ABSTRACT
Non-Lactobacillus-dominated vaginal microbiota has been associated with poor gynecologic health and complications during pregnancy. Lactobacilli and especially Lactobacillus crispatus associate with good reproductive health and dominate the microbiota during healthy pregnancy. We examined whether the composition of vaginal microbiota at the time of fresh embryo transfer (ET) has an impact on the success of in vitro fertilization (IVF) and whether vaginal microbiota changes from IVF-ET to early pregnancy within individuals. Vaginal swab samples were collected from subfertile women at the time of IVF-ET (n = 76) and at the eighth gestational week (n = 21) from those who achieved clinical pregnancy. The microbiota composition was analyzed using 16S rRNA gene amplicon sequencing. L. crispatus was more abundant among the 30 women who achieved clinical pregnancy (46.9% vs. 19.1%, q = 0.039) and the 26 women who had live birth (43.3% vs. 23.1%, q = 0.32) compared to those who did not. Lactobacilli, mainly L. crispatus (76.2%), dominated all early pregnancy samples. Microbiota remained the same, i.e., Lactobacillus-dominated type in 52% (11/21), shifted from one Lactobacillus-dominated type to another in 24% (5/21), or shifted from mixed community to Lactobacillus-dominated type in 24% (5/21) women, but never from Lactobacillus dominance to non-lactobacilli dominance. Our results emphasize the role of L. crispatus in the success of IVF-ET and in early pregnancy. During pregnancy, the microbiota shifted toward L. crispatus dominance even if it was undetectable before pregnancy, indicating that most women hold a reservoir of this beneficial Lactobacillus in their reproductive tract.
IMPORTANCE
Infertility is a global public health issue which leads many couples to seek fertility treatments, of which in vitro fertilization (IVF) is considered to be the most effective. Still, only about one-third of the women achieve live birth after the first IVF embryo transfer (IVF-ET). Factors affecting embryo implantation are poorly known, but the female reproductive tract microbiota may play a key role. Our study confirms the beneficial role of vaginal lactobacilli, especially Lactobacillus crispatus, in the probability of achieving clinical pregnancy and live birth following IVF-ET. Our findings regarding the intra-individual shift of vaginal microbiota between non-pregnancy and pregnancy states are novel and provide new information about the dynamics of microbiota in the early steps of human reproduction. These findings may help clinicians in their attempts to optimize the conditions for ET by microbiota screening or modulation and timing the ET when the microbiota is the most favorable.
KEYWORDS: infertility, L. crispatus, embryo transfer, vaginal microbiota, IVF
INTRODUCTION
Infertility is a global public health issue affecting up to 15% of couples at the childbearing age (1). This leads to many couples seeking infertility treatments of which in vitro fertilization (IVF) is considered to be the most effective (2). However, only a few IVF cycles lead to pregnancy and childbirth, since only about 30%–35% of women achieve live birth after IVF–embryo transfer (IVF-ET) (3, 4). For a successful IVF-ET, the fertilized egg or blastocyst must be able to attach and implant to a healthy endometrium. Along with the quality of the embryo (5) and the receptivity of the endometrium (6), the IVF-ET outcome may also be affected by the microbiota of the female reproductive tract (7).
Lactobacilli are known to be the most abundant bacteria in a healthy endometrial (8) and vaginal (9) microbiota, and the presence of other microbes than lactobacilli on the tip of ET catheters has been associated with poor outcomes of IVF (10, 11). Also, in vaginal smears examined by microscopy, the dominance of other bacteria than lactobacilli has been associated with a lower clinical pregnancy rate (12, 13) and live birth rate (14) following IVF treatment.
Studies using high-throughput DNA sequencing techniques have suggested that Lactobacillus-dominated endometrial (15, 16), cervical (17), and vaginal (7, 18) microbiota may have higher pregnancy rates after IVF-ET, compared to women with non-Lactobacillus dominance. In particular, Lactobacillus crispatus has been associated with a successful IVF-ET outcome (19, 20). In turn, vaginal dysbiosis, characterized by the dominance of other bacteria than lactobacilli, has been associated with recurrent implantation failure (21). Gardnerella vaginalis, Fannyhessea vaginae, and Prevotella bivia are bacteria typically associated with bacterial vaginosis (22, 23) and have also been associated with lower pregnancy rates in IVF (12), whereas low diversity of the vaginal microbiota, reflecting Lactobacillus dominance, has been implicated in higher clinical pregnancy rates in IVF (24). However, some studies have found opposite results regarding the effect of Lactobacillus dominance on IVF-ET results (25 – 27). The discrepant results may originate from relatively small sample sizes or differences in the clinical or other background characteristics of the study populations (24, 25).
The composition of microbiota is affected by pregnancy, mainly due to the increasing levels of circulating estrogen (28). During pregnancy, the composition of vaginal microbiota shifts toward Lactobacillus dominance (29) and changes postpartum, as serum estrogen levels fall dramatically (28, 30). In particular, L. crispatus has been the most abundant species in the vaginal microbiota in the first (31) and third (32) trimesters, whereas early miscarriage (33) and preterm birth (PTB) (34) have been associated with the non-Lactobacillus-dominated microbiota. The intra-individual transition in the composition of the vaginal microbiota during IVF-ET and between early pregnancy has not been explored before.
The aims of this study were to investigate whether the composition of the vaginal microbiota at the time of the fresh embryo transfer is associated with the probability of achieving a clinical pregnancy and live birth and how the vaginal microbiota composition changes between the time of the IVF-ET and early pregnancy.
RESULTS
Cohort characteristics
A total of 76 women underwent fresh IVF-ET cycles, of which 30 (39.5%) achieved clinical pregnancy, and two (2.6%) had biochemical pregnancy. None had an ectopic pregnancy. Four of the 30 (13.3%) women with clinical pregnancy ended up in a miscarriage, and altogether, 26 women (34.2%) had live births. Women who achieved clinical pregnancy were significantly younger than women who did not, as were the women with live births (Tables 1 and 2). Also, women with clinical pregnancy (86.7% vs 63.0%, P = 0.02) and live birth (92.3% vs 62.0%, P = 0.005) were more often nulliparous (no prior deliveries) compared with women with no clinical pregnancy or live birth. There were no significant differences between the women who achieved clinical pregnancy or live birth compared to those who did not in terms of body mass index (BMI), duration of infertility, smoking habits, and education. We also compared clinical factors that might have an impact on the success of implantation, including serum anti-Müllerian hormone (AMH) level, endometrium thickness, embryo stage, and other related clinical variables, between the pregnancy, and non-pregnancy groups and found no significant differences (Table 1).
TABLE 1.
