ABSTRACT
We characterized carbapenemase-producing organism (CPO) detected in municipal wastewater to better understand the epidemiology of CPOs in the community. In total, 36 samples were collected at six sampling sites every other month from December 2020 to October 2021. CPOs were not recovered from influent taken from inlet A receiving separated sewer line, treated effluents, and river water samples upstream and downstream of the effluent outlet. By contrast, 75 CPOs were detected in all influent samples taken from inlets B and C receiving combined sewer lines collecting both domestic/industrial wastewater and rainwater runoff. Aeromonas caviae was the dominant species (25/75, 33.3%), and the other 11 Aeromonas spp. together accounted for 48% of CPOs. The remaining 39 Enterobacterales strains mainly comprised 17 Klebsiella spp. and 10 Raoultella spp. CPOs carrying bla GES carbapenemase genes were overwhelmingly dominant, accounting for 72 of 75 isolates, including two isolates harboring both bla GES-24 and bla IMP-1 (96%), followed by three bla IMPs-positive isolates, where those carbapenemase genes were mainly carried in diverse class 1 integrons. Among bla GES variants, including six new variants (bla GES-47, bla GES-48, bla GES-49, bla GES-50, bla GES-51, and bla GES-54), bla GES-5 was detected in 28 CPOs, with Aeromonas spp. accounting for 53.6% of these organisms. Quantitative analysis revealed that the repeated detection of bla GES-48-positive A. caviae ST1056 from both inlets B and C ranked the total number of this bacterial clone highest in the wastewater influent. In summary, our study revealed the high prevalence and persistence of diverse bla GES carbapenemase genes among CPOs isolated from influent inlets connected to combined sewer systems.
IMPORTANCE
The emergence and spread of carbapenemase-producing organisms (CPOs) represent a global health threat because they are associated with limited treatment options and poor clinical outcomes. Wastewater is considered a hotspot for the evolution and dissemination of antimicrobial resistance. Thus, analyses of municipal wastewater are critical for understanding the circulation of these CPOs and carbapenemase genes in local communities, which remains scarcely known in Japan. This study resulted in several key observations: (i) the vast majority of bla GES genes, including six new bla GES variants, and less frequent bla IMP genes were carbapenemase genes encountered exclusively in wastewater influent; (ii) the most dominant CPO species were Aeromonas spp., in which a remarkable diversity of new sequence types was observed; and (iii) CPOs were detected from combined sewer wastewater, but not from separate sewer wastewater, suggesting that the load of CPOs from unrecognized environmental sources could greatly contribute to their detection in influent wastewater.
KEYWORDS: carbapenemase, wastewater, bla GESs , bla IMPs , Aeromonas spp., Enterobacterales, class 1 integron
INTRODUCTION
Antimicrobial resistance (AMR) represents a major global problem linked to increasing concerns across human, animal, and environmental settings. Carbapenems remain the last-line antimicrobials for treating multidrug-resistant microorganism infections. Thus, the emergence and spread of carbapenemase-producing organisms (CPOs) represent a global health threat because they are associated with limited treatment options and poor clinical outcomes. Clinically important carbapenemases belonging to different Ambler classes include class A carbapenemase Klebsiella pneumoniae carbapenemase (KPC) type, class B metallo-β-lactamases IMP-type metallo-β-lactamase (IMP) type, Verona integron-encoded metallo-β-lactamase (VIM) type, and New Delhi metallo-β-lactamase (NDM) type, and class D carbapenemase OXA-48 like. Among the class A β-lactamases of the Guiana extended spectrum β-lactamase (GES) type, those with an amino acid substitution of Gly170Ser within the Ω-loop region, such as GES-24 and GES-5, exhibit carbapenem-hydrolyzing activities. The relatively low carbapenem minimum inhibitory concentrations (MICs) of some isolates of GES carbapenemase producers in combination with the lack of available selective inhibitors specific for GES carbapenemases may make it difficult to detect these enzymes, leading to an underestimation of the true prevalence of GES carbapenemase-producing isolates. These carbapenemase genes are often located in mobile genetic elements (MGEs) such as plasmids, transposons, and integrons, thereby facilitating their rapid spread among Enterobacterales and other Gram-negative bacteria (1). The acquisition of MGEs by high-risk bacterial clones with adaptive traits in humans and environments with accumulating virulence and resistance genes plays critical roles in the successful dissemination of carbapenemase genes (2). The geographic distribution of CPOs is variable. Generally, the high endemicity of certain carbapenemases is associated with specific regions or countries, such as the KPC type in the USA, Israel, Greece, and Italy; NDM type in the Indian subcontinent; OXA-48 like in Turkey, the Middle East, and North Africa; and IMP type in East Asian countries including Japan (3, 4). Moreover, several factors, such as international travel/migration, repatriation of patients, food import, and wildlife migration from areas of high endemicity, can accelerate the extensive spread of CPOs to neighboring regions, surrounding countries, and other continents (5 – 8), leading to constant changes in local and global epidemiology.
In Japan, the prevalence of carbapenemase-producing Enterobacterales among carbapenem-resistant Enterobacterales (CRE) clinical isolates remained flat at 16.5%–17.6% in 2018–2020 [Pathogen surveillance of carbapenem-resistant Enterobacteriaceae, 2020 (In Japanese). Infect. Agents Sruveillance Rep. 2022, 43, 215–216, available online at https://www.niid.go.jp/niid/ja/cre-m/cre-iasrd/11520-511d01.html]. Japan has been characterized as endemic for the IMP type (approximately 85% of isolates), whereas the NDM type, KPC type, and OXA-48 like are sporadically detected. Among IMP-type enzymes, IMP-1 is the most prevalent, whereas IMP-6 producers, which originally exhibited a regional-specific distribution while causing large-scale outbreaks, have expanded their areas of distribution across Japan (9, 10). With increasing concerns about CPO spread, regional surveillance is essential to understand their epidemiology.
Analyses of wastewater containing bacteria mainly of human origin could be helpful for better understanding the community prevalence of antimicrobial-resistant bacteria, AMR genes, and associated MGEs (11). Recent studies revealed the presence of carbapenemase-encoding genes, such as bla KPC, bla NDM, bla IMP, and bla OXA-48 in wastewater influent in different countries, and the concordance of their prevalence in wastewater and human isolates was generally demonstrated (12 – 14). Meanwhile, the predominance of bla GES carbapenemase genes mainly harbored by Aeromonas, Enterobacter, and Kluyvera spp. in wastewater influent has been reported in Finland, where bla GES has rarely been detected in clinical isolates (15). GES carbapenemases are relatively rare among clinical isolates, whereas they are increasingly detected in aquatic environments (16). Nonetheless, outbreaks of GES-5-producing Pseudomonas aeruginosa and Serratia marcescens have been reported in Japan (17, 18). In a very recent study, we found that bla GES-24 and bla GES-5, which are mainly carried by Klebsiella and Enterobacter spp., are the only carbapenemase genes detected in wastewater effluent from a hospital with no history of detection of clinical GES carbapenemase-producing isolates (19). Notably, these bla GES genes were mostly embedded in new class 3 integrons, although they are generally associated with class 1 integrons in clinically relevant Enterobacterales (19, 20). These findings prompted us to investigate the epidemiology of CPOs in local communities.
In this study, quantitative monitoring of CPOs in influent from a municipal wastewater treatment plant (WWTP) was conducted to investigate their presence, distribution, and dynamics. This WWTP receives sewer water in a combined sewer system that primarily collects domestic/industrial wastewater mixed with urban runoff rainwater, as well as in the separated sewer system that carries only domestic/industrial wastewater via different inlets. Therefore, the influent of these inlets was analyzed to assess the environmental impact of the CPO load from the combined sewer system. Additionally, wastewater effluent and river water samples (upstream and downstream of the effluent outlet) were also included in the analysis to better understand whether the effluent is a potential source of CPO release into the environment.
RESULTS
Detection of CPOs in the influent and effluent of a WWTP and river water samples
In total, 36 samples were collected at six sampling sites once every 2 months between December 2020 and October 2021. CPOs were detected in all six raw influent samples taken from inlets B (pH, 7.3 ± 0.1; temperature, 18.3 ± 3.0) and C (pH, 7.3 ± 0.1; temperature, 19.5 ± 3.7) receiving combined sewer lines. No CPOs were recovered from the raw influent of inlet A (pH, 7.4 ± 0.1; temperature, 18.5 ± 3.4) receiving separated sewer lines, treated effluent (pH, 7.0 ± 0.2; temperature, 19.5 ± 3.6) from the WWTP outlet, and river water samples (upstream: pH, 7.0 ± 0.8; temperature, 13.3 ± 3.5; downstream: pH, 6.9 ± 0.7; temperature, 13.8 ± 3.7; Table S1).
A total of 75 CPOs were selected as representative strains of bacterial populations, estimated as colony-forming units (CFU) per milliliter, sharing similar colony morphologies, the same bacterial species, and the same carbapenemase genes. Table 1 shows bacterial species based on average nucleotide identity based on MUMmer (ANIm) analyses, carbapenemase genes, sequence types (STs), and strain numbers for 75 CPOs identified in the raw influent of inlets B (28 isolates) and C (47 isolates). Aeromonas caviae was the dominant species (25/75, 33.3%), and 11 other Aeromonas spp. (four Aeromonas veronii bv. veronii isolates, three Aeromonas hydrophila subsp. hydrophila isolates, two Aeromonas taiwanensis isolates, and one isolate each of Aeromonas dhakensis and Aeromonas allosaccharophila) collectively accounted for 48% of CPOs. Other isolates included seven isolates each of Klebsiella michiganensis and Raoultella ornithinolytica; five isolates each of Klebsiella pneumoniae subsp. pneumoniae and Klebsiella quasipneumoniae subsp. similipneumoniae; three isolates each of Citrobacter freundii and Raoultella planticola; two isolates each of Enterobacter cloacae subsp. cloacae, Enterobacter kobei, and Enterobacter roggenkampii; and one isolate each of Citrobacter braakii, Citrobacter portucalensis, and Kluyvera cryocrescens.
TABLE 1.
