Main Text
Tick‐borne encephalitis virus (TBE) is an RNA virus, a member of the flaviridae family, transmitted by tick bites or consumption of unpasteurized dairy products from infected animals. 1 TBE has a median incubation period of 8 days (range 2–48 days), 1 is symptomatic in about 33.3% of cases, 1 and follows a biphasic course in 72–87%. 1 The first viremic stage lasts 2–7 days, 1 with unspecific symptoms such as fever, headache, and myalgias, 2 followed by a 2–10 day afebrile period. 1 In the second stage, there is recrudescence of fever and signs/symptoms of CNS infection/inflammation. 2 Imaging and neuropathological studies show tropism for the thalamus, basal ganglia, and cerebellum 3 , 4 circuits known to be involved in the pathogenesis of movement disorders.
Table 1 shows a review of the case series describing movement disorders in TBE. None of which were designed to evaluate involuntary movements in a systematic manner.
TABLE 1.
Review of the involuntary movements described in case series of TBE patients
| Article | Country | Number of patients | Tremor | Ataxia | Parkinsonism | Dystonia | Myoclonus | Other |
|---|---|---|---|---|---|---|---|---|
| Grygorczuk S. et al., 2015 5 | Poland | 152 | 10 (7%) | 37 (24%) | ‐ | ‐ | ‐ | No reports of chorea, opsoclonus, athetosis, ballismus or tics. |
| Kaiser R. et al., 1999 6 | Germany | 656 | 31 (4.7%) | 119 (18%) | ‐ | ‐ | ‐ | |
| Radzišauskienė D. et al., 2017 7 | Lithuania | 712 | 338 (47.5%) | 579 (81.3%) | 11 (1.5%) | ‐ | ‐ | |
| Czupryna P. et al., 2010 8 | Poland | 687 | ‐ | 88 (14.1%) | ‐ | ‐ | ‐ | |
| Karelis G. et al., 2012 9 | Latvia | 228 | ‐ | 78 (34.1%) | ‐ | ‐ | ‐ | |
| Barp N. et al., 2020 10 | Italy | 148 | 41 (28%) | 67 (45%) | <1% | ‐ | <1% | |
| Kaiser R., 2000 11 | Germany | 731 | 29 (4%) | 164 (22%) | ‐ | ‐ | ‐ | |
| Kaiser R. et al., 2011 12 | Germany | 81 | 2 (2.4%) | 45 (55%) | ‐ | ‐ | ‐ | |
| Gunther G, et al., 1997 13 | Sweden | 85 | 8 (9.4%) | 22 (26%) | ‐ | ‐ | ‐ | |
| Lenhard T. et al., 2016 14 | Germany | 111 | ‐ | 17 (15%) | ‐ | ‐ | ‐ | |
| Kohlmaier B. et al., 2021 15 | European multicenter study | 555 | 210 (38%) | 206 (37%) | ‐ | ‐ | ‐ | |
| Radzišauskienė D. et al., 2020 16 | Lithuania | 1040 | 526 (51%) | 819 (79%) | 69 (7%) | ‐ | ‐ | |
| Anić K. et al., 1998 17 | Croatia | 92 | 20 (22%) | 40 (43%) | ‐ | ‐ | ‐ | |
| Logar M. et al., 2000 18 | Slovenia | 60 | 30 (50%) | ‐ | ‐ | ‐ | ‐ | |
| Mickienė A. et al., 2002 19 | Lithuania | 133 | 29 (22%) | 35 (26%) | ‐ | ‐ | ‐ | |
| Krech T. 1980 20 | Germany | 58 | 2 (3%) | 7 (12%) | ‐ | 1 (1.5%) | ‐ | |
| Radsel‐Medvescek A. et al., 1982 21 | Slovenia | 50 | 8 (16%) | 24 (48%) | ‐ | ‐ | ‐ | |
| Haglund M. et al., 1996 22 | Sweeden | 114 | 13 (11%) | 1 (0.8%) | ‐ | ‐ | ||
| Czupryna P. et al., 2018 23 | Poland | 1072 | ‐ | 64 (6%) | ‐ | ‐ | ‐ | |
| Grygorczuk G. et al., 2023 24 | Poland | 96 | ‐ | 25 (26%) | ‐ | ‐ | ‐ | |
