ABSTRACT
Tripartite efflux systems transport antimicrobial agents, toxic metabolites, and siderophores from Gram-negative cells into the environment. For example, the main siderophore pyoverdine of the soil bacterium Pseudomonas putida KT2440 is secreted into the environment via the PvdRT-OpmQ and MdtABC-OpmB systems. Here, we looked for efflux systems that might be involved in the secretion of pyoverdine in addition to the latter two systems. Screening of different efflux systems revealed that parX (encoding the periplasmic adapter protein of the ParXY system) is of particular importance for bacterial growth under iron limitation. Further analysis showed that the deletion of parX impairs the production and secretion of pyoverdine, causing the observed growth effect. The effects were dependent on the presence of other tripartite efflux systems and the conditions of iron limitation. The results suggest that ParXY not only plays a role in antibiotic resistance, as shown previously, but also influences the secretion of siderophores in a network of overlapping activities of different tripartite efflux systems.
IMPORTANCE
Gram-negative bacteria from the Pseudomonas group are survivors in various environmental niches. For example, the bacteria secrete siderophores to capture ferric ions under deficiency conditions. Tripartite efflux systems are involved in the secretion of siderophores, which are also important for antibiotic resistance. For one of these efflux systems, the resistance-nodulation-cell division transporter ParXY from the model organism Pseudomonas putida KT2440, we show that it influences the secretion of the siderophore pyoverdine in addition to its already known involvement in antibiotic resistance. Phenotypically, its role in pyoverdine secretion is only apparent when other pyoverdine secretion systems are inactive. The results confirm that the different tripartite efflux systems have overlapping substrate specificities and can at least partially functionally substitute for each other, especially in important physiological activities such as supplying the cell with iron ions. This fact must be taken into account when developing specific inhibitors for tripartite efflux systems.
KEYWORDS: RND transporter, pyoverdine, ParXY, PP_3455, PP_3456, iron, siderophore, PvdRT-OpmQ, MdtABC-OpmB, MexAB, Pseudomonas putida KT2440, chloramphenicol
INTRODUCTION
Iron is essential as a trace element for almost every organism on earth, including bacteria, fungi, plants, and animals (1). Its importance ranges from crucial processes such as bacterial development to oxygen transport in the metabolism of invertebrates (2). It is a cofactor in iron-sulfur clusters and many enzymes such as nitrogenase (3), cytochrome c, hemoglobin, or catalase (4). However, the transition from vital to toxic is reached rather quickly (1): excess iron promotes the formation of harmful reactive oxygen species through the Fenton reaction, which can destroy Fe-S clusters and cause DNA damage (4, 5).
Iron-specific transporters can take up soluble ferrous (Fe2+) ions. However, Fe2+ is readily oxidized under aerobic conditions to the ferric form (Fe3+), which forms insoluble iron hydroxide (4). Fe3+ can be scavenged by complexation with siderophores. Bacteria, fungi, and plants are all capable of producing these iron chelators (6), which can have different structures and molecular sizes and differ in their functional groups. Synthesis and secretion of siderophores in bacteria are regulated at the transcriptional level in response to iron availability by extracytoplasmic sigma factors, which in turn are tightly controlled by the ferric uptake regulator (Fur) (7). While strains such as Escherichia coli (8) and Pseudomonas aeruginosa (5) produce more than one siderophore, Pseudomonas putida KT2440 produces pyoverdine as its only siderophore. It has a high affinity for ferric ions (kd of 10−32 M−1) (9) and can chelate ferric ions from abiotic and biotic sources (2). Pyoverdine is produced by cytoplasmic non-ribosomal peptide synthetases (NRPS) and matures in the periplasm to the final fluorescent structure (7, 10, 11). Although the mechanisms of siderophore synthesis, maturation, and uptake have been extensively studied (7), the secretion mechanisms and systems involved are only partially understood (12 – 15).
Gram-negative bacteria utilize ATP-binding cassettes (ABC) and resistance-nodulation-cell division (RND) efflux systems to enable the secretion of siderophores. These systems cross the inner membrane, periplasm, and outer membrane (16). Accordingly, the systems consist of proteins in the inner and outer membranes, connected by a periplasmic adaptor protein (17). These efflux systems contribute to microbial resistance to antibiotics and other toxic compounds (18 – 20). At the same time, they are crucial for microbial physiology, including bacterial cell communication, colonization, intracellular survival, and virulence (18, 20). The regulation of these transport systems involves a highly complex interplay of global and local transcriptional regulators and two-component systems (18, 20).
The involvement of the ABC and RND systems in the secretion of siderophores has been extensively studied in Escherichia coli (21). Here, AcrAB, AcrAD, and MdtABC, each together with TolC, a multifunctional outer membrane channel, form three RND systems involved in the secretion of the siderophore enterobactin (Ent) (21). In pseudomonads, the tripartite efflux system PvdRT-OpmQ (ABC type) is responsible for the secretion of newly synthesized and recycled siderophore pyoverdine. In P. aeruginosa, this is the only efflux system identified to date (13, 14, 22). In P. putida KT2440, previous studies have shown that in addition to PvdRT-OpmQ, the tripartite efflux system MdtABC-OpmB (RND type) is also involved in the secretion of the siderophore (15). First biochemical evidence for an interaction of the PvdRT-OpmQ system of P. putida KT2440 with pyoverdine has been recently presented, reconfirming the involvement of this system in siderophore transport (23). Inactivation of these transport systems leads to the inhibition of pyoverdine secretion and, thus, the growth of the bacterium in the presence of iron deficiency. The inhibition is partial, suggesting that at least one other system is involved in pyoverdine secretion or can take over the tasks of the deleted systems (2, 7, 15).
Here, we search for transport systems in addition to PvdRT-OpmQ and MdtABC-OpmB, which are important for the growth of P. putida KT2440 under iron limitation and might contribute to pyoverdine secretion. In the first experiment, genes encoding components of tripartite efflux pumps or outer membrane porins were individually deleted in a P. putida KT2440-derived strain, lacking functional PvdRT-OpmQ and MdtABC-OpmB (Δpm). A comparison of the growth of the resulting mutants under iron-replete and iron-deficient conditions revealed that the RND-type efflux system ParXY is crucial for growth under iron limitation. Subsequently, more detailed analyses of the role of ParXY revealed that the RND system influences growth, pyoverdine production, and secretion. The influence of ParXY depends on the presence of other efflux systems and the exact conditions of iron limitation in the culture medium, indicating a crucial role for this system in pyoverdine secretion under iron-scarce conditions.
RESULTS AND DISCUSSION
Screening for efflux systems and outer membrane porins critical for P. putida KT2440 growth under iron limitation
The tripartite efflux systems PvdRT-OpmQ and MdtABC-OpmB were previously suggested to be involved in pyoverdine secretion in P. putida KT2440 (15). Here, we show that P. putida KT2440 (wild type), P. putida ΔpvdRT-opmQΔmdtA (Δpm), and P. putida 3E2 [no biosynthesis of pyoverdine (24)] grew under iron-rich conditions [King’s B (KB) medium] with only small differences. However, under strong iron limitation [KB plus 1 mM 2′2-bipyridyl (Bip)], the growth of the Δpm strain was inhibited compared to the wild type but significantly better than that of strain 3E2 (Fig. 1). The results are in agreement with our previous observations (15) and suggest that other systems besides PvdRT-OpmQ and MdtABC-OpmB may contribute to the secretion of pyoverdine.
