Abstract
Background
Real-world data regarding the impact of onabotulinumtoxinA on healthcare resource utilization and costs for post-stroke spasticity are scarce.
Objective
To compare differences in 12-month healthcare resource utilization and costs before and after post-stroke spasticity management including onabotulinumtoxinA.
Methods
This retrospective claims analysis of IBM MarketScan Commercial and Medicare Supplemental databases included adults with ≥ 1 onabotulinumtoxinA claim for post-stroke spasticity (1 January 2010 to 30 June 2018) and continuous enrolment for ≥ 12 months pre- and post-index (first onabotulinumtoxinA claim date). All-cause and spasticity-related healthcare resource utilization and costs were compared 12 months pre- and post-index (McNemar’s χ2 test or paired t-test). A subgroup analysis assessed effect of stroke-to-index interval on costs.
Results
Among 735 patients, mean (standard deviation) stroke-date-to-index-date interval was 284.5 (198.8) days. Decreases were observed post-index for mean all-cause outpatient (62.9 vs 60.5; p ≤ 0.05) and emergency department visits (1.1 vs 0.8; p ≤ 0.0001), and hospital admissions (1.5 vs 0.4; p ≤ 0.0001). Increase in prescription fills (43.0 vs 53.7) was seen post-index. Post-index decreases in all-cause (–66%) and spasticity-related (–51%) costs were driven by reduced inpatient care costs. Findings were consistent regardless of stroke-date-to-index-date interval.
Conclusion
Significant reductions in healthcare resource utilization and costs were observed after 1 year of post-stroke spasticity management including onabotulinumtoxinA. Long-term studies are needed to establish causality.
LAY ABSTRACT
Post-stroke spasticity is debilitating and can potentially increase healthcare costs. OnabotulinumtoxinA is approved to treat spasticity; however, little is known about its effect on costs for real-world stroke patients in the USA. This study compared differences in use of healthcare resources and costs 12 months before and after treatment including onabotulinumtoxinA for post-stroke spasticity. Adults with at least 1 claim for onabotulinumtoxinA for post-stroke spasticity between 1 January 2010 and 30 June 2018 in the IBM MarketScan Commercial and Medicare Supplemental databases were included. Among 735 patients, mean time from stroke to starting onabotulinumtoxinA was 284.5 days. Compared with before starting onabotulinumtoxinA, outpatient and emergency department visits and hospital admissions per patient decreased after starting onabotulinumtoxinA. Overall costs decreased by 66% and spasticity-related costs by 51%. Cost reductions were observed regardless of the time between stroke and starting onabotulinumtoxinA. Long-term studies are needed to determine whether these reductions were caused by onabotulinumtoxinA treatment.
Key words: botulinum toxins, type A; cost analysis; health resources; muscle spasticity; stroke
Post-stroke spasticity (PSS) is a debilitating condition characterized by intermittent or sustained involuntary muscle activation resulting from a central sensorimotor network lesion in the brain or spinal cord (1, 2). Spasticity occurs in approximately 20–40% of patients in the first 6 months to 1 year following a stroke (3–8), and 2–13% of patients develop disabling spasticity (9). For a patient in the chronic phase of stroke recovery, the presence of spasticity represents a barrier to positive outcomes from subacute stroke rehabilitation and is associated with a negative impact on quality of life (2, 8). Following discharge from hospital after a stroke, patients with spasticity have longer stays in the inpatient rehabilitation clinic than patients without spasticity; in 1 prospective study, 53.2% of patients with spasticity had an inpatient stay longer than 5 weeks compared with 35.8% of patients without spasticity (3).
Overall, the economic burden of stroke care is high, particularly in patients with a greater level of functional impairment and during the first 1–3 months following a stroke (10–12). Healthcare costs are 2–4 times higher for stroke survivors with spasticity than for those without spasticity (13, 14). PSS is also associated with symptoms such as pain, contractures, immobilization in abnormal limb postures, and development of pressure sores (2, 4, 15). Furthermore, PSS increases caregiver burden, productivity loss, and indirect costs associated with caring for patients with spasticity (16).