Background and clinical characteristics of the study population according to the achievement of clinical pregnancy
| Variable | Pregnancy (n = 30) | Non-pregnancy (n = 46) | P-value |
|---|---|---|---|
| Age (years) | 0.01 | ||
| Mean (SD [range]) | 32.9 (4.3 [23–39]) | 35.1 (3.6 [24–40]) | |
| BMI (kg/m2) | 0.91 | ||
| Mean (SD [range]) | 24.9 (3.8 [19.8–32.5]) | 24.8 (4.2 [17.4–36.0]) | |
| Duration of infertility (months) a | 0.49 | ||
| Mean (SD [range]) | 40.1 (27.6 [12–156]) | 46.0 (30.2 [12–134]) | |
| Cause of infertility, n (%) | |||
| Endometriosis (n = 20) | 10 (33.3) | 10 (21.7) | |
| Male factor (n = 11) | 2 (6.7) | 9 (19.6) | |
| Tubal factor (n = 16) | 5 (16.7) | 11 (23.9) | |
| Anovulation (n = 6) | 4 (13.3) | 2 (4.3) | |
| Unexplained (n = 23) | 9 (30.0) | 14 (30.4) | |
| Type of infertility, n (%) | 0.08 | ||
| Primary (n = 44) | 21 (70.0) | 23 (50.0) | |
| Secondary (n = 32) | 9 (30.0) | 23 (50.0) | |
| Prior delivery, n (%) | 0.02 | ||
| Yes (n = 21) | 4 (13.3) | 17 (37.0) | |
| No (n = 55) | 26 (86.7) | 29 (63.0) | |
| Prior extrauterine pregnancy, n (%) a | 1 | ||
| Yes (n = 6) | 2 (6.7) | 4 (8.9) | |
| No (n = 69) | 28 (93.3) | 41 (91.1) | |
| Prior miscarriage, n (%) a | 0.65 | ||
| Yes (n = 17) | 6 (20.0) | 11 (24.4) | |
| No (n = 58) | 24 (80.0) | 34 (75.6) | |
| Smoking status, n (%) a | 0.39 | ||
| Current/former smoker (n = 33) | 15 (50.0) | 18 (40.0) | |
| Non-smoker (n = 42) | 15 (50.0) | 27 (60.0) | |
| Level of education, n (%) a | 0.91 | ||
| Low (comprehensive school, vocational secondary school) (n = 16) | 6 (20.7) | 10 (21.7) | |
| High (upper secondary school, university) (n = 59) | 23 (79.3) | 36 (78.3) | |
| AFC | 0.22 | ||
| Mean (SD [range]) | 17.7 (10.8 [1–49]) | 14.3 (6.7 [4–34]) | |
| AMH (ng/mL) b | 0.53 | ||
| Mean (SD [range]) | 2.6 (1.9 [0.1–6.4)] | 2.3 (1.6 [0.4–8.4]) | |
| Stimulation protocol, n (%) | 0.79 | ||
| Agonist (n = 52) | 20 (66.7) | 32 (69.6) | |
| Antagonist (n = 24) | 10 (33.3) | 14 (30.4) | |
| Endometrium thickness (mm) | 0.77 | ||
| Mean (SD [range]) | 10.3 (2.7 [3.7–17.4]) | 10.2 (2.4 [3.3–15.6]) | |
| The number of oocytes retrieved | 0.59 | ||
| Mean (SD [range]) | 9.9 (5.6 [3–22]) | 9.5 (6.5 [2–27]) | |
| The number of mature oocytes b | 0.74 | ||
| Mean (SD [range]) | 8.2 (4.9 [3–19]) | 8.1 (5.5 [1–22]) | |
| Fertilization, n (%) | 0.12 | ||
| Conventional IVF (n = 56) | 25 (83.3) | 31 (67.4) | |
| ICSI (n = 20) | 5 (16.7) | 15 (32.6) | |
| The number of normally fertilized eggs | 0.3 | ||
| Mean (SD [range]) | 5.8 (4.0 [1–15]) | 5.3 (4.5 [0–16]) | |
| Age of embryo transfered (days) | 0.82 | ||
| Mean (SD [range]) | 3.1 (0.9 [2–5]) | 3.2 (1.0 [2–5]) | |
| Stage of embryo | 0.75 | ||
| Cleavage stage (n = 62) | 25 (83.3) | 37 (80.4) | |
| Blastocyst (n = 14) | 5 (16.7) | 9 (19.6) |
Data missing in one case.
Data missing in five cases.
TABLE 2.
Background characteristics of the study population according to the achievement of live birth
| Variable | Live birth (n = 26) | No live birth (n = 50) | P-value |
|---|---|---|---|
| Age (years) | |||
| Mean (SD [range]) | 32.5 (4.2 [23–38]) | 35.1 (3.6 [24–40]) | 0.005 |
| BMI (kg/m2) | |||
| Mean (SD [range]) | 25.2 (3.9 [19.8–32.5]) | 24.7 (4.1 [17.4–36.0]) | 0.54 |
| Duration of infertility (months) a | |||
| Mean (SD [range]) | 39.7 (28.3 [12–156]) | 45.7 (29.7 [12–134]) | 0.48 |
| Cause of infertility, n (%) | |||
| Endometriosis (n = 20) | 9 (34.6) | 11 (22.0) | |
| Male factor (n = 11) | 2 (7.7) | 9 (18.0) | |
| Tubal factor (n = 16) | 5 (19.2) | 11 (22.0) | |
| Anovulation (n = 6) | 4 (15.4) | 2 (4.0) | |
| Unexplained (n = 23) | 6 (23.1) | 17 (34.0) | |
| Type of infertility, n (%) | 0.02 | ||
| Primary (n = 44) | 20 (76.9) | 24 (48.0) | |
| Secondary (n = 32) | 6 (23.1) | 26 (52.0) | |
| Prior delivery, n (%) | 0.005 | ||
| Yes (n = 21) | 2 (7.7) | 19 (38.0) | |
| No (n = 55) | 24 (92.3) | 31 (62.0) | |
| Prior extrauterine pregnancy, n (%) a | 1 | ||
| Yes (n = 6) | 2 (7.7) | 4 (8.2) | |
| No (n = 69) | 24 (92.3) | 45 (91.8) | |
| Prior miscarriage, n (%) a | 0.09 | ||
| Yes (n = 17) | 3 (11.5) | 14 (28.6) | |
| No (n = 58) | 23 (88.5) | 35 (71.4) | |
| Smoking status, n (%) a | 0.78 | ||
| Current/former smoker (n = 33) | 12 (7.7) | 21 (42.9) | |
| Non-smoker (n = 42) | 14 (53.8) | 28 (57.1) | |
| Level of education, n (%) a | 0.69 | ||
| Low (comprehensive school, vocational secondary school) (n = 16) | 6 (24.0) | 10 (20.0) | |
| High (upper secondary school, university) (n = 59) | 19 (76.0) | 40 (80.0) |
Data missing in one case.
Vaginal microbiota at the time of fresh IVF-ET
After taxonomic annotations and quality filtration (>500 reads, median of 27,794 reads), 75 samples were available for the bacterial analysis. One sample from a woman who achieved clinical pregnancy and live birth after IVF-ET was not available for analysis due to low read count.
First, associations between background and clinical variables and the vaginal microbiota were analyzed with permutational analysis of variance (PERMANOVA). The microbiota composition was associated with clinical pregnancy (R 2 = 0.07, P = 0.00007), woman’s age (R 2 = 0.05, P = 0.001), gravidity (R 2 = 0.09, P = 0.008), parity (R 2 = 0.04, P = 0.01), and history of preterm birth (R 2 = 0.03, P = 0.008). Based on these results as well as the clinical differences presented in Tables 1 and 2, age, parity, and gravidity were used to adjust the microbiota comparisons between the IVF success groups in order to study their independent association with microbiota composition. Other background variables, including prior urogenital infections, smoking, use of probiotics or antibiotics in the past 3 months, or the level of education, had no impact on the vaginal microbiota variation. Also, clinical variables such as AMH, infertility diagnosis, or factors related to the IVF treatment did not explain the microbiota variation (https://github.com/SchahzadSaqib/HEMI).