Fig 1.
Influence of different tripartite efflux systems and outer membrane porins on the growth of P. putida KT2440 and derived mutants. (A) Cells were grown under iron-rich conditions (KB medium) and (B) strong iron limitation (KB plus 1 mM Bip). Cultures were started at an OD600 of 0.08 and grown in a CLARIOstar Plus plate reader. For this figure, the AUC was determined using a baseline corresponding to the initial OD600. Mutants were generated in the genetic background of the Δpm strain lacking functional PvdRT-OpmQ and MdtABC-OpmB complexes. Consequently, the gene number on the x-axis indicates the third deletion introduced into the strain. In the case of ΔT6SS, we introduced the Δpm deletions into a strain without functional T6SSs [ΔtssA1ΔtssM2ΔtssM4 (25)]. For growth analysis, mutants were always grown together with the wild-type strain, the strain Δpm (light gray), and the pyoverdine non-producer 3E2 (dark gray). Under iron-deplete conditions, the largest growth defects were observed for strains 3E2 and ΔpmΔparX (Δpp3455) (cyan). Mean values of a minimum of at least four biological replicates are shown. ANOVA and Dunnett’s T3 multiple comparison tests (α 0.05) were used for statistical analysis. *0.0332; **0.0021; ***<0.0002; and ****<0.0001.
In search of these other transport systems, genes encoding components of tripartite efflux systems [PP_0166 (paxA), PP_0804, PP_0906 (mexV), PP_1264 (fusBCD), PP_1516 (mexJ), PP_2064 (mexH), PP_2818 (mexD), PP_3302, PP_3426 (mexF), PP_3455 (parX), PP_3549 (emrA), PP_5173 (triC)] or outer membrane porins [PP_1798, PP_4519, PP_4923 (all tolC-like), PP_1019 (oprB-I), PP_1273, PP_2069, PP_2558, PP_3427 (oprN)] were individually deleted in the Δpm strain (23). Furthermore, based on a previous report on the involvement of type 6 secretion systems (T6SSs) in pyoverdine secretion by Pseudomonas taiwanesis (26), we introduced the Δpm mutations into a P. putida KT2440R-derived strain (ΔT6SS) lacking all three T6SSs (25). All resulting mutants grew about equally well under iron-rich conditions (KB medium) (Fig. 1A). However, under strong iron limitation (KB plus 1 mM Bip), a strain with the triple deletion ΔpmΔparX (Δpp3455) showed the most striking effect besides strain 3E2 on growth compared to the Δpm strain. In fact, the area under the curve (AUC) used here as a growth parameter was for strain ΔpmΔparX about 40% of that of the Δpm strain. However, in contrast to the non-producer 3E2 (2% of Δpm), the ΔpmΔparX strain showed significant growth (Fig. 1B). Minor but statistically significant effects on the growth of the Δpm strain were caused by the additional deletion of PP_0166 (paxA), PP_3302 (RND family transporter), PP_3426, PP_3427 (inner and outer membrane components of the MexEF-OprN system), or PP_4519 and PP_4923 (tolC-like) (Fig. 1B). While the exact mechanism behind these effects is unknown, it should be noted that in KB plus 1 mM Bip medium, changes in the complex network of efflux systems may affect not only the circuits associated with iron acquisition (e.g., secretion of pyoverdine) but also resistance to toxic Bip. Indeed, the tripartite efflux systems TtgABC and MexEF-OprN were previously shown to be involved in the resistance to Bip (19, 27). Due to the strong iron-dependent impact of the parX deletion on the growth of the Δpm strain, we focus below on clarifying the role of the gene (gene product) under iron limitation.
Impact of the deletion of parX on chloramphenicol resistance of P. putida KT2440
The parX (PP_3455) gene encodes a periplasmic adaptor protein typically found in tripartite efflux systems of the RND type. The gene is in an operon with parY (PP_3456), the inner membrane component of the system. As the operon does not contain a gene encoding an outer membrane porin, parXY is expected to utilize the outer membrane components of other tripartite efflux systems (e.g., TtgC of TtgABC) (28). The amino acid sequences of ParX and ParY are indeed similar to the periplasmic (TtgA) and inner membrane components (TtgB) of the TtgABC system (Fig. S1). The ParXY system confers resistance to the antibiotics cefepime, aminoglycosides, and fluoroquinolones (28). Moreover, chloramphenicol treatment stimulates the expression of parX in P. putida strain KT2440R (28, 29), implicating ParXY in the transport of structurally diverse substrates.
To independently validate the loss of function of the ParXY system, we tested the effect of deletion of parX on chloramphenicol resistance under our conditions. We confirmed that deletion of the gene significantly increased the resistance of P. putida KT2440 to chloramphenicol (Fig. S2). Although biochemical evidence for direct functional interactions between ParXY and TtgABC is lacking, the results support the idea that the different tripartite efflux systems in P. putida KT2440 and other strains not only have overlapping substrate specificities but also interact functionally.
Impact of parX on colony morphology
Next, we analyzed the impact of the deletion of parX on the morphology of colonies (growth, color, and fluorescence) grown on KB agar plates with and without Bip. All strains (e.g., wild type, ΔparX, Δpm, ΔpmΔparX, pyoverdine non-producer 3E2) developed colonies of about the same size on KB agar (Fig. S3). Despite this observation, the colony of strain ΔpmΔparX was less fluorescent than the other strains but more fluorescent than pyoverdine non-producer 3E2. On KB plus 1 mM Bip, the colony development of strain ΔpmΔparX was poor compared to the other strains, and strain 3E2 did not grow at all (Fig. S3). For complementation, gene parX was cloned into pSEVA224 (30), and the colony morphology assay (CMA) was performed with cells transformed with either pSEVA224 (pSEVA) or pSEVA224-parX (pSEVA-parX) (Fig. 2). On KB agar plates, cells of strain ΔpmΔparX transformed with the empty plasmid were less fluorescent than the colonies of strain Δpm transformed with the same plasmid. Plasmid-based expression of parX in the ΔpmΔparX strain restored fluorescence to levels comparable to the Δpm strain. On KB plus 1 mM Bip plates, colony development of strain ΔpmΔparX was poor, and fluorescence was low compared to the two other strains. Again, plasmid-based expression of parX in the ΔpmΔparX strain restored colony development and fluorescence to levels comparable to the Δpm strain. This result confirms that the deletion of parX in the Δpm strain affects colony morphology on KB and KB plus 1 mM Bip plates and suggests a growth defect under iron limitation. On the contrary, deletion of parX alone in the wild type has no effect on colony morphology.
Fig 2.