Botulinum toxins type A (BoNT/A), including onabotulinumtoxinA, are indicated for the treatment of upper- and lower-limb spasticity based on randomized controlled clinical trials that demonstrated reductions in predetermined disability parameters, including pain, and reductions in caregiver burden (17–22). In the observational, real-world Adult Spasticity International Registry (ASPIRE) study, onabotulinumtoxinA treatment for PSS demonstrated high patient and clinician satisfaction with treatment efficacy (23). OnabotulinumtoxinA treatment also helped patients participate in therapy/exercise, reduced Disability Assessment Scale scores (dressing, hygiene, limb posture, pain), and reduced patient-reported spasticity-related pain (23). Studies have suggested that BoNT/A treatment is cost-effective and reduces disease burden, as evidenced by increased quality-adjusted life years, in patients with PSS (24, 25). However, there is a paucity of information regarding the impact of onabotulinumtoxinA treatment on healthcare resource utilization (HRU) and costs for patients with PSS.
The primary objective of this retrospective claims data analysis was to assess the impact of comprehensive spasticity management including onabotulinumtoxinA treatment on HRU and costs among patients diagnosed with PSS by comparing differences in 12-month HRU and costs before vs after initiation of onabotulinumtoxinA treatment.
METHODS
Study design
The study design was a retrospective analysis of claims data from the IBM MarketScan Commercial and Medicare Supplemental databases (2009–2019). The IBM MarketScan Commercial database consists of health insurance claims for inpatient, outpatient, and outpatient pharmacy visits and enrolment data from employers and health plans across the USA, whereas the Medicare Supplemental database includes enrolment records and claims data for inpatient, outpatient, ancillary, and drug claims for retirees in the USA with Medicare supplemental insurance. These databases comprise integrated, patient-level health records that provide a comprehensive view of HRU and costs, with de-identified records of more than 270 million individual patients linked via unique identifiers to provide robust, longitudinal data, which are geographically representative of the US population (26).
Patients were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and Tenth Revision, Clinical Modification (ICD-10-CM), and procedure codes (Table SI). The first onabotulinumtoxinA claim for PSS identified in the study period was assigned as the index date. HRU and costs were compared between the 12-month pre- and post-index periods.
Patient records were de-identified in agreement with US guidelines for patient confidentiality, including the 1996 Health Insurance Portability and Accountability Act, as certified by an independent third party. This study did not require institutional review board approval because only de-identified patient records were used and no individually identifiable data were collected, used, or transmitted.
Patient selection
Eligible patients were adults ≥ 18 years with ≥ 1 onabotulinumtoxinA claim for PSS between 1 January 2010, and 30 June 2018, and continuous enrolment for ≥ 12 months before and after the index date. Key exclusion criteria were spasticity unrelated to stroke; any use of onabotulinumtoxinA prior to index date; use of other toxins at any time during the study period; documented evidence of contracture within 12 months prior to index date; and acute stroke within 12 months following the index date.
Study outcomes
All-cause and spasticity-related HRU were assessed 12 months before and after index treatment with onabotulinumtoxinA and included outpatient visits, emergency department (ED) visits, hospitalizations, and prescriptions. All-cause and spasticity-related costs were evaluated 12 months before and after index treatment with onabotulinumtoxinA and included costs overall and by category (i.e. outpatient, ED, inpatient, and pharmacy). Spasticity-related costs were determined using inpatient stay records that included any of the ICD-10-CM codes noted in Table SI as the primary diagnosis. Service codes for other outpatient claims (occupational therapy and physical therapy) are shown in Table SII. Because stroke itself can be a high-cost event and may overwhelm any meaningful differences in HRU and costs, a sensitivity analysis was conducted to evaluate all-cause and spasticity-related costs for subgroups stratified by time between index date and the most recent stroke diagnosis (≤ 180 vs > 180 days and ≤ 365 vs > 365 days).
