ABSTRACT
The microbial communities of disease vectors may represent a key feature in several biological functions and thus deserve special attention in light of climate change and the consequent need to develop novel control strategies. Nevertheless, vector-borne microbial networks are still poorly understood. Assessing vectors’ microbial interactions and climatic dependencies may contribute to better estimating pathogen transmission characteristics and public health risks. In a climatically representative country-wide survey, Ixodes ricinus ticks were collected from 17 locations in Hungary. Using shotgun metagenomic sequencing, the bacteriome composition was analyzed by investigating the relationship between the abundance of nymphs and females in various climatic environments. Bacterial composition on the genus level revealed a significant difference between the samples from females and nymphs. Within the core bacteriome, females and nymphs showed significant variation in the following genera: Arsenophonus, Bacillus, Candidatus Midichloria, Rhodococcus, Sphingomonas, Staphylococcus, and Wolbachia. Among females, according to temperature strata, the following were found differentiating: Curtobacterium, Pseudomonas, and Sphingomonas. There was no genus with a significant difference in precipitation categories for females. Curtobacterium showed significant variation between temperature and Bacillus and Curtobacterium for various precipitation levels in the nymphs. The composition of vector-borne bacteriome members showed significant alterations at sampling points with different climatic conditions and development stages of the tick hosts. Our findings not only pave the way toward understanding tick-borne bacterial networks and interdependencies but also shed light on the high potential for the presence of a possible biological tick control species, the tick parasitoid, Ixodiphagus hookeri based on related bacteriome patterns.
IMPORTANCE
Climate-sensitive disease vectors, such as ticks, respond to the environment with changes in their microbiome. These changes can affect the emergence or re-emergence of various vector-borne pathogens, such as the causative agent of Lyme borreliosis (LB) or tick-borne encephalitis. This aspect is particularly emphasized in light of climate change. The climatically representative assessment of microbiome differences in various developmental stages of the most common Central European tick species, Ixodes ricinus, deepens our understanding of the potential climatic factors behind microbial relative abundance and interaction changes. This knowledge can support the development of novel disease vector control strategies.
KEYWORDS: Ixodes ricinus, bacteriome, climate, female, nymph
INTRODUCTION
Almost all aspects of human life are directly or indirectly affected by the Earth’s climate system dynamics. Many organisms, such as arthropods being poikilotherm organisms, are particularly sensitive to climate factors (1). Recent estimates suggest that around 80% of the world’s human population is at risk of one or more vector-borne disease(s) (2). The geographical distribution of arthropod vectors is changing due to climate change (3, 4). The response of ticks and dipteran vectors to the changing climate appears to differ (5). While bivalves respond to short-term weather and climate changes with rapid responses, ticks are affected by spatiotemporal averages of climate variation rather than short-term or localized climate variability. This suggests that changes in the risk of disease spread by dipterans can be expected in the short-term, while the prevalence of tick-borne diseases (TBDs) may be expected over a more extended period of time (5). Ixodes ricinus is the primary vector of the most prevalent TBDs in Europe, tick-borne encephalitis, and Lyme borreliosis (LB). Although reports on the prevalence of these TBDs do not show consistent global trends (6, 7), the European I. ricinus populations do. As a consequence of climate change, I. ricinus has been found at extreme altitudes and latitudes other than its former range and its population has shifted further north within the European continent (4, 8, 9). Similarly, due to some recently studied adaptation processes (10), the geographic range of LB can expand to non-endemic areas, and also to higher altitudes and latitudes (6, 7). In consideration of climatic factors, a shift toward a more thermophilic tick fauna has also been described in Hungary (11).
The eukaryotic, and as such, tick-borne microbiota impacts numerous biological functions of the host causing a variety of detrimental, neutral, or beneficial effects. In addition, certain bacterial assemblages can affect tick-borne pathogens of public health importance. Thus, understanding the changes in tick microbiota associated with the climatic environment could aid in the deeper understanding of tick biology (12, 13). Because the tick bacteriota contains a number of climate-dependent, environment-dependent components, bacterial interaction patterns may also be determined by climatic factors (14). In our work, we investigated the diversity of the bacteriome of I. ricinus samples from nymphs and females from wetter or drier and cooler or warmer environments based on a climatically representative survey in Hungary.
MATERIALS AND METHODS
Sampling design and sample collection
As the study’s main goal was to understand the natural bacteriome differences in I. ricinus, we designed the sampling to be representative of Hungary. In order to achieve this, we identified sampling points representative of climatic conditions (15). In Hungary, there are 175 local administrative units (LAU 1), for each of these units (geographical areas), we calculated the 10-year average of the yearly growing degree days (GDD) with base 10°C and the total yearly precipitation for each of the years. Meteorology data for the period of 2008 to 2017 was gathered from the ERA-Interim reanalysis data repository (16) with a spatial resolution of 0.125°. For each of the two environmental variables, binary categories were defined: GDD with classes “cooler” and “warmer” and precipitation with classes “less” and “more”. Regarding GDD, the lower two quartiles were classified as “cooler” and the upper two quartiles as “warmer”. For precipitation, the yearly means below the country-wide median were defined as “less” and scores above the median as “more”. Each LAU 1 was categorized with its own climatic variables. By stratified spatial random sampling (17, 18), 20 LAUs were chosen as sampling areas. The strata’s sample size was proportional to the stratifying GDD and precipitation categories’ country-wide frequency to be representative. All data management and analysis were performed in the R environment (19). A forest edge to sample from was identified within each of the 20 selected LAUs. Between 23/3/2019 and 20/5/2019, questing ticks were collected by flagging and dragging. In the laboratory (BSL2), ticks from the frozen samples were classified taxonomically. Ten nymphs and ten females of I. ricinus were selected randomly per sampling site. Since we could not collect the minimum of ten females and ten nymphs at three sampling sites, only the samples collected at the remaining 17 sites were included in the sequencing and downstream analyses (Fig. 1). Before DNA extraction, the ticks were washed twice with 99.8% alcohol.
Fig 1.
Climate category spatial pattern and sampling points. The color of the sylvan areas is defined by the climatic categories based on GDD and precipitation over the period 2008–2017. The points are relatively homogeneous within Hungary geographically.
DNA extraction and metagenomics library preparation
For sequencing, we formed pools of nymphs per sampling site and did the same for females. Ticks were subjected to DNA extraction using a Qiagen DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and the supplementary protocol designated for the purification of total DNA from ticks for the detection of Borrelia DNA according to the manufacturer’s instructions. The blackPREP Tick DNA/RNA Kit (Analytik Jena GmbH) was used for the DNA isolation. Isolated total metagenome DNA was used for library preparation. In vitro, fragment libraries were prepared using the NEBNext Ultra II DNA Library Prep Kit for Illumina. Paired-end fragment reads were generated on an Illumina NextSeq sequencer using TG NextSeq 500/550 High Output Kit v2 (300 cycles). Primary data analysis (base-calling) was carried out with Bbcl2fastq software (v2.17.1.14, Illumina).
Bioinformatic analysis
After merging the paired-end reads by PEAR (20), quality-based filtering and trimming were performed by TrimGalore (v.0.6.6, https://github.com/FelixKrueger/TrimGalore), with a setting of 20 as a quality threshold, retaining reads longer than 50 bp only. The remaining reads after deduplication by VSEARCH (21) were taxonomically classified using Kraken2 (k = 35) (22) with a database created (26/03/2022) from the NCBI RefSeq complete archaeal, bacterial, and viral genomes. For taxon assignment, the confidence 0.5 parameter was used to obtain more precise hits. Core bacteria was defined as the relative abundance of agglomerated counts on genus levels above 1% in at least one of the samples. The taxon classification data were managed in R (19) using functions of package phyloseq (23), microbiome (24), and metacoder (25). The pre-processed reads were assembled to contigs by MEGAHIT (v1.2.9) (26) using default settings. The contigs were also classified taxonomically by Kraken2 with the same database as above. The assembly-generated contigs that were classified to a pathogen bacteria genus by Kraken2 we reclassified by BLAST (27) on the representative prokaryotic genomes (downloaded on 16/6/2022). For each contig, the longest and the smallest e-value hit were kept and reported.