Colony morphology assay for the ΔpmΔparX strain complemented with parX. (A) CMA under iron-replete conditions for strains Δpm transformed pSEVA224 without parX (control) and ΔpmΔparX transformed with pSEVA224 or pSEVA-parX. (B) CMA of the strains under iron-deplete conditions. Cells were pre-grown in KB medium containing 50 µg/mL kanamycin overnight at 30°C and continuous shaking. Ten microliters of the pre-culture were spotted onto KB agar plates containing 0.5 mM IPTG (induction of parX expression) and 50 µg/mL kanamycin without (left) and with the addition of 1 mM Bip (right). Plates were incubated for 18 h at 30°C and imaged using a BioRad Gel Doc XR + Gel Documentation System and trans-UV. Montages were generated using Fiji (31).
The importance of parX for growth in different liquid media
For growth experiments in liquid culture, the following media were chosen for cultivation: KB (6.57 ± 0.71 µM iron), KB plus 1 mM Bip (strong iron limitation), and casamino acid (CAA) (1.45 ± 0.19 µM iron). CAA was selected because it does not contain any known toxic compound (i.e., Bip or cetyltrimethylammonium bromide) whose presence could interfere with activities of tripartite efflux and other systems, as previously shown for Bip and the TtgABC and MexEF systems (19).
The effects of parX deletion on growth under different iron availability conditions in liquid culture are shown in Fig. 3. In KB medium, wild type and strains ΔparX, Δpm, and ΔpmΔparX grew well and showed no significant differences in terms of lag phase and growth rate (Fig. 3A). In KB plus 1 mM Bip, the combined deletion of genes of the Pvd and Mdt efflux systems (Δpm) resulted in a prolongation of the lag phase and a reduction of the specific growth rate (Fig. 3B and D). Both effects were significantly enhanced by the additional deletion of parX (strain ΔpmΔparX) (Fig. 3B and D). In contrast, individual deletion of parX had no significant effect on growth dynamics compared to the wild type. Growth of the 3E2 strain was severely impaired in KB plus 1 mM Bip (Fig. 3B and D). Under weak iron limitation (CAA), the deletions had qualitatively similar, however, weaker effects on growth (Fig. 3C). Statistically, only the combined inactivation of all three efflux systems (ΔpmΔparX) resulted in significantly altered growth parameters in the CAA medium. The latter strain grew very similar to strain 3E2 in this medium (Fig. 3C and D).
Fig 3.
Impact of parX deletion on the growth of P. putida KT2440 and derived mutants. (A) Iron-rich conditions (KB medium) and (B) strong iron limitation (KB plus 1 mM Bip) were used to grow strains P. putida KT2440 (WT), ΔparX, Δpm, ΔpmΔparX, and the pyoverdine non-producer 3E2. Bacteria were incubated with shaking for 18 h in a Tecan infinite M200 Pro plate reader. (C) For growth under weak iron limitation (CAA medium), glass flasks containing 35 mL medium were inoculated with the given strain (start OD600 = 0.1), incubated at 30°C with constant shaking, and the cell density (OD600) was measured hourly. (D) The specific growth rate of each strain was determined using data from the exponential phase of the growth curve under conditions of strong iron limitation (B) and weak iron limitation (C). Mean values of a minimum of three biological replicates are shown. ANOVA and Tukey’s multiple comparisons tests (α 0.05) were used for statistical analysis.
For complementation, the growth of strains Δpm and ΔpmΔparX transformed with pSEVA or pSEVA-parX were analyzed in KB plus 1 mM Bip (Fig. 4A) and in the CAA medium (Fig. 4B). Under strong iron limitation, the growth of the ΔpmΔparX strain transformed with the empty plasmid was impaired (prolonged lag phase, decreased growth rate) compared to the Δpm strain containing the same plasmid, as observed above with the strains without plasmid (Fig. 3B and D). However, the growth dynamics of the ΔpmΔparX strain expressing parX from plasmid pSEVA-parX were almost identical to those of strain Δpm with pSEVA, indicating that parX expression is sufficient for complementation. Similarly, the growth of strain ΔpmΔparX with pSEVA was impaired in the CAA medium compared to strain Δpm with pSEVA. Here, the expression of parX from pSEVA-parX stimulated the growth rate of strain ΔpmΔparX to values identical to those of strain Δpm while the lag phase was still slightly prolonged (Fig. 4B; Fig. S4). The prolonged lag phase in CAA medium, which is more nutrient-poor compared to KB, could be due to a temporal and/or quantitative imbalance of parX expression by the plasmid-based system. Thereby, it is possible that an insufficient amount of complementing adapter protein ParX is produced, leading to the prolonged lag phase. Taken together, the results confirm that the efflux system ParXY is crucial for growth under iron limitation when functional PvdRT-OpmQ and MdtABC-OpmB efflux systems are absent.
Fig 4.
Expression of parX from plasmid pSEVA224 complements the phenotype of P. putida KT2440 with the triple deletion ΔpmΔparX. (A) Strains containing the pSEVA224 plasmid encoding the adapter protein ParX (cyan) or empty plasmids (control, light, and medium gray) were grown in a 96-well plate with (A) KB medium supplemented with 1 mM 2′2-bipyridyl or (B) CAA medium. Media contained 0.5 mM IPTG for gene expression and kanamycin (50 mg/mL) for plasmid maintenance. Growth was started with an initial OD600 of 0.1, cultures were continuously shaken, and the OD600 was monitored for 20 h. (B) Growth rates were calculated from the exponential growth phase and are shown in Fig. S4. Mean values were calculated from a minimum of three biological replicates.
Influence of parX on the secretion of pyoverdine
Is the parX-dependent growth defect of the ΔpmΔparX strain under iron limitation related to an altered pyoverdine secretion? To answer this question, we compared the amounts of pyoverdine secreted into the culture medium (CAA) during the cultivation of the ΔpmΔparX strain with those of the wild-type, 3E2, ΔparX, and the Δpm strains. Because impaired pyoverdine secretion may be associated with the accumulation of the siderophore in the periplasm, we also determined the amounts of pyoverdine in the periplasm of the strains. In agreement with previous results (15), we found that the average amount of pyoverdine released into the CAA medium by the Δpm strain (normalized to the OD600) was significantly reduced compared with the wild type. Additional deletion of parX further reduced the amount of pyoverdine secreted. Deletion of parX alone in the wild type had no significant effect on the secretion of pyoverdine (Fig. 5A). After 24 h of growth, the difference in the amount of secreted pyoverdine between the strain Δpm and ΔpmΔparX became even more obvious (Fig. S5A). The phenotype was accompanied by a pale color of the ΔpmΔparX culture (Fig. S5B).
Fig 5.
