ABSTRACT
Currently, whole-genome sequencing (WGS) data have not shown strong concordance with Escherichia coli susceptibility profiles to the commonly used β-lactam/β-lactamase inhibitor (BL/BLI) combinations: ampicillin-sulbactam (SAM), amoxicillin-clavulanate (AMC), and piperacillin-tazobactam (TZP). Progressive resistance to these BL/BLIs in the absence of cephalosporin resistance, also known as extended-spectrum resistance to BL/BLI (ESRI), has been suggested to primarily result from increased copy numbers of bla TEM variants, which is not routinely assessed in WGS data. We sought to determine whether addition of gene amplification could improve genotype-phenotype associations through WGS analysis of 147 E. coli bacteremia isolates with increasing categories of BL/BLI non-susceptibility ranging from ampicillin (AMP) susceptibility to being fully resistant to all three BL/BLIs. Consistent with a key role of bla TEM in ESRI, 112/134 strains (84%) with at least ampicillin non-susceptibility encoded bla TEM. Evidence of bla TEM amplification (i.e., bla TEM gene copy number estimates > 2×) was present in 40/112 (36%) strains. There were positive correlations between bla TEM copy numbers with minimum inhibitory concentrations of AMC and TZP (P < 0.05) but not for SAM (P = 0.09). The diversity of β-lactam resistance mechanisms, including non-ceftriaxone hydrolyzing bla CTX-M variants, bla OXA-1, and ampC and bla TEM strong promoter mutations, was greater in AMC- and TZP-non-susceptible strains but rarely observed within SAM- and AMP-non-susceptible isolates. Our study indicates that comprehensive analysis of WGS data, including β-lactamase-encoding gene amplification, can help categorize E. coli with AMC or TZP non-susceptibility but that discerning the transition from SAM susceptibility to SAM non-susceptibility using genetic data requires further refinement.
IMPORTANCE
The increased feasibility of whole-genome sequencing has generated significant interest in using such molecular diagnostic approaches to characterize difficult-to-treat, antimicrobial-resistant (AMR) infections. Nevertheless, there are current limitations in the accurate prediction of AMR phenotypes based on existing AMR gene database approaches, which primarily correlate a phenotype with the presence/absence of a single AMR gene. Our study utilized a large cohort of cephalosporin-susceptible Escherichia coli bacteremia samples to determine how increasing the dosage of narrow-spectrum β-lactamase-encoding genes in conjunction with other diverse β-lactam/β-lactamase inhibitor (BL/BLI) genetic determinants contributes to progressively more severe BL/BLI phenotypes. We were able to characterize the complexity of the genetic mechanisms underlying progressive BL/BLI resistance including the critical role of β-lactamase encoding gene amplification. For the diverse array of AMR phenotypes with complex mechanisms involving multiple genomic factors, our study provides an example of how composite risk scores may improve understanding of AMR genotype/phenotype correlations.
KEYWORDS: ESRI, progressive AMR resistance, BL/BLI, AMR gene amplification
INTRODUCTION
Rapid and accurate characterization of antimicrobial-resistant (AMR) infections is necessary to combat their increasing threat to public health (1, 2). There has been recent interest from both clinical and research standpoints in using whole-genome sequencing (WGS) to predict antimicrobial susceptibility patterns as molecular diagnostic approaches become more feasible (3, 4). When AMR is driven by a single gene, such as bla CTX-M for an extended-spectrum β-lactamase phenotype, AMR database query approaches generally detect a strong concordance between WGS-predicted and observed phenotypes (5). However, discordant genotype-phenotype predictions can occur due to complicated resistance mechanisms that involve multiple contributing genetic factors (6). Such issues have been consistently observed when trying to use WGS to predict the antimicrobial susceptibility pattern of β-lactamase/β-lactamase inhibitor (BL/BLI) combinations (5, 7, 8).
Although recent years have seen the introduction of such novel BL/BLI combinations as ceftolozane/tazobactam and ceftazidime/avibactam into clinical practice, the vast majority of BL/BLIs currently used are ampicillin-sulbactam (SAM), amoxicillin-clavulanate (AMC), and piperacillin-tazobactam (TZP) (9 – 11). Given the clinical impact of Escherichia coli and its highly varied susceptibility profile to these three BL/BLI combinations, E. coli is among the most well-studied organisms in terms of BL/BLI resistance (8, 12 – 21). Cephalosporinases and carbapenemases that hydrolyze broad-spectrum β-lactams generally also inactivate SAM, AMC, and TZP (22). There has been increasing interest in characterizing cephalosporin-susceptible E. coli strains with varying susceptibility patterns amongst these three common BL/BLI combinations (13 – 16, 23). Nevertheless, the characterization of these increasingly more severe BL/BLI-resistant phenotypes using WGS to detect potential associations in well-defined cohorts is less understood.
To date, most studied E. coli ceftriaxone-susceptible, BL/BLI-resistant strains harbor class A or class D beta-lactamases such as bla TEM-1B or bla OXA-1 (13 – 15). It has been suggested that the BL/BLI resistance amongst such strains evolves in a gradient fashion—from being ampicillin-sulbactam non-susceptible (SAM-NS) to being amoxicillin-clavulanate non-susceptible (AMC-NS) to being piperacillin-tazobactam non-susceptible (TZP-NS) (13, 24). Based on studies of a limited number of strains under laboratory passage as well as of clinical isolates, bla TEM amplification has been proposed as a major mechanism of what has been called extended-spectrum resistance to BL/BLIs (ESRI) (13 – 15, 18, 21, 23). However, there are many other genetic determinants that can contribute to reduced susceptibility to BL/BLIs such as augmented expression of bla TEM due to promoter mutations (25, 26), de-repression of the chromosomal ampC gene (27, 28), decreased outer membrane permeability (20, 29), inhibitor-resistant TEM variants (30), and CTX-M enzymes with decreased cephalosporin affinity but increased TZP hydrolysis activity (31). Furthermore, it is not clear how these BL/BLI genetic determinants contribute to BL/BLI resistance across the full ESRI spectrum.
There has been an in-depth examination of E. coli BL/BLI resistance mechanisms with regard to each respective BL/BLI combination. For example, Noguchi et al. used a phenotypic approach and found that hyperproduction of TEM-1 and altered cell membrane permeability accounted for a large proportion of SAM resistance (20). Conversely, WGS-based analyses of AMC and TZP resistance showed a complex variety of mechanisms including bla TEM-1 overexpression as well as increased copy numbers of various bla-encoding enzymes (8, 16). A critical missing piece in understanding the role of genetic analyses in BL/BLI resistance is the lack of WGS investigation of a large number of cephalosporin-susceptible E. coli isolates with varying BL/BLI resistance profiles. Thus, we performed WGS of 147 ceftriaxone-susceptible E. coli which had BL/BLI resistance phenotypes ranging from ampicillin (AMP) susceptibility to TZP non-susceptibility. We sought to determine whether genetic analysis of known BL/BLI resistance mechanisms could be used to classify among the various phenotypic ESRI categories. Our data suggest that progressive ESRI is driven in part by bla TEM amplification but that many complex mechanisms detectable by WGS analysis also contribute to AMC and TZP non-susceptibility. The lack of ability to separate SAM susceptibility from SAM non-susceptibility using WGS data, including analysis of the bla TEM copy number, indicates that improvements of these genotype/phenotype correlations may require alternative approaches to current AMR genetic database query methods.