Statistical analysis
HRU was determined by counting claims that occurred within the 12-month pre- and post-index period for inpatient admissions, outpatient visits, ED visits, and prescriptions filled. For HRU, number of visits/admissions per patient, visits/admissions per patient with at least one visit/admission, number of prescriptions filled per patient, and numbers of prescriptions filled per patient with at least one prescription fill were summarized via continuous statistics including mean, standard deviation (SD), median, and interquartile range (IQR). HRU counts were also summarized categorically by visit/admission as patients with ≥ 1 utilized, ≥ 2 utilized, and ≥ 3 utilized. Unadjusted costs were calculated for the 12 months pre- and post-index date including means (SD) and first and third quartiles across mutually exclusive categories. Mean costs per patient were reported for the following categories: outpatient visits, ED visits, inpatient admissions, and prescriptions filled, as well as a total cost calculated by summing all these categories. HRU and costs were then compared between the pre-and post-index periods within the onabotulinumtoxinA-treated cohort. The significance of observed differences in HRU and cost outcomes measured continuously were measured using the paired t-test (mean) and Wilcoxon signed rank test (median). Pre- and post-index period comparisons were analysed using McNemar’s χ2 test (categorical variables) and the paired t-test (continuous variables).
RESULTS
Cohort attrition and baseline characteristics
Of 352,064 patients identified with an initial spasticity diagnosis between 1 January 2010 and 30 June 2018, 2,696 patients also received onabotulinumtoxinA for PSS during this period, and a total of 735 patients met all eligibility requirements and were included in this analysis. Cohort attrition is shown in Table SIII. More than half (54%) of the patients were male, with a mean age at index date of 60.7 years, and 65% were enrolled in a commercial insurance plan (vs 35% in Medicare) (Table I). The most common comorbidities were cerebrovascular disease, hemiplegia, diabetes without chronic complication, and peripheral vascular disease (Table I).
Table I.
Patient demographics and baseline characteristics
| Baseline characteristics | OnabotulinumtoxinA (N = 735) |
|---|---|
| Male, n (%) | 394 (54) |
| Age at index date, mean (SD) | 60.7 (13.9) |
| Insurance type, n (%) | |
| Commercial | 479 (65.2) |
| Medicare | 256 (34.8) |
| Patients with stroke diagnosis prior to first onabotulinumtoxinA claima (index date), n (%) | 542 (73.7) |
| ≤ 180 days | 181 (33.4) |
| > 180 days | 361 (66.6) |
| ≤ 365 days | 420 (77.5) |
| > 365 days | 122 (22.5) |
| Charlson comorbidities within 12 months of index date, n (%) | |
| Cerebrovascular diseaseb | 735 (100) |
| Hemiplegiab | 694 (94.4) |
| Diabetes without chronic complication | 214 (29.1) |
| Peripheral vascular disease | 176 (23.9) |
| Chronic pulmonary disease | 139 (18.9) |
| Congestive heart failure | 128 (17.4) |
| Diabetes with chronic complication | 65 (8.8) |
| Any malignancy | 65 (8.8) |
| Renal disease | 61 (8.3) |
| Myocardial infarction | 60 (8.2) |
| Mild liver disease | 32 (4.4) |
| Dementia | 31 (4.2) |
| Peptic ulcer disease | 16 (2.2) |
| Rheumatic disease | 11 (1.5) |
| Metastatic solid tumour | 9 (1.2) |
| Moderate or severe liver disease | 3 (0.4) |
| AIDS/HIV | 1 (0.1) |
For the remainder of patients (26%), it was not possible to confirm stroke diagnosis based on medical history despite diagnosis of post-stroke spasticity and, therefore, they were omitted from sensitivity analysis.
These components of the Charlson Comorbidity Index were also represented among the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Codes for inclusion in this study.
SD: standard deviation.
Mean (SD) time from stroke to initial spasticity diagnosis was 27.1 (85.9) days, and mean (SD) time from stroke diagnosis to index date was 284.5 (198.8) days. A total of 542 patients had a confirmed diagnosis of stroke prior to the index date and were included in the sensitivity analysis; of these patients, the majority had been diagnosed with stroke more than 6 months (67%; n = 361) but less than 1 year (77%; n = 420) before the index date (Table I).
Healthcare resource utilization outcomes
Overall, utilization of all-cause outpatient services was similar in the pre- and post-index periods (mean (SD) 62.9 (42.9) and 60.5 (46.9) visits, respectively; p = 0.16) (Fig. 1). Significant reductions were observed in the use of outpatient primary care and other specialists (p ≤ 0.05) in the post-index period, whereas there were significant increases in outpatient visits to a neurologist and for physical medicine and occupational therapy (p ≤ 0.05) (Fig. 1). Overall spasticity-related outpatient visits increased in the post-index period (mean (SD) 15.7 (23.8) and 18.9 (26.0) visits per patient in the pre- and post-index periods, respectively, p ≤ 0.05), with significant increases observed for neurologist, physical medicine, and occupational therapy visits (Fig. 1).