Statistical analysis
The within-subject ( ) diversity was assessed using the numbers of observed species (richness) and the Inverse Simpson’s Index (evenness). These indices were calculated in 1,000 iterations of rarefied operational taxonomic unit (OTU) tables with a sequencing depth of 158. The average over the iterations was taken for each sample. The -diversity expressed by Inverse Simpson’s Index was compared between the conditions using linear models. Comparing the female and nymph samples collected, a mixed-effect model was applied to manage the repeated measurements by sampling site as a random factor.
The between-subject ( ) diversity was assessed by UniFrac distance (28) based on the relative abundances of bacteria species. Using this measure, principal coordinate analysis (PCoA) ordination was applied to visualize the samples’ variance. To examine statistically whether the bacterial species composition differed by strata Permutational Multivariate Analysis of Variance (PERMANOVA (29)) was performed using the package vegan (30) in R (19).
The abundance differences in core bacteriome between groups were analyzed by a negative binomial generalized model of DESeq2 package (31) in R (19). This approach was applied following the recommendation of Weiss et al. (32). None of the compared groups had more than 20 samples, and their average library size ratio was less than 10. According to the multiple comparisons, the FDR-adjusted P-value less than 0.05 was considered significant. The SparCC correlation coefficient quantified the relationship among the relative abundances of bacterial species (33, 34). The statistical tests were two-sided.
RESULTS
After the basic demography of the samples, we present the -diversity of the full bacteriome. From the analysis of the core bacteriome, we report the species that are part of it, the -diversity, and the differences of the genera level expressed relative abundances. The Supplementary Material summarizes species of core bacteriome, the detected pathogen bacteria, and the correlations of bacteria genera (Fig. S1).
Among the ticks collected at the sampling sites, the median proportion of nymphs was 76.52% (IQR: 19.33), females 15.32% (IQR: 8.58), and males 7.91% (IQR: 11.47).
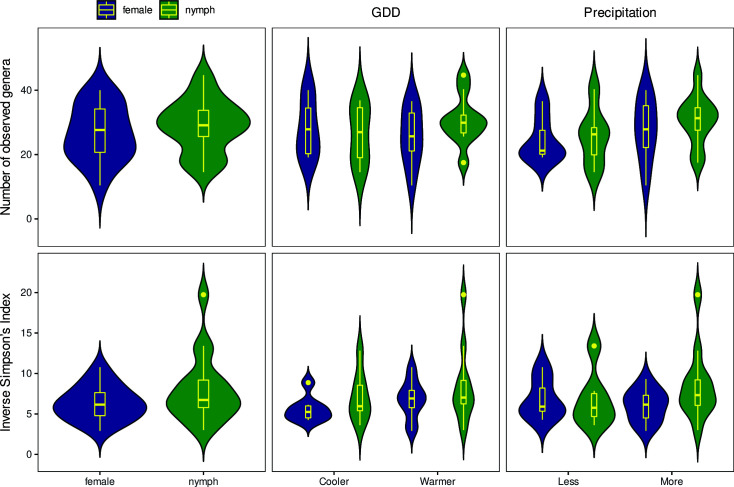
The numbers of observed species and the Inverse Simpson’s Index -diversity metrics by strata are shown in Fig. 2. Alpha diversity showed no significant difference between groups in either metric. For the comparison of females and nymphs, the P-value was 0.138. Within females, the P-values obtained by comparing groups from colder and warmer environments and drier and wetter environments were P = 0.562 and P = 0.577, respectively. Within nymphs, the comparative metrics were P = 0.174 and P = 0.309, respectively.
Fig 2.
Richness and evenness of Ixodes ricinus bacteriome by sample groups. The number of observed species (richness) and the Inverse Simpson’s Index (evenness) as -diversity metrics are presented in the form of a violin and box plot combination. These indices were calculated from 1,000 iterations of rarefied OTU tables with a sequencing depth of 158. The average over the iterations was taken for each sampling site. The violin plot shows the probability density of metrics, while the box plot marks the outliers, median, and IQR.
Core bacteriome
The core bacteriome is composed of the genera shown in Fig. 3. For the list of species identified within genera see Supplementary Material.
Fig 3.
Core bacteriome composition of Ixodes ricinus samples. The relative abundance is plotted for the females and nymphs. Besides the bacterial genera of the core bacteriome, the environmental condition (GDD and precipitation) categories of sampling locations are also marked.
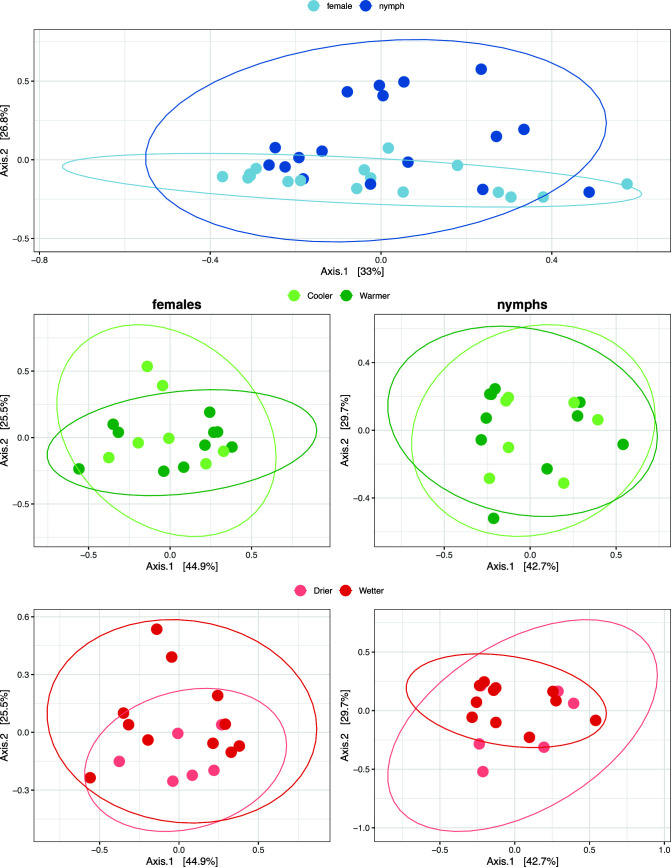
The variability of the samples’ core bacteriome genus profiles ( -diversity) is visualized by PCoA ordination (Fig. 4) based on weighted UniFrac distance. The PERMANOVA analysis of the bacterial composition on the genus level revealed a significant (P = 0.005) difference between the samples originating from females and nymphs. The core bacteriome of female samples showed no significant distance between either GDD (P = 0.444) or precipitation (P = 0.244) categories. Similarly, there was no significant difference between groups within nymphs (GDD P = 0.108, precipitation P = 0.722).
Fig 4.
PCoA plots of -diversity estimated based on the core bacteriome of Ixodes ricinus samples.
Abundance differences
The abundance differences (log2 median fold change, Log2FC) of groups per taxon comparison are summarized in Fig. 5. Comparing females and nymphs, the following genera showed significant (adjusted P = 0.05) differences in abundance: Arsenophonus, Bacillus, Candidatus Midichloria, Rhodococcus, Sphingomonas, Staphylococcus, Wolbachia. In the female samples, according to GDD, the following were found differentiating: Curtobacterium, Pseudomonas, and Sphingomonas. There was no genus with a significant difference in precipitation categories in females. In the nymphs, Curtobacterium showed a significant variation between GDD groups and Bacillus and Curtobacterium between precipitation levels.
Fig 5.
Core bacteriome abundance fold changes by taxonomic ranks. The colors represent the Log2FC of the median abundances of the compared groups. Subfigure (a) shows the ratio of the abundances in females comparing nymphs as a reference. Figure (b) compares female samples from warmer areas to cooler ones. Figure (c) compares samples from drier areas to those from wetter areas among females. Comparisons of GDD groups in nymphs are shown in figure (d), while comparisons of precipitation groups are shown in figure (e).
