Abstract
Significance:
Retinal neurons are vulnerable to disease and injury, which can result in neuronal death and degeneration leading to irreversible vision loss. The human retina does not regenerate to replace neurons lost to disease or injury. However, cells within the retina of other animals are capable of regenerating neurons, and homologous cells within the mammalian retina could potentially be prompted to do the same. Activating evolutionarily silenced intrinsic regenerative capacity of the mammalian retina could slow, or even reverse, vision loss, leading to an improved quality of life for millions of people.
Recent Advances:
During development, neurons in the retina are generated progressively by retinal progenitor cells, with distinct neuron types born over developmental time. Many genes function in this process to specify the identity of newly generated neuron types, and these appropriate states of gene expression inform recent regenerative work. When regeneration is initiated in other vertebrates, including birds and fish, specific signaling pathways control the efficiency of regeneration, and these conserved pathways are likely to be important in mammals as well.
Critical Issues:
Using insights from development and from other animals, limited regeneration from intrinsic cell types has been demonstrated in the mammalian retina, but it is able only to generate a subset of partially differentiated retinal neuron types.
Future Directions:
Future studies should aim at increasing the efficiency of regeneration, activating regeneration in a targeted fashion across the retina, and improving the ability to generate specific types of retinal neurons to replace those lost to disease or injury. Antioxid. Redox Signal. 39, 1039–1052.
Keywords: retina, regeneration, gene therapy, neurodegeneration
Introduction
The human retina is an intricately organized collection of millions of neurons and glia whose primary purpose is to collect visual information and relay it to visual processing centers in the brain (Fig. 1). Within the retina, light is detected by the rod photoreceptors, and the wavelength of light is detected by cone photoreceptors. Rods and cones transmit signals to retinal interneurons, the horizontal cells (HCs), bipolar cells (BCs), and amacrine cells (ACs), which integrate information about changes in and across their receptive fields, including motion, direction, and changes in illumination.
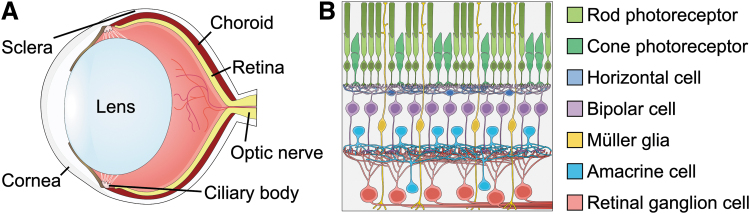
FIG. 1.
The retina is the light-sensing tissue of the eye. (A) Light passes through the cornea and is focused by the lens to strike the retina, which sits at the back of the eye. (B) Light is detected by photoreceptors, and information about the visual field is passed through horizontal, bipolar, and amacrine interneurons, then to RGCs, which transmit this information to the brain via the optic nerve. RGC, retinal ganglion cell.
These interneurons, in turn, transmit signals to retinal ganglion cells (RGCs), the output projection neurons of the retina, which integrate information and send it on to central targets in the thalamus and midbrain. Retinal neurons are supported by several types of glial cells, including Müller glia, nerve fiber layer astrocytes, astrocytes, oligodendrocytes, and others through the optic nerve and optic tract.
Like other regions of the mammalian nervous system, the retina is vulnerable to injury and disease, and, once neurons die, they are not replaced, leading to a permanent loss of visual function. Neuronal death can be caused by acute injury to neurons, as in hypoxia following central or branch retinal artery occlusion, or to their axons, as in traumatic optic nerve injury, where RGCs degenerate following damage to their axons.
More commonly, cell death and resultant vision loss is secondary to chronic damage incurred from the retinal or optic nerve microenvironment (World Health Organization, 2019). In the case of glaucoma, a disease that affects an estimated 76 million people worldwide, elevated intraocular pressure is associated with progressive degeneration of RGCs, and, to a much lesser extent, other retinal neurons, causing progressive visual field loss and eventually leading to blindness (Stein et al., 2021; Tham et al., 2014). Retinal vascular blockade, as in artery or vein occlusions, or vascular ischemia, as in diabetic retinopathy, are also common causes of retinal neuron death (Stitt et al., 2016).
Retinal blood vessels also grow abnormally in the case of age-related macular degeneration, leading to death of retinal neurons and the adjacent retinal pigment epithelium (RPE); age-related macular degeneration is the most common cause of vision impairment in older adults, affecting an estimated 200 million worldwide (Mitchell et al., 2018). Other genetic causes of photoreceptor cell death, such as retinitis pigmentosa, are individually rare, but quite common in aggregate.
Given the impact of vision loss due to retinal neuron death, neuron replacement in the retina, whether by transplantation or by recruitment of endogenous progenitors, is a major goal of regenerative medicine. For neuron replacement by transplantation, neurons must be produced in vitro in sufficient quantities from induced pluripotent stem cells, then transplanted and integrated into the retina.
Stimulating intrinsic tissue regeneration bypasses the need to exogenously transplant new neurons, instead relying on progenitors in situ, or on transdifferentiated cell populations, to produce new neurons where they are needed. In both cases, it is necessary to understand the processes of neuron-type-specific development and circuit integration to promote the production of desired neuron types and position them to integrate and function in retinal circuits.
Retinal cell replacement from intrinsic progenitors is an attractive strategy for preventing or curing vision loss. Producing patient-specific cells in vitro and transplanting them across an entire retina in sufficient numbers to attain meaningful functional recovery remains technically challenging. Even setting aside the technical challenges of performing these transplants, the cost of this procedure, if performed with patient-specific cells, would likely remain prohibitively high, given the operational infrastructure required for personalized cell-based therapeutics.
In contrast, intrinsic regenerative strategies may benefit from the availability of safe and highly efficient gene delivery across large areas of the retina using adeno-associated viruses (AAV) (Chan et al., 2017). AAV-mediated gene delivery is approved by the Food and Drug Administration to restore gene function, and thereby vision, in patients with Leber's congenital amaurosis (Bainbridge et al., 2015). Therefore, existing AAV vectors could possibly enable genetic manipulation and subsequent reprogramming of endogenous cells in the retina to generate new retinal neurons.
Despite the clear benefits of regeneration from endogenous cells in the retina, significant knowledge gaps remain (Bassett and Wallace, 2012; Brzezinski and Reh, 2015). Some developmental gene expression pathways that specify particular retinal neuron subtypes are known, but others remain to be discovered. Even when important transcriptional regulators of subtype specification have been identified, knowledge of the progenitors that produce a given neuron type is not currently sufficient to allow directed differentiation of pure populations of specific subtypes (Cepko, 2014), and much work remains to integrate foundational work on the sequence of gene expression events and the subsequent coordination of downstream genetic programs in normally developing retinal neuron types (Clark et al., 2019; Sajgo et al., 2017).
Important proof-of-principle examples of intrinsic regeneration have been demonstrated, but they are inefficient and generate limited and incompletely differentiated cell types (Hoang et al., 2020; Jorstad et al., 2017). For any claim of regeneration from endogenous precursors, it is critical to rigorously demonstrate true regeneration, rather than neuron sparing or mislabeling of non-degenerated endogenous neurons (Blackshaw and Sanes, 2021).
In this review, we examine the basis, state of current knowledge, and future challenges of intrinsic neuron regeneration in the retina. We discuss the molecular processes that regulate generation of retinal neuron types during early development, as well as the implications of developmental knowledge for regeneration. We review evidence of regeneration across species and consider insights from non-mammalian species that may be relevant for mammalian retinal regeneration.
Regeneration in the mammalian retina could potentially be achieved by induction of differentiation from any of several cell populations, and we discuss the merits and challenges of each. Finally, we identify challenges that remain to translating existing and future model organism work to clinical applications.
Specification and Differentiation of Retinal Neurons
Over the course of development, neurons are generated from retinal progenitors, acquiring distinct type-specific identities, a process that is tightly regulated by transcriptional controls that specify and maintain these identities. After migrating to the appropriate retinal layer, these neurons produce elaborate dendritic trees and establish axonal projections to assemble into circuits. Retinal progenitor cells (RPCs) progressively generate different neuron types, then finally produce Müller glial cells. The RPCs generate neurons in a temporally stereotyped progression of type identities, beginning with RGCs, ACs, and cones, followed by HCs and BCs, and ending with rod photoreceptors.
As newly postmitotic neurons emerge from the cell cycle, they begin to express characteristic transcription factors, which set neuron-type-specific genetic programs into motion to definitively establish their nascent identity. Finally, all retinal neurons must form circuits with specific partners of various identities, and RGCs must establish a long-distance axonal projection that enables the targeted transfer of information from the eye to central visual centers.