Fig. 1.
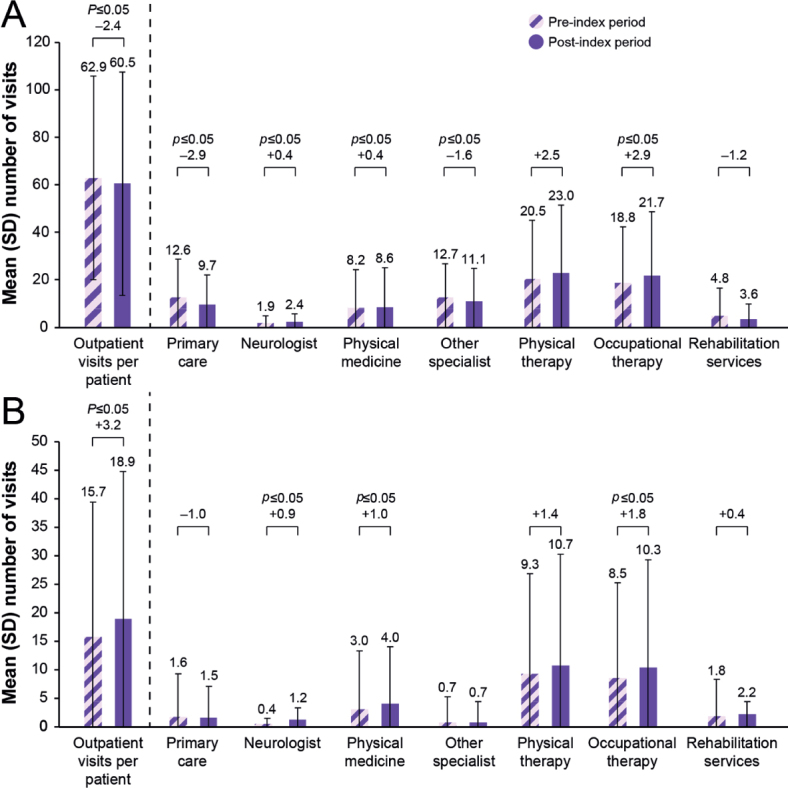
Mean numbers of (A) all-cause and (B) spasticity-related outpatient visits during the 12 months before and after onabotulinumtoxinA treatment, overall and by outpatient visit type. SD: standard deviation. p-values were determined by using the paired t-test. p-values ≤ 0.05 were significant.
ED visits and hospital admissions 12 months before and after the first onabotulinumtoxinA treatment claim are shown in Fig. 2. Mean (SD) frequency of all-cause visits to the ED (1.1 (1.6) vs 0.8 (1.4); p ≤ 0.0001) and hospital admissions (1.5 (1.5) vs 0.4 (0.8); p ≤ 0.0001) per patient decreased significantly in the post-index vs the pre-index period. Significantly fewer patients had spasticity-related hospitalizations in the post-index period than in the pre-index period (Fig. 2).
Fig. 2.
