ABSTRACT
The bacterial pathogen Neisseria gonorrhoeae is an urgent global health problem due to increasing numbers of infections, coupled with rampant antibiotic resistance. Vaccines against gonorrhea are being prioritized to combat drug-resistant N. gonorrhoeae. Meningococcal serogroup B vaccines such as four-component meningococcal B vaccine (4CMenB) are predicted by epidemiology studies to cross-protect individuals from natural infection with N. gonorrhoeae and elicit antibodies that cross-react with N. gonorrhoeae. Evaluation of vaccine candidates for gonorrhea requires a suite of assays for predicting efficacy in vitro and in animal models of infection, including the role of antibodies elicited by immunization. Here, we present the development and optimization of assays to evaluate antibody functionality after immunization of mice: antibody binding to intact N. gonorrhoeae, serum bactericidal activity, and opsonophagocytic killing activity using primary human neutrophils [polymorphonuclear leukocytes (PMNs)]. These assays were developed with purified antibodies against N. gonorrhoeae and used to evaluate serum from mice that were vaccinated with 4CMenB or given alum as a negative control. Results from these assays will help prioritize gonorrhea vaccine candidates for advanced preclinical to early clinical studies and will contribute to identifying correlates and mechanisms of immune protection against N. gonorrhoeae.
KEYWORDS: Neisseria gonorrhoeae, antibody, vaccine, serum bactericidal activity, opsonophagocytic killing, imaging flow cytometry, complement
INTRODUCTION
Gonorrhea is a prominent global health threat, with 82.4 million cases estimated worldwide in 2020 (1). Challenges to the control of gonorrhea include increasing numbers of cases in the United States and worldwide, the high percentage of asymptomatic infections, and limited treatment options due to increasing resistance to the antibiotics used to treat gonorrhea (1–3). Untreated gonorrhea or where there is treatment failure has significant consequences for human health, including pelvic inflammatory disease leading to chronic pelvic pain, infertility, the life-threatening risk of ectopic pregnancy, and arthritis (4). Moreover, individuals with gonorrhea have an increased risk of both acquiring and spreading other sexually transmitted infections including HIV and chlamydia (5, 6). These issues have prompted organizations including the World Health Organization and US Centers for Disease Control and Prevention to call for the development of vaccines for gonorrhea (3, 7). Epidemiological modeling predicts that even a partially protective vaccine would help reduce the prevalence of gonorrhea, especially in populations experiencing high levels of infection (8–11).
Despite decades of effort, an effective gonorrhea vaccine has not yet been developed. The bacterium that causes gonorrhea, Neisseria gonorrhoeae, undergoes extensive phase and antigenic variation, as well as allelic recombination of immunodominant surface structures such as type IV pili, opacity-associated proteins, lipooligosaccharide, and the PorB porin (12). Further, antibodies against the reduction-modifiable protein on the gonococcal surface mask the presentation of PorB to protect it from immune recognition (13). Immunologically, acute gonorrhea is characterized by an inflammatory response in which neutrophils are recruited to sites of mucosal infection but do not clear the bacteria (14). The sustained neutrophilic inflammatory response may lead to tissue damage rather than control of infection (15). Neither the mechanisms nor the correlates of immune protection for gonorrhea are defined, since infected individuals do not generate protective immunity against subsequent infections (12).
For the related pathogen Neisseria meningitidis, bactericidal antibodies are the correlate of protection for vaccines that target the capsular polysaccharide or other surface-exposed components. A similar assumption has been made for N. gonorrhoeae, which like N. meningitidis is predominantly an extracellular pathogen. At mucosal surfaces, N. gonorrhoeae would be exposed to antibodies in secretions and inflammatory serum transudates, and bacteria that translocate to blood for disseminated infection would encounter antibodies in systemic circulation. Antibodies can protect against infection by extracellular pathogens by facilitating complement-mediated deposition on the pathogen surface that leads to lysis and/or opsonophagocytic killing and also by blocking essential functions of the pathogen such as adherence or nutrient acquisition (16, 17). The complement system is a cascade of highly regulated proteolytic activities that culminate in the deposition of C3b on the microbial surface (18). C3b and its degradation products serve as opsonins that bind complement receptors on phagocytic cells to lead to microbial uptake and intracellular killing. C3b is also part of the C5 convertase that is required for the formation of the membrane attack complex, a pore comprised of C5b-C9 that leads to microbial rupture. Degradation products C3a, C4a, and C5a increase vascular permeability, and C5a has chemotactic activity for phagocytes, which together enhance immune cell recruitment and activation when encountering microbes. The importance of the complement system in Neisserial pathogenesis is reflected in the ~1,000–10,000-fold increased susceptibility of individuals with complement deficiencies to invasive meningococcal and gonococcal disease (19).
The introduction of reverse vaccinology and the successful creation and deployment of vaccines against serogroup B Neisseria meningitidis have reinvigorated efforts to develop a gonorrhea vaccine. In 2017, immunization with an outer membrane vesicle (OMV)-based meningococcal serogroup B vaccine was reported to reduce the risk of gonorrhea in individuals in New Zealand with an estimated efficacy of 31%, based on a retrospective epidemiological study, without affecting susceptibility to chlamydia (20). The vaccinated individuals also had a lower hospitalization rate due to gonorrhea (21). Similarly, a different meningococcal B OMV vaccine markedly reduced gonorrhea rates in Cuba by retrospective analysis (22, 23). In 2015, the four-component meningococcal B vaccine (4CMenB; trade name Bexsero) was licensed for use in the US. It is comprised of OMV from the New Zealand serogroup B vaccine strain, along with three recombinant antigens [Neisserial adhesin A (NadA), the factor H binding protein fused to the GNA2091 antigen, and Neisserial heparin binding antigen fused to the GNA1030 antigen] (24). These three antigens were included in part for their ability to elicit antibodies that conferred serum bactericidal activity against serogroup B N. meningitidis (25). 4CMenB vaccination reduces the duration of colonization by N. gonorrhoeae in a female mouse genital tract infection model (26). Antibodies raised in response to 4CMenB and other meningococcal OMV-based immunogens in mice, rabbits, and humans have been shown to cross-react against antigens in N. gonorrhoeae lysates (26–28). Reports since the New Zealand and Cuba studies suggest a 32%–46% reduced incidence of gonorrhea in individuals vaccinated with 4CMenB compared with those who were not, while the incidence of chlamydia in these cohorts was unaffected by vaccination (29–32). 4CMenB is in several Phase III clinical trials for its efficacy against gonorrhea [recently summarized in (33)], and additional OMV-based vaccines, including those with detoxified LPS, are being evaluated preclinically (28). Both defined and novel surface-exposed components of N. gonorrhoeae, including protein and lipooligosaccharide antigens, are being evaluated preclinically for their vaccine potential (16, 17, 34–37).
Antibody-mediated serum bactericidal activity (SBA) has long been considered as a correlate of protection for vaccines against N. meningitidis (38–41), and SBA titers were used to prioritize antigens for incorporation into 4CMenB (25). While there is less consensus about the contribution of opsonophagocytic killing activity (OPKA) to meningococcal vaccine efficacy, OPKA may help protect individuals whose antibody titer is not sufficient to evoke SBA and in those with terminal complement deficiencies who cannot mount SBA (40, 42–44). Assays to examine SBA and OPKA in N. gonorrhoeae are being developed (45, 46). The premise for both SBA and opsonophagocytosis is that vaccine-elicited antibody binds to the surface of bacteria, but the direct evaluation of bacterial antibody binding is not typically performed in the context of intact pathogens. Instead, isolated antigens are used in enzyme-linked immunosorbent assays (ELISA) or similar in vitro approaches, but the antigens may not be in the conformation, membrane environment, and density as found on the bacterial surface.
In this report, we present results from three assays to interrogate the functional antibody response against N. gonorrhoeae that is elicited by immunization of mice: measurement of antibody binding to the bacterial surface by imaging flow cytometry, SBA assay, and OPKA assay using primary human polymorphonuclear leukocytes (PMNs; predominantly neutrophils). Alternative analyses for complement C3 deposition on N. gonorrhoeae and for opsonophagocytosis of N. gonorrhoeae by HL-60 promyelocytes using flow cytometry are also presented. As proof of concept, we show that the serum from mice that are vaccinated with meningococcal 4CMenB elicits stronger functional antibody responses than serum from alum-treated mice. Results from these assays will support the quest for a gonorrhea vaccine and will allow the discovery of correlates and mechanisms of immune protection against N. gonorrhoeae.
MATERIALS AND METHODS
Bacterial strains and growth conditions
The following N. gonorrhoeae strains were used for this study: FA1090 (47), F62 (48), FA19 (49), MS11 (50), and H041 (WHO X) (51, 52). N. gonorrhoeae was routinely grown on gonococcal medium base (BD Difco) plus Kellogg’s supplement I and 1.25 µM Fe(NO3)3 (53) [gonococcal base (GCB)] plates for 16 h at 37°C in 5% CO2.
Human sera as complement sources
For SBA, IgG/IgM-depleted pooled normal human serum (Igd-NHS, PelFreez Catalog #34010, Lot #28341) was used as the complement source. C6-depleted Normal Human Serum (Complement Technology, Catalog #A323) was used as the complement source for OPKA and opsonophagocytosis. Serum was heat inactivated by incubation at 56°C for 30 min.
Antibodies
See Table 1 for antibodies used in this study.
TABLE 1.
Antibodies used in this study
| Target | Antibody/clone name | Species, isotype | Labela | Concentration (mg/ml) | Source | Usea | Comments |
|---|---|---|---|---|---|---|---|
| 2C7 | Anti-N. gonorrhoeae LOS | IgG3 | (-) | 0.28 mg/ml | S. Ram, UMass | IFC, C3 deposition | Hybridoma supernatant |
| 6B4 | Anti-N. gonorrhoeae LOS | IgM, κ | (-) | 0.33 mg/ml | S. Ram, UMass | IFC, SBA | Hybridoma supernatant |
| CD11b, human | M1/70 | Rat IgG2b, κ | PE | 0.2 mg/ml | Biolegend | Flow cytometry | Catalog # 101207 |
| N. gonorrhoeae | Rabbit | (-) | 4-5 mg/ml | Meridian | IFC, SBA, OPKA | Catalog #B65111R | |
| IgG, rabbit | AffiniPure, Fc-fragment specific | Goat | AF488 | 1.5 mg/ml | Jackson Immuno Research | IFC | Catalog # 111-545-008 |
| IgG, mouse | Fcy fragment specific | Goat | AF488 | 1.5 mg/ml | Jackson Immuno Research | IFC | Catalog # 115-545-164 |
| IgM, mouse | µ-Chain specific | Goat | AF647 | 1.4 mg/ml | Jackson Immuno Research | IFC | Catalog # 115-606-075 |
| C3, human | Goat IgG | FITC | 7 mg/ml | MP Biomedical | IFC | Catalog # 55167 | |
| Isotype control for mouse IgG | E1D5H | Mouse IgG3 | FITC | 2.5 mg/ml | Cell Signaling Technology | IFC | Catalog # 37988 |
| Isotype control for mouse IgM | Clone MM-30 | Mouse IgMκ | APC | 2-3 mg/ml | Biolegend | IFC | Catalog # 401630 |
| Isotype control for goat anti-human C3 | Goat IgG | (-) | 1 mg/ml | R&D Systems | IFC | Catalog # AB-108-C |
AF, AlexaFluor; APC, allophycocyanin; FITC, fluorescein isothiocyanate; IFC, imaging flow cytometry; OPKA, opsonophagocytic killing; PE, phycoerythrin; SBA, serum bactericidal activity.
Mouse sera
All animal experiments were conducted at the Uniformed Services University according to guidelines established by the Association for the Assessment and Accreditation of Laboratory Animal Care using a protocol approved by the University’s Institutional Animal Care and Use Committee. Female Balb/c mice were vaccinated subcutaneously with 250 µL 4CMenB (GlaxoSmithKline) or given an equal volume of alum control three times, separated by 3-week intervals (days 0, 21, and 42). On day 63, sera were collected, pooled, and snap frozen at −80°C. Mouse sera were heat inactivated at 56°C for 30 min before use in functional assays. Only female mice were used in order to be compatible with vaccine efficacy studies in the future, since gonococcal infection has only been successfully established in female mice (54).