Neurons in the retina are produced by RPCs, a population of multipotent progenitors. The RPCs begin to proliferate around the end of the second post-conception week in mice and the seventh in humans (Mellough et al., 2019). Early fate-mapping studies demonstrated that RPCs are multipotent, as sparse permanent labeling of clones during development generally results in clones containing several different neuron types (Turner and Cepko, 1987).
Later work has established the existence of a pool of uncommitted RPCs from which progenitors are continuously recruited (Clark et al., 2019), beginning to produce biased percentages of neuron types due to their expression of transcription factors, including Prox1, Atoh7, and Ascl1 (Brzezinski et al., 2012; Brzezinski et al., 2011; Dyer et al., 2003). As in other areas of the nervous system, the potential of RPCs to generate neuron types becomes progressively restricted over time, and late RPCs lack the capacity to produce subtypes from earlier in development (Belliveau and Cepko, 1999).
Expression of the transcription factor Otx2 by late progenitors causes them to produce specific late cell fates (Brzezinski and Reh, 2015), but the competence of late progenitors to produce early cell fates can be restored with expression of the transcription factor Ikzf1 (Elliott et al., 2008). Understanding the relationship between transcription factor controls and progenitor competence states, as well as the plasticity of these competence states, is critically important for initiating and directing neuronal regeneration in the retina.
Retinal neurons are generated from RPCs in a temporally defined order, with different types produced in sequential overlapping waves (Bassett and Wallace, 2012). The RGCs are first to be produced, beginning around embryonic day (E) 11 in mice, followed by HCs, ACs, cone photoreceptors, rod photoreceptors, and BCs (Rapaport et al., 2004) (Fig. 2). The RPCs conclude their proliferative lifetime by producing Müller glia, the primary glial type of the retina (Goldman, 2014).
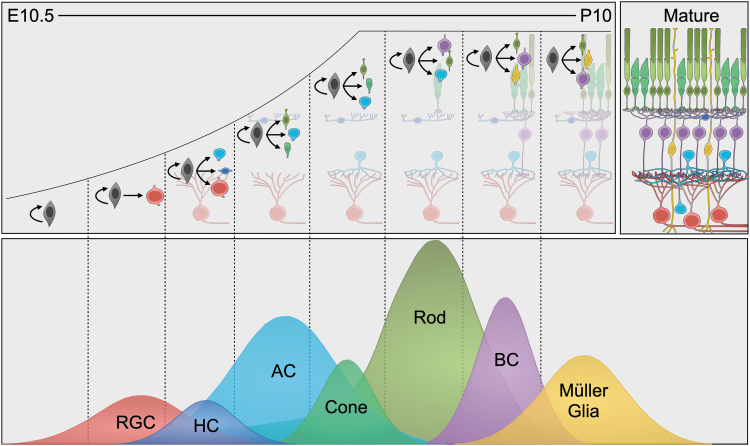
FIG. 2.
Retinal neuron types are born in overlapping waves during development. (Top) Retinal progenitor cells produce retinal neurons starting during mid-embryogenesis in the mouse, and they continue producing neurons and self-renewing until the second week after birth. (Bottom) Approximate timeline and relative proportion of cell type birth over time.
These waves last several days and are highly overlapping, so a neuron type cannot be identified based solely on its birthdate, but some types are born generally later or earlier than others. Neuron types that are generated closer to each other temporally often appear to be evolutionarily and molecularly more closely related with each other than with other cells; for example, progenitors that typically generate ACs and HCs will instead produce RGCs with the loss of Ptf1a expression (Fujitani et al., 2006), and progenitors generate either BCs or rods according to expression levels of Vsx2 and Prdm1 (Goodson et al., 2020; Livne-bar et al., 2006).
Even subtype diversity within a neuron type can be generated in a temporally stereotyped fashion, with progression through subtypes in a defined order, as is the case for BCs (West et al., 2022), ACs (Cherry et al., 2009), and RGCs (Shekhar et al., 2022). These molecular pathways that direct neuron type specification during development can potentially be exploited to favor intrinsic regeneration of one type or another.
Transcription factor regulatory networks, and the neuron-type-specific downstream molecular differentiation programs they set in motion, specify and consolidate the identity of retinal neurons. These identity-specifying transcription factors were largely originally identified by loss-of-function studies and continue to be investigated in large-scale single-cell-sequencing studies, along with their downstream target genes.
The development of RGCs and their molecular regeneration have been extensively studied. During early RPC proliferation, transcription factors Pax6 and Vsx2 act respectively to promote or inhibit Atoh7 expression (Riesenberg et al., 2009; Vitorino et al., 2009). Atoh7 is necessary for RGC differentiation, and in the absence of Atoh7, RGCs are almost completely absent from the retina (Brown et al., 2001; Wang et al., 2001).
Atoh7 begins to be expressed in RPCs, beginning to transition to a neurogenic state, conferring competence to generate RGCs on nascent RPCs (Wu et al., 2021). The Sox family group C factors Sox4 and Sox11 are expressed in early postmitotic RGCs, establishing their initial specification (Chang et al., 2017). After being specified, RGCs begin to express Brn3b and Isl1, establishing their identity and initiating essential RGC-specific gene programs (Erkman et al., 1996; Gan et al., 1996; Wu et al., 2015). The fate of RGCs remains somewhat plastic even after the onset of Brn3b expression, as, for example, Brn3b acts antagonistically to Ptf1a to control the relative specification of RGCs and ACs (Ohsawa and Kageyama, 2008; Prasov and Glaser, 2012).
Further transcription factors, including Tbr1 and Satb1, act to specify RGC subtypes and their unique dendritic patterns after a general RGC identity is established (Liu et al., 2018; Peng et al., 2017). Importantly, nearly all of these data have been generated in mice, whose complement of RGC subtypes does not match that of humans and other primates (Goetz et al., 2022; Peng et al., 2019), although it shows broad agreement with data from zebrafish (Kölsch et al., 2021).
Following their differentiation into distinct types, neurons in the retina must partner with their neighbors and assemble into functional visual circuits to convey visual information to the brain. The timed generation of different retinal neuron types from progenitors affects circuit development. Since RGCs and ACs are among the first neurons to be generated during development, they are the first neurons to begin forming circuits (Morgan et al., 2018).
Photoreceptors form synapses with HCs, and BCs add themselves to these synapses once they differentiate (Hoon et al., 2014). A major challenge for intrinsic regeneration is to instruct newly born neurons to integrate into mature circuits, which may be relatively preserved, degenerated, or remodeled, depending on the retinal disease state. Retinal ganglion cells face the additional challenge of extending a long axon through the optic nerve head, into the optic nerve, and toward targets in the brain, including optic tectum and the lateral geniculate nucleus of thalamus. Along the way, RGC axons respond to numerous guidance cues, some secreted and others mediated by cellular contact (Erskine and Herrera, 2007). A further challenge for regenerative efforts is to drive RGC output connectivity across much larger distances than exist during development, and in the absence of most developmental axon-pathfinding cues.
Retinal Regeneration in Vertebrate Model Organisms
Although retinal regeneration does not occur after injury or degeneration in mammals, regeneration is possible in many non-mammalian vertebrate species, and mechanisms of regeneration in these animals can provide insight into possible strategies for directing mammalian retinal regeneration. Regeneration has been observed in a number of species, including species across birds, fish, and amphibians, but the primary models used for retinal regeneration research are zebrafish and chicken (Fig. 3). Ongoing work comparing gene expression and regenerative pathways between species continues to produce potential strategies for improving the regenerative capacity of the mammalian retina.
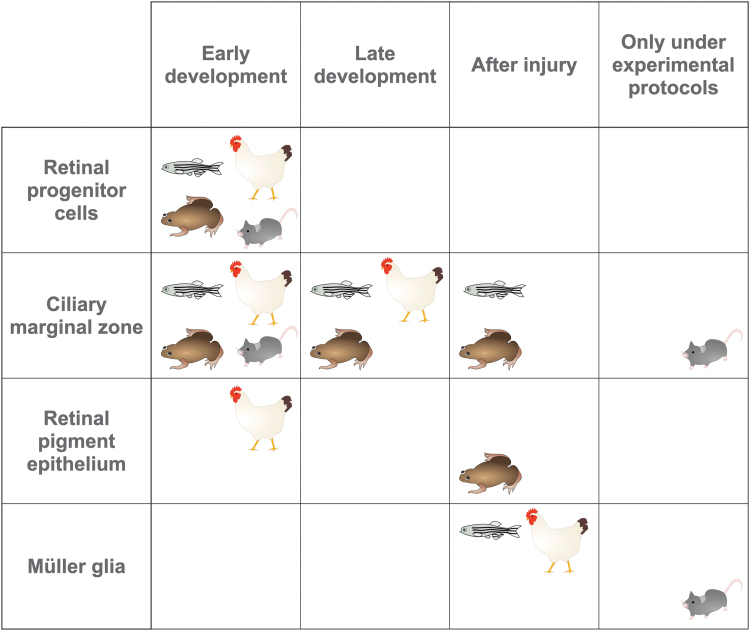
FIG. 3.
