Abstract
Brain tumours are the most common solid neoplasm in children, posing a significant challenge in oncology due to the limited range of treatment. Intraoperative magnetic resonance imaging (iMRI) has recently emerged to aid surgical intervention in neurosurgery resection with the potential to delineate tumour boundaries. This narrative literature review aimed to provide an updated evaluation of the clinical implementation of iMRI in paediatric neurosurgical resection, with an emphasis on the extent of brain tumour resection, patient outcomes and its drawbacks. Databases including MEDLINE, PubMed, Scopus and Web of Science were used to investigate this topic with key terms: paediatric, brain tumour, and iMRI. Exclusion criteria included literature comprised of adult populations and the use of iMRI in neurosurgery in the absence of brain tumours. The limited body of research evaluating the clinical implementation of iMRI in paediatric cohorts has been predominantly positive. Current evidence demonstrates the potential for iMRI use to increase rates of gross total resection (GTR), assess the extent of resection, and improve patient outcomes, such as progression‐free survival. Limitations regarding the use of iMRI include prolonged operation times and complications associated with head immobilisation devices. iMRI has the potential to aid in the achievement of maximal brain tumour resection in paediatric patients. Future prospective randomised controlled trials are necessary to determine the clinical significance and benefits of using iMRI during neurosurgical resection for clinical management of brain neoplasms in children.
Keywords: Brain neoplasm, intraoperative magnetic resonance imaging, paediatric, surgery
Brain tumours are the most common solid neoplasm in children, posing a significant challenge in oncology due to the limited range of treatment. Intraoperative magnetic resonance imaging (iMRI) has recently emerged to aid surgical intervention in neurosurgery resection. This literature review evaluated the clinical implementation of iMRI in paediatric neurosurgical resection.

Introduction
Brain tumours are the most common solid neoplasm in children, with a variety of histological tumour presentations affecting the paediatric population, including astrocytomas, medulloblastomas and ependymomas. 1 Low‐grade tumours account for a large proportion of epileptogenic lesions, 2 with epilepsy being the most common neurological disorder affecting the paediatric population. 3 Paediatric brain tumours are a key challenge in modern oncology as available treatments are limited, 4 thereby resulting in high symptomatic burden 5 and poor patient prognosis. 6 , 7
Surgical resection is an important avenue in the treatment of paediatric brain tumours. 8 Magnetic resonance imaging (MRI) is used for surgical planning, 9 and plays a role in determining the extent of tumour volume to be resected, 9 , 10 , 11 as well as post‐operative evaluations. 12 Recently, intraoperative MRI (iMRI) has emerged to aid surgical intervention by providing real‐time data to delineate tumour boundaries during surgery, with the potential to maximise the extent of resection (EOR). 13 , 14 , 15 EOR is defined as the proportion of the tumour volume removed during surgery. 16 Use of high‐field strength iMRI has been reported to increase EOR and improve patient survival within the adult population, 17 , 18 , 19 , 20 , 21 with increasing work demonstrating high‐field iMRI as a potential surgical adjunct in the management of paediatric brain tumours. 22 , 23 , 24 , 25 , 26 EOR plays a major role in treating paediatric brain tumours and has been recognised as an integral predictive factor for survival in children. 27 Gross total resection (GTR), defined as the EOR that achieves complete surgical removal of tumour mass, 28 can be curative in a large proportion of paediatric tumour types. 29 Importantly, in low‐grade gliomas, GTR has been recognised as an independent positive prognostic factor. 30 Associated improved patient outcomes can be measured by progression‐free survival (PFS), 31 and further, Engel Score (Grade I–IV) is used to define epilepsy seizure freedom post‐resection. 32 Length of hospital stay is another important consideration for the implementation of iMRI regarding patient outcomes, with a longer hospital stay associated with an increased risk of infection. 33 , 34
Despite accumulating evidence demonstrating the utility of iMRI in brain tumour resections in adults, 17 , 18 , 19 , 20 , 21 studies evaluating its use in the paediatric population are not extensive, and there are relatively few literature reviews. Notably, a meta‐analysis 35 identified 11 studies reporting the EOR and safety outcomes in paediatric patients, but data were limited to papers published prior to 2017 and focused on low‐grade gliomas. The last five years have seen a continuous advancement of technology and continuing interest in the study of paediatric patients undergoing iMRI‐assisted brain tumour resection surgeries, including some large cohort studies. 36 , 37 Therefore, the objective of this narrative literature review was to provide an updated synthesis on the use of iMRI in paediatric brain tumour resections. The main considerations were EOR and patient outcomes, with further investigation into the drawbacks of the clinical implementation of iMRI.
Methods
A narrative review was undertaken to provide a broad scope of the available literature. A search was conducted on four databases: MEDLINE, PubMed, Scopus and Web of Science using a combination of key words: pediatric*, paediatric*, child, childhood, children, infant*, adolescent*, juvenile*, young adult*, teen, teenager*, neonatal*, neonate*, brain tumo?r*, cancer, neoplasm, malignancy, malignancies, neurosurgery, neurosurgeries, brain surgery, brain surgeries, mass, masses, intraoperative magnetic resonance imaging, intraoperative MRI, iMRI, ioMRI. The keyword search employed appropriate truncations and Boolean operators. The search limits included: mean age of study participants as <18 years old, human studies, English language, and 2016–2022 publication years. Additional references were sourced through a manual check of the cited reference lists. The eligibility criteria used to filter the retrieved studies were the paediatric population (for this review, identified as <18 years old) and use of iMRI in the surgical removal of brain tumours. The literature selection process is outlined in Figure 1. The full‐text articles that met the eligibility criteria were reviewed by two authors independently. The relevant data were extracted, then summarised and organised thematically into tables. From this, a written discussion was generated, and conclusions that were relevant to the aim were drawn.
Figure 1.