DISCUSSION
Our shotgun sequencing-based microbiome analysis assesses an in-depth characterization of I. ricinus nymphs and adult females collected from a climatically representative set of sampling points in Hungary. Our study revealed a comprehensive picture of the bacterial diversity and associations in various host categories in I. ricinus ticks over the various climatic regions of Hungary.
While I. ricinus ticks had previously been linked with relatively higher alpha diversity scores than other common tick species (35), no significant difference was observed among the nymph and adult female stages or warmer-colder and drier-wetter areas of origin. Carpi and colleagues found that the bacterial communities of geographically distant ticks of the same developmental stage differ more from those from the same regions (36). Moreover, Batool and colleagues found that in Ukraine, a neighboring country to Hungary with an area around 6.5 times as big, the alpha diversity analyses demonstrated differences in tick microbiota patterns of various administrative regions (37). For the dissimilarity of the samples’ core bacteriome genus profiles (beta diversity), inter-regional comparison of developmental stages (females or nymphs) produced significant differences, while developmental stage-wise testing of the climatic condition-associated localization did not. Similarly, Batool and colleagues described that tick sex comparisons resulted in significant differences on various beta-diversity tests regardless of the area of origin (37). While these results are interesting to compare, the different study models and testing methods should be considered.
During the tick-collection phase of our study, three categories of ticks were evaluated for metagenome sequencing: nymphs, adult females, and adult males. Nevertheless, the sequencing of adult males was rejected as the number of males was lower than nymphs and adult females at all sampling points. Sex ratio shifts by ticks are not uncommon (38, 39). The elucidation of the reason for the observed shift toward females in the adult life stage in our study would require further investigation. Nonetheless, the presence of certain maternally inherited genera, namely Arsenophonus, Rickettsia, Spiroplasma, and Wolbachia in the metagenomes is noteworthy. These genera are described to induct parthenogenesis, feminize or kill males, and, thus, manipulate the reproduction of their host species toward the production of daughters (40, 41). Sex ratio skewness may be adaptive from the perspective of upper-generation ticks since it diminishes the competition of related males in tick-dense localities by reducing their numbers while increasing the number of related females that can be fertilized by the smaller male population (42, 43).
Besides the members of the core bacteriome of nymphs and adult females, reads deriving from further bacterial genera with relatively lower abundance rates but high clinical relevance (pathogens), such as Anaplasma, Bartonella, Borrelia, Borreliella, and Ehrlichia have also been detected. While Anaplasma phagocytophylum, the cause of Anaplasmosis has previously been associated with the presence of a tick parasitoid, Ixodiphagus hookeri, based on its lifestyle and its mode of hunting (44), its positive correlation with the genera of Arsenophonus and Wolbachia, endosymbionts of I. hookeri, was not affirmed (13). Within our study, the geolocations where Anaplasma sp. occurred did not match the detection points I. hookeri in Hungary described in a recent study (45). I. ricinus ticks are considered to be the vector of the Borrelia burgdorferi sensu lato complex, which is responsible for LB (46 – 48). Despite the high incidence rates of Lyme disease in Europe (49), Borreliella burgdorferi itself was not detected in our samples, while other species of Borrelia and Borreliella were identified. Since the causative agent of Lyme disease is normally mentioned as B. burgdorferi sensu lato (50, 51), referring to this species broadly, involving other members of the genera as well, the presence of Borrelia garinii, Borrelia valaisiana, Borrelia afzelii, Borrelia coriaceae and Borrelia miyamotoi can also be associated with this common disease. Bartonella spp. and Ehrlichia spp. are pathogens that are also often isolated in European settings (52 – 54). The presence of pathogens may influence the composition of tick microbiota (55).
Some genera constituting the core bacteriome, namely Arsenophonus, Candidatus Midichloria, Rickettsia, and Wolbachia are believed to be maternally inherited or strongly tick-associated due to direct or indirect reasons, while the members of Bacillus, Curtobacterium, Cutibacterium, Mycobacteroides, Pseudomonas, Rhodococcus, Sphingomonas, Staphylococcus, and Stenotrophomonas are related to soil, water, plants, skin or mucosa of vertebrates, thus may rather be acquired from the environment of the ticks (13, 13, 37, 56, 57). The representatives of the environmental genera may either participate in ticks’ transient or long-term microbiota. Several environmental, tick gut, or surface bacteria cause opportunistic infections in humans, especially in patients with an immunocompromised history (58, 59). Even though certain bacteria, such as cutibacteria and staphylococci, may be considered bacterial contaminants from the sample processing steps (13), according to a recent study, the effects of a possible, minor level contamination are not noticeable in the overall relative bacterial abundance (37). Nevertheless, it is possible that some environmental genera were only present at the cuticle of the collected ticks regardless of the repeated laboratory washing steps with 99.8% alcohol.
Except for Cutibacterium, Mycobacteroides, Rickettsia, and Stenotrophomonas, genera constituting the core bacteriome showed statistically significant alterations in the examined tick groups differing in life stage and climatic condition-associated geographical origins. This finding is plausible considering the fact that both the life stage and the season-associated climatic conditions are very important in the composition of the tick bacteriome (60, 61).
The members of Rickettsia are maternally inherited or transstadially passed symbionts (62, 63) that may cause tick-borne infections (62) and have been described to be dominant in tick microbiomes in several studies (36, 64 – 68), However, high rickettsial abundance rates are not necessarily present in the ticks. Certain studies presented relatively low rickettsial genome fragment counts (37). The reason for these alterations is that the appearance rates of Rickettsia spp. vary between the geographical populations of ticks. According to a recent study, the members of Rickettsia are more abundant in ticks from forests (57, 69), where ticks included in the present study are also derived. Considering that nymphal rickettsial counts were high in other studies as well (64), while male ticks are normally associated with relatively fewer members of the genus Rickettsia (64, 65), the number of rickettsias seems to decrease throughout the life of male ticks gradually. This theory is in line with a study on microbiome changes during tick ontogeny (70). On the other hand, certain rickettsial species are also associated with male-killing, which may also cause the decreasing numbers associated with male adult ticks (71). Rickettsia helvetica and Rickettsia monacensis, two species identified in our samples as well, have been connected with the presence of adult I. hookeri wasps which are the parasitoids of ticks. The reason for the association may either be related to the role of the parasitoid wasps in the circulation of rickettsias among ticks or with digested bacterial DNA in the wasp body lumen (72).
Even though the members of Mycobacteroides are considered as either long-term or transient environmentally derived residents of the tick microbiota, recent studies revealed that certain species might be able to multiply inside the host and be transovarially inherited (56, 73). Nevertheless, the relative abundance of the species seems dependent on the geographic region (66). In our study, a statistically non-significant shift could have been observed in the direction of cooler and dryer regions. Considering climatic tendencies, Thapa and colleagues reported higher mycobacterial genome fragment counts in ticks from Texas than from Massachusetts (66), which shows a contrary mycobacterial temperature preference. The observed differences may be caused by the different species-level Mycobacterium composition in the U.S. and Europe. Since several Mycobacterium species may be pathogenic to humans or animals, this finding may have a public health significance, especially in the light of current climate change trajectories.
Cutibacterium and Stenotrophomonas are common environmental bacteria that have been associated with ticks in several studies (13, 35, 37). Cutibacterium is strongly associated with ticks in forests (69), where our I. ricinus ticks are derived from. Stenotrophomonas maltophilia, a species identified in our samples, is an opportunistic pathogen often isolated from the infections of immunocompromised individuals (59).
The majority of the genera constituting the core bacteriome were found to demonstrate significant differences among various life stages and climatic conditions associated with tick host groups. With the abundance rates of specifically tick-associated genera, Arsenophonus, Candidatus Midichloria, and Wolbachia, the significant differences among adult female ticks and nymphs were clearly explainable.