Influence of the parX deletion on the secretion and periplasmic accumulation of pyoverdine of P. putida KT2440 and derived mutants. (A) Relative amounts of pyoverdine in the culture supernatants (extracellular pyoverdine). Cells were grown by shaking in CAA medium at 30°C for 7 h. One milliliter of culture was taken from each flask and centrifuged (15,700 × g for 3 min). Pyoverdine was determined based on its fluorescence (λ ex = 400 nm, λ em = 455 nm, Tecan infinite M200 Pro plate reader). Values were normalized to the OD600. (B) Relative amounts of pyoverdine in the periplasm of cells (intracellular pyoverdine). Aliquots of cells grown and shaken in CAA medium at 30°C for 3 h (Fig. 3C) were analyzed on agarose pads with a Leica DMi8 inverted microscope. Pyoverdine fluorescence was observed using a Leica DFC365 FX camera (CFP channel, exposure time: 250 ms; gain: 1.5). Fiji (31) and MicrobeJ (32) tools were used to analyze the intracellular fluorescence of each individual strain for a total of 350 cells. Relative units for the corrected mean of intracellular fluorescence in the cyan channel are shown. Mean values of a minimum of three biological replicates are shown. ANOVA and Tukey’s multiple comparisons tests (α 0.05) were used for statistical analysis. (C) Representative images in phase contrast and of fluorescence microscopy of WT, ΔparX, Δpm, and ΔpmΔparX.
The reduced amounts of pyoverdine secreted by the Δpm and ΔpmΔparX strains compared to the wild type were accompanied by increased levels of pyoverdine in the periplasm of both mutants (Fig. 5B). Remarkably, pyoverdine accumulation was slightly but significantly increased in strain ΔpmΔparX compared to strain Δpm. Deletion of parX alone in the wild type again had no significant effect on the amount of pyoverdine in the periplasm (Fig. 5B and C). These results suggest that an additional deletion of parX in the genetic background of the Δpm strain further impairs pyoverdine secretion. It is unclear whether ParXY itself is capable of transporting pyoverdine or whether this is an indirect effect. In addition, in the presence of the PvdRT-OpmQ and MdtABC-OpmB systems, a secretion of pyoverdine by the ParXY system does not seem to play a role.
Effect of externally added FeCl3, CuSO4, and pyoverdine on growth of strain ΔpmΔparX
To further test whether parX plays a role in iron acquisition via pyoverdine, the effect of externally added FeCl3 (Fig. 6A) and pyoverdine (Fig. 6B) on the growth of the ΔpmΔparX strain was analyzed in comparison to the wild-type and the Δpm strains. Since pyoverdines are also known to bind Cu2+, CuSO4 was included in the analysis. Growth experiments were performed in CAA medium in a 96-well microtiter plate format. The addition of 1 µM FeCl3 (Fig. 6A) stimulated the growth of the ΔpmΔparX strain to levels comparable to that of the Δpm strain. In contrast, the addition of 1 µM CuSO4 did not significantly increase the growth of the mutants (Fig. 6B). Supplementation with 10 µM pyoverdine yielded the best growth for the ΔpmΔparX strain (Fig. 6B). Since the addition of iron or pyoverdine stimulates the growth of the ΔpmΔparX strain, the defects caused by the mutations (including the parX deletion) are likely to be due to disturbances in pyoverdine-dependent iron uptake. Similar to the effects shown in CAA medium, the growth defects of mutants from Fig. 1 and 3 in KB supplemented with 1 mM Bip could also be complemented by the addition of FeCl3 at concentrations close to the Bip complex (one Fe3+ interacts with three Bip) to KB 1 mM Bip medium (Fig. S6). In agreement with the results in CAA medium, these data support the idea that the observed parX effects are not due to changes in Bip resistance but to iron supply.
Fig 6.
Rescue of ΔparX phenotype mediated by the addition of external FeCl3 and pyoverdine. (A) WT P. putida KT2440, Δpm, and triple deletion ΔpmΔparX were grown in CAA medium for 20 h. For deletion mutants Δpm and triple deletion ΔpmΔparX, the medium was supplemented with 1 µM of FeCl3. (B) Additionally, WT P. putida KT2440, Δpm, and triple deletion ΔpmΔparX were grown in CAA medium without (black) and with the addition of either 1 µM FeCl3 (medium gray), 1 µM CuSO4 (dark gray), or 10 µM pyoverdine (light gray). The AUC for all tested conditions was determined using the mean initial OD600 of each curve as a baseline: cells were grown for 20 h with continuous shaking with an orbital amplitude of 2, and optical density was measured in a Tecan infinite M200 Pro plate reader. Mean values for a minimum of three biological replicates are shown. ANOVA and Dunnett’s T3 multiple comparisons tests (α 0.05) were used for statistical analysis, always comparing against CAA medium. *0.0332 and **0.0021.
Dependence of parX expression on iron availability
If ParXY indeed plays a role in siderophore-mediated iron uptake, the question arises whether the expression of the corresponding operon is dependent on iron availability. To answer this question, the promoter region of the parXY operon was fused to the lux gene cluster in plasmid pBBR1-MCS5-lux (28). Subsequently, the luminescence of P. putida KT2440 transformed with the resulting plasmid was analyzed (Fig. 7). Based on the high luminescence values measured compared to the negative control (reporter plasmid lacking the parXY promoter region), the parXY operon was induced in all media used. However, there were significant differences between the luminescence values that correlated with iron availability. For example, the addition of 10 µM FeCl3 to the CAA medium resulted in an approximately sevenfold reduction in luminescence. The addition of 1 mM Bip to the KB medium resulted in a twofold increase in luminescence compared to KB alone. The results suggest that iron limitation stimulates the expression of the parXY operon. The mechanism behind this stimulation is not known. Unlike the promoters of pvdRT-opmQ and mdtABC-opmB, the parXY operon was not predicted to have a binding site for the Fur-dependent sigma factor PvdS (33). Instead, parXY expression was thought to be controlled by a two-component system (encoded by genes PP_3453 and PP_3454) immediately upstream of the operon (28). The stimulus perceived by the two-component system is not known. Based on sequence similarities with the RstBA system of E. coli, it has been speculated that PP_3453/PP_3454 interacts with another two-component system similar to PhoPQ, which senses fluctuations in extracellular Mg2+ concentration (28). Clearly, further studies are needed to understand the role of PP_3453/PP_3454.
Fig 7.

Impact of iron availability on parXY expression in P. putida KT2440. Expression was analyzed based on a transcriptional fusion of the promoter region of parXY with the luxCDABE operon in plasmid pBBR1 (34). Cells of P. putida KT2440 were cultivated in KB, KB plus 1 mM Bip, CAA, or CAA medium supplemented with 10 µM FeCl3, and luminescence was detected when the OD600 reached a value of 0.4 (exponential growth phase). Mean values of at least three biological replicates are shown. Mean values for respective measurements with the empty vector are 4.5 × 104 (KB), 1.0 × 105 (KB 1 mM Bip), 1.0 × 105 (CAA), and 6.6 × 104 (CAA 10 µM FeCl3). ANOVA and Tukey’s multiple comparisons tests (α 0.05) were used for statistical analysis of KB vs KB 1 mM Bip or CAA vs CAA + 10 µM FeCl3, respectively.