Retinal neurons are produced from several progenitor populations across different species. Species displayed represent fish (zebrafish), amphibians (frog), birds (chicken), and mammals (mouse), and they distinguish between early (embryonic/larval) development and late (postnatal/post-hatch) development.
Fish
The fish retina has long been used as a model organism capable of generating neurons postnatally (Hitchcock et al., 2004), and it is able to functionally regenerate even following severe injury (Maier and Wolburg, 1979; Wan and Goldman, 2016). Postnatal neurogenesis in fish is largely a function of stem cells in the ciliary marginal zone (CMZ) (Raymond, 1986), and neurogenesis in this region largely recapitulates earlier developmental processes in the central retina (Lenkowski and Raymond, 2014).
Following damage to the retina, however, the major regenerative cells are Müller glia, which de-differentiate in response to injury, re-enter the cell cycle, and divide asymmetrically to self-renew and produce progenitors capable of generating neurons of all identities (Bernardos et al., 2007; Fausett and Goldman, 2006; Nagashima et al., 2013). Following injury, zebrafish Müller glia follow a process similar to RPCs during development (Lahne et al., 2021). They become reactive and express Ascl1 (Ramachandran et al., 2010) and begin to express many transcription factors, such as Sox2 and Pax6, that also direct differentiation from RPCs during development (Gorsuch et al., 2017; Thummel et al., 2010). Restoration of retinal architecture after injury in zebrafish depends on the extent of the injury, with more extensive injuries requiring more time for repair than modest injuries (Sherpa et al., 2014).
In addition, circuits are restored with remarkable precision, with specific neuron subtypes often re-establishing appropriate synaptic connectivity following regeneration; for example, H3-type HCs continue to prefer partnering with ultraviolet cones over other cone types (Yoshimatsu et al., 2016). Mechanisms of regeneration after injury in zebrafish suggest that Müller glial regeneration can be leveraged in other species, that intrinsic mechanisms may regulate some aspects of endogenous regeneration and circuit formation, and that developmental knowledge can provide significant insight into regeneration from Müller glia.
Chicken
Retinal regeneration in chickens is fundamentally similar to regeneration in fish, and it primarily involves the same cell populations, neural stem cells in the CMZ and Müller glia (Fischer, 2005). During early embryonic development, differentiation of retinal pigment epithelium into neurons is also observed, but this capacity is lost after a few days (Coulombre and Coulombre, 1965; Park and Hollenberg, 1993). Multipotent progenitors in the CMZ remain competent to produce neurons in post-hatch chicks and adult birds, though at reduced rates compared with those observed during development, and proliferation of CMZ progenitors is not increased when the retina is damaged (Fischer and Reh, 2001).
The capacity of Müller glia to generate neurons following injury was originally identified in post-hatch chicks before being confirmed in other model organisms (Fischer and Reh, 2001). However, Müller-glia-mediated regeneration is modest in chicks compared with zebrafish, and most cells produced by proliferation of Müller glia do not differentiate into neurons (Fischer and Reh, 2003). Still, the modest neurogenic capacity of chick Müller glia is potentially an advantage to its use as a model organism, since identifying strategies to overcome intrinsic resistance to neurogenesis in the chick could suggest strategies useful in mammals (El-Hodiri et al., 2022).
Frog
Retinal development in frog species, especially Xenopus laevis, has been extensively studied due to the well-known ability of frog RGC axons to regenerate and re-establish connections with neurons in the optic tectum (Attardi and Sperry, 1963). The frog retina is primarily produced from progenitors in the CMZ (Moshiri et al., 2004), which produces new neurons during development and in the early post-metamorphosis period.
Although RGC axons continue to regenerate after injury in mature X. laevis, RGC neurogenesis is reduced in adult compared with juvenile frogs (Taylor et al., 1989). The retina is capable of regeneration from both CMZ and RPE in post-metamorphosis X. laevis after surgical retinectomy (Yoshii et al., 2007), and some neuron types can be regenerated from Müller glia following mechanical injury or photoreceptor ablation (Langhe et al., 2017).
Regeneration in other frog species can differ from X. laevis; for example, in Xenopus tropicalis, regeneration after injury occurs primarily from cells in the CMZ, rather than the RPE (Miyake and Araki, 2014). In Rana pipiens, neurogenesis can be induced following injury (Reh, 1987), but although some RGC axons regenerate after optic nerve crush, approximately half the RGC population dies within several months of this type of injury (Beazley et al., 1986), which suggests that regenerative ability may differ considerably between anuran species and between experimental injury paradigms.
Mouse
Regeneration does not occur spontaneously in the post-injury adult mammalian retina, but some specific protocols enable the generation of new neurons in the post-developmental mouse retina, primarily from Müller glia. For example, based on work discussed earlier, where zebrafish Müller glia activate Ascl1 in response to injury and re-enter the cell cycle (Ramachandran et al., 2010), mouse Müller glia infected in vitro with Ascl1-expressing virus were found to divide and begin expressing progenitor- and neuron-specific markers (Pollak et al., 2013).
Transgenic Ascl1 expression similarly induces Müller glia to produce neurons in vivo in response to damage in young mice (Ueki et al., 2015), and neuron production can be induced even in older mice with the addition of histone deacetylase and STAT pathway inhibitors (Jorstad et al., 2020; Jorstad et al., 2017). An initiating damage event is not required if Müller glial reprogramming is induced by overexpression of Ascl1 and Atoh1 (Todd et al., 2021).
An alternate protocol for reprogramming adult mouse Müller glia in the absence of damage relies on the overexpression of transcription factors Ikzf1 and Ikzf4, which results in the direct conversion of Müller glia into cells that resemble cone photoreceptors (Boudreau-Pinsonneault et al., 2023). Existing protocols remain relatively inefficient, and future work remains to improve the efficiency of neuron production, from Müller glia or from other populations.
Given that the existence and efficiency of endogenous neuron production following injury vary so widely between model organisms, comparing the regenerative process between species is a particularly attractive approach to screen for genes and pathways responsible for promoting or inhibiting regeneration. In a remarkably comprehensive recent study (Hoang et al., 2020), gene expression and chromatin state were compared at different intervals relative to injury between chick, zebrafish, and mouse retinas.
Müller glia were found to express networks of genes that direct states of rest, reactivity, and proliferation. In zebrafish and chick, Müller glia alter their gene expression from genes characteristic of the resting state to genes characteristic of the reactive state after injury, then express genes characteristic of the proliferative state, although chick Müller glia are less efficient at proceeding to the proliferative state than zebrafish Müller glia.
Similarly, mouse Müller glia change their gene expression profiles from resting to reactive following injury, but then proceed to an alternate resting state characterized by a gene expression profile that is different from the initial resting state, and different from expression states found in zebrafish. Repressing the nuclear factor I family transcription factors Nfia, Nfib, and Nfix, which regulate Müller glial fate specification during development (Clark et al., 2019), and which are expressed at high levels in the post-injury alternate resting state, allowed Müller glia to self-renew and produce neurons post-injury.
This work suggests that suppressing genes and pathways that promote a return to quiescence in mouse Müller glia may be crucial for improving the efficiency of mammalian retinal regeneration.
Candidate Progenitor Populations for Directed Intrinsic Neurogenesis
Several potential sources of intrinsically generated retinal neurons exist, and these niches produce some neurons during development and, to varying degrees, after injury (Fig. 4). Stem cells in the CMZ are a possible source of neurons after the normal period of development, as they can generate neurons in zebrafish and chick. The RPE is also a potential source of neurons, as it can generate neurons in the amphibian and chick retina.
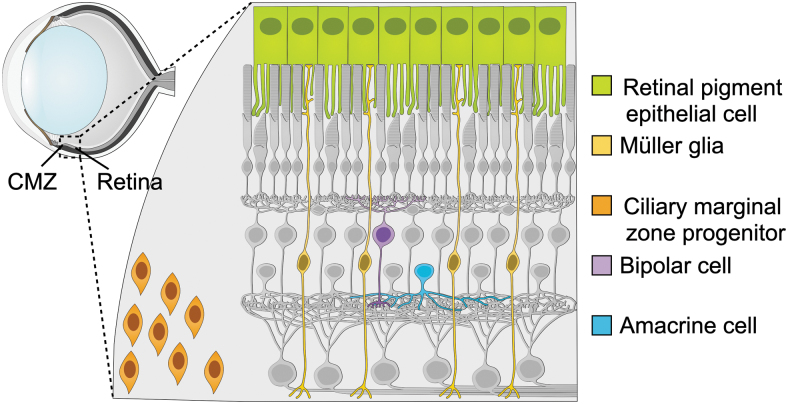
FIG. 4.