Antibody binding to N. gonorrhoeae by imaging flow cytometry
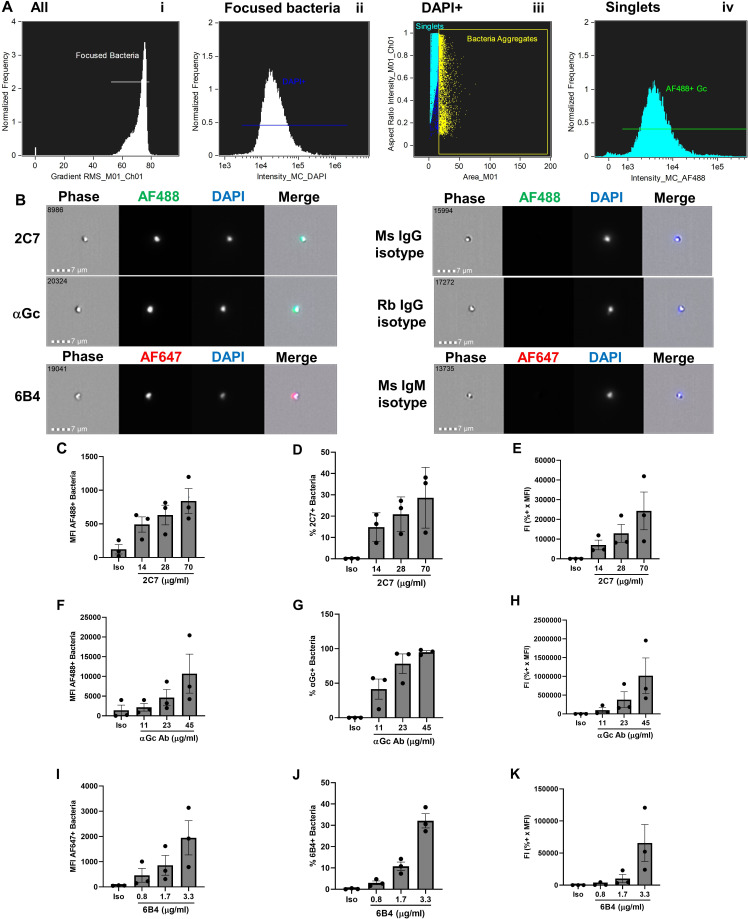
N. gonorrhoeae was swabbed from GCB agar into gonococcal base liquid media (GCBL) with Kellogg’s supplement I and 1.25 µM Fe(NO3)3 • 9 H2O (53, 55) to an optical density at 550 nm (OD550) of 0.392, pelleted by centrifugation at 10,000 × g for 3 min, and resuspended in 1 mL of RPMI medium + 2% bovine serum albumin (BSA; heat shock fraction, protease free, essentially globulin free, pH 7, Sigma, Catalog #A3059) to a concentration of ~2 × 108 CFU/mL. Twenty-five microliters of bacterial suspension was added to a 96-well V-bottom plate (Sarstedt, Catalog # 82.1583.001) and incubated with 25 µL of heat-inactivated mouse serum or control monoclonal antibodies (2C7, 6B4) or polyclonal rabbit anti-N. gonorrhoeae (GC; Meridian, # B65111R) at the indicated concentrations for 30 min at 37°C, 5% CO2. 2C7 was incubated at final concentrations of 70 µg/mL, 28 µg/mL, and 14 µg/mL; 6B4 was incubated at final concentrations of 3.3 µg/mL, 1.65 µg/mL, and 0.825 µg/mL; and rabbit anti-GC was incubated at final concentrations of 45 µg/mL, 22.5 µg/mL, and 11.25 µg/mL. Bacteria were washed three times by centrifugation using RPMI + 10% heat-inactivated fetal bovine serum (HI-FBS). Secondary antibodies were added at a final concentration of 7.5 µg/mL and incubated with bacteria for 30 min at 37°C, 5% CO2. Samples were washed once with PBS, and pellets were resuspended in 2% paraformaldehyde (PFA) containing 5 µg/mL 4′, 6-diamidino-2-phenylindole (DAPI). Samples were analyzed by imaging flow cytometry using ImagestreamX Mk II with INSPIRE software (Luminex Corporation). Alexa Fluor 488 fluorescence was detected with excitation at 488 nm and emission collected with a 480–560-nm filter. Alexa Fluor 647 fluorescence was detected with a 658-nm laser excitation, and emission was collected with a 660–740-nm filter. DAPI fluorescence was detected with excitation at 405 nm and emission collected with a 420–505-nm filter. Single-color samples for Alexa Fluor 488, Alexa Fluor 647, and DAPI were collected without brightfield to create a compensation matrix for each experiment. Gating strategy is presented in Fig. 1.
Fig 1.
Antibody binding to the surface of N. gonorrhoeae by imaging flow cytometry. (A) Gating strategy. N. gonorrhoeae strain FA1090 was incubated with rabbit anti-N. gonorrhoeae antibody and AlexaFluor 488 (AF488)-coupled anti-rabbit antibody, fixed, and incubated with DAPI. On the ImagestreamX imaging flow cytometer, samples were gated on (I) Root mean square (RMS) of >52, then (ii) focused bacteria, and then (iii) DAPI+ singlets, from which AF488-positive fluorescence was measured (iv). (B) Representative examples of individual N. gonorrhoeae of strain FA1090 that bind mouse (Ms) 2C7 anti-lipooligosaccharide IgG3, polyclonal rabbit (Rb) anti-N. gonorrhoeae IgG (αGc), and mouse 6B4 anti-lipooligosaccharide IgM (left) but not corresponding isotype controls (right). Number in the upper left corner of each phase image indicates the identity of the particle captured by the imaging flow cytometer. Measurements of mean fluorescence intensity (MFI) (C, F, I), percent-positive bacteria (D, G, J), and fluorescence index (FI = MFI x percent positive) (E, H, K) for N. gonorrhoeae incubated with the indicated concentrations of 2C7 (C–E), αGc (F–H), and 6B4 (I–K) or highest concentration of each isotype control (iso). Bars indicate the mean ± SD of three independent experiments, with each biological replicate as one data point.
Serum bactericidal activity assay
N. gonorrhoeae was swabbed from GCB agar into liquid media and diluted to an OD550 of 0.07. The bacterial suspension was diluted 1:2,500 into Hank’s Balanced Salt Solution with Ca2+ and Mg2+ (HBSS; Gibco, Catalog #14025–092) + 2% BSA to yield ~5 × 104 CFU/mL. Twenty microliters of bacterial suspension was added to a 96-well V-bottom plate and incubated with 20 µL of heat-inactivated mouse serum or control antibodies at indicated concentrations or HBSS + 2% BSA, for 15 min at 37°C, 5% CO2. Igd-NHS was added in HBSS + 2% BSA (40 µL) to yield the percent of active complement indicated in Table 2 for each of the five strains used in this study. Samples were mixed with a multichannel pipettor and incubated for 45 min at 37°C, 5% CO2. GCBL (100 µL) was added to each sample and mixed vigorously with a multichannel pipettor, and 20 µL of suspension was plated on GCB agar. CFUs were enumerated after overnight incubation at 37°C, 5% CO2.
TABLE 2.
Serum bactericidal activity elicited by 4CMenB vaccination
| Strain | SBA titer of alum controla | SBA titer of 4CMenBa | Normalized SBA titerb | % NHSc | Positive control Abd |
|---|---|---|---|---|---|
| FA1090 | <5 | 40 | >8 | 2% | 6B4 |
| MS11 | <5 | 160 | >32 | 0.50% | 6B4 |
| H041 (WHO X) | <5 | 80 | >16 | 0.50% | 6B4 |
| F62 | <5 | 40 | >8 | 4% | Rabbit anti-N. gonorrhoeae |
| FA19 | 10 | 80 | 8 | 1% | 6B4 |
Highest dilution that gave ≥50% killing.
SBA titer of 4CMenB SBA titer of alum control.
Percentage of Ig-depleted pooled normal human serum used as complement source.
Antibody used as positive control to elicit SBA.
Percent bacterial killing was determined by the following equation:
Samples with >50% killing at the indicated concentration of antibody or dilution of mouse serum were considered positive.
PMN opsonophagocytic killing assay
Venous blood was drawn from healthy human subjects who gave informed consent, in accordance with a protocol approved by the University of Virginia Institutional Review Board for Health Sciences Research (#13909). Erythrocytes were removed from heparinized blood by dextran sedimentation, and PMNs were isolated from the remaining cells using Ficoll-Hypaque, followed by hypotonic lysis of the remaining erythrocytes as described (2). The remaining cells were >95% granulocytes by morphology. PMNs were resuspended in Dulbecco’s phosphate buffered saline (D-PBS, without Ca2+ and Mg2+) plus 0.1% dextrose. PMNs were kept on ice and used within 30 min of isolation. Each experimental replicate used PMNs from a different subject.
N. gonorrhoeae was swabbed from GCB agar into liquid media and diluted to an OD550 of 0.07. The bacterial suspension was diluted 1:2,500 into Hank’s Balanced Salt Solution, with Ca2+ and Mg2+ (HBSS) + 2% BSA to yield ~5 × 104 CFU/mL. Twenty microliters of bacterial suspension was added to a 96-well V-bottom plate and incubated with 20 µL of heat-inactivated mouse serum at indicated dilutions or control antibodies for 30 min at 37°C, 5% CO2. C6-depleted pooled normal human serum was added (60 µL) to yield a 5% final serum concentration. PMNs (100 µL) at 2 × 106 /mL in HBSS + 2% BSA were added, mixed, briefly centrifuged together (600 × g, 4 min), and incubated for 2 h at 37°C, 5% CO2. Samples were mixed well with a multichannel pipettor, and 20 µL of each suspension was plated onto GCB agar. CFU were enumerated after overnight incubation at 37°C, 5% CO2, and reported as CFU/20 µL, or as percent bacteria killed by the following equation:
PMN opsonophagocytosis of N. gonorrhoeae by imaging flow cytometry
Agar-grown FA1090 N. gonorrhoeae (1 × 108 CFU) was incubated with 1.5 µL Tag-IT Violet (TIV) (BioLegend, Catalog # 425101) in 100 µL 1× PBS + 5 mM MgSO4. N. gonorrhoeae (1 × 104 CFU) diluted in 20 µL of HBSS + 2% BSA was mixed with 20 µL rabbit anti-N. gonorrhoeae antibody (Meridian B65111R) diluted 1:50 in of HBSS + 2% BSA (final antibody concentration 50 µg/mL) for 30 min. Bacteria were then incubated with 60 µL 5% C6-depleted NHS and 2 × 106 primary human PMNs in a total volume of 400 µL to yield a 200:1 PMN:bacterium ratio and centrifuged at 600 × g for 4 min. After 2 h at 37°C, 5% CO2, cells were fixed without permeabilization, lifted with a cell scraper, blocked in 10% normal goat serum (Gibco, 16210–064) in PBS, and stained with AF647-labeled goat anti-rabbit antibody to detect extracellular bacteria. Intracellular bacteria were defined as those that were TIV+ and AF647−. Samples were processed for imaging flow cytometry using ImagestreamX Mk II with INSPIRE software (Luminex Corporation), and data were analyzed using IDEAS software.
C3 deposition on N. gonorrhoeae
Strain FA1090 bacteria were prepared as the above, with 25 µL of 2C7 (1:2 dilution, 0.14 mg/mL) added as the opsonic antibody for 20 min at 37°C, 5% CO2. After two washes with RPMI + 10% HI-FBS, C6-depleted NHS (in RPMI + 2% BSA) was added to the indicated final concentration and incubated for 15 mins at 37°C, 5% CO2. Bacteria were washed and then incubated with FITC-conjugated goat IgG anti-human C3 (1:5000 dilution) for 15 min at 37°C, 5% CO2. Bacteria were washed once with PBS and resuspended in 2% PFA containing 5 µg/mL DAPI. Samples were analyzed by imaging flow cytometry using ImagestreamX Mk II with INSPIRE software. FITC fluorescence was detected with excitation at 488 nm and emission collected with a 480–560-nm filter. DAPI fluorescence was detected as the above. Single-color samples for FITC and DAPI were collected without brightfield to create a compensation matrix for each experiment. The gating strategy for antibody binding to N. gonorrhoeae was also used for evaluating C3 deposition.
