ABSTRACT
Polymicrobial airway infections in persons with cystic fibrosis (pwCF) can positively or negatively impact the course of disease. A major CF pathogen, Pseudomonas aeruginosa, often establishes a chronic infection leading to lung deterioration. Interestingly, the presence of certain oral commensal streptococci is correlated with improved outcomes for pwCF. We previously reported that hydrogen peroxide production by these commensals combined with nitrite generates reactive nitrogen intermediates (RNI), which inhibit P. aeruginosa in vitro. In this study, we utilized a rat co-infection lung model to assess whether oral commensal-generated RNI can restrict the pathogenesis of a CF isolate of P. aeruginosa. We report that the oral commensal Streptococcus parasanguinis and nitrite reduce P. aeruginosa-induced host inflammation in wild-type rats. To better recapitulate CF-specific airway conditions, we used a bronchial epithelial cell culture model to gain a better understanding of how S. parasanguinis and nitrite may influence P. aeruginosa burden during a CF infection. Hence, we co-infected wild-type and cystic fibrosis transconductance regulator (CFTR) channel-deficient bronchial epithelial cells with P. aeruginosa and S. parasanguinis with or without nitrite. Strikingly, S. parasanguinis reduced the bacterial burden of P. aeruginosa without nitrite, promoted epithelial cell viability, and stimulated nitrite production in the wild-type and CFTR-deficient epithelial cells, where nitrite induction was more apparent in the CFTR mutant cells. Taken together, our study demonstrates that the commensal S. parasanguinis may provide protection against P. aeruginosa-induced inflammation and cell death, as well as modulate nitrite flux in airway epithelial cells.
IMPORTANCE
Respiratory infections are a leading cause of morbidity and mortality in people with cystic fibrosis (CF). These infections are polymicrobial in nature with overt pathogens and other colonizing microbes present. Microbiome data have indicated that the presence of oral commensal bacteria in the lungs is correlated with improved outcomes. We hypothesize that one oral commensal, Streptococcus parasanguinis, inhibits CF pathogens and modulates the host immune response. One major CF pathogen is Pseudomonas aeruginosa, a Gram-negative, opportunistic bacterium with intrinsic drug resistance and an arsenal of virulence factors. We have previously shown that S. parasanguinis inhibits P. aeruginosa in vitro in a nitrite-dependent manner through the production of reactive nitrogen intermediates. In this study, we demonstrate that while this mechanism is evident in a cell culture model of the CF airway, an alternative mechanism by which S. parasanguinis may improve outcomes for people with CF is through immunomodulation.
KEYWORDS: Pseudomonas aeruginosa, Streptococcus parasanguinis, nitrosative stress, airway inflammation, commensal
INTRODUCTION
Cystic fibrosis (CF) is a genetic condition in which the cystic fibrosis transmembrane conductance regulator channel (CFTR) is absent, has reduced function, or is nonfunctional (1 – 4). As an anion channel, CFTR plays a myriad of functions; however, its function to maintain ion balance within the airway is paramount to clearance of mucus and lung function. Due to reduced mucociliary clearance and impaired immune cell function, airway infections in persons with cystic fibrosis (pwCF) can persist for decades and are often polymicrobial in nature (5 – 9). Some microbes colonize the lung directly from the environment, while other microbes may first colonize sites upstream of the lungs such as the sinuses or the oral cavity and then translocate to the lung (10 – 15). These diverse microbes originating from various environments interact together through mechanisms that may be synergistic, antagonistic, direct, or indirect (16 – 27). Multiple studies have indicated that the presence of oral commensal bacteria in the lung, particularly streptococci, is associated with positive outcomes, including improved lung function and reduced pathogen burden (14, 15, 28 – 31). Many oral commensal streptococci are known to inhibit oral pathogens through the production of hydrogen peroxide (H2O2). While H2O2 is intrinsically antimicrobial, it can also react with other metabolites found throughout the body, such as nitrite. Nitrate and nitrite are acquired through the diet wherein oral anaerobic bacteria reduce nitrate to nitrite, nitric oxide, and nitrogen (32 – 34). Nitrite can also react with H2O2 to form reactive nitrogen intermediates (RNIs) that are antimicrobial. This commensal-generated RNI antagonism has been documented to inhibit oral pathogens as well as the CF pathogen Pseudomonas aeruginosa (18, 35, 36).
Nitrite is not only an important intermediate for the production of RNI, which can inhibit microbes, but its reduction to nitric oxide by anaerobic bacteria in the oral cavity and the lung is known to be an important regulator of vascular tone and the immune system itself (32 – 34, 37 – 39). Nitric oxide is abundantly produced by neutrophils and macrophages during inflammatory responses as an antimicrobial agent. Additionally, bronchial epithelial cells are also known to produce nitric oxide (40). Several studies have indicated that excess nitric oxide downregulates the host immune response to act as a negative feedback to inflammation (41, 42). Interestingly, nitrite and nitrotyrosine (an indicator of the presence of RNI) have been correlated with improved lung function in pwCF (43, 44).
Previously, our laboratory has shown that Streptococcus parasanguinis, an oral commensal that is prevalent in clinically stable pwCF, can inhibit P. aeruginosa in a nitrite-dependent manner. This work indicated that P. aeruginosa is sensitive to RNIs in vitro and in a Drosophila melanogaster infection model (20), that the presence of S. parasanguinis alters the P. aeruginosa denitrification response (18, 45), and that CF isolates of P. aeruginosa are particularly sensitive to these RNIs (18). Thus, antagonism of P. aeruginosa through RNI may be a mechanism that explains why oral commensal streptococci are correlated with improved outcomes in pwCF.
While previous studies indicate a promising avenue for the treatment of debilitating infections in pwCF, the possibility of these commensal-generated RNI inhibiting P. aeruginosa in a mammalian lung has not yet been investigated. Furthermore, the impact of S. parasanguinis on the host immune response is unknown. Several oral commensal bacteria have been demonstrated to have anti-inflammatory properties in the oral cavity and the lung (46 – 50). Thus, the impact of S. parasanguinis on the CF lung may be twofold: inhibiting P. aeruginosa through RNI and modulating the host immune response to reduce hyper-inflammation. Given the intrinsic immune dysfunction that results in nonproductive inflammation and host damage in CF, the contributions of commensal bacteria on inflammation are important to understand (51).
In an effort to define the role of S. parasanguinis on airway infection with P. aeruginosa, the goals of this study were to (i) determine if S. parasanguinis-generated RNI can inhibit P. aeruginosa in the mammalian lung, (ii) determine the impact of S. parasanguinis, nitrite, and co-infection on the host immune response to P. aeruginosa, and (iii) determine the impact on CFTR on RNI-dependent antagonism of P. aeruginosa. To accomplish this, we employed a wild-type rat lung infection model as well as wild-type and CF bronchial epithelial cell models to assess these interactions in vivo and in vitro. Our findings demonstrate that (i) both S. parasanguinis and nitrite reduce host inflammation to P. aeruginosa, (ii) S. parasanguinis inhibits P. aeruginosa in a bronchial epithelial cell infection model, and (iii) S. parasanguinis increases CF bronchial epithelial cell extracellular nitrite and promotes cell viability. Taken together, this work illustrates that the commensal S. parasanguinis may provide protection from P. aeruginosa in the airway through immunomodulation and potentially nitrite induction.