RESULTS
Distribution of ESRI groups amongst CRO-S Escherichia coli BSI isolates
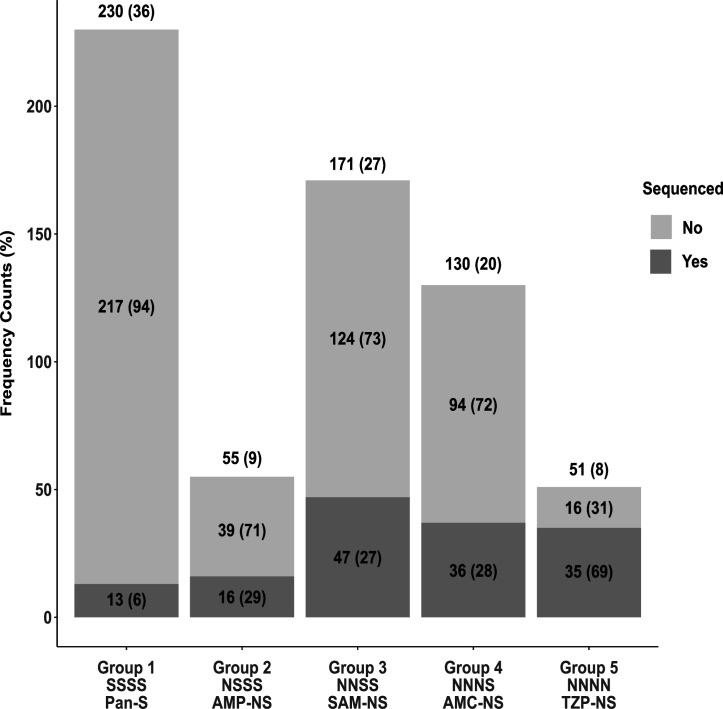
We identified 1,026 E. coli bloodstream isolates from May 1st, 2015, to April 30th, 2020. Of these, 389 (38%) had a ceftriaxone (CRO) MIC ≥4 mg/L. Consistent with a high rate of BL/BLI resistance among CRO-resistant isolates, 91% and 76% were non-susceptible to SAM and AMC, respectively. We selected the remaining 637 (62%) E. coli isolates in our sampling frame with a CRO MIC < 4 mg/L to study cephalosporin-susceptible, BL/BLI-associated resistance mechanisms (Fig. S1A). We grouped isolates by their susceptibility patterns according to CLSI guidelines as follows: β-lactam pan-susceptible (PAN-S; Group 1), ampicillin non-susceptible (AMP-NS; Group 2), ampicillin-sulbactam non-susceptible (Group 3), amoxicillin-clavulanate non-susceptible (Group 4), and piperacillin-tazobactam non-susceptible (Group 5) as shown in Fig. 1.
Fig 1.
Summary of ceftriaxone-susceptible (i.e., CRO MIC < 4 mg/L) Escherichia coli bloodstream isolates (n = 637) stratified by increasing BL/BLI-resistant phenotype (Groups 1–5) along with the number of sequenced isolates from each group. Unique BSI samples are grouped into progressively more resistant BL/BLI phenotypes from being pan-susceptible (Group 1) to being resistant to all four drug combinations in our study (Group 5). Frequency counts above each bar give group totals with the percentage of total population in parenthesis [e.g., there were 230 Group 1 isolates out of the total 637 (36%)]. We further split groups into sequenced (n = 147) (dark gray) vs non-sequenced (light gray) with frequency counts and percentages of each respective group labeled (e.g., 13 out of 230 Group 1 isolates were sequenced [6%]). N, non-susceptible-susceptible; S, susceptible; Pan-S, pan-susceptible; AMP-NS, ampicillin non-susceptible; SAM-NS, ampicillin-sulbactam non-susceptible; AMC, amoxicillin-clavulanate non-susceptible; TZP-NS, piperacillin-tazobactam non-susceptible.
The plural majority (230/637; 36%) of CRO-susceptible E. coli BSI isolates were pan-susceptible to the studied β-lactams (Group 1). The next most common isolates were SAM-NS (Group 3; 27%) and AMC-NS (Group 4; 20%) whereas the lowest frequencies were found within AMP-NS (Group 2; 9%) and TZP-NS (Group 5; 8%). We next looked at the minimum inhibitory concentration (MIC) distributions for each respective BL/BLI across each of the BL/BLI groups (Fig. S1B). For AMP and TZP antimicrobial susceptibility testing (AST), there was a bifurcation of susceptible and resistant isolates with very few isolates near the respective MIC breakpoints (Fig. S1B). Conversely, for SAM and AMC AST, there were large numbers of isolates, which had intermediate resistant phenotypes, which likely reflects a more diffuse spectrum of resistance for SAM and AMC vs. AMP and TZP (Fig. S1B).
From the CRO-susceptible E. coli BSI cohort, we selected 147 isolates for whole-genome sequencing across the spectrum of BL/BLI susceptibilities with the group distribution of sequenced strains relative to the total cohort shown in Fig. 1. We under- and over-sampled Group 1 and Group 5, respectively, whereas Groups 2, 3, and 4 had similar proportions of isolates sequenced within each group respective to the total cohort (148/637; 23%) as shown in Fig. 1.
Exogenous β-lactamase gene detection contribution to BL/BLI genetic determinants
The majority of isolates which were at least AMP-NS had ≥1 exogenous β-lactamase encoding gene detected (129/134; 96%). The bla TEM variants were the most commonly identified exogenous β-lactamase encoding genes being present in 84% (112/134) of AMP-NS or greater strains with the non-inhibitor-resistant TEM (N-IRT) gene, bla TEM-1B, accounting for the vast majority (n = 110) of the N-IRT detected. The bla OXA-1 gene was the second most commonly identified exogenous β-lactamase-encoding gene being present in 19/134 strains (14%). Previously characterized inhibitor-resistant TEM (IRT) variants were quite rare, with bla TEM-31 and bla TEM-40 each found once. Other rare β-lactamase-encoding genes included bla SHV-1 (n = 1), bla CARB-2 (n = 2), bla HER-3 (n = 1), bla LAP-2 (n = 1), and two bla CTX-M variants further described below.
For both AMP-NS and SAM-NS strains, nearly all strains carried bla TEM-1 alone with a single SAM-NS strain containing bla CARB-2 whereas bla LAP and bla CARB-2 were present in two AMC-NS strains. Conversely, strains in the TZP-NS had the most diverse array of β-lactamase-encoding genes including two strains with IRT β-lactamase variants (n = 2) and CTX-M enzymes (n = 2), respectively. One CTX-M enzyme was a derivative of CTX-M-15 (i.e., CTX-M-189) with an S133G polymorphism whereas the other was a derivative of CTX-M-27 (i.e., CTX-M-255) and contained a G239S polymorphism. Both of these polymorphisms have been shown in an experimental model to engender resistance to β-lactamase inhibitors but also to diminish the ability to hydrolyze cephalosporins (31). There was a statistically significant difference in exogenous β-lactamase gene content between the groups (χ 2, P < 0.05). Specifically, AMP-NS strains were significantly less likely to contain any exogenous β-lactamase whereas TZP-NS strains were more likely to contain bla OXA-1 (Fisher’s exact test, P < 0.001 for each). When only Groups 2 through 4 (i.e., AMP-NS, SAM-NS, and AMC-NS) were analyzed, no statistically significant differences in β-lactamase-encoding gene content were observed. Thus, we conclude that the fully susceptible and fully resistant groups can be distinguished from the other groups using exogenous β-lactamase content alone, but the middle three groups cannot.
Contribution of additional mechanisms to BL/BLI non-susceptibility
Given that exogenous β-lactamase-encoding genes only separated the fully susceptible and fully resistant strains from the remainder of the cohort, we next focused on other factors previously associated with BL/BLI resistance (8). First, E. coli strains encode an AmpC β-lactamase which is typically transcriptionally silenced but can become active in the presence of promoter mutations (27). We identified three strains, all in AMC-NS isolates, which contained ampC promoter variations previously associated with AMC resistance (27). Similarly, we assessed for variation in the bla TEM promoter region and found nine instances of strong bla TEM promoter variants (i.e., eight Pa/Pb and one P5) (25, 26). For eight of the nine cases, strains were in the TZP-NS group with the remaining strain having a borderline TZP MIC of 16 mg/L indicating that bla TEM promoter mutations were associated with TZP non-susceptibility. Finally, we analyzed the OmpC and OmpF contents of our cohort inasmuch as variation in the outer membrane protein profile has been associated with BL/BLI resistance (20). Only four strains had predicted inactivating ompC (n = 1) or ompF (n = 3) mutations, and the strains were present in varied groups (one in AMP-NS, one in SAM-NS, and two in TZP-NS). Thus, 16 strains (11%) had genetic changes predicted to increase ampC or bla TEM expression or eliminate ompC/ompF production, which have been previously associated with BL/BLI resistance.