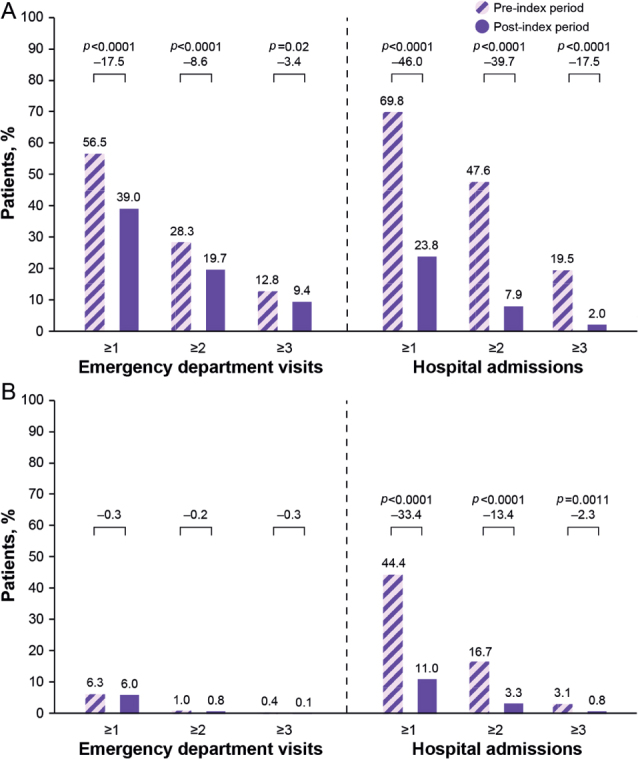
Proportions of patients with ≥ 1, ≥ 2, or ≥ 3 (A) all-cause and (B) spasticity-related emergency department visits or hospital admissions during the 12 months before and after onabotulinumtoxinA treatment for post-stroke spasticity.
Mean (SD) frequency of all-cause pharmacy fills was 43.0 (30.0) pre-index and 53.7 (39.7) post-index. There was a mean (SD) of 7.1 (8.2) spasticity-related pharmacy fills in the pre-index period and 10.1 (11.2) in the post-index period (p ≤ 0.05).
Cost outcomes
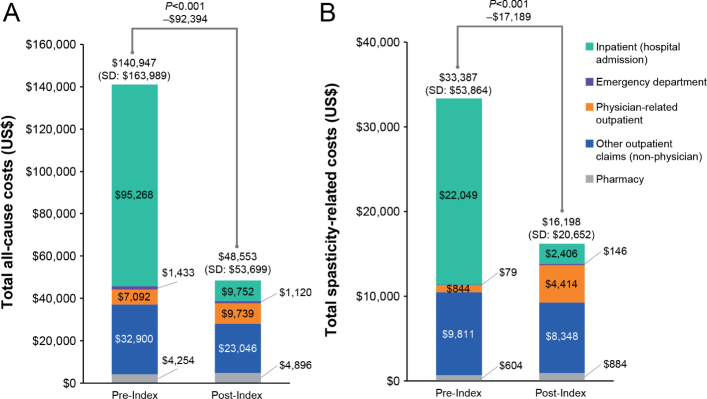
Compared with the 12 months before index onabotulinumtoxinA treatment, there was a 66% decrease in total all-cause costs (pre-index: United States dollars ($)140,947; post-index: $48,553; p < 0.001) and a 51% decrease in total spasticity-related costs (pre-index: $33,387; post-index: $16,198; p < 0.001) observed in the post-index period, driven largely by a reduction in inpatient costs (Fig. 3). Increases in outpatient physician costs and pharmacy costs in the post-index period were offset by the decrease in inpatient costs (Fig. 3).
Fig. 3.
(A) Total all-cause and (B) spasticity-related costs during the 12 months before and after onabotulinumtoxinA treatment, overall and by cost category. SD: standard deviation. Codes for other outpatient claims (outpatient claims and physical therapy claims) are described in Table SII.
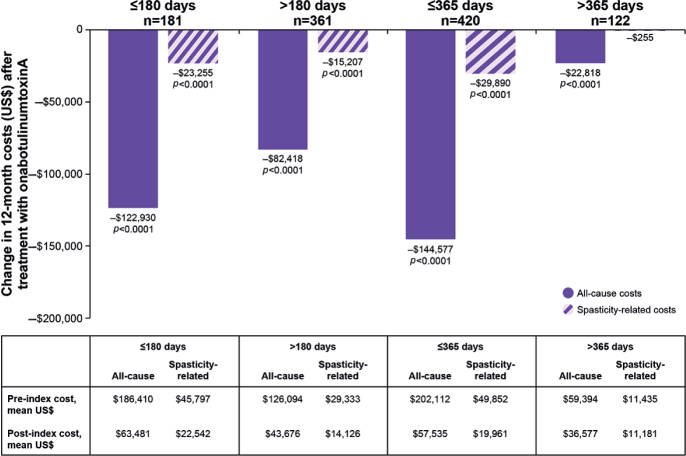
In the sensitivity analysis that evaluated change in 12-month costs before and after onabotulinumtoxinA treatment based on length of time from stroke diagnosis, there was a reduction in all-cause and spasticity-related costs in the post-index period for nearly all time-from-stroke-to-treatment subgroups (p < 0.0001) (Fig. 4). These cost savings were maintained even in the subgroup of patients whose index date was most distant from the stroke event (> 365 days; p < 0.0001), although the magnitude of the reduction in costs was lessened further away from the stroke diagnosis (Fig. 4).