RESULTS
S. parasanguinis overproduces hydrogen peroxide in synthetic CF sputum
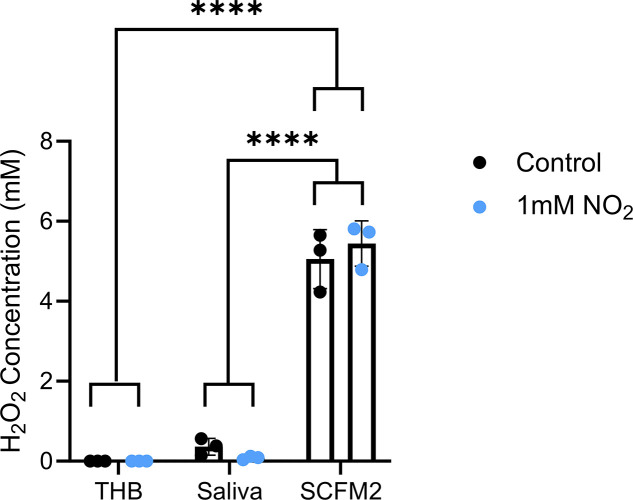
We previously demonstrated that RNI-mediated inhibition of P. aeruginosa by S. parasanguinis is H2O2 dependent in vitro. Regulation of H2O2 production in oral streptococci is typically dependent on nutritional availability, although other factors such as pH and oxygen are known to alter H2O2 production (52 – 56). Although much is known about H2O2 production by oral commensals within the context of the oral cavity, it is currently unknown whether conditions in the CF airway support the optimal production of H2O2 after an oral commensal translocates from the oral cavity to the respiratory tract. Therefore, we questioned whether H2O2 production is differentially regulated in the oral cavity versus the CF lung. S. parasanguinis utilizes pyruvate oxidase (PoxL, a homolog of SpxB in other streptococci) to produce H2O2 (20). Thus, H2O2 production was measured in the laboratory medium Todd-Hewitt Broth (THB), synthetic saliva, and synthetic CF sputum (SCFM2). H2O2 production was markedly increased when S. parasanguinis was grown in SCFM2 compared to THB and artificial saliva, independent of the presence of nitrite (Fig. 1). Concordantly, poxL expression was decreased in artificial saliva compared to THB but was increased in SCFM2 (S1 A-B). When each medium was supplemented with 1 mM nitrite, poxL expression decreased in both THB and artificial saliva but was increased in SCFM2 (S1 C-E). These data indicate that S. parasanguinis upregulates H2O2 production when it translocates from the oral cavity to the lung and that our established mechanism of RNI generation is likely possible in the context of the CF airway.
Fig 1.
S. parasanguinis overproduces hydrogen peroxide in synthetic CF sputum. Hydrogen peroxide was measured in THB, artificial saliva, and SCFM2 with or without 1 mM nitrite. n = 3; error bars represent standard deviation, ****P < 0.0001 [two-way analysis of variance (ANOVA), Tukey post hoc test].
S. parasanguinis attenuates P. aeruginosa-induced tissue inflammation
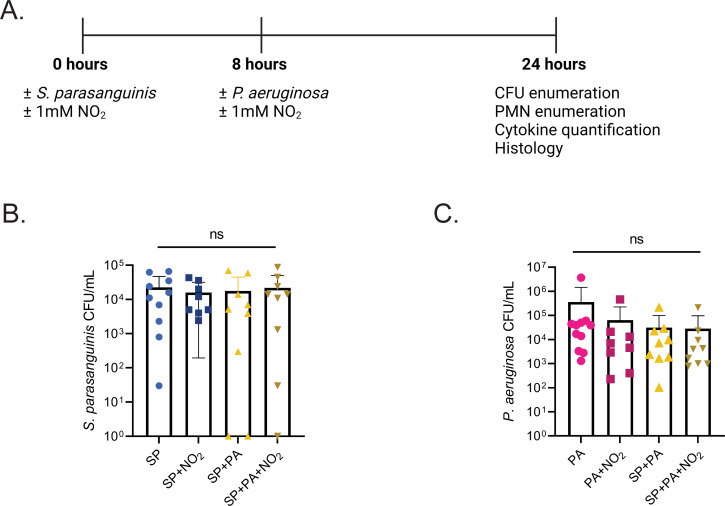
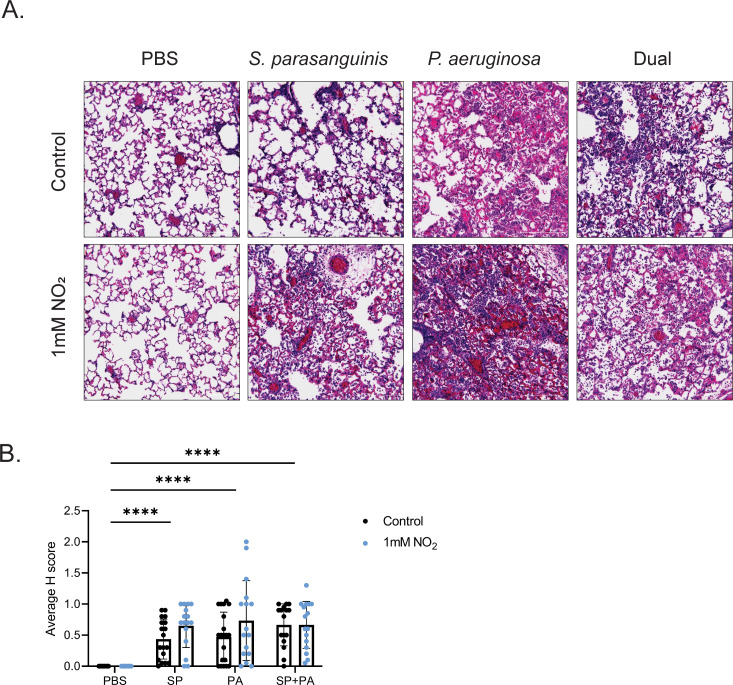
Given that S. parasanguinis produces high concentrations of H2O2 in SCFM2, we hypothesized that this overproduction would contribute to enhanced P. aeruginosa inhibition during airway infection in the presence of nitrite. Six- to eight-week-old wild-type Sprague-Dawley rats were infected intranasally with S. parasanguinis (FW213), P. aeruginosa (mPA08-31, a mucoid CF isolate), or both S. parasanguinis and P. aeruginosa with or without 1 mM nitrite. As polymicrobial infections tend to be sequential in nature and that oral commensals colonize the CF lung before P. aeruginosa, S. parasanguinis was dosed 24 hours before sacrifice, while P. aeruginosa was dosed 16 hours before sacrifice (Fig. 2A). S. parasanguinis colonized the rat lung at ~103 CFU/mL regardless of treatment, indicating that S. parasanguinis can survive alongside P. aeruginosa and can colonize the lung at 24 hours (Fig. 2B). Interestingly, the addition of nitrite, S. parasanguinis, or both nitrite and S. parasanguinis did not significantly reduce P. aeruginosa burden in the lung (Fig. 2C). Next, we examined lung tissue to determine whether S. parasanguinis and nitrite influenced tissue damage and inflammation in the presence of a P. aeruginosa infection. Histology sections from infected lungs were blindly scored for severity of inflammation using neutrophil influx and tissue damage as parameters. Histological analysis revealed that a single-species S. parasanguinis infection induced mild inflammation compared to the phosphate-buffered saline (PBS) control, and this inflammation was slightly increased with the addition of nitrite. As expected, a single-species P. aeruginosa infection resulted in a greater increase in inflammation compared to S. parasanguinis, and the addition of nitrite further promoted P. aeruginosa-induced tissue damage. S. parasanguinis reduced P. aeruginosa-induced airway damage with or without the addition of nitrite (Fig. 3A; Fig. S2). However, there were no significant differences in histological scores (H-score) between any of the infection groups with both S. parasanguinis and P. aeruginosa (Fig. 3B). Taken together, these data indicate that while S. parasanguinis may reduce P. aeruginosa-induced tissue damage, this protection is variable, and the addition of nitrite at the concentration of 1 mM may induce moderate inflammation.
Fig 2.
S. parasanguinis colonizes the mammalian lung. (A) Rat infection scheme. Rats were inoculated intranasally with or without S. parasanguinis (SP) or 1 mM nitrite. Eight hours later, rats were inoculated with or without P. aeruginosa (PA) or nitrite. After 24 hours, rats were sacrificed for downstream experiments. (B) S. parasanguinis CFU recovered from rats 24 hours post infection. (C) P. aeruginosa CFU recovered from rats 24 hours post infection. n = 8–11, ns, P > 0.05, (Kruskal-Wallis test, Dunett’s post hoc test).
Fig 3.
S. parasanguinis attenuates P. aeruginosa tissue damage. (A) Representative images of hematoxylin and eosin staining from the worst scored sections from each infection group. (B) Average H scores of lung tissue sections for PBS, S. parasanguinis (SP), P. aeruginosa (PA), and dual animals with or without nitrite. n = 8–11; error bars represent standard deviation. ****P < 0.0001 (two-way ANOVA, Tukey’s post hoc test).