Effect of the deletion of genes encoding components of PvdRT-OpmQ, MdtABC-OpmB, and ParXY on gene expression
It is well known that tripartite efflux systems have overlapping substrate specificities and can functionally substitute for each other (18, 27, 35). In this context, we hypothesized that the loss of one or more efflux systems involved in pyoverdine secretion leads to the stimulation of the expression of genes of alternative efflux systems as compensation. To determine whether the PvdRT-OpmQ, MdtABC-OpmB, and ParXY efflux systems affect each other on the one hand and pyoverdine synthesis in terms of gene expression on the other, the promoters of the operons of these systems and of the pyoverdine synthesis gene pvdL (encodes a non-ribosomal peptide synthetase) were individually fused to the lux gene cluster in pBBR1-MCS5-lux (34). Subsequent analysis of gene expression by luminescence measurements in wild type and mutants with single or combined gene deletions revealed no or only relatively minor effects (Fig. 8). For example, although the results from above showed that the ParXY system became physiologically important when PvdRT-OpmQ and MdtABC-OpmB were not functional, the individual or combined deletion of genes of the latter two systems had only very little effect on the expression of parXY (Fig. 8A). Only inactivation of the MdtABC-OpmB system by gene deletion stimulated expression of PvdRT-OpmQ system genes and vice versa (Fig. 8A), confirming the above hypothesis. Since expression of pyoverdine-related genes is controlled by sigma factors (e.g., PvdS), which in turn are regulated by Fur (15, 36, 37), a reduced secretion of pyoverdine and, as a consequence, a reduced uptake of iron into cells may affect Fur signaling and stimulate expression of the remaining functional efflux system. Contrary to the promoters of pvdRT-opmQ and mdtABC-opmB, the parXY operon was not predicted to have a PvdS binding site (33).
Fig 8.
Influence of the deletion of parX, mdtA, and pvdRT-opmQ on the expression of genes encoding tripartite efflux systems and a pyoverdine synthesis gene. Cells of P. putida KT2440 (WT) and the given derived mutants were transformed with pBBR1 (34), containing transcriptional fusions of the promoter region of the investigated efflux systems or the NRPS pvdL with the luxCDABE operon. Cells were cultivated in CAA medium, and the influence of the deletion of individual efflux systems (A) on the activities of the promoter of parXY (P parXY ), mdtABC-opmB (P mdtABC-opmB ), and pvdRT-opmQ (P pvdRT-opmQ ) and (B) on pvdL (P pvdL ) was tested. The graphs represent the absolute data (AU) of luciferase activity at an optical density of OD600 = 0.4 (exponential phase). Mean values of at least three biological replicates are shown. ANOVA and Dunnett’s multiple comparisons tests (α 0.05) with the WT as a control strain were used for statistical analysis.
Surprisingly, deletion of parX resulted in approximately twofold reduced expression of the mdtABC-opmB operon (Fig. 8A). Similarly, expression of pvdL was approximately twofold reduced upon parX deletion, while deletion of the two other efflux systems (strain Δpm) had no impact on pvdL expression (Fig. 8B). The physiological significance of the latter phenomena is unclear. For the ΔpmΔparX mutant, one could speculate that reduced pyoverdine production in the absence of three (putative) pyoverdine secretion systems prevents the accumulation of pyoverdine in the periplasm at toxic concentrations. The accumulation of intracellular siderophores can indeed have drastic effects on cell viability (38).
Conclusion
This study showed that the ParXY system is crucial for growth under conditions of iron limitation when the pyoverdine-secreting tripartite systems PvdRT-OpmQ and MdtABC-OpmB are inactive. The growth defect is associated with decreased secretion of pyoverdine into the environment and accumulation of the siderophore in the periplasm. Growth of a mutant in which all three efflux systems were inactivated by gene deletion was restored to wild type levels by the addition of ferric iron or pyoverdine. In addition, expression of the parXY operon in the wild type was stimulated by iron limitation via an as yet unknown mechanism. Overall, the results suggest that ParXY contributes to the secretion of pyoverdine in addition to PvdRT-OpmQ and MdtABC-OpmB. Considering the strength of the phenotypes of mutants with individual and combined gene deletions of the three systems [this publication and reference (15)], PvdRT-OpmQ seems to be the main pyoverdine secretion system of P. putida KT2440, followed by MdtABC-OpmB. Since the deletion of parX alone does not have a significant effect on growth under iron limitation as well as pyoverdine secretion, it remains questionable whether the proposed ability of ParXY to secrete pyoverdine in the presence of the other two tripartite efflux systems is of physiological significance. However, if required, the ParXY system might be able to contribute to the secretion of siderophores with the help of TtgC or another outer membrane porin (Fig. 9). Nevertheless, to unequivocally demonstrate that the ParXY system is able to contribute to the secretion of the siderophore pyoverdine, biochemical evidence of direct binding and transport by the system is required, as has been provided in initial experiments for the PvdRT-OpmQ system (23). Finally, the increased resistance of the ΔparX strain to chloramphenicol suggests functional interactions of ParXY with other RND efflux systems (e.g., TtgABC). Taken together, the results are consistent with the idea that individual or combined deletion of tripartite efflux systems can have major effects on the physiological state of bacteria. This was particularly evident in a recent analysis in which six RND systems were simultaneously removed in P. aeruginosa (17). The resulting cells were characterized by iron starvation and modified lipid A.
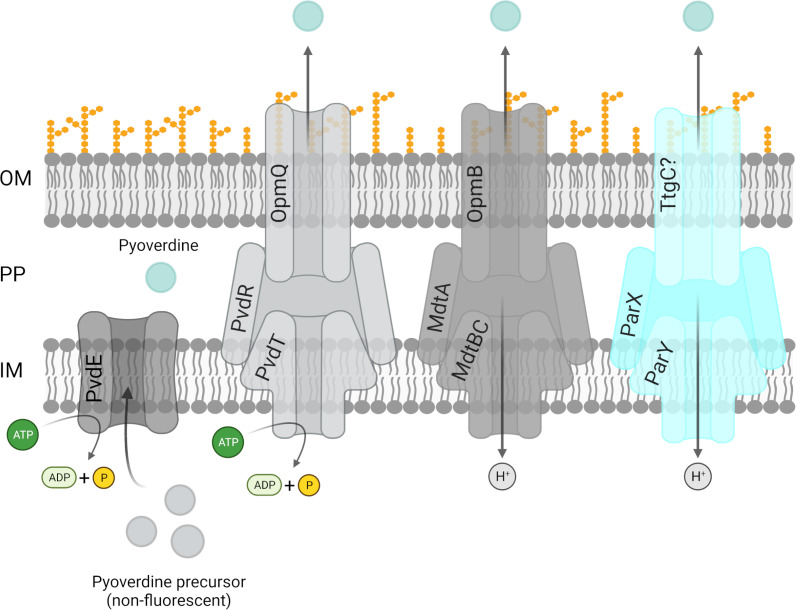
Fig 9.
Proposed model for efflux pumps involved in pyoverdine secretion in P. putida KT2440. Pyoverdine precursor is synthesized in the cytosol and transported into the periplasmic space (PP) via the ABC transporter PvdE. Several efflux systems are proposed to be involved in this process: PvdRT-OpmQ, MdtABC-OpmB (15), and ParXY-OMP. Initial biochemical evidence for an interaction of PvdRT-OpmQ with pyoverdine has recently been published (23).