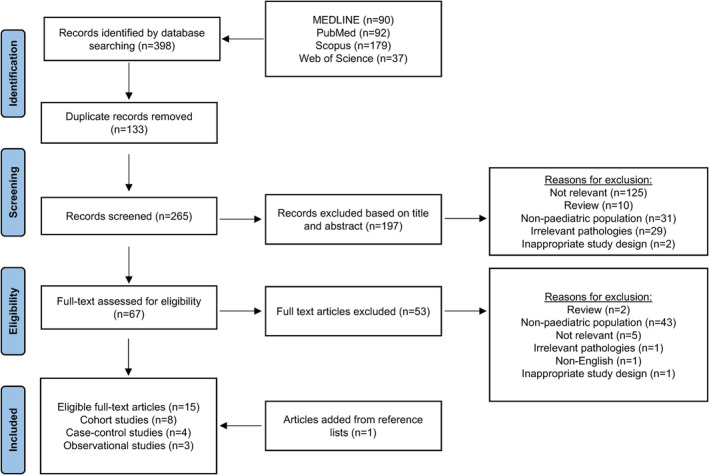
PRISMA flowchart depicting the literature search strategy and study selection process.
Results
Study characteristics
A total of 15 studies were included in this literature review (Fig. 1). Key study characteristics are summarised in Table 1. There were eight cohort studies, four case‐control studies and three studies that did not specify a study design and were broadly defined as observational studies. There were eight retrospective studies, one prospective study and six studies that retrospectively analysed prospectively and retrospectively collected data. Retrospective studies collated data from databases and patient medical records. Of the 15 studies, 14 studies were conducted in a single centre, while one study 37 included patients from six facilities across North America. Studies were conducted in various countries: United States (n = 4), Germany (n = 3), Canada (n = 3), Netherlands (n = 2), United Kingdom (n = 2), and Singapore (n = 1). The studies varied in sample size, ranging from 10 to 1341 paediatric patients. While the studies were published between 2016 and 2022, the reported neurosurgical procedures were performed and follow‐up data were collected between 1991 and 2020.
Table 1.
Summary of key study characteristics of included studies examining extent of resection (EOR), complications and patient outcomes following brain tumour removal surgeries under intraoperative magnetic resonance imaging (iMRI) guidance in paediatric patients.
| Study | Study design | Sample size | iMRI strength | Findings |
|---|---|---|---|---|
|
Avula et al. (2021) 42 |
Single centre, prospective case–control study | n = 20 patients | 3T | 100% GTR where GTR was intended (11 cases) and 100% partial resection where partial resection was intended (9 cases), which is seen on both iMRI and 24–72 hour post‐operative MRI scans |
| Sunderland et al. (2021) 44 | Single centre, cohort study that retrospectively analysed prospective data | n = 30 patients (20 for tumour resection, 25 procedures) | High‐field strength | GTR: 94.2% with iMRI and 37.5% without iMRI (P = 0.014) |
| Eid et al. (2020) 39 | Single centre, retrospective case‐control study | n = 80 patients (45 in iMRI, 35 in non‐iMRI) | 1.5T or 3T |
GTR: 73.3% with iMRI and 62.2% without iMRI (P = 0.46) Frequency of poor seizure outcome: 11.1% with iMRI and 28.6% without iMRI (P = 0.048) |
| Low et al. (2020) 45 | Single centre, cohort study that retrospectively analysed prospective data | n = 35 patients | 1.5T |
100% GTR where GTR was intended with iMRI 100% STR where STR was intended with iMRI |
| Day and Scott (2019) 43 | Single centre, retrospective cohort study | n = 53 patients, 59 procedures | 1.5T | iMRI was useful in guiding the EOR in 54.4% of cases. Ultimate determinants of patient outcome were tumour genetics and behaviour, regardless of EOR |
| Karsy et al. (2019) 37 | Multi‐centre, cohort study that retrospectively analysed prospectively and retrospectively collected data | n = 314 patients (280 for tumour resection) | 1.5T or 3T |
55.7% GTR, 8.6% NTR, 11.1% STR, 24.6% unknown EOR with iMRI 46.8% additional surgery with iMRI 87.9% of post‐iMRI tumour positive on pathological analysis 8.2% mortality No significant difference in PFS or OS between patients who underwent additional resection after iMRI and those who did not. No significant difference in OS of PFS between lesion grades |
| Saint‐Martin et al. (2019) 50 | Single centre, retrospective observational study | n = 101 patients, 115 procedures | High‐field strength | 13 of 115 iMRI‐assisted cases (11%) were complicated by a hyperacute arterial ischemic infarct. 4 of which were detected on intraoperative DWI scans |
| Dias et al. (2018) 46 | Single centre, cohort study that retrospectively analysed prospectively and retrospectively collected data | n = 17 patients | 3T | In 71% of cases, tumour resection continued after iMRI. iMRI showed GTR in 4 cases, and residual tumour in 1 case |
| Khan et al. (2018) 51 | Single centre, retrospective observational study | n = 895 procedures | 3T | 28 of 895 tumour resection cases resulted in strokes, 11 of which used iMRI. Out of 11 cases, iMRI only detected 5 infarcts (45%). Strokes undetected on iMRI were deep arterial strokes |
| Tejada et al. (2018) 36 | Single centre, cohort study that retrospectively analysed prospectively collected data | n = 255 procedures (175 for tumour resection) | 3T | iMRI identified residual tumour in 27.5% of cases where GTR was intended and 56% where STR was planned. All 20 epilepsy patients achieved complete seizure freedom |
| van Tonder et al. (2018) 41 | Single centre, retrospective cohort study | n = 10 patients, 11 procedures | High‐field strength |
50% GTR, 30% NTR, 20% STR (~30% residual tumour volume) with iMRI 100% of patients had a good seizure outcome (Engle score I or II) 80% achieved complete seizure freedom No new neurological complications reported |
| Zaazoue et al. (2018) 49 | Single centre, retrospective observational study | n = 940 patients (599 for tumour resection) | 1.5T | DORO clamp head immobilisation device used in iMRI‐assisted procedures. 7 patients suffered depressed skull fractures due to DORO clamp. All 7 patients had brain neoplasms |
| Giordano et al. (2017) 47 | Single centre, retrospective cohort study | n = 75 patients, 82 procedures | 1.5T |
In glioma cases: 56% GTR, 26% STR, 18% NTR with iMRI, 27% additional surgery with iMRI due to the detection of residual tumour No intraoperative complications reported with the use of iMRI |
| Roder et al. (2016) 48 | Single centre, case–control study that retrospectively analysed prospectively collected data | n = 65 patients (31 iMRI, 34 non‐iMRI) | 1.5T |
GTR: 71% with iMRI and 41% without iMRI (P = 0.05) Smaller residual on 3‐month post‐operative scans with iMRI 90% PFS in cohort with complete resection, 30% PFS with incomplete resection (P < 0.001) No relevant iMRI‐related or infectious complications reported |
| Warsi et al. (2016) 40 | Single centre, retrospective case–control study | n = 78 procedures (39 iMRI, 39 non‐iMRI) | 3T | In well‐defined cases, 88.9% GTR with iMRI, 100% GTR without iMRI. Good seizure outcomes were more common in iMRI group across lesion types. iMRI does not significantly improve outcomes in paediatric epilepsy surgery |
DWI, diffusion‐weighted imaging; EOR, extent of resection; GTR, gross total resection; iMRI, intraoperative magnetic resonance imaging; MRI, magnetic resonance imaging; NTR, near‐total resection; OS, overall survival; PFS, progression‐free survival; STR, subtotal resection.