S. parasanguinis reduces neutrophil infiltration and pro-inflammatory cytokines in dual infection
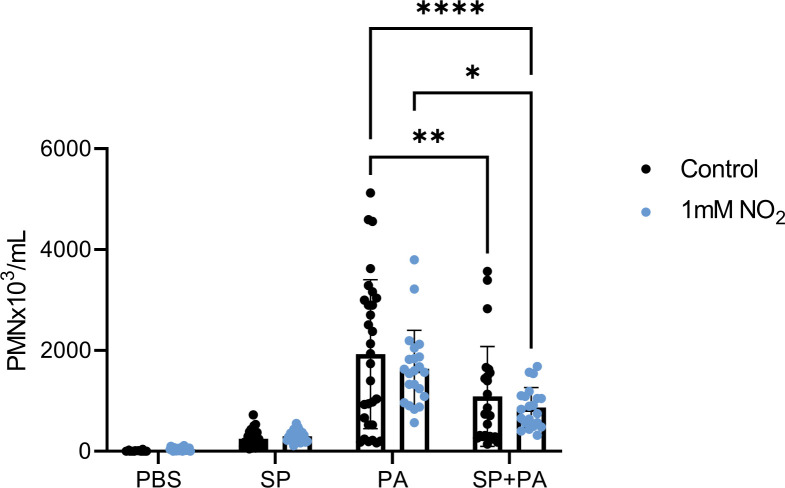
While S. parasanguinis did not significantly reduce P. aeruginosa burden, we hypothesized that S. parasanguinis could reduce lung inflammation associated with P. aeruginosa infection. Therefore, we measured polymorphonuclear cells (PMNs) and cytokines in bronchoalveolar lavage fluid to assess the innate immune response. S. parasanguinis did not induce significantly more PMNs than PBS controls (Fig. 4). As expected, P. aeruginosa infection led to a marked increase of PMNs (Fig. 4). When S. parasanguinis was dosed before P. aeruginosa infection with or without nitrite, however, PMNs were reduced by 44% and 55%, respectively, compared to P. aeruginosa alone (Fig. 4).
Fig 4.
S. parasanguinis reduces P. aeruginosa-induced neutrophilia. PMNs from bronchoalveolar lavage fluid were quantified in single and dual S. parasanguinis (SP) and P. aeruginosa (PA) infected rats. n = 8–10 biological replicates with three technical replicates; error bars represent standard deviation. Significant outliers were identified using the ROUT method and removed. ****P < 0.0001 (two-way ANOVA, Tukey’s post hoc test).
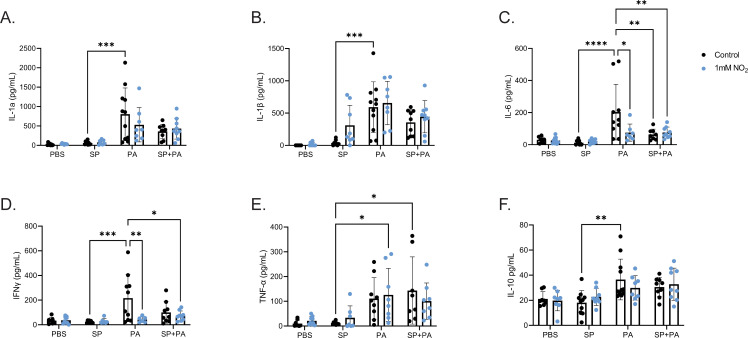
Next, we measured cytokines IL-1α, IL-1β, IFN-γ, IL-6, TNF-α, and IL-10 in the bronchial alveolar lavage fluid (BALF) in response to S. parasanguinis, P. aeruginosa, or both with or without nitrite. S. parasanguinis alone did not elicit any significant increases from PBS in any of these cytokines (Fig. 5A through F). As expected, P. aeruginosa infection markedly increased all cytokine production (Fig. 5A through F). Interestingly, the presence of nitrite alone reduced both IL-6 and IFN-γ production in P. aeruginosa-infected animals (Fig. 5C and D). The presence of both S. parasanguinis and nitrite also significantly reduced IL-6 and IFN-γ production in P. aeruginosa-infected animals (Fig. 5C and D). As expected, all cytokines were significantly positively correlated with PMN counts (Fig. S3A through F). In dual-infected animals, pro-inflammatory cytokine production was negatively correlated with higher S. parasanguinis to P. aeruginosa ratio (Fig. S4). Overall, these data demonstrate that S. parasanguinis can reduce pro-inflammatory markers during a P. aeruginosa infection.
Fig 5.
S. parasanguinis reduces P. aeruginosa-induced cytokine production. Cytokines IL-1a (A), IL-1B (B), IL-6 (C), IFN-γ (D), TNF-a (E), and IL-10 (F) were measured via enzyme-linked immunosorbent assay (ELISA) from bronchoalveolar lavage fluid in single and dual S. parasanguinis (SP)- and P. aeruginosa (PA)-infected rats. n = 8–11; error bars represent standard deviation. Significant outliers were identified using the ROUT method and removed. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (two-way ANOVA, Tukey’s post hoc test).
S. parasanguinis inhibits P. aeruginosa in a cell infection model
Our rat infection data indicated that S. parasanguinis reduces host inflammation in response to P. aeruginosa despite a lack of impact on clearance of P. aeruginosa in wild-type animals. To study the impact of H2O2, nitrite, single, and dual infections at the epithelial cellular level in the presence and absence of a functional epithelial CFTR, we employed a cell culture model infection with 16HBE and cystic fibrosis bronchial epithelial (CFBE) cell lines. In addition to wild-type S. parasanguinis, a pyruvate oxidase deletion mutant, poxL, which is deficient in H2O2 production (20), was employed to assess the impact of H2O2 on P. aeruginosa colonization and epithelial cell viability. In addition to mPA08, we also used the laboratory-adapted, non-mucoid PAO1 strain of P. aeruginosa for three reasons: (i) given the mild effect (based on the comparable H-scores to the commensal) of mPA08 on lung histology, we questioned the impact S. parasanguinis would have on a more virulent isolate like PAO1; (ii) we previously demonstrated that CF isolates of P. aeruginosa, such as mPA08, have increased sensitivity to S. parasanguinis- generated RNI in vitro compared to non-CF isolates such as PAO1; and (iii) oral commensals are more abundant in younger pwCF and would encounter P. aeruginosa strains that more closely resemble PAO1 genotypically and phenotypically.