Fig. 4.
Change in 12-month costs before and after onabotulinumtoxinA treatment based on length of time from stroke diagnosis. p-values < 0.0001 were significant for difference in costs before and after treatment with onabotulinumtoxinA.
DISCUSSION
There is a high economic burden associated with stroke care (10–12), and healthcare costs are increased substantially for PSS (13, 14). BoNT/A (including onabotulinumtoxinA) is an effective treatment for PSS (19, 22, 23), and prior studies have assessed its cost-effectiveness for treating this condition (24, 25). However, there is currently little information about the impact of onabotulinumtoxinA treatment for PSS on overall HRU and costs for patients with stroke in real-world settings. In this analysis of data from a US claims database, we assessed HRU and costs before and after initiation of comprehensive spasticity management including onabotulinumtoxinA in patients with PSS. A strength of this analysis is the use of an administrative claims database allowing access to HRU and cost data from a large number of patients in a real-world setting across the USA.
There were significant reductions in all-cause ED visits and hospitalizations in the 12 months following treatment with onabotulinumtoxinA for PSS vs the 12-month pre-index period, as well as significant reductions in PSS-related hospital admissions. Overall, utilization of all-cause outpatient services was similar in the pre- and post-index periods, but there was variability by type. Significant increases in visits to neurologists, physical medicine specialists, and occupational therapists were observed, but significant reductions in primary care and other specialist visits were also observed. Total all-cause and spasticity-related costs decreased in the post-index period, driven largely by reductions in costs for inpatient care. Costs were reduced in the post-index period irrespective of length of time from stroke diagnosis (onabotulinumtoxinA treatment initiation within or after 180 days following stroke and within or after 365 days following stroke).
The increases in certain types of outpatient visits (i.e. neurologist, physical medicine specialist, occupational therapist) and pharmacy costs are consistent with patients initiating BoNT/A treatment for PSS and indicate that patients are receiving increased care related to this medical issue. In patients who received onabotulinumtoxinA treatment, these increases were more than offset by the observed decreases in other categories of HRU and costs, as indicated by the overall decreases in total all-cause and spasticity-related costs.
These findings are consistent with prior resource use and cost-effectiveness modelling studies of BoNT/A treatment vs usual care (routine physical/occupational therapy) or oral therapy (24, 25). In 2005, Ward et al. (25) developed a decision-tree model to analyse data from a Delphi panel survey involving 15 UK clinicians and patients with flexed-wrist/clenched-fist spasticity. Notwithstanding the bias inherent in relying on expert opinion for outcome assessment, treatment with BoNT/A as first- or second-line therapy was 1.8–1.2 times more cost-effective than oral therapy in terms of number of successfully treated months per year (i.e. months in which benefit was sufficient to continue treatment) (25). Fewer nurse hours, but more physiotherapist hours, were required for patients receiving BoNT/A than for those receiving oral therapy (25).
In 2013, Doan et al. (24) developed a simulation model based on clinical trial data to compare the cost of usual care plus onabotulinumtoxinA with usual care alone for the treatment of upper-limb PSS. Although usual care plus onabotulinumtoxinA treatment increased direct medical costs compared with usual care alone, the combination therapy was associated with reduced patient disability, increased quality-adjusted life-years, and reduced caregiver burden, and was considered cost-effective in this patient population (24).