Synthesis of findings
Across all 15 studies, the most common brain tumours resected were low‐grade gliomas, with sub‐types including astrocytomas and gangliogliomas. Of the included studies, 12 studies reported the EOR, which is often categorised as GTR, subtotal resection or partial resection, and seven studies reported patient outcomes (Table 1). Several studies discussed the drawbacks of iMRI, with two studies focusing on stroke complications observed in iMRI‐assisted surgeries of paediatric patients (Table 1).
The method of reporting tumour types was inconsistent across the studies. Brain tumours were stratified based on their anatomical location, specific tumour type or World Health Organization (WHO) grade (I–IV). 38 There were three studies 39 , 40 , 41 where iMRI was used for epilepsy surgeries, with two of these studies 39 , 40 incorporating other neurological lesions in addition to brain tumours, such as malformations of cortical development and mesial temporal sclerosis. While Eid et al. 39 listed the specific pathologies, their findings did not distinguish between brain tumours and other lesions. In contrast, Warsi et al. 40 broadly classified their pathologies on whether they were well‐defined, poorly defined or diffuse hemispheric cases. Furthermore, two studies 36 , 42 defined paediatric as up to 21 years old, with an additional two studies 40 , 43 including patients up to 26 years old. However, the mean age across all studies ranged from 5.83 to 13.1 years, and thus were included in the review.
Discussion
Rates of gross total resection
Within the identified studies, GTR was a common goal in the iMRI‐assisted paediatric neurosurgeries (Table 1). 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 There were three case–control studies that compared rates of GTR in procedures with and without iMRI. 39 , 44 , 48 Sunderland et al. 44 found that a significantly higher proportion of cases achieved GTR in iMRI (94.2%) compared to conventional surgeries without iMRI (37.5%). Similarly, Roder et al. 48 reported that GTR rates significantly increased by 30% following iMRI implementation. Volumetric analysis at the 3‐month follow‐up further demonstrated significantly lower rates of residual tumour volume in the iMRI cohort (29%) compared to non‐iMRI cases (59%). 48 They observed a trend towards higher accuracy of the operating surgeons' assessment of whether GTR had been achieved, with post‐operative imaging confirming the achievement of GTR in 81% of iMRI procedures compared to only 50% of non‐iMRI cases. 48 Eid et al. 39 also reported higher rates of GTR for iMRI cases (73.3%) compared to that of non‐iMRI cases (65.7%), although no statistical significance was found. In their volumetric analysis, however, the reduction of residual tumour volume was statistically significant, with iMRI decreasing residual tumour mass by 11.7%. 39 These studies suggest that iMRI is successful in increasing GTR rates in paediatric brain tumour resections. 39 , 44 , 48
Impact on extent of resection
Studies indicate that iMRI can improve resection rates beyond the surgical goal. 36 , 37 , 41 , 43 , 47 , 48 iMRI led to the surgical decision to remove additional tissue in up to 46.8% of cases (Table 1). 36 , 37 , 41 , 43 , 47 , 48 Following iMRI usage, Giordano et al. 47 demonstrated an increase of EOR from partial (<90% of tumour volume) to subtotal (≥90% of tumour volume) resection in 50% of glioma cases where partial resection had been planned. Tejada et al. 36 found that in 79.5% of cases, only one iMRI scan was required to improve the EOR beyond the surgical goal. Furthermore, iMRI effectively demonstrated where residual tumour was strongly adhered to vascular structures or close to the eloquent tissue, thereby assisting surgeons to recognise when resection should be ceased. 47 , 48 iMRI appears to be a valuable surgical adjunct to achieve maximal resection in children. However, none of these studies conducted statistical analyses.
Effect of tumour anatomy
Few studies assessed the impact of tumour anatomy on the utility of iMRI in paediatric tumour resections. 43 , 47 , 48 Roder et al. 48 reported that the border zone to the eloquent tissue was a limiting factor in achieving GTR. Day and Scott 43 observed that iMRI aided in additional resection in such cases where the tumour was adjacent to the eloquent areas, as well as when tumour borders could not be visualised or the tumour was deep within the brain. They concluded that iMRI was most effective in cases where tumours exhibited contrast enhancement and were located in anatomically accessible regions of the brain. 43 Giordano et al. 47 found that iMRI was particularly useful in the resection of histological entities that could not be microscopically differentiated from normal tissues, such as gangliomas and ependymomas; however, statistical analysis was not conducted.