Several potential sources of endogenous regeneration exist within the mammalian retina. Neurons could be produced from cells in the retinal pigment epithelium (green) or lingering progenitors in the ciliary marginal zone (orange), as in other vertebrates. Current protocols can provoke neurogenesis from Müller glia (yellow). Existing mature neurons could potentially acquire functions of closely related neuron types, such as amacrine cells (blue) for RGCs, or bipolar cells (purple) for rods.
Closely related mature neurons could also serve as a source of directly converted neurons; for example, ACs could potentially be engineered to express genes characteristic of RGCs, or BCs to express genes characteristic of rod photoreceptors. Müller glia are the most likely source of significant numbers of neurons, given their ability to re-enter the cell cycle and produce retinal neurons in many vertebrate species.
Ciliary marginal zone
Progenitors in the CMZ produce neurons postnatally in many vertebrates, and similar cells at the retinal margin could serve as an endogenous progenitor source in mammals. In larval frogs, these progenitors are a major source of new neurons for the duration of development (Reh and Constantine-Paton, 1983), and they respond to injury by producing appropriate neuron types according to those that were lost (Reh and Tully, 1986; Reh, 1987).
Similarly, the fish CMZ is a major source of neurons produced during development, and the progenitors in this zone seem to be responsive to neuron types lost to injury (Lamba et al., 2008; Lenkowski and Raymond, 2014). Unlike populations in amphibians and fish, progenitors in the CMZ of chickens do not increase neuron production in response to injury, although these cells do produce new neurons in the postnatal animal (Fischer and Reh, 2000).
These progenitors do not appear to be a major contributing source of neurons in adult mammals; neurogenesis in the mouse retina is completed during the second postnatal week, although a limited number of proliferating cells can be identified at the retinal margin. In mice lacking the Shh receptor Ptc, however, actively dividing cells persist at the retinal margin for approximately 3 months, and these cells produce photoreceptors and other neurons in response to injury (Moshiri and Reh, 2004).
As development in the retina proceeds centrifugally, from the center out toward the margins, the CMZ retains some characteristics and remains molecularly distinct from the rest of the retina (Gautam et al., 2021), even in mammals, and might, therefore, be more easily induced to return to a neurogenic state.
Retinal pigment epithelium
The RPE could also serve as a source of newly generated neurons, as is seen in embryonic chicks. The RPE is a monolayer of pigmented cells that line the space between the retina and the choroid. The primary functions of the RPE are to absorb photons that are not detected by photoreceptors and to support photoreceptors by recycling retinal for phototransduction, by phagocytosing shed photoreceptor outer segments, by delivering nutrients from the bloodstream, and by secreting growth and immunosuppressive factors necessary for the health of photoreceptors and inner retina (Strauss, 2005).
In some amphibian systems, including frogs and newts, the RPE is capable of regenerating retinal neurons in vitro after dissociation (Kuriyama et al., 2009; Reh and Nagy, 1987; Stone, 1950). Limited neurogenesis occurs from the RPE in chicken embryos, and although this capacity is lost during later development (Coulombre and Coulombre, 1965), it can be augmented with the addition of various growth factors (Park and Hollenberg, 1991).
Although the RPE is capable of limited focal proliferation after separation from the retina in mammals, new neurons are not produced (Park and Hollenberg, 1993). Nonetheless, the RPE is derived from neuroectodermal tissue during early eye development (Chiba, 2014), and this shared developmental origin with the retina makes it a promising candidate source of neuron-producing progenitors.
Differentiated neurons
Closely related differentiated neuron types represent an intriguing potential source of a desired neuron population, especially with increasing knowledge of the evolutionary, developmental, and molecular relationships between neuron types. For example, retinal progenitors can be redirected from the production of ACs to the production of RGCs based on the expression of Ptf1a (Fujitani et al., 2006), and RGCs and ACs cluster closely together according to gene expression (Macosko et al., 2015).
Similarly, progenitors generate either BCs or rods according to expression levels of Vsx2 and Prdm1 (Goodson et al., 2020; Livne-bar et al., 2006), and these cell types are closely related by cell trajectory analysis in developmental single-cell RNA sequencing datasets (Clark et al., 2019). The overall similarity of the genetic programs directing differentiation of related neuron types suggests that the genetic and epigenetic barriers to reprogramming particular neuron types to their closest relatives might be relatively low.
Müller glia
In vertebrate species with injury-induced retinal regeneration, intrinsic progenitors, primarily Müller glia, are competent to produce neurons of all types following injury. In chicks, production of specific neuron types after injury appears to be responsive to the type of neuron that was lost (Fischer and Reh, 2002). A similar neuron-type responsiveness occurs in zebrafish, although neurons of the appropriate type are not always produced in the proper ratios and can be overproduced in response to severe injuries (Sherpa et al., 2008).
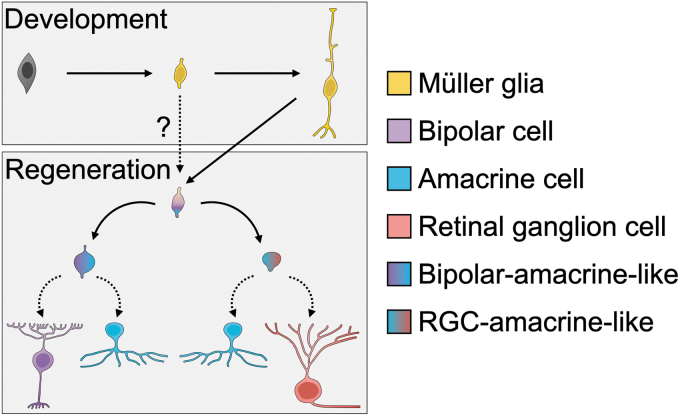
In contrast, mouse Müller glia produce only limited neuron types in response to current injury-and-regeneration protocols, and regenerated neurons are often not fully differentiated when examined by gene expression profiling (Fig. 5). These protocols produce neurons with gene expression and morphology characteristic of rods, cones, BCs, ACs, and immature RGCs. There is considerable interest in guiding Müller glia to generate mature RGCs, but this has proved challenging.
FIG. 5.
A model of developmental trajectories available to Müller-glia-derived neurons in mammals. During development (top panel), late retinal progenitor cells produce nascent Müller glia, which develop into mature Müller glia. For regeneration to occur (bottom panel), Müller glia must enter a general neurogenic state (top), which may bear a resemblance to their developmental state (dotted arrow and question mark). According to the protocol delivered, they seem to be capable primarily of producing bipolar/amacrine-like neurons (left path) or RGC/amacrine-like neurons (right path). Additional factors may be necessary to enable a true bipolar, amacrine, or RGC fate (bottom row).
Rod and cone photoreceptor-like cells can be produced by mouse Müller glia following injury. A handful of cells with gene expression characteristic of rods is produced following N-methyl-D-aspartate (NMDA) injury, Ascl1 overexpression, and histone deacetylase treatment (Jorstad et al., 2017). Overexpression of Ikzf1 and Ikzf4 by retinal electroporation produces cells with morphology and laminar position characteristic of cones, and gene expression characteristic of cones as well as rods (Boudreau-Pinsonneault et al., 2023).
Rods are produced in more substantial numbers after treatment with AAV expressing GFAP-driven beta-catenin, followed by overexpression of Otx2, Crx, and Nrl (Yao et al., 2018). However, AAV-mediated gene expression under the control of a GFAP mini-promoter lacks Müller glia specificity in the presence of ectopic transcription factors, and therefore it is possible that these experiments labeled existing photoreceptors (Le et al., 2022; Leib et al., 2022).
Many neurons produced by Müller glia following reprogramming protocols in mice demonstrate gene expression, morphology, and laminar position characteristic of BCs, or of a hybrid bipolar-amacrine-like cell. The neurons produced after NMDA injury, Ascl1 overexpression, and histone deacetylase treatment appear to be a bipolar-amacrine-like hybrid based on gene expression from single-cell RNA sequencing. At a morphological level, most of these neurons appear similar to BCs, and a minority appear similar to ACs (Jorstad et al., 2017).
Similarly, after NMDA injury and subsequent repression of Nfia, Nfib, and Nfix, Müller glia produce primarily bipolar-like cells, with a minority of amacrine-like cells, by both gene expression and morphology (Hoang et al., 2020). Overexpression of Ikzf1 and Ikzf4 by retinal electroporation produces several distinct bipolar-like populations by gene expression (Boudreau-Pinsonneault et al., 2023), which map to distinct gene-expression-defined bipolar subtypes (Shekhar et al., 2016).