Claims database analyses ensure the benefit of large, diverse sample sizes with longitudinal follow-up and lack of selection bias; however, certain limitations should be considered when interpreting the results of this study, owing to the type of data available. One limitation of the current analysis is the potential bias associated with the small cohort of 735 patients who received onabotulinumtoxinA for PSS and met all inclusion criteria. There are healthcare access issues at play in this regard, as there is a variable level of comfort with using toxins as a treatment for PSS across patients and healthcare providers. We chose to limit the attrition to patients with 12 months of continuous enrolment before and after initial spasticity diagnosis to check for use of onabotulinumtoxinA in the 12 months before spasticity diagnosis. This was to identify true new starts to onabotulinumtoxinA. A significant number of patients received onabotulinumtoxinA within 12 months of stroke diagnosis, and this contributed to the high pre-period costs. Spasticity-related costs would not be impacted by HRU and cost of the stroke event itself. Nevertheless, the lack of a random study sample and requirement for included patients to have continuous plan enrolment may limit the generalizability of the study findings. Furthermore, because claims data do not include key confounding information about stroke and spasticity severity, we were unable to develop and compare outcomes to an appropriately matched cohort of patients not receiving onabotulinumtoxinA treatment, given that patients not receiving onabotulinumtoxinA treatment are likely to have less severe spasticity. Consequently, it is difficult to isolate the contribution of onabotulinumtoxinA treatment to the overall cost of care for these patients. In addition, the current cost analysis does not take into account the potential for other benefits of effective treatment for PSS, including gains in patient quality of life and functioning and decreases in caregiver burden, which also can have important indirect economic consequences (16).
In conclusion, all-cause and spasticity-related HRU and costs decreased significantly in the 12 months following initiation of spasticity management including onabotulinumtoxinA treatment, suggesting that onabotulinumtoxinA may help to alleviate the economic burden associated with PSS, although this study did not demonstrate causality between onabotulinumtoxinA treatment and HRU and cost reductions. In the sensitivity analysis, cost savings in the post-index period relative to the pre-index period were largely maintained irrespective of time between stroke and initiation of onabotulinumtoxinA. The data presented reflect HRU in the USA, and similar data have been used in other countries to support the value of onabotulinumtoxinA treatment in the management of spasticity (24, 25). Further long-term controlled studies would be needed to establish a causative role for onabotulinumtoxinA treatment in the declining HRU and costs associated with PSS observed in this retrospective claims database analysis.
Supplementary Material
ACKNOWLEDGEMENTS
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. Medical writing support was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and was funded by AbbVie.
Footnotes
Conflicts of interest and funding disclosure. AE has served as scientific advisor to and has received research support from Allergan (now AbbVie) and Ipsen. MV-G has been a paid consultant or on an advisory board for AbbVie, Ipsen, Medtronic, and Merz. She has a research grant from Ipsen. LB and KM-W are employees of Curta, Inc, which received financial support from AbbVie for the conduct of this study. DO is an employee of Genesis Research, which received financial support from AbbVie for the conduct of this study. AT is a former employee of AbbVie, currently employed by Curta Consulting, and may hold AbbVie stock. PG is a former employee of AbbVie, currently employed by BioCryst Pharmaceuticals, and may hold AbbVie stock.
This study was sponsored by AbbVie.
REFERENCES
- 1.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005; 27: 2–6. [DOI] [PubMed] [Google Scholar]
- 2.Wissel J, Ri S. Assessment, goal setting, and botulinum neurotoxin a therapy in the management of post-stroke spastic movement disorder: updated perspectives on best practice. Expert Rev Neurother 2022; 22: 27–42. [DOI] [PubMed] [Google Scholar]
- 3.Urban PP, Wolf T, Uebele M, Marx JJ, Vogt T, Stoeter P, et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010; 41: 2016–2020. [DOI] [PubMed] [Google Scholar]