| Veje M. et al., 2016 25 | Sweden | 96 | 15 (16%) | 13 (14%) | ‐ | ‐ | ‐ | |
| Velay A. et al., 2017 26 | France | 54 | 15 (28%) | 16 (30%) | ‐ | ‐ | ‐ | |
| Lämmli, B. et al., 2000 27 | Switzerland | 57 | 12 (21%) | ‐ | ‐ | ‐ |
Note: Methodology: In June 2023, we performed a search of the relevant literature using the MEDLINE database using the terms “tick‐borne encephalitis” with the following additional search terms: “Movement Disorders,” “Hyperkinetic,” “Hypokinetic,” “Parkinsonism,” “Tremor,” “Ataxia,” “Dystonia,” “Myoclonus,” “Opsoclonus,” “Chorea,” “Athetosis,” “Ballismus,” and “Tics”. References were checked for potentially missing relevant articles. Studies including only pediatric patients were excluded.
We present a case of a patient with TBE who developed a complex pattern of involuntary movements.
Case Report
A 44‐year‐old female presented with an eight‐day history of fever (38.0–39.2°C), bilateral frontal throbbing headache of moderate intensity, photophobia, nausea, and myalgias. She denied vomiting, and worsening with decubitus or Valsalva maneuvers. Symptoms progressed over the course of 1 week to sleepiness and language disturbance (unintelligible speech and impaired comprehension). She was a seasonal migrant worker in grape harvesting in Aigle (Switzerland) and returned to Portugal 1 month before hospital admission. In retrospect, she was bitten by a tick in Switzerland. The patient denied other relevant medical history or medications.
The initial neurological exam disclosed mild somnolence, moderate inattentiveness, global aphasia, and nuchal rigidity. Examination of the cranial nerves, muscular strength and sensibility was unremarkable. No involuntary movements or appendicular ataxia were present. On the third day of hospital admission, there was a significant clinical improvement of alertness and language but she developed a complex pattern of involuntary movements: stimulus‐sensitive perioral myoclonus and of the upper limbs, mild bilateral bradykinesia and mild appendicular/axial rigidity, dystonic posturing of the head (right laterocollis), hands and fingers, slight bilateral cerebellar ataxia more predominant on the right (Video 1). Cognitive evaluation disclosed disorientation in time, mild inattentiveness, mild anomic aphasia, working memory and short‐term memory impairment, executive dysfunction and visuospatial deficits.
VIDEO 1.
Segment 1: Facial myoclonus predominantly on the left side; Segment 2: Distal stimulus sensitive myoclonus of the upper limbs, slight dystonic posturing of the fingers; Segment 3: Bilateral sight ataxia, more marked on the right side; Segment 4: Mild parkinsonism, more marked on the left upper limb.
The CSF showed pleocytosis (95 μL/L|97% mononuclear cells), and hyperproteinorrachia (1.31 mg/dL), so empirical therapy with acyclovir and ceftriaxone was started. Table 2 shows the results of the investigations performed.
TABLE 2.