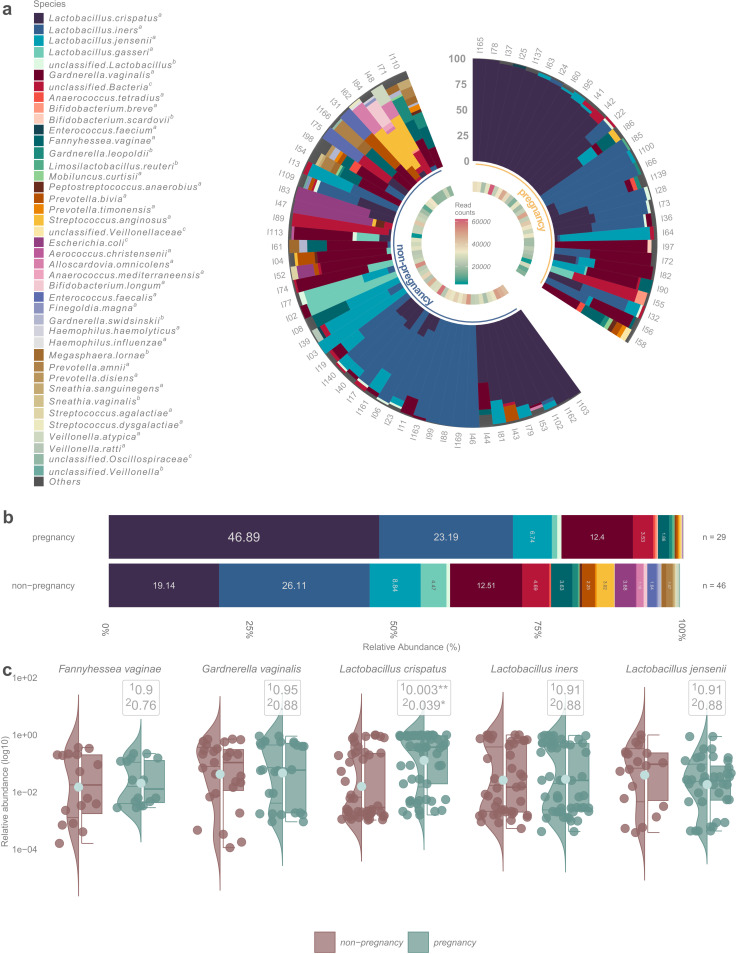
Lactobacillus-dominated (relative abundance >50%) vaginal microbiota was detected in 50 (66.7%) women at the time of IVF-ET (https://github.com/SchahzadSaqib/HEMI). Richness and diversity were on average lower for women who achieved clinical pregnancy, but the difference was not statistically significant (https://github.com/SchahzadSaqib/HEMI). L. crispatus was the most abundant species in the vaginal samples of women who achieved clinical pregnancy and live birth, whereas Lactobacillus iners was the most abundant species in those who failed to become pregnant (Fig. 1 and 2). Statistical analysis showed that the relative abundance of L. crispatus was significantly higher in the pregnancy vs non-pregnancy group (46.9% vs 19.1%, q = 0.003) and the result remained similar when adjusted for age, parity, and gravidity (Fig. 1). The relative abundance of L. iners (23.2% vs 26.1%, q = 0.91), Lactobacillus jensenii (6.7% vs 8.8%, q = 0.91), G. vaginalis (12.4% vs 12.5%, q = 0.95), and F. vaginae (1.9% vs 3.6%, q = 0.9) was slightly lower among women who achieved pregnancy compared to women who did not, but the differences were small.
Fig 1.
(a) Relative abundances of bacterial taxa in all 75 samples, divided based on pregnancy/non-pregnancy after fresh IVF-ET. (bB Stacked bar plot demonstrating the mean bacterial abundances of samples of women who achieved clinical pregnancy (n = 29) and women who did not (n = 46). (c) Violin plots showing the distribution of taxa between the groups. 1The non-adjusted value and 2after adjusting for age, parity, and gravidity.
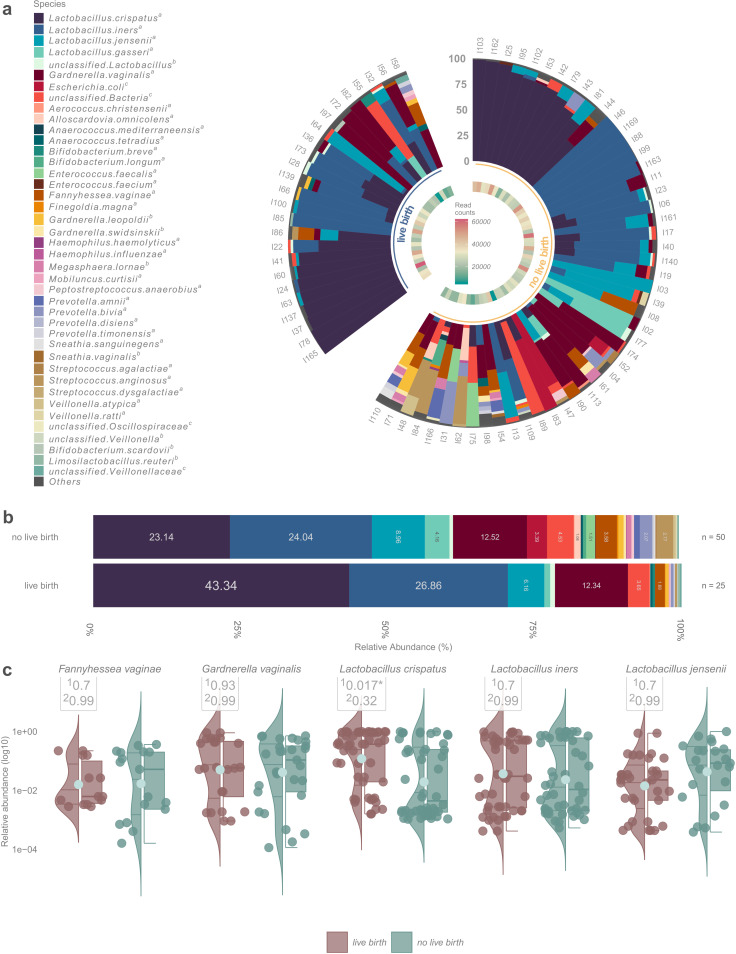
Fig 2.
(a) Relative abundances of bacterial taxa in all 75 samples, divided based on the no live birth/live birth after fresh IVF-ET. (b) Stacked bar plot demonstrating the mean bacterial abundances of samples of women who achieved live birth (n = 25) and women who did not (n = 50). (c) Violin plots showing the distribution of taxa between the groups. 1The non-adjusted value and 2after adjusting for age, parity, and gravidity.
In women who had live births, the relative abundance of L. crispatus was significantly higher compared to women with no pregnancy or live births (43.3% vs 23.1%, q = 0.017). However, when adjusted for age, parity, and gravidity the difference was not statistically significant (q = 0.32) (Fig. 2).
In the non-pregnancy group, P. bivia (8.70% vs 3.45%) and Streptococcus anginosus (6.52% vs 3.45%) were more prevalent and abundant (mean relative abundances 2.25% vs 0.39% for P. bivia and 3.02% vs 0.31% for S. anginosus) than in the pregnancy group, but the results were not tested for significance due to low overall prevalence in the cohort.