Overall, current protocols for inducing neurogenesis from mouse Müller glia appear to strongly favor producing neurons with characteristics of BCs, which could suggest that these protocols drive Müller glia to de-differentiate into progenitors that resemble developmentally late-stage retinal progenitors that are competent only to produce primarily BCs and ACs.
Since RGCs are clinically important, and degenerate in glaucoma and other optic neuropathies leading to irreversible blindness, driving their regeneration from intrinsic progenitors is of great interest in the field. Robust reprogramming of Müller glia to a RGC identity has been reported (Zhou et al., 2020), but this protocol relies on AAV expressing GFAP-driven expression of Ptbp1, an RNA-binding protein, and off-target labeling of endogenous RGCs cannot be ruled out.
Performing these experiments using a genomic GFAP-driven Cre recombinase failed to replicate the effect of Ptbp1 in reprogramming Müller glia to RGCs, and conditional deletion of Ptbp1 using a Müller-glia-specific inducible Cre recombinase did not induce a regenerative response (Le et al., 2022). In other work, reprogramming protocols using Ascl1 and Atoh1 overexpression produce neurons with gene expression profiles consistent with immature RGCs, with increased expression of Sox4 and Sox11.
These neurons do not appear to fully mature as RGCs, however, and do not express genes characteristic of mature RGCs, such as Brn3b or Rbpms, even after several weeks, nor do they extend axons into the optic nerve (Todd et al., 2021). Nonetheless, this protocol represents a promising direction for Müller glial reprogramming in the mouse retina.
Müller glia likely represent the most promising source of substantial numbers of regenerated neurons in the mammalian retina. Nonetheless, future work is necessary to identify more efficient of neurogenic induction, and accomplishing this might require additional approaches, such as knocking down genes that maintain transcriptional profiles or safeguard chromatin states anchoring Müller glia in their differentiated state. Eventually, it will be important to develop protocols that provide therapeutic benefits for human patients while maintaining a favorable safety profile.
Challenges for Translating Intrinsic Regeneration to Human Patients
Although intrinsic regeneration represents a promising strategy for repairing retinal damage in patients with irreversible vision loss due to neuronal degeneration or injury, many hurdles remain. Foundationally, it is necessary for those in the field to rigorously demonstrate that each proposed method for stimulating regeneration from intrinsic progenitors represents true regeneration, excluding inadvertent labeling of undamaged endogenous cells.
It will be crucial to cause neurons to be generated in sufficient numbers to ameliorate degeneration or injury and, further, to ensure that they integrate into circuits in acceptable subtype ratios and establish appropriate output connectivity and function. Since the environment surrounding intrinsic progenitors affects their propensity for neurogenesis, these methods must be demonstrated to be effective in animal models that faithfully recapitulate human diseases of the eye. Finally, methods to activate intrinsic regeneration must be established to be safe and effective, as well as practical for use in clinical settings.
A fundamental challenge of investigating and stimulating regeneration from intrinsic progenitors is to demonstrate conclusively that any results represent true regeneration, and not accidental labeling of healthy endogenous cells of the same type. Most methods to distinguish endogenous cells from newly generated cells, including nucleoside analogue labeling, chromosomal sex, and constitutive reporter gene expression, can be used for transplantation experiments, but not for intrinsic regeneration experiments.
Even using these methods, cell fusion and protein transfer artifacts have long been problematic for demonstrating regeneration from transplanted cells (Alvarez-Dolado et al., 2003; Santos-Ferreira et al., 2016; Santos-Ferreira et al., 2015). Therefore, when regeneration is attempted from endogenous progenitors, it is even more difficult to exclude alternative interpretations for positive results (Blackshaw and Sanes, 2021).
As described earlier, some recent high-profile claims of intrinsic regeneration in the retina (Yao et al., 2018; Zhou et al., 2020) have been called into question given that virally delivered glial mini-promoters seem not to drive expression faithfully in only glial cells in the presence of ectopic transcription factors (Hoang et al., 2022; Le et al., 2022; Leib et al., 2022).
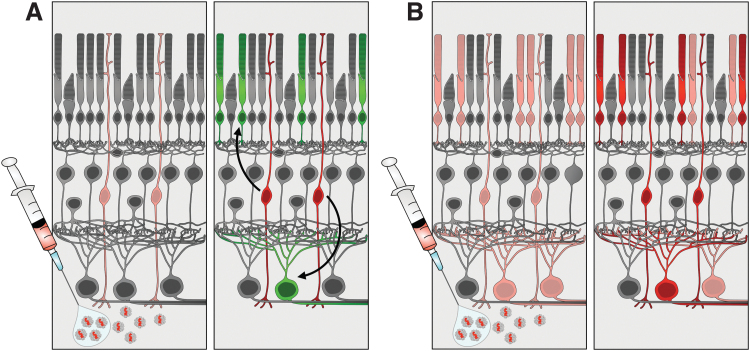
This causes labeling of endogenous neurons with the glial fibrillary acidic protein (GFAP)-promoter-driven payload, leading to the appearance of newly born neurons derived from GFAP-expressing mature Müller glial cells, although these are actually healthy endogenous neurons generated normally during development (Fig. 6). To demonstrate that genuine intrinsic regeneration occurs, immunostaining with individual cell-type-specific markers is not sufficient, and transcriptomic profiling should be the gold standard instead.
FIG. 6.
Promiscuous viral infection and/or expression can confound reports of intrinsic regeneration. (A) In the preferred case, virus only transduces and becomes activated in Müller glia (pink) (left panel), and therefore neurogenesis from labeled Müller glia (red) is the exclusive source of labeled mature neurons (green) (right panel). (B) In the alternate case, virus may transduce and become active in several retinal cell types (pink), even if inefficiently (left panel), and labeled mature neurons (red) may be directly transduced rather than produced by neurogenesis (right panel).
Single-cell RNA sequencing is a particularly useful method for this purpose, since it is likely that regenerated neurons will display at least some heterogeneity and gene expression differences from endogenous neurons, even if reprogramming and regeneration work well. In addition, transgenic reagents, which are subject to regulation by a more complete promoter environment in the genome, are likely to provide better cell-type-specific expression and fidelity than promoter fragments provided by viral or plasmid delivery. Once regeneration is rigorously confirmed, though, it will be important to develop tools for reprogramming that can be used in humans rather than relying on transgenic lines.
Once regeneration from intrinsic progenitors can be meaningfully achieved, it will be necessary to determine whether neurons are regenerated in complements of subtypes that are appropriate for the human retina. Fundamentally, it will be important to identify whether regeneration occurs across functional subtypes, or whether bias exists in the subtypes of retinal neurons regenerated; if bias does exist, the subtypes of neurons generated may not be adequately equipped to restore vision in human patients.
Subtypes of RGCs, for example, are differentially vulnerable to injury in the mouse (Tran et al., 2019), and project to different visual target areas in the brain in zebrafish (Kölsch et al., 2021; Robles et al., 2014), indicating that precise ratios of particular subtypes may be required to restore vision faithfully. Moreover, humans and other primates have RGC subtypes that do not map neatly onto those types identified in model organism studies, for example, with a predominance of image-forming foveal midget ganglion cells (Peng et al., 2019). Using protocols derived from work in model organisms, therefore, may not produce RGC subtypes that are adequately tuned to re-establish human vision.
A further obstacle to meaningful regeneration is for newly generated neurons to integrate into existing circuitry and, in the case of RGCs, extend long-distance axons to connect with visual centers in the brain. Neurons in the zebrafish retina are capable of functionally integrating into circuitry following regeneration from Müller glia, and that regenerated neurons can maintain the same preferences for synaptic partners held by neurons of that type generated during development (McGinn et al., 2017; Yoshimatsu et al., 2016), though they do not always do so with perfect fidelity (D'Orazi et al., 2016).
Circuit plasticity within the mouse retina after partial degeneration would suggest that newly generated neurons may be capable of finding and establishing synapses with their typical partners, given sufficient synaptic space for them to explore (Beier et al., 2018), and, indeed, some neurons generated from Müller glia in the mouse respond to light, indicating that they have likely integrated into the adult retina (Todd et al., 2021).
The RGC axon extension and target selection is likely to present a significant challenge to regenerative efforts, with RGCs needing to extend an axon into the optic nerve and connect with visual targets in the brain in the absence of developmental cues and over a much longer physical distance than is present developmentally. To produce a fully regenerated and functional RGC from endogenous Müller glia, it will be necessary to integrate discoveries from studying intrinsic rebirth of RGCs with discoveries from studying the regenerative capacity of injured RGC axons (Williams et al., 2020).
The identification of transcriptional programs and pathways that allow limited regeneration of mammalian RGCs following injury (Jacobi et al., 2022; Tian et al., 2022; Winter et al., 2022) is likely to offer useful insight into critical genetic controls over regeneration from endogenous progenitors.