Seventy-five strains harboring carbapenemase genes identified in raw influents of inlets B and C a
| Sampling date (month/year) | Dec-20 | Feb-21 | Apr-21 | Jun-21 | Aug-21 | Oct-21 | Dec-20 | Feb-21 | Apr-21 | Jun-21 | Aug-21 | Oct-21 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inlet | B | C | |||||||||||||||
| Water temperature | 17°C | 13°C | 17°C | 20°C | 22°C | 21°C | 18°C | 13°C | 18°C | 21°C | 24°C | 23°C | |||||
| pH | 7.47 | 7.41 | 7.29 | 7.36 | 7.37 | 7.06 | 7.53 | 7.29 | 7.28 | 7.09 | 7.19 | 7.17 | |||||
| Bacterial species | Integron | Carbapenemases | Others | ST b | Klebsiella capsule locus (KL) type | Strain (single nucleotide polymorphisms [SNPs]) d | Strain (single nucleotide polymorphisms [SNPs]) d | ||||||||||
| A. allosaccharophila | 3 | GES-24 | TEM-1B | 1,180 c | 10CC9 | ||||||||||||
| A. caviae | 1 | IMP-10 | 1,061 c | 6BC7 | |||||||||||||
| 3 | GES-5 | 1,015 c | 12BC1 | 8BC4 | 10BC1 | ||||||||||||
| (365) | (19) | ||||||||||||||||
| 1 | GES-5 | 1,057 c | 4CC9 | ||||||||||||||
| 1 | GES-5 | MOX + VEB-1 | 1,059 c | 10BC8 | 2CC7 | 4CC12 | |||||||||||
| (31) | (19) | ||||||||||||||||
| 1 | GES-5 | MOX + VEB-1 | 1,176 c | 10CC10 | |||||||||||||
| 1 | GES-5 | MOX | 1,178 c | 6BC8 | 6CC6 | ||||||||||||
| (59) | |||||||||||||||||
| 3 | GES-6 | 1,182 c | 6CC8 | ||||||||||||||
| 1 | GES-24 | 1,199 c | 6BC6 | ||||||||||||||
| 1 | GES-24 | 1,179 c | 10BC6 | ||||||||||||||
| 1 | GES-24 + IMP-1 | 1,062 c | 6CC5 | ||||||||||||||
| 1 | GES-48 a | 1,056 c | 2BC3 | 4BC2 | 6BC4 | 8BC3 | 10BC4 | 2CC8 | 4CC5 | 6CC12 | 10CC8 | ||||||
| (274) | (9) | (63) | (211) | (63) | (60) | (9) | (8) | ||||||||||
| 1 | GES-48 a | 1,182 c | 8CC4 | ||||||||||||||
| A. dhakensis | 1 | GES-24 | 518 | 10CC4 | |||||||||||||
| A. hydrophila subsp. hydrophila | 1 | GES-5 | 1,181 c | 6CC13 | |||||||||||||
| 1 | GES-24 | 1,177 c | 8CC6 | ||||||||||||||
| 1 | GES-24 + IMP-1 | 860 c | 8CC5 | ||||||||||||||
| A. taiwanensis | 3 | GES-5 | 1,055 c | 2BC2 | 4BC3 | ||||||||||||
| (835) | |||||||||||||||||
| A. veronii bv. veronii | 1 | GES-5 | 1,054 c | 12BC2 | 2BC1 | ||||||||||||
| (30) | |||||||||||||||||
| 1 | GES-24 | 1,058 c | 2CC6 | ||||||||||||||
| 1 | GES-49 a | 1,060 c | 6BC9 | ||||||||||||||
| C. braakii | 1 | GES-5 | 110 | 8BC2 | |||||||||||||
| C. portucalensis | 1 | GES-51 a | 166 | 10CC7 | |||||||||||||
| C. freundii | 1 | GES-24 | CTX-M-3 + TEM-1B | 22 | 4CC11 | ||||||||||||
| 1 | IMP-1 | CTX-M-3 + TEM-1B | 116 | 12CC1 | 2CC2 | ||||||||||||
| (12) | |||||||||||||||||
| E. cloacae subsp. cloacae | 1 | GES-4 | 1,821 c | 8BC1 | 10BC2 | ||||||||||||
| (19) | |||||||||||||||||
| E. kobei | 1 | GES-4 | 1,822 c | 6CC1 | |||||||||||||
| 1 | GES-54 a | 520 | 6BC5 | ||||||||||||||
| E. roggenkampii | 1 | GES-4 | 1,059 | 4CC4 | |||||||||||||
| 1 | GES-24 | MCR-9 | 433 | 6BC1 | |||||||||||||
| K. cryocrescens | 1 | GES-6 | −— | 6BC3 | |||||||||||||
| K. michiganensis | 1 | GES-4 | 95 | 12CC2 | 4CC2 | 6CC2 | 8CC1 | ||||||||||
| (15) | (13) | (24) | |||||||||||||||
| 1 | GES-47 a | 135 | 2CC1 | ||||||||||||||
| 1 | GES-50 a | 210 | 2BC4 | 10BC5 | |||||||||||||
| (65) | |||||||||||||||||
| K. pneumoniae subsp. pneumoniae | 1 | GES-5 | 2,791 | KL48 | 6BC2 | 4CC3 | 6CC4 | 10CC2 | |||||||||
| (100) | (138) | (74) | |||||||||||||||
| 1 | GES-48 a | 76 | KL35 | 10CC1 | |||||||||||||
| K. quasipneumoniae subsp. similipneumoniae | 1 | GES-4 | 6,101 c | KL159 | 6CC9 | ||||||||||||
| 1 | GES-5 | 1,822 | KL48 | 4CC10 | 6CC7 | 10CC6 | |||||||||||
| (30) | (59) | ||||||||||||||||
| 1 | GES-48 a | 6,102 c | KL140 | 8CC2 | |||||||||||||
| R. planticola | 1 | GES-5 | – | 12CC3 | 2CC3 | 4CC1 | |||||||||||
| (115) | (116) | ||||||||||||||||
| R. ornithinolytica | 1 | GES-4 | – | 4CC7 | 6CC3 | 8CC3 | 10CC5 | ||||||||||
| (111) | (7) | (30) | |||||||||||||||
| 1 | GES-5 | – | 2CC4 | 6CC10 | |||||||||||||
| (66) | |||||||||||||||||
| 1 | GES-24 | – | 4BC1 | ||||||||||||||
GES variants newly identified in this study.
ST, sequence type.
ST newly assigned in this study.
SNPs in comparison to the genome of the first strain (in parentheses).
Some isolates of A. taiwanensis, A. dhakensis, A. allosaccharophila, E. roggenkampii, E. kobei, K. michiganensis, K. quasipneumoniae subsp. similipneumoniae, and R. planticola were initially identified as A. caviae, A. hydrophila, A. veronii, Enterobacter asburiae, Enterobacter bugandensis, Klebsiella oxytoca, K. pneumoniae, and R. ornithinolytica, respectively, by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS; data not shown).
bla GES carbapenemase genes were predominant among the 75 CPOs
The predominance of bla GESs genes (72/75 isolates, 96%) was observed across all 18 bacterial species identified in this study (Table 1). bla GES-5 was detected in 28 of 75 (37.3%) isolates, and a high frequency (53.6%) of bla GES-5-positive Aeromonas spp. was noted. In addition, a new variant bla GES-48 (NG_074709.1) and bla GES-24 were primarily detected in Aeromonas spp., corresponding to 10 of 12 (83.3%) bla GES-48-positive isolates and 8 of 11 (72.7%) bla GES-24-positive isolates, including two bla GES-24- and bla IMP-1-positive isolates. Meanwhile, bla GES-4, the second most commonly encountered carbapenemase gene (13/75, 17.3%), was harbored by bacterial species belonging to genera other than Aeromonas.
In addition to bla GES-48, five other variants, namely bla GES-47, bla GES-49, bla GES-50, bla GES-51, and bla GES-54 (NG_074708.1, NG_076643.1, NG_077975.1, NG_077976.1, and NG_081003.1, respectively), were newly identified in CPOs. The phylogenetic tree constructed from the amino acid sequences of 56 GES variants (Beta-Lactamase Database, available online at http://bldb.eu/BLDB.php?prot=A#GES, accessed on 10 December 2022) classified them broadly into three major clades (Fig. S1). The 32 GES variants mostly sharing a Gly170Ser substitution formed the largest clade, mainly comprising three subclades: one consisting of six GES variants including GES-4, GES-24, and four newly identified variants (GES-47, GES-48, GES-50, and GES-54), one consisting of 15 GES variants including GES-5 and one newly identified variant (GES-51), and one consisting of seven GES variants including GES-6 and one newly identified variant (GES-49). Compared to the GES-4 sequence, GES-47, GES-48, GES-50, and GES-54 harbored Lys104Glu/His116Tyr, Ala21Ser/Lys104Glu, Ala6Thr/Lys104Glu, and Thr109Met mutations, respectively, according to the Ambler numbering scheme (21). GES-51 and GES-49 harbored Ala6Val from GES-5 and GES-6, respectively. Twenty-three new STs were identified among the CPOs, including 19 STs for Aeromonas spp. and 2 STs each for Enterobacter spp. and K. quasipneumoniae subsp. similipneumoniae.
As shown in Table 1, the same species belonging to the same STs and harboring the same carbapenemase genes were repeatedly isolated; specifically, A. caviae ST1056 carrying bla GES-48 (8−274 SNP differences), A. caviae ST1059 carrying bla GES-5 (19 and 31 SNP differences), and K. pneumoniae subsp. pneumoniae ST2791 carrying bla GES-5 (74−138 SNP differences) were detected in nine, three, and four samples, respectively, across inlets B and C. bla GES-5-positive A. caviae ST1015 (19 and 365 SNP differences), A. taiwanensis ST1055 (835 SNP difference), and A. veronii bv. veronii ST1054 (30 SNP difference) and bla GES-4-positive E. cloacae subsp. cloacae ST1821 (19 SNP difference) were detected in three, two, two, and two samples, respectively, only from inlet B, whereas bla GES-5-positive K. quasipneumoniae subsp. similipneumoniae ST1822 (30 and 59 SNP differences), R. planticola (115 and 116 SNP differences), and R. ornithinolytica (66 SNP difference), bla GES-4-positive K. michiganensis ST95 (13–24 SNP differences) and R. ornithinolytica (7–111 SNP differences), and bla IMP-1-positive C. freundii ST116 (12 SNP difference) were detected in three, three, two, four, four, and two samples, respectively, only from inlet C.