Association of bla TEM amplification and BL/BLI resistance
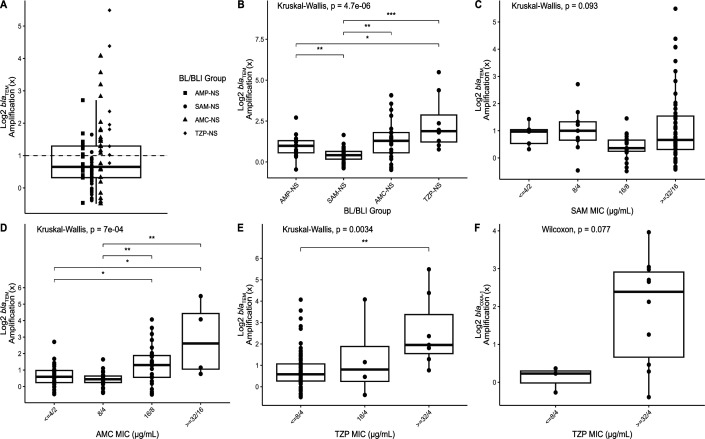
Increased TEM-1 activity has previously been shown to be an integral aspect of progressive BL/BLI resistance (16, 18) with mechanisms involving increased transcription due to promoter variation and/or increase in bla TEM-1 copy number (18, 25, 26). The median copy number estimate of bla TEM was 1.57× with a maximum of 45× observed (Fig. 2A). Taking a cut-off of 2.0-fold DNA coverage as evidence of amplification (32), 40/115 bla TEM NIR-containing strains (35%) had bla TEM amplification. To understand the relationship more clearly between bla TEM amplifications and BL/BLI resistance, we focused on the 91 strains that only contained N-IRT genes as a β-lactam resistance mechanism (i.e., no other exogenous β-lactamase, no ampC/bla TEM promoter variation, and no ompC/ompF disruptions). We found statistically significant increases in bla TEM amplification levels between SAM-NS and TZP-NS isolates (Fig. 2B). However, even for groups where there was a statistically significant difference, there remained an overlap such that bla TEM-1 amplifications did not clearly distinguish between the various resistance profiles (Fig. 2B). Consistent with the inability of bla TEM-1 amplifications to distinguish between strains from AMP-NS and SAM-NS, there was no significant correlation between SAM MIC and bla TEM-1 amplification (P = 0.09; Fig. 2C). However, AMC and TZP MICs were significantly associated with bla TEM-1 amplification (P < 0.01; Fig. 2D and E). When bla TEM amplification > 2× was considered as a potential β-lactam mechanism, there was a statistically significant difference in β-lactamase content between SAM-NS and AMC-NS (Fisher’s exact test, P < 0.001) whereas AMP-NS and SAM-NS continued to have similar mechanisms (Fisher’s exact test, P = 0.17). Thus, these data suggest that bla TEM-1 copy numbers help distinguish AMC resistant from AMC-susceptible strains but do not assist with assessing SAM susceptibility.
Fig 2.
Log2 transformed bla copy number variant (CNV) estimates for BL/BLI isolates with a single exogenous β-lactamase gene after excluding isolates with non-exogenous BL/BLI genetic determinant factors. (A) CNV estimates for bla TEM-1 for 91 E. coli isolates stratified by BL/BLI group indicated by shape. Horizontal dashed line indicates cut-off for 2× CNV; (B) bla TEM-1 CNV across each of the BL/BLI groups; (C) bla TEM-1 CNV by SAM MIC; (D) bla TEM-1 CNV by AMC MIC; (E) bla TEM-1 CNV by TZP MIC; (F) bla OXA-1 CNV by TZP MIC. Global P-values for Kruskal-Wallis or Wilcoxon rank-sum tests are reported above each respective panel. For global P-value <0.05, pairwise Wilcoxon rank-sum test adjusted P-values are reported as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
As previously noted, bla OXA-1-containing strains were only present in AMC-NS and TZP-NS isolates with the vast majority of bla OXA-1-containing strains belonging to TZP-NS (16/19). The three bla OXA-1 AMC-NS strains did not contain another BL/BLI resistance mechanism whereas six TZP-NS strains contained both bla OXA-1 and bla TEM-1, and one TZP-NS strain had a TEM-promoter variation, bla OXA-1, and bla TEM-1. Similar to bla TEM-1, an increase in bla OXA-1 CNV estimates was commonly observed in our cohort with 11/19 strains (57%) having >2.0× normalized coverage depths. When strains containing only bla OXA-1 as a β-lactam-resistant mechanism were considered, there was an increase in CNV positively correlated with TZP MIC albeit this did not reach statistical significance (Fig. 2F). Consistent with these data, all strains with a bla OXA-1 copy number ≥2× were in the fully resistant category.
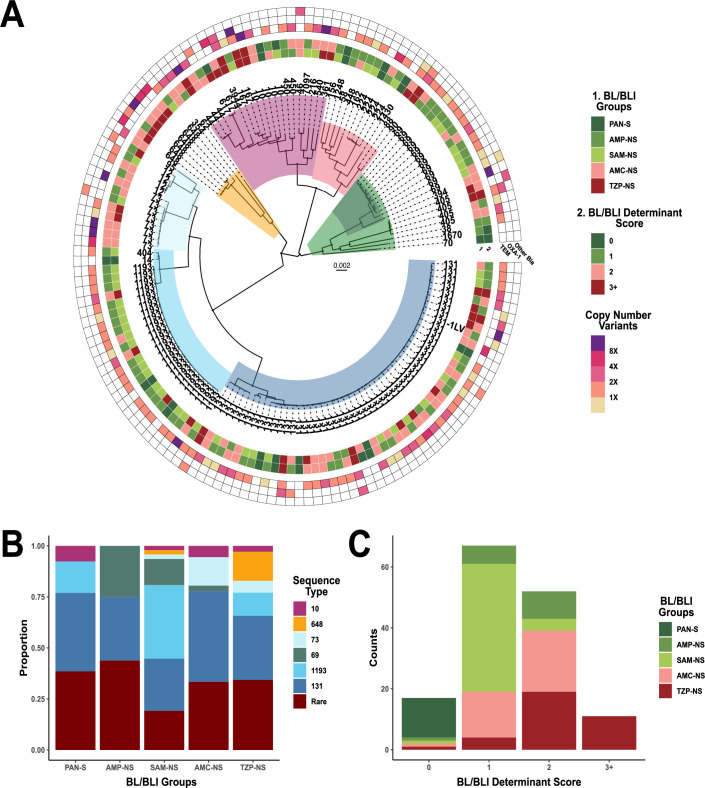
Correlations of BL/BLI genetic determinants with BL/BLI groups across the population structure
A core gene alignment-inferred maximum-likelihood phylogeny is presented in Fig. 3A. There were eight core gene-inferred clusters identified through the hierBAPS algorithm (33) that are highlighted in the phylogeny (Fig. 3A). Each of the cluster levels was associated with previously established phylogroups (34) or more closely related sequence types (STs) with highly related STs labeled on branch tips grouping together (Fig. 3A). The majority of isolates in this CRO-S cohort belonged to the B2 clade (60%; 88/147) although a total of 37 distinct STs were present. Strains of the pandemic ST131 clade (33%; 49/147) or the emergent ST1193 (16%; 23/147) were the most frequently observed STs with both belonging to the B2 phylogroup. There was only one other ST with more than 10 observations, which was ST69 (7%; 11/147). Other STs comprising of five or more strains included ST73 (n = 8), ST648 (n = 6), and ST10 (n = 5). We grouped STs with less than five strains together as “Rare STs,” and such strains accounted for 31% of the cohort (n = 45). When comparing all STs comprising of ≥5 isolates, there was a statistically significant relationship between ST and AMR grouping (χ 2 simulated P < 0.001) (Fig. 3B). Specifically, ST648 strains were more likely to be TZP-NS, and ST1193 strains were more likely to be SAM-NS (pairwise Fisher’s test adj. P < 0.05). Conversely, ST131 strains were not statistically significantly associated with any particular susceptibility profile. While not statistically significant, similar trends can be seen in Fig. 3A, where ST73/ST12 isolates (hierBAPS level 7; light blue) have 85% of isolates with AMC-NS or TZP-NS phenotypes whereas ST69 has more susceptible patterns with 91% of isolates having SAM-NS or a more susceptible phenotype.