In some cases, iMRI was of limited use due to tumour anatomy. 43 Day and Scott 43 found that well‐defined tumours did not require iMRI to achieve the surgical goal. Tumours invading the brainstem, hypothalamus, or basal ganglia were shown to benefit most from visual and anatomical cues for resection as opposed to iMRI guidance. 43 Imaging characteristics of the tumour limited the ability of iMRI to improve resection, such as when tumours did not enhance relative to their surrounding structures. 43 They observed that residual tissue of non‐enhancing tumours were indistinguishable from surgically‐induced cytotoxic oedema of the tumour bed as the intraoperative signal enhancement of the oedema was identical to that of the tumour in the pre‐operative scans, limiting additional tumour resection. 43 This phenomenon was particularly pronounced in patients with low‐grade gliomas, oligoastrocytomas and other benign tumours. 43 In a case of reoperation, glial scar tissue displayed similar enhancement to residual tumour mass in the intraoperative scan and was resected in error. 43 Similarly, in a case observed by Roder et al., 48 the radiologist was unable to distinguish between a residual tumour tissue and a glial scar tissue, thereby hindering effective tumour removal. Ultimately, Day and Scott 43 suggested that tumour characteristics can influence the utility of iMRI.
Given the varied usefulness of iMRI due to tumour anatomy, WHO graded I–IV tumours have the potential to differentially affect the surgical outcomes of iMRI‐guided paediatric brain tumour resections. 37 , 44 Sunderland et al. 44 found that paediatric thalamic tumours grades were not associated with achieving GTR or subtotal resection. However, GTR was more achievable in low‐grade tumours, particularly pilocytic astrocytoma. 44 Karsy et al. 37 found no significant differences in EOR among WHO grades I–IV paediatric gliomas. Both studies failed to draw a clear conclusion on the relationship between iMRI and tumour grade. Further studies are needed to determine the effect of tumour grade on the ability of iMRI to improve EOR as it has the potential to influence the cases in which iMRI could be implemented.
Clinical outcomes
Progression‐free survival in brain tumour resection
Studies investigating the effect of iMRI on PFS or overall survival in paediatric brain tumours were limited. Only one study was identified, which focused on low‐grade gliomas. 48 In a case‐control study with 65 patients (31 iMRI, 34 non‐iMRI), Roder et al. 48 demonstrated a significant correlation between improved PFS rates and achieving GTR in both iMRI and non‐iMRI procedures, with identification of a trend towards improved rates of PFS with iMRI use. However, Day and Scott 43 postulated that the ultimate determinant of patient outcome was tumour genetics and behaviour regardless of EOR. Nevertheless, Roder et al. 48 provided a promising indication that iMRI is a valuable tool for assessing EOR and improving PFS in paediatric brain tumour resections.
Seizure outcome in epilepsy surgeries
Of the identified studies, three investigated the use of iMRI during epilepsy surgery and its effect on seizure outcomes. 39 , 40 , 41 Warsi et al. 40 demonstrated that iMRI use has the potential to improve seizure outcomes (Engel scores I, II) compared to cases with no iMRI use, regardless of the lesion type. They found that complete seizure freedom was achieved in a higher proportion in the iMRI group (74.4%) compared to the non‐iMRI group (61.5%), although this did not reach statistical significance. 40 Eid et al. 39 found that iMRI significantly mitigated poor seizure outcomes (Engel scores III, IV) in cases of focal paediatric epilepsy (odds ratio: 0.84), thereby increasing the efficacy of neurosurgical treatment in these cases. The residual volume was directly correlated to the odds of a poor outcome. 39 This may indicate that any improvement in the EOR would lower the odds of a poor Engel score outcome (III, IV). 39 A cohort study by van Tonder et al. 41 revealed that iMRI was useful in achieving seizure freedom rates of 80% in paediatric hypothalamic hamartoma resection. They compared their data to previous literature where the same surgical technique was used without iMRI, and found that seizure freedom was achieved in 60% of cases. 41 However, no statistical analyses were conducted. 41 It is important to note, however, that the studies by Eid et al. 39 and Warsi et al. 40 also included paediatric patients with other neurological lesions, including malformations of cortical development and mesial temporal sclerosis. These studies did not distinguish between seizure outcomes based on pathology type. 39 , 40 This may limit the finding's generalisability to populations comprised mainly of paediatric epilepsy patients with brain tumours.
Prevalence of neurological deficits
The prevalence of neurological deficits is a key concern in iMRI‐assisted paediatric neurosurgeries as GTR involves operating close to a functional tissue. 40 , 43 , 47 Roder et al. 48 reported higher rates of transient neurological deficits (resolving within 3 months) in iMRI cases (42%) compared to non‐iMRI cases (32%). However, iMRI cases (1%) had lower rates of persistent neurological deficits (beyond 3 months) than non‐iMRI cases (9%). 48 The differences in both transient and persistent rates of neurological deficits between the iMRI and non‐iMRI cases did not reach statistical significance. 48 Conversely, when examining the rates of neurological deficits between brain tumour types in iMRI procedures, Giordano et al. 47 reported no complications due to iMRI. However, since the data were collected upon admission and discharge, the follow‐up period was too short to draw clinically significant conclusions. 47
It has been found that iMRI can increase EOR, bringing the resection margin closer to a healthy tissue. 44 , 48 Tejada et al. 36 examined whether this affected the rates of neurological deficits by comparing gross total and subtotal resection cohorts. They found no significant difference in the outcomes between the cohorts, with the rate of neurological deficits at 11% and 13%, respectively. 36 They also found that the surgical decision on whether to continue resecting after using iMRI did not affect the rates of neurological deficits. 36 The factors that affected the rate of neurological deficits in this study were the tumour WHO grade and location, with high‐grade tumours (15%) and tumours of infratentorial location (14%) having the highest prevalence of neurological complications. 36
Length of hospital stay
Length of hospital stay was found to be shorter in neurosurgical resections using iMRI (7.0 days) compared to non‐iMRI procedures (8.8 days). 48 However, the tumour grade was found to have a greater impact on the length of hospital stay than the use of iMRI, with Karsy et al. 37 demonstrating that paediatric patients with WHO grade I tumours had a significantly shorter stay (5.6 days) than those with WHO grade IV tumours (29 days).