Results of the investigation performed
| Infectious screening | |
|---|---|
| CSF | Negative: CSF bacterial, mycobacterial and fungal cultures; VDRL; TPHA; Wright test; PCR screening for viral meningitis (Herpes simplex 1; Herpes simplex 2; Varicela‐zoster; Enterovirus; Parechovirus; Adenovirus); PCR for bacterial meningitis (Neisseria meningitidis; Haemophilus influenzae; Haemophilus influenzae B; Streptococcus pneumoniae); PCR for zoonosis (Anaplasma phagocytophilum; Ehrlichia chaffeensis, E. muris; Borrelia burgdorferi s. l., B. miyamotoi, B. hermsii; Coxiella burnetii; Babesia microti, Babesia divergens; Rickettsia spp; tick‐borne encephalitis virus); borrelia anti‐bodies; CMV; Mycobacterium tuberculosis DNA |
| Normal values: ADA | |
| Serum | Negative: Blood cultures, Wright test; rose bengal; serologies for HIV, Hepatitis B and C; borrelia anti‐bodies; PCR for zoonosis (Anaplasma phagocytophilum; Ehrlichia chaffeensis, E. muris; Borrelia burgdorferi s. l., B. miyamotoi, B. hermsii; Coxiella burnetii; Babesia microti, Babesia divergens; Rickettsia spp; tick‐borne encephalitis virus); Herpes silplex 1 and 2 DNA |
| Auto‐imune/inflamatory/Paraneoplasic | |
| Serum | Negative: Indirect imunoflurescence for ANAS and ANCAS; lupic anti‐coagulant; antibodies anti: cadiolipin; Beta‐2 glycoprotein; MPO; PR3; Mi; Ku; RNP; Sm; SSA; Ro 52; SSB; Scl 70; PM‐Scl; Jo‐1; CENP B; PCNA; dsDNA; nucleossomes; histones; ribossome P, M2; DFS 70); thyroglobulin; thyroid peroxidase. |
| Negative anti‐neuronal antibodies (Yo (PCA‐1); Hu (ANNA‐1); Ri (ANNA‐2); Tr (DNER); GAD65; zic4 Titin; SOX1; Recoverin; PNMA2 (Ma2/Ta); CV2/CRMP5; amphiphysin; NMDA) | |
| Normal: ACE; Erytocyte sedimentation rate | |
| CSF | Negative: Olygoclonal bands; Antineuronal antibodies (NMDA; LGI1; CASPR2; AMPA receptors; GABAb and GABAa; VGKC) |
| Metabolic | |
| Serum | Normal values: Vitamine B12; folic acid; Thyroid hormones |
| Neoplasic | |
| Serum | Normal values: b‐HCG; Beta‐2 microglobulin |
| Imaging | Mamography, mamary ecography and pelvic ecography were normal. |
Initial CSF and serum infectious screening was negative, which included PCR panels for common neurotropic virus and zoonosis (including TBE) and the initial antimicrobial therapy was stopped. Anti‐neuronal antibodies were negative. Brain MRI (on the third day of hospital admission) demonstrated bilateral thalamic and left temporal medial T2/FLAIR hyperintensities, with no restricted diffusion or contrast enhancement (Fig. 1). EEG at admission showed moderate encephalopathy with no epileptiform activity. EEG at day 5 of hospital admission was normal.
Figure 1.
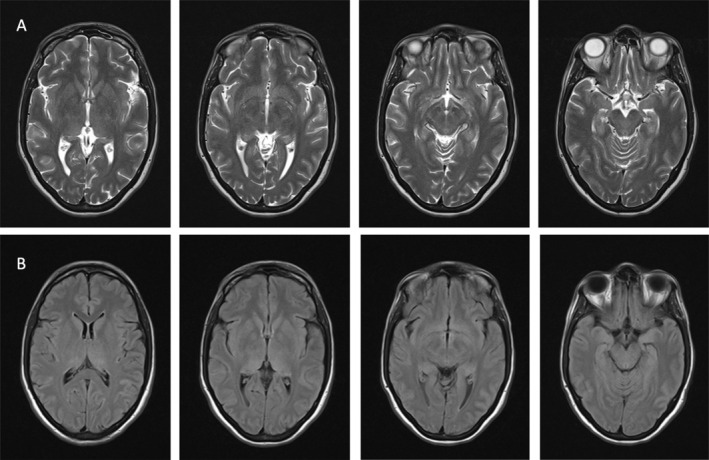
Brain MRI 1.5 Tesla, Axial cuts. Panel (A) T2 sequence; Panel (B) FLAIR; Both sequences show Bilateral Hyperintensities of the thalamus and left medial temporal lobe.