Phylogenetic relationships among 75 CPOs and their AMR-associated gene profiles
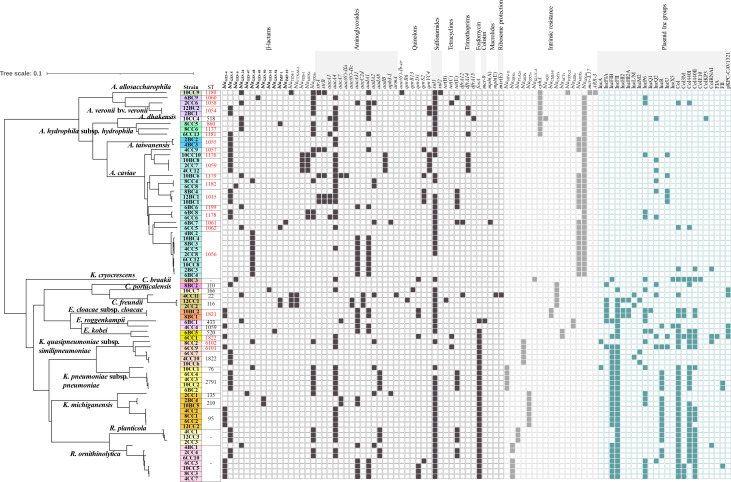
As presented in Fig. 1, whole-genome clustering via Population Partitioning Using Nucleotide K-mers (PopPUNK) analyses separated the genomes of 75 CPOs into distinct clades that were congruent with their bacterial species and STs. bla CTX-M-3 was harbored by one ST22 strain (4CC11) and two ST116 strains (12CC1 and 2CC2) of C. freundii. Three strains of A. caviae ST1059 (10BC8, 2CC7, and 4CC12) and one strain of A. caviae ST1176 (10CC10) harbored bla VEB-1 and bla MOX, the latter of which was also harbored by two strains of A. caviae ST1178 (6BC8 and 6CC6). One E. roggenkampii ST433 strain (6BC1) carried mcr-9. aacA4 and sul1 were found in 42 (56%) and 57 (76%) isolates, respectively. fosA was harbored by 30 of 39 (76.9%) Enterobactelares isolates. cphA encoding metallo-β-lactamase, which is intrinsic to Aeromonas spp., was detected in A. hydrophila subsp. hydrophila, A. veronii bv. veronii, A. dhakensis, and A. allosaccharophila, but it was not detected in A. caviae and A. taiwanensis.
Fig 1.
Phylogenetic relationship of 75 CPOs. Core genome neighbour-joining tree (left) was generated by PopPUNK. Strain numbers and their STs are indicated at the right of the tree, with new STs marked in red. The presence (filled squares) or absence (open squares) of antimicrobial resistance-associated genes and plasmid Inc groups among strains is shown.
Antimicrobial susceptibility of 75 CPOs
The MICs of antimicrobials against 75 CPOs are shown in Table S2. Among the 36 Aeromonas stains, 31 GES carbapenemase-producing strains were susceptible to imipenem (MIC ≤ 0.25–4 µg/mL) and meropenem (MIC ≤ 0.12–4 µg/mL). The remaining five strains were resistant to imipenem (A. hydrophila subsp. hydrophila strains 6CC13 and 8CC6 producing GES-5 and GES-24, respectively) and/or meropenem (A. hydrophila subsp. hydrophila strain 8CC5 producing GES-24 and IMP-1, 8CC6, A. caviae 6CC5 producing GES-24 and IMP-1, and A. caviae 6BC7 producing IMP-10). Among the 39 Enterobacterales strains, the MICs of imipenem and meropenem varied between ≤0.25 and >8 µg/mL and between ≤0.12 and >8 µg/mL, respectively. Cephalosporin resistance was noted in three strains of C. freundii, one of which produced GES-24 and CTX-M-3 extended-spectrum β-lactamase (ESBL) and the other two produced IMP-1 and CTX-M-3 ESBL.
The transformants carrying the new bla GES variants, bla GES-47, bla GES-48, bla GES-49, bla GES-50, bla GES-51, and bla GES-54, exhibited slightly higher MICs for imipenem (0.5–2 µg/mL) and meropenem (0.25–1 µg/mL) than NEB10-beta electrocompetent Escherichia coli (Table 2). They also had high MICs for ampicillin (>16 µg/mL), piperacillin (MIC 32 to >64 µg/mL), cefazolin (>16 µg/mL), cefotiam (>4 µg/mL), ceftazidime (2 to >16 µg/mL), cefpodoxime (>4 µg/mL), cefmetazole (32 to >32 µg/mL), and flomoxef (8 to >16 µg/mL).
TABLE 2.
MICs of antimicrobials for carbapenemase-producing organisms harboring newly identified bla GES genes, and their transformants a
| Antimicrobial agents | MICs (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bla GES-47 | bla GES-48 | bla GES-49 | bla GES-50 | bla GES-51 | bla GES-54 | — | |||||||
|
K. michiganensis
2CC1 |
E. coli
NEB10-beta |
A. A. caviae
2CC8 |
E. coli
NEB10-beta |
A. A. veronii bv. veronii6BC9 |
E. coli
NEB10-beta |
K. michiganensis
10BC5 |
E. coli
NEB10-beta |
C. portucalensis 10CC7 |
E. coli
NEB10-beta |
E. kobei
6BC5 |
E. coli
NEB10-beta |
E. coli
NEB10-beta |
|
| Ampicillin | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | ≤2 |
| Piperacillin | >64 | >64 | 16 | >64 | >64 | >64 | 32 | 64 | 64 | 32 | 32 | 32 | ≤4 |
| SAM | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | >16–8 | ≤2–1 |
| TZP | >64–4 | >64–4 | 16-4 | 32–4 | >64–4 | 64–4 | 8-4 | 8-4 | 16-4 | 32–4 | 16-4 | 8-4 | ≤4–4 |
| Cefazolin | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | 1 |
| Cefotiam | >4 | >4 | >4 | >4 | >4 | >4 | 4 | >4 | >4 | >4 | >4 | >4 | ≤0.5 |
| Ceftazidime | >16 | 4 | 8 | 16 | >16 | >16 | 2 | 2 | 4 | 8 | 16 | >16 | ≤0.5 |
| Cefpodoxime | >4 | >4 | >4 | >4 | >4 | >4 | ≤1 | >4 | >4 | >4 | 4 | >4 | ≤1 |
| Ceftriaxone | 4 | ≤1 | 16 | 8 | 16 | 8 | ≤1 | ≤1 | 2 | 2 | 2 | 4 | ≤1 |
| Cefepime | 4 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 |
| Cefmetazole | >32 | 32 | >32 | >32 | >32 | >32 | 16 | 32 | >32 | 32 | >32 | 32 | ≤1 |
| Flomoxef | >16 | 8 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | 16 | 16 | ≤2 |
| Aztreonam | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 |
| Imipenem | 2 | 0.5 | ≤0.25 | 2 | 2 | 0.5 | 0.5 | 2 | 4 | 2 | ≤0.25 | 0.5 | ≤0.25 |
| Meropenem | 4 | 0.25 | 0.5 | 1 | 1 | 0.5 | 0.25 | 1 | 4 | 0.5 | 0.5 | 0.5 | ≤0.12 |
| Gentamicin | ≤2 | ≤2 | ≤2 | 4 | 8 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 |
| Amikacin | ≤8 | ≤8 | ≤8 | ≤8 | ≤8 | ≤8 | ≤8 | 32 | ≤8 | ≤8 | ≤8 | ≤8 | ≤8 |
| Minocycline | ≤2 | ≤2 | ≤2 | ≤2 | 4 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | 8 | ≤2 | ≤2 |
| Levofloxacin | 0.5 | ≤0.12 | 1 | ≤0.12 | 1 | ≤0.12 | 0.5 | ≤0.12 | 0.5 | ≤0.12 | 1 | ≤0.12 | ≤0.12 |
| Fosfomycin | ≤32 | ≤32 | 64 | ≤32 | ≤32 | ≤32 | ≤32 | ≤32 | ≤32 | ≤32 | 128 | ≤32 | ≤32 |
| SXT | ≤9.5–0.5 | ≤9.5–0.5 | 19-1 | ≤9.5–0.5 | >38/2 | ≤9.5–0.5 | ≤9.5–0.5 | ≤9.5–0.5 | ≤9.5–0.5 | ≤9.5–0.5 | 19-1 | ≤9.5–0.5 | ≤9.5–0.5 |
MIC, minimum inhibitory concentration; SAM, ampicillin-sulbactam; SXT, sulfamethoxazole-trimethoprim; TZP, piperacillin-tazobactam.
bla GES-48-positive A. caviae ST1056 occurred at the highest total densities in the influent
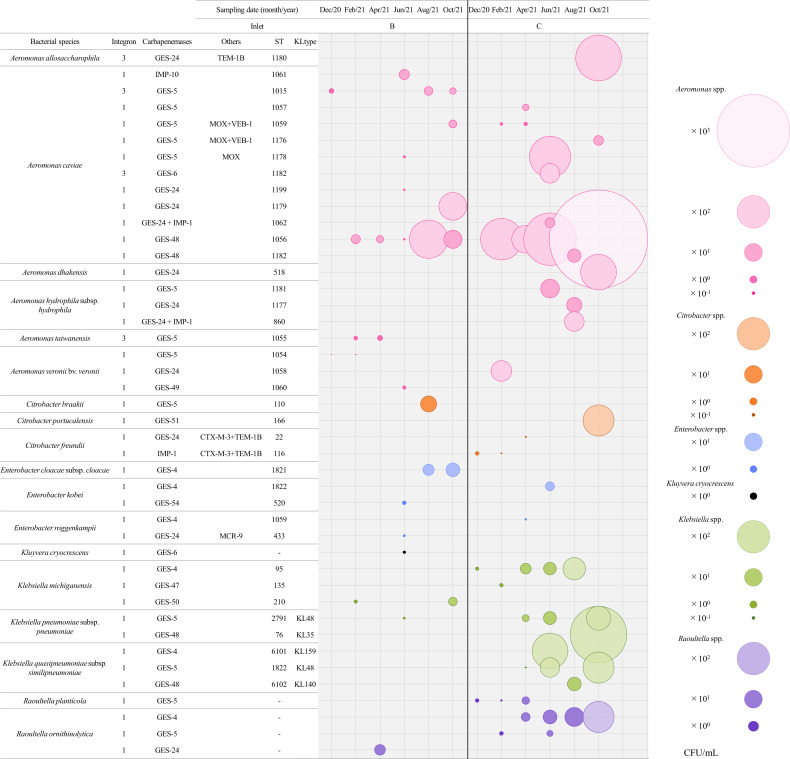
The abundance (CFU/mL) of CPOs measured bimonthly at inlets B and C is shown in Fig. 2. The bacterial counts of CPOs ranged from 1.0 × 10−1 to 3.8 × 102 CFU/mL and from 5.0 × 10−1 to 2.5 × 103 CFU/mL for the influent from inlets B and C, respectively, and significant differences in the overall CPO abundance were detected between samples from these two inlets (Wilcoxon signed-rank sum test, P = 0.00056). When examining seasonal differences in the abundance of CPOs, no significant differences were detected in samples at each inlet (Friedman test). On a total abundance level, A. caviae ST1056 lineage isolates harboring class 1 integron-associated bla GES-48, which were detected in almost every sampling month at both inlets, represented the most abundant clone (Fig. 2). The occurrence of class 1 integron-associated bla GES-48 was observed among A. caviae ST1182, K. pneumoniae subsp. pneumoniae ST76, and K. quasipneumoniae subsp. similipneumoniae ST6102 lineage isolates in the last two sampling months (August and October 2021). Our conjugation experiments between A. caviae ST1056 or A. caviae ST1182 as donor strains and K. pneumoniae subsp. pneumoniae ATCC13883 (Rifr) or E. coli χ1037 (Rifr) as the recipient strain failed to obtain any transconjugants harboring bla GES-48.