Arsenophonus, which showed significantly lower abundance rates in females than in nymphs, is a widespread, mainly insect-associated bacterial genus with a wide spectrum of either parasitic or symbiotic host relations (57, 74). While the high number of Arsenophonus, more precisely Arsenophonus nasoniae associated reads could suggest the dominance of this genus in the nymph microbiota; its presence is associated with I. hookeri, a parasitoid wasp of ticks that is supposed to have a wide geographical range worldwide. I. hookeri oviposits to larval and nymphal hard tick hosts. Its eggs can only develop in engorging or engorged nymphs (75, 76). If the tick immune system-borne encapsulation of the I. hookeri eggs is not successful, the eggs hatch. Larvae start consuming the tick tissues and thus cause the death of the host (75). Furthermore, Bohacsova and colleagues found that A. nasoniae, an endosymbiont of encyrtid wasps identified in high numbers in our nymph samples, is only detectable in tick nymphs parasitized by the wasp (72). According to these findings, nymphs harboring A. nasoniae do not often reach the adult stage due to the parasitoid wasp, I. hookeri. Thus A. nasoniae deriving reads demolish from the adult tick population specifically due to the death of nymph hosts. While the developmental stage dependence of I. hookeri-associated A. nasoniae is beyond question; the median abundance of this bacterial genus also showed differences in nymph groups of different climatical regions. Nymphs collected from warmer and rainier areas harbored more reads deriving from Arsenophonus spp. than ones collected from warmer areas. Attempts have been made to use I. hookeri as a means of biological control of ticks for approximately 100 years (76) and the consideration of climatic conditions as underlying causes for unstable control technique success rates may improve current biological control methods. Moreover, A. nasoniae is described to be male-killing in several wasp species (77), but in I. hookeri adult wasp males were also infected by A. nasoniae, although the emergence ratio of males to females was 1:3.6 in the infected populations (72). Nevertheless, the presence of this bacterium may underlie decreased I. hookeri numbers at certain habitats or could even have contributed to insufficient abundance rates by biological tick control purposes, attempted mass releases of the parasitoid wasps in the past (78, 79).
The genus of Wolbachia, having a significant, strong abundance rate shift to nymphs is reported to share several characteristics with Arsenophonus that affect tick life and, potentially, tick population size. One of the identified species, Wolbachia pipientis, is strongly related to the presence of I. hookeri (80, 81). Thus, the reason for the high number of wolbachial reads in nymphs may be the same as in the case of the Arsenophonus genus. Wolbachia spp. are also known to kill male hosts in some host insect species selectively (40, 82), while in others it appears to be non-male-killing (83). To the best of our knowledge, no studies exist on the effect of Wolbachia spp. on I. hookeri. Furthermore, similarly to the case of Arsenophonus, warmer and rainier areas had a slight positive effect on the number of wolbachial reads in nymphs although this effect was weaker than in the case of Arsenophonus. Due to the nymphal loads of reads from Arsenophonus and Wolbachia, the prevalence of I. hookeri in the samples and at the sampling points in Hungary can be strongly hypothesized. Evidence for the presence of these parasitoid wasps has recently been discovered in Hungary (45).
By Candidatus Midichloria, the strong, statistically significant difference that was observed for females among adult female and nymph ticks is in agreement with the results of other research groups and has an explanation in the scientific literature. Studies exploring the inter-sex microbiome differences of adult I. ricinus ticks (35, 37, 84, 85) demonstrated much higher abundance rates of Candidatus Midichloria in females than in males independent of the regions of tick collection. The unique reason for this is that Candidatus Midichloria mitochondrii (CMM) invades the mitochondria of the cells within the ovaries of the female ticks. Despite the multiplication of the bacteria that consumes many ovarian mitochondria, the tick oocytes are expected to develop normally. CMM is described as being vertically transferred to all eggs (84, 86). Even though the nymphal sex ratio of ticks may not be exactly 1:1 (87), according to the available evidence, the presence of CMM does not result in sex ratio distortion in ticks (84). Simultaneously, it has been observed that CMM is transferred to both male and female larvae, but later, during the nymph stage, its specialization occurs toward females (85). Thus, a possible reason for the difference in CMM abundances among females and nymphs may be that many nymphs are males. Furthermore, the multiplication of CMM appears to increase after engorgement (85). Accordingly, the relatively low numbers of CMM might also be associated with the lack of engorgement among the nymphs collected for our study. Since tick collections occurred between the end of March and the middle of May, which represents the beginning of the activity period of nymphs (88), information on the overall engorgement status based on CMM counts appears to be realistic.
With the environmentally derived bacterial groups (Bacillus, Curtobacterium, Pseudomonas, Rhodococcus, Sphingomonas, and Staphylococcus), the explanation of statistically significant life stage and climatic condition-wise differences in the bacterial composition appeared to be present.
Bacilli inhabit a broad range of environments, ranging from soils to insect guts and some species can be pathogens (36). The finding that the number of reads deriving from bacilli was significantly higher in nymphs than in adult females may originate from the choice to assess adult female ticks and exclude adult males from the study. While nymphs appeared in the study as a mixed population of males and females, the adult males collected at the sampling points were not represented due to their relatively low number compared to adult females. A study on the microbiome of Rhipicephalus sanguineus ticks describes a strong shift of Bacillus spp. toward the male tick population. Since our study only contained males in the nymph population, the observed shift may represent the male-relatedness to Bacillus spp. The nature of the relatedness of male ticks and Bacillus spp. has not yet been characterized (64). Nonetheless, Bacillus spp. are described not to be present in every tick microbiota (66, 89) and the detection rates of the members of this genus appear to show significant regional differences (37). Within our study, reads deriving from Bacillus spp. showed significantly higher abundances in nymphs from areas with more precipitation. Furthermore, Fernández-Ruvalcaba and colleagues reported that Bacillus thuringiensis strains significantly elevated mortality and demolished oviposition and egg hatch among adult, pesticide-resistant Rhipicephalus microplus ticks (90). Moreover, certain strains of Bacillus wiedmannii are known, while B. thuringiensis strains are even commercialized as insecticides or nematicides for biological pest control (91, 92), although the entomopathogenic effect of environmental strains proved to be more present (93). Both above-mentioned Bacillus species were present in our samples, which can decrease I. ricinus numbers, although potentially in both age groups. The key to the biopesticide characteristics of these bacilli is the formation of functional crystalline Cry proteins that are specific toxins. Other entomopathogenic bacteria such as Bacillus cereus, also present within our samples, are opportunistic as they act in secondary, non-specific pathways facilitated by certain weakening motives, such as the presence of Cry proteins (92). Thus, the shift in Bacillus abundance toward nymphs rather appears to occur due to the sex determination of the adult ticks included in the study.
Although within our samples, significantly more Rhodococcus reads are derived from adults than from nymphs, this result may be based on the following factor. The dominating species, Rhodococcus fascians, is a common bacterial phytopathogen that interacts with a broad array of plants, causing their malformation (94). Older ticks may have had more opportunities for encounters with these environmental bacteria than younger ones. Higher Rhodococcus read counts in adults align with René-Martellet and colleagues’ findings (64).
Association with Staphylococcus spp. that were significantly more abundant in adult females than in nymphs may be explained with similar reasoning. Staphylococci are common findings related to ticks (56, 65) that often appear on the skin and mucous membranes of the hosts of the ticks as well (56). Ticks carrying staphylococci may have already engorged and thus encountered these bacteria. Considering that the number of engorged nymphs appeared to be relatively low within our samples according to the reasons explained by the genus Candidatus Midichloria, fewer opportunities to encounter the members of this genus by nymphs are possible. Moreover, the relative abundance of Staphylococcus spp. also appears to be dependent on the region of tick collection (66) and, as described earlier, staphylococcal hits may also derive from contamination (37).