Rate of repeat surgery
Cohort studies by Giordano et al. 47 and Tejada et al. 36 reported low reoperation rates in iMRI‐assisted cases, ranging from 0% to 0.7% of patients. Conversely, Warsi et al. 40 reported higher numbers of repeat surgeries performed in the iMRI group (0.77%) compared to the non‐iMRI group (0.26%). All three studies reported repeat surgical rates on various pathologies, including non‐tumour related cases 36 , 40 , 47 but Giordano et al. 47 and Tejada et al. 36 had a larger proportion of brain tumour cases within their cohort. This may account for the discrepancy between studies, as types of pathology may play a role in the success of iMRI.
Intraoperative magnetic resonance imaging as an initial post‐operative scan
The final iMRI scan is defined as the scan after which no further resection is performed. 42 A preliminary prospective study by Avula et al. 42 suggested that utilising the final iMRI scan to determine the EOR may be more beneficial than the conventional 24–72 hour post‐operative MRI scan as they found no difference in determining the EOR between both scans. This application of the final iMRI scan reduces the need for additional anaesthesia or sedation involved in conventional post‐operative scanning, and improves quality of care as the post‐operative MRI scan may be delayed for unstable patients. 36 , 42 , 48 In the case of non‐enhancing tumours, Avula et al. 42 found that the final iMRI scan was better at distinguishing tumour remnants from post‐operative changes compared to the post‐operative MRI scan. Post‐operative changes, such as surgically‐induced cytotoxic oedema and tissue injuries, produced abnormal T2/Fluid‐attenuated inversion recovery (FLAIR) hyperintensities on the post‐operative scan. 42 Such changes were absent on the final iMRI scan. 42 Conversely, Day & Scott 43 observed cases of non‐enhancing tumours appearing hyperintense on T2 and FLAIR intraoperative scans, and the residual tumours were indistinguishable from surgically‐induced oedema. Therefore, the viability of the final iMRI as an initial post‐operative scan appears to be influenced by the characteristic appearance of the tumour, particularly in non‐enhancing tumours that rely on T2/FLAIR signal change for diagnosis. 43 , 48 Additionally, in the case of contrast‐enhancing lesions, Roder et al. 48 identified the possibility that a healthy tissue could be labelled as a tumour on iMRI scans due to contrast medium leakage across the blood–brain barrier during resection, presenting a possibility of a false‐positive for residual tumour on the final iMRI scan. This phenomenon was not observed in the 24–72 hour post‐operative scan. 48
The study by Avula et al. 42 may not contain a true representation of the clinical population due to the exclusion of paediatric patients who required additional anaesthesia or sedation during post‐operative MRI scans. Other studies were not designed to investigate the utility of iMRI as a substitute for an initial post‐operative scan. 43 , 48 Collectively, these limitations may explain why contradictions exist between findings. The use of a final iMRI scan as an initial post‐operative scan remains controversial. Therefore, a more rigorous approach with the inclusion of a larger sample size is recommended to further validate the findings.
Combined use of intraoperative neurophysiologic monitoring and intraoperative magnetic resonance imaging
Dias et al. 46 demonstrated that iMRI can be used in conjunction with intraoperative neurophysiologic monitoring (IONM), enabling neurosurgeons to determine the resection limit of the eloquent areas by assessing both function and morphology throughout the procedure. IONM involves the placement of electrodes along the body to stimulate and receive electrical signals, which enables continuous evaluation of neural pathways. 46 Dias et al. 46 observed that IONM recordings remained reliable after being exposed to 3T iMRI, allowing for the safe achievement of maximal EOR by avoiding resection into functional tissue. However, two of seven patients experienced first‐degree skin burns when electrodes were placed in the shoulder. 46 This was due to proximity of the electrodes to the iMRI head coil, which has the highest radiofrequency field of the iMRI machine. 46 To avoid skin burns, shoulder electrodes can be moved to hip level. 46 When the surgical goal is indicated, electrodes may be removed prior to the final iMRI scan to minimise the risk of skin burns. 46 Despite these risk minimising strategies, the clinical setting in Dias et al. 46 may differ from other facilities, and therefore, the possibility of thermal injuries cannot be excluded.
Potential drawbacks and safety risks of intraoperative magnetic resonance imaging
Prolonged operation times
Studies reported that iMRI prolonged operation times of paediatric brain tumour resection surgeries by 30–112 minutes compared to procedures without intraoperative scans. 36 , 39 , 40 , 43 , 48 This extended time was due to longer pre‐operative and pre‐scanning preparation times, and the iMRI scanning time but varied depending on tumour type and location. 43 , 47 , 48 Potential complications relating to prolonged operation time include infections, wound‐related problems, neurological deficits, and pressure and heat‐related damages. 36 , 41 Despite the extended time, there was no association between iMRI use and increased rates of surgical complications. 36 , 39 , 40 , 43 , 48 For example, Tejada et al. 36 found similar complication rates between iMRI cases (2.5%) and surgeries without iMRI (2.6%). Supporting this, retrospective cohort studies have reported very low complication rates 41 , 43 or no complications when using iMRI in paediatric neurosurgeries. 47 However, these complications were reported as an overall rate, and not specifically attributed to prolonged operation times.