Fig 3.
BL/BLI genetic determinant distribution across ESRI population. (A) Core gene alignment inferred maximum-likelihood phylogeny of 147 CRO-S E. coli bloodstream isolates. The tree is mid-point rooted with the timescale indicating mean nucleotide substitutions per site. Clades are shaded by core hierarchical population structure using the hierBAPS algorithm with each clade strongly associated with one or more phylogroups. Branch tips are labeled by the respective sequence type. Inner rings 1 and 2 correspond to BL/BLI groups and BL/BLI determinant scores, respectively, as labeled in the legend. Outer ring corresponds to bla TEM, bla OXA-1, and other bla gene copy number variants with copy number estimates color coded as indicated in the legend. (B) Stacked bar chart of the total proportion of STs across each of the BL/BLI groups. (C) Stacked bar chart of frequency counts of BL/BLI groups across each of the BL/BLI determinant scores.
We further characterized each of the BL/BLI groups by the number of BL/BLI genetic determinants, which could range from 0 to 10 (see Materials and Methods for details) (Fig. 3C). Each of these BL/BLI genetic determinants was based on the presence/absence of genetic features that have been previously identified as contributing to BL/BLI resistance (13, 20, 25, 27, 31, 35). The number of unique combinations of BL/BLI genetic determinants across each of the BL/BLI phenotype groups is shown in Fig. S2. All PAN-S isolates (n = 13) had no identifiable BL/BLI determinants whereas almost all isolates (130/134; 97%) with AMP-NS or higher had at least one genetic determinant identified (Fig. S2). Interestingly, all isolates in our cohort with 3+ BL/BLI determinants (n = 10) were TZP-NS (Fig. 3C). Importantly, there was a positive monotonic relationship (ρ = 0.62, P = 2.2e−16) between the BL/BLI group status and the BL/BLI genetic determinant score, thus underscoring how increasing numbers of BL/BLI genetic determinants impact BL/BLI non-susceptibility. Nevertheless, there was a similar distribution of BL/BLI genetic determinant scores across Group 2 and Group 3 isolates (Table S1), further highlighting the difficulty in discriminating genetic differences across these isolates.
Finally, we next sought to combine genetic determinants associated with BL/BLI resistance along with our phylogenomic structure to assess the odds of being in a greater or lesser BL/BLI non-susceptibility group using ordinal logistic regression methods. Table 1 provides an overview of BL/BLI and population-level covariate relationships with the odds of being in each BL/BLI phenotypic group. There were no statistically significant associations observed between the increasing BL/BLI group and ST or phylogroup; however, there was a weak association between Cluster 2, predominantly consisting of B1/C isolates that had 0.3 times (95% CI: 0.1–0.9) lesser odds of belonging to a higher (i.e., less susceptible) BL/BLI group compared with Cluster one isolates (ST131). When looking at the relationships between increasing BL/BLI non-susceptibility with the composite BL/BLI genetic determinant score, for every one unit increase in BL/BLI genetic determinant, the odds of being in a higher BL/BLI group were 10.9 times (95% CI: 6.2–20.2) greater than a lower BL/BLI group (Table 1). Importantly, there were no associations between N-IRT gene carriage and the BL/BLI group (OR = 1.1; 95% CI: 0.5–2.4) whereas bla TEM amplification, bla OXA-1 presence, and other non-TEM bla amplifications all appeared to contribute to increasing odds of BL/BLI non-susceptibility. Thus, these data suggest that the presence and amplification of narrow-spectrum β-lactamases are the primary drivers of the progressive BL/BLI phenotype.
TABLE 1.
Ordinal logistic regression to measure covariate associations with BL/BLI groups
| Feature | Mean (SD)/n (%) | OR a | 95% CI b |
|---|---|---|---|
| BL/BLI genetic determinant score c | 1.4 (0.9) | 10.9 | 6.2–20.2 |
| N-IRT presence | 110 (74.8) | 1.1 | 0.5–2.4 |
| bla TEM ≥ 2× | 40 (27.2) | 2.8 | 1.4–5.4 |
| bla OXA-1 | 19 (12.9) | 33.6 | 9.0–125.1 |
| Other bla ≥ 2× | 14 (9.5) | 67.8 | 8.5–543.5 |
| Hierarchical cluster d | |||
| Cluster 2 (B1/C) | 12 (8.2) | 0.3 | 0.1–0.9 |
| Cluster 3 (D–ST405-like) | 7 (4.8) | 0.8 | 0.2–3.8 |
| Cluster 4 (D–ST69-like) | 12 (8.2) | 0.3 | 0.1–1.0 |
| Cluster 5 (B2–ST1193-like) | 25 (17.0) | 0.5 | 0.2–1.1 |
| Cluster 6 (A) | 20 (13.6) | 1.9 | 0.7–5.0 |
| Cluster 7 (B2–ST73-like) | 13 (8.8) | 2.1 | 0.8–5.9 |
| Cluster 8 (F–ST648-like) | 8 (5.4) | 4.5 | 1.0–20.9 |
OR, odds ratio.
CI, confidence interval.
BL/BLI genetic determinant score composite variable of 10 binary-coded variables (see Materials and Methods).
Each group is compared with Cluster 1 which is B2 phylogroup ST131 isolates; parenthesis includes phylogroup and most common ST group where applicable.
DISCUSSION
The increasing affordability and availability of WGS data have markedly increased the understanding of bacterial AMR to the point where it has been postulated that such analysis might augment or even replace routine phenotypic susceptibility testing (36). For fast-growing bacteria, such as Enterobacterales, WGS has shown good concordance with the AMR phenotype for many “bug-drug” combinations, but in general, WGS has not accurately predicated Enterobacterales susceptibility to commonly used BL/BLIs such as SAM, AMC, and TZP (5, 7, 37). Herein, we sought to determine whether a comprehensive WGS analysis, including assessment of the bla copy number, could separate cephalosporin-susceptible E. coli based on BL/BLI resistance patterns. Our data indicate that an increase in copy numbers of narrow-spectrum β-lactamases does occur with progressive BL/BLI resistance but accounts for only part of this process in conjunction with an accumulation of a variety of other mechanisms (13).
A key strength of our study was using WGS data to analyze a phenotypically diverse cohort of E. coli bloodstream isolates from across the spectrum of BL/BLI resistance in contrast to previous studies which investigated a single BL/BLI combination (8, 16, 20). Moreover, we focused on cephalosporin-susceptible strains and integrated analyses of bla gene copy numbers to elucidate the recently proposed concept of extended-spectrum resistance to BL/BLI (13). This approach allowed for an augmented understanding of how each β-lactam resistance mechanism contributes to the spectrum of BL/BLI resistance in clinical isolates. For example, mutations in the ampC promoter were associated with resistance through AMC but not TZP whereas mutations in the bla TEM promoter were observed only in fully resistant strains except for a single isolate with a TZP MIC of 16/4 mg/L which would be considered susceptible dose dependent (SDD) by current CLSI MIC breakpoints. Similarly, although bla OXA-1 has been associated with TZP resistance (16), we observed that strains in which bla OXA-1 was the only β-lactamase and did not have an increase in copy number remained TZP susceptible although resistant to AMC and SAM suggesting a necessary gene dosage and/or OXA-1 activity effect for progressive resistance. Given that bla OXA-1 amplification is clearly associated with TZP resistance (16, 17), these and other data raise concerns regarding the clinical efficacy of TZP therapy of bla OXA-1-containing strains even if they test initially susceptibility given the capacity of such strains to readily increase the bla OXA-1 copy number (38).