The differential diagnosis of encephalitis with bilateral T2/FLAIR thalamic hyperintensities includes infectious disorders, auto‐immune/paraneoplastic encephalitis, and auto‐immune/inflammatory disorders (such as ADEM and neuro‐behçet), and lymphoma. Flavivirus‐associated encephalitis have a particular tropism for the basal ganglia.
The patient's initial non‐specific symptomatic prodrome, tick‐bite history and clinical improvement without of immunotherapy supported the diagnostic hypothesis of viral infection. Laboratory results were not suggestive of other causes.
Therefore serum and CSF serologies were obtained for vector‐transmitted virus, which were positive for TBE.
At 6 months follow‐up, the patient showed slight facial stimulus sensitive myoclonus predominantly on the left hemiface; myoclonus of the hands and mild asymmetrical parkinsonism, worse on the right (Video 2). Cognitive assessment was normal. Brain MRI and CSF were unremarkable.
VIDEO 2.
Segment 1: Palpebral myokymia, facial stimulus sensitive myoclonus predominantly on the left hemiface; slight right laterocollis; Segment 2: Bilateral stimulus sensitive distal myoclonus of the upper limbs; Segment 3: Kinetic tremor of the upper limbs Segment 4: Mild parkinsonism more marked on the right hemibody.
Discussion
Up to 20.4% 2 of secondary movement disorders are associated with infectious diseases, either as a direct consequence of damage to the basal ganglia circuit, 2 abnormal circuit reorganization, 2 or as immediate/delayed immune responses to the infectious agent. 2 Our patient developed a complex pattern of involuntary movements with imaging showing bilateral thalamic lesions (although we cannot exclude the involvement of the subthalamus as well). The development of complex movement disorders probably involves the disruption of the functional network of circuits 7 connecting the striatum, subthalamic, dentate nuclei, the cerebral cortex, and the thalamus which acts as a relay for both the input and output to the basal ganglia circuit. 7
In case series of TBE patients, tremor has been reported in 2.4–51% 6 , 16 cases; ataxia in 6–81.3%, 7 , 23 parkinsonism in 0.8–7% 7 , 16 and dystonia in 1.5%. 20 There were no reports of tics, opsoclonus, or chorea although the majority of patients were not evaluated by neurologists. Chorea, 28 palatal myoclonus, 29 and cervical dystonia 20 have been described in one case, respectively.
To our knowledge, this is the first case of TBE described in Portugal. Despite not being an endemic virus in Portugal, due to its long incubation period, it should be included in the differential diagnosis of meningoencephalitis/meningitis in emigrants from endemic regions. PCR CSF and serum determinations are usually positive only in the first viremic phase, 1 as such serological studies (which remain elevated thought the disease course) should be obtained in all patients. 1
Movement disorders in TBE patients are currently insufficiently characterized and may be underreported. Our case shows a complex pattern of involuntary movements that developed later in the disease course and had significant improvement at 1 month, which emphasizes the importance of serial clinical evaluations.
Author Roles
Research project: A. Conception, B. Organization, C. Execution;
Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
Manuscript Preparation: A. Writing of the first draft, B. Review and Critique;
M.V.: 1A,1B, 1C, 3A
A.C.: 1B, 1C, 3B
M.M.: 1B, 1C, 3B
R.R.: 1A, 1B, 1C, 3B
A.G.V.: 1B, 1C, 3B.
Disclosures
Ethical Compliance Statement: We are submitting a clinical case, as such no authorization by the ethics committee of our hospital was required, all clinicians involved in this patient care complied with the ethics norms by the Portuguese Ordem dos Médicos. Written informed consent was obtained with the patient for documentation of the neurological examination in video, for the submission of the clinical case and videos to a peer‐reviewed medical journal. We confirm that all authors have read the Journal's Ethical Publication Guidelines and affirm that the work is consistent with those guidelines.