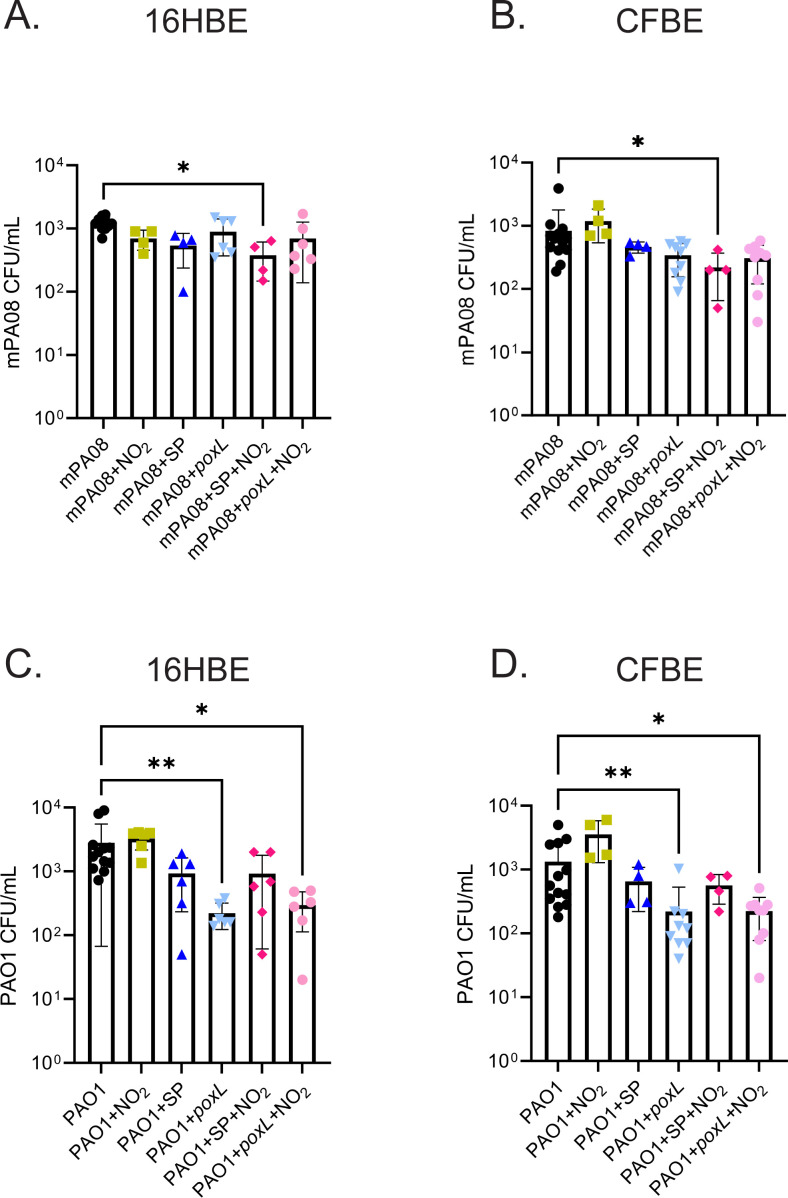
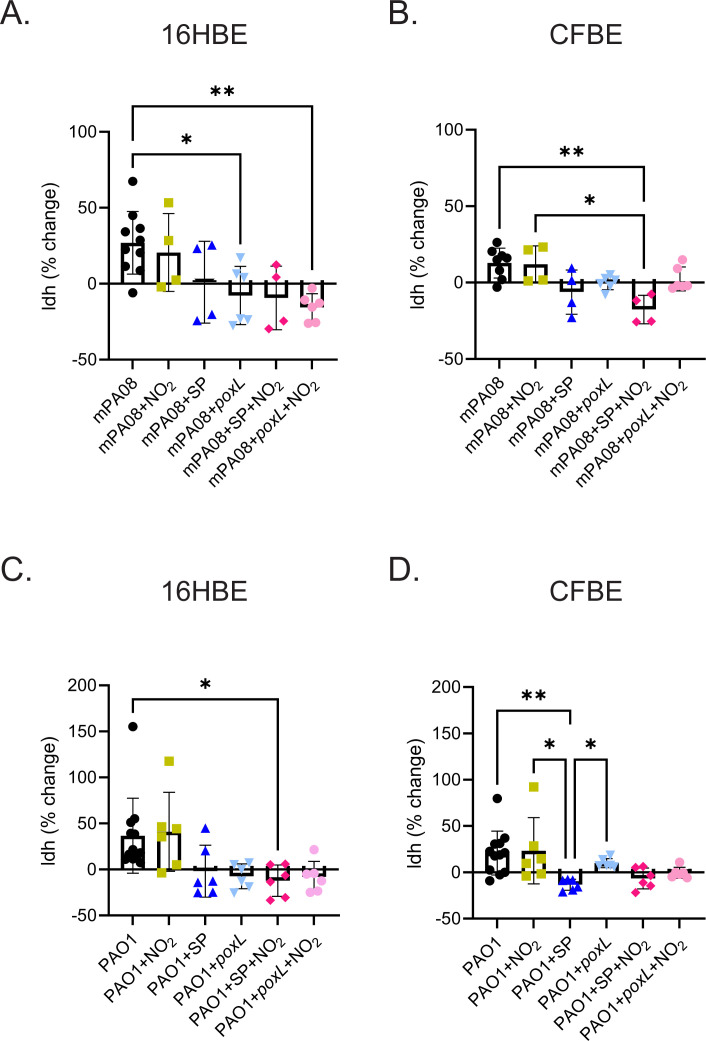
Bronchial epithelial cells grown at air-liquid interface were incubated with S. parasanguinis for 2 hours before incubation with P. aeruginosa mPA08 or PAO1 for 4 hours. mPA08 CFUs were reduced in the presence of S. parasanguinis and nitrite in both 16HBE and CFBE cell lines, and S. parasanguinis poxL was required for optimal inhibition of this clinical isolate (Fig. 6A and B). PAO1 was slightly inhibited by wild-type S. parasanguinis with or without nitrite. Interestingly, this inhibition was further increased by the poxL mutant (with or without nitrite) in both epithelial cell types, indicating a novel H2O2-independent mechanism of P. aeruginosa inhibition during cell infection. S. parasanguinis wild-type and poxL mutant CFUs were unchanged regardless of treatment and cell type (S5). Prior to and following infection, cell viability was assayed via lactate dehydrogenase (LDH) quantification and normalized to percentage change from prior to post infection. S. parasanguinis alone or in the presence of nitrite did not induce LDH release from either cell type, suggesting that S. parasanguinis elicits little damage to the lung epithelium in the absence of immune cells (Fig. S6A and B). Moreover, the loss of poxL did not induce LDH release in 16HBE or CFBE cells, with the exception of CFBEs treated with nitrite; however, this increase was not significant (Fig. S6B). In contrast, cells that were infected with mPA08 or PAO1 had markedly increased LDH release (Fig. 7). Strikingly, the presence of S. parasanguinis with P. aeruginosa abrogated this LDH release in both cell types (Fig. 7). Collectively, these data indicate that in a non-CF and CF specific condition, S. parasanguinis can reduce P. aeruginosa burden and increase cell viability in the presence or absence of hydrogen peroxide production.
Fig 6.
S. parasanguinis inhibits P. aeruginosa in a bronchial epithelial cell infection model. Wild-type or CF bronchial epithelial cells were infected with either mPA08 or PAO1 strains of P. aeruginosa with or without S. parasanguinis (SP) or the poxL mutant and/or 0.5 mM nitrite. P. aeruginosa CFU were enumerated after 6-hour incubation. (A) 16HBE cells infected with mPA08, (B) CFBE cells infected with mPA08, (C) 16HBE cells infected with PAO1, and (D) CFBE cells infected with PAO1. n = 4–10; error bars represent standard deviation. *P < 0.05, **P < 0.01 (A, one-way ANOVA; B–D, Kruskal-Wallis test, Tukey’s post hoc test).
Fig 7.
S. parasanguinis increases cell viability in bronchial epithelial cells. Wild-type or CF bronchial epithelial cells were infected with either mPA08 or PAO1 strains of P. aeruginosa with or without S. parasanguinis (SP) or the poxL mutant and/or 0.5 mM nitrite. Lactate dehydrogenase was measured before and after infection and normalized. (A) 16HBE cells infected with mPA08, (B) CFBE cells infected with mPA08, (C) 16HBE cells infected with PAO1, and (D) CFBE cells infected with PAO1. n = 4–10; error bars represent standard deviation. *P < 0.05, **P < 0.01 (A, one-way ANOVA; B–D, Kruskal-Wallis test, Tukey’s post hoc test).
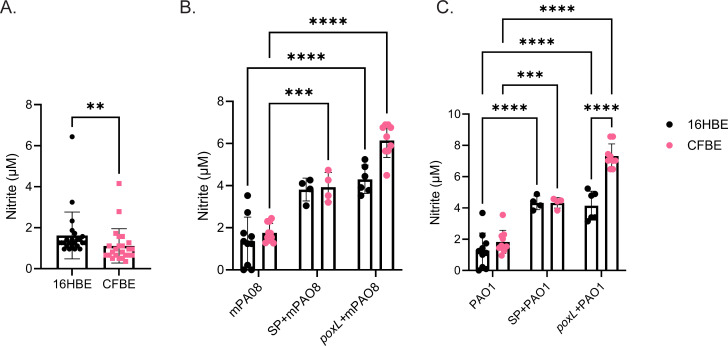
S. parasanguinis increases extracellular nitrite in CFBE cells
Nitrite was quantified before and after infections in both 16HBE and CFBE cells. At baseline, extracellular nitrite in CFBEs was 31% less than by the 16HBEs (Fig. 8A). Interestingly, when infected with P. aeruginosa, extracellular nitrite was relatively unchanged. However, when cells were exposed to S. parasanguinis (+/−poxL), extracellular nitrite was significantly increased in both CFBE and 16HBE cells (Fig. 8B and C). The presence of S. parasanguinis increased CFBE extracellular nitrite to that of 16HBE, suggesting that S. parasanguinis can modulate NOx flux in bronchial epithelial cells (Fig. 8B and C; Fig. S7).