HL-60 opsonophagocytosis assay
HL-60 cells (ATCC Catalog # CCL-240) were maintained in Iscove’s modified Dulbecco’s medium (IMDM, Corning Catalog #15-016-CV) with 20% heat-inactivated fetal bovine serum (HyClone, Catalog # SH30071.03), Glutamax (Gibco, Catalog #35050-061), and antibiotic-antimycotic (Gibco, Catalog #15240-062). Cells were cultured to a density of 1.5 × 105/ mL and not allowed to exceed 1 × 106 /mL. For differentiation, cells were centrifuged in 15 mL conical tubes at 300 × g for 7 min and then resuspended at 1 × 106 /mL in growth medium containing 1.25% dimethyl sulfoxide (DMSO, Sigma, Catalog #D2650) for 3 days at 37°C, 5% CO2. Cells were then changed into growth medium without DMSO for an additional 2 days at 37°C, 5% CO2. Cells were washed 3× in RPMI and resuspended at 2 × 107 /mL in RPMI + 2% BSA. Under these differentiation conditions, HL-60 were >90% viable and >70% CD11b+ by flow cytometry.
N. gonorrhoeae was labeled with Tag-IT Violet, then washed, and resuspended at 1.6 × 109 CFU. Bacteria (12.5 µL for 2 × 106/well) were added to a V-bottom 96-well plate. 2C7 IgG (12.5 µL, 0.28 mg/mL) was added, and the plate was incubated for 30 min at 37°C, 5% CO2. Samples were washed three times before C6-depleted NHS (50 µL of 4% for 1% final) and differentiated HL-60 cells (100 µL of 2 × 107 /mL) were added and incubated for 1 h at 37°C, in 5% CO2. Samples were washed one time, then Zombie Green (BioLegend, Catalog #423111, 1:1,000) was added to measure and gate on the % live HL-60 cells, and anti-mouse/human CD11b PE (BioLegend, Catalog #101207, 1:50) was added to measure and gate on differentiated HL-60 cells. Samples were incubated for 30 min on ice, washed 1× in PBS and fixed with 2% PFA.
Analysis of 1 × 104 cells per sample was performed by imaging flow cytometry using ImagestreamX Mk II with INSPIRE software (Luminex Corporation). A scatter plot with Area_M01 on the X axis and Aspect Ratio_M01 on the Y axis was used to identify single-HL-60 cells with Area_M01 between 200 and 400 and Aspect Ratio_M01 between 0.6 and 1. From the singlets gate, focused cells were identified with Brightfield Gradient RMS values over 50. Dead cells with Zombie Green intensity over 1e4 were excluded. From the live cell population, differentiated HL-60s were defined as cells positive for CD11b PE. Using a non-differentiated control, an intensity over 5e3 was considered CD11b+. Tag-it Violet fluorescence was measured in the live, differentiated cell population. Cells with TIV intensity over 6e3 were considered associated with bacteria. Zombie Green fluorescence was detected with excitation at 488 nm and emission collected with a 480–560-nm filter. PE fluorescence was detected with excitation at 561 nm and emission collected with a 560–595-nm filter. Tag-IT Violet fluorescence was detected with excitation at 405 nm and emission collected with a 420–505 nm-filter. Single-color samples for Zombie Green, PE, and Tag-it Violet were collected without Brightfield to create a compensation matrix for each experiment.
Statistics
Independent biological replicates were conducted with different bacterial cultures on different days, different preparations of HL-60 cells, and/or different human subjects’ PMNs. Statistical comparisons were made as indicated in the figure legends.
RESULTS
Assay development
Antibody binding to the surface of N. gonorrhoeae
Imaging flow cytometry was applied to assess the binding of antibodies to antigens on the surface of live N. gonorrhoeae, with the goal to use immunized serum as the antibody source. Imaging flow cytometry has a slower flow rate than conventional flow cytometry and can better distinguish small particles like individual N. gonorrhoeae (diameter ~0.5 µm) from bacterial aggregates, whose fluorescence could skew results. Our group has previously used imaging flow cytometry to measure binding of the N-terminal domain of human carcinoembryonic antigen-related cell adhesion receptors to opacity protein adhesins on N. gonorrhoeae (56), binding of complement iC3b to fluorescent beads (57), and binding of complement C4b-binding protein to N. gonorrhoeae (58). As proof of concept, live N. gonorrhoeae strain FA1090 was incubated with one of three purified antibodies: 2C7, a mouse monoclonal IgG3 antibody that recognizes an epitope including the β chain lactose of Neisseria lipooligosaccharide (59, 60); a polyclonal rabbit IgG raised against N. gonorrhoeae (61); and 6B4, a mouse monoclonal IgM antibody that recognizes the lacto-N-tetraose α chain of Neisseria lipooligosaccharide (59, 62). Bacteria were washed, incubated with appropriate fluorescently coupled secondary antibodies, resuspended in fixative, counterstained with DAPI, and analyzed by imaging flow cytometry. Primary antibodies of the same isotype with the same secondary antibodies were used as negative controls for background fluorescence. The gating strategy is depicted in Fig. 1A: (i) Focused particles were identified as those with high gradient RMS, which is a measure of image sharpness based on pixelation. (ii) Within this gate, DAPI+ particles (N. gonorrhoeae) were identified. (iii) DAPI+ bacterial aggregates, as defined by their area and aspect ratio, were excluded from further analysis. (iv) The intensity of AF488 IgG+ DAPI+ singlets was quantified. Examples of antibody-positive bacteria are presented on the left of Fig. 1B, and matching isotype controls on the right.
Both 2C7 and the anti-Neisseria gonorrhoeae polyclonal rabbit IgG elicited a concentration-dependent increase in fluorescence of N. gonorrhoeae, while there was no-to-marginal detection of fluorescence of the isotype control in each condition (Fig. 1C through H). The mouse IgM that recognizes the lacto-N-tetraose α chain of Neisseria lipooligosaccharide, 6B4, also elicited a concentration-dependent increase in the fluorescence of N. gonorrhoeae (Fig. 1I through K). The magnitude of fluorescence was calculated in three ways: (i) mean fluorescence intensity (Fig. 1C, F, I), (ii) percent-positive bacteria (e.g., in the green gate in Fig. 1A iv) (Fig. 1D, G and J), and (iii) fluorescence index, calculated as the MFI multiplied by the percent-positive bacteria (Fig. 1E, H and K). The use of hybridoma supernatants of 2C7 and 6B4 precluded us from incubating N. gonorrhoeae with higher concentrations of antibody; hence, the percent-positive bacteria in Fig. 1D and J do not reach saturation. However, these lower concentrations may be more reflective of specific antibodies in serum from immunized animals. Thus, (imaging) flow cytometry can be used to measure the binding of antibodies to the surface of N. gonorrhoeae, a prerequisite for functional antibody activities.
Serum bactericidal activity assay for N. gonorrhoeae
SBA is a defined correlate of protection for vaccination against extracellular Gram-negative bacteria, including the related N. meningitidis (39, 40, 63–65). We sought to develop a SBA platform with N. gonorrhoeae to allow for the screening of serum from immunized and control animals in a rapid and reproducible manner. To establish this platform, we used 6B4 IgM antibody, since pentameric IgM is a potent initiator of the classical complement cascade. Igd-NHS was then added as the source of complement. HBSS served as the medium for incubation. The use of Igd-NHS removes any potential contribution of naturally occurring antibodies in human serum that cross-react with N. gonorrhoeae. CFU were enumerated after overnight growth.
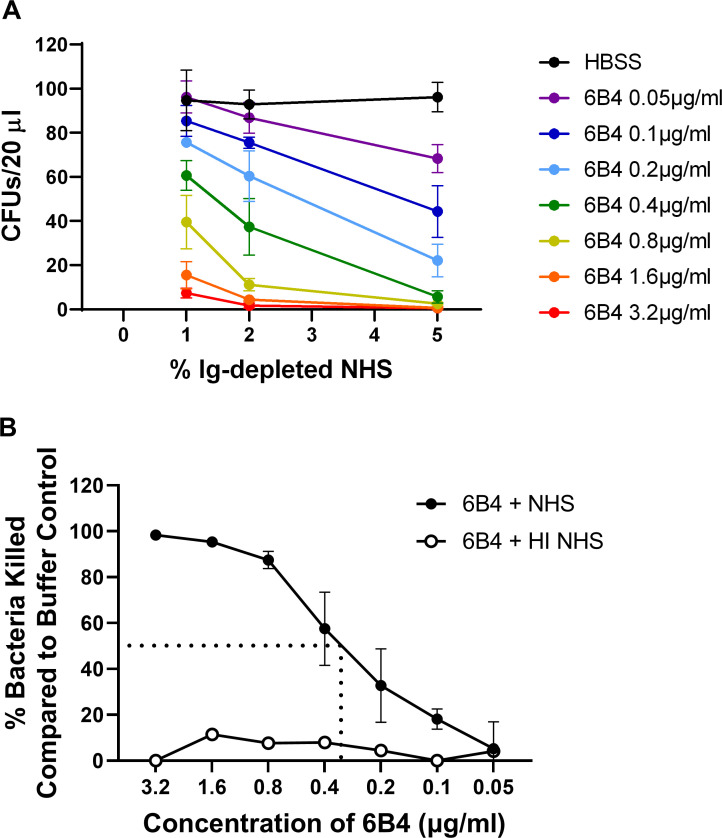
Using strain FA1090, there was an antibody- and complement-dependent effect on N. gonorrhoeae viability (Fig. 2A). As expected for this serum-resistant strain, there was no effect of NHS on bacterial viability in the absence of 6B4 (black line, Fig. 2A). At each concentration of NHS, increasing concentrations of 6B4 led to reduced bacterial survival. At the highest concentrations of 6B4, few to no CFU were enumerated (red, orange lines, Fig. 2A); there was a concentration-dependent decrease in bacterial killing as the concentration of antibody decreased (Fig. 2A). A concentration of 2% NHS and 6B4 hybridoma supernatant at 0.2 µg/mL yielded approximately 50% bacterial survival compared with the HBSS control (light-blue vs. black symbols, Fig. 2A). We selected 2% NHS as the concentration of complement source that yielded the greatest dynamic range of SBA against strain FA1090. Similar calculations were made for other strains of N. gonorrhoeae (Table 2).
Fig 2.
N. gonorrhoeae serum bactericidal assay. (A) N. gonorrhoeae strain FA1090 (103 CFU) was incubated with the indicated concentration of 6B4 or HBSS buffer, followed by the addition of the indicated concentration of Ig-depleted normal human serum. Bacterial CFU from 20 µL of the incubation medium were enumerated after overnight growth on GCB agar. (B) Bacteria killed were expressed as a percentage of the buffer (HBSS) control and graphed relative to the concentration of 6B4 in 2% NHS (solid circles). The dotted lines indicate the extrapolation to the 6B4 concentration that yields 50% bacterial killing. The SBA titer, or the lowest concentration that yielded ≥50% killing, was calculated as 0.4 µg/mL (green). Five percent heat-inactivated (HI) NHS, the highest concentration of NHS that was tested, served as a negative control (open circles). Bars indicate the mean ± SD of three independent experiments, with each biological replicate as one data point.