Fig 2.
Babble plots showing the abundance (CFU/mL) of CPOs detected in raw influents at inlets B and C. The CFU/mL values were obtained by quantifying organisms of the same bacterial species belonging to the same STs and harboring the same carbapenemase genes.
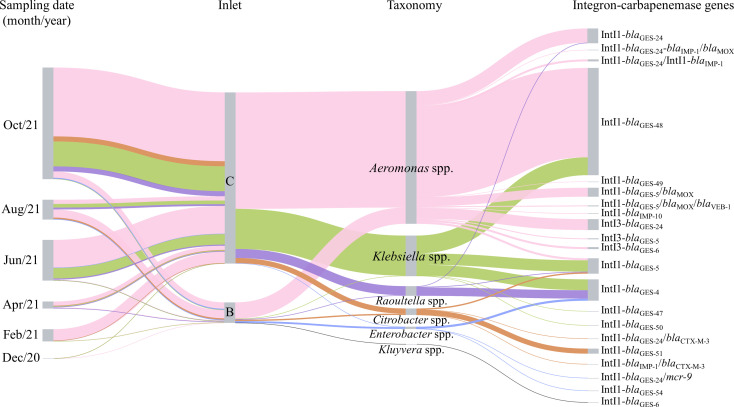
The distribution and linkages among sampling months, wastewater inlets, bacterial genera, and integrons and their associated carbapenemase genes as depicted by the Sankey diagram revealed that Aeromonas spp. carrying bla GES-5, bla GES-6, bla GES-24, bla GES-48, and bla GES-49 embedded in class 1 or 3 integrons were the most abundant CPOs in the municipal wastewater influent (Fig. 3), followed by Klebsiella spp. carrying bla GES-4, bla GES-5, bla GES-47, bla GES-48, and bla GES-50 embedded in class 1 integrons.
Fig 3.
Sankey diagram showing the distribution of the bacterial genera and carbapenemase and integrase genes detected in raw influents across the six sampling months and two inlets. The width of each node is proportional to the abundance (CFU/mL) of the CPOs.
Virulence and heavy metal resistance traits of Aeromonas spp
Whole-virulome analysis predicted the presence of virulence genes encoding adherence factors [lateral flagella, mannose-sensitive hemagglutinin (Msh) type IV pilus, polar flagella, type IV Tap pili, and type I fimbriae], secretion systems [type II secretion system (T2SS), type III secretion system, and type VI secretion system (T6SS)], and toxins (aerolysin AerA/cytotoxic enterotoxin Act, extracellular hemolysin AHH1, heat-stable cytotonic enterotoxin Ast, and hemolysin HlyA) among different lineage isolates of Aeromonas spp. (Fig. S2). The isolates shared Msh type IV pili, polar flagella, type IV Tap pili, T2SS, and hemolysin HlyA genes. Additionally, 6 of the 11 A. caviae clones (ST1056, ST1061, ST1062, ST1178, ST1179, and ST1199), all A. veronii bv. veronii clones (ST1054, ST1058, and ST1060), and an A. taiwanensis clone (ST1055) had lateral flagellar genes. The T6SS gene was discovered among 4 of the 11 A. caviae clones (ST1056, ST1061, ST1062, and ST1179), all A. hydrophila subsp. hydrophila clones (ST860, ST1177, and ST1181), and an A. dhakensis clone (ST518). The latter four clones also harbored AerA/Act and AHH1 toxin genes. Heavy metal resistance genes for copper (copA), arsenic (arsB), and zinc (zntAB) were found among Aeromonas spp. isolates (Fig. S2). In addition, mercuric resistance genes (merAEPRT) were harbored by all three clones of A. hydrophila subsp. hydrophila, five A. caviae clones, one of three clones of A. veronii bv. veronii, and one A. allosaccharophila clone.
Genetic context of bla GES and bla IMP genes
Exploration of the genetic environment surrounding the bla GES and bla IMP genes in 75 CPOs revealed that these genes were carried in different genetic locations (Table 3). In 23 of 28 bla GES-5-positive isolates, bla GES-5 was located in the first gene cassette position immediately downstream of the class 1 integron−integrase gene (intI1). Among them, 14 intI1−bla GES-5-positive isolates harbored class 1 integrons containing the qacEΔ1−sul1 region in the 3′-conserved segment (3′ CS). The class 1 integron with the structure intI1−bla GES-5−bla OXA-1042−qacL−3′ CS was shared by two strains each of A. caviae ST1178 (6BC8 and 6CC6) and A. veronii bv. veronii ST1054 (12BC2 and 2BC1), whereas intI1−bla GES-5−aacA4−catB10−bla OXA-4−aadA8−3′ CS was shared by four K. pneumoniae subsp. pneumoniae ST2791 strains (4CC3, 6BC2, 6CC4, and 10CC2), three R. planticola strains (12CC3, 2CC3, and 4CC1), and two R. ornithinolytica strains (2CC4 and 6CC10). Two E. cloacae subsp. cloacae ST1821 strains (8BC1 and 10BC2) and one E. roggenkampii ST1059 strain (4CC4) harbored the class 1 integron containing intI1–bla GES-4–aacA31–aadA1–3′ CS, which was extremely similar to intI1–bla GES-4–aacA31–aadA1–aadA1–33′ CS contained by four R. ornithinolytica strains (4CC7, 6CC3, 8CC3, and 10CC5). The bla GES-48 gene embedded in a class 1 integron with the structure intI1–bla GES-48–aacA31–aadA1–aadA1–33′ CS was shared by four A. caviae ST1056 strains (2CC8, 4CC5, 8BC3, and 10BC4), whereas one strain (4BC2) from the same lineage carried intI1–bla GES-48 –ISPa59–3′ CS. The genetic context of bla GES-48 with intI1–bla GES-48 –bla OXA-129– aadA2–3′ CS shared by one K. pneumoniae subsp. pneumoniae ST76 strain (10CC1) and one K. quasipneumoniae subsp. similipneumoniae ST6102 strain (8CC2) was different from those found in A. caviae strains. Of note, the linkage of bla GES-24, bla GES-6, and bla GES-5 with rare class 3 integrons was observed in one A. allosaccharophila strain (ST1180), one A. caviae strain (ST1182), three A. caviae strains (ST1015), and two A. taiwanensis strains (ST1055), and the latter six strains carried Tn402-like class 3 integrons possessing the complete tniABQR transposition module.
TABLE 3.