Inter-individual comparison of microbiota at the time of the fresh IVF-ET embryo and at early pregnancy
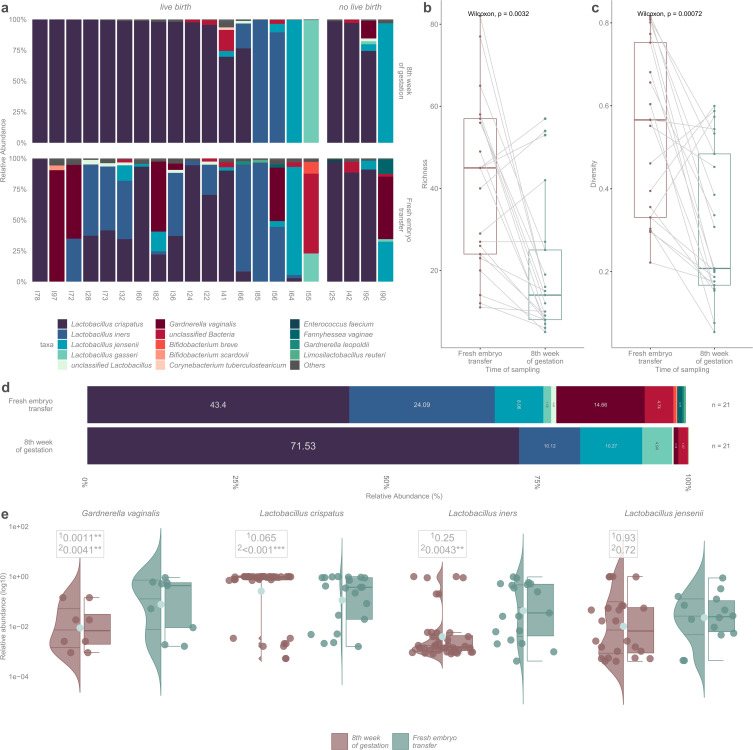
Vaginal samples were additionally collected at the eighth gestational week from 21 of the 30 women who achieved clinical pregnancy after ET. Nine samples were not available due to human error or because the ultrasound examination was performed elsewhere than in our research centers. Richness and diversity were significantly lower in samples at the eighth week of gestation (P = 0.0032; P = 0.00072, Fig. 3b and c). All pregnancy samples at 8 wk were dominated by lactobacilli, mainly by L. crispatus (n = 16, 76.2%). Two women (9.5%) had L. iners–dominated vaginal microbiota, two (9.5%) were dominated by L. jensenii, and one (4.8%) was dominated by Lactobacillus gasseri (Fig. 3). The relative abundance of L. crispatus was higher in pregnancy samples compared to the samples taken at ET (71.5% vs 43.4%, q = 0.065). The relative abundance of L. iners (24.1% vs 10.1%, q = 0.25) and G. vaginalis (14.7% vs 0.8%, q = 0.001) decreased between the IVF-ET and early pregnancy. After adjusting the results for age, parity, and gravidity, the relative abundance for L. crispatus (q < 0.001) and L. iners (q = 0.004) increased between the IVF-ET and early pregnancy (Fig. 3).
Fig 3.
(a) Stacked bar plot demonstrating the inter-individual comparison of the microbiota of 21 women at the time of the ET and at early pregnancy. (b) Paired boxplot for richness comparisons between the longitudinal samples. (c) Paired boxplot for diversity comparisons between the longitudinal samples. (d) Stacked bar plot demonstrating the mean relative bacterial abundances between the samples taken from 21 women at the time of the fresh embryo transfer and at early pregnancy. (e) Violin plots showing the distribution of taxa between the different time points. 1The non-adjusted value and 2after adjusting for age, parity, and gravidity.
When comparing the vaginal samples taken at the time of the fresh IVF-ET and at early pregnancy in the same individuals, eight women had L. crispatus dominance in both samples, two women had L. iners dominance, and one woman had L. jensenii dominance in paired samples (Fig. 3). Altogether, 10 women showed a shift in their microbiota profiles between these two time points. In five women, microbiota dominance changed from L. iners to L. crispatus, in three women from G. vaginalis to L. crispatus, in one woman from G. vaginalis to L. jensenii, and in one woman from unclassified bacteria to L. gasseri.
DISCUSSION
This study shows that the vaginal microbiota profile may influence the results of IVF-ET, as the dominance of L. crispatus at the time of the fresh IVF-ET was associated with a higher clinical pregnancy rate and higher live birth rate. In addition, a shift toward Lactobacillus dominance in early pregnancy was observed in women who successfully became pregnant after fresh IVF-ET.
Our results regarding the benefit of lactobacilli (35), especially on L. crispatus, and the success of implantation are in line with many earlier studies (7, 20). Koedooder et al. showed that women who had higher relative abundance of L. crispatus in their vaginal samples had higher chance of clinical pregnancy (7). Based on this finding, they developed an algorithm to predict the success of the first fresh IVF-ET based on the relative abundance of L. crispatus on the vaginal microbiota prior to the IVF cycle. However, they sampled women within 2 months before the analyzed IVF cycle, not at the time of IVF-ET. Another study consisting of women undergoing IVF with donated oocytes reported that women who achieved clinical pregnancy or live birth after fresh IVF-ET had higher abundance of L. crispatus compared to those who did not (19). There are also studies varying in sampling points during the IVF cycle and the type of embryo transferred (fresh or frozen) that have not found the composition of vaginal microbiota to affect the probability of achieving clinical pregnancy or live birth rate after IVF treatment (27, 36). Contradictory findings between different studies may also be explained by ethnically or geographically different study populations, since the vaginal microbial composition variates between different ethnic groups (9, 37).
The beneficial role of lactobacilli, especially L. crispatus, has been shown in several studies (38 – 40). L. crispatus possesses the enzymatic machinery to debranch and ferment the polymers derived from glycogen from the vaginal epithelium, producing lactic acid and hydrogen peroxide (H2O2), lowering the pH of the vagina (41, 42). Nevertheless, the function of hydrogen peroxide in the lower genital tract has still been controversial (43). Coupled with the secretion of bacteriocins, this acidic environment prevents the growth of other microbes and protects against sexually transmitted and other opportunistic infections (42, 44, 45). Lactic acid has also been shown to have immunomodulatory properties, particularly the suppression of inflammation and induction of anti-inflammatory compounds (42, 46, 47). Shortage of lactobacilli, in turn, has been associated with increased levels of proinflammatory cytokines (48). Hence, the vaginal microbiota overall can regulate the local immune environment and therefore contribute to the manifestation of various clinical phenotypes.
In the present study, G. vaginalis, P. bivia, and S. anginosus were more prevalent in the group that did not achieve pregnancy. This is in agreement with a previous study showing an increased relative abundance of Streptococcus and Gardnerella in women who failed to achieve a clinical pregnancy after fresh IVF-ET (49). Streptococci are often linked to aerobic vaginitis (AV) (50), a vaginal inflammation characterized by the disturbance of lactobacilli-dominated microbiota and increased amount of various aerobic bacteria (51), whereas G. vaginalis, F. vaginae, and P. bivia are known to be associated with bacterial vaginosis (BV) (22, 23). Both BV (12) and chronic endometritis (52) are suggested to decrease the likelihood of achieving clinical pregnancy in IVF-ET, whereas endometrial microbiota dominated by lactobacilli have been associated with higher implantation and ongoing pregnancy rates compared to non-lactobacilli-dominated microbiota (15). BV is known to compromise mucosal health in the vagina by the formation of high oxidative stress, degradation of mucin, and formation of polymicrobial biofilm initiated by G. vaginalis (53). While there are no studies on endometrial microbiota in BV, the intra-individual vaginal and endometrial bacterial communities have been observed to be closely related to each other (39, 54), and it is therefore likely that our results on the microbiota of vagina reflect the microbial environment of the uterine cavity as well. In parallel with the direct effects on the mucosa, non-Lactobacillus-dominated microbiota in the endometrium may trigger an inflammatory response that negatively affects embryo implantation and the likelihood of pregnancy, as immune mediators are tightly regulated during the implantation of the blastocyst to the endometrial epithelium (8, 15).
All vaginal samples taken at the eighth gestational week were dominated by lactobacilli in our study, even if the microbiota during the time of the IVF-ET in the same individual was non-Lactobacillus dominance. This result is in line with an earlier study, which showed that the relative abundance of L. crispatus in the vaginal samples of pregnant women is higher compared to those of non-pregnant women (29). However, this was a case–control study, and intra-individual changes in the microbiota composition between the non-pregnant and pregnant states were not studied. We showed that the relative abundances of L. iners and G. vaginalis were lower in early pregnancy samples than at the time of IVF-ET, indicating a shift in the composition of vaginal microbiota. In some women, L. iners–dominated microbiota shifted toward L. crispatus dominance between IVF-ET and early pregnancy, suggesting a shift toward a more stable microbiota type.
L. iners is a bacterium that can be dominant in both healthy women and in those with dysbiosis. By lacking the ability to produce D-lactic acid and hydrogen peroxide and by producing proteins mediating the adhesion of pathogens to the host cells, L. iners seems to provide less protection against the invasion of pathogens (55). Despite being a Lactobacillus species with beneficial characteristics such as the production of lactic acid, L. iners–dominated vaginal microbiota has been suggested to associate with BV, sexually transmitted infections, and even adverse pregnancy outcomes including PTB (55).