A major surprising finding of our study was that whereas there was a progressive increase in bla TEM copy numbers moving from SAM-NS to AMC-NS to TZP-NS strains, there was conversely a small decrease in bla TEM copy numbers between AMP-NS (Group 2) and SAM-NS (Group 3) isolates. Such data were even more surprising given that bla TEM-1 was nearly universal and the only β-lactam resistance mechanism for both groups, leading us to hypothesize that increased TEM-1 production due to gene amplification would be the major mechanism distinguishing these two groups (13). Differentiation between AMP-NS and SAM-NS E. coli has not been systemically studied using WGS data such as has been done for AMC-NS and TZP-NS isolates (8, 13, 15, 16, 19), and thus, we are limited in our ability to benchmark our data against others. Noguchi et al. studied SAM-resistant isolates from Japan using phenotypic assays and found some relationship between increased TEM-1 activity, decreased membrane permeability, and SAM resistance (20). Consistent with our data, they found only a small percentage of strains had inactivating mutations in ompC or ompF. Thus, together with other data regarding E. coli responses to antimicrobials, we hypothesize that bla TEM-1 harboring E. coli develops SAM-NS through an increase in TEM-1 activity that is not detectable via assessment of the gene copy number when the organism is grown in the absence of antimicrobial pressure, along with decreased SAM entry through outer membrane changes which are not due to defined genetic mutations. As E. coli moves from SAM-NS to AMC-NS to TZP-NS, the resistance mechanisms then become both more diverse and genetically fixed in the population such that they become detectable using WGS-based methodologies. Thus, barring identification of new genetic mechanisms that can separate AMP-NS and SAM-NS isolates, it seems unlikely that WGS methods using bacteria grown in the absence of antimicrobial pressure will be able to accurately separate these two groups. Consistent with data from other groups (8, 16), WGS analyses are likely to be useful in detecting AMC and TZP resistance in E. coli but need to consider a large group of potential mechanisms, including single-nucleotide polymorphisms (SNPs), which are additive in nature and thus much more difficult to predict relative to ESBL phenotypes primarily driven by single genes. There is growing interest to create “mechanism agnostic” machine learning models that can classify AST phenotypes based on detecting associations with SNPs and gene presence/absence after controlling for population structure (39 – 42).
There are several limitations to our study worth noting. We sought to provide a “real-world” assessment of using WGS to analyze BL/BLI resistance and thus did not recapitulate the phenotypic data nor did we assess BL/BLI phenotypes comparing different methodologies such as broth microdilution vs. agar dilution. Thus, given the difficulties in the accuracy of BL/BLI susceptibilities using automated methodologies (24), it is likely that there was some misclassification of organisms into the various groups, particularly for those whose MICs were near the susceptibility breakpoints. Additionally, we used the WGS data to assess for known β-lactam resistance mechanisms and thus cannot be certain that other mechanisms were not present. Furthermore, bacterial outer-membrane remodeling and changes in efflux pump activity can further affect susceptibility to multiple classes of antibiotics and cannot be easily assessed using the WGS-based methods we employed in our study (43). These aforementioned changes may lead to phenomena known as antibiotic tolerance as well as heteroresistant subpopulations that can render significant challenges to antibiotic susceptibility interpretations (44 – 46). Further work is necessary to elucidate the contributions of these phenomena to treatment failure and how to effectively incorporate these factors into prediction models (47, 48). Even though we assessed nearly 150 strains, our sample sizes for some groups, such as group 2, were relatively small which could have limited our power to detect statistically significant differences in various mechanisms. Finally, we only sequenced strains from a single center meaning we do not know the generalizability of our findings although our data are in accord with other data from geographically distant locales (13 – 15, 18, 21).
In summary, we present herein a comprehensive WGS analysis of a large cohort of cephalosporin-susceptible E. coli strains from across the susceptibility spectrum of commonly used BL/BLIs. Our data both support and add to the complexity of the progressive ESRI spectrum proposed by Rodriguez-Villodres et al. (13). Our data suggest that the addition of β-lactam copy number and promoter variation assessment does assist with the delineation of AMC-NS and TZP-NS but not SAM-NS suggesting that the initial movement of E. coli along the ESRI spectrum may not be detectable using methods that query AMR databases.
MATERIALS AND METHODS
Bacterial strains and AST characterization
Escherichia coli bloodstream isolates are collected weekly through an IRB-approved protocol and stored at −80°C in 40% glycerol stocks. Initial antimicrobial susceptibility testing is performed on a Vitek2 (bioMérieux) automated platform through the University of Texas MD Anderson Cancer Center clinical microbiology laboratory. Additional AST was performed on selected candidate isolates to determine cohort inclusion using gradient ETest strips (Liofilchem) for SAM, AMC, and TZP, respectively, on MHB plates. MIC breakpoints were determined based on the current CLSI guidelines as established in M100-ED33 (49). Isolates with “intermediate” susceptibilities to the respective BL/BLI were grouped with isolates with interpretations of “resistant” within the same BL/BLI group and thus defined as non-susceptible to that particular BL/BLI combination. Further AST information is included in Table S2.
Whole-genome sequencing of bacterial isolates
Isolates with no indication of contamination and that fit into one of five groups based on AST confirmation were sub-cultured in LB and incubated for 3 hours at 37°C with 225 rpm agitation. A culture cell pellet was used to perform genomic DNA extraction using the QIAGEN DNeasy Blood & Tissue Kit following the manufacturer’s instructions. The quality of gDNA was assessed through a measure of gDNA concentration with the Qubit 4 fluorometer (Invitrogen) and high molecular weight with the 4200 Tapestation (Agilent) system using the Genomic DNA ScreenTape assay. The respective quality controlled (QC) gDNA was submitted to the MDACC Advanced Technology Genomics Core (ATGC) for library preparation and short-read, paired-end (150 × 2) whole-genome sequencing using the Illumina NovaSeq6000 instrument. Samples were multiplexed, barcoded, and sequenced to achieve coverage depths > 100×.
Short-read sequencing data QC and copy number quantification
Short-read data were QC’d and processed through a bespoke pipeline (W. Shropshire, spades_pipeline, GitHub: https://github.com/wshropshire/SPAdes_pipeline). Briefly, paired-end fastq reads have Illumina adaptors and low-quality bases trimmed using Trimmomatic-v0.39 with a seed match (16 bp) that allows up to two mismatches, a sliding window of 4 bp, minimum average quality = 15, and minimum length of 36 bp. Trimmed fastq reads were then quality assessed using fastqc-v0.11.9. Sequencing QC information as well as BioSample IDs are provided in Table S2.
Trimmed reads were inputted into the copy number variant quantification tool (CONVICT; W. Shropshire; GitHub: https://github.com/wshropshire/convict). A core-gene control file, which is created through the panaroo pan_reference.fa file, is used to normalize coverage depths. CONVICT uses a coverage-depth normalization approach whereby the coverage-depth of target genes is divided by the coverage-depth of control genes to give an estimation of the ploidy-agnostic copy number. In the first step of the CONVICT algorithm, reads are aligned with a target set of antimicrobial resistance genes determined by kmerresistance (50, 51) with the resfinder database (v4.0) (52, 53). Aligned reads are then sorted with Samtools into a corresponding bam file. Coverage measurements are determined by pileup.sh from the BBMAP suite of tools in bins of 100 bases along the length of a target gene. Bins are removed or maintained in an iterative process by comparing coverage-depth of a given bin to the mean coverage-depth of all bins for the respective gene until a specific coefficient of variation (CV = 0.175) is met. Coverage-depth for control genes is calculated in the same way as for target genes. Copy numbers are then estimated by dividing the coverage-depth of target genes by the coverage-depth of control genes. Complete convict-estimated copy number results are provided in Table S2.