The finding that the members of another environmental bacterial genus, Sphingomonas (13 ) were identified with significantly higher abundance rates in nymphs than in adults is controversial to the hypotheses of Rhodoccus spp., Staphylococcus spp., and to the related findings of other authors (70). Another finding was that among adult females, the abundance rate of Sphingomonas spp. was significantly higher in warmer sampling areas than in cooler localities, while by nymphs, the temperature-wise difference was not relevant. Interestingly, another research group found that Sphingomonas spp. were much more abundant in adult ticks kept at 4°C than by those at 20°C, 30°C, or 37°C. Moreover, according to their study, Sphingomonas was among the most abundant bacterial genera at 4°C by males (65). The reason for this discrepancy with our results on temperature-wise abundance may rest on the species-level Sphingomonas composition. Additionally, interactions with other bacterial groups may also underlie our findings. In any case, further studies would be required to elucidate the findings of Sphingomonas populations and confirm or invalidate our hypotheses.
Statistically significant climatic condition-wise alterations in adult female and nymphal stage ticks only appeared in environmental bacterial genera, namely Curtobacterium and Pseudomonas. While the former appeared significantly more abundantly in warmer environments both for adult females and nymphs, a significant preference for little precipitation was observable in nymphs. This finding may be relevant in light of climate change, mostly because of a dominant species, Curtobacterium flaccumfaciens which is a phytopathogenic bacteria with economic significance (95) and has also been isolated from a child with fatal septicemia (96).
Reads from the genus of Pseudomonas were detected with significantly greater abundances at areas with higher GDD in adult females, while temperatures appeared not to influence the relative abundance of Pseudomonas spp. in nymphs. The formation of biofilms might explain this finding as some Pseudomonas strains have better biofilm formation capacities at warmer temperatures (97, 98). Thus, adult ticks from warmer environments that have already survived at least one summer, according to our knowledge of the tick life cycle, may harbor more bacteria from the Pseudomonas genus that managed to form biofilms and so became more steadily present. According to a study, the egg wax composition of the cattle tick, Rhipicephalus microplus is able to inhibit the biofilm formation of Pseudomonas aeruginosa (99). Thus, certain mechanisms may influence the abundance of Pseudomonas species at earlier tick life stages. The exact composition of Pseudomonas strains should be examined to evaluate this hypothesis as the genus is very versatile. At the same time, Pseudomonas spp. have been related to both nymphs and adults in other publications (37), while certain studies report more stable Pseudomonas spp. appearance rates in males than in females (35, 66).
All the above-mentioned bacterial groups share another factor, namely the interaction among the bacterial community members, this may be responsible for the microbial pattern assessed. In order to elucidate the possible influence of certain bacterial co-occurrences, the correlation analysis of the bacterial genera was performed in each development stage and climatic category. Positive correlations among the taxa of microbial communities may be interpreted as the reflection of shared habitat or environmental condition preferences, cooperative activities, such as cross-feeding (100), or the representation of functional guilds performing complementary or similar functions (101). In contrast, negative correlations may indicate competition for limiting resources, niche partitioning, inequivalent resistance to losses, or active negative interactions (100, 101). In contrast to other studies (13), the number of positive and negative correlations was balanced within our samples.
All samples considered, Arsenophonus and Wolbachia correlated positively, which is assumed to have occurred due to the presence of I. hookeri. Furthermore, Rickettsia was observed to be negatively correlated with Curtobacterium spp. in several development stages and climatic groups, which may indicate positive public health consequences and a possible step toward a future tick control tool. In contrast, Candidatus Midichloria spp. which were previously detected in positive correlation with Rickettsia spp. (13) showed no correlation in our samples. Juxtaposing another publication in the field (13), neither the Pseudomonas and Rickettsia nor the Bacillus and Rickettsia pair showed any correlation. Correlations among environmental bacteria of various climatic categories are likely based on their similar environmental preferences and the previously mentioned interaction types. Due to the seasonal variability of environmental bacteria, the number of tick-borne, potentially pathogenic bacteria may increase or decrease correspondingly with the taxa of environmental origins (13). Accordingly, the presence or absence of certain environmental taxa may reflect the temporal dynamics of certain pathogens. The public health significance of this finding is particularly significant in light of climate change and potentially varying climatic conditions around the globe. Nevertheless, the interpretation of co-occurrence patterns and the nature of these correlations require further studies as abundance shifts depend on multiple factors. Thus, bacterial and parasitological interconnections are not exclusively responsible for the variations. Moreover, our results were obtained from the sequencing of entire tick individuals and thus lacked finer, e.g., organ scale considerations of the correlating taxa.
Conclusion
Here we reported the identification of the I. ricinus bacteriome-associated findings in adult females and nymphs collected from a climatically representative set of sampling points in Hungary. These results allowed us to show that (i) the I. ricinus bacteriome is dependent on the temperature and precipitation history of the geolocation of sampling; (ii) the I. ricinus bacteriome is not stable in the developmental stages of the ticks; (iii) based on the bacteriome patterns identified, the identified developmental stage-wise alterations may be associated with the presence of certain tick parasitoids that exclude the option of reaching the adult age; (iv) TBD pathogens are widely distributed at the climatically representative sampling points.
In the future, developmental stage and climate-associated microbial differences and correlations identified in this ecosystem study could be confirmed with experimental approaches and complemented with further metagenome studies to achieve sufficient data volumes of tick microbial inter-relatedness and exploit them as promising resources for novel tick control strategies.
ACKNOWLEDGMENTS
The research was supported by the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 874735, VEO) to A.G.T., M.P., and N.S. The European Union’s Horizon 2020 research and innovation program supports the project under Grant Agreement No. 874735 (VEO).
N.S. takes responsibility for the integrity of the data and the accuracy of the data analysis. N.S. and R.F. conceived the concept of the study. E.K., G.M., and M.G. performed sample collection and procedures. A.G.T., M.P., and N.S. participated in the bioinformatic and statistical analysis. A.G.T., H.Y., L.M., and N.S. participated in the drafting of the manuscript. A.G.T., G.M., N.S., and O.K. carried out the manuscript’s critical revision for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Norbert Solymosi, Email: solymosi.norbert@gmail.com.
Anna Moniuszko-Malinowska, Uniwersytet Medyczny w Bialymstoku, Bialystok, Poland .
DATA AVAILABILITY
The short-read data of samples are publicly available and accessible through PRJNA828115 from the NCBI Sequence Read Archive (SRA).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01243-23.