During a normal pregnancy, the vaginal microbiota profile is more stable than in the non-pregnant state (29). High levels of estrogen produced by the ovaries and the placenta promote glycogen stratification in the vaginal epithelial cells, which in turn induces the growth and proliferation of lactobacilli (56). In our study, bacterial communities remained in the same Lactobacillus-dominated type (52% of women), shifted from one Lactobacillus-dominated microbiota type to another (24%), or shifted from mixed community to Lactobacillus-dominant type (24%), but never from Lactobacillus dominance to non-lactobacilli-dominated microbiota type. This reflects the importance of lactobacilli in the vaginal ecosystem during early pregnancy protecting the uterus from ascending pathogens and the risk of miscarriage and PTB.
Our results regarding the intra-individual microbiota changes address a pivotal translational question of how to increase the carriage and abundance of lactobacilli that promote gynecological and reproductive health. In the current sample of 21 women, all possessed a Lactobacillus-dominant microbiota in favorable conditions, i.e., in the presence of high estrogen and resultant vaginal glycogen during pregnancy. Of these women, as high as 76% had L. crispatus–dominated microbiota during pregnancy, respectively. This indicates that most women carry L. crispatus in their vagina. Hence, approaches aiming to promote the existing strains deserve more attention as an efficient and safe option for microbiota restoration with, for example, prebiotics, over the current approaches on orally or vaginally supplemented probiotics, antibiotics, or vaginal microbiota transplants (57). Probiotic supplementation with Lactobacillus species has shown some benefit in maintaining healthy vaginal microbiota when used orally or vaginally (58, 59), although controversial results have been found as well (60). The only study that has examined the effect of probiotics in IVF detected no difference in the pregnancy rates between the vaginal probiotic and control groups (61). Similarly, the use of probiotics during the last 3 months did not have a significant effect on the outcome of the IVF treatment in our study.
Many studies suggest that the vaginal and endometrial ecosystems are not separate but share microbes (39, 54, 62). Thus, analyzing the microbial composition of vaginal microbiota at the time of IVF-ET could potentially be used as a screening test for the endometrium’s favorability for the implantation. We have shown before that the vaginal microbiota profile largely reflects endometrial microbiota profile intra-individually (39). Screening of vaginal microbiota at the time of IVF-ET would enable performing the ET in a cycle where the vaginal and accordingly the endometrial microbiota would be the most favorable for the implantation. However, further research is needed to evaluate how to promote the most favorable microbiota for implantation.
Strengths of this study include a prospective study design, uniform treatment and sample collection protocols in both participating tertiary centers, and state-of-the art methods for the analysis of the vaginal microbiota. The main limitation of our study was the small sample size and that only one IVF cycle was analyzed. Women had also different causes for their subfertility, and subjects with systemic diseases were not excluded, which may cause background variability. However, our study population was ethnically homogenous and composed of white women, which decreases inter-subject variation but may limit the generalizability of our results.
There is growing evidence that the vaginal microbiota may have an impact on reproductive health and the success of fertility treatments. Our results confirm the beneficial effect of L. crispatus on the outcome of the IVF treatment, particularly on the success of fresh embryo transfer. Our study is one of the few studies analyzing the outcome of fresh embryo transfer, and the samples were taken exactly at the time of the ET. To our knowledge, this is the first study to examine the intra-individual transition between the composition of vaginal microbiota during IVF-ET, i.e., before pregnancy and in early pregnancy.
In conclusion, vaginal microbiota and especially the presence of L. crispatus are positively associated with IVF-ET outcomes. Moreover, we report a shift of the vaginal microbiota toward Lactobacillus domination in early pregnancy also in women that had a non-Lactobacillus-dominated microbiota before pregnancy, suggesting that most women have an endogenous reservoir of lactobacilli with a potential to be promoted rather than supplemented externally. Our study provides novel information on the natural development of microbiota during early pregnancy and opens new research avenues regarding the optimization of the vaginal and endometrial microbiota.
MATERIALS AND METHODS
Study population
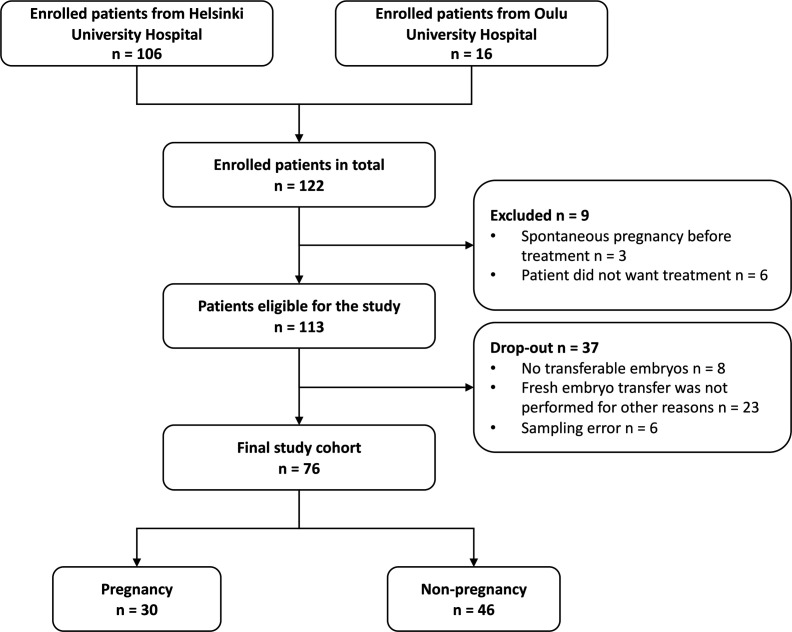
This study was conducted in the Reproductive Medicine Unit, Helsinki University Hospital (Helsinki, Finland), and in Oulu University Hospital (Oulu, Finland). We recruited a total of 122 subfertile women undergoing one IVF treatment cycle with their own oocytes: 106 at Helsinki University Hospital between November 2019 and September 2021 and 16 at Oulu University Hospital between January 2020 and December 2020 (Fig. 4).
Fig 4.
Flowchart of the study population.
The patient recruitment was performed at the outpatient clinic visit, where the IVF treatment was planned 1–4 months prior to the treatment cycle. The inclusion criteria were women aged under 40 y with a male partner aged under 60 y, body mass index (BMI) under 35 kg/m2, and over 12 months of infertility. Six participants decided to forgo the planned treatment for personal reasons, and three had spontaneous pregnancy before IVF stimulation started. Of the remaining 113 women, 31 were excluded because there were no embryos after the stimulation cycle, or fresh embryo transfer was not performed for other reasons. In six women, samples were not collected due to human error. The reasons for withdrawal are summarized in https://github.com/SchahzadSaqib/HEMI.
The final study cohort was composed of 76 women between 23 yr and 40 yr of age who underwent one IVF stimulation cycle with either gonadotropin-releasing hormone (GnRH) agonist (n = 52) or antagonist (n = 24) protocol and underwent fresh embryo transfer. The main outcomes were clinical pregnancy rate, defined as the visualization of a gestational sac in an ultrasound at the eighth week of gestation, and live birth. Based on the outcome of the treatment, the population was divided into the non-pregnancy and pregnancy subgroups.
All women were asked to complete a background questionnaire about their gynecological history, sexual habits, previous infections, antibiotic and probiotic use, and educational status. Clinical data, including the outcome of the treatment, were obtained from the hospital’s patient registry.