Genetic contexts of bla GESs and bla IMPs in carbapenemase-producing organisms
| Bacterial species | Sequence type | Carbapenemase gene | Strain | Genetic context |
|---|---|---|---|---|
| A. allosaccharophila | 1,180 | bla GES-24 | 10CC9 | intI3–bla OXA-17 –bla GES-24 |
| A. caviae | 1,061 | bla IMP-10 | 6BC7 | intI1–bla IMP-10 –bla IMP-10 |
| 1,015 | bla GES-5 | 12BC1 | tniABQR–intI3–bla GES-5 –aacA4 | |
| 8BC4 | ||||
| 10BC1 | tniABQR–intI3–bla GES-5 –bla GES-5 –aacA4 | |||
| 1,057 | bla GES-5 | 4CC9 | intI1–bla GES-5 | |
| 1,059 | bla GES-5 | 2CC7 | intI1–bla GES-5 | |
| 4CC12 | ||||
| 10BC8 | ||||
| 1,176 | bla GES-5 | 10CC10 | intI1–bla GES-5 –bla OXA-17 –ISPa25–qacEΔ1–sul1 | |
| 1,178 | bla GES-5 | 6BC8 | intI1–bla GES-5 –bla OXA-1042 –qacL–qacEΔ1–sul1 | |
| 6CC6 | ||||
| 1,182 | bla GES-6 | 6CC8 | tniABQR–intI3–bla GES-6 –aadA2–aacA4 | |
| 1,199 | bla GES-24 | 6BC6 | intI1–bla GES-24 –aacA31–qacL–qacEΔ1–sul1 | |
| 1,179 | bla GES-24 | 10BC6 | intI1–aacA7–bla GES-24 | |
| 1,062 | bla GES-24/bla IMP-1 | 6CC5 | intI1–bla GES-24 –aacA4–aacA4–bla IMP-1–sul1 | |
| 1,056 | bla GES-48 | 2BC3 | intI1–bla GES-48–aacA31–aadA1–aadA1 | |
| 6BC4 | ||||
| 10CC8 | ||||
| 2CC8 | intI1–bla GES-48 –aacA31–aadA1–aadA1–qacEΔ1–sul1 | |||
| 4CC5 | ||||
| 8BC3 | ||||
| 10BC4 | ||||
| 4BC2 | intI1–bla GES-48–ISPa59–qacEΔ1–sul1 | |||
| 6CC12 | intI1–bla GES-48 –aacA31–aadA1 | |||
| 1,182 | bla GES-48 | 8CC4 | intI1–bla GES-48 | |
| A. dhakensis | 518 | bla GES-24 | 10CC4 | intI1–bla GES-24 –aacA4–catB–aadA1–qacEΔ1–sul1 |
| A. hydrophila subsp. hydrophila | 1,181 | bla GES-5 | 6CC13 | intI1–bla GES-5 –bla OXA-1042 |
| 1,177 | bla GES-24 | 8CC6 | intI1–bla GES-24 –bla GES-24 | |
| 860 | bla GES-24 | 8CC5 | intI1–aacA4–catB8–bla GES-24 –qacEΔ1–sul1 | |
| bla IMP-1 | intI1–bla IMP-1 –bla OXA-1053 | |||
| A. taiwanensis | 1,055 | bla GES-5 | 2BC2 | tniABQR–intI3–bla GES-5 –aacA4 |
| 4BC3 | tniABQR–intI3–bla GES-5 | |||
| A. veronii bv. veronii | 1,054 | bla GES-5 | 12BC2 | intI1–bla GES-5 –bla OXA-1042 –qacL–qacEΔ1–sul1 |
| 2BC1 | ||||
| 1,058 | bla GES-24 | 2CC6 | intI1–bla GES-24 –aacA31 | |
| 1,060 | bla GES-49 | 6BC9 | intI1–bla GES-49 –aacA4–bla OXA-129 –qacEΔ1–sul1 | |
| C. braakii | 110 | bla GES-5 | 8BC2 | intI1–bla GES-5 |
| C. portucalensis | 166 | bla GES-51 | 10CC7 | intI1–bla GES-51 –aacA4–qacEΔ1–sul1 |
| C. freundii | 22 | bla GES-24 | 4CC11 | intI1–bla GES-24 |
| 116 | bla IMP-1 | 12CC1 | intI1–bla IMP-1 –aac(6')–IIc–qacEΔ1–sul1 | |
| 2CC2 | ||||
| E. cloacae subsp. cloacae | 1,821 | bla GES-4 | 8BC1 | intI1–bla GES-4 –aacA31–aadA1–qacEΔ1–sul1 |
| 10BC2 | ||||
| E. kobei | 1,822 | bla GES-4 | 6CC1 | intI1–bla GES-4 |
| 520 | bla GES-54 | 6BC5 | intI1–bla GES-54 –aacA31–aadA1–qacEΔ1–sul1 | |
| E. roggenkampii | 1,059 | bla GES-4 | 4CC4 | intI1–bla GES-4 –aacA31–aadA1–qacEΔ1–sul1 |
| 433 | bla GES-24 | 6BC1 | intI1–aacA7–bla GES-24 –catB6–aacA31–qacEΔ1–sul1 | |
| K. cryocrescens | bla GES-6 | 6BC3 | intI1–bla GES-6 –aacA4–ORF–qacEΔ1–sul1 | |
| K. michiganensis | 95 | bla GES-4 | 12CC2 | intI1–bla GES-4 –aacA4–catB–ORF–qacEΔ1–sul1 |
| 4CC2 | ||||
| 6CC2 | ||||
| 8CC1 | ||||
| 135 | bla GES-47 | 2CC1 | intI1–bla GES-47 | |
| 210 | bla GES-50 | 2BC4 | intI1–aacA7–bla GES-50 –catB6–aacA31–qacEΔ1–sul1 | |
| 10BC5 | ||||
| K. pneumoniae subsp. pneumoniae | 2,791 | bla GES-5 | 4CC3 | intI1–bla GES-5 –aacA4–catB10–bla OXA-4 –aadA8–qacEΔ1–sul1 |
| 6BC2 | ||||
| 6CC4 | ||||
| 10CC2 | ||||
| 76 | bla GES-48 | 10CC1 | intI1–bla GES-48 –bla OXA-129 –aadA2–qacEΔ1–sul1 | |
| K. quasipneumoniae subsp. similipneumoniae | 6,101 | bla GES-4 | 6CC9 | intI1–aacA4–IS91–intI1–bla GES-4 |
| 1,822 | bla GES-5 | 4CC10 | intI1–bla GES-5 | |
| 6CC7 | ||||
| 10CC6 | ||||
| 6,102 | bla GES-48 | 8CC2 | intI1–bla GES-48 –bla OXA-129 –aadA2–qacEΔ1–sul1 | |
| R. planticola | bla GES-5 | 12CC3 | intI1–bla GES-5 –aacA4–catB10–bla OXA-4 –aadA8–qacEΔ1–sul1 | |
| 2CC3 | ||||
| 4CC1 | ||||
| R. ornithinolytica | bla GES-4 | 4CC7 | intI1–bla GES-4 –aacA31–aadA1–aadA1–qacEΔ1–sul1 | |
| 6CC3 | ||||
| 8CC3 | ||||
| 10CC5 | ||||
| bla GES-5 | 2CC4 | intI1–bla GES-5 –aacA4–catB10–bla OXA-4 –aadA8–qacEΔ1–sul1 | ||
| 6CC10 | ||||
| bla GES-24 | 4BC1 | intI1–bla GES-24 –aadA2–aacA31–qacEΔ1–sul1 |
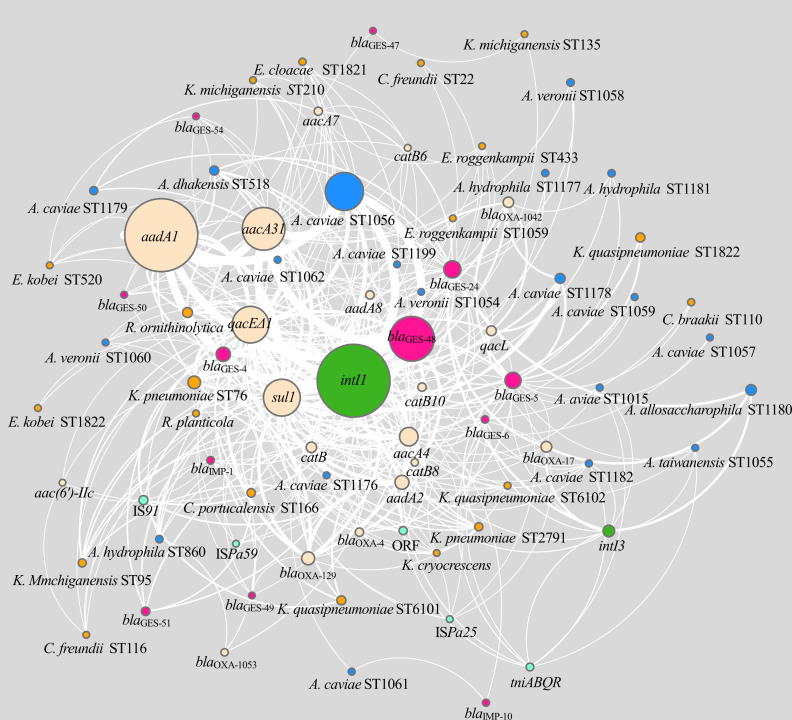
Figure 4 shows the network consisting of 78 nodes and 777 edges depicting the co-occurrence patterns among bla GES and bla IMP gene cassettes in class 1 and 3 integrons and bacterial lineages. The topological analysis revealed that the top 15 ranking genes (intI1, sul1, qacEΔ1, aacA4, bla GES-5, aacA31, aadA1, bla GES-24, bla GES-48, bla GES-4, aadA8, bla OXA-4, catB10, intI3, and aadA2) with node degrees exceeding the average value and higher betweenness centrality and closeness centrality values (greater than the median of the nodes in the network) were considered hub nodes. Clustering analysis by MCODE identified three important cluster subnetworks of highly intraconnected nodes. The first cluster, consisting of 77 nodes and 774 edges with an MCODE score of 9.692, mainly depicted densely connected nodes of class 1 integron-associated genes (intI1, qacEΔ1, and sul1), carbapenemase genes, other resistance genes, and diverse host species and lineages. The second cluster (32 nodes and 99 edges with an MCODE score of 3.879) featured a significant relationship among carbapenemase genes (bla GES-24, bla GES-48, bla GES-54, and bla IMP-1), several other resistance genes, and mainly Aeromonas and Enterobacter spp. as host species. The third cluster (11 nodes and 27 edges with an MCODE score of 3.333) mainly featured a relationship among bla GES-48, aadA2, and Klebsiella spp.
Fig 4.
Network analysis depicting co-occurrence relationship among bla GESs and bla IMPs (pink nodes), class 1 and 3 integron-integrase genes, intI1 and intI3 (green nodes), gene cassette composition in these integrons (beige nodes), and species and lineages of Enterobacterales (orange nodes) and the genus Aeromonas (blue nodes).
DISCUSSION
This study revealed the occurrence and persistence of CPOs comprising a diverse range of bacterial species and lineages in wastewater influent from inlets B and C connected to combined sewer systems collecting both primarily domestic/industrial wastewater and urban runoff rainwater. CPOs carrying bla GES carbapenemase genes were overwhelmingly dominant, accounting for 72 of 75 isolates (96%). Our finding is congruent with previous reports documenting the predominance of bla GES genes among CPOs in raw wastewater samples from WWTPs (15, 22). However, our results are unique in that various bla GES variants, including six newly identified variants, were associated with CPOs, and Aeromonas spp. were the most dominant CPO species. In addition, CPOs were not detected in any raw influent from inlet A connected to the separated sewer systems carrying only domestic/industrial wastewater. Thus, the load of water washing the ground surface, including rainwater, contaminated with CPOs carrying bla GES carbapenemase genes from unrecognized environmental sources could contribute significantly to their detection in the wastewater influent from inlets B and C. In particular, in recent years, the volume of runoff has often exceeded the capacity of the combined sewer system due to localized heavy rainfall resulting from abnormal weather conditions. This can lead to the backflow of wastewater, resulting in the spread of CPOs throughout the entire system. However, one limitation of the study is the lack of data on this type of water monitoring. The bla GES carbapenemase genes (bla GES-5, bla GES-6, bla GES-16, and bla GES-24) carried by diverse Enterobacterales and non-Enterobacterales species have been documented in aquatic environments, such as urban streams (23), river water (24, 25), coastal water (26, 27), urban ponds (28), lake water (29), wastewater (30), and hospital sewage (19, 31, 32). In this study, we could not identify potential sources of the bla GES carbapenemase genes. However, the possible presence of these CPOs of environmental origin is also supported by the findings that Aeromonas spp., which are ubiquitous in aquatic environments and soils and capable of rapidly colonizing various niches and hosts (33), permitting them to serve as potential environmental reservoir bacteria for carbapenemase genes, were the most predominant CPO species harboring bla GES carbapenemase genes. bla GES genes remain rare in clinical isolates, accounting for 2.1% (5/240 isolates) among isolates positive for carbapenemase genes according to the carbapenem-resistant Enterobacteriaceae surveillance in the Infectious Agents Surveillance System, 2020, in Japan (available online at https://www.niid.go.jp/niid/ja/cre-m/cre-iasrd/11520-511d01.html). Thus, the normal human microbiota is unlikely to be a source of the bla GES carbapenemase genes primarily detected in wastewater influent in our study.