Bacterial genus abundance correlations.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Huey RB, Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am Nat 158:204–210. doi: 10.1086/321314 [DOI] [PubMed] [Google Scholar]
- 2. World health organization . 2017. Global vector control response 2017-2030
- 3. Stocker T, Qin D, Plattner G, Tignor M, Allen S, Boschung J. 2013. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Climate change 5:1–1552. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_all_final.pdf. [Google Scholar]
- 4. Semenza JC, Suk JE. 2018. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett 365:fnx244. doi: 10.1093/femsle/fnx244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogden NH, Lindsay LR. 2016. Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol 32:646–656. doi: 10.1016/j.pt.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 6. Hubálek Z. 2009. Epidemiology of lyme borreliosis. Lyme borreliosis 37:31–50. doi: 10.1159/000213069 [DOI] [PubMed] [Google Scholar]
- 7. Rizzoli A, Hauffe H, Carpi G, Vourc H G, Neteler M, Rosa R. 2011. Lyme Borreliosis in Europe. Euro Surveill 16:27. doi: 10.2807/ese.16.27.19906-en [DOI] [PubMed] [Google Scholar]
- 8. Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, Lindgren E. 2012. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors 5:8. doi: 10.1186/1756-3305-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George J-C, Golovljova I, Jaenson TGT, Jensen J-K, Jensen PM, Kazimirova M, Oteo JA, Papa A, Pfister K, Plantard O, Randolph SE, Rizzoli A, Santos-Silva MM, Sprong H, Vial L, Hendrickx G, Zeller H, Van Bortel W. 2013. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 6:1. doi: 10.1186/1756-3305-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samanta K, Azevedo JF, Nair N, Kundu S, Gomes-Solecki M. 2022. Infected ixodes scapularis nymphs maintained in prolonged questing under optimal environmental conditions for one year can transmit Borrelia burgdorferi (Borreliella genus novum) to uninfected hosts. Microbiol Spectr 10:e0137722. doi: 10.1128/spectrum.01377-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hornok S, Kováts D, Horváth G, Kontschán J, Farkas R. 2020. Checklist of the hard tick (acari: ixodidae) fauna of Hungary with emphasis on host-associations and the emergence of Rhipicephalus sanguineus. Exp Appl Acarol 80:311–328. doi: 10.1007/s10493-019-00461-6 [DOI] [PubMed] [Google Scholar]
- 12. Narasimhan S, Fikrig E. 2015. Tick microbiome: the force within. Trends Parasitol 31:315–323. doi: 10.1016/j.pt.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lejal E, Chiquet J, Aubert J, Robin S, Estrada-Peña A, Rue O, Midoux C, Mariadassou M, Bailly X, Cougoul A, Gasqui P, Cosson JF, Chalvet-Monfray K, Vayssier-Taussat M, Pollet T. 2021. Temporal patterns in Ixodes ricinus microbial communities: an insight into tick-borne microbe interactions. Microbiome 9:153. doi: 10.1186/s40168-021-01051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cabezas-Cruz A. 2021. Can the impact of climate change on the tick microbiome bring a new epidemiological landscape to tick-borne diseases? CABI Climate Change Series:46–49. doi: 10.1079/9781789249637.0000 [DOI] [Google Scholar]
- 15. Papp M, Békési L, Farkas R, Makrai L, Judge MF, Maróti G, Tőzsér D, Solymosi N. 2022. Natural diversity of the honey bee (Apis mellifera) gut bacteriome in various climatic and seasonal states. PLoS One 17:e0273844. doi: 10.1371/journal.pone.0273844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dee DP, Uppala SM, Simmons AJ, Berrisford P, Poli P, Kobayashi S, Andrae U, Balmaseda MA, Balsamo G, Bauer P, et al. 2011. The ERA-interim reanalysis: configuration and performance of the data assimilation system. Quart J Royal Meteoro Soc 137:553–597. doi: 10.1002/qj.828 [DOI] [Google Scholar]
- 17. Stevens DL, Olsen AR. 2004. Spatially balanced sampling of natural resources. Journal of the American Statistical Association 99:262–278. doi: 10.1198/016214504000000250 [DOI] [Google Scholar]
- 18. Kincaid TM, Olsen AR, Weber MH. 2019. Spsurvey: spatial survey design and analysis. R package version 4.1.0. doi: 10.18637/jss.v105.i03 [DOI] [Google Scholar]
- 19. R Core Team . 2022. R: a language and environment for statistical computing. Vienna, Austria. Available from: https://www.R-project.org [Google Scholar]
- 20. Zhang J, Kobert K, Flouri T, Stamatakis A. 2014. PEAR: a fast and accurate Illumina paired-end reAd mergeR. Bioinformatics 30:614–620. doi: 10.1093/bioinformatics/btt593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wood DE, Lu J, Langmead B. 2019. Improved metagenomic analysis with Kraken 2. Genome Biol 20:257. doi: 10.1186/s13059-019-1891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMurdie PJ, Holmes S. 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lahti L, Shetty S. 2012-2019. microbiome R package. doi: 10.18129/B9.bioc.microbiome [DOI] [Google Scholar]
- 25. Foster ZSL, Sharpton TJ, Grünwald NJ. 2017. Metacoder: an R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput Biol 13:e1005404. doi: 10.1371/journal.pcbi.1005404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li D, Liu C-M, Luo R, Sadakane K, Lam T-W. 2015. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:1674–1676. doi: 10.1093/bioinformatics/btv033 [DOI] [PubMed] [Google Scholar]
- 27. Coordinators NR. 2016. Database resources of the National center for biotechnology information. Nucleic Acids Res. 44:D7–D19. doi: 10.1093/nar/gkv1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x [DOI] [Google Scholar]
- 30. Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D. 2019. Vegan: community ecology package. Available from: https://CRAN.R-project.org/package=vegan
- 31. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss S, Xu ZZ, Peddada S, Amir A, Bittinger K, Gonzalez A, Lozupone C, Zaneveld JR, Vázquez-Baeza Y, Birmingham A, Hyde ER, Knight R. 2017. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 5:27. doi: 10.1186/s40168-017-0237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedman J, Alm EJ. 2012. Inferring correlation networks from genomic survey data. PLoS Comput Biol 8:e1002687. doi: 10.1371/journal.pcbi.1002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurtz ZD, Müller CL, Miraldi ER, Littman DR, Blaser MJ, Bonneau RA. 2015. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput Biol 11:e1004226. doi: 10.1371/journal.pcbi.1004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Portillo A, Palomar AM, de Toro M, Santibáñez S, Santibáñez P, Oteo JA. 2019. Exploring the bacteriome in anthropophilic ticks: to investigate the vectors for diagnosis. PLoS One 14:e0213384. doi: 10.1371/journal.pone.0213384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho LP, Drautz DI, Rizzoli A, Schuster SC. 2011. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS One 6:e25604. doi: 10.1371/journal.pone.0025604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batool M, Blazier JC, Rogovska YV, Wang J, Liu S, Nebogatkin IV, Rogovskyy AS. 2021. Metagenomic analysis of individually analyzed ticks from Eastern Europe demonstrates regional and sex-dependent differences in the microbiota of Ixodes ricinus. Ticks Tick Borne Dis 12:101768. doi: 10.1016/j.ttbdis.2021.101768 [DOI] [PubMed] [Google Scholar]
- 38. Fourie LJ, Belozerov VN, Kok DJ. 1996. Sex ratio characteristics in Ixodes rubicundus (acari: ixodidae), the karoo paralysis tick. Exp Appl Acarol 20:477–481. doi: 10.1007/BF00053311 [DOI] [PubMed] [Google Scholar]
- 39. Van Oosten AR, Duron O, Heylen DJA. 2018. Sex ratios of the tick Ixodes arboricola are strongly female-biased, but there are no indications of sex-distorting bacteria. Ticks Tick Borne Dis 9:307–313. doi: 10.1016/j.ttbdis.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 40. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of Invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 41. Engelstädter J, Hurst GDD. 2009. The Ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst 40:127–149. doi: 10.1146/annurev.ecolsys.110308.120206 [DOI] [Google Scholar]