Short-read assembly, database query, and phylogenetics
Trimmed reads are then used as input for short-read genome assemblies using SPAdes-v.3.15.5 using the –isolate parameter (54). Short-read assemblies were used as input to AMRfinder Plus (v3.11.11) using database version 2023–04-17.1 to confirm AMR variant alleles (55) detected with the short-read-based convict tool. We used known strong bla TEM promoter variants (25) and ampC promoter variants (27) that have been experimentally characterized as database input into a variant annotator tool (Selvalakshmi27, VariantAnnotator, https://github.com/Selvalakshmi27/VariantAnnotator). Each variant was confirmed using blast searches as well as visually inspected on SnapGene-v5.0.8. Genome assemblies were first annotated using Prokka-v1.14.6 (56) Annotated .gff files and were used to produce the core gene alignment file using panaroo-v1.2.10 (57). We used the MAFT-v7.505 aligner (58), with the panaroo parameters in strict mode and a core gene threshold of greater than 99% present within the population (n = 147). The core gene alignment file was then used to generate a maximum-likelihood (ML) phylogeny using IQtree2- 2.2.0-beta. Parameterization included 1,000 replicates for the SH approximate likelihood ratio test, 1,000 replicates for ultrafast bootstrap (59), and model optimization using ModelFinder (60). The optimized ML model was an unrestricted model with optimized base frequencies using ML with a FreeRate of heterogeneity chosen according to BIC. A core gene alignment file was used as input to investigate the population structure using an implementation of the hierarchical population clustering tool, RhierBAPS (33), with a max depth of hierarchical search = 2 and max populations = 30. The ggtree-v3.3.1 R package was used for tree visualization and mapping metadata to each individual tree branch-tip (61).
Statistics
Genetic determinants were coded into 10 binary variables to create a cumulative BL/BLI genetic determinant score (Table S3). These variables included N-IRT presence, TEM gene amplification (≥2×), IRT gene, strong bla TEM promoter variant, ampC promoter variant, bla OXA-1, bla CTX-M, other bla gene, other bla gene amplification (≥2×), and predicted Omp mutations based on previous research (13, 20, 25, 27, 31, 35). A Spearman’s rho coefficient was calculated to test for the relationship between BL/BLI groups and the BL/BLI genetic determinant score. Global Wilcoxon rank sum or Kruskal-Wallis tests were performed to detect significant differences in log-transformed copy numbers between groups. For global comparisons with significant P-values, pairwise comparisons using Wilcoxon rank sum exact test were performed with a false discovery rate P-value adjustment method. Ordinal logistic regression was performed using the BL/BLI group as an ordinal outcome and various genetic features as independent covariates with the MASS R package (v.7.3–54) and the “polr” function with a proportional odds logistic regression model. The brant test was performed to determine if parallel regression assumption held for each model using the “brant” R package (v0.3–0). Statistical analysis was performed using R-v4.0.4.
ACKNOWLEDGMENTS
The authors acknowledge the support of the High-Performance Computing for Research Facility at the University of Texas MD Anderson Cancer Center for providing computational resources that have contributed to the research results reported in this paper. The MDACC clinical microbiology lab does a fantastic job identifying and transferring these organisms of interest to us, which, as always, is much appreciated.
Core grant CA016672(ATGC) and NIH 1S10OD024977-01 grant provide funding for the Advanced Technology Genomics Core (ATGC) sequencing facility at MD Anderson Cancer Center. The National Institute of Allergy and Infectious Diseases (NIAID) T32 AI141349 Training Program in Antimicrobial Resistance supports the work of W.C.S. The NIAID R21AI151536 and P01AI152999 NIAID grants support the work on this project for S.A.S.
Contributor Information
Samuel A. Shelburne, Email: sshelburne@mdanderson.org.
Krisztina M. Papp-Wallace, JMI Laboratories, North Liberty, Iowa, USA
DATA AVAILABILITY
Whole-genome sequencing short-read data have been submitted to BioProject PRJNA924946 and PRJNA836696. R scripts for analyses can be made available upon request from the corresponding author.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02221-23.
Fig. S1 and S2.
Tables S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. 2017. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 376:2235–2244. doi: 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Kader DA, Anwar S, Hussaini H, Jones Amaowei EE, Rasuli SF, Hussain N, Kaddo S, Memon A. 2022. Systematic review on the effects of prompt antibiotic treatment on survival in septic shock and sepsis patients in different hospital settings. Cureus 14:e32405. doi: 10.7759/cureus.32405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alleweldt F, Kara Ş, Best K, Aarestrup FM, Beer M, Bestebroer TM, Campos J, Casadei G, Chinen I, Van Domselaar G, Dominguez C, Everett HE, Fouchier RA, Grant K, Green J, Höper D, Johnston J, Koopmans MP, Oude Munnink BB, Myers R, Nadon C, Patel A, Pohlmann A, Pongolini S, Reimer A, Thiessen S, Wylezich C. 2021. Economic evaluation of whole genome sequencing for pathogen identification and surveillance – results of case studies in Europe and the Americas 2016 to 2019. Euro Surveill 26:1900606. doi: 10.2807/1560-7917.ES.2021.26.9.1900606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon LG, Elliott TM, Forde B, Mitchell B, Russo PL, Paterson DL, Harris PNA. 2021. Budget impact analysis of routinely using whole-genomic sequencing of six multidrug-resistant bacterial pathogens in Queensland, Australia. BMJ Open 11:e041968. doi: 10.1136/bmjopen-2020-041968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shelburne SA, Kim J, Munita JM, Sahasrabhojane P, Shields RK, Press EG, Li X, Arias CA, Cantarel B, Jiang Y, Kim MS, Aitken SL, Greenberg DE. 2017. Whole-genome sequencing accurately identifies resistance to extended-spectrum beta-lactams for major gram-negative bacterial pathogens. Clin Infect Dis 65:738–745. doi: 10.1093/cid/cix417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doyle RM, O’Sullivan DM, Aller SD, Bruchmann S, Clark T, Coello Pelegrin A, Cormican M, Diez Benavente E, Ellington MJ, McGrath E, Motro Y, Phuong Thuy Nguyen T, Phelan J, Shaw LP, Stabler RA, van Belkum A, van Dorp L, Woodford N, Moran-Gilad J, Huggett JF, Harris KA. 2020. Discordant bioinformatic predictions of antimicrobial resistance from whole-genome sequencing data of bacterial isolates: an inter-laboratory study. Microb Genom 6:e000335. doi: 10.1099/mgen.0.000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hujer AM, Long SW, Olsen RJ, Taracila MA, Rojas LJ, Musser JM, Bonomo RA. 2020. Predicting β-lactam resistance using whole genome sequencing in Klebsiella pneumoniae: the challenge of β-lactamase inhibitors. Diagn Microbiol Infect Dis 98:115149. doi: 10.1016/j.diagmicrobio.2020.115149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies TJ, Stoesser N, Sheppard AE, Abuoun M, Fowler P, Swann J, Quan TP, Griffiths D, Vaughan A, Morgan M, Phan HTT, Jeffery KJ, Andersson M, Ellington MJ, Ekelund O, Woodford N, Mathers AJ, Bonomo RA, Crook DW, Peto TEA, Anjum MF, Walker AS. 2020. Reconciling the potentially irreconcilable? genotypic and phenotypic amoxicillin-clavulanate resistance in Escherichia coli. Antimicrob Agents Chemother 64:e02026-19. doi: 10.1128/AAC.02026-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grigg C, Jackson KA, Barter D, Czaja CA, Johnston H, Lynfield R, Vagnone PS, Tourdot L, Spina N, Dumyati G, Cassidy PM, Pierce R, Henkle E, Prevots DR, Salfinger M, Winthrop KL, Toney NC, Magill SS. 2023. Antimicrobial use in US hospitals: comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin Infect Dis 72:1784–1792. doi: 10.1093/cid/ciad214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ControlCfD, Prevention . 2021. Outpatient antibiotic prescriptions—United States, 2021. CDC. Atlanta, GA, USA: [Google Scholar]