IVF treatment summary and sample collection
Women were treated by either GnRH agonist or antagonist stimulation protocol based on their individual medical backgrounds. In the agonist protocol, patient’s own GnRH production was suppressed using GnRH agonist nafarelin (Synarela) or leuprorelin (Procren) before ovarian stimulation. Once successful suppression was achieved, daily injections with follitropin alfa (Gonal-F) or menotropin (Menopur) were started to stimulate the development of the follicles. In the antagonist protocol, follitropin alfa injections were started from cycle day 2 or 3. In the midfollicular phase of the stimulation (5th–6th day of cycle), GnRH antagonist (ganirelix acetate) (Orgalutran or Fyremadel) was initiated to prevent spontaneous luteinizing hormone (LH) secretion and ovulation. Follicle development was monitored by transvaginal ultrasound examination. When the mean diameter of follicles was over 17 mm, with the presence of two or more follicles, ovulation was triggered by administering hCG injection (Ovitrelle or Pregnyl). Before oocyte retrieval, 13 (17.1%) women who had endometriomas received a single-dose cefuroxime 1.5 mg intravenously. Oocytes were retrieved 36 h after ovulation trigger using vaginal ultrasound-guided aspiration and fertilized by either conventional IVF or intracytoplasmic sperm injection (ICSI). ICSI was used for 20 couples when low sperm count was confirmed. Fresh embryo transfer was performed according to the recommended elective single-elective transfer protocol 2–5 d after oocyte retrieval, under transabdominal ultrasound guidance. Luteal phase support began 48 h after oocyte retrieval and continued for 12 d.
Vaginal swab samples were collected with sterile flocked swabs (FLOQSwabs, Copan spa, Italy) by healthcare professionals at the time of fresh embryo transfer and at the eighth gestational week from those women who got pregnant. Lubricants were not used during sampling. Samples were severed to 1.5 mL Eppendorf tubes which were frozen at −20°C immediately after sampling and further moved to −80°C within 4 wk.
DNA extraction and sequencing
Using a bead-beating method, the bacterial DNA was extracted from the vaginal swabs and subjected to quality control checks, followed by amplification, index PCR, and Illumina MiSeq sequencing as described previously (63). The targeted V3–V4 region of the 16S rRNA gene was amplified using the following primers: 341F 5′-CCTACGGGNGGCWGCAG-3′ and 785Rev 5′-GACTACHVGGGTATCTAATCC-3′).
After initial quality control, the paired-end sequencing data were processed using the 16S rRNA gene workflow of the dada2 R package (v1.26) (64), obtaining amplicon sequence variants (ASVs). Next, species-level taxonomic annotations were assigned using NCBI BLAST and the nucleotide database as part of the taxminer R package workflow (65).
As a supplemental analysis based mainly on the dominance or absence of Lactobacillus spp. in the vaginal microbiota, the taxonomic profiles were sorted into community state types (CSTs) using the VALENCIA classification tool (66) (https://github.com/SchahzadSaqib/HEMI).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 27 (IBM Corporation, Armonk, NY, USA) software for univariate data and R (v4.2.2, Rstudio). A two-sample t-test and Mann–Whitney U test were used to compare the continuous background variables between the pregnancy and non-pregnancy groups; Pearson d (χ2) and Fisher’s exact tests were used to compare the categorical variables. The relative abundance of individual bacterial taxa of the subgroups of clinical outcomes was tested for significance using the “GroupTest” function implementing generalized linear models using negative binomial distribution of the mare R package (67). “Presence/observation” of a bacterium was defined as >5%, and “dominance” was defined as >50% of the relative abundance in the sample. “Prevalence” was defined as the presence of the microbe above a threshold of 1% of the sample across all samples. All reported P-values from the “Grouptest” function were false discovery rate (FDR) adjusted for multivariable comparisons and reported as q-values. Richness (“specnumber” function), alpha diversity (“diversity” function, Simpson index), and permutational analysis of variance for associations between background variables and the microbiota (“adonis2” function) were obtained with the vegan R package (68). Richness and alpha diversity were compared between the groups using the Wilcoxon test.
ACKNOWLEDGMENTS
We are grateful to the women who participated in this study. Midwives Riina Meltemutmut, Heta Railo, and Ira Larsen are deeply acknowledged for their invaluable work in helping to schedule and organize patient recruitment and sampling.
The European Union’s Horizon 2020 research and innovation programme (H2020 MSCA Sweet Crosstalk project under grant agreement No. 814102; to AS) and the Department of Obstetrics and Gynaecology, Helsinki University Hospital (No. TYH2020401), funded the study. Funds from the Finnish Medical Foundation supported T.H. and from the Academy of Finland supported I.K.
T.H., K.K., K.L., M.H.-N., P.N., I.K., S.V., and A.S. conceived and designed the study. T.H., K.L., and M.N. collected the samples, and T.H. and S.V. collected the clinical data. A.S. was responsible for the microbiota analyses. S.S., T.H., and S.V. analyzed the data. S.V. and T.H. wrote the first draft of the manuscript. All authors made critical reviews and approved the final version of the manuscript.
All authors declare no conflict of interest.
Contributor Information
Anne Salonen, Email: anne.salonen@helsinki.fi.
Diyan Li, Chengdu University, Chengdu, Sichuan, China .
ETHICS APPROVAL
The study was approved by the ethical committee of the Hospital District of Helsinki and Uusimaa (HUS/512/2019) and performed in accordance with the principles of the Helsinki Declaration. Written informed consent was obtained from all participants prior to sample collection, and participation was voluntary.
DATA AVAILABILITY
The sequencing data generated within this study has been deposited to the European Nucleotide Archive (ENA), project accession number PRJEB61794. All R scripts, supplemental material, and basic metadata used for data processing, statistical analysis, and visualization have been deposited to GitHub (https://github.com/SchahzadSaqib/HEMI) where they are publicly available. Additional information can be made available by the corresponding author upon reasonable request. For data protection reasons, full clinical data cannot be made public.