- 42. Werren JH. 1980. Sex ratio adaptations to local mate competition in a parasitic wasp. Science 208:1157–1159. doi: 10.1126/science.208.4448.1157 [DOI] [PubMed] [Google Scholar]
- 43. Poulin R. 2011. Evolutionary ecology of parasites. In Evolutionary Ecology of parasites. Princeton university press. [Google Scholar]
- 44. Krawczyk AI, Bakker JW, Koenraadt CJM, Fonville M, Takumi K, Sprong H, Demir S. 2020. Tripartite interactions among Ixodiphagus hookeri, Ixodes ricinus and deer: differential interference with transmission cycles of tick-borne pathogens. Pathogens 9:339. doi: 10.3390/pathogens9050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tóth AG, Farkas R, Gyurkovszky M, Krikó E, Solymosi N. 2023. First detection of Ixodiphagus hookeri (hymenoptera: encyrtidae) in Ixodes ricinus ticks (acari: ixodidae) from multiple locations in Hungary. Sci Rep 13:1624. doi: 10.1038/s41598-023-28969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richtrová E, Míchalová P, Lukavská A, Navrátil J, Kybicová K. 2022. Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in urban green areas in Prague. Ticks Tick Borne Dis 13:102053. doi: 10.1016/j.ttbdis.2022.102053 [DOI] [PubMed] [Google Scholar]
- 47. Marchant A, Le Coupanec A, Joly C, Perthame E, Sertour N, Garnier M, Godard V, Ferquel E, Choumet V. 2017. Infection of Ixodes ricinus by Borrelia burgdorferi sensu lato in peri-urban forests of France. PLoS One 12:e0183543. doi: 10.1371/journal.pone.0183543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Díaz P, Arnal JL, Remesar S, Pérez-Creo A, Venzal JM, Vázquez-López ME, Prieto A, Fernández G, López CM, Panadero R, Benito A, Díez-Baños P, Morrondo P. 2017. Molecular identification of Borrelia spirochetes in questing Ixodes ricinus from Northwestern Spain. Parasit Vectors 10:615. doi: 10.1186/s13071-017-2574-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marques AR, Strle F, Wormser GP. 2021. Comparison of lyme disease in the United States and Europe. Emerg. Infect. Dis 27:2017–2024. doi: 10.3201/eid2708.204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hubálek Z, Halouzka J. 1998. Prevalence rates of Borrelia burgdorferi sensu lato in host-seeking Ixodes ricinus ticks in Europe. Parasitol Res 84:167–172. doi: 10.1007/s004360050378 [DOI] [PubMed] [Google Scholar]
- 51. Gern L. 2008. Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: life in the wilds. Parasite 15:244–247. doi: 10.1051/parasite/2008153244 [DOI] [PubMed] [Google Scholar]
- 52. Jenkins A, Kristiansen BE, Allum AG, Aakre RK, Strand L, Kleveland EJ, van de Pol I, Schouls L. 2001. Borrelia burgdorferi sensu lato and Ehrlichia spp. in Ixodes ticks from Southern Norway. J Clin Microbiol 39:3666–3671. doi: 10.1128/JCM.39.10.3666-3671.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Csokai J, Klas EM, Heusinger A, Müller E. 2017. Occurrence of Ehrlichia canis in dogs living in germany and comparison of direct and indirect diagnostic methods. Tierarztl Prax Ausg K Kleintiere Heimtiere 45:301–307. doi: 10.15654/TPK-170065 [DOI] [PubMed] [Google Scholar]
- 54. Regier Y, Komma K, Weigel M, Kraiczy P, Laisi A, Pulliainen AT, Hain T, Kempf VAJ. 2019. Combination of microbiome analysis and serodiagnostics to assess the risk of pathogen transmission by ticks to humans and animals in central Germany. Parasit Vectors 12:11. doi: 10.1186/s13071-018-3240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, Ansari JM, Jefferson KK, Cava F, Jacobs-Wagner C, Fikrig E. 2017. Pathogen-mediated manipulation of Arthropod Microbiota to promote infection. Proc Natl Acad Sci U S A 114:E781–E790. doi: 10.1073/pnas.1613422114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Egyed L, Makrai L. 2014. Cultivable internal bacterial flora of ticks isolated in Hungary. Exp Appl Acarol 63:107–122. doi: 10.1007/s10493-013-9762-y [DOI] [PubMed] [Google Scholar]
- 57. Duron O, Binetruy F, Noël V, Cremaschi J, McCoy KD, Arnathau C, Plantard O, Goolsby J, Pérez de León AA, Heylen DJA, Van Oosten AR, Gottlieb Y, Baneth G, Guglielmone AA, Estrada-Peña A, Opara MN, Zenner L, Vavre F, Chevillon C. 2017. Evolutionary changes in symbiont community structure in ticks. Mol Ecol 26:2905–2921. doi: 10.1111/mec.14094 [DOI] [PubMed] [Google Scholar]
- 58. Bagdure SR, Fisher MA, Ryan ME, Khasawneh FA. 2012. Rhodococcus erythropolis encephalitis in patient receiving rituximab. Emerg Infect Dis 18:1377–1379. doi: 10.3201/eid1808.110434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Burtis JC, Sullivan P, Levi T, Oggenfuss K, Fahey TJ, Ostfeld RS. 2016. The impact of temperature and precipitation on blacklegged tick activity and lyme disease incidence in endemic and emerging regions. Parasit Vectors 9:606. doi: 10.1186/s13071-016-1894-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gil JC, Helal ZH, Risatti G, Hird SM. 2020. Ixodes scapularis microbiome correlates with life stage, not the presence of human pathogens, in ticks submitted for diagnostic testing. PeerJ 8:e10424. doi: 10.7717/peerj.10424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parola P, Paddock CD, Raoult D. 2005. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18:719–756. doi: 10.1128/CMR.18.4.719-756.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kurtti TJ, Felsheim RF, Burkhardt NY, Oliver JD, Heu CC, Munderloh UG. 2015. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int J Syst Evol Microbiol 65:965–970. doi: 10.1099/ijs.0.000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. René-Martellet M, Minard G, Massot R, Tran Van V, Valiente Moro C, Chabanne L, Mavingui P. 2017. Bacterial microbiota associated with Rhipicephalus sanguineus (sl) ticks from France, Senegal and Arizona. Parasit Vectors 10:416. doi: 10.1186/s13071-017-2352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thapa S, Zhang Y, Allen MS. 2019. Effects of temperature on bacterial microbiome composition in Ixodes scapularis ticks. Microbiologyopen 8:e00719. doi: 10.1002/mbo3.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thapa S, Zhang Y, Allen MS. 2019. Bacterial microbiomes of Ixodes scapularis ticks collected from Massachusetts and Texas, USA. BMC Microbiol 19:138. doi: 10.1186/s12866-019-1514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kmet’ V, Čaplová Z. 2021. An update on the Ixodes Ricinus Microbiome. J Microbiol Biotechnol Food Sci 2021:1340–1342. doi: 10.15414/jmbfs.2019.8.6.1340-1342 [DOI] [Google Scholar]
- 68. Jiao J, Lu Z, Yu Y, Ou Y, Fu M, Zhao Y, Wu N, Zhao M, Liu Y, Sun Y, Wen B, Zhou D, Yuan Q, Xiong X. 2021. Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in inner Mongolia, China. Parasit Vectors 14:287. doi: 10.1186/s13071-021-04740-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Estrada-Peña A, Cabezas-Cruz A, Pollet T, Vayssier-Taussat M, Cosson J-F. 2018. High throughput sequencing and network analysis disentangle the microbial communities of ticks and hosts within and between ecosystems. Front Cell Infect Microbiol 8:236. doi: 10.3389/fcimb.2018.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zolnik CP, Prill RJ, Falco RC, Daniels TJ, Kolokotronis S-O. 2016. Microbiome changes through ontogeny of a tick pathogen vector. Mol Ecol 25:4963–4977. doi: 10.1111/mec.13832 [DOI] [PubMed] [Google Scholar]