The regenerative capacity of endogenous cells is affected by their environment and the method of injury used in model organisms, and, therefore, identifying methods of intrinsic regeneration that produce robust regeneration in the context of human retinal disease processes is crucially important. In zebrafish, regeneration is most often induced by protocols that ablate neurons acutely, such as light damage or drug-induced toxicity (Lahne et al., 2020).
These methods induce a robust regenerative response in the zebrafish retina, and, thus, they have served as a tremendously useful starting point for investigating intrinsic regeneration in the mammalian retina, but they may not translate well to human diseases that progressively cause neuron death over a period of years. Human disease states may alter the retinal microenvironment into which regenerated neurons are born and could affect their development by affecting the retinal vasculature, health of RPE and other support cells, and immune system activation, among other factors.
It may be necessary to identify mammalian models that more closely approximate the disease processes that occur in the human retina, such as RGC death in response to increased intraocular pressure to model glaucoma (Zhang et al., 2019). Inducing disease and subsequent regeneration in large animals, including non-human primates, will also be a necessary step toward demonstrating the feasibility and safety of these approaches.
A final hurdle to directing regeneration from intrinsic progenitors to cure human disease is the ability to place the initiation and control of regeneration in the hands of a clinician. This is challenging first in terms of the logistical requirements for targeted gene delivery in humans; work in zebrafish and mice allows investigators to use transgenic lines that activate a virally delivered payload only under specific conditions of gene expression, but this is not possible in humans.
Gene delivery to neurons in the retina through subretinal injection of AAV is an attractive strategy, and one that has already been approved by the Food and Drug Administration for the treatment of inherited retinal disease (Bainbridge et al., 2015). It would be useful, in this case, simply to place AAV-delivered genes under the control of a cell-type-specific promoter, such as the GFAP promoter to target Müller glia, but the fidelity of small promoter fragments is limited, as discussed earlier (Le et al., 2022; Leib et al., 2022).
This issue could potentially be mitigated by also using an engineered viral capsid that preferentially infects particular cell types, such as the ShH10 variant of AAV6 that primarily infects Müller glia (Klimczak et al., 2009). The content of virally delivered reprogramming factors will likely determine whether gene delivery in the clinic must be stringently restricted only to specific cell types, or whether some off-target infection can be tolerated.
Conclusion
Retinal regeneration from intrinsic progenitors holds significant promise as a strategy to repair vision in patients. Building on lessons from retinal development in mammals, and from regeneration after injury in fish and birds, some groups have demonstrated proof of the principle that regeneration of retinal neurons may be possible from intrinsic Müller glia in the mammalian retina.
Future work may focus on other potential endogenous sources of new neurons. However, considerable work will be necessary to increase the efficiency of regeneration, to ensure the generation of particular complements of desired neuron types, and to develop safe and effective tools for translating these discoveries into clinical use.
Authors' Contribution
M.B.W. and L.C.G. prepared the figures; all authors wrote and edited the article.
Abbreviations Used
- AAV
adeno-associated virus
- AC
amacrine cell
- Ascl1
ashaete-scute basic helix-loop-helix family transcription factor 1
- Atoh1
atonal basic helix-loop-helix transcription factor 7
- Atoh7
atonal basic helix-loop-helix transcription factor 7
- BC
bipolar cell
- Brn3b
POU homeodomain class 4 homeobox 2 transcription factor
- CMZ
ciliary marginal zone
- Crx
cone-rod homeobox
- E
embryonic day
- GFAP
glial fibrillary acidic protein
- HC
horizontal cell
- Ikzf1
Ikaros zinc finger transcription factor 1
- Ikzf4
Ikaros zinc finger transcription factor 4
- Isl1
Islet 1
- Nfia
nuclear factor 1a
- Nfib
nuclear factor 1b
- Nfix
nuclear factor 1x
- NMDA
N-methyl-D-aspartate
- Nrl
neural retina leucine zipper
- Otx2
orthodenticle homeobox 2
- P
postnatal day
- Pax6
paired box 6
- Prdm1
PR domain zinc finger protein 1
- Prox1
prospero homeobox 1
- Ptbp1
polypyrimidine tract binding protein 1
- Ptc
patched
- Ptf1a
pancreas associated transcription factor 1a
- Rbpms
RNA binding protein, mRNA processing factor
- RGC
retinal ganglion cell
- RNA
ribonucleic acid
- RPC
retinal progenitor cell
- RPE
retinal pigment epithelium
- Satb1
special AT-rich binding homeobox protein 1
- Shh
Sonic hedgehog
- ShH10
AAV variant derived from shuffled (ShH) library
- Sox2
sex-determining-region-of-Y-chromosome-box transcription factor 2
- Sox4
sex-determining-region-of-Y-chromosome-box transcription factor 4
- Sox11
sex-determining-region-of-Y-chromosome-box transcription factor 11
- STAT
signal transducer and activator of transcription
- Tbr1
T-box brain transcription factor 1
- Vsx2
visual system homeobox 2
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This review was supported by funding from the National Eye Institute (K99 EY031403 to Mollie Woodworth; U24 EY029903 to Jeffrey Goldberg; Stanford Vision Research Core P30 EY026877 to Jeffrey Goldberg), the Knights Templar Eye Foundation (KTEF Early Career Starter Grant to Luciano Greig), Stanford Maternal and Child Health Research Institute (Instructor K Award to Mollie Woodworth; Pilot Early Career Grant to Luciano Greig), Research to Prevent Blindness (Institutional Grant to Jeffrey Goldberg), and the Gilbert Family Foundation (Gilbert Vision Restoration Initiative to Jeffrey Goldberg).
References
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003;425(6961):968–973; doi: 10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- Attardi DG, Sperry RW. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol 1963;7:46–64; doi: 10.1016/0014-4886(63)90093-1 [DOI] [PubMed] [Google Scholar]
- Bainbridge JWB, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on leber's congenital amaurosis. N Engl J Med 2015;372(20):1887–1897; doi: 10.1056/nejmoa1414221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci 2012;35(9):565–573; doi: 10.1016/j.tins.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Beazley LD, Darby JE, Perry VH. Cell death in the retinal ganglion cell layer during optic nerve regeneration for the frog rana pipiens. Vision Res 1986;26(4):543–556; doi: 10.1016/0042-6989(86)90003-9 [DOI] [PubMed] [Google Scholar]
- Beier C, Palanker D, Sher A. Stereotyped synaptic connectivity is restored during circuit repair in the adult mammalian retina. Curr Biol 2018;28(11):1818–1824.e2; doi: 10.1016/j.cub.2018.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau MJ, Cepko CL. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 1999;126(3):555–566; doi: 10.1242/dev.126.3.555 [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, et al. Late-stage neuronal progenitors in the retina are radial muller glia that function as retinal stem cells. J Neurosci 2007;27(26):7028–7040; doi: 10.1523/JNEUROSCI.1624-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S, Sanes JR. Turning lead into gold: Reprogramming retinal cells to cure blindness. J Clin Invest 2021;131(3):e146134; doi: 10.1172/jci146134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau-Pinsonneault C, David LA, Lourenço Fernandes JA, et al. Direct neuronal reprogramming by temporal identify factors. Proc Natl Acad Sci 2023;120(19):e221216820; doi: 10.1101/2021.07.05.451124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, et al. Math5 is required for retinal ganglion cell and optic nerve formation. Dev Camb Engl 2001;128(13):2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Kim EJ, Johnson JE, et al. Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development 2011;138(16):3519–3531; doi: 10.1242/dev.064006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Prasov L, Glaser T. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Dev Biol 2012;365(2):395–413; doi: 10.1016/j.ydbio.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski JA, Reh TA. Photoreceptor cell fate specification in vertebrates. Development 2015;142(19):3263–3273; doi: 10.1242/dev.127043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci 2014;15(9):615–627; doi: 10.1038/nrn3767 [DOI] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 2017;20(8):1172–1179; doi: 10.1038/nn.4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-C, Hertz J, Zhang X, et al. Novel regulatory mechanisms for the SoxC transcriptional network required for visual pathway development. J Neurosci 2017;37(19):4967–4981; doi: 10.1523/jneurosci.3430-13.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry TJ, Trimarchi JM, Stadler MB, et al. Development and diversification of retinal amacrine interneurons at single cell resolution. Proc Natl Acad Sci USA 2009;106(23):9495–9500; doi: 10.1073/pnas.0903264106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba C. The retinal pigment epithelium: An important player of retinal disorders and regeneration. Exp Eye Res 2014;123:107–114; doi: 10.1016/j.exer.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Clark BS, Stein-O'Brien GL, Shiau F, et al. Single-Cell RNA-Seq analysis of retinal development identifies NFI factors as regulating mitotic exit and late-born cell specification. Neuron 2019;102(6):1111–1126.e5; doi: 10.1016/j.neuron.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol 1965;12(1):79–92; doi: 10.1016/0012-1606(65)90022-9 [DOI] [PubMed] [Google Scholar]