Head immobilisation devices
While iMRI was not associated with increased complication rates, some complications were still observed that were related to iMRI‐compatible head immobilisation devices. 43 , 49 Various iMRI‐compatible immobilisation devices exist, including the DORO clamp used by Zaazoue et al. 49 and the NORAS OR head holder used by Tejada et al., 36 both of which provide three‐point fixation. Zaazoue et al. 49 found that seven of 599 paediatric patients with brain tumours experienced depressed skull fractures during iMRI‐assisted tumour resection due to the immobilisation device used. These fractures occurred at the pin site, with the majority (n = 5) in the temporal region. 49 Zaazoue et al. 49 attributed these fractures to the placement of the pins, as well as reduced cranial vault thickness caused by the presence of brain tumours. Therefore, the thin squamous temporal bone should be avoided during pinning. 49 Due to these fractures, six of seven patients developed epidural haematomas, four of which suffered long‐term motor deficits. 49 Furthermore, Day and Scott 43 reported one case of sudden cerebral swelling, which resulted from problematic head‐holder pin placement. Zaazoue et al. 49 recommended that increasing the number of pins and adjusting the placement of their contact points with the skull to evenly distribute the forces would help reduce the pressure for a given pin, thereby lowering the risk of fractures and other device‐related complications. Tejada et al. 36 implemented the NORAS OR head holder, which utilised five to seven pins in patients older than two years. In patients under two years old, pins were avoided by using a cushion with the head holder. 36 They observed no complications during their iMRI‐assisted surgeries, 36 corroborating the recommendation made by Zaazoue et al. 49 Together, these findings indicate that while the frequency of complications relating to immobilisation devices was low, they can still occur and lead to severe consequences. Zaazoue et al. 49 have highlighted the lack of widely accepted guidelines for the use of head immobilisation devices. The absence of a standardised protocol emphasises the need for further research to elucidate optimal head immobilisation devices in iMRI‐assisted paediatric neurosurgeries to maintain patient safety and prevent associated complications. Meanwhile, the ability to detect such complications intraoperatively and address these events while still under general anaesthesia can be considered an advantage of iMRI. 49
Limitations on patient positioning
Studies have reported other equipment‐related limitations of iMRI. 47 , 49 Giordano et al. 47 found that the surgical table configuration in iMRI‐assisted surgeries limited certain patient positions. They reported challenges with the prone position due to the fixed pin pattern of the head immobilisation device. 47 However, no intraoperative complications were observed. 47 Zaazoue et al. 49 found that the bore size of their iMRI scanner (60 cm) made it challenging to position the immobilisation device without obstructing the bore. Conversely, Tejada et al. 36 observed no problems resulting from the patient positioning or the immobilisation device obstructing the bore. They attributed this to the relatively larger bore size (65 cm) and NORAS OR head coil used. 36 These findings suggest that the relative size of the immobilisation device compared to the bore size is an important consideration when using iMRI for paediatric neurosurgeries.
Difficulties in intraoperative magnetic resonance imaging scan interpretation
Day and Scott 43 found difficulties in interpreting iMRI scans due to inappropriate head coil size. In one case, the patient's head was too large for the coil, resulting in degraded images that were difficult to interpret. 43 Likewise, Giordano et al. 47 reported that the head immobilisation unit used in their series was designed for adult‐sized heads, rather than paediatric‐sized patients, but no image degradation was reported. Nevertheless, this suggests the need to investigate head immobilisation devices and coils specifically designed to accommodate paediatric patients. Studies have also found difficulties in interpreting intraoperative diffusion‐weighted imaging (DWI) scans due to susceptibility artefacts, related to the presence of air, pins and blood artefacts. 48 , 50 Roder et al. 48 and Avula et al. 42 emphasised the importance of irrigating the surgical cavity with saline continuously prior to scanning to ensure the highest possible quality final iMRI for accurate tumour remnant determination.
Insensitivity to stroke
There is evidence suggesting that iMRI has low sensitivity for detecting strokes during paediatric brain tumour resection, with sensitivity ranging from 30% 50 to 45.5%. 51 Khan et al. 51 reported that 28 of 895 elective tumour resection procedures resulted in strokes. In 11 of these cases, iMRI was implemented. 51 However, iMRI detected only five (45.5%) infarcts, with the remaining six undetected strokes being deep arterial infarcts. 51 Similarly, Saint‐Martin et al. 50 observed that of 115 iMRI‐assisted procedures, 13 reported hyperacute arterial infarctions (11%), four (30%) of which were detected in intraoperative DWI scans. These infarcts were either small in size or misinterpreted as susceptibility artefacts, and were later confirmed as infarcts in early post‐operative MRI scans. 50 Furthermore, minimally diffuse signal intensity changes resulting from hyperacute infarcts may inhibit the detection of an intraoperative ischaemic event on an iMRI scan. 50 Importantly, study design may contribute to the differing sensitivity reported for iMRI in stroke detection. For example, Saint‐Martin et al. 50 had a single radiologist reviewing all iMRI scans, which may introduce bias. Based on the identified studies, stroke is not a rare intraoperative complication, occurring in up to 11% of the paediatric brain tumour resections reported, 50 and intraoperative DWI may underestimate the extent of infarction. 50 , 51 Therefore, a negative iMRI may not exclude an infarction. 51 As a hyperacute infarction may complicate a brain tumour resection, 50 a more comprehensive investigation may be beneficial to gain a better understanding of this subject.
Limitations
This literature review, along with the included studies, possess some limitations. Studies were retrieved from four databases using set search terms, and some studies may not have been identified. To compensate for this, manual searches were conducted. Internal validity may have been reduced by various factors. For example, small sample size limits statistical power, and information bias may be present in retrospective studies as data were not collected for the purpose of the study. Additionally, the lack of control groups in studies investigating patient outcomes made it challenging to determine the efficacy of the intervention, thereby reducing internal validity. While studies were published within the last five years, data were collected from 1991 to 2020. Technological advancements over the data collection period may limit the external validity as findings may not be applicable to current practice. Non‐standardised protocols between institutions and individual cases reduced the external validity. Furthermore, the interaction of surgical equipment in the proximity of the magnetic field is beyond the scope of this review.
Future directions
To solidify our conclusions regarding whether iMRI should be widely adopted in paediatric brain tumour resection surgeries, there is a need to conduct prospective research. Future research models should incorporate larger cohort sizes extracted from a diverse range of hospitals and centres, as well as determine the effect size and perform statistical analyses comparing iMRI and non‐iMRI groups. Since procedures and technical factors of iMRI differ between institutions, further research is required to determine the optimal protocol. Studies should aim to determine which tumour types iMRI should be used for, and which equipment should be used in conjunction with iMRI. Furthermore, cost‐benefit analyses should be performed to justify the clinical implementation of iMRI in paediatric neurosurgeries.