Fig 8.
S. parasanguinis increases extracellular nitrite in CF bronchial epithelial cells. (A) Extracellular nitrite was measured in 16HBE and CFBE cells; n = 24. **P < 0.01 (Mann-Whitney test). After infection with mPA08 (B) or PAO1 (C) with or without S. parasanguinis (SP) or the poxL mutant, extracellular nitrite was measured. Error bars represent standard deviation. n = 4–10; ***P < 0.001, ****P < 0.0001 (two-way ANOVA, Tukey’s post hoc test).
DISCUSSION
Chronic P. aeruginosa infections contribute to severe lung decline and loss of lung function in pwCF. Recent literature indicates that the translocation of commensal streptococci from the oral cavity to the CF lung mitigates the effects of P. aeruginosa-induced inflammation and lung damage (50). Expanding on previous in vitro studies from our laboratory that demonstrate oral streptococci utilize nitrite to inhibit P. aeruginosa, we sought to understand whether the protective effects of S. parasanguinis and nitrite are active within the context of a mammalian lung and CF-relevant infection. In this study, we used a rat co-infection model and a wild-type and CFTR-deficient cell culture model to assess the protective effects of S. parasanguinis and nitrite. Initially, we tested whether H2O2 production by S. parasanguinis is active in the context of the CF airway because we previously published that nitrite-dependent inhibition of P. aeruginosa is also H2O2 dependent (20). Using synthetic media that mimic the environmental conditions of the oral cavity and airway, we observed an increase in H2O2 production, suggesting that S. parasanguinis increases H2O2 production when translocating from the oral cavity to the lung. In a rat respiratory co-infection model, P. aeruginosa burden did not decrease in the presence of S. parasanguinis and nitrite. However, the presence of S. parasanguinis significantly decreased PMN infiltration, IL-6, and IFN-γ production in response to P. aeruginosa. Strikingly, we also found that nitrite alone decreased IL-6 and IFN-γ production to P. aeruginosa, although it was also associated with mild inflammation in the lung as measured by histology score. Using wild-type and CF bronchial epithelial cell culture models of infection, we found that S. parasanguinis can inhibit the CF isolate of P. aeruginosa through RNI but inhibit the non-CF isolate of P. aeruginosa independently of both H2O2 and RNI. Additionally, S. parasanguinis reduced extracellular lactate dehydrogenase concentrations, suggesting that S. parasanguinis reduces cell damage by P. aeruginosa. Finally, we found that CFBEs have reduced extracellular nitrite compared to 16BE at baseline; however, the presence of S. parasanguinis restored CFBE extracellular nitrite to wild-type levels. In summary, our data indicate that S. parasanguinis can safeguard the airway from P. aeruginosa-induced inflammation and damage and also alter NOx production by airway epithelial cells.
Multiple microbiome studies have indicated that the presence of certain oral commensal streptococci is associated with decreased disease burden in CF (14, 15, 28 – 31, 57, 58) [reviewed by Scott and O’Toole (27)]. While this association has been well established, mechanisms through which these streptococci decrease disease burden are relatively unclear, and many mechanisms are specific to species or strains. One possibility is the antagonism of CF pathogens by streptococci. Our laboratory has previously demonstrated that H2O2-producing streptococci, such as S. parasanguinis, can inhibit P. aeruginosa through the generation of RNI (18, 20). Another possible mechanism is that these streptococci can modulate the host immune response. Several studies assessing immune modulation in the context of the oral cavity have elucidated a myriad of mechanisms through which streptococci can change host cell behavior (47). One mechanism is through production of H2O2 wherein H2O2 activates nuclear factor erythroid 2-related factor 2 (Nrf2) which inhibits NF-kB transcription (59). Studies elucidating the response of airway epithelial cells to oral streptococci are limited. Tony-Odigie et al. demonstrated that certain strains of S. mitis and S. oralis can reduce IL-8 expression and production in response to P. aeruginosa in human airway epithelial cells (BEAS-2B) (50). Furthermore, using an ex vivo lung infection system, it was found that S. mitis also reduces production of MCP-1, RANTES, GM-CSF, and TNF-α in response to P. aeruginosa. The presence of S. mitis reduced mTOR signaling, NOD-like receptor signaling, and Toll-like receptor signaling compared to P. aeruginosa infection alone. Taken together, these studies suggest that S. parasanguinis may modulate the host immune response away from hyper-inflammation, although several different mechanisms are possible. Further studies are warranted to establish the mechanism(s) through which S. parasanguinis modulates the host immune response during respiratory colonization.
Our results also indicated that the addition of nitrite alone decreased IL-6 and IFN-γ but did not change PMN infiltration or histology scores in our rat infection model. Studies have indicated that (i) pwCF have reduced exhaled nitric oxide compared to healthy controls and (ii) the presence of reactive nitrogen intermediates are positively correlated with lung function in pwCF (43, 44, 60). The role of nitric oxide (NO) on immune regulation is complex, where NO from inducible nitric oxide synthase in airway cells can either positively or negatively regulate cytokine production via NF-kB depending on host inflammatory signals, the concentration of NO, and duration of NO exposure [reviewed by Bayarri et al. (40)]. Furthermore, cytokine signaling can influence production of nitric oxide. Importantly, nitric oxide is readily oxidized into nitrite, and nitrite can later be converted back to nitric oxide through a variety of processes (61, 62). Given our high concentration of administered nitrite to rats (1 mM), it is possible that this induced negative feedback responses to IL-6 and IFN-γ to P. aeruginosa but may have exacerbated tissue damage when given with S. parasanguinis or P. aeruginosa (Fig. 3A; Fig. S2). Taken together, more studies assessing the host immune response to various concentrations of nitrite in combination with infection are warranted.
Infection of 16HBE or CFBE airway cells elucidated several strain-specific nuances between the non-CF isolate, PAO1, and the CF isolate, mPA08, in our system. As expected, we found that mPA08 was inhibited by the presence of both S. parasanguinis and nitrite, indicating that RNI is required for this inhibition in both healthy and CF cells. Surprisingly, we found that PAO1 was most inhibited by the poxL mutant of S. parasanguinis, indicating an RNI-independent mechanism of P. aeruginosa inhibition. Given the many changes that P. aeruginosa undergoes through evolution in the CF airway, it is possible that CF isolates are not sensitive to this RNI-independent inhibition, whereas non-CF isolates are sensitive (9, 63 – 65). RNI-independent mechanisms of P. aeruginosa inhibition by S. parasanguinis have not been demonstrated in vitro, so it is possible that S. parasanguinis stimulates host cells, in an H2O2-independent manner, to produce antimicrobials that non-CF isolates are more sensitive to than CF isolates. For example, LL-37, a cationic antimicrobial peptide, inhibits nonmucoid P. aeruginosa to a greater extent than mucoid P. aeruginosa (66).
Our cell data indicate that S. parasanguinis can increase extracellular nitrite concentrations in CF bronchial epithelial cells to that of wild-type cells. Literature assessing the roles commensal bacteria have on NOx flux is limited, indicating a significant gap in knowledge. However, given the inhibition of P. aeruginosa by S. parasanguinis in the absence of nitrite in our cell infection model, it is tempting to speculate (i) that S. parasanguinis may inhibit P. aeruginosa in a nitrite-independent manner, perhaps through host cell modulation or (ii) S. parasanguinis stimulates nitric oxide production by host cells which may inhibit P. aeruginosa on its own or react with H2O2 to form other RNIs that have been shown to inhibit P. aeruginosa.