To calculate the SBA titer, strain FA1090 was incubated with increasing dilutions of 6B4 prior to exposure to 2% NHS. Fig. 2B reports the percentage of N. gonorrhoeae killed, relative to bacteria incubated with the buffer control, for the indicated concentrations of 6B4. The SBA titer was calculated as the lowest concentration of antibody in which ≥50% of the bacteria were killed (dotted line showing interpolation for 50%; SBA titer = 0.4 µg/mL, Fig. 2B). Of note, there was no appreciable killing of N. gonorrhoeae when heat-inactivated NHS was provided (white symbols, Fig. 2B), indicating that bactericidal activity required active complement. A similar approach was used to establish the SBA titer for four other strains of N. gonorrhoeae commonly used for biomedical experimentation and vaccine development: MS11, H041 (WHO X), F62, and FA19. The concentration of Ig-depleted NHS and the positive control antibody for each of these strains is presented in Table 2. Of note, we measured positive SBA titers not only for the serum-sensitive strains F62, MS11, and H041 but also for the two highly serum-resistant strains FA1090 and FA19.
For evaluating immunized and control mouse serum, our plan for measuring and reporting SBA is as follows: (i) Determine the SBA titer for the serum from control mice. (ii) Measure bacterial killing by the immunized serum, starting with 2× the dilution of the SBA titer for the serum from control mice. (iii) If >50% of the bacteria are killed by the immunized serum at this dilution, conduct SBA over a full range of dilutions of serum and calculate the SBA titer. (iv) Report the results as a normalized SBA titer. If there is no increased SBA with the immunized serum, report as a normalized SBA titer of 2. This approach allows for the efficient analysis of many samples while conserving reagents, in particular the limited amount of serum that can be obtained from individual mice.
Antibody-elicited opsonophagocytic killing activity against N. gonorrhoeae
Gonorrhea is typified by influx of neutrophils at the site of infection (14), and opsonophagocytic killing may contribute to the efficacy of N. meningitidis vaccines (42, 66, 67). To assess the contribution of immunization-elicited antibodies in opsonophagocytic killing of N. gonorrhoeae, we established an in vitro assay for opsonophagocytic killing activity using primary human PMNs from the venous blood of healthy human subjects. As proof of concept, N. gonorrhoeae was first incubated with an anti-N. gonorrhoeae rabbit polyclonal IgG (see Fig. 1), followed by the addition of C6-depleted NHS as a complement source. PMNs were then added at a ratio of 200 per bacterium, and after 2 h of coincubation, CFU were enumerated. C6-depleted serum, which cannot make the membrane attack the complex and cannot elicit SBA, was used to allow the effect of PMN-dependent killing to be selectively assessed. Binding and phagocytosis of N. gonorrhoeae by PMNs under the OPKA conditions were examined by imaging flow cytometry, using bacteria prelabeled with the fluorophore Tag-IT Violet. After incubation, PMNs were fixed but not permeabilized, and an Alexa Fluor 647-coupled antibody was used to detect extracellular bacteria. As shown in the representative images in Fig. 3A, N. gonorrhoeae was phagocytosed by PMNs (Tag-IT Violet+, Alexa Fluor 647−). At the MOI used, approximately 4% of all PMNs had associated N. gonorrhoeae, and 1.8% of all PMNs had phagocytosed bacteria.
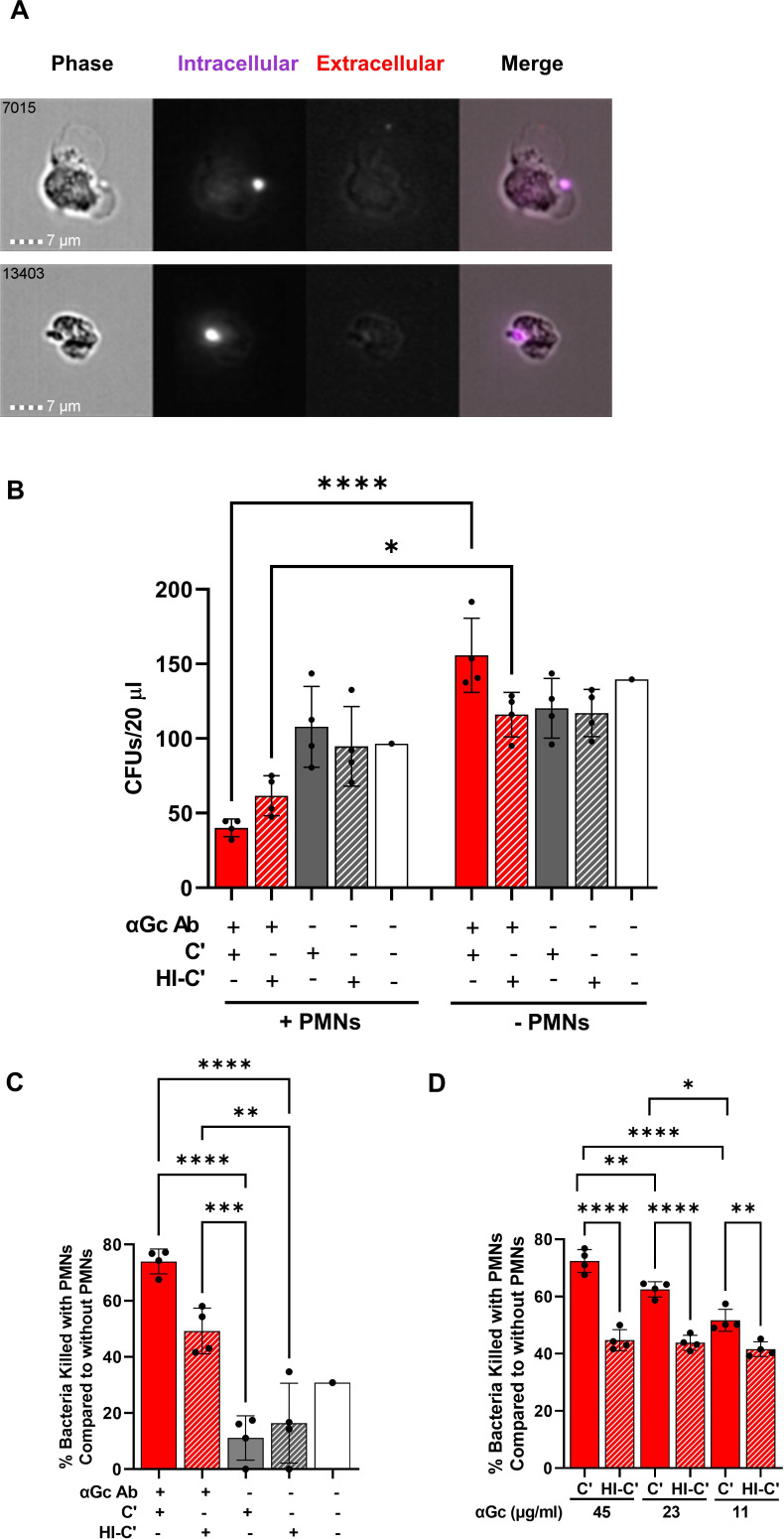
Fig 3.
Opsonophagocytic killing assay with primary human PMNs. (A) Examples of two PMNs that underwent opsonophagocytosis of Tag-IT Violet-labeled (purple) N. gonorrhoeae strain FA1090, in the presence of C6-depleted NHS and rabbit anti-N. gonorrhoeae antibody (50 µg/mL), analyzed by imaging flow cytometry. PMNs were fixed and stained with Alexa Fluor 647-coupled anti-N. gonorrhoeae antibody to detect bound but not phagocytosed bacteria (red). The number in the upper left corner of each phase image indicates the identity of the cell captured by the imaging flow cytometer. (B) N. gonorrhoeae strain FA1090 was mixed with rabbit anti-N. gonorrhoeae antibody or buffer control as the above, except not fluorescently labeled. C6-depleted normal human serum that was untreated (C’) or heat inactivated (HI-C’) was added to 5% final concentration, along with primary human PMNs (+PMNs) or buffer (−PMNs). CFUs were enumerated from 20 µL of the incubation mix. Red solid bar with PMNs is the full opsonophagocytic condition. (C) Results are as in B except each condition was expressed as a percentage of the enumerated CFU for the same condition in the absence of PMNs. (D) Results are as in B except with the indicated concentrations of rabbit anti-N. gonorrhoeae antibody and presented as the percent of bacteria killed compared with bacterial killing when heat-inactivated serum was used. In B–D, results presented are the average ± SD from four independent experiments using different human subjects’ PMNs, with each biological replicate as one data point. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001, and ****P ≤ 0.0001 by one-way ANOVA followed by Holm-Šidák multiple comparisons test.
For OPKA, CFU were enumerated for strain FA1090 N. gonorrhoeae with or without antibody, in the presence of intact or heat-inactivated C6-depleted NHS, in the presence or absence of PMNs from three unrelated subjects. Addition of antibody significantly reduced the viability of N. gonorrhoeae exposed to PMNs compared with those that were not, whether active complement or heat-inactivated complement was used (compare solid red bars with and without PMNs, and red hatched bars with and without PMNs, Fig. 3B). Reductions in bacterial viability in the presence of PMNs were not significant in other conditions (Fig. 3B). These observations agree with a previous report on the effect of IgG on phagocytic killing of N. gonorrhoeae (61).
To take into account any differences in viability that occurred independently of PMNs (right side, Fig. 3B), CFU in the experimental condition was divided by CFU from the same conditions without PMNs, subtracted from one to convert into a fraction of bacteria killed, and multiplied by 100%. From these results, we measured a statistically significant decrease in N. gonorrhoeae viability when antibody, complement, and PMNs were all present, relative to antibody alone, active complement alone, or neither (Fig. 3C). The effect of antibody was concentration dependent, with statistically significant increased bacterial survival with increasing dilutions of the opsonic antibody (Fig. 3D). However, this was dependent on active complement, because there was no difference in the magnitude of bacteria killed by PMNs at these different antibody concentrations when heat-inactivated serum was used as complement source (Fig. 3D). We conclude that N. gonorrhoeae-specific IgG in the presence of active complement and PMNs elicits measurable OPKA.
Alternative assays for examining functional antibody contributions to control of N. gonorrhoeae
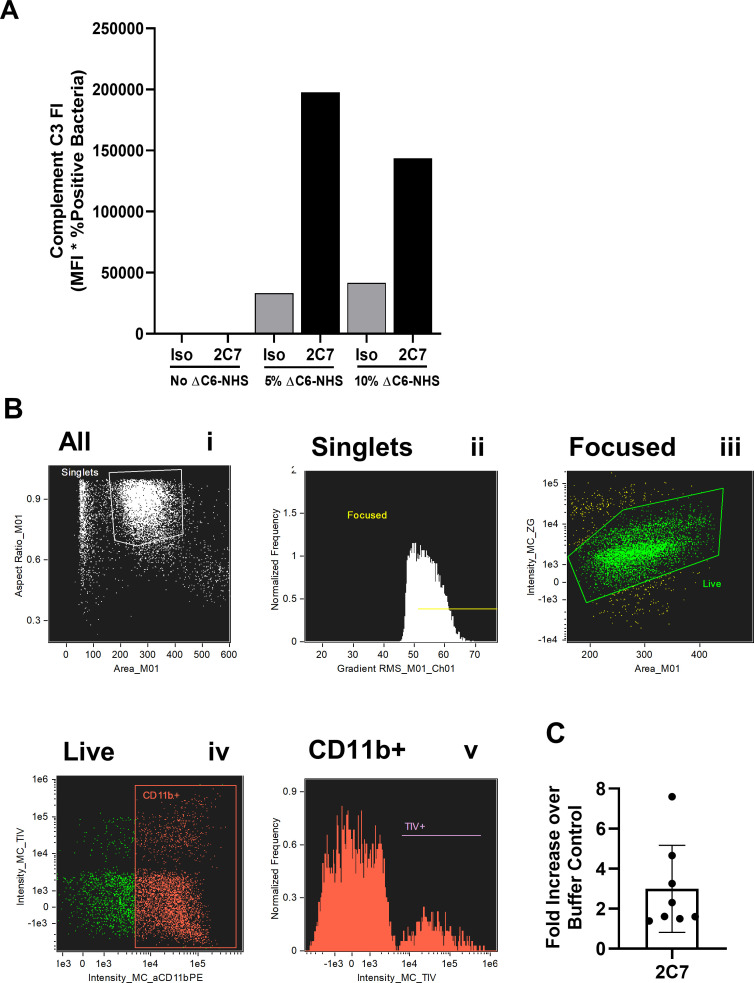
The premise of the SBA and OPKA assays is that antibodies facilitate complement deposition on N. gonorrhoeae, leading to membrane attack complex formation and opsonophagocytosis, respectively. An alternative readout of these activities is deposition of complement component 3 (C3) on the bacterial surface, which occurs downstream of the classical complement cascade. We evaluated the ability of the monoclonal anti-LOS antibody 2C7 and C6-depleted NHS to elicit C3 deposition on strain FA1090 N. gonorrhoeae using imaging flow cytometry, since 2C7 has been previously reported to increase C3 on the surface of N. gonorrhoeae (60, 68). C6-depleted NHS was used to avoid bacterial lysis, which could confound quantitation of bacterial C3 positivity. Bacteria were mixed with 2C7 antibody or isotype control, after which C6-depleted NHS at the indicated concentrations or buffer were added, following the initial steps outlined above for OPKA. Bacteria were then fixed and stained with a fluorescent antibody that recognizes all forms of human C3, including the opsonins C3b and iC3b. The intensity of C3 binding was calculated as the fluorescence index (percent positive × MFI). C3 deposition was only detected in the presence of C6-depleted NHS and was markedly increased when 2C7 was added, compared with isotype control (Fig. 4A). This assay can be used to demonstrate increased complement deposition on N. gonorrhoeae that is promoted by vaccine-elicited antibodies.