Conclusion
The use of iMRI has the potential to achieve maximal brain tumour resection in paediatric patients. The limited body of research evaluating the clinical implementation of iMRI in paediatric cohorts has been predominantly positive. Current evidence demonstrates the potential for iMRI use to safely increase rates of GTR, assess the extent of brain tumour resection and improve patient outcomes. Limitations regarding the use of iMRI include prolonged operation times and complications associated with head immobilisation devices. In the future, prospective randomised controlled trials are necessary to assess the clinical significance and benefits of using iMRI during neurosurgical resection for clinical management of brain neoplasms in children. Findings from such research will help determine whether iMRI should be widely adopted in paediatric brain tumour resection surgeries.
Acknowledgements
We wish to acknowledge Matea Arambasic, Amanda Gough and Renee Maher for their contribution to the inception of this paper. We would also like to thank Dr Yobelli Jimenez for her advice and support in the preparation of this literature review.
Data Availability Statement
Data sharing is not applicable to this article as this is a literature review based on published literature and no new data were created.
References
- 1. Nejat F, El Khashab M, Rutka JT. Initial management of childhood brain tumors: neurosurgical considerations. J Child Neurol 2008; 23: 1136–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prayson RA. Tumours arising in the setting of paediatric chronic epilepsy. Pathology 2010; 42: 426–431. [DOI] [PubMed] [Google Scholar]
- 3. Friedman MJ, Sharieff GQ. Seizures in children. Pediatr Clin North Am 2006; 53: 257–277. [DOI] [PubMed] [Google Scholar]
- 4. Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: current management, biological insights, and future directions. J Neurosurg Pediatr 2019; 23: 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Ruiter MA, van Mourik R, Schouten‐van Meeteren AYN, Grootenhuis MA, Oosterlaan J. Neurocognitive consequences of a paediatric brain tumour and its treatment: a meta‐analysis. Dev Med Child Neurol 2013; 55: 408–417. [DOI] [PubMed] [Google Scholar]
- 6. Udaka YT, Packer RJ. Pediatric brain tumors. Neurol Clin 2018; 36: 533–556. [DOI] [PubMed] [Google Scholar]
- 7. Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low‐grade astrocytoma treatment in childhood. Cancer 2006; 106: 396–402. [DOI] [PubMed] [Google Scholar]
- 8. Shinjo D, Matsumoto K, Terashima K, et al. Volume effect in paediatric brain tumour resection surgery: analysis of data from the Japanese national inpatient database. Eur J Cancer 2019; 109: 111–119. [DOI] [PubMed] [Google Scholar]
- 9. Dimou S, Battisti RA, Hermens DF, Lagopoulos J. A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg Rev 2013; 36: 205–214. [DOI] [PubMed] [Google Scholar]
- 10. Shalan ME, Soliman AY, Nassar IA, Alarabawy RA. Surgical planning in patients with brain glioma using diffusion tensor MR imaging and tractography. Egypt J Radiol Nucl Med 2021; 52: 110. [Google Scholar]
- 11. Yan J‐L, van der Hoorn A, Larkin TJ, Boonzaier NR, Matys T, Price SJ. Extent of resection of peritumoral diffusion tensor imaging–detected abnormality as a predictor of survival in adult glioblastoma patients. J Neurosurg 2017; 126: 234–241. [DOI] [PubMed] [Google Scholar]
- 12. Kessler AT, Bhatt AA. Brain tumour post‐treatment imaging and treatment‐related complications. Insights Imaging 2018; 9: 1057–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chicoine MR, Lim CC, Evans JA, et al. Implementation and preliminary clinical experience with the use of ceiling mounted mobile high field intraoperative magnetic resonance imaging between two operating rooms. Acta Neurochir Suppl 2011; 109: 97–102. [DOI] [PubMed] [Google Scholar]
- 14. Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high‐field intraoperative MRI guidance. Neuro Oncol 2011; 13: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fountain DM, Bryant A, Barone DG, et al. Intraoperative imaging technology to maximise extent of resection for glioma: a network meta‐analysis. Cochrane Database Syst Rev 2021; 1: CD013630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karschnia P, Vogelbaum MA, van den Bent M, et al. Evidence‐based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer 2021; 149: 23–33. [DOI] [PubMed] [Google Scholar]
- 17. Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 2011; 12: 997–1003. [DOI] [PubMed] [Google Scholar]
- 18. Wu JS, Gong X, Song YY, et al. 3.0‐T intraoperative magnetic resonance imaging‐guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple‐blind, parallel‐controlled trial. Neurosurgery 2014; 61(Suppl 1): 145–154. [DOI] [PubMed] [Google Scholar]
- 19. Nimsky C, Fujita A, Ganslandt O, Von Keller B, Fahlbusch R. Volumetric assessment of glioma removal by intraoperative high‐field magnetic resonance imaging. Neurosurgery 2004; 55: 358–370. [DOI] [PubMed] [Google Scholar]
- 20. Gong X, Yao C‐J, Yuan S‐W, et al. 3.0T iMRI‐guided resection of eloquent high‐grade gliomas: preliminary results of a randomised controlled trial. Lancet 2015; 386: S11. [Google Scholar]
- 21. Sankar T, Moore NZ, Johnson J, et al. Magnetic resonance imaging volumetric assessment of the extent of contrast enhancement and resection in oligodendroglial tumors: clinical article. J Neurosurg 2012; 116: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 22. Choudhri AF, Klimo P, Auschwitz TS, Whitehead MT, Boop FA. 3T Intraoperative MRI for management of pediatric CNS neoplasms. AJNR Am J Neuroradiol 2014; 35: 2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah MN, Leonard JR, Inder G, et al. Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr 2012; 9: 259–264. [DOI] [PubMed] [Google Scholar]