- 71. Hornett EA, Kageyama D, Hurst GDD. 2022. Sex determination systems as the interface between male-killing bacteria and their hosts. Proc Biol Sci 289:20212781. doi: 10.1098/rspb.2021.2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bohacsova M, Mediannikov O, Kazimirova M, Raoult D, Sekeyova Z. 2016. Arsenophonus nasoniae and rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS One 11:e0149950. doi: 10.1371/journal.pone.0149950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferreira J da S, Souza Oliveira DA, Santos JP, Ribeiro CCDU, Baêta BA, Teixeira RC, Neumann A da S, Rosa PS, Pessolani MCV, Moraes MO, Bechara GH, de Oliveira PL, Sorgine MHF, Suffys PN, Fontes ANB, Bell-Sakyi L, Fonseca AH, Lara FA. 2018. Ticks as potential vectors of Mycobacterium leprae: use of tick cell lines to culture the bacilli and generate transgenic strains. PLoS Negl Trop Dis 12:e0007001. doi: 10.1371/journal.pntd.0007001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nováková E, Hypsa V, Moran NA. 2009. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiol 9:143. doi: 10.1186/1471-2180-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hu R, Hyland KE. 1998. Effects of the feeding process of Ixodes scapularis (acari: ixodidae) on embryonic development of its parasitoid, Ixodiphagus hookeri (hymenoptera: encyrtidae). J Med Entomol 35:1050–1053. doi: 10.1093/jmedent/35.6.1050 [DOI] [PubMed] [Google Scholar]
- 76. Collatz J, Selzer P, Fuhrmann A, Oehme RM, Mackenstedt U, Kahl O, Steidle JLM. 2011. A hidden beneficial: biology of the tick-wasp Ixodiphagus hookeri in Germany. Journal of Applied Entomology 135:351–358. doi: 10.1111/j.1439-0418.2010.01560.x [DOI] [Google Scholar]
- 77. Taylor GP, Coghlin PC, Floate KD, Perlman SJ. 2011. The host range of the male-killing symbiont Arsenophonus nasoniae in filth fly parasitioids. J Invertebr Pathol 106:371–379. doi: 10.1016/j.jip.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 78. Larrousse F, King AG, Wolbach SB. 1928. The overwintering in Massachusetts of Ixodiphagus caucurtei. Science 67:351–353. doi: 10.1126/science.67.1735.351 [DOI] [PubMed] [Google Scholar]
- 79. Alfeev N. 1946. The utilization of Hunterellus hookeri How. for the control of the ticks, Ixodes ricinus L. and Ixodes persulcatus P. Sch. with reference to peculiarities of their metamorphosis under conditions of the Province of Lenningrad. Review of Applied Entomology 34:108–109. [Google Scholar]
- 80. Tijsse-Klasen E, Braks M, Scholte EJ, Sprong H. 2011. Parasites of vectors – Ixodiphagus hookeri and its Wolbachia symbionts in ticks in the Netherlands. Parasit Vectors 4:228. doi: 10.1186/1756-3305-4-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Plantard O, Bouju-Albert A, Malard M-A, Hermouet A, Capron G, Verheyden H. 2012. Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the hymenoptera endoparasitoid Ixodiphagus hookeri. PLoS One 7:e30692. doi: 10.1371/journal.pone.0030692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fukui T, Kawamoto M, Shoji K, Kiuchi T, Sugano S, Shimada T, Suzuki Y, Katsuma S. 2015. The endosymbiotic bacterium Wolbachia selectively kills male hosts by targeting the masculinizing gene. PLoS Pathog 11:e1005048. doi: 10.1371/journal.ppat.1005048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Arai H, Lin SR, Nakai M, Kunimi Y, Inoue MN. 2020. Closely related male-killing and nonmale-killing Wolbachia strains in the oriental tea tortrix Homona magnanima. Microb Ecol 79:1011–1020. doi: 10.1007/s00248-019-01469-6 [DOI] [PubMed] [Google Scholar]
- 84. Lo N, Beninati T, Sassera D, Bouman EAP, Santagati S, Gern L, Sambri V, Masuzawa T, Gray JS, Jaenson TGT, Bouattour A, Kenny MJ, Guner ES, Kharitonenkov IG, Bitam I, Bandi C. 2006. Widespread distribution and high prevalence of an alpha-Proteobacterial symbiont in the tick Ixodes ricinus. Environ Microbiol 8:1280–1287. doi: 10.1111/j.1462-2920.2006.01024.x [DOI] [PubMed] [Google Scholar]
- 85. Sassera D, Lo N, Bouman EAP, Epis S, Mortarino M, Bandi C. 2008. "Candidatus Midichloria" endosymbionts bloom after the blood meal of the host, the hard tick Ixodes ricinus. Appl Environ Microbiol 74:6138–6140. doi: 10.1128/AEM.00248-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sacchi L, Bigliardi E, Corona S, Beninati T, Lo N, Franceschi A. 2004. A symbiont of the tick Ixodes ricinus invades and consumes mitochondria in a mode similar to that of the parasitic bacterium Bdellovibrio bacteriovorus. Tissue Cell 36:43–53. doi: 10.1016/j.tice.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 87. Pinter A, Labruna MB, Faccini JLH. 2002. The sex ratio of Amblyomma cajennense (acari: ixodidae) with notes on the male feeding period in the laboratory. Vet Parasitol 105:79–88. doi: 10.1016/s0304-4017(01)00650-1 [DOI] [PubMed] [Google Scholar]
- 88. Levi T, Keesing F, Oggenfuss K, Ostfeld RS. 2015. Accelerated phenology of blacklegged ticks under climate warming. Philos Trans R Soc Lond B Biol Sci 370:20130556. doi: 10.1098/rstb.2013.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Overbeek L, Gassner F, van der Plas CL, Kastelein P, Nunes-da Rocha U, Takken W. 2008. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. FEMS Microbiol Ecol 66:72–84. doi: 10.1111/j.1574-6941.2008.00468.x [DOI] [PubMed] [Google Scholar]
- 90. Fernández-Ruvalcaba M, Peña-Chora G, Romo-Martínez A, Hernández-Velázquez V, de la Parra AB, De La Rosa DP. 2010. Evaluation of Bacillus thuringiensis pathogenicity for a strain of the tick, Rhipicephalus microplus, resistant to chemical pesticides. J Insect Sci 10:186. doi: 10.1673/031.010.14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lazarte JN, Lopez RP, Ghiringhelli PD, Berón CM. 2018. Bacillus wiedmannii biovar thuringiensis: a specialized mosquitocidal pathogen with plasmids from diverse origins. Genome Biol Evol 10:2823–2833. doi: 10.1093/gbe/evy211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ehling-Schulz M, Lereclus D, Koehler TM. 2019. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol Spectr 7:7–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Szczepańska A, Kiewra D, Guz-Regner K. 2018. Sensitivity of Ixodes ricinus (L., 1758) and Dermacentor reticulatus (Fabr., 1794) ticks to Bacillus thuringiensis isolates: preliminary study. Parasitol Res 117:3897–3902. doi: 10.1007/s00436-018-6096-z [DOI] [PubMed] [Google Scholar]
- 94. Vereecke D, Burssens S, Simón-Mateo C, Inzé D, Van Montagu M, Goethals K, Jaziri M. 2000. The Rhodococcus fascians-plant interaction: morphological traits and biotechnological applications. Planta 210:241–251. doi: 10.1007/PL00008131 [DOI] [PubMed] [Google Scholar]
- 95. Osdaghi E, Young AJ, Harveson RM. 2020. Bacterial wilt of dry beans caused by Curtobacterium flaccumfaciens pv. flaccumfaciens: a new threat from an old enemy. Mol Plant Pathol 21:605–621. doi: 10.1111/mpp.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Francis MJ, Doherty RR, Patel M, Hamblin JF, Ojaimi S, Korman TM. 2011. Curtobacterium flaccumfaciens septic arthritis following puncture with a coxspur hawthorn thorn. J Clin Microbiol 49:2759–2760. doi: 10.1128/JCM.00340-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Donnarumma G, Buommino E, Fusco A, Paoletti I, Auricchio L, Tufano MA. 2010. Effect of temperature on the shift of Pseudomonas fluorescens from an environmental microorganism to a potential human pathogen. Int J Immunopathol Pharmacol 23:227–234. doi: 10.1177/039463201002300120 [DOI] [PubMed] [Google Scholar]
- 98. Kim S, Li X-H, Hwang H-J, Lee J-H. 2020. Thermoregulation of Pseudomonas aeruginosa biofilm formation. Appl Environ Microbiol 86:e01584-20. doi: 10.1128/AEM.01584-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zimmer KR, Macedo AJ, Nicastro GG, Baldini RL, Termignoni C. 2013. Egg wax from the cattle tick Rhipicephalus (Boophilus) microplus inhibits Pseudomonas aeruginosa biofilm. Ticks Tick Borne Dis 4:366–376. doi: 10.1016/j.ttbdis.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 100. Long RA, Azam F. 2001. Antagonistic interactions among marine pelagic bacteria. Appl Environ Microbiol 67:4975–4983. doi: 10.1128/AEM.67.11.4975-4983.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eiler A, Heinrich F, Bertilsson S. 2012. Coherent dynamics and association networks among lake bacterioplankton taxa. ISME J 6:330–342. doi: 10.1038/ismej.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial genus abundance correlations.
Data Availability Statement
The short-read data of samples are publicly available and accessible through PRJNA828115 from the NCBI Sequence Read Archive (SRA).