Fig 4.
Alternative assays for complement-mediated functional antibody activity against N. gonorrhoeae. (A) Complement C3 deposition. N. gonorrhoeae strain FA1090 was incubated with 2C7 or HBSS in the absence or presence of the indicated concentrations of C6-depleted (ΔC6) NHS. Bacteria were fixed, stained with a FITC-labeled anti-human C3 antibody, counterstained with DAPI, and processed for flow cytometry. The fluorescence index was calculated as in Fig. 1. One representative experiment is reported. (B and C) Opsonophagocytic assay with differentiated HL-60 cells. N. gonorrhoeae strain FA1090 was labeled with Tag-IT Violet and then incubated with 2C7 or isotype control, followed by exposure to C6-depleted NHS and differentiated HL-60 cells. After 2 h, cells were fixed, stained with anti-CD11b for differentiation and Zombie Green for viability, and processed for imaging flow cytometry. Gating strategy: (i) singlets with size and aspect ratio indicative of intact cells; (ii) focused singlets; (iii) live cells; (iv) CD11b + cells. In (v), the fluorescence intensity of TIV+ N. gonorrhoeae was measured in the CD11b+ gate. (C) The fold increase in opsonophagocytosis of N. gonorrhoeae by dHL-60 cells elicited by 2C7 compared with the buffer control is reported. Bars represent the mean ± SD of eight independent experiments conducted on different days, with each biological replicate as one data point.
Experiments with primary PMNs require human subject approval and have the potential for person-to-person variability. As an alternative to human blood cells, we used the human promyelocytic cell line HL-60, differentiated toward a neutrophil-like phenotype by treatment with DMSO (dHL-60). HL-60 cells have been used extensively for assaying OPKA against S. pneumoniae and other Gram-positive bacteria (69–71). However, in our hands, dHL-60 cells do not kill N. gonorrhoeae (57). As a surrogate, we established an imaging flow cytometry assay using Tag-IT Violet-labeled N. gonorrhoeae, where we hypothesized the intensity of Tag-IT Violet fluorescence of dHL-60 would be proportional to the extent of phagocytosis. dHL-60 cells were incubated with N. gonorrhoeae, IgG antibody against N. gonorrhoeae (in this case, 2C7), and C6-depleted NHS. Cells were then fixed and stained with dyes to assess cellular viability (Zombie Green) and differentiation (CD11b upregulation). The percentage of dHL-60 cells that were Tag-IT Violet+ and the mean fluorescence intensity for Tag-IT Violet+ cells was determined from the viable, CD11b+ focused singlet population (Fig. 4B). While 2C7 elicited increased opsonophagocytosis compared with the buffer (no antibody or complement) control, the spread of the biological replicates was greater than expected for a cell line and greater than that observed for OPKA with human PMNs (Fig. 4C; compared with data points on the solid red bar, Fig. 3C). Given the expenses of cell culture and flow cytometry, the inconsistent dHL-60 response, and ability of primary human PMNs to kill N. gonorrhoeae in a complement- and antibody-dependent manner, our preference is to use human PMNs for evaluating antibodies elicited by immunization.
Assay optimization: the 4CMenB meningococcal serogroup B vaccine elicits functional antibody responses against multiple strains of N. gonorrhoeae
The reverse vaccinology-engineered meningococcal serogroup B vaccine 4CMenB has been shown to elicit antibodies in humans and mice that recognize N. gonorrhoeae and protect mice from gonococcal infection (26, 27, 29). To examine the ability of 4CMenB to promote functional antibody responses against N. gonorrhoeae, mice were vaccinated with 3 × 250 µL of 4CMenB or alum as a control, on days 0, 21, and 42. Serum was collected on day 63 post-immunization, pooled, heat-treated to inactivate mouse complement, and assessed for antibody binding, SBA, and OPKA, using the human sera described above as the complement sources.
Antibody binding
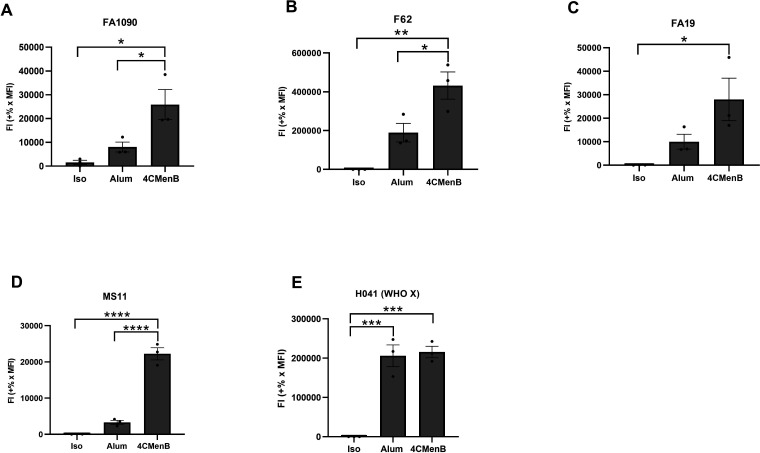
Five different strains of N. gonorrhoeae were assessed for 4CMenB-elicited antibody binding to the bacterial surface, reported as fluorescence index. Here, N. gonorrhoeae was mixed with serum from 4CMenB-vaccinated mice or alum-treated mice as the control, prior to the staining procedure described for Fig. 1. Purified antibody that recognizes the given strain of N. gonorrhoeae served as positive control (see Table 2) and isotype antibody as negative control. For the tested strains except H041, the fluorescence index of IgG bound to N. gonorrhoeae was markedly increased when using serum from 4CMenB-vaccinated mice, compared with alum-treated mice or the isotype control (Fig. 5). Among the four strains with increased binding with 4CMenB, there were differences in both the fluorescence index and the magnitude of the difference between 4CMenB-vaccinated and alum-treated mice, with strain F62 having the most robust binding of IgG from 4CMenB and strains FA19 and MS11 having the least (F62>H041>FA1090>FA19~MS11). However, strain MS11 showed the greatest difference in binding of serum IgG from 4CMenB-vaccinated compared with alum-treated mice, while H041 showed essentially no difference. The reason for the lack of specific 4CMenB IgG binding to H041 is not clear, but it is possible that greater differences between the vaccinated and control sera would be observed at higher dilutions. Moreover, the serum from the vaccinated vs. alum-treated mice may recognize different antigens on N. gonorrhoeae. The imaging flow cytometry findings presented here could help inform future studies to identify the gonococcal antigens cross-reacting with 4CMenB using comparative proteomic analyses (34).
Fig 5.
4CMenB vaccination of mice elicits cross-reactive antibodies that bind the surface of multiple strains of N. gonorrhoeae. Bacteria of the indicated strain background were incubated with a 1:10 dilution of pooled serum from 4CMenB-vaccinated or alum-treated mice or mouse IgG isotype control. Bacteria were fixed, processed for imaging flow cytometry, and fluorescence index calculated as in Fig. 1. Bars indicate the mean ± SD of three independent experiments, with each biological replicate as one data point. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001, and ****P ≤ 0.0001 by ordinary one-way ANOVA with Tukey’s multiple comparison’s test.
Serum bactericidal activity
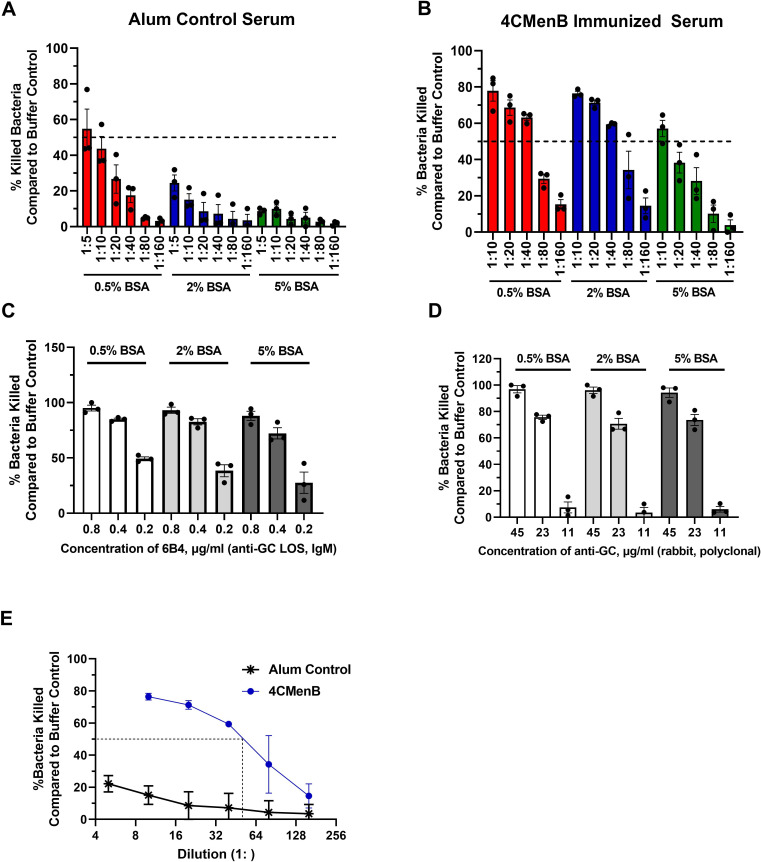
Strain FA1090 was subjected to SBA as in Fig. 2, using pooled serum from 4CMenB-vaccinated and alum-treated mice. Unlike purified antibodies, we found that serum from the alum-treated mice killed N. gonorrhoeae in the presence of active complement (not shown). We hypothesized that the presence of non-specific or low-affinity antibodies was responsible for SBA elicited by the unimmunized controls. To mitigate this effect, BSA at concentrations ranging from 0.5% to 5% was added to the assay buffer (HBSS). While 0.5% BSA did not prevent killing of the strain FA1090 by the serum from alum-treated mice at dilutions up to 1:10 (red bars, Fig. 6A), addition of 2% BSA reduced the SBA background of alum-treated mouse serum (blue bars, Fig. 6A) without impeding the SBA elicited by the serum from 4CMenB-vaccinated mice (blue bars, Fig. 6B). Five percent BSA further reduced the background of the serum from alum-treated mice but also reduced SBA elicited by the 4CMenB-vaccinated serum (green bars, Fig. 6A and B). These effects were specific to the presence of serum, since addition of BSA did not affect the SBA of the two positive control antibodies, 6B4 (Fig. 6C) and rabbit IgG anti-N. gonorrhoeae (Fig. 6D). Based on these results, going forward, HBSS + 2% BSA was used as the diluent for SBA assays.
Fig 6.