In this study, we described the occurrence of multiple bla GES variants, with bla GES-5 being the most prevalent in 36 Aeromonas spp. strains mostly belonging to 19 newly identified STs and in 39 Enterobacterales strains including Enterobacter, Klebsiella, and Raoultella spp. Furthermore, six variants of bla GES carbapenemase genes, namely bla GES-47, bla GES-48, bla GES-49, bla GES-50, bla GES-51, and bla GES-54, were newly identified. Compared to the sequence of GES-1, GES-49 and GES-54 shared the amino acid substitution Glu104Lys; GES-47, GES-48, GES-50, and GES-54 shared the amino acid substitution Met62Thr; and an amino acid change at position 6 was shared by GES-49 and GES-51 (Ala6Val) and GES-50 (Ala6Thr), in addition to the Gly170Ser substitution identified in all six bla GES variants. The Gly170Ser substitution within the Ω-loop region displays carbapenem-hydrolyzing activity (34). This substitution has also been associated with increased catalytic efficiency against cephamycin (cefoxitin), whereas Glu104Lys was linked to enhanced hydrolytic activity toward oxyimino-cephalosporins (35). The Met62Thr substitution does not significantly affect the MICs of β-lactams for E. coli clones (36). The Ala6Thr substitution in the leader peptide was associated with higher MICs for β-lactams (cefotaxime, aztreonam, and imipenem) (37). The phenotypic effects of these resistance-related substitutions did not vary substantially, as all E. coli transformants gaining these new bla GES variants showed susceptibility or intermediate resistance to imipenem and meropenem with MIC ranges of 0.5–2 and 0.25–1 µg/mL, respectively.
Class 1 integrons, being capable of capturing AMR genes, play a particularly important role in the acquisition and dissemination of multidrug resistance among Gram-negative bacteria (38). bla GES and bla IMP genes are frequently associated with class 1 integrons in Enterobacterales, Pseudomonas, and Aeromonas spp. of clinical and aquatic environmental origin (17, 20, 30, 39). These carbapenemase genes have been less commonly associated with class 3 integrons, but our recent study revealed the linkage of bla GES-24, which was predominant among CPO isolates in hospital wastewater, with rare Tn402-like class 3 integrons (19). In the present study, bla GES and/or bla IMP genes carried by 29 Aeromonas spp. and 39 Enterobacterales strains were embedded in class 1 integrons, whereas bla GES genes (bla GES-5, bla GES-6, and bla GES-24) carried by the remaining seven Aeromonas spp. strains were mostly embedded in Tn402-like class 3 integrons. Although structural diversity of gene cassette arrays was noted in the class 1 integrons carried by these 68 CPOs, several shared gene cassettes were persistent in wastewater environment, including one between A. veronii bv. veronii ST1054 and A. caviae ST1178 strains, one among K. pneumoniae subsp. pneumoniae ST2791, R. planticola, and R. ornithinolytica, and one between E. cloacae subsp. cloacae ST1821 and E. roggenkampii ST1059 (R. ornithinolytica also carried an extremely similar one). A. caviae ST1015 and A. taiwanensis ST1055 strains shared the class 3 integron tniABQR–intI3–bla GES-5–aacA4. Thus, these integrons, by association with transposons and plasmids, might contribute to the dissemination of bla GES carbapenemase genes through horizontal gene transfer events (40) within different species of Aeromonas or Enterobacter/Klebsiella/Raoultella.
Quantitative analysis of CPOs revealed that the repeated detection of bla GES-48-positive A. caviae ST1056 from both inlets B and C ranked the total number of this bacterial clone highest in the wastewater influent, suggesting the constant presence of this clone in the combined sewer wastewater. Biofilm formation mediated by polar flagella, lateral flagella, T6SS, etc. might also allow this clone to become a resident organism in the sewer system. However, there might be a missing link to explain the occurrence of the bla GES-48-positive A. caviae ST1056 strains and the bla GES-5-positive A. caviae ST1059, A. caviae ST1178, and K. pneumoniae subsp. pneumoniae ST2791 strains in influent from both inlets B and C. Interestingly, eight of nine A. caviae ST1056 strains harbored identical or extremely similar class 1 integron cassettes around the bla GES-48 genes (intI1–bla GES-48–aacA31–aadA1–aadA1–qacEΔ1–sul1), which were different from the structure of class 1 integron with bla GES-48 carried by the remaining A. caviae ST1056 strain (intI1–bla GES-48–ISPa59–qacEΔ1–sul1) and by one each of K. pneumoniae subsp. pneumoniae ST76 and K. quasipneumoniae subsp. similipneumoniae ST6102 strains (intI1–bla GES-48–bla OXA-129–aadA2–qacEΔ1–sul1). bla GES-48 could not be transferred via conjugation between A. caviae ST1056 and K. pneumoniae subsp. pneumoniae ATCC13883 or E. coli χ1037; thus, bla GES-48 harbored by A. caviae ST1056 is likely associated with an Aeromonas-specific plasmid.
In this study, a representative colony was selected from a minimum of three colonies with the same colony morphology, the same bacterial species, and the same carbapenemase genes. This selection process of representative colonies can result in the successful detection of different ST types within the same CPO species sharing the same carbapenemase genes and the successful tracking of the presence of CPOs belonging to multiple STs in wastewater influents. However, these representative colonies are not guaranteed to be an exact match to the corresponding bacterial populations in terms of bacterial species and genetic types, which may limit the quantitative detection of CPOs under the conditions studied.
In summary, our study revealed the high prevalence and persistence of bla GES carbapenemase genes among CPOs isolated from influent inlets connected to combined sewer systems. Aeromonas spp. carrying bla GES-5, bla GES-6, bla GES-24, bla GES-48, and bla GES-49 embedded in class 1 or 3 integrons stood out as the most abundant CPOs, followed by Klebsiella spp. carrying bla GES-4, bla GES-5, bla GES-47, bla GES-48, and bla GES-50 embedded in class 1 integrons. The presence of diverse integrons, bla GES variants, and host clones of Aeromonas spp., Enterobacter spp., Klebsiella spp., and Raoultella spp. represents the plasticity of these genetic elements, which might allow integrons to capture and disseminate such bla GES variants and facilitate the adaptability of CPOs to the environment. This study shed light on the great potential of the environment in holding bla GES carbapenemase genes and promoting their genetic variability.
MATERIALS AND METHODS
Sample collection and bacterial isolation
One sample each of raw influent from three different inlets, namely, inlet A receiving separated sewer lines and inlets B and C receiving combined sewer lines, and treated effluent from the WWTP outlet were collected once every 2 months on rain-free days from December 2020 to October 2021 from the municipal WWTP located in Matsumoto City, Nagano Prefecture, Japan (Fig. S3). The WWTP processes approximately 82,200 m3 of wastewater per day from 124,700 inhabitants, comprising 43,980, 24,540, and 56,180 inhabitants for inlets A, B, and C, respectively. Simultaneously, river water samples from sites 1.35-km upstream (36°13′54.7″N, 137°57′11.0″E) and 0.56-km downstream (36°14′56.0″N, 137°57′4.7″E) from the effluent outlet were collected (Table S1). Each sample (approximately 1,000 mL) was placed in cleaned polypropylene bottles and immediately transported in a cooler box with sufficient ice packs to our laboratory for processing within 2 h after obtaining the samples. A volume of 250 mL from the influent was concentrated 100-fold via centrifugation at 3,500 rpm for 20 min, after which the pellet was suspended in 2.5 mL of sterile phosphate-buffered saline (PBS, pH 7.4). For effluent and river water samples, 600 mL were filtered through a 0.45 µM membrane (HAWP04700; Millipore). Then, the filter was cut into small pieces and placed in 6 mL of sterile PBS to prepare a bacterial cell suspension. Finally, a 1,000× concentrated suspension was obtained via centrifugation at 15,000 rpm for 10 min.
The 100× concentrated influent samples were serially diluted using sterile PBS, and 100 µL of each dilution was spread onto MacConkey agar (Eiken Chemical Co., Tokyo, Japan) containing 1 µg/mL imipenem (Sigma-Aldrich Japan, Tokyo, Japan) and CHROMagar mSuperCARBA (Kanto Chemical, Tokyo, Japan), followed by incubation at 37°C overnight. In the same manner, 100 µL aliquots of 1,000× concentrated suspensions obtained from effluent and river water samples were spread directly on the same agar plates.
For each sample, bacterial colonies exhibiting similar morphological features were counted using only agar plates yielding approximately 300 visible colonies, enabling us to calculate the number of CFU per milliliter. Then, a minimum of three well-isolated colonies from the bacterial population with similar colony morphology were individually subcultured onto Mueller−Hinton agar (Eiken) and subjected to MALDI-TOF MS with the Bruker BioTyper database and software version 3.1 (Bruker Daltonics Japan, Yokohama, Japan) using a cutoff of ≥2.00 for species-level identification. Intrinsically carbapenem-resistant bacterial species, including Morganella morganii, Providencia rettgeri, and Stenotrophomonas maltophilia, were excluded from further analysis after PCR confirmation of the absence of acquired carbapenemase genes.
Detection of carbapenemase genes
The carbapenemase genes bla IMP , bla NDM , bla KPC , bla GES, and bla OXA-48 were screened by PCR and identified by DNA sequencing (19). After confirming by MALDI-TOF MS that at least three colonies representing a population of bacteria with similar colony morphology were the same bacterial species and harbored the same carbapenemase genes, a representative strain was selected from these colonies. Plural representative isolates identified as K. pneumoniae sharing the same carbapenemase genes obtained from the same agar plate were further differentiated by analyzing rpoB sequences (41).
Antimicrobial susceptibility testing
The MICs of the carbapenemase-producing isolates were determined by the broth microdilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) using Dry Plate DP41 (Eiken), and the results were interpreted using CLSI document M100-ED32 (42). The MICs of faropenem (Sigma-Aldrich), ertapenem (Fujifilm Wako Pure Chemical Co., Osaka, Japan), colistin (Fujifilm), and tigecycline (Tokyo Chemical Industry Co., Tokyo, Japan) were determined using in-house prepared panels according to the CLSI broth microdilution method. E. coli ATCC25922 was used as a quality control strain.