- 4.Wissel J, Schelosky LD, Scott J, Christe W, Faiss JH, Mueller J. Early development of spasticity following stroke: a prospective, observational trial. J Neurol 2010; 257: 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundström E, Terént A, Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol 2008; 15: 533–539. [DOI] [PubMed] [Google Scholar]
- 6.Menoux D, Jousse M, Quintaine V, Tlili L, Yelnik AP. Decrease in post-stroke spasticity and shoulder pain prevalence over the last 15 years. Ann Phys Rehabil Med 2019; 62: 403–408. [DOI] [PubMed] [Google Scholar]
- 7.Katoozian L, Tahan N, Zoghi M, Bakhshayesh B. The onset and frequency of spasticity after first ever stroke. J Natl Med Assoc 2018; 110: 547–552. [DOI] [PubMed] [Google Scholar]
- 8.Hotter B, Padberg I, Liebenau A, Knispel P, Heel S, Steube D, et al. Identifying unmet needs in long-term stroke care using in-depth assessment and the Post-Stroke Checklist – the Managing Aftercare for Stroke (MAS-I) study. Eur Stroke J 2018; 3: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology 2013; 80: S13–S19. [DOI] [PubMed] [Google Scholar]
- 10.Lekander I, Willers C, von Euler M, Lilja M, Sunnerhagen KS, Pessah-Rasmussen H, et al. Relationship between functional disability and costs one and two years post stroke. PLoS One 2017; 12: e0174861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care 2010; 16: 525–533. [PubMed] [Google Scholar]
- 12.Pucciarelli G, Rebora P, Arisido MW, Ausili D, Simeone S, Vellone E, et al. Direct cost related to stroke: a longitudinal analysis of survivors after discharge from a rehabilitation hospital. J Cardiovasc Nurs 2020; 35: 86–94. [DOI] [PubMed] [Google Scholar]
- 13.Lundström E, Smits A, Borg J, Terént A. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity: the first year after the event. Stroke 2010; 41: 319–324. [DOI] [PubMed] [Google Scholar]
- 14.Raluy-Callado M, Cox A, MacLachlan S, Bakheit AM, Moore AP, Dinet J, et al. A retrospective study to assess resource utilization and costs in patients with post-stroke spasticity in the United Kingdom. Curr Med Res Opin 2018; 34: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 15.Bavikatte G, Subramanian G, Ashford S, Allison R, Hicklin D, Gill CE, et al. Early identification, intervention and management of post-stroke spasticity: expert consensus recommendations. Prevalence of spasticity in nursing home residents. J Cent Nerv Syst Dis 2021; 13: 11795735211036576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganapathy V, Graham GD, DiBonaventura MD, Gillard PJ, Goren A, Zorowitz RD. Caregiver burden, productivity loss, and indirect costs associated with caring for patients with poststroke spasticity. Clin Interv Aging 2015; 10: 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016; 86: 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wissel J, Ward AB, Erztgaard P, Bensmail D, Hecht MJ, Lejeune TM, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med 2009; 41: 13–25. [DOI] [PubMed] [Google Scholar]
- 19.Rosales RL, Chua-Yap AS. Evidence-based systematic review on the efficacy and safety of botulinum toxin-a therapy in post-stroke spasticity. J Neural Transm (Vienna) 2008; 115: 617–623. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LC, Price CI, van Wijck FM, Shackley P, Steen N, Barnes MP, et al. Botulinum toxin for the upper limb after stroke (BoTULS) trial: effect on impairment, activity limitation, and pain. Stroke 2011; 42: 1371–1379. [DOI] [PubMed] [Google Scholar]
- 21.Bhakta BB, Cozens JA, Chamberlain MA, Bamford JM. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial. J Neurol Neurosurg Psychiatry 2000; 69: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brashear A, Gordon MF, Elovic E, Kassicieh VD, Marciniak C, Do M, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med 2002; 347: 395–400. [DOI] [PubMed] [Google Scholar]
- 23.Francisco GE, Jost WH, Bavikatte G, Bandari DS, Tang SFT, Munin MC, et al. Individualized onabotulinumtoxinA treatment for upper limb spasticity resulted in high clinician- and patient-reported satisfaction: long-term observational results from the ASPIRE study. PM R 2020; 12: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doan QV, Gillard P, Brashear A, Halperin M, Hayward E, Varon S, et al. Cost-effectiveness of onabotulinumtoxinA for the treatment of wrist and hand disability due to upper-limb post-stroke spasticity in Scotland. Eur J Neurol 2013; 20: 773–780. [DOI] [PubMed] [Google Scholar]
- 25.Ward A, Roberts G, Warner J, Gillard S. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med 2005; 37: 252–257. [DOI] [PubMed] [Google Scholar]
- 26.IBM MarketScan Research Databases for life sciences researchers Somers, NY, USA: IBM Corporation; 2021. [Last accessed: 23 October 2023] Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.