Serum bactericidal activity elicited by 4CMenB vaccination against N. gonorrhoeae. (A and B) N. gonorrhoeae strain FA1090 was incubated with the indicated concentrations of BSA in the presence of the indicated dilution of pooled serum from alum-treated (A) or 4CMenB-vaccinated (B) mice. Serum bactericidal activity was measured using 2% Ig-depleted NHS as complement source and reported as percent bacteria killed relative to the buffer control. (C and D) N. gonorrhoeae strain FA1090 was mixed with the indicated concentration of BSA and 6B4 (C) or rabbit anti-N. gonorrhoeae (GC) antibody (D). The percentage of bacteria killed relative to buffer control was calculated as in A and B. Based on the results, 2% Ig-depleted NHS, 2% BSA, and 0.4 µg/mL 6B4 or 22.5 µg/mL rabbit anti-N. gonorrhoeae antibody were selected as positive control conditions for FA1090 N. gonorrhoeae; similar calculations were made for other N. gonorrhoeae strains with the positive control antibodies in Table 2. (E) SBA activity measured using strain FA1090 N. gonorrhoeae, 2% BSA, the indicated dilutions of pooled serum from 4CMenB-vaccinated (circle) or alum-treated control (asterisk) mice, and 2% Ig-depleted NHS. SBA titers for all five strains of N. gonorrhoeae are reported in Table 2. Bars indicate the mean ± SD of three independent experiments, with each biological replicate as one data point.
We next determined the SBA titer of 4CMenB-vaccinated serum against strain FA1090 and four other strains of N. gonorrhoeae using 2% BSA in the assay. The 4CMenB SBA response was more potent than the alum control in each case, with a calculated SBA titer of 40 for 4CMenB against FA1090 compared with <4 for alum (Fig. 6E). SBA titers for 4CMenB ranged from 40 to 160 against MS11, H041, F62, and FA19 compared with <5 for their respective alum controls (except for FA19, whose alum SBA titer was 10) (Table 2). The normalized SBA titer, or the SBA titer elicited by the vaccinated serum (e.g., 4CMenB) divided by the SBA titer elicited by the control serum (e. g., alum treated), is also presented in Table 2 for each strain. These findings show that 4CMenB vaccination elicits a humoral response that promotes SBA against multiple strains of N. gonorrhoeae.
Opsonophagocytic killing assay
We applied the OPKA assay with primary human PMNs to strain FA1090 N. gonorrhoeae that was incubated with serum from mice vaccinated with 4CMenB or treated with alum. The number of PMNs and the volume of mouse serum that would be needed precluded us from calculating an OPKA titer. Instead, we selected a single dilution (1:10) of vaccine-elicited or control serum for OPKA measurements and expressed vaccine-elicited OPKA as a fold increase over its biological control. As in the SBA, 2% BSA was added to N. gonorrhoeae prior to adding the mouse serum.
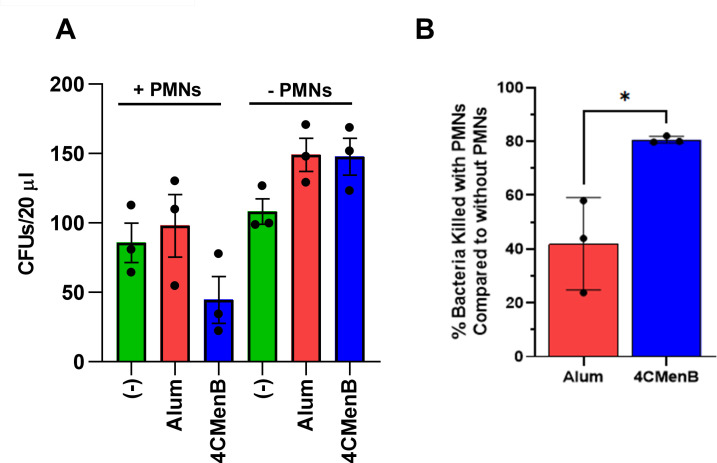
Exposure to C6-depleted NHS, serum from 4CMenB-vaccinated mice, and human PMNs reduced survival of N. gonorrhoeae relative to all other conditions (blue bar on the left, Fig. 7A). The serum from alum-treated mice had no effect on OPKA compared with bacteria that were not incubated with any mouse serum (red bar vs. green bar on the left, Fig. 7A). In the absence of PMNs, there was no reduction in bacterial CFU (right half of Fig. 7A). In fact, non-heat-inactivated mouse serum supported N. gonorrhoeae outgrowth, potentially due to the presence of heat-sensitive nutrients that N. gonorrhoeae can consume (red and blue bars, right half of Fig. 7A).
Fig 7.
Serum from 4CMenB-vaccinated mice enhances opsonophagocytic killing of N. gonorrhoeae by primary human PMNs. (A) N. gonorrhoeae strain FA1090 was incubated with 2% BSA and 1:10 dilution of pooled serum from 4CMenB-vaccinated (blue) or alum-treated control (red) mice or no serum (−) (green). Five percent C6-depleted human serum that was intact (C’) or heat inactivated (HI-C’) was then added along with primary human PMNs (left side) or buffer control (right side). After 2 h, CFUs were enumerated from the infection mix after overnight growth. (B) The percentage of bacteria killed by OPKA with 4CMenB-vaccinated serum relative to the alum control is presented. Bars indicate the mean ± SD of three independent experiments, with each biological replicate as one data point from a different subject’s PMNs. *P < 0.025, Student’s t test.
We conducted the OPKA on serum from 4CMenB-vaccinated and alum-treated mice in the presence or absence of PMNs and with intact C6-depleted NHS in three independent experiments, each with a different subjects’ PMNs. CFUs were enumerated from each condition as in Fig. 7A. For each mouse serum condition, the CFUs enumerated with PMNs were divided by the CFUs enumerated without PMNs, subtracted from one to represent as fraction of bacteria killed in the presence of PMNs, and multiplied by 100%. As shown in Fig. 7B, there was a statistically significant, reproducible increase in OPKA-mediated killing of strain FA1090 N. gonorrhoeae by 4CMenB-vaccinated mouse serum compared with serum from alum-treated mice. However, the magnitude of this effect was approximately a twofold increase in killing, compared with the potency of SBA (see Fig. 6), which was approximately 10-fold. We conclude that 4CMenB vaccination elicits a measurable but small effect on opsonophagocytic killing of N. gonorrhoeae by PMNs.
Taken together, these results show that 4CMenB vaccination of mice elicits antibodies that bind the surface of N. gonorrhoeae and promotes SBA and OPKA. These findings align with ongoing investigations into the cross-protective efficacy of OMV-containing vaccines like 4CMenB against N. gonorrhoeae and serve as methods for further investigation of these vaccines’ mechanisms of action and correlates of protection.
DISCUSSION
Preclinical efforts to develop vaccines for gonorrhea warrant the establishment of assays that report the functional, protective activities associated with vaccination, particularly in animal models. Although N. gonorrhoeae is a human-restricted pathogen, female mice can be experimentally infected with N. gonorrhoeae, and murine models of cervico-vaginal and ascending gonococcal infection are being used to test candidate vaccines against gonorrhea (72). To facilitate the investigation of immune correlates for candidate gonorrhea vaccines in the mouse model, here, we report the development of assays that measure functions associated with vaccine-elicited antibodies in mice: antibody binding to the gonococcal surface, SBA using Ig-depleted pooled normal human serum, and OPKA using C6-depleted pooled normal human serum and primary human PMNs. We report outcomes from these assays using purified antibodies with known gonococcal surface-binding characteristics as proof of concept and as positive controls. We extended these assays to demonstrate that the 4CMenB meningococcal vaccine elicits functional antibodies in mice that bind N. gonorrhoeae and elicit cidal activities. These assays can be applied in the context of preclinical gonorrhea vaccine development to measure and compare functional outcomes elicited by different vaccine formulations in mice and review these results in light of clearance rates and colonization loads in individual mice that are immunized and challenged with N. gonorrhoeae. Ultimately, this information can contribute to the quest to define correlates and surrogates of immunity and mechanisms of immune protection in humans with gonorrhea.
This study presents, to the best of our knowledge, the first time a suite of assays has been developed to measure antibody binding, SBA, and OPKA using multiple monoclonal and polyclonal antibodies and normal human serum as the complement source, for evaluating multiple lineages of N. gonorrhoeae. As our goal was to incorporate these assays into the workflow for gonorrhea vaccine development, we prioritized rigor and reproducibility considerations. A single stock of each bacterial strain was maintained at −80°C and cultured under the same conditions for each experiment. Antibody binding, SBA, and OPKA assays were conducted in 96-well plates to minimize volumes of bacteria, cells, human serum as complement source, and mouse serum as antibody source. The same positive control antibodies were included in every run of each experiment for comparison between days and human subjects and to ensure all reagents are functioning properly. A single lot of human serum as complement source was purchased in bulk to ensure consistency for the duration of these experiments. The human serum was purchased prealiquoted in small volumes to avoid repeated freezing and thawing of complement, which is heat labile. Human serum was used as exogenous complement source to mimic conditions in human gonorrheal secretions, including the presence of components that modulate complement- and antibody-mediated activities. These components include the complement-inhibitory factor H and complement C4b-binding protein, where only the human proteins bind to N. gonorrhoeae (19). For SBA, we used Ig-depleted normal human serum as complement source to remove any potentially cross-reactive endogenous human antibodies from the assay. BSA was added to the SBA and OPKA to minimize background non-specific or low-affinity antibody binding to N. gonorrhoeae and amplify differences in the context of immunization, which may explain differences in the magnitude of our SBA results with those reported by Leduc et al. (26). With a number of publications on SBA and OPKA for gonorrhea vaccines, studies that directly compare the SBA and OPKA assays here to ones published by other groups are needed for the field to reach consensus on preclinical vaccine evaluation for N. gonorrhoeae (26, 45, 46).
Functional antibody studies for N. gonorrhoeae have particular challenges. First, N. gonorrhoeae is notoriously phase and antigenically variable, which can affect day-to-day experimental outcomes. We did not select for any bacterial subpopulations from the variable bacterial stocks, choosing instead for a diverse stock of bacteria in each experiment to reflect the variants that would emerge in human infection (73, 74). These variations could explain why it was not possible to obtain 100% antibody-positive bacteria by imaging flow cytometry. Second, flow cytometry, particularly imaging flow cytometry, is expensive and time-consuming in sample processing, data acquisition, and analysis. ELISA is typically used as a surrogate for antibody binding to bacteria and is an approach being taken by others in our consortium. However, some antibodies may differ in binding to immobilized epitopes and binding to the bacterial surface, especially if the antigens are membrane proteins that achieve different conformations in the bacterial outer membrane. Future studies will compare the results obtained with imaging flow and ELISA for antigens of interest. Third, while CFU enumeration is an accurate way to monitor antibody-mediated control of bacteria via SBA and OPKA, it takes time and effort to plate and count colonies after overnight growth. The latter could be accelerated using automated colony counting. Our attempts to use the metabolic dye alamarBlue as a surrogate for colony counting for SBA did not yield substantial differences between sensitive and resistant conditions with the low numbers of N. gonorrhoeae used for SBA, which agrees with the finding from Clow et al. that a luminescence-based alternative for SBA was not as quantitative as CFU enumeration (75). However, these alternative approaches could be optimized in the future to enable higher-throughput analyses of vaccine candidates, as used for N. meningitidis (76). Fourth, OPKA requires the use of primary human PMNs, but not all investigators can readily source human blood cells. We developed the opsonophagocytic flow cytometry assay with the HL-60 human promyelocyte cell line, which has been used extensively for measuring OPKA against Gram-positive bacteria like S. pneumoniae, to avoid the need for primary blood cells. However, results with differentiated HL-60 cells were less consistent than anticipated. Murine PMNs could also be isolated by peritoneal lavage for in vitro OPKA to match the murine antibodies elicited by immunization in this preclinical model (77). Fifth, we used C6-depleted NHS as the OPKA complement source to decouple the direct bactericidal effect of serum from phagocyte-mediated killing but recognize that these activities may be synergistic in the context of infection. Finally, these assays measure the serum antibody response to N. gonorrhoeae, which may not accurately reflect the nature of the immune response to the bacteria at the mucosal surfaces they inhabit. To address this issue, we are exploring if the volume and antibody titer in vaginal washes from immunized mice are sufficient for use in the functional antibody studies.