Transformation experiments
Transformation of NEB 10-beta electrocompetent E. coli (DH10B derivative, New England Biolabs, Tokyo, Japan) with plasmid DNA extracted from each of the carbapenemase-producing isolates harboring new variants of the bla GES genes (bla GES-47, bla GES-48, bla GES-49, bla GES-50, bla GES-51, and bla GES-54) was performed by electroporation. Transformants were selected on LB agar plates containing ampicillin (50 µg/mL), and the presence of bla GES genes and the absence of other β-lactamase genes were confirmed by PCR and sequencing. The MICs for the transformants were determined in the same manner described above.
Transferability of bla GES-48 from A. caviae to Enterobacterales
The broth mating assay was used to investigate the transferability of bla GES-48 from the parental isolates, A. caviae ST1056 strain 2BC3 and A. caviae ST1182 strain 8CC4 to K. pneumoniae subsp. pneumoniae ATCC13883 (Rifr) or E. coli χ1037 (Rifr). Conjugation was performed at 25°C and 37°C, and transconjugants were selected on rifampicin-containing (100 µg/mL) LB agar plates supplemented with faropenem (16 µg/mL) and ampicillin (50 µg/mL) for the former and latter recipients, respectively.
WGS and bioinformatics
Genomic DNA was extracted using lysozyme and proteinase K and purified using an Agencourt AMpure XP kit (Beckman-Coulter Life Sciences, Brea, CA, USA) according to the manufacturer’s instructions. To construct DNA libraries, 5× whole-genome sequencing (WGS) Fragmentation Mix and 5× WGS Ligase Mix (Enzymatics, Beverly, MA, USA) were used. Pooled libraries were subjected to 350- to 800-bp size selection using the BluePippin system (Sage Science, Inc., Beverly, MA, USA). The pooled libraries were sequenced on the Illumina HiSeq X Five platform (Illumina Inc., San Diego, CA, USA) using the 150-bp paired-end method. A small portion of the CPOs were subjected to WGS by the 150-bp paired-end method using the NovaSeq6000, MiSeq, and MiniSeq platforms (Illumina). The de novo assembly of short reads was conducted using Shovill v1.1.0 (https://github.com/tseemann/shovill).
Pairwise ANIm was calculated using JspeciesWS (http://jspecies.ribohost.com/jspeciesws/), and 95%–96% ANI was used as the threshold for defining species. A. allosaccharophila CECT 4199 (GCA_000819685.1), A. caviae NCTC 12244 (GCA_900476005.1), A. dhakensis F2S2-1 (GCA_001673685.1), A. hydrophila subsp. hydrophila ATCC 7966 (GCA_000014805.1), A. taiwanensis LMG 24683 (GCA_000820165.1), A. veronii bv. veronii CECT 4257 (GCA_000820225.1), C. braakii ATCC 51113 (GCA_002075345.1), C. freundii ATCC 8090 (GCA_011064845.1), C. portucalensis A60 (GCA_002042885.1), E. cloacae subsp. cloacae ATCC 13047 (GCA_000025565.1), E. kobei DSM 13645 (GCA_001729765.1), E. roggenkampii DSM 16690 (GCA_001729805.1), K. cryocrescens NBRC 102467 (GCA_001571285.1), K. michiganensis 10–5242 (GCA_000247835.1), K. pneumoniae subsp. pneumoniae ATCC 13883 (GCA_000788015.1), K. quasipneumoniae subsp. similipneumoniae ATCC 700603 (GCA_003181175.1), R. ornithinolytica ATCC 31898 (GCA_001598295.1), and R. planticola ATCC 33531 (GCA_000735435.1) served as the reference genomes were used for ANIm analyses. Assembled genomes were scanned against ResFinder 4.1, PlasmidFinder 2.1, and CSI Phylogeny 1.4 available from the Center for Genomic Epidemiology (http://www.genomicepidemiology.org). Multilocus sequence typing (MLST) was performed using mlst v2.22.0 (https://github.com/tseemann/mlst). The capsule and outer lipopolysaccharide loci of K. pneumoniae species complex isolates were typed using Kaptive v2.0.0 (https://github.com/katholt/kaptive). In Aeromonas spp., virulence-associated genes were analyzed using VFanalyzer from the virulence factors database (http://www.mgc.ac.cn/VFs/), and heavy metal resistance genes were explored manually using WGS data.
Genome annotation was achieved using Prokka 1.14.6 (https://github.com/tseemann/prokka). Whole-genome clustering based on k-mer mash distances among genomes was conducted using PopPUNK (43) on a Galaxy ARIES-based platform (Galaxy Version 1.1). iTOL v6 (https://itol.embl.de/) was used to annotate and visualize the tree. The genetic context of the carbapenemase gene was investigated by PCR mapping and sequencing. The co-occurrence relationships among integrons, resistance genes, and bacterial strains were visualized in a network using Cytoscape version 3.9.1 (44). The Cytoscape plugin cytoHubba (v0.1) (45) and MCODE (v2.0.2) (46) were used to predict hub nodes based on topological parameters and the identification of highly intraconnected clusters in a network, respectively. New STs were assigned by the Institut Pasteur MLST database or PubMLST database. New allele numbers for bla GES carbapenemase genes were assigned by NCBI.
Statistical analysis
The abundance of CPOs was not normally distributed according to the D’Agostino−Pearson omnibus normality test; thus, the occurrence of CPOs in influent samples from inlets B and C was compared using the nonparametric Wilcoxon signed-rank sum test. The Friedman test was used to investigate differences in the occurrence of CPOs between sampling months. P ≤ 0.05 indicated statistical significance.
ACKNOWLEDGMENTS
The authors would like to thank Enago (www.enago.com) for manuscript review and editing support.
This work was supported by AMED under Grant Number 22fk0108132h0103.
We declare no conflicts of interest.
Contributor Information
Yo Sugawara, Email: suga-yo@niid.go.jp.
Noriyuki Nagano, Email: naganon@shinshu-u.ac.jp.
Krisztina M. Papp-Wallace, JMI Laboratories, North Liberty, Iowa, USA
DATA AVAILABILITY
All raw and assembled sequence data for 75 CPOs have been deposited in the DDBJ/EMBL/GenBank database under the umbrella project PRJDB14710 that encompasses two primary submission projects, PRJDB14712 and PRJDB13738. The complete nucleotide sequences of bla GES-47, bla GES-48, bla GES-49, bla GES-50, bla GES-51, and bla GES-54 have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence database under accession numbers NG_074708.1, NG_074709.1, NG_076643.1, NG_077975.1, NG_077976.1, and NG_081003.1, respectively.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.02188-23.
Supplementary data.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Diene SM, Rolain J-M. 2014. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin Microbiol Infect 20:831–838. doi: 10.1111/1469-0691.12655 [DOI] [PubMed] [Google Scholar]
- 2. Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- 3. Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansen GT. 2021. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect Dis Ther 10:75–92. doi: 10.1007/s40121-020-00395-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armand-Lefèvre L, Andremont A, Ruppé E. 2018. Travel and acquisition of multidrug-resistant Enterobacteriaceae. Med Mal Infect 48:431–441. doi: 10.1016/j.medmal.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 6. Hassing RJ, Alsma J, Arcilla MS, van Genderen PJ, Stricker BH, Verbon A. 2015. International travel and acquisition of multidrug-resistant Enterobacteriaceae: a systematic review. Euro Surveill 20. doi: 10.2807/1560-7917.ES.2015.20.47.30074 [DOI] [PubMed] [Google Scholar]
- 7. Morrison BJ, Rubin JE. 2015. Carbapenemase producing bacteria in the food supply escaping detection. PLoS One 10:e0126717. doi: 10.1371/journal.pone.0126717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loest D, Uhland FC, Young KM, Li X-Z, Mulvey MR, Reid-Smith R, Sherk LM, Carson CA. 2022. Carbapenem-resistant Escherichia coli from shrimp and salmon available for purchase by consumers in Canada: a risk profile using the Codex framework. Epidemiol Infect 150:e148. doi: 10.1017/S0950268822001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamoto N, Asada R, Kawahara R, Hagiya H, Akeda Y, Shanmugakani RK, Yoshida H, Yukawa S, Yamamoto K, Takayama Y, Ohnishi H, Taniguchi T, Matsuoka T, Matsunami K, Nishi I, Kase T, Hamada S, Tomono K. 2017. Prevalence of, and risk factors for, carriage of carbapenem-resistant Enterobacteriaceae among hospitalized patients in Japan. J Hosp Infect 97:212–217. doi: 10.1016/j.jhin.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 10. Yamagishi T, Matsui M, Sekizuka T, Ito H, Fukusumi M, Uehira T, Tsubokura M, Ogawa Y, Miyamoto A, Nakamori S, Tawa A, Yoshimura T, Yoshida H, Hirokawa H, Suzuki S, Matsui T, Shibayama K, Kuroda M, Oishi K. 2020. A prolonged multispecies outbreak of IMP-6 carbapenemase-producing Enterobacterales due to horizontal transmission of the IncN plasmid. Sci Rep 10:4139. doi: 10.1038/s41598-020-60659-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aarestrup FM, Woolhouse MEJ. 2020. Using sewage for surveillance of antimicrobial resistance. Science 367:630–632. doi: 10.1126/science.aba3432 [DOI] [PubMed] [Google Scholar]
- 12. Pärnänen KMM, Narciso-da-Rocha C, Kneis D, Berendonk TU, Cacace D, Do TT, Elpers C, Fatta-Kassinos D, Henriques I, Jaeger T, Karkman A, Martinez JL, Michael SG, Michael-Kordatou I, O’Sullivan K, Rodriguez-Mozaz S, Schwartz T, Sheng H, Sørum H, Stedtfeld RD, Tiedje JM, Giustina SVD, Walsh F, Vaz-Moreira I, Virta M, Manaia CM. 2019. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci Adv 5:eaau9124. doi: 10.1126/sciadv.aau9124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blaak H, Kemper MA, de Man H, van Leuken JPG, Schijven JF, van Passel MWJ, Schmitt H, de Roda Husman AM. 2021. Nationwide surveillance reveals frequent detection of carbapenemase-producing Enterobacterales in Dutch municipal wastewater. Sci Total Environ 776:145925. doi: 10.1016/j.scitotenv.2021.145925 [DOI] [Google Scholar]