Funding Sources and Conflict of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
Acknowledgments
None.
References
- 1. Taba P, Schmutzhard E, Forsberg P, et al. EAN consensus review on prevention, diagnosis and management of tick‐borne encephalitis. Eur J Neurol 2017;24(10):e1214–e1261. [DOI] [PubMed] [Google Scholar]
- 2. Schmutzhard E, Poewe W. Movement disorders in encephalitis. In: Poewe W, Jankovic J, eds. Movement Disorders in Neurologic and Systemic Disease. Cambridge, England: Cambridge University Press; 2014:293–313. [Google Scholar]
- 3. Pichler A, Sellner J, Harutyunyan G, et al. Magnetic resonance imaging and clinical findings in adults with tick‐borne encephalitis. J Neurol Sci 2017;15(375):266–269. [DOI] [PubMed] [Google Scholar]
- 4. Gupta N, Pandey S. Post‐thalamic stroke movement disorders: a systematic review. Eur Neurol 2018;79(5–6):303–314. [DOI] [PubMed] [Google Scholar]
- 5. Grygorczuk S, Parczewski M, Moniuszko A, et al. Increased concentration of interferon lambda‐3, interferon beta and interleukin‐10 in the cerebrospinal fluid of patients with tick‐borne encephalitis. Cytokine 2015;71(2):125–131. [DOI] [PubMed] [Google Scholar]
- 6. Kaiser R. The clinical and epidemiological profile of tick‐borne encephalitis in southern Germany 1994‐98: a prospective study of 656 patients. Brain 1999;122(Pt 11):2067–2078. [DOI] [PubMed] [Google Scholar]
- 7. Radzišauskienė D, Žagminas K, Ašoklienė L, et al. Epidemiological patterns of tick‐borne encephalitis in Lithuania and clinical features in adults in the light of the high incidence in recent years: a retrospective study. Eur J Neurol 2018;25(2):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czupryna P, Moniuszko A, Pancewicz SA, Pancewicz SA, Grygorczuk S, Kondrusik M, Zajkowska J. Tick‐borne encephalitis in Poland in years 1993–2008‐epidemiology and clinical presentation. A retrospective study of 687 patients. Eur J Neurol 2011. May;18(5):673–679. [DOI] [PubMed] [Google Scholar]
- 9. Karelis G, Bormane A, Logina I, Lucenko I, Suna N, Krumina A, Donaghy M. Tick‐borne encephalitis in Latvia 1973‐2009: epidemiology, clinical features and sequelae. Eur J Neurol 2012;19(1):62–68. [DOI] [PubMed] [Google Scholar]
- 10. Barp N, Trentini A, Di Nuzzo M, et al. Clinical and laboratory findings in tick‐borne encephalitis virus infection. Parasite Epidemiol Control 2020;19(10):e00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaiser R. Epidemiology and progress of early summer meningoencephalitis in Baden‐Württemberg between 1994 and 1999. A prospective study of 731 patients. Dtsch Med Wochenschr 2000;125(39):1147–1153. [DOI] [PubMed] [Google Scholar]
- 12. Kaiser R. Long‐term prognosis of patients with primary myelitic manifestation of tick‐borne encephalitis: a trend analysis covering 10 years. Nervenarzt 2011;82(8):1020–1025. [DOI] [PubMed] [Google Scholar]
- 13. Günther G, Haglund M, Lindquist L. Tick‐bone encephalitis in Sweden in relation to aseptic meningo‐encephalitis of other etiology: a prospective study of clinical course and outcome. J Neurol 1997;244(4):230–238. [DOI] [PubMed] [Google Scholar]