MATERIALS AND METHODS
Bacterial strains and cultivation
Escherichia coli and P. putida strains and plasmids used for this study are listed in Tables S1 and S2. Pseudomonas strains were cultivated in casamino acid medium containing 0.5% casein hydrolysate, 6.77 mM K2HPO4, and 1.02 mM MgSO4xH2O under aerobic conditions by constant shaking at 180 rpm at 30°C (24). For colony morphology analysis, overnight cultures of cells were grown in King’s B medium (39) containing 2% peptone, 0.15% K2HPO4, 0.15% MgSO4x(7H2O), and 1% (wt/vol) glycerol. Cells transformed with plasmids were grown in the presence of 50 µg/mL kanamycin (pSEVA224, pNPTS138-R6KT) or 30 µg/mL gentamycin (pBBR1-MCS5-lux). For measurements of growth and luminescence in the 96-well format, the gentamycin concentration was reduced to 15 µg/mL. Plasmid-based gene expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). For storage, cells were grown in lysogeny broth LB medium (1% tryptone/peptone, 1% NaCl, 0.5% yeast extract) overnight, supplemented with glycerol freezing medium (48.75% glycerol, 12 mM KCl, 12 mM NaCl, 0.3 mM MgSO4), frozen in liquid nitrogen, and kept at −80°C. For agar plates, culture media were supplemented with 1.5% agar and poured into Petri dishes. In addition, P. putida KT2440 and derived strains were grown on Cetrimide agar plates (40). After inoculation, colonies developed at 30°C overnight. The resulting plates were kept at 4°C.
Generation of plasmids and mutants
Oligonucleotides used during this study are listed in Table S3. For the generation of deletion mutants listed in Table S1, homologous recombination with the pNPTS138-R6KT system (41) was used, as described by Henríquez et al. (15). For complementation, the gene PP_3455 (parX) was amplified by PCR and cloned into the multiple cloning site (MCS) of pSEVA224 (30). For gene expression analyses, putative promoter regions were determined using the BPROM tool (42). Identified regions were cloned into pBBR1-MCS5-luxCDABE (34). PCR and sequencing were used to confirm the final plasmids and strains.
Growth analyses
For the initial growth analysis in Fig. 1, cells from overnight cultures in KB medium were used to inoculate KB medium with and without the addition of 1 mM 2′2-bipyridyl in a 96-well plate (200 µL). Growth was monitored using a CLARIOstar Plus (BMG LABTECH) with hourly measurements of the optical density at 600 nm (OD600). P. putida KT2440 (WT) served as a control strain for each setup. The AUC was determined using GraphPad Prism version 9.4.1 and a minimum of four biological replicates.
For further growth analysis in CAA medium in flasks, P. putida strains were initially grown in LB medium for 8 h. The resulting cells were used to start an overnight culture in CAA medium. This step helps the cells to adapt to iron limitation. Centrifugation (15,700 × g, 3 min) was used to obtain the cells from CAA cultures. Cells were washed and used to inoculate 35 mL of fresh CAA medium (initial OD600 = 0.05). Growth curves were recorded under continuous shaking at 180 rpm and 30°C, and the OD600 was measured. Complementation, recovery experiments with FeCl3, CuSO4, or pyoverdine, and chloramphenicol susceptibility testing were performed in a 96-well plate (Greiner) using a Tecan Infinite M200 Pro plate reader or a CLARIOstar Plus (BMG LABTECH) without initial washing. The growth rate was calculated from the exponential phase of the growth curve. For growth recovery experiments using purified pyoverdine, the siderophore was extracted following a modified protocol based on Meyer et al. (43), as recently described in Stein et al. (23). All recovery experiments were performed in CAA and KB medium. For CAA, FeCl3 and CuSO4 were added at a final concentration of 1 µM, whereas pyoverdine was supplemented at 10 µM prior to measurements. For KB, the medium contained 1 mM 2′2-bipyridyl and 100, 200, 300, or 400 µM of FeCl3. Susceptibility testing towards chloramphenicol was performed in Mueller Hinton (MH) medium (Sigma-Aldrich). Here, pre-cultures in MH medium were used to inoculate MH medium containing 0, 40, 80, 100, 120 to 140 µg/mL chloramphenicol (96-well plates, 200 µL medium per well, initial OD600 = 0.1, orbital amplitude of 2).
Colony morphology assay
With some modifications, the experiment was conducted according to the protocol of Sakhtah et al. (44). Here, 10 µL of overnight cultures in KB medium was spotted onto KB plates with and without 1 mM of the iron chelator 2′2-bipyridyl and incubated at 30°C for 18 h. Agar plates were supplemented with 0.5 mM IPTG and 50 µg/mL of kanamycin for complementation assays. After incubation, the colonies were photographed under natural and UV light (BioRad Gel Doc XR + Gel Documentation System). Final images were generated using Fiji (31).
Determination of pyoverdine in the supernatant
Pseudomonas putida strains were grown in CAA medium for 7 h. A 1 mL aliquot of the culture was centrifuged (15,700 × g, 3 min). The fluorescence of the resulting supernatant was determined with Tecan infinite M200 Pro plate reader using an excitation wavelength λ ex of 400 nm and an emission wavelength λem of 455 nm. Values were normalized by the OD600 of the original culture.
Total reflection X-ray fluorescence
To analyze the element composition, total X-ray fluorescence was performed on a Bruker T-Star following a protocol described by Höhner et al. (45). For iron determination in bacterial media, the protocol was adapted as follows: media were mixed with an internal standard solution in a 1:1 ratio to a final concentration of 1 mg/mL gallium, 50 mg/mL scandium, and 0.2% polyvinyl alcohol. Subsequently, samples were dried on quartz-glass carriers (Bruker). Iron concentrations were determined via total X-ray fluorescence with Mo-K excitation at 50 kV using gallium as the standard element.
Single-cell fluorescence analysis
Pseudomonas putida strains were grown in CAA medium for 3 h, harvested, and washed by centrifugation (15,700 × g, 3 min). The resulting cells were spotted on agar pads (2% agarose), and imaging was performed with a Leica DMi8 inverted microscope equipped with a Leica DFC365 FX camera (Wetzlar, Germany). Phase contrast and cyan filter with a λex of 436 nm and a λem of 480 nm with a 40 nm bandwidth and an exposure of 250 ms, gain 4, and 100% intensity were used to capture the images. Fluorescence quantification and image analysis were done using Fiji (31) and MicrobeJ (32) tools.
Luciferase activity assay
Overnight cultures (5 mL LB medium with 30 µg/mL gentamycin, incubated at 30°C) of P. putida transformed with plasmids pBBR1-P parXY -lux, pBBR1-P mdtABC-opmB-lux, pBBR1-P pvdRT-opmQ-lux, pBBR1-PPP_4243-lux (PvdL), or pBBR1-MCS5-lux (control) were used to inoculate CAA medium containing 15 µg/mL gentamycin (96-well plate format, 200 µL medium per well, initial OD600 = 0.1). Plates were incubated in a CLARIOstar Plus (BMG LABTECH) plate reader at 30°C for 20 h and double orbital shaking with 600 rpm. After subtracting the background signal, the luminescence values measured at an OD600 of 0.4 (exponential phase) were plotted individually for P. putida strains containing either control or plasmids with indicated promoter regions.