- D'Orazi FD, Zhao X-F, Wong RO, et al. Mismatch of synaptic patterns between neurons produced in regeneration and during development of the vertebrate retina. Curr Biol 2016;26(17):2268–2279; doi: 10.1016/j.cub.2016.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, et al. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 2003;34(1):53–58; doi: 10.1038/ng1144 [DOI] [PubMed] [Google Scholar]
- El-Hodiri HM, Campbell WA, Kelly LE, et al. Nuclear Factor I in neurons, glia and during the formation of müller glia-derived progenitor cells in avian, porcine and primate retinas. J Comp Neurol 2022;530(8):1213–1230; doi: 10.1002/cne.25270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J, Jolicoeur C, Ramamurthy V, et al. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron 2008;60(1):26–39; doi: 10.1016/j.neuron.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, et al. Role of transcription factors a Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 1996;381(6583):603–606; doi: 10.1038/381603a0 [DOI] [PubMed] [Google Scholar]
- Erskine L, Herrera E. The retinal ganglion cell axon's journey: Insights into molecular mechanisms of axon guidance. Dev Biol 2007;308(1):1–14; doi: 10.1016/j.ydbio.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for 1 tubulin-expressing muller glia in regeneration of the injured zebrafish retina. J Neurosci 2006;26(23):6303–6313; doi: 10.1523/jneurosci.0332-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res 2005;24(2):161–182; doi: 10.1016/j.preteyeres.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol 2000;220(2):197–210; doi: 10.1006/dbio.2000.9640 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci 2001;4(3):247–252; doi: 10.1038/85090 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol 2002;251(2):367–379; doi: 10.1006/dbio.2002.0813 [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Potential of müller glia to become neurogenic retinal progenitor cells: Retinal müller glia as a source of stem cells. Glia 2003;43(1):70–76; doi: 10.1002/glia.10218 [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, et al. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development 2006;133(22):4439–4450; doi: 10.1242/dev.02598 [DOI] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, et al. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci USA 1996;93(9):3920–3925; doi: 10.1073/pnas.93.9.3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Hamashima K, Chen Y, et al. Multi-species single-cell transcriptomic analysis of ocular compartment regulons. Nat Commun 2021;12(1):5675; doi: 10.1038/s41467-021-25968-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz J, Jessen ZF, Jacobi A, et al. Unified classification of mouse retinal ganglion cells using function, morphology, and gene expression. Cell Rep 2022;40(2):111040; doi: 10.1016/j.celrep.2022.111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci 2014;15(7):431–442; doi: 10.1038/nrn3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson NB, Park KU, Silver JS, et al. Prdm1 overexpression causes a photoreceptor fate-shift in nascent, but not mature, bipolar cells. Dev Biol 2020;464(2):111–123; doi: 10.1016/j.ydbio.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsuch RA, Lahne M, Yarka CE, et al. Sox2 regulates müller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a. Exp Eye Res 2017;161:174–192; doi: 10.1016/j.exer.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, et al. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res 2004;23(2):183–194; doi: 10.1016/j.preteyeres.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Hoang T, Kim DW, Appel H, et al. Genetic loss of function of Ptbp1 does not induce glia-to-neuron conversion in retina. Cell Rep 2022;39(11):110849; doi: 10.1016/j.celrep.2022.110849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Wang J, Boyd P, et al. Gene regulatory networks controlling vertebrate retinal regeneration. Science 2020;370(6519):eabb8598; doi: 10.1126/science.abb8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Okawa H, Santina LD, et al. Functional architecture of the retina: Development and disease. Prog Retin Eye Res 2014;42:44–84; doi: 10.1016/j.preteyeres.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi A, Tran NM, Yan W, et al. Overlapping transcriptional programs promote survival and axonal regeneration of injured retinal ganglion cells. Neuron 2022;110(16):2625–2645.e7; doi: 10.1016/j.neuron.2022.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, et al. Stimulation of functional neuronal regeneration from müller glia in adult mice. Nature 2017;548(7665):103; doi: 10.1038/nature23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Todd L, et al. STAT signaling modifies Ascl1 chromatin binding and limits neural regeneration from muller glia in adult mouse retina. Cell Rep 2020;30(7):2195–2208.e5; doi: 10.1016/j.celrep.2020.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimczak RR, Koerber JT, Dalkara D, et al. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat müller cells. PLoS One 2009;4(10):e7467; doi: 10.1371/journal.pone.0007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölsch Y, Hahn J, Sappington A, et al. Molecular classification of zebrafish retinal ganglion cells links genes to cell types to behavior. Neuron 2021;109(4):645–662.e9; doi: 10.1016/j.neuron.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama F, Ueda Y, Araki M. Complete reconstruction of the retinal laminar structure from a cultured retinal pigment epithelium is triggered by altered tissue interaction and promoted by overlaid extracellular matrices. Dev Neurobiol 2009;69(14):950–958; doi: 10.1002/dneu.20745 [DOI] [PubMed] [Google Scholar]
- Lahne M, Brecker M, Jones SE, et al. The regenerating adult zebrafish retina recapitulates developmental fate specification programs. Front Cell Dev Biol 2021;8:617923; doi: 10.3389/fcell.2020.617923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Nagashima M, Hyde DR, et al. Reprogramming müller glia to regenerate retinal neurons. Annu Rev Vis Sci 2020;6(1):1–23; doi: 10.1146/annurev-vision-121219-081808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: A view from the eye. Cell Stem Cell 2008;2(6):538–549; doi: 10.1016/j.stem.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhe R, Chesneau A, Colozza G, et al. Müller glial cell reactivation in xenopus models of retinal degeneration. Glia 2017;65(8):1333–1349; doi: 10.1002/glia.23165 [DOI] [PubMed] [Google Scholar]
- Le N, Appel H, Pannullo N, et al. Ectopic insert-dependent neuronal expression of GFAP promoter-driven AAV constructs in adult mouse retina. Front Cell Dev Biol 2022;10:914386; doi: 10.3389/fcell.2022.914386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib D, Chen YH, Monteys AM, et al. Limited astrocyte-to-neuron conversion in the mouse brain using NeuroD1 overexpression. Mol Ther 2022;30(3):982–986; doi: 10.1016/j.ymthe.2022.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res 2014;40:94–123; doi: 10.1016/j.preteyeres.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Reggiani JDS, Laboulaye MA, et al. Tbr1 instructs laminar patterning of retinal ganglion cell dendrites. Nat Neurosci 2018;21(5):659–670; doi: 10.1038/s41593-018-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne-bar I, Pacal M, Cheung MC, et al. Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc Natl Acad Sci USA 2006;103(13):4988–4993; doi: 10.1073/pnas.0600083103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 2015;161(5):1202–1214; doi: 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Wolburg H. Regeneration of the goldfish retina after exposure to different doses of ouabain. Cell Tissue Res 1979;202(1):99–118; doi: 10.1007/bf00239223 [DOI] [PubMed] [Google Scholar]
- McGinn TE, Mitchell DM, Meighan PC, et al. Restoration of dendritic complexity, functional connectivity, and diversity of regenerated retinal bipolar neurons in adult zebrafish. J Neurosci 2017;38(1):120–136; doi: 10.1523/jneurosci.3444-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellough CB, Bauer R, Collin J, et al. An integrated transcriptional analysis of the developing human retina. Development 2019;146(2):dev169474; doi: 10.1242/dev.169474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet 2018;392(10153):1147–1159; doi: 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- Miyake A, Araki M. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: A study of retinal regeneration in a novel animal model. Dev Neurobiol 2014;74(7):739–756; doi: 10.1002/dneu.22169 [DOI] [PubMed] [Google Scholar]
- Morgan J, Wong R. Development of Cell Types and Synaptic Connections in the Retina. 2018. Available from: https://webvision.med.utah.edu/book/part-vi-development-of-cell-types-and-synaptic-connections-in-the-retina/development-of-cell-types-and-synaptic-connections-in-the-retina/ [Last accessed: June 27, 2023].
- Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol 2004;48(8–9):1003–1014; doi: 10.1387/ijdb.041870am [DOI] [PubMed] [Google Scholar]
- Moshiri A, Reh TA. Persistent progenitors at the retinal margin of Ptc+/- mice. J Neurosci 2004;24(1):229–237; doi: 10.1523/jneurosci.2980-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013;140(22):4510–4521; doi: 10.1242/dev.090738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res 2008;1192:90–98; doi: 10.1016/j.brainres.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Induction of retinal regeneration in vivo by growth factors. Dev Biol 1991;148(1):322–333; doi: 10.1016/0012-1606(91)90341-Y [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Growth factor-induced retinal regeneration in vivo. Int Rev Cytol 1993;146:49–74; doi: 10.1016/s0074-7696(08)60379-4 [DOI] [PubMed] [Google Scholar]
- Peng Y-R, Shekhar K, Yan W, et al. Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell 2019;176(5):1222–1237.e22; doi: 10.1016/j.cell.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y-R, Tran NM, Krishnaswamy A, et al. Satb1 Regulates Contactin 5 to pattern dendrites of a mammalian retinal ganglion cell. Neuron 2017;95(4):869–883.e6; doi: 10.1016/j.neuron.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, et al. ASCL1 reprograms mouse müller glia into neurogenic retinal progenitors. Development 2013;140(12):2619–2631; doi: 10.1242/dev.091355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasov L, Glaser T. Dynamic expression of ganglion cell markers in retinal progenitors during the terminal cell cycle. Mol Cell Neurosci 2012;50(2):160–168; doi: 10.1016/j.mcn.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates müller glia dedifferentiation and retinal regeneration through a Lin-28-Dependent, Let-7 MicroRNA signalling pathway. Nat Cell Biol 2010;12(11):1101–1107; doi: 10.1038/ncb2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Wong LL, Wood ED, et al. Timing and topography of cell genesis in the rat retina. J Comp Neurol 2004;474(2):304–324; doi: 10.1002/cne.20134 [DOI] [PubMed] [Google Scholar]
- Raymond P. Movement of retinal terminals in goldfish optic tectum predicted by analysis of neuronal proliferation. J Neurosci 1986;6(9):2479–2488; doi: 10.1523/jneurosci.06-09-02479.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh T. Cell-specific regulation of neuronal production in the larval frog retina. J Neurosci 1987;7(10):3317–3324; doi: 10.1523/jneurosci.07-10-03317.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Constantine-Paton M. Qualitative and quantitative measures of plasticity during the normal development of the rana pipiens retinotectal projection. Dev Brain Res 1983;10(2):187–200; doi: 10.1016/0165-3806(83)90136-0 [DOI] [PubMed] [Google Scholar]
- Reh TA, Nagy T. A possible role for the vascular membrane in retinal regeneration in rana catesbienna tadpoles. Dev Biol 1987;122(2):471–482; doi: 10.1016/0012-1606(87)90311-3 [DOI] [PubMed] [Google Scholar]
- Reh TA, Tully T. Regulation of tyrosine hydroxylase-containing amacrine cell number in larval frog retina. Dev Biol 1986;114(2):463–469; doi: 10.1016/0012-1606(86)90210-1 [DOI] [PubMed] [Google Scholar]
- Riesenberg AN, Le TT, Willardsen MI, et al. Pax6 Regulation of Math5 during mouse retinal neurogenesis. Genesis 2009;47(3):175–187; doi: 10.1002/dvg.20479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Laurell E, Baier H. The retinal projectome reveals brain-area-specific visual representations generated by ganglion cell diversity. Curr Biol 2014;24(18):2085–2096; doi: 10.1016/j.cub.2014.07.080 [DOI] [PubMed] [Google Scholar]
- Sajgo S, Ghinia MG, Brooks M, et al. Molecular codes for cell type specification in Brn3 retinal ganglion cells. Proc Natl Acad Sci USA 2017;114(20):E3974–E3983; doi: 10.1073/pnas.1618551114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Llonch S, Borsch O, et al. Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nat Commun 2016;7(1):13028; doi: 10.1038/ncomms13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Postel K, Stutzki H, et al. Daylight vision repair by cell transplantation. Stem Cells 2015;33(1):79–90; doi: 10.1002/stem.1824 [DOI] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 2016;166(5):1308–1323.e30; doi: 10.1016/j.cell.2016.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Whitney IE, Butrus S, et al. Diversification of multipotential postmitotic mouse retinal ganglion cell precursors into discrete types. eLife 2022;11:e73809; doi: 10.7554/eLife.73809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa T, Fimbel SM, Mallory DE, et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol 2008;68(2):166–181; doi: 10.1002/dneu.20568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa T, Lankford T, McGinn TE, et al. Retinal regeneration is facilitated by the presence of surviving neurons. Dev Neurobiol 2014;74(9):851–876; doi: 10.1002/dneu.22167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults—screening, diagnosis, and management. JAMA 2021;325(2):164–174; doi: 10.1001/jama.2020.21899 [DOI] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 2016;51:156–186; doi: 10.1016/j.preteyeres.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Stone LS. The role of retinal pigment cells in regenerating neural retinae of adult salamander eyes. J Exp Zool 1950;113(1):9–31; doi: 10.1002/jez.1401130103 [DOI] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev 2005;85(3):845–881; doi: 10.1152/physrev.00021.2004 [DOI] [PubMed] [Google Scholar]
- Taylor JS, Jack JL, Easter SS. Is the capacity for optic nerve regeneration related to continued retinal ganglion cell production in the frog? Eur J Neurosci 1989;1(6):626–638; doi: 10.1111/j.1460-9568.1989.tb00368.x [DOI] [PubMed] [Google Scholar]
- Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014;121(11):2081–2090; doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res 2010;90(5):572–582; doi: 10.1016/j.exer.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Cheng Y, Zhou S, et al. Core Transcription programs controlling injury-induced neurodegeneration of retinal ganglion cells. Neuron 2022;110(16):2607–2624.e8; doi: 10.1016/j.neuron.2022.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Hooper MJ, Haugan AK, et al. Efficient stimulation of retinal regeneration from müller glia in adult mice using combinations of proneural BHLH transcription factors. Cell Rep 2021;37(3):109857; doi: 10.1016/j.celrep.2021.109857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NM, Shekhar K, Whitney IE, et al. Single-cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron 2019;104(6):1039–1055.e12; doi: 10.1016/j.neuron.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature 1987;328(6126):131–136; doi: 10.1038/328131a0 [DOI] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, et al. Transgenic expression of the proneural transcription factor Ascl1 in müller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci USA 2015;112(44):13717–13722; doi: 10.1073/pnas.1510595112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino M, Jusuf PR, Maurus D, et al. Vsx2 in the zebrafish retina: Restricted lineages through derepression. Neural Develop 2009;4(1):14; doi: 10.1186/1749-8104-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Goldman D. Retina regeneration in zebrafish. Curr Opin Genet Dev 2016;40:41–47; doi: 10.1016/j.gde.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, et al. Requirement for Math5 in the development of retinal ganglion cells. Genes Dev 2001;15(1):24–29; doi: 10.1101/gad.855301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Lapan SW, Lee C, et al. Spatiotemporal patterns of neuronal subtype genesis suggest hierarchical development of retinal diversity. Cell Rep 2022;38(1):110191; doi: 10.1016/j.celrep.2021.110191 [DOI] [PubMed] [Google Scholar]
- Williams PR, Benowitz LI, Goldberg JL, et al. Axon regeneration in the mammalian optic nerve. Annu Rev Vis Sci 2020;6(1):195–213; doi: 10.1146/annurev-vision-022720-094953 [DOI] [PubMed] [Google Scholar]
- Winter CC, He Z, Jacobi A. Axon regeneration: A subcellular extension in multiple dimensions. Cold Spring Harb Perspect Biol 2022;14(3):a040923; doi: 10.1101/cshperspect.a040923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World Report on Vision. World Health Organization: Geneva; 2019. [Google Scholar]
- Wu F, Bard JE, Kann J, et al. Single cell transcriptomics reveals lineage trajectory of retinal ganglion cells in wild-type and Atoh7-null retinas. Nat Commun 2021;12(1):1465; doi: 10.1038/s41467-021-21704-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Kaczynski TJ, Sethuramanujam S, et al. Two transcription factors, Pou4f2 and Isl1, are sufficient to specify the retinal ganglion cell fate. Proc Natl Acad Sci USA 2015;112(13):E1559–E1568; doi: 10.1073/pnas.1421535112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Qiu S, Wang YV, et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 2018;560(7719):484–488; doi: 10.1038/s41586-018-0425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, et al. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol 2007;303(1):45–56; doi: 10.1016/j.ydbio.2006.11.024 [DOI] [PubMed] [Google Scholar]
- Yoshimatsu T, D'Orazi FD, Gamlin CR, et al. Presynaptic partner selection during retinal circuit reassembly varies with timing of neuronal regeneration in vivo. Nat Commun 2016;7(1):10590; doi: 10.1038/ncomms10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li L, Huang H, et al. Silicone oil-induced ocular hypertension and glaucomatous neurodegeneration in mouse. eLife 2019;8:e45881; doi: 10.7554/eLife.45881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Su J, Hu X, et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell 2020;181(3):590–603.e16; doi: 10.1016/j.cell.2020.03.024 [DOI] [PubMed] [Google Scholar]