- 14. Teban-Man A, Szekeres E, Fang P, Klümper U, Hegedus A, Baricz A, Berendonk TU, Pârvu M, Coman C. 2022. Municipal wastewaters carry important carbapenemase genes independent of hospital input and can mirror clinical resistance patterns. Microbiol Spectr 10:e0271121. doi: 10.1128/spectrum.02711-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tiwari A, Paakkanen J, Österblad M, Kirveskari J, Hendriksen RS, Heikinheimo A. 2022. Wastewater surveillance detected carbapenemase enzymes in clinically relevant Gram-negative bacteria in Helsinki, Finland; 2011-2012. Front Microbiol 13:887888. doi: 10.3389/fmicb.2022.887888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cherak Z, Loucif L, Moussi A, Rolain J-M. 2021. Carbapenemase-producing Gram-negative bacteria in aquatic environments: a review. J Glob Antimicrob Resist 25:287–309. doi: 10.1016/j.jgar.2021.03.024 [DOI] [PubMed] [Google Scholar]
- 17. Hishinuma T, Tada T, Kuwahara-Arai K, Yamamoto N, Shimojima M, Kirikae T. 2018. Spread of GES-5 carbapenemase-producing Pseudomonas aeruginosa clinical isolates in Japan due to clonal expansion of ST235. PLoS One 13:e0207134. doi: 10.1371/journal.pone.0207134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakanishi N, Komatsu S, Iwamoto T, Nomoto R. 2022. Characterization of a novel plasmid in Serratia marcescens harbouring blaGES-5 isolated from a nosocomial outbreak in Japan. J Hosp Infect 121:128–131. doi: 10.1016/j.jhin.2021.11.022 [DOI] [PubMed] [Google Scholar]
- 19. Takizawa S, Soga E, Hayashi W, Sakaguchi K, Koide S, Tanabe M, Denda T, Sugawara Y, Yu L, Kayama S, Sugai M, Nagano Y, Nagano N. 2022. Genomic landscape of blaGES-5- and blaGES-24-harboring Gram-negative bacteria from hospital wastewater: emergence of class 3 integron-associated blaGES-24 genes. J Glob Antimicrob Resist 31:196–206. doi: 10.1016/j.jgar.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 20. Pedersen T, Sekyere JO, Govinden U, Moodley K, Sivertsen A, Samuelsen Ø, Essack SY, Sundsfjord A. 2018. Spread of plasmid-encoded NDM-1 and GES-5 carbapenemases among extensively drug-resistant and pandrug-resistant clinical Enterobacteriaceae in Durban, South Africa. Antimicrob Agents Chemother 62:e02178-17. doi: 10.1128/AAC.02178-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambler RP, Coulson AF, Frère JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. 1991. A standard numbering scheme for the class A β-lactamases. Biochem J 276 ( Pt 1):269–270. doi: 10.1042/bj2760269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Araújo S, Sousa M, Tacão M, Baraúna RA, Silva A, Ramos R, Alves A, Manaia CM, Henriques I. 2021. Carbapenem-resistant bacteria over a wastewater treatment process: carbapenem-resistant Enterobacteriaceae in untreated wastewater and intrinsically-resistant bacteria in final effluent. Sci Total Environ 782:146892. doi: 10.1016/j.scitotenv.2021.146892 [DOI] [Google Scholar]
- 23. Manageiro V, Ferreira E, Caniça M, Manaia CM. 2014. GES-5 among the β-lactamases detected in ubiquitous bacteria isolated from aquatic environment samples. FEMS Microbiol Lett 351:64–69. doi: 10.1111/1574-6968.12340 [DOI] [PubMed] [Google Scholar]
- 24. Teixeira P, Tacão M, Pureza L, Gonçalves J, Silva A, Cruz-Schneider MP, Henriques I. 2020. Occurrence of carbapenemase-producing Enterobacteriaceae in a Portuguese river: blaNDM, blaKPC and blaGES among the detected genes. Environ Pollut 260:113913. doi: 10.1016/j.envpol.2020.113913 [DOI] [PubMed] [Google Scholar]
- 25. de Araujo CFM, Silva DM, Carneiro MT, Ribeiro S, Fontana-Maurell M, Alvarez P, Asensi MD, Zahner V, Carvalho-Assef APD. 2016. Detection of carbapenemase genes in aquatic environments in Rio de Janeiro, Brazil. Antimicrob Agents Chemother 60:4380–4383. doi: 10.1128/AAC.02753-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montezzi LF, Campana EH, Corrêa LL, Justo LH, Paschoal RP, da Silva I, Souza M do C, Drolshagen M, Picão RC. 2015. Occurrence of carbapenemase-producing bacteria in coastal recreational waters. Int J Antimicrob Agents 45:174–177. doi: 10.1016/j.ijantimicag.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 27. Paschoal RP, Campana EH, Corrêa LL, Montezzi LF, Barrueto LRL, da Silva IR, Bonelli RR, Castro L de S, Picão RC. 2017. Concentration and variety of carbapenemase producers in recreational coastal waters showing distinct levels of pollution. Antimicrob Agents Chemother 61:e01963-17. doi: 10.1128/AAC.01963-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teixeira P, Pinto N, Henriques I, Tacão M. 2022. KPC-3-, GES-5-, and VIM-1-producing Enterobacterales isolated from urban ponds. Int J Environ Res Public Health 19:5848. doi: 10.3390/ijerph19105848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gomi R, Matsumura Y, Tanaka M, Ihara M, Sugie Y, Matsuda T, Yamamoto M. 2022. Emergence of rare carbapenemases (FRI, GES-5, IMI, SFC and SFH-1) in Enterobacterales isolated from surface waters in Japan. J Antimicrob Chemother 77:1237–1246. doi: 10.1093/jac/dkac029 [DOI] [PubMed] [Google Scholar]
- 30. Gomi R, Matsuda T, Yamamoto M, Chou P-H, Tanaka M, Ichiyama S, Yoneda M, Matsumura Y. 2018. Characteristics of carbapenemase-producing Enterobacteriaceae in wastewater revealed by genomic analysis. Antimicrob Agents Chemother 62:e02501-17. doi: 10.1128/AAC.02501-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maehana S, Eda R, Hirabayashi A, Niida N, Nakamura M, Furukawa T, Ikeda S, Kojima F, Sakai K, Sei K, Kitasato H, Suzuki M. 2021. Natural factories that manufacture antimicrobial resistance genes: quadruple blaGES-carrying plasmids in Aeromonas and Pseudomonas species. Int J Antimicrob Agents 57:106327. doi: 10.1016/j.ijantimicag.2021.106327 [DOI] [PubMed] [Google Scholar]
- 32. White L, Hopkins KL, Meunier D, Perry CL, Pike R, Wilkinson P, Pickup RW, Cheesbrough J, Woodford N. 2016. Carbapenemase-producing Enterobacteriaceae in hospital wastewater: a reservoir that may be unrelated to clinical isolates. J Hosp Infect 93:145–151. doi: 10.1016/j.jhin.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 33. Lamy B, Baron S, Barraud O. 2022. Aeromonas: the multifaceted middleman in the One Health world. Curr Opin Microbiol 65:24–32. doi: 10.1016/j.mib.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 34. Walther-Rasmussen J, Høiby N. 2007. Class A carbapenemases. J Antimicrob Chemother 60:470–482. doi: 10.1093/jac/dkm226 [DOI] [PubMed] [Google Scholar]
- 35. Kotsakis SD, Miriagou V, Tzelepi E, Tzouvelekis LS. 2010. Comparative biochemical and computational study of the role of naturally occurring mutations at Ambler positions 104 and 170 in GES β-lactamases. Antimicrob Agents Chemother 54:4864–4871. doi: 10.1128/AAC.00771-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wachino J, Doi Y, Yamane K, Shibata N, Yagi T, Kubota T, Ito H, Arakawa Y. 2004. Nosocomial spread of ceftazidime-resistant Klebsiella pneumoniae strains producing a novel class A β-lactamase, GES-3, in a neonatal intensive care unit in Japan. Antimicrob Agents Chemother 48:1960–1967. doi: 10.1128/AAC.48.6.1960-1967.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bontron S, Poirel L, Nordmann P. 2015. In vitro prediction of the evolution of GES-1 β-lactamase hydrolytic activity. Antimicrob Agents Chemother 59:1664–1670. doi: 10.1128/AAC.04450-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, Chen D, Bian H, Li Y, Yu G. 2015. Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob 14:45. doi: 10.1186/s12941-015-0100-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uechi K, Tada T, Sawachi Y, Hishinuma T, Takaesu R, Nakama M, Nakasone I, Kirikae T, Fujita J. 2018. A carbapenem-resistant clinical isolate of Aeromonas hydrophila in Japan harbouring an acquired gene encoding GES-24 β-lactamase. J Med Microbiol 67:1535–1537. doi: 10.1099/jmm.0.000842 [DOI] [PubMed] [Google Scholar]
- 40. Gillings MR. 2017. Lateral gene transfer, bacterial genome evolution, and the Anthropocene. Ann N Y Acad Sci 1389:20–36. doi: 10.1111/nyas.13213 [DOI] [PubMed] [Google Scholar]
- 41. Diancourt L, Passet V, Verhoef J, Grimont PAD, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clinical and Laboratory Standards Institute . 2022. Performance standards for antimicrobial susceptibility testing. CLSI document M100-Ed32. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43. Lees JA, Harris SR, Tonkin-Hill G, Gladstone RA, Lo SW, Weiser JN, Corander J, Bentley SD, Croucher NJ. 2019. Fast and flexible bacterial genomic epidemiology with PopPUNK. Genome Res 29:304–316. doi: 10.1101/gr.241455.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chin C-H, Chen S-H, Wu H-H, Ho C-W, Ko M-T, Lin C-Y. 2014. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 8 Suppl 4:S11. doi: 10.1186/1752-0509-8-S4-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bader GD, Hogue CWV. 2003. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 4:2. doi: 10.1186/1471-2105-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data.
Data Availability Statement
All raw and assembled sequence data for 75 CPOs have been deposited in the DDBJ/EMBL/GenBank database under the umbrella project PRJDB14710 that encompasses two primary submission projects, PRJDB14712 and PRJDB13738. The complete nucleotide sequences of bla GES-47, bla GES-48, bla GES-49, bla GES-50, bla GES-51, and bla GES-54 have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence database under accession numbers NG_074708.1, NG_074709.1, NG_076643.1, NG_077975.1, NG_077976.1, and NG_081003.1, respectively.