Statistical analysis and figure visualization
GraphPad Prism version 9.4.1 for Windows (GraphPad Software, San Diego, California, USA) was used to plot and statistically analyze the data. Ordinary one-way ANOVA, Tukey’s multiple comparison test, Dunnett’s (T3) multiple comparisons test, Kruskal-Wallis test, two-way ANOVA, and Šídák’s multiple comparisons tests were used as indicated. The generation of figures was done using Affinity Designer version 1.10.4.1198 for Windows.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tania Henríquez for the generation of strains P. putida KT2440 Δpm and Δmdt. The proposed model was generated using Biorender.com.
Research and the APC in the group of H.J. were supported by the Deutsche Forschungsgemeinschaft, projects JU333/6-1, and the Faculty of Biology, LMU Munich. The funder had no role in the study design, data collection, and interpretation or the decision to submit the work for publication.
P. putida strain 3E2 and P. putida strain KT2440R ΔtssA1ΔtssM2ΔtssM3 (ΔT6SS) were kindly provided by Pierre Cornelis (Vrije Universiteit Brussels, Belgium) and Alain Filloux (Imperial College London), respectively.
N.V.S. and H.J. planned and supervised the experiments; N.V.S., M.E., F.B., and S.S. generated the strains and plasmids and performed growth curves; N.V.S. and S.S. conducted pyoverdine measurements; N.V.S. performed microscopy and image evaluation; N.V.S. and F.B. performed the luciferase assays; N.V.S., M.E., and F.B. performed the susceptibility tests and rescue experiments; L.H. and H.-H.K. performed TXRF measurements; N.V.S. and H.J. wrote the manuscript; H.-H.K. and H.J. supervised and acquired funding. All authors reviewed and approved the manuscript.
Contributor Information
Heinrich Jung, Email: hjung@lmu.de.
Giordano Rampioni, Universita degli Studi Roma Tre Dipartimento di Scienze, Rome, Italy .
DATA AVAILABILITY
Supporting data, additional oligonucleotide sequences, and findings of this study are available from the corresponding authors for provable reasons.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02300-23.
Tables S1 to S3 and Fig. S1 to S6.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Lasocki S, Gaillard T, Rineau E. 2014. Iron is essential for living! Crit Care 18:678. doi: 10.1186/s13054-014-0678-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poole K, Heinrichs DE, Neshat S. 1993. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol 10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x [DOI] [PubMed] [Google Scholar]
- 3. Rubio LM, Ludden PW. 2008. Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu Rev Microbiol 62:93–111. doi: 10.1146/annurev.micro.62.081307.162737 [DOI] [PubMed] [Google Scholar]
- 4. Cornelis P, Wei Q, Andrews SC, Vinckx T. 2011. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 3:540–549. doi: 10.1039/c1mt00022e [DOI] [PubMed] [Google Scholar]
- 5. Cunrath O, Geoffroy VA, Schalk IJ. 2016. Metallome of Pseudomonas aeruginosa: a role for siderophores. Environ Microbiol 18:3258–3267. doi: 10.1111/1462-2920.12971 [DOI] [PubMed] [Google Scholar]
- 6. Ahmed E, Holmström SJM. 2014. Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208. doi: 10.1111/1751-7915.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ringel MT, Brüser T. 2018. The biosynthesis of pyoverdines. Microb Cell 5:424–437. doi: 10.15698/mic2018.10.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Searle LJ, Méric G, Porcelli I, Sheppard SK, Lucchini S. 2015. Variation in siderophore biosynthetic gene distribution and production across environmental and faecal populations of Escherichia coli. PLoS One 10:e0117906. doi: 10.1371/journal.pone.0117906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer J.M., Abdallah M.A.. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. Microbiology 107:319–328. doi: 10.1099/00221287-107-2-319 [DOI] [Google Scholar]
- 10. Yeterian E, Martin LW, Guillon L, Journet L, Lamont IL, Schalk IJ. 2010. Synthesis of the siderophore pyoverdine in Pseudomonas aeruginosa involves a periplasmic maturation. Amino Acids 38:1447–1459. doi: 10.1007/s00726-009-0358-0 [DOI] [PubMed] [Google Scholar]
- 11. Baune M, Qi Y, Scholz K, Volmer DA, Hayen H. 2017. Structural characterization of pyoverdines produced by Pseudomonas putida KT2440 and Pseudomonas taiwanensis VLB120. Biometals 30:589–597. doi: 10.1007/s10534-017-0029-7 [DOI] [PubMed] [Google Scholar]
- 12. Hannauer M, Braud A, Hoegy F, Ronot P, Boos A, Schalk IJ. 2012. The PvdRT-OpmQ efflux pump controls the metal selectivity of the iron uptake pathway mediated by the siderophore pyoverdine in Pseudomonas aeruginosa. Environ Microbiol 14:1696–1708. doi: 10.1111/j.1462-2920.2011.02674.x [DOI] [PubMed] [Google Scholar]
- 13. Hannauer M, Yeterian E, Martin LW, Lamont IL, Schalk IJ. 2010. An efflux pump is involved in secretion of newly synthesized siderophore by Pseudomonas aeruginosa. FEBS Lett 584:4751–4755. doi: 10.1016/j.febslet.2010.10.051 [DOI] [PubMed] [Google Scholar]
- 14. Yeterian E, Martin LW, Lamont IL, Schalk IJ. 2010. An efflux pump is required for siderophore recycling by Pseudomonas aeruginosa. Environ Microbiol Rep 2:412–418. doi: 10.1111/j.1758-2229.2009.00115.x [DOI] [PubMed] [Google Scholar]
- 15. Henríquez T, Stein NV, Jung H. 2019. PvdRT-OpmQ and MdtABC-OpmB efflux systems are involved in pyoverdine secretion in Pseudomonas putida KT2440. Environ Microbiol Rep 11:98–106. doi: 10.1111/1758-2229.12708 [DOI] [PubMed] [Google Scholar]
- 16. Ramos J-L, Sol Cuenca M, Molina-Santiago C, Segura A, Duque E, Gómez-García MR, Udaondo Z, Roca A. 2015. Mechanisms of solvent resistance mediated by interplay of cellular factors in Pseudomonas putida. FEMS Microbiol Rev 39:555–566. doi: 10.1093/femsre/fuv006 [DOI] [PubMed] [Google Scholar]
