisporicin lantibiotic gene cluster from Microbispora corallina, Doctoral thesis, University of East Anglia: [Google Scholar]
- 55. Tsai C-Y, Loh JMS, Proft T. 2016. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7:214–229. doi: 10.1080/21505594.2015.1135289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pereira MF, Rossi CC, da Silva GC, Rosa JN, Bazzolli DMS. 2020. Galleria mellonella as an infection model: An in-depth look at why it works and practical considerations for successful application. Pathog Dis 78:ftaa056. doi: 10.1093/femspd/ftaa056 [DOI] [PubMed] [Google Scholar]
- 57. Ménard G, Rouillon A, Cattoir V, Donnio P-Y. 2021. Galleria mellonella as a suitable model of bacterial infection: past, present and future. Front Cell Infect Microbiol 11:782733. doi: 10.3389/fcimb.2021.782733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoneyama F, Ohno K, Imura Y, Li M, Zendo T, Nakayama J, Matsuzaki K, Sonomoto K. 2011. Lacticin Q-mediated selective toxicity depending on physicochemical features of membrane components. Antimicrob Agents Chemother 55:2446–2450. doi: 10.1128/AAC.00808-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoneyama F, Imura Y, Ohno K, Zendo T, Nakayama J, Matsuzaki K, Sonomoto K. 2009. Peptide-lipid huge toroidal pore, a new antimicrobial mechanism mediated by a lactococcal bacteriocin, lacticin Q. Antimicrob Agents Chemother 53:3211–3217. doi: 10.1128/AAC.00209-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Netz DJA, Bastos M do C de F, Sahl HG. 2002. Mode of action of the antimicrobial peptide aureocin A53 from staphylococcus aureus. Appl Environ Microbiol 68:5274–5280. doi: 10.1128/AEM.68.11.5274-5280.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uzelac G, Kojic M, Lozo J, Aleksandrzak-Piekarczyk T, Gabrielsen C, Kristensen T, Nes IF, Diep DB, Topisirovic L. 2013. A Zn-dependent metallopeptidase is responsible for sensitivity to lsbb, a class II leaderless bacteriocin of lactococcus lactis subsp. lactis bgmn1-5. J Bacteriol 195:5614–5621. doi: 10.1128/JB.00859-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jabés D, Brunati C, Candiani G, Riva S, Romanó G, Donadio S. 2011. Efficacy of the new Lantibiotic NAI-107 in experimental infections induced by multidrug-resistant gram-positive pathogens. Antimicrob Agents Chemother 55:1671–1676. doi: 10.1128/AAC.01288-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1a and b, showing two Kaplan-Meier plots.