- 11. Thompson ND, Stone ND, Magill SS. 2021. Antimicrobial use in a cohort of US nursing homes, 2017. JAMA 326:676. doi: 10.1001/jama.2021.8975 [DOI] [PubMed] [Google Scholar]
- 12. Rajer F, Allander L, Karlsson PA, Sandegren L. 2022. Evolutionary trajectories toward high-level β-lactam/β-Lactamase inhibitor resistance in the presence of multiple β-lactamases. Antimicrob Agents Chemother 66:e0029022. doi: 10.1128/aac.00290-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodríguez-Villodres Á, Gil-Marqués ML, Álvarez-Marín R, Bonnin RA, Pachón-Ibáñez ME, Aguilar-Guisado M, Naas T, Aznar J, Pachón J, Lepe JA, Smani Y. 2019. Extended-spectrum resistance to beta-lactams/beta-lactamase inhibitors (ESRI) evolved from low-level resistant Escherichia Coli. J Antimicrob Chemother 75:77–85. doi: 10.1093/jac/dkz393 [DOI] [PubMed] [Google Scholar]
- 14. Hansen KH, Andreasen MR, Pedersen MS, Westh H, Jelsbak L, Schønning K. 2019. Resistance to piperacillin/tazobactam in Escherichia coli resulting from extensive IS26-associated gene amplification of blaTEM-1 . J Antimicrob Chemother 74:3179–3183. doi: 10.1093/jac/dkz349 [DOI] [PubMed] [Google Scholar]
- 15. Hubbard ATM, Mason J, Roberts P, Parry CM, Corless C, van Aartsen J, Howard A, Bulgasim I, Fraser AJ, Adams ER, Roberts AP, Edwards T. 2020. Piperacillin/tazobactam resistance in a clinical isolate of Escherichia coli due to IS26-mediated amplification of blaTEM-1B . Nat Commun 11:4915. doi: 10.1038/s41467-020-18668-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards T, Heinz E, van Aartsen J, Howard A, Roberts P, Corless C, Fraser AJ, Williams CT, Bulgasim I, Cuevas LE, Parry CM, Roberts AP, Adams ER, Mason J, Hubbard ATM. 2022. Piperacillin/tazobactam-resistant, cephalosporin-susceptible Escherichia coli bloodstream infections are driven by multiple acquisition of resistance across diverse sequence types. Microb Genom 8:000789. doi: 10.1099/mgen.0.000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shropshire WC, Aitken SL, Pifer R, Kim J, Bhatti MM, Li X, Kalia A, Galloway-Peña J, Sahasrabhojane P, Arias CA, Greenberg DE, Hanson BM, Shelburne SA. 2021. IS26-mediated amplification of blaOXA-1 and blaCTX-M-15 with concurrent outer membrane porin disruption associated with de novo carbapenem resistance in a recurrent bacteraemia cohort. J Antimicrob Chemother 76:385–395. doi: 10.1093/jac/dkaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schechter LM, Creely DP, Garner CD, Shortridge D, Nguyen H, Chen L, Hanson BM, Sodergren E, Weinstock GM, Dunne WM, van Belkum A, Leopold SR. 2018. Extensive gene amplification as a mechanism for piperacillin-tazobactam resistance in Escherichia coli. mBio 9:e00583-18. doi: 10.1128/mBio.00583-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Livermore DM, Day M, Cleary P, Hopkins KL, Toleman MA, Wareham DW, Wiuff C, Doumith M, Woodford N. 2019. OXA-1 beta-lactamase and non-susceptibility to penicillin/beta-lactamase inhibitor combinations among ESBL-producing Escherichia coli. J Antimicrob Chemother 74:326–333. doi: 10.1093/jac/dky453 [DOI] [PubMed] [Google Scholar]
- 20. Noguchi T, Matsumura Y, Kanahashi T, Tanaka M, Tsuchido Y, Matsumura T, Nakano S, Yamamoto M, Nagao M, Ichiyama S. 2019. Role of TEM-1 β-lactamase in the predominance of ampicillin-sulbactam-nonsusceptible Escherichia coli in Japan. Antimicrob Agents Chemother 63:e02366-18. doi: 10.1128/AAC.02366-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waltner-Toews RI, Paterson DL, Qureshi ZA, Sidjabat HE, Adams-Haduch JM, Shutt KA, Jones M, Tian G-B, Pasculle AW, Doi Y. 2011. Clinical characteristics of bloodstream infections due to ampicillin-sulbactam-resistant, non-extended- spectrum-beta-lactamase-producing Escherichia coli and the role of TEM-1 hyperproduction. Antimicrob Agents Chemother 55:495–501. doi: 10.1128/AAC.00797-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bush K, Bradford PA. 2016. β-lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdelraouf K, Chavda KD, Satlin MJ, Jenkins SG, Kreiswirth BN, Nicolau DP. 2020. Piperacillin-tazobactam-resistant/third-generation cephalosporin-susceptible Escherichia coli and Klebsiella pneumoniae isolates: resistance mechanisms and in vitro-in vivo discordance. Int J Antimicrob Agents 55:105885. doi: 10.1016/j.ijantimicag.2020.105885 [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez-Villodres Á, Gutiérrez Linares A, Gálvez-Benitez L, Pachón J, Lepe JA, Smani Y. 2021. Semirapid detection of piperacillin/tazobactam resistance and extended-spectrum resistance to β-lactams/β-lactamase inhibitors in clinical isolates of Escherichia coli. Microbiol Spectr 9:e0080121. doi: 10.1128/Spectrum.00801-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lartigue MF, Leflon-Guibout V, Poirel L, Nordmann P, Nicolas-Chanoine M-H. 2002. Promoters P3, Pa/Pb, P4, and P5 upstream from bla TEM genes and their relationship to β-lactam resistance. Antimicrob Agents Chemother 46:4035–4037. doi: 10.1128/AAC.46.12.4035-4037.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou K, Tao Y, Han L, Ni Y, Sun J. 2019. Piperacillin-tazobactam (TZP) resistance in Escherichia coli due to hyperproduction of TEM-1 β-lactamase mediated by the promoter Pa/Pb. Front Microbiol 10:833. doi: 10.3389/fmicb.2019.00833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peter-Getzlaff S, Polsfuss S, Poledica M, Hombach M, Giger J, Böttger EC, Zbinden R, Bloemberg GV. 2011. Detection of AmpC beta-lactamase in Escherichia coli: comparison of three phenotypic confirmation assays and genetic analysis. J Clin Microbiol 49:2924–2932. doi: 10.1128/JCM.00091-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Oh CE, Choi EH, Lee HJ. 2013. The impact of the increased use of piperacillin/tazobactam on the selection of antibiotic resistance among invasive Escherichia coli and Klebsiella pneumoniae isolates. Int J Infect Dis 17:e638–43. doi: 10.1016/j.ijid.2013.01.030 [DOI] [PubMed] [Google Scholar]
- 29. Beceiro A, Maharjan S, Gaulton T, Doumith M, Soares NC, Dhanji H, Warner M, Doyle M, Hickey M, Downie G, Bou G, Livermore DM, Woodford N. 2011. False extended-spectrum beta-lactamase phenotype in clinical isolates of Escherichia coli associated with increased expression of OXA-1 or TEM-1 penicillinases and loss of porins. J Antimicrob Chemother 66:2006–2010. doi: 10.1093/jac/dkr265 [DOI] [PubMed] [Google Scholar]
- 30. Salverda MLM, De Visser JAGM, Barlow M. 2010. Natural evolution of TEM-1 beta-lactamase: experimental reconstruction and clinical relevance. FEMS Microbiol Rev 34:1015–1036. doi: 10.1111/j.1574-6976.2010.00222.x [DOI] [PubMed] [Google Scholar]