In summary, our data indicate that S. parasanguinis increases H2O2 production when grown in artificial CF sputum. Despite this, we did not observe any nitrite-dependent inhibition of P. aeruginosa in an acute rat respiratory infection model. However, we found that S. parasanguinis significantly reduced PMN burden in the BALF, as well as production of IL-6 and IFN-γ following P. aeruginosa infection. Using a bronchial epithelial cell infection model, we found that S. parasanguinis can inhibit P. aeruginosa in both wild-type and CF-derived cell lines. Furthermore, the presence of S. parasanguinis reduced cell damage as compared to P. aeruginosa infection alone as assayed by lactate dehydrogenase release. Additionally, we found that S. parasanguinis can increase extracellular nitrite in CF bronchial epithelial cells, a novel factor that may further explain the intricate role commensals have in CF polymicrobial infections. Taken together, the improved outcomes in pwCF colonized with oral commensal streptococci such as S. parasanguinis may be explained through both microbial antagonism and through modulation of host inflammatory factors.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and reagents
S. parasanguinis FW213 and its poxL deletion mutant (20) were maintained on Todd Hewitt Agar and broth (THB) and grown at 37°C with 5% CO2. Pseudomonas aeruginosa PAO1 and mPA08-31 were maintained on Pseudomonas isolation agar (PIA) and grown in lysogeny broth (LB) at 37°C shaking at 200 rpm. Artificial saliva was prepared following the recipe described by Silva et al. (67): 2 g/L yeast extract, 5 g/L peptone, 2 g/L glucose, 1 g/L gastric porcine mucin (Sigma-Aldrich, St. Louis, MO, USA), 0.35 g/L NaCl, 0.2 g/L CaCl2, and 0.2 g/L KCl (67). Artificial cystic fibrosis sputum (SCFM2) was prepared as previously described by Turner et al. (68).
Hydrogen peroxide quantification
S. parasanguinis overnight cultures were subcultured to an absorbance at 600 nm of 0.5. Five milliliters of THB, artificial saliva, and SCFM2 were inoculated at 1:1,000, and cultures were grown statically for 16 hours at 37°C with 5% CO2. Hydrogen peroxide was quantified using the Amplex Red reagent (Thermo Fisher Scientific, Waltham, MA, USA).
Real-time quantitative PCR
Using the same conditions as above, RNA was extracted using the Zymo Directzol kit (Irvine, CA, USA). Quantitative PCR was performed using primers 16S: 5′-GAGAGATGGACCTGCGTTGT-3′ and 5′-GCCGAAGATTCCCTACTGCT-3′ and poxL primers: 5′-CTACTCAATCGACGTCGGTAAC-3′ and 5′-TGTCGCAAAGAGTGGAGATG-3′. The delta-delta Ct method was used to standardize gene expression to 16S (69).
Rat infection and CFU enumeration
Six- to eight-week-old Sprague-Dawley rats were infected sequentially with S. parasanguinis followed by P. aeruginosa mPA08-31 8 hours later. Rats were infected intranasally with 300-µL overnight culture (~107 CFU/mL S. parasanguinis and mPA08). After 24 hours, rats were sacrificed via anesthetization with CO2 followed by cervical dislocation. Broncho-alveolar lavage with 4-mL Hanks Balanced Salt Solution (Thermo Fisher Scientific, Waltham, MA, USA) was performed for polymorphonuclear cell enumeration and cytokine quantification. Right lungs were harvested and homogenized in 1-mL PBS for CFU enumeration. Lung homogenate was serially diluted and plated on PIA. Homogenate was grown overnight at 37°C with 5% CO2 and was enumerated the following day. Left lungs were inflated with formalin for histological analysis. All rat infection protocols were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committees (IACUC protocol 21546).
Histological analysis
Left lungs were inflated and stored in 4% formalin (Thermo Fisher Scientific, Waltham, MA, USA) at 4°C until processing at UAB pathology core where sections were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). H&E-stained sections were evaluated by a board-certified surgical pathologist (L.N.) and graded in a blind fashion. Imaging was performed using a Cytation 5 microscope (Agilent Bio Tech) at 100× magnification. Parameters for scoring included inflammatory cell influx into the airways and alveolar wall damage. Tissues were scored on a scale from 0 to 3 where a score of 0 indicates no inflammatory cell influx/damage, 1 indicates rare inflammatory cell influx (mild damage), 2 indicates dense inflammatory cell influx with intact alveolar walls (moderate damage), and 3 indicates dense inflammatory cells with undefined alveolar walls (severe damage).
Polymorphonuclear cell enumeration and cytokine quantification
Bronchoalveolar lavage fluid from rat lungs was centrifuged at 800 rpm for 4 min at 4°C. Five-hundred-microliter aliquots of supernatant were devoted to ELISA analyses and stored at −80°C until use. Rat ELISAs for IL-1α, IL-1β, IL-6, IFN-γ, TNF-α, and IL-10 were performed following manufacturer’s protocols (R&D Systems, Minneapolis, MN, USA). The cell pellet was resuspended in 500-µL PBS and diluted before centrifugation with Cytospin centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) at 600 rpm for 10 min. Slides were stained with Kwik-Diff (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s protocol. PMNs were enumerated using three representative fields from each spot.
Cell infection, CFU enumeration, nitrite quantification, and cell viability assays
Human bronchial epithelial cell lines 16HBE (70) and cystic fibrosis bronchial epithelial cells [CFBE4lo-, referred to as CFBE (71)] were maintained on Gibco MEM (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) and 100 IU/mL penicillin-streptomycin at 37°C with 5% CO2 (Thermo Fisher Scientific, Waltham, MA, USA). Cells were seeded on 12-well transwell plates (Corning, Corning, NY, USA) at 5 × 105 cells/mL and grown with media apically and basally for 1 week. After the first week, apical media were removed, and cells were grown at air-liquid interface for 1 week.
Cells were infected first with overnight S. parasanguinis culture brought to a final absorbance at 600 nm of 0.5 (~106 CFU/mL) for 2 hours. Following this, overnight PAO1 and mPA08 were diluted to an OD of 0.1 and serially diluted 1:100,000 (~103 CFU/mL) and added to cells for 4 hours. Following the 6-hour total infection, cells were washed twice with PBS, scraped, and serially diluted for CFU enumeration. Nitrite was quantified before and after cell infection using the Griess Assay (Promega, Madison, WI, USA). Prior to and after cell infection, lactate dehydrogenase was measured and normalized, with change in LDH release following infection reported (Promega, Madison, WI, USA).
Statistical analysis
All statistical analyses were performed on GraphPad Prism (GraphPad Prism version 9.5.1 for Windows GraphPad Software, San Diego, CA, USA). Normality was assessed using the Shapiro-Wilk test. Further analyses were performed where indicated.
ACKNOWLEDGMENTS
We thank Dr. Susan Birket for providing the mPA08-31 P. aeruginosa strain and animal training.
This work was supported by grants awarded to J.S. from the National Institute of Dental and Craniofacial Research/NIDCR (R00DE025913) and the National Institute of General Medical Sciences (R35GM142748). This work was also funded by a UAB Cystic Fibrosis Research Center Pilot grant (P30DK072482) awarded to J.S. and start-up funds from the UAB Department of Microbiology. J.B. was supported by the NIDCR/Dental Academic Research Training Program (T90DE022736) and is now supported by an NIH/NIDCR F31DE031508 National Research Service Award. S.S. was supported by the Alabama Louis Stokes for Minority Participation fellowship funded by the National Science Foundation (1806130) and the National Heart, Lung, and Blood Institute (NHLBI) T32 UAB pre-doctoralpre-doctoral training program in lung diseases (T32HL 134640-03) and is now supported by an NIH/NHLBI 1F31HL162487-01 National Research Service Award. M.M. is supported by a Cystic Fibrosis Foundation Postdoctoral Fellowship (MCDANI23F0).
The authors declare no competing financial interests.
Contributor Information
Jessica A. Scoffield, Email: jscoff@uab.edu.
Justin R. Kaspar, The Ohio State University College of Dentistry, Columbus, Ohio, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02198-23.