- 14. Lenhard T, Ott D, Jakob NJ, et al. Predictors, neuroimaging characteristics and long‐term outcome of severe European tick‐borne encephalitis: a prospective cohort study. PloS One 2016;11(4):e0154143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kohlmaier B, Schweintzger NA, Sagmeister MG. Clinical characteristics of patients with tick‐borne encephalitis (TBE): a European multicentre study from 2010 to 2017. Microorganisms 2021;9(7):1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radzišauskienė D, Urbonienė J, Kaubrys G. The epidemiology, clinical presentation, and predictors of severe tick‐borne encephalitis in Lithuania, a highly endemic country: a retrospective study of 1040 patients. PloS One 2020;15(11):e0241587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anić K, Soldo I, Perić L, Karner I, Barac B. Tick‐borne encephalitis in eastern Croatia. Scand J Infect Dis 1998;30(5):509–512. [DOI] [PubMed] [Google Scholar]
- 18. Logar M, Arnez M, Kolbl J, Avsic‐Zupanc T, Strle F. Comparison of the epidemiological and clinical features of tick‐borne encephalitis in children and adults. Infection 2000;28(2):74–77. [DOI] [PubMed] [Google Scholar]
- 19. Mickiene A, Laiskonis A, Günther G. Tickborne encephalitis in an area of high endemicity in Lithuania: disease severity and long‐term prognosis. Clin Infect Dis 2002;35(6):650–658. [DOI] [PubMed] [Google Scholar]
- 20. Krech T. TBE foci in Switzerland. Int J Med Microbiol 2002;291(Suppl 33):30–33. [DOI] [PubMed] [Google Scholar]
- 21. Radšel‐Medvešček A, Marolt‐Gomišček M, Povše‐Trojar M, et al. Late sequels after tick‐borne meningoencephalitis in patients treated at the Hospital for Infectious Diseases University Medical Centre of Ljubljana during the period 1974‐1975. In: Vesenjak‐Hirjan J, ed. Arbnoviruses in the Mediterranean Countries. Vol 1980. Stuttgart: Zbl Bakt; 1982:281–284. [Google Scholar]
- 22. Haglund M, Forsgren M, Lindh G, et al. A 10‐year follow‐up study of tick‐borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy. Scand J Infect Dis 1996;28(3):217–224. [DOI] [PubMed] [Google Scholar]
- 23. Czupryna P, Grygorczuk S, Krawczuk K, et al. Sequelae of tick‐borne encephalitis in retrospective analysis of 1072 patients. Epidemiol Infect 2018;146(13):1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grygorczuk S, Osada J, Sulik A, et al. Associations of the cerebrospinal fluid lymphocyte population with a clinical presentation of tick‐borne encephalitis. Ticks Tick Borne Dis. 2023;14(5):102204. [DOI] [PubMed] [Google Scholar]
- 25. Veje M, Nolskog P, Petzold M, Bergström T, Lindén T, Peker Y, Studahl M. Tick‐borne encephalitis sequelae at long‐term follow‐up: a self‐reported case‐control study. Acta Neurol Scand 2016;134(6):434–441. [DOI] [PubMed] [Google Scholar]
- 26. Velay A, Solis M, Kack‐Kack W, et al. A new hot spot for tick‐borne encephalitis (TBE): a marked increase of TBE cases in France in 2016. Ticks Tick Borne Dis 2018;9(1):120–125. [DOI] [PubMed] [Google Scholar]
- 27. Lämmli B, Müller A, Ballmer PE. Late sequelae of early summer meningoencephalitis. Schweiz Med Wochenschr 2000;130(24):909–915. [PubMed] [Google Scholar]
- 28. Zajkowska J, Moniuszko A, Czupryna P, et al. Chorea and tick‐borne encephalitis. Poland Emerg Infect Dis 2013;19(9):1544–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pogorzelski R, Drozdowski W, Rogowski M. Symptomatic palatal myoclonus with ear click after tick‐borne meningoencephalitis. Eur Arch Otorhinolaryngol 2006;263(8):711–713. [DOI] [PubMed] [Google Scholar]