- 31. Rosenkilde CEH, Munck C, Porse A, Linkevicius M, Andersson DI, Sommer MOA. 2019. Collateral sensitivity constrains resistance evolution of the CTX-M-15 β-lactamase. Nat Commun 10:618. doi: 10.1038/s41467-019-08529-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shropshire WC, Konovalova A, McDaneld P, Gohel M, Strope B, Sahasrabhojane P, Tran CN, Greenberg D, Kim J, Zhan X, Aitken S, Bhatti M, Savidge TC, Treangen TJ, Hanson BM, Arias CA, Shelburne SA. 2022. Systematic analysis of mobile genetic elements mediating Β-lactamase gene amplification in noncarbapenemase-producing carbapenem-resistant Enterobacterales bloodstream infections. mSystems 7:e0047622. doi: 10.1128/msystems.00476-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tonkin-Hill G, Lees JA, Bentley SD, Frost SDW, Corander J. 2018. RhierBAPS: an R implementation of the population clustering algorithm hierBAPS. Wellcome Open Res 3:93. doi: 10.12688/wellcomeopenres.14694.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clermont O, Christenson JK, Denamur E, Gordon DM. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5:58–65. doi: 10.1111/1758-2229.12019 [DOI] [PubMed] [Google Scholar]
- 35. Oteo J, González-López JJ, Ortega A, Quintero-Zárate JN, Bou G, Cercenado E, Conejo MC, Martínez-Martínez L, Navarro F, Oliver A, Bartolomé RM, Campos J, Spanish Network for Research in Infectious Diseases (REIPI) . 2014. Inhibitor-resistant TEM- and OXA-1-producing Escherichia coli isolates resistant to amoxicillin-clavulanate are more clonal and possess lower virulence gene content than susceptible clinical isolates. Antimicrob Agents Chemother 58:3874–3881. doi: 10.1128/AAC.02738-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yee R, Simner PJ. 2022. Next-generation sequencing approaches to predicting antimicrobial susceptibility testing results. Clin Lab Med 42:557–572. doi: 10.1016/j.cll.2022.09.011 [DOI] [PubMed] [Google Scholar]
- 37. Vanstokstraeten R, Piérard D, Crombé F, De Geyter D, Wybo I, Muyldermans A, Seyler L, Caljon B, Janssen T, Demuyser T. 2023. Genotypic resistance determined by whole genome sequencing versus phenotypic resistance in 234 Escherichia coli isolates. Sci Rep 13:449. doi: 10.1038/s41598-023-27723-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) . 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. doi: 10.1001/jama.2018.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ren Y, Chakraborty T, Doijad S, Falgenhauer L, Falgenhauer J, Goesmann A, Hauschild A-C, Schwengers O, Heider D, Birol I. 2022. Prediction of antimicrobial resistance based on whole-genome sequencing and machine learning. Bioinformatics 38:325–334. doi: 10.1093/bioinformatics/btab681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim JI, Maguire F, Tsang KK, Gouliouris T, Peacock SJ, McAllister TA, McArthur AG, Beiko RG. 2022. Machine learning for antimicrobial resistance prediction: current practice, limitations, and clinical perspective. Clin Microbiol Rev 35:e0017921. doi: 10.1128/cmr.00179-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Avershina E, Sharma P, Taxt AM, Singh H, Frye SA, Paul K, Kapil A, Naseer U, Kaur P, Ahmad R. 2021. AMR-diag: neural network based genotype-to-phenotype prediction of resistance towards beta-lactams in Escherichia coli and Klebsiella pneumoniae Comput Struct Biotechnol J 19:1896–1906. doi: 10.1016/j.csbj.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim J, Greenberg DE, Pifer R, Jiang S, Xiao G, Shelburne SA, Koh A, Xie Y, Zhan X, Langille M. 2020. VAMPr: VAriant mapping and prediction of antibiotic resistance via explainable features and machine learning. PLoS Comput Biol 16:e1007511. doi: 10.1371/journal.pcbi.1007511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosas NC, Lithgow T. 2022. Targeting bacterial outer-membrane remodelling to impact antimicrobial drug resistance. Trends Microbiol 30:544–552. doi: 10.1016/j.tim.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 44. Murtha AN, Kazi MI, Schargel RD, Cross T, Fihn C, Cattoir V, Carlson EE, Boll JM, Dörr T. 2022. High-level carbapenem tolerance requires antibiotic-induced outer membrane modifications. PLoS Pathog 18:e1010307. doi: 10.1371/journal.ppat.1010307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Band VI, Crispell EK, Napier BA, Herrera CM, Tharp GK, Vavikolanu K, Pohl J, Read TD, Bosinger SE, Trent MS, Burd EM, Weiss DS. 2016. Antibiotic failure mediated by a resistant subpopulation in enterobacter cloacae. Nat Microbiol 1:16053. doi: 10.1038/nmicrobiol.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Band VI, Hufnagel DA, Jaggavarapu S, Sherman EX, Wozniak JE, Satola SW, Farley MM, Jacob JT, Burd EM, Weiss DS. 2019. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nat Microbiol 4:1627–1635. doi: 10.1038/s41564-019-0480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Band VI, Weiss DS. 2019. Heteroresistance: a cause of unexplained antibiotic treatment failure? PLoS Pathog 15:e1007726. doi: 10.1371/journal.ppat.1007726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34 [DOI] [PubMed] [Google Scholar]
- 49. CLSI . 2023. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 50. Clausen P, Aarestrup FM, Lund O. 2018. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinformatics 19:307. doi: 10.1186/s12859-018-2336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clausen P, Zankari E, Aarestrup FM, Lund O. 2016. Benchmarking of methods for identification of antimicrobial resistance genes in bacterial whole genome data. J Antimicrob Chemother 71:2484–2488. doi: 10.1093/jac/dkw184 [DOI] [PubMed] [Google Scholar]
- 52. Florensa AF, Kaas RS, Clausen P, Aytan-Aktug D, Aarestrup FM. 2022. ResFinder - an open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb Genom 8:000748. doi: 10.1099/mgen.0.000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF, Fagelhauer L, Chakraborty T, Neumann B, Werner G, Bender JK, Stingl K, Nguyen M, Coppens J, Xavier BB, Malhotra-Kumar S, Westh H, Pinholt M, Anjum MF, Duggett NA, Kempf I, Nykäsenoja S, Olkkola S, Wieczorek K, Amaro A, Clemente L, Mossong J, Losch S, Ragimbeau C, Lund O, Aarestrup FM. 2020. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother 75:3491–3500. doi: 10.1093/jac/dkaa345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Feldgarden M, Brover V, Gonzalez-Escalona N, Frye JG, Haendiges J, Haft DH, Hoffmann M, Pettengill JB, Prasad AB, Tillman GE, Tyson GH, Klimke W. 2021. AMRFinderPlus and the reference gene catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci Rep 11:12728. doi: 10.1038/s41598-021-91456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 57. Tonkin-Hill G, MacAlasdair N, Ruis C, Weimann A, Horesh G, Lees JA, Gladstone RA, Lo S, Beaudoin C, Floto RA, Frost SDW, Corander J, Bentley SD, Parkhill J. 2020. Producing polished prokaryotic pangenomes with the panaroo pipeline. Genome Biol 21:180. doi: 10.1186/s13059-020-02090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. doi: 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. doi: 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu G, Smith DK, Zhu H, Guan Y, Lam TT, McInerny G. 2017. Ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36. doi: 10.1111/2041-210X.12628 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2.
Tables S1 to S3.
Data Availability Statement
Whole-genome sequencing short-read data have been submitted to BioProject PRJNA924946 and PRJNA836696. R scripts for analyses can be made available upon request from the corresponding author.