Fig. S1 to S7
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. 1989. Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073–1080. doi: 10.1126/science.2570460 [DOI] [PubMed] [Google Scholar]
- 2. Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N. 1989. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245:1059–1065. doi: 10.1126/science.2772657 [DOI] [PubMed] [Google Scholar]
- 3. Riordan JR, Rommens JM, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, Drumm ML, Iannuzzi MC, Collins FS, Tsui L-C. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073. doi: 10.1126/science.2475911 [DOI] [PubMed] [Google Scholar]
- 4. Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, Skach WR, Cutting GR, Frizzell RA, Sheppard DN, Cyr DM, Sorscher EJ, Brodsky JL, Lukacs GL. 2016. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 27:424–433. doi: 10.1091/mbc.E14-04-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khanolkar RA, Clark ST, Wang PW, Hwang DM, Yau YCW, Waters VJ, Guttman DS. 2020. Ecological succession of polymicrobial communities in the cystic fibrosis airways. mSystems 5:e00809-20. doi: 10.1128/mSystems.00809-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A 109:5809–5814. doi: 10.1073/pnas.1120577109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahboubi MA, Carmody LA, Foster BK, Kalikin LM, VanDevanter DR, LiPuma JJ. 2016. Culture-based and culture-independent bacteriologic analysis of cystic fibrosis respiratory specimens. J Clin Microbiol 54:613–619. doi: 10.1128/JCM.02299-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carmody LA, Caverly LJ, Foster BK, Rogers MAM, Kalikin LM, Simon RH, VanDevanter DR, LiPuma JJ. 2018. Fluctuations in airway bacterial communities associated with clinical states and disease stages in cystic fibrosis. PLoS One 13:e0194060. doi: 10.1371/journal.pone.0194060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jelsbak L, Johansen HK, Frost A-L, Thøgersen R, Thomsen LE, Ciofu O, Yang L, Haagensen JAJ, Høiby N, Molin S. 2007. Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect Immun 75:2214–2224. doi: 10.1128/IAI.01282-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Favero MS, Carson LA, Bond WW, Petersen NJ. 1971. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science 173:836–838. doi: 10.1126/science.173.3999.836 [DOI] [PubMed] [Google Scholar]
- 11. Green SK, Schroth MN, Cho JJ, Kominos SK, Vitanza-jack VB. 1974. Agricultural plants and soil as a reservoir for Pseudomonas aeruginosa. Appl Microbiol 28:987–991. doi: 10.1128/am.28.6.987-991.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan NH, Ishii Y, Kimata-Kino N, Esaki H, Nishino T, Nishimura M, Kogure K. 2007. Isolation of Pseudomonas aeruginosa from open ocean and comparison with freshwater, clinical, and animal isolates. Microb Ecol 53:173–186. doi: 10.1007/s00248-006-9059-3 [DOI] [PubMed] [Google Scholar]
- 13. Hansen SK, Rau MH, Johansen HK, Ciofu O, Jelsbak L, Yang L, Folkesson A, Jarmer HØ, Aanæs K, von Buchwald C, Høiby N, Molin S. 2012. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J 6:31–45. doi: 10.1038/ismej.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O’Toole GA. 2012. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol 194:4709–4717. doi: 10.1128/JB.00566-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuthbertson L, Walker AW, Oliver AE, Rogers GB, Rivett DW, Hampton TH, Ashare A, Elborn JS, De Soyza A, Carroll MP, Hoffman LR, Lanyon C, Moskowitz SM, O’Toole GA, Parkhill J, Planet PJ, Teneback CC, Tunney MM, Zuckerman JB, Bruce KD, van der Gast CJ. 2020. Lung function and microbiota diversity in cystic fibrosis. Microbiome 8:45. doi: 10.1186/s40168-020-00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x [DOI] [PubMed] [Google Scholar]
- 17. Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, Surette MG. 2008. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A 105:15070–15075. doi: 10.1073/pnas.0804326105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baty JJ, Huffines JT, Stoner SN, Scoffield JA. 2022. A commensal streptococcus dysregulates the Pseudomonas aeruginosa nitrosative stress response. Front Cell Infect Microbiol 12:817336. doi: 10.3389/fcimb.2022.817336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scoffield JA, Duan D, Zhu F, Wu H. 2017. A commensal streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS Pathog 13:e1006300. doi: 10.1371/journal.ppat.1006300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scoffield JA, Wu H. 2015. Oral streptococci and nitrite-mediated interference of Pseudomonas aeruginosa. Infect Immun 83:101–107. doi: 10.1128/IAI.02396-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camus L, Briaud P, Vandenesch F, Moreau K. 2021. How bacterial adaptation to cystic fibrosis environment shapes interactions between Pseudomonas aeruginosa and Staphylococcus aureus. Front Microbiol 12:617784. doi: 10.3389/fmicb.2021.617784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Limoli DH, Warren EA, Yarrington KD, Donegan NP, Cheung AL, O’Toole GA. 2019. Interspecies interactions induce exploratory motility in Pseudomonas aeruginosa. Elife 8:e47365. doi: 10.7554/eLife.47365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Price CE, Brown DG, Limoli DH, Phelan VV, O’Toole GA. 2020. Exogenous alginate protects Staphylococcus aureus from killing by Pseudomonas aeruginosa. J Bacteriol 202:e00559-19. doi: 10.1128/JB.00559-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDaniel MS, Schoeb T, Swords WE. 2020. Cooperativity between Stenotrophomonas maltophilia and Pseudomonas aeruginosa during polymicrobial airway infections. Infect Immun 88:e00855-19. doi: 10.1128/IAI.00855-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindgren NR, Novak L, Hunt BC, McDaniel MS, Swords WE. 2022. Nontypeable haemophilus influenzae infection Impedes Pseudomonas aeruginosa colonization and persistence in mouse respiratory tract. Infect Immun 90:e0056821. doi: 10.1128/IAI.00568-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jean-Pierre F, Vyas A, Hampton TH, Henson MA, O’Toole GA. 2021. One versus many: polymicrobial communities and the cystic fibrosis airway. mBio 12:e00006-21. doi: 10.1128/mBio.00006-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scott JE, O’Toole GA. 2019. The Yin and Yang of Streptococcus lung infections in cystic fibrosis: a model for studying polymicrobial interactions. J Bacteriol 201:e00115-19. doi: 10.1128/JB.00115-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flight WG, Smith A, Paisey C, Marchesi JR, Bull MJ, Norville PJ, Mutton KJ, Webb AK, Bright-Thomas RJ, Jones AM, Mahenthiralingam E, McAdam AJ. 2015. Rapid detection of emerging pathogens and loss of microbial diversity associated with severe lung disease in cystic fibrosis. J Clin Microbiol 53:2022–2029. doi: 10.1128/JCM.00432-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ronan NJ, Einarsson GG, Twomey M, Mooney D, Mullane D, NiChroinin M, O’Callaghan G, Shanahan F, Murphy DM, O’Connor OJ, Shortt CA, Tunney MM, Eustace JA, Maher MM, Elborn JS, Plant BJ. 2018. CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with Ivacaftor. Chest 153:395–403. doi: 10.1016/j.chest.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 30. Acosta N, Heirali A, Somayaji R, Surette MG, Workentine ML, Sibley CD, Rabin HR, Parkins MD. 2018. Sputum microbiota is predictive of long-term clinical outcomes in young adults with cystic fibrosis. Thorax 73:1016–1025. doi: 10.1136/thoraxjnl-2018-211510 [DOI] [PubMed] [Google Scholar]
- 31. Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Zhang Y, Surendra A, Gong Y, Elizabeth Tullis D, Yau YCW, Waters VJ, Hwang DM, Guttman DS. 2015. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5:10241. doi: 10.1038/srep10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burleigh MC, Liddle L, Monaghan C, Muggeridge DJ, Sculthorpe N, Butcher JP, Henriquez FL, Allen JD, Easton C. 2018. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic Biol Med 120:80–88. doi: 10.1016/j.freeradbiomed.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 33. Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, Bryan NS. 2014. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One 9:e88645. doi: 10.1371/journal.pone.0088645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. 2005. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci 113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x [DOI] [PubMed] [Google Scholar]