- 24. Yousaf J, Avula S, Abernethy LJ, Mallucci CL. Importance of intraoperative magnetic resonance imaging for pediatric brain tumor surgery. Surg Neurol Int 2012; 3(Suppl 2): S65–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lam CH, Hall WA, Truwit CL, Liu H. Intra‐operative MRI‐guided approaches to the pediatric posterior fossa tumors. Pediatr Neurosurg 2001; 34: 295–300. [DOI] [PubMed] [Google Scholar]
- 26. Levy R, Cox RG, Hader WJ, Myles T, Sutherland GR, Hamilton MG. Application of intraoperative high‐field magnetic resonance imaging in pediatric neurosurgery. J Neurosurg Pediatr 2009; 4: 467–474. [DOI] [PubMed] [Google Scholar]
- 27. Mislow JMK, Golby AJ, Black PM. Origins of intraoperative MRI. Neurosurg Clin N Am 2009; 20: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han Q, Liang H, Cheng P, Yang H, Zhao P. Gross total vs. subtotal resection on survival outcomes in elderly patients with high‐grade glioma: a systematic review and meta‐analysis. Front Oncologia 2020; 10: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogiwara H, Bowman RM, Tomita T. Long‐term follow‐up of pediatric benign cerebellar astrocytomas. Neurosurgery 2012; 70: 40–47. [DOI] [PubMed] [Google Scholar]
- 30. Albert FK, Forsting M, Sartor K, Adams H‐P, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 1994; 34: 45–61. [DOI] [PubMed] [Google Scholar]
- 31. Lamborn KR, Yung WK, Chang SM, et al. Progression‐free survival: an important end point in evaluating therapy for recurrent high‐grade gliomas. Neuro Oncol 2008; 10: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Racz A, Becker AJ, Quesada CM, et al. Post‐surgical outcome and its determining factors in patients operated on with focal cortical dysplasia type II‐A retrospective monocenter study. Front Neurol 2021; 12: 666056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One 2018; 13: e0195901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients' in‐hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res 2015; 15: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wach J, Banat M, Borger V, Vatter H, Haberl H, Sarikaya‐Seiwert S. Intraoperative MRI‐guided Resection in pediatric brain tumor surgery: a meta‐analysis of extent of resection and safety outcomes. J Neurol Surg A Cent Eur Neurosurg 2021; 82: 64–74. [DOI] [PubMed] [Google Scholar]
- 36. Tejada S, Avula S, Pettorini B, Henningan D, Abernethy L, Mallucci C. The impact of intraoperative magnetic resonance in routine pediatric neurosurgical practice—a 6‐year appraisal. Childs Nerv Syst 2018; 34: 617–626. [DOI] [PubMed] [Google Scholar]
- 37. Karsy M, Akbari SH, Limbrick D, et al. Evaluation of pediatric glioma outcomes using intraoperative MRI: a multicenter cohort study. J Neurooncol 2019; 143: 271–280. [DOI] [PubMed] [Google Scholar]
- 38. WHO Classification of Tumours Edicatorial Board . World Health Organization Classification of Tumours of the Central Nervous System, 5th edn. International Agency for Research on Cancer, Lyon, 2021. [Google Scholar]
- 39. Eid H, Crevier‐Sorbo G, Moreau JT, et al. Eight‐year experience with 3‐T intraoperative MRI integration in focal pediatric epilepsy surgery: impact on extent of resection, residual volumes, and seizure outcomes. Am J Roentgenol 2020; 214: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 40. Warsi NM, Lasry O, Farah A, et al. 3‐T intraoperative MRI (iMRI) for pediatric epilepsy surgery. Childs Nerv Syst 2016; 32: 2415–2422. [DOI] [PubMed] [Google Scholar]
- 41. van Tonder L, Burn S, Iyer A, et al. Open resection of hypothalamic hamartomas for intractable epilepsy revisited, using intraoperative MRI. Childs Nerv Syst 2018; 34: 1663–1673. [DOI] [PubMed] [Google Scholar]
- 42. Avula S, Jaspan T, Pizer B, et al. Comparison of intraoperative and post‐operative 3‐T MRI performed at 24–72 h following brain tumour resection in children. Neuroradiology 2021; 63: 1367–1376. [DOI] [PubMed] [Google Scholar]
- 43. Day EL, Scott RM. The utility of intraoperative MRI during pediatric brain tumor surgery: a single‐surgeon case series. J Neurosurg Pediatr 2019; 24: 577–583. [DOI] [PubMed] [Google Scholar]
- 44. Sunderland G, Foster MT, Pizer B, Hennigan D, Pettorini B, Mallucci C. Evolution of surgical attitudes to paediatric thalamic tumours: the alder hey experience. Childs Nerv Syst 2021; 37: 2821–2830. [DOI] [PubMed] [Google Scholar]
- 45. Low SYY, Lim EHL, Loh LE, et al. Use of an offsite intraoperative MRI operating theater for pediatric brain tumor surgery: experience from a Singapore children's hospital. World Neurosurg 2020; 135: e28–e35. [DOI] [PubMed] [Google Scholar]
- 46. Dias S, Sarnthein J, Jehli E, Neidert MC, Regli L, Bozinov O. Safeness and utility of concomitant intraoperative monitoring with intraoperative magnetic resonance imaging in children: a pilot study. World Neurosurg 2018; 115: e637–e644. [DOI] [PubMed] [Google Scholar]
- 47. Giordano M, Samii A, Lawson McLean AC, et al. Intraoperative magnetic resonance imaging in pediatric neurosurgery: safety and utility. J Neurosurg Pediatr 2017; 19: 77–84. [DOI] [PubMed] [Google Scholar]
- 48. Roder C, Breitkopf M, Bisdas S, et al. Beneficial impact of high‐field intraoperative magnetic resonance imaging on the efficacy of pediatric low‐grade glioma surgery. Neurosurg Focus 2016; 40: E13. [DOI] [PubMed] [Google Scholar]
- 49. Zaazoue MA, Bedewy M, Goumnerova LC. Complications of head immobilization devices in children: contact mechanics, and analysis of a single institutional experience. Neurosurgery 2018; 82: 678–685. [DOI] [PubMed] [Google Scholar]
- 50. Saint‐Martin C, Apuzzo S, Salman A, Farmer JP. Hyperacute infarct on intraoperative diffusion imaging of pediatric brain tumor surgery. Can J Neurol Sci 2019; 46: 550–558. [DOI] [PubMed] [Google Scholar]
- 51. Khan NR, Moore K, Basma J, et al. Ischemic stroke following elective craniotomy in children. J Neurosurg Pediatr 2018; 23: 355–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as this is a literature review based on published literature and no new data were created.