REFERENCES
- 1. Cui W. 2010. Mother or nothing: the agony of infertility. Bull World Health Organ 88:881–882. doi: 10.2471/BLT.10.011210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elzeiny H, Garrett C, Toledo M, Stern K, McBain J, Baker HWG. 2014. A randomised controlled trial of intra-uterine insemination versus in vitro fertilisation in patients with idiopathic or mild male infertility. Aust N Z J Obstet Gynaecol 54:156–161. doi: 10.1111/ajo.12168 [DOI] [PubMed] [Google Scholar]
- 3. Smith ADAC, Tilling K, Nelson SM, Lawlor DA. 2015. Live-birth rate associated with repeat in vitro fertilization treatment cycles. JAMA 314:2654–2662. doi: 10.1001/jama.2015.17296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Høyer S, Kesmodel US, Aagaard J. 2021. Conditional and cumulative live birth rates after blastocyst transfer. Eur J Obstet Gynecol Reprod Biol 261:46–51. doi: 10.1016/j.ejogrb.2021.03.037 [DOI] [PubMed] [Google Scholar]
- 5. Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, Xu K, Rosenwaks Z. 2017. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril 107:664–670. doi: 10.1016/j.fertnstert.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 6. Macklon N. 2017. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil Steril 108:9–14. doi: 10.1016/j.fertnstert.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 7. Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD, Poort L, Cuypers WJSS, Beckers NGM, Broekmans FJM, Cohlen BJ, den Hartog JE, Fleischer K, Lambalk CB, Smeenk JMJS, Budding AE, Laven JSE. 2019. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 34:1042–1054. doi: 10.1093/humrep/dez065 [DOI] [PubMed] [Google Scholar]
- 8. Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahçeci M, Barrionuevo MJ, Taguchi S, Puente E, Dimattina M, Lim MW, Meneghini G, Aubuchon M, Leondires M, Izquierdo A, Perez-Olgiati M, Chavez A, Seethram K, Bau D, Gomez C, Valbuena D, Vilella F, Simon C. 2022. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 10:1. doi: 10.1186/s40168-021-01184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108 Suppl 1:4680–4687. doi: 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egbase PE, al-Sharhan M, al-Othman S, al-Mutawa M, Udo EE, Grudzinskas JG. 1996. Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum Reprod 11:1687–1689. doi: 10.1093/oxfordjournals.humrep.a019470 [DOI] [PubMed] [Google Scholar]
- 11. Fanchin R, Harmas A, Benaoudia F, Lundkvist U, Olivennes F, Frydman R. 1998. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil Steril 70:866–870. doi: 10.1016/s0015-0282(98)00277-5 [DOI] [PubMed] [Google Scholar]
- 12. Haahr T, Jensen JS, Thomsen L, Duus L, Rygaard K, Humaidan P. 2016. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod 31:795–803. doi: 10.1093/humrep/dew026 [DOI] [PubMed] [Google Scholar]
- 13. Ji L, Peng C, Bao X. 2022. Effect of vaginal flora on clinical outcome of frozen embryo transfer. Front Cell Infect Microbiol 12:987292. doi: 10.3389/fcimb.2022.987292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng H, He D, Hu L, Abdullah RK, Zhang L, Jiang B, Xie H, Liu N. 2022. Non-lactobacillus dominance of the vagina is associated with reduced live birth rate following IVF/ICSI: a propensity score-matched cohort study. Arch Gynecol Obstet 305:519–528. doi: 10.1007/s00404-021-06171-y [DOI] [PubMed] [Google Scholar]
- 15. Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí J, Pellicer A, Ramon D, Simon C. 2016. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 215:684–703. doi: 10.1016/j.ajog.2016.09.075 [DOI] [PubMed] [Google Scholar]
- 16. Moreno I, Garcia-Grau I, Bau D, Perez-Villaroya D, Gonzalez-Monfort M, Vilella F, Romero R, Simón C. 2020. The first glimpse of the endometrial microbiota in early pregnancy. Am J Obstet Gynecol 222:296–305. doi: 10.1016/j.ajog.2020.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hao X, Li P, Wu S, Tan J. 2021. Association of the cervical microbiota with pregnancy outcome in a subfertile population undergoing in vitro fertilization: a case-control study. Front Cell Infect Microbiol 11:654202. doi: 10.3389/fcimb.2021.654202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernabeu A, Lledo B, Díaz MC, Lozano FM, Ruiz V, Fuentes A, Lopez-Pineda A, Moliner B, Castillo JC, Ortiz JA, Ten J, Llacer J, Carratala-Munuera C, Orozco-Beltran D, Quesada JA, Bernabeu R. 2019. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet 36:2111–2119. doi: 10.1007/s10815-019-01564-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vergaro P, Tiscornia G, Barragán M, García D, Rodriguez A, Santaló J, Vassena R. 2019. Vaginal microbiota profile at the time of embryo transfer does not affect live birth rate in IVF cycles with donated oocytes. Reprod Biomed 38:883–891. doi: 10.1016/j.rbmo.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 20. Bui BN, van Hoogenhuijze N, Viveen M, Mol F, Teklenburg G, de Bruin J-P, Besselink D, Brentjens LS, Mackens S, Rogers MRC, Steba GS, Broekmans F, Paganelli FL, van de Wijgert JHHM. 2023. The endometrial microbiota of women with or without a live birth within 12 months after a first failed IVF/ICSI cycle. Sci Rep 13:3444. doi: 10.1038/s41598-023-30591-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu M, Zhang X, Liang Y, Lin S, Qian W, Fan S, Fidel PL, Lin X. 2020. Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. mBio 11:mBio doi: 10.1128/mBio.03242-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwebke JR, Muzny CA, Josey WE. 2014. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis 210:338–343. doi: 10.1093/infdis/jiu089 [DOI] [PubMed] [Google Scholar]
- 23. Castro J, Rosca AS, Muzny CA, Cerca N. 2021. Atopobium vaginae and Prevotella bivia are able to incorporate and influence gene expression in a pre-formed Gardnerella vaginalis biofilm. Pathogens 10:247. doi: 10.3390/pathogens10020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haahr T, Humaidan P, Elbaek HO, Alsbjerg B, Laursen RJ, Rygaard K, Johannesen TB, Andersen PS, Ng KL, Jensen JS. 2019. Vaginal microbiota and in vitro fertilization outcomes: development of a simple diagnostic tool to predict patients at risk of a poor reproductive outcome. J Infect Dis 219:1809–1817. doi: 10.1093/infdis/jiy744 [DOI] [PubMed] [Google Scholar]
- 25. Hashimoto T, Kyono K. 2019. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J Assist Reprod Genet 36:2471–2479. doi: 10.1007/s10815-019-01630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang R, Zhou G, Wu L, Huang X, Li Y, Luo B, Zhu H, Huang W. 2021. The microbial composition of lower genital tract may affect the outcome of in vitro fertilization-embryo transfer. Front Microbiol 12:729744. doi: 10.3389/fmicb.2021.729744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reschini M, Benaglia L, Ceriotti F, Borroni R, Ferrari S, Castiglioni M, Guarneri D, Porcaro L, Vigano’ P, Somigliana E, Uceda Renteria S. 2022. Endometrial microbiome: sampling, assessment, and possible impact on embryo implantation. Sci Rep 12:8467. doi: 10.1038/s41598-022-12095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nunn KL, Witkin SS, Schneider GM, Boester A, Nasioudis D, Minis E, Gliniewicz K, Forney LJ. 2021. Changes in the vaginal microbiome during the pregnancy to postpartum transition. Reprod Sci 28:1996–2005. doi: 10.1007/s43032-020-00438-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. 2014. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2:4. doi: 10.1186/2049-2618-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown R, Teoh TG, Holmes E, Nicoholson JK, Marchesi JR, Bennett PR. 2015. The vaginal microbiome during pregnancy and the postpartum period in a european population. Sci Rep 5:8988. doi: 10.1038/srep08988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nasioudis D, Forney LJ, Schneider GM, Gliniewicz K, France MT, Boester A, Sawai M, Scholl J, Witkin SS. 2017. The composition of the vaginal microbiome in first trimester pregnant women influences the level of autophagy and stress in vaginal epithelial cells. J Reprod Immunol 123:35–39. doi: 10.1016/j.jri.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 32. Kervinen K, Holster T, Saqib S, Virtanen S, Stefanovic V, Rahkonen L, Nieminen P, Salonen A, Kalliala I. 2022. Parity and gestational age are associated with vaginal microbiota composition in term and late term pregnancies. EBioMedicine 81:104107. doi: 10.1016/j.ebiom.2022.104107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al‐Memar M, Bobdiwala S, Fourie H, Mannino R, Lee YS, Smith A, Marchesi JR, Timmerman D, Bourne T, Bennett PR, MacIntyre DA. 2020. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG 127:264–274. doi: 10.1111/1471-0528.15972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown RG, Marchesi JR, Lee YS, Smith A, Lehne B, Kindinger LM, Terzidou V, Holmes E, Nicholson JK, Bennett PR, MacIntyre DA. 2018. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med 16:9. doi: 10.1186/s12916-017-0999-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diaz-Martínez MDC, Bernabeu A, Lledó B, Carratalá-Munuera C, Quesada JA, Lozano FM, Ruiz V, Morales R, Llácer J, Ten J, Castillo JC, Rodríguez A, Nouni-García R, López-Pineda A, Moliner B, Bernabeu R. 2021. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J Clin Med 10:4063. doi: 10.3390/jcm10184063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haahr T, Zacho J, Bräuner M, Shathmigha K, Skov Jensen J, Humaidan P. 2019. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. BJOG 126:200–207. doi: 10.1111/1471-0528.15178 [DOI] [PubMed] [Google Scholar]