OMV-based meningococcal serogroup B vaccines exhibit cross-protection of humans from gonorrhea infection and hospitalization (9, 20, 21, 23, 29, 31). Our results show that immunization of mice with the 4CMenB meningococcal vaccine elicits antibodies that bind the surface of diverse strains of N. gonorrhoeae and promote SBA and OPKA. These findings align with reports that mice vaccinated with 4CMenB are significantly less likely to remain vaginally colonized with N. gonorrhoeae (26) and that 4CMenB vaccination elicits antibodies that cross-react with N. gonorrhoeae antigens (27). We find it intriguing that the magnitude of the SBA titer did not correlate with the degree of antibody binding to the bacterial surface. Strain MS11, which had the highest SBA titer for 4CMenB-vaccinated serum, also had the lowest bacterial binding, and H041 did not show any specific increase in binding of antibody from 4CMenB-vaccinated serum, yet vaccination elicited SBA 16-fold above the alum-treated control (see Fig. 5 and Table 2). Factors that may contribute to this discrepancy include the efficacy of the subclass of antibodies that bind to the bacterial surface to elicit SBA, the presence of other immunoglobulin subtypes in the serum that differentially elicit SBA on different strains (e.g., IgM), the abundance and conformation of the antigen on the surface of each strain, and the intrinsic resistance of different strains to complement-mediated lysis.
Gonorrhea vaccine research has greatly accelerated in recent years, with several candidate vaccines now in the discovery stage (17, 78, 79). Immune parameters including antibody levels elicited by these different vaccine candidates are being measured, and validated functional assays are needed to assess the importance of antibody-mediated protection in experimental challenge models. At this time, we do not know which candidate vaccine antigens elicit protective effects, nor do we know what immune parameters are protective (17). Excitingly, many different vaccine candidates are entering preclinical evaluation in the mouse model, including purified proteins, proteins refolded into nanodiscs, peptides presented on virus-like particles, or OMV-based strategies. As results from these candidates become available, we and others will be able to rigorously test the hypothesis that mice that show enhanced clearance of N. gonorrhoeae have a stronger functional antibody response in bacterial antibody binding, SBA, and/or OPKA. When these antigens are identified, their contribution to the vaccine-elicited functional antibody response can be confirmed using N. gonorrhoeae that is engineered to be lacking the antigen of interest. Future efforts will be directed at developing functional antibody assays for evaluating serum from vaccinated vs. unvaccinated humans that is collected from clinical trials, such as those ongoing for 4CMenB [including NCT05294588 and (80)).
There are several important outcomes from applying functional antibody assays to gonococcal vaccine discovery. First, we will directly test the hypothesis that vaccine-elicited antibodies are protective against N. gonorrhoeae in experimental murine infection. This hypothesis is generally accepted for N. meningitidis, where capsular polysaccharides in some serogroups elicit a protective antibody response and where SBA was used to identify the antigens to include in 4CMenB but remains to be demonstrated for N. gonorrhoeae. Second, we will identify the antigens in N. gonorrhoeae that elicit serum antibody responses and the protective nature of those responses. These findings can help prioritize antigens to include or exclude in new gonococcal vaccines. For instance, antibodies against the Rmp antigen are detrimental to protective antibody responses against pathogenic Neisseria (13, 81). Third, we will test how outcomes in bacterial antibody binding, SBA, and OPKA correlate with one another and with other measures of vaccine efficacy, such as IgG titer and T cell responses. Finally, we will identify parameters that are predictive of a protective response against experimental infection of mice with N. gonorrhoeae. These parameters can be used to prioritize gonococcal vaccine antigens for further preclinical testing and optimization and ultimately for introduction into humans in phased clinical trials. These studies can help achieve the pressing need for vaccines against gonorrhea to mitigate antibiotic resistance and improve global public health.
ACKNOWLEDGMENTS
We thank Asya Smirnov and Stephanie Ragland for their initial contributions in the development of these protocols. We thank Michael Solga of the UVA Flow Cytometry Core Facility (RRID: SCR_017829) for the expert advice on imaging flow cytometry, Sanja Arandjelovic for the advice on growing and differentiating HL-60 cells, and Sanjay Ram and Peter Rice for the gifts of 2C7 and 6B4 monoclonal antibodies. We thank all members of the Gonorrhea Vaccine Cooperative Research Center, in particular Margaret Bash, Alex Duncan, and Kathryn Matthias, as well as Sanjay Ram and Lisa Lewis, for thoughtful discussions on assay development and reporting.
This work was supported by NIH U19 AI144180, NIH U01 AI162457, R01 AI097312, and R21 AI157539.
Contributor Information
Alison K. Criss, Email: akc2r@virginia.edu.
Kimberly A. Kline, Universite de Geneve, Geneva, Switzerland
REFERENCES
- 1. World Health Organization . 2021. Gonorrhoea: Latest antimicrobial global surveillance results and guidance for vaccine development. Available from: https://www.who.int/news/item/22-11-2021-gonorrhoea-antimicrobial-resistance-results-and-guidance-vaccine-development
- 2. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021. https://www.cdc.gov/std/statistics/2021/default.htm. [Google Scholar]
- 3. Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, Dillon J-AR, Ramon-Pardo P, Bolan G, Wi T. 2019. World health organization global gonococcal antimicrobial surveillance program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 16:412. doi: 10.1071/SH19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Unemo M, Seifert HS, Hook EW III, Hawkes S, Ndowa F, Dillon J-AR. 2019. Gonorrhoea. Nat Rev Dis Primers 5:79. doi: 10.1038/s41572-019-0128-6 [DOI] [PubMed] [Google Scholar]
- 5. Lawson RM. 2020. Sexually transmitted infections and human immunodeficiency virus. Nurs Clin North Am 55:445–456. doi: 10.1016/j.cnur.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 6. Leonard CA, Schoborg RV, Low N, Unemo M, Borel N. 2019. Pathogenic interplay between Chlamydia trachomatis and Neisseria gonorrhoeae that influences management and control efforts-more questions than answers. Curr Clin Micro Rpt 6:182–191. doi: 10.1007/s40588-019-00125-4 [DOI] [Google Scholar]
- 7. Deal CD, Wetzler LM, Feavers IM, Gray-Owen SD, Jerse AE, Rice PA. 2016. Summary and recommendations from the National Institute of allergy and infectious diseases (NIAID) workshop “Gonorrhea vaccines: the way forward. Clin Vaccine Immunol 23:656–663. doi: 10.1128/CVI.00230-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craig AP, Gray RT, Edwards JL, Apicella MA, Jennings MP, Wilson DP, Seib KL. 2015. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 33:4520–4525. doi: 10.1016/j.vaccine.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Longtin J, Dion R, Simard M, Betala Belinga J-F, Longtin Y, Lefebvre B, Labbé A-C, Deceuninck G, De Wals P. 2017. Possible impact of wide-scale vaccination against serogroup B Neisseria meningitidis on gonorrhea incidence rates in one region of Quebec. Open Forum Infect Dis 4:S734–S735. doi: 10.1093/ofid/ofx180.002 [DOI] [Google Scholar]
- 10. Whittles LK, Didelot X, White PJ. 2022. Public health impact and cost-effectiveness of gonorrhoea vaccination: an integrated transmission-dynamic health-economic modelling analysis. Lancet Infect Dis 22:1030–1041. doi: 10.1016/S1473-3099(21)00744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Régnier SA, Huels J. 2014. Potential impact of vaccination against Neisseria meningitidis on Neisseria gonorrhoeae in the United States: results from a decision-analysis model. Hum Vaccin Immunother 10:3737–3745. doi: 10.4161/hv.36221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quillin SJ, Seifert HS. 2018. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 16:226–240. doi: 10.1038/nrmicro.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plummer FA, Chubb H, Simonsen JN, Bosire M, Slaney L, Maclean I, Ndinya-Achola JO, Waiyaki P, Brunham RC. 1993. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J Clin Invest 91:339–343. doi: 10.1172/JCI116190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmer A, Criss AK. 2018. Gonococcal defenses against antimicrobial activities of neutrophils. Trends Microbiol 26:1022–1034. doi: 10.1016/j.tim.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens JS, Criss AK. 2018. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol 25:13–21. doi: 10.1097/MOH.0000000000000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurakis SA, Cornelissen CN. 2022. Recent progress towards a gonococcal vaccine. Front Cell Infect Microbiol 12:881392. doi: 10.3389/fcimb.2022.881392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rice PA, Shafer WM, Ram S, Jerse AE. 2017. Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development. Annu Rev Microbiol 71:665–686. doi: 10.1146/annurev-micro-090816-093530 [DOI] [PubMed] [Google Scholar]
- 18. Ricklin D, Hajishengallis G, Yang K, Lambris JD. 2010. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797. doi: 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis LA, Ram S.. 2020. Complement interactions with the pathogenic Neisseriae: clinical features, deficiency states, and evasion mechanisms. FEBS Lett doi: 10.1002/1873-3468.13760. [DOI] [PubMed] [Google Scholar]
- 20. Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, Black S. 2017. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 390:1603–1610. doi: 10.1016/S0140-6736(17)31449-6 [DOI] [PubMed] [Google Scholar]
- 21. Paynter J, Goodyear-Smith F, Morgan J, Saxton P, Black S, Petousis-Harris H. 2019. Effectiveness of a group B outer membrane Vesicle Meningococcal vaccine in preventing hospitalization from gonorrhea in New Zealand: a retrospective cohort study. Vaccines (Basel) 7:5. doi: 10.3390/vaccines7010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azze RFO. 2019. A meningococcal B vaccine induces cross-protection against gonorrhea. Clin Exp Vaccine Res 8:110–115. doi: 10.7774/cevr.2019.8.2.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ochoa-Azze RF. 2018. Cross-protection induced by VA-MENGOC-BC(R) vaccine. Hum Vaccin Immunother 14:1064–1068. doi: 10.1080/21645515.2018.1438028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30:B87–97. doi: 10.1016/j.vaccine.2012.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seib KL, Zhao X, Rappuoli R. 2012. Developing vaccines in the era of genomics: a decade of reverse vaccinology. Clin Microbiol Infect 18:109–116. doi: 10.1111/j.1469-0691.2012.03939.x [DOI] [PubMed] [Google Scholar]
- 26. Leduc I, Connolly KL, Begum A, Underwood K, Darnell S, Shafer WM, Balthazar JT, Macintyre AN, Sempowski GD, Duncan JA, Little MB, Rahman N, Garges EC, Jerse AE. 2020. The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae. PLoS Pathog 16:e1008602. doi: 10.1371/journal.ppat.1008602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Semchenko EA, Tan A, Borrow R, Seib KL. 2019. The serogroup B meningococcal vaccine bexsero elicits antibodies to Neisseria gonorrhoeae. Clin Infect Dis 69:1101–1111. doi: 10.1093/cid/ciy1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matthias KA, Connolly KL, Begum AA, Jerse AE, Macintyre AN, Sempowski GD, Bash MC. 2022. Meningococcal detoxified outer membrane vesicle vaccines enhance gonococcal clearance in a murine infection model. J Infect Dis 225:650–660. doi: 10.1093/infdis/jiab450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruxvoort KJ, Lewnard JA, Chen LH, Tseng HF, Chang J, Veltman J, Marrazzo J, Qian L. 2023. Prevention of Neisseria gonorrhoeae with meningococcal B vaccine: a matched cohort study in Southern California. Clin Infect Dis 76:e1341–e1349. doi: 10.1093/cid/ciac436 [DOI] [PubMed] [Google Scholar]
- 30. Wang B, Giles L, Andraweera P, McMillan M, Almond S, Beazley R, Mitchell J, Lally N, Ahoure M, Denehy E, Koehler A, Flood L, Marshall H. 2022. Effectiveness and impact of the 4CMenB vaccine against invasive serogroup B meningococcal disease and gonorrhoea in an infant, child, and adolescent programme: an observational cohort and case-control study. Lancet Infect Dis 22:1011–1020. doi: 10.1016/S1473-3099(21)00754-4 [DOI] [PubMed] [Google Scholar]