- 35. Scoffield J, Michalek S, Harber G, Eipers P, Morrow C, Wu H. 2019. Dietary nitrite drives disease outcomes in oral polymicrobial infections. J Dent Res 98:1020–1026. doi: 10.1177/0022034519855348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huffines JT, Scoffield JA. 2020. Disruption of Streptococcus mutans and Candida albicans synergy by a commensal streptococcus. Sci Rep 10:19661. doi: 10.1038/s41598-020-76744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. 2013. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MacMicking J, Xie QW, Nathan C. 1997. Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350. doi: 10.1146/annurev.immunol.15.1.323 [DOI] [PubMed] [Google Scholar]
- 39. Galkina SI, Golenkina EA, Viryasova GM, Romanova YM, Sud’ina GF. 2019. Nitric oxide in life and death of neutrophils. Curr Med Chem 26:5764–5780. doi: 10.2174/0929867326666181213093152 [DOI] [PubMed] [Google Scholar]
- 40. Bayarri MA, Milara J, Estornut C, Cortijo J. 2021. Nitric oxide system and bronchial epithelium: more than a barrier. Front Physiol 12:687381. doi: 10.3389/fphys.2021.687381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meldrum DR, Shames BD, Meng X, Fullerton DA, McIntyre RC, Grover FL, Harken AH. 1998. Nitric oxide downregulates lung macrophage inflammatory cytokine production. Ann Thorac Surg 66:313–317. doi: 10.1016/s0003-4975(98)00525-6 [DOI] [PubMed] [Google Scholar]
- 42. Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. 2001. Biphasic regulation of NF-κB activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol 166:3873–3881. doi: 10.4049/jimmunol.166.6.3873 [DOI] [PubMed] [Google Scholar]
- 43. Balint B, Kharitonov SA, Hanazawa T, Donnelly LE, Shah PL, Hodson ME, Barnes PJ. 2001. Increased nitrotyrosine in exhaled breath condensate in cystic fibrosis. Eur Respir J 17:1201–1207. doi: 10.1183/09031936.01.00072501 [DOI] [PubMed] [Google Scholar]
- 44. Grasemann H, Ioannidis I, Tomkiewicz RP, de Groot H, Rubin BK, Ratjen F. 1998. Nitric oxide metabolites in cystic fibrosis lung disease. Arch Dis Child 78:49–53. doi: 10.1136/adc.78.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scoffield JA, Wu H. 2016. Nitrite reductase is critical for Pseudomonas aeruginosa survival during co-infection with the oral commensal Streptococcus parasanguinis. Microbiology (Reading) 162:376–383. doi: 10.1099/mic.0.000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaci G, Goudercourt D, Dennin V, Pot B, Doré J, Ehrlich SD, Renault P, Blottière HM, Daniel C, Delorme C. 2014. Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl Environ Microbiol 80:928–934. doi: 10.1128/AEM.03133-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baty JJ, Stoner SN, Scoffield JA. 2022. Oral commensal streptococci: gatekeepers of the oral cavity. J Bacteriol 204:e0025722. doi: 10.1128/jb.00257-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mathieu E, MacPherson CW, Belvis J, Mathieu O, Robert V, Saint-Criq V, Langella P, Tompkins TA, Thomas M. 2020. Oral primo-colonizing bacteria modulate inflammation and gene expression in bronchial epithelial cells. Microorganisms 8:1094. doi: 10.3390/microorganisms8081094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock REW. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76:4163–4175. doi: 10.1128/IAI.00188-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tony-Odigie A, Wilke L, Boutin S, Dalpke AH, Yi B. 2022. Commensal bacteria in the cystic fibrosis airway microbiome reduce P. aeruginosa induced inflammation. Front Cell Infect Microbiol 12:824101. doi: 10.3389/fcimb.2022.824101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lara-Reyna S, Holbrook J, Jarosz-Griffiths HH, Peckham D, McDermott MF. 2020. Dysregulated signalling pathways in innate immune cells with cystic fibrosis mutations. Cell Mol Life Sci 77:4485–4503. doi: 10.1007/s00018-020-03540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng L, Itzek A, Chen Z, Kreth J. 2011. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl Environ Microbiol 77:4318–4328. doi: 10.1128/AEM.00309-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zheng L, Chen Z, Itzek A, Ashby M, Kreth J. 2011. Catabolite control protein A controls hydrogen peroxide production and cell death in Streptococcus sanguinis. J Bacteriol 193:516–526. doi: 10.1128/JB.01131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng L, Itzek A, Chen Z, Kreth J. 2011. Oxygen dependent pyruvate oxidase expression and production in Streptococcus sanguinis. Int J Oral Sci 3:82–89. doi: 10.4248/IJOS11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. García-Mendoza A, Liébana J, Castillo AM, de la Higuera A, Piédrola G. 1993. Evaluation of the capacity of oral streptococci to produce hydrogen peroxide. J Med Microbiol 39:434–439. doi: 10.1099/00222615-39-6-434 [DOI] [PubMed] [Google Scholar]
- 56. Barnard JP, Stinson MW. 1999. Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii. Infect Immun 67:6558–6564. doi: 10.1128/IAI.67.12.6558-6564.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zemanick ET, Wagner BD, Robertson CE, Ahrens RC, Chmiel JF, Clancy JP, Gibson RL, Harris WT, Kurland G, Laguna TA, McColley SA, McCoy K, Retsch-Bogart G, Sobush KT, Zeitlin PL, Stevens MJ, Accurso FJ, Sagel SD, Harris JK. 2017. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur Respir J 50:1700832. doi: 10.1183/13993003.00832-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frey DL, Boutin S, Dittrich SA, Graeber SY, Stahl M, Wege S, Herth FJF, Sommerburg O, Schultz C, Mall MA, Dalpke AH. 2021. Relationship between airway dysbiosis, inflammation and lung function in adults with cystic fibrosis. J Cyst Fibros 20:754–760. doi: 10.1016/j.jcf.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 59. Tang YL, Sim TS, Tan KS. 2022. Oral streptococci subvert the host innate immune response through hydrogen peroxide. Sci Rep 12:656. doi: 10.1038/s41598-021-04562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meng QH, Springall DR, Bishop AE, Morgan K, Evans TJ, Habib S, Gruenert DC, Gyi KM, Hodson ME, Yacoub MH, Polak JM. 1998. Lack of inducible nitric oxide synthase in bronchial epithelium: a possible mechanism of susceptibility to infection in cystic fibrosis. J Pathol 184:323–331. doi: [DOI] [PubMed] [Google Scholar]
- 61. Zhao X-J, Wang L, Shiva S, Tejero J, Myerburg MM, Wang J, Frizzell S, Gladwin MT. 2013. Mechanisms for cellular NO oxidation and nitrite formation in lung epithelial cells. Free Radic Biol Med 61:428–437. doi: 10.1016/j.freeradbiomed.2013.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lundberg JO, Weitzberg E, Gladwin MT. 2008. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7:156–167. doi: 10.1038/nrd2466 [DOI] [PubMed] [Google Scholar]
- 63. Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di Serio C, Döring G, Tümmler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180:138–145. doi: 10.1164/rccm.200812-1943OC [DOI] [PubMed] [Google Scholar]
- 64. Feltner JB, Wolter DJ, Pope CE, Groleau M-C, Smalley NE, Greenberg EP, Mayer-Hamblett N, Burns J, Déziel E, Hoffman LR, Dandekar AA. 2016. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 7:e01513-16. doi: 10.1128/mBio.01513-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boucher JC, Yu H, Mudd MH, Deretic V. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun 65:3838–3846. doi: 10.1128/iai.65.9.3838-3846.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Malhotra S, Limoli DH, English AE, Parsek MR, Wozniak DJ, Bomberger JM, Goldberg J, Vasil M. 2018. Mixed communities of mucoid and nonmucoid Pseudomonas aeruginosa exhibit enhanced resistance to host antimicrobials. mBio 9:e00275-18. doi: 10.1128/mBio.00275-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silva S, Pires P, Monteiro DR, Negri M, Gorup LF, Camargo ER, Barbosa DB, Oliveira R, Williams DW, Henriques M, Azeredo J. 2013. The effect of silver nanoparticles and nystatin on mixed biofilms of Candida glabrata and Candida albicans on acrylic. Med Mycol 51:178–184. doi: 10.3109/13693786.2012.700492 [DOI] [PubMed] [Google Scholar]
- 68. Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. 2015. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci U S A 112:4110–4115. doi: 10.1073/pnas.1419677112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 70. Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. 1994. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 10:38–47. doi: 10.1165/ajrcmb.10.1.7507342 [DOI] [PubMed] [Google Scholar]
- 71. Goncz KK, Kunzelmann K, Xu Z, Gruenert DC. 1998. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum Mol Genet 7:1913–1919. doi: 10.1093/hmg/7.12.1913 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S7