- 17. Adamiak JW, Jhawar V, Bonifay V, Chandler CE, Leus IV, Ernst RK, Schweizer HP, Zgurskaya HI. 2021. Loss of RND-type multidrug efflux pumps triggers iron starvation and lipid A modifications in Pseudomonas aeruginosa. Antimicrob Agents Chemother 65:e0059221. doi: 10.1128/AAC.00592-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li XZ, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henríquez T, Stein NV, Jung H. 2020. Resistance to bipyridyls mediated by the TtgABC efflux system in Pseudomonas putida KT2440. Front Microbiol 11:1974. doi: 10.3389/fmicb.2020.01974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Housseini B Issa K, Phan G, Broutin I. 2018. Functional mechanism of the efflux pumps transcription regulators from Pseudomonas aeruginosa based on 3D structures. Front Mol Biosci 5:57. doi: 10.3389/fmolb.2018.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horiyama T, Nishino K. 2014. AcrB, AcrD, and MdtABC multidrug efflux systems are involved in enterobactin export in Escherichia coli. PLoS ONE 9:e108642. doi: 10.1371/journal.pone.0108642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imperi F, Tiburzi F, Visca P. 2009. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 106:20440–20445. doi: 10.1073/pnas.0908760106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stein NV, Eder M, Brameyer S, Schwenkert S, Jung H. 2023. The ABC transporter family efflux pump PvdRT-OpmQ of Pseudomonas putida KT2440: purification and initial characterization. FEBS Lett 597:1403–1414. doi: 10.1002/1873-3468.14601 [DOI] [PubMed] [Google Scholar]
- 24. Matthijs S, Laus G, Meyer J-M, Abbaspour-Tehrani K, Schäfer M, Budzikiewicz H, Cornelis P. 2009. Siderophore-mediated iron acquisition in the entomopathogenic bacterium Pseudomonas entomophila L48 and its close relative Pseudomonas putida KT2440. Biometals 22:951–964. doi: 10.1007/s10534-009-9247-y [DOI] [PubMed] [Google Scholar]
- 25. Bernal P, Allsopp LP, Filloux A, Llamas MA. 2017. The Pseudomonas putida T6SS is a plant warden against phytopathogens. ISME J 11:972–987. doi: 10.1038/ismej.2016.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen WJ, Kuo TY, Hsieh F-C, Chen P-Y, Wang C-S, Shih Y-L, Lai Y-M, Liu J-R, Yang Y-L, Shih M-C. 2016. Involvement of type VI secretion system in secretion of iron chelator pyoverdine in Pseudomonas taiwanensis. Sci Rep 6:32950. doi: 10.1038/srep32950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henriquez T, Baldow T, Lo YK, Weydert D, Brachmann A, Jung H. 2020. Involvement of MexS and MexEF-OprN in resistance to toxic ion chelators in Pseudomonas putida KT2440. Microorganisms 8:1782. doi: 10.3390/microorganisms8111782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puja H, Comment G, Chassagne S, Plésiat P, Jeannot K. 2020. Coordinate overexpression of two RND efflux systems, ParXY and TtgABC, is responsible for multidrug resistance in Pseudomonas putida. Environ Microbiol 22:5222–5231. doi: 10.1111/1462-2920.15200 [DOI] [PubMed] [Google Scholar]
- 29. Fernández M, Conde S, de la Torre J, Molina-Santiago C, Ramos J-L, Duque E. 2012. Mechanisms of resistance to chloramphenicol in Pseudomonas putida KT2440. Antimicrob Agents Chemother 56:1001–1009. doi: 10.1128/AAC.05398-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva-Rocha R, Martínez-García E, Calles B, Chavarría M, Arce-Rodríguez A, de Las Heras A, Páez-Espino AD, Durante-Rodríguez G, Kim J, Nikel PI, Platero R, de Lorenzo V. 2013. The standard European vector architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res 41:D666–D675. doi: 10.1093/nar/gks1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ducret A, Quardokus EM, Brun YV. 2016. Microbej, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. doi: 10.1038/nmicrobiol.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swingle B, Thete D, Moll M, Myers CR, Schneider DJ, Cartinhour S. 2008. Characterization of the PvdS-regulated promoter motif in Pseudomonas syringae pv. tomato DC3000 reveals regulon members and insights regarding PvdS function in other pseudomonads. Mol Microbiol 68:871–889. doi: 10.1111/j.1365-2958.2008.06209.x [DOI] [PubMed] [Google Scholar]
- 34. Gödeke J, Heun M, Bubendorfer S, Paul K, Thormann KM. 2011. Roles of two Shewanella oneidensis MR-1 extracellular endonucleases. Appl Environ Microbiol 77:5342–5351. doi: 10.1128/AEM.00643-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li XZ, Nikaido H. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64:159–204. doi: 10.2165/00003495-200464020-00004 [DOI] [PubMed] [Google Scholar]
- 36. Pasqua M, Visaggio D, Lo Sciuto A, Genah S, Banin E, Visca P, Imperi F. 2017. Ferric uptake regulator Fur is conditionally essential in Pseudomonas aeruginosa. J Bacteriol 199:22. doi: 10.1128/JB.00472-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leoni L, Orsi N, de Lorenzo V, Visca P. 2000. Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J Bacteriol 182:1481–1491. doi: 10.1128/JB.182.6.1481-1491.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones CM, Wells RM, Madduri AVR, Renfrow MB, Ratledge C, Moody DB, Niederweis M. 2014. Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. Proc Natl Acad Sci U S A 111:1945–1950. doi: 10.1073/pnas.1311402111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307. [PubMed] [Google Scholar]
- 40. Brown VI, Lowbury EJ. 1965. Use of an improved cetrimide agar medium and other culture methods for Pseudomonas aeruginosa. J Clin Pathol 18:752–756. doi: 10.1136/jcp.18.6.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lassak J, Henche A-L, Binnenkade L, Thormann KM. 2010. ArcS, the cognate sensor kinase in an atypical Arc system of Shewanella oneidensis MR-1. Appl Environ Microbiol 76:3263–3274. doi: 10.1128/AEM.00512-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solovyev V, Salamov A. 2011. Automatic annotation of microbial genomes and metagenomic sequences, p 61–78. In Metagenomics & its applications in agriculture, biomedicine & environmental studies may 2011 Ed. Nova Science Publishers. [Google Scholar]
- 43. Meyer J-M, Stintzi A, De Vos D, Cornelis P, Tappe R, Taraz K, Budzikiewicz H. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology (Reading) 143 ( Pt 1):35–43. doi: 10.1099/00221287-143-1-35 [DOI] [PubMed] [Google Scholar]
- 44. Sakhtah H, Koyama L, Zhang Y, Morales DK, Fields BL, Price-Whelan A, Hogan DA, Shepard K, Dietrich LEP. 2016. The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proc Natl Acad Sci U S A 113:E3538–E3547. doi: 10.1073/pnas.1600424113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Höhner R, Tabatabaei S, Kunz H-H, Fittschen U. 2016. A rapid total reflection X-ray fluorescence protocol for micro analyses of ion profiles in Arabidopsis thaliana. Spectrochim Acta Part B At Spectrosc 125:159–167. doi: 10.1016/j.sab.2016.09.013 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 to S6.
An accounting of the reviewer comments and feedback.
Data Availability Statement
Supporting data, additional oligonucleotide sequences, and findings of this study are available from the corresponding authors for provable reasons.