- 37. Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA, Forney LJ. 2007. Differences in the composition of vaginal microbial communities found in healthy caucasian and black women. ISME J 1:121–133. doi: 10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]
- 38. Sun D, Zhao X, Pan Q, Li F, Gao B, Zhang A, Huang H, Xu D, Cheng C. 2022. The association between vaginal microbiota disorders and early missed abortion: a prospective study. Acta Obstet Gynecol Scand 101:960–971. doi: 10.1111/aogs.14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peuranpää P, Holster T, Saqib S, Kalliala I, Tiitinen A, Salonen A, Hautamäki H. 2022. Female reproductive tract microbiota and recurrent pregnancy loss: a nested case-control study. Reprod Biomed 45:1021–1031. doi: 10.1016/j.rbmo.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 40. Haahr T, Clausen TD, Thorsen J, Rasmussen MA, Mortensen MS, Lehtimäki J, Shah SA, Hjelmsø MH, Bønnelykke K, Chawes BL, Vestergaard G, Jacobsson B, Larsson P-G, Brix S, Sørensen SJ, Bisgaard H, Stokholm J. 2022. Vaginal dysbiosis in pregnancy associates with risk of emergency caesarean section: a prospective cohort study. Clin Microbiol Infect 28:588–595. doi: 10.1016/j.cmi.2021.08.028 [DOI] [PubMed] [Google Scholar]
- 41. van der Veer C, Hertzberger RY, Bruisten SM, Tytgat HLP, Swanenburg J, de Kat Angelino-Bart A, Schuren F, Molenaar D, Reid G, de Vries H, Kort R. 2019. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: implications for in vivo dominance of the vaginal microbiota. Microbiome 7:49. doi: 10.1186/s40168-019-0667-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. France M, Alizadeh M, Brown S, Ma B, Ravel J. 2022. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol 7:367–378. doi: 10.1038/s41564-022-01083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu B, Tao Z, Edupuganti L, Serrano MG, Buck GA. 2022. Roles of the microbiota of the female reproductive tract in gynecological and reproductive health. Microbiol Mol Biol Rev 86:e0018121. doi: 10.1128/mmbr.00181-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stoyancheva G, Marzotto M, Dellaglio F, Torriani S. 2014. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol 196:645–653. doi: 10.1007/s00203-014-1003-1 [DOI] [PubMed] [Google Scholar]
- 45. Ceccarani C, Foschi C, Parolin C, D’Antuono A, Gaspari V, Consolandi C, Laghi L, Camboni T, Vitali B, Severgnini M, Marangoni A. 2019. Diversity of vaginal microbiome and metabolome during genital infections. Sci Rep 9:14095. doi: 10.1038/s41598-019-50410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Delgado-Diaz DJ, Tyssen D, Hayward JA, Gugasyan R, Hearps AC, Tachedjian G. 2019. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front Cell Infect Microbiol 9:446. doi: 10.3389/fcimb.2019.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, Cone RA, Tachedjian G. 2015. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 6:164. doi: 10.3389/fphys.2015.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campisciano G, Zanotta N, Licastro D, De Seta F, Comar M. 2018. In vivo microbiome and associated immune markers: new insights into the pathogenesis of vaginal dysbiosis. Sci Rep 8:2307. doi: 10.1038/s41598-018-20649-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karaer A, Doğan B, Günal S, Tuncay G, Arda Düz S, Ünver T, Tecellioğlu N. 2021. The vaginal microbiota composition of women undergoing assisted reproduction: a prospective cohort study. BJOG 128:2101–2109. doi: 10.1111/1471-0528.16782 [DOI] [PubMed] [Google Scholar]
- 50. Donders GGG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. 2002. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG 109:34–43. doi: 10.1111/j.1471-0528.2002.00432.x [DOI] [PubMed] [Google Scholar]
- 51. Donders GGG, Bellen G, Grinceviciene S, Ruban K, Vieira-Baptista P. 2017. Aerobic vaginitis: no longer a stranger. Res Microbiol 168:845–858. doi: 10.1016/j.resmic.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 52. Moreno I, Simon C. 2018. Relevance of assessing the uterine microbiota in infertility. Fertil Steril 110:337–343. doi: 10.1016/j.fertnstert.2018.04.041 [DOI] [PubMed] [Google Scholar]
- 53. Chee WJY, Chew SY, Than LTL. 2020. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact 19:203. doi: 10.1186/s12934-020-01464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, Xie H, Chen X, Zeng C, Wen B, Zeng L, Du H, Tang H, Xu C, Xia Y, Xia H, Yang H, Wang J, Wang J, Madsen L, Brix S, Kristiansen K, Xu X, Li J, Wu R, Jia H. 2017. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 8:875. doi: 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng N, Guo R, Wang J, Zhou W, Ling Z. 2021. Contribution of Lactobacillus iners to vaginal health and diseases: a systematic review. Front Cell Infect Microbiol 11:792787. doi: 10.3389/fcimb.2021.792787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boskey ER, Cone RA, Whaley KJ, Moench TR. 2001. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod 16:1809–1813. doi: 10.1093/humrep/16.9.1809 [DOI] [PubMed] [Google Scholar]
- 57. Moumne O, Hampe ME, Montoya-Williams D, Carson TL, Neu J, Francois M, Rhoton-Vlasak A, Lemas DJ. 2021. Implications of the vaginal microbiome and potential restorative strategies on maternal health: a narrative review. J Perinat Med 49:402–411. doi: 10.1515/jpm-2020-0367 [DOI] [PubMed] [Google Scholar]
- 58. Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, Bruce AW, Reid G. 2006. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect 8:1450–1454. doi: 10.1016/j.micinf.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 59. Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, Mehrabany EV. 2014. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis 18:79–86. doi: 10.1097/LGT.0b013e31829156ec [DOI] [PubMed] [Google Scholar]
- 60. Buggio L, Somigliana E, Borghi A, Vercellini P. 2019. Probiotics and vaginal microecology: fact or fancy? BMC Womens Health 19:25. doi: 10.1186/s12905-019-0723-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gilboa Y, Bar-Hava I, Fisch B, Ashkenazi J, Voliovitch I, Borkowski T, Orvieto R. 2005. Does intravaginal probiotic supplementation increase the pregnancy rate in IVF-embryo transfer cycles? Reprod Biomed 11:71–75. doi: 10.1016/s1472-6483(10)61301-6 [DOI] [PubMed] [Google Scholar]
- 62. Wang J, Li Z, Ma X, Du L, Jia Z, Cui X, Yu L, Yang J, Xiao L, Zhang B, Fan H, Zhao F. 2021. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat Commun 12:4191. doi: 10.1038/s41467-021-24516-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Virtanen S, Rantsi T, Virtanen A, Kervinen K, Nieminen P, Kalliala I, Salonen A. 2019. Vaginal microbiota composition correlates between pap smear microscopy and next generation sequencing and associates to socioeconomic status. Sci Rep 9:7750. doi: 10.1038/s41598-019-44157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Virtanen S, Saqib S, Kanerva T, Nieminen P, Kalliala I, Salonen A. 2021. Metagenome-validated parallel Amplicon sequencing and text mining-based annotations for simultaneous profiling of bacteria and fungi: Vaginal Microbiome and Mycobiota in healthy women. In review. doi: 10.21203/rs.3.rs-321778/v1 [DOI]
- 66. France MT, Ma B, Gajer P, Brown S, Humphrys MS, Holm JB, Waetjen LE, Brotman RM, Ravel J. 2020. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. doi: 10.1186/s40168-020-00934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Korpela K. 2016. R package version 1.0. Mare: Microbiota Analysis in R Easily. Available from: https://github.com/katrikorpela/mare
- 68. Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Solymos P, Stevens MHH, Szoecs E, et al. 2022. R package version 2.6-4. Vegan: Community Ecology Package. Available from: https://CRAN.R-project.org/package=vegan
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing data generated within this study has been deposited to the European Nucleotide Archive (ENA), project accession number PRJEB61794. All R scripts, supplemental material, and basic metadata used for data processing, statistical analysis, and visualization have been deposited to GitHub (https://github.com/SchahzadSaqib/HEMI) where they are publicly available. Additional information can be made available by the corresponding author upon reasonable request. For data protection reasons, full clinical data cannot be made public.