- 31. Raccagni AR, Galli L, Spagnuolo V, Bruzzesi E, Muccini C, Bossolasco S, Ranzenigo M, Gianotti N, Lolatto R, Castagna A, Nozza S. 2023. Meningococcus B vaccination effectiveness against Neisseria gonorrhoeae infection in people living with HIV: a case-control study. Sex Transm Dis 50:247–251. doi: 10.1097/OLQ.0000000000001771 [DOI] [PubMed] [Google Scholar]
- 32. Abara WE, Bernstein KT, Lewis FMT, Schillinger JA, Feemster K, Pathela P, Hariri S, Islam A, Eberhart M, Cheng I, Ternier A, Slutsker JS, Mbaeyi S, Madera R, Kirkcaldy RD. 2022. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: a retrospective observational study. Lancet Infect Dis 22:1021–1029. doi: 10.1016/S1473-3099(21)00812-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belcher T, Rollier CS, Dold C, Ross JDC, MacLennan CA. 2023. Immune responses to Neisseria gonorrhoeae and implications for vaccine development. Front Immunol 14:1248613. doi: 10.3389/fimmu.2023.1248613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zielke RA, Wierzbicki IH, Baarda BI, Gafken PR, Soge OO, Holmes KK, Jerse AE, Unemo M, Sikora AE. 2016. Proteomics-driven antigen discovery for development of vaccines against gonorrhea. Mol Cell Proteomics 15:2338–2355. doi: 10.1074/mcp.M116.058800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu T, McClure R, Harrison OB, Genco C, Massari P. 2019. Integrated bioinformatic analyses and immune characterization of new Neisseria gonorrhoeae vaccine antigens expressed during natural mucosal infection. Vaccines (Basel) 7:153. doi: 10.3390/vaccines7040153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parzych EM, Gulati S, Zheng B, Bah MA, Elliott STC, Chu JD, Nowak N, Reed GW, Beurskens FJ, Schuurman J, Rice PA, Weiner DB, Ram S. 2021. Synthetic DNA delivery of an optimized and engineered monoclonal antibody provides rapid and prolonged protection against experimental gonococcal infection. mBio 12:e00242-21. doi: 10.1128/mBio.00242-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gulati S, Pennington MW, Czerwinski A, Carter D, Zheng B, Nowak NA, DeOliveira RB, Shaughnessy J, Reed GW, Ram S, Rice PA. 2019. Preclinical efficacy of a lipooligosaccharide peptide mimic candidate gonococcal vaccine. mBio 10:e02552-19. doi: 10.1128/mBio.02552-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus I. the role of humoral antibodies. J Exp Med 129:1307–1326. doi: 10.1084/jem.129.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McIntosh EDG, Bröker M, Wassil J, Welsch JA, Borrow R. 2015. Serum bactericidal antibody assays - the role of complement in infection and immunity. Vaccine 33:4414–4421. doi: 10.1016/j.vaccine.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 40. Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, Miller E, Plikaytis B, van Alphen L, Poolman J, Rappuoli R, Danzig L, Hackell J, Danve B, Caulfield M, Lambert S, Stephens D. 2006. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report, Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson A, Lepow IH. 1979. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science 205:298–299. doi: 10.1126/science.451601 [DOI] [PubMed] [Google Scholar]
- 42. Granoff DM. 2009. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine 27 Suppl 2:B117–25. doi: 10.1016/j.vaccine.2009.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ross SC, Rosenthal PJ, Berberich HM, Densen P. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis 155:1266–1275. doi: 10.1093/infdis/155.6.1266 [DOI] [PubMed] [Google Scholar]
- 44. Perkins BA, Jonsdottir K, Briem H, Griffiths E, Plikaytis BD, Hoiby EA, Rosenqvist E, Holst J, Nokleby H, Sotolongo F, Sierra G, Campa HC, Carlone GM, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger JD, Broome CV. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis 177:683–691. doi: 10.1086/514232 [DOI] [PubMed] [Google Scholar]
- 45. Gulati S, Rice PA, Ram S. 2019. Complement-dependent serum bactericidal assays for Neisseria gonorrhoeae. Methods Mol Biol 1997:267–280. doi: 10.1007/978-1-4939-9496-0_16 [DOI] [PubMed] [Google Scholar]
- 46. Semchenko EA, Jen F-C, Jennings MP, Seib KL. 2022. Assessment of serum bactericidal and opsonophagocytic activity of antibodies to gonococcal vaccine targets. Methods Mol Biol 2414:363–372. doi: 10.1007/978-1-0716-1900-1_19 [DOI] [PubMed] [Google Scholar]
- 47. Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis 169:532–537. doi: 10.1093/infdis/169.3.532 [DOI] [PubMed] [Google Scholar]
- 48. Shafer WM, Joiner K, Guymon LF, Cohen MS, Sparling PF. 1984. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis 149:175–183. doi: 10.1093/infdis/149.2.175 [DOI] [PubMed] [Google Scholar]
- 49. Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. 2003. A Gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seifert HS, Ajioka RS, Marchal C, Sparling PF, So M. 1988. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature 336:392–395. doi: 10.1038/336392a0 [DOI] [PubMed] [Google Scholar]
- 51. Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vincent LR, Kerr SR, Tan Y, Tomberg J, Raterman EL, Dunning Hotopp JC, Unemo M, Nicholas RA, Jerse AE. 2018. In vivo -selected compensatory mutations restore the fitness cost of mosaic penA Alleles that confer ceftriaxone resistance in Neisseria gonorrhoeae. mBio 9:e01905–17. doi: 10.1128/mBio.01905-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. KELLOGG DS, PEACOCK WL, DEACON WE, BROWN L, PIRKLE DI. 1963. Neisseria gonorrhoeae. I. virulence genetically linked to clonal variation. J Bacteriol 85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, Garvin LE. 2011. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front Microbiol 2:107. doi: 10.3389/fmicb.2011.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ragland SA, Criss AK. 2019. Protocols to interrogate the interactions between Neisseria gonorrhoeae and primary human neutrophils. Methods Mol Biol 1997:319–345. doi: 10.1007/978-1-4939-9496-0_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Werner LM, Palmer A, Smirnov A, Belcher Dufrisne M, Columbus L, Criss AK. 2020. Imaging flow cytometry analysis of CEACAM binding to Opa-expressing Neisseria gonorrhoeae. Cytometry A 97:1081–1089. doi: 10.1002/cyto.a.24037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smirnov A, Daily KP, Gray MC, Ragland SA, Werner LM, Johnson MB, Eby JC, Hewlett EL, Taylor RP, Criss AK. 2023. Phagocytosis via complement receptor 3 enables microbes to evade killing by neutrophils. J Leukoc Biol 114:1–20. doi: 10.1093/jleuko/qiad028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Werner LM, Alcott A, Mohlin F, Ray JC, Belcher Dufrisne M, Smirnov A, Columbus L, Blom AM, Criss AK. 2023. Neisseria gonorrhoeae co-opts C4b-binding protein to enhance complement-independent survival from neutrophils. PLoS Pathog 19:e1011055. doi: 10.1371/journal.ppat.1011055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mandrell R, Schneider H, Apicella M, Zollinger W, Rice PA, Griffiss JM. 1986. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun 54:63–69. doi: 10.1128/iai.54.1.63-69.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. 1996. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis 174:1223–1237. doi: 10.1093/infdis/174.6.1223 [DOI] [PubMed] [Google Scholar]
- 61. Johnson MB, Criss AK. 2013. Neisseria gonorrhoeae phagosomes delay fusion with primary granules to enhance bacterial survival inside human neutrophils. Cell Microbiol 15:1323–1340. doi: 10.1111/cmi.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mandrell RE, Griffiss JM, Macher BA. 1988. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med 168:107–126. doi: 10.1084/jem.168.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lewis LA, Ram S. 2014. Meningococcal disease and the complement system. Virulence 5:98–126. doi: 10.4161/viru.26515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, Galeotti CL, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood DW, Jeffries AC, Saunders NJ, Granoff DM, Venter JC, Moxon ER, Grandi G, Rappuoli R. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816–1820. doi: 10.1126/science.287.5459.1816 [DOI] [PubMed] [Google Scholar]
- 65. Gotschlich EC, Goldschneider I, Artenstein MS. 1969. Human immunity to the meningococcus. V. the effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med 129:1385–1395. doi: 10.1084/jem.129.6.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Plested JS, Granoff DM. 2008. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol 15:799–804. doi: 10.1128/CVI.00036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martinez J, Pilishvili T, Barnard S, Caba J, Spear W, Romero-Steiner S, Carlone GM. 2002. Opsonophagocytosis of fluorescent polystyrene beads coupled to Neisseria meningitidis serogroup A, C, Y, or W135 polysaccharide correlates with serum bactericidal activity. Clin Diagn Lab Immunol 9:485–488. doi: 10.1128/CDLI.9.2.485-488.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chakraborti S, Lewis LA, Cox AD, St Michael F, Li J, Rice PA, Ram S. 2016. Phase-variable heptose I glycan extensions modulate efficacy of 2C7 vaccine antibody directed against Neisseria gonorrhoeae lipooligosaccharide. J Immunol 196:4576–4586. doi: 10.4049/jimmunol.1600374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol 13:165–169. doi: 10.1128/CVI.13.2.165-169.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 4:415–422. doi: 10.1128/cdli.4.4.415-422.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fleck RA, Romero-Steiner S, Nahm MH. 2005. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol 12:19–27. doi: 10.1128/CDLI.12.1.19-27.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Connolly KL, Pilligua-Lucas M, Gomez C, Costenoble-Caherty AC, Soc A, Underwood K, Macintyre AN, Sempowski GD, Jerse AE. 2021. Preclinical testing of vaccines and therapeutics for gonorrhea in female mouse models of lower and upper reproductive tract infection. J Infect Dis 224:S152–S160. doi: 10.1093/infdis/jiab211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest 93:2744–2749. doi: 10.1172/JCI117290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med 179:911–920. doi: 10.1084/jem.179.3.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Clow F, O’Hanlon CJ, Christodoulides M, Radcliff FJ. 2019. Feasibility of using a luminescence-based method to determine serum bactericidal activity against Neisseria gonorrhoeae. Vaccines (Basel) 7:191. doi: 10.3390/vaccines7040191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mountzouros KT, Howell AP. 2000. Detection of complement-mediated antibody-dependent bactericidal activity in a fluorescence-based serum bactericidal assay for group B Neisseria meningitidis. J Clin Microbiol 38:2878–2884. doi: 10.1128/JCM.38.8.2878-2884.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu H, Jerse AE. 2006. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect Immun 74:4094–4103. doi: 10.1128/IAI.00433-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sikora AE, Gomez C, Le Van A, Baarda BI, Darnell S, Martinez FG, Zielke RA, Bonventre JA, Jerse AE. 2020. A novel gonorrhea vaccine composed of MetQ lipoprotein formulated with CpG shortens experimental murine infection. Vaccine 38:8175–8184. doi: 10.1016/j.vaccine.2020.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fegan JE, Calmettes C, Islam EA, Ahn SK, Chaudhuri S, Yu R-H, Gray-Owen SD, Moraes TF, Schryvers AB. 2019. Utility of hybrid transferrin binding protein antigens for protection against pathogenic Neisseria species. Front Immunol 10:247. doi: 10.3389/fimmu.2019.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thng C, Semchenko EA, Hughes I, O’Sullivan M, Seib KL. 2023. An open-label randomised controlled trial evaluating the efficacy of a meningococcal serogroup B (4CMenB) vaccine on Neisseria gonorrhoeae infection in gay and bisexual men: the mengo study protocol. BMC Public Health 23:607. doi: 10.1186/s12889-023-15516-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rosenqvist E, Musacchio A, Aase A, Høiby EA, Namork E, Kolberg J, Wedege E, Delvig A, Dalseg R, Michaelsen TE, Tommassen J. 1999. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect Immun 67:1267–1276. doi: 10.1128/IAI.67.3.1267-1276.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]