Abstract
Purpose
To report prevalence and risk factor associations for age-related macular degeneration (AMD) and AMD features from multimodal retinal grading in a multidisciplinary longitudinal population-based study of aging in Northern Ireland.
Study design
Population-based longitudinal cohort study.
Methods
Retinal imaging at the Norther Ireland Cohort for the Longitudinal Aging Study health assessment included stereo Colour Fundus Photography (CFP) (Canon CX-1, Tokyo, Japan) and Spectral-Domain Optical Coherence Tomography (SD-OCT) ((Heidelberg Retinal Angopgraph (HRA)+OCT; Heidelberg Engineering, Heidelberg, Germany). Medical history and demographic information was obtained during a home interview. Descriptive statistics were used to describe the prevalence of AMD and individual AMD features. Multiple imputation followed by multiple regression modelling was used to explore risk factor associations including relationships with AMD genetic risk score.
Results
Retinal images from 3386 participants were available for analysis. Mean age of the sample was 63.4 (SD 9.01, range: 36–99). Population weighted prevalence of AMD using colour grading in those over 55 years was: no drusen: 6 0.4%; drusen <63 μm: 15.9%; drusen 63–125 µm: 13.7%; drusen >125 µm or pigmentary changes: 8.3%; late AMD: 1.6%. Prevalence of AMD features in those over 55 years was: OCT drusen 27.5%, complete outer retinal pigment epithelium and outer retinal atrophy (cRORA) on OCT was 4.3%, reticular drusen 3.2% and subretinal drusenoid deposits 25.7%. The genetic risk score was significantly associated with drusen and cRORA but less so for SDD alone and non-significant for hyperpigmentation or vitelliform lesions.
Conclusions
Multimodal imaging-based classification has provided evidence of some divergence of genetic risk associations between classical drusen and SDD. Our findings support an urgent review of current AMD severity classification systems.
Keywords: epidemiology, genetics, imaging, macula, retina
WHAT IS ALREADY KNOWN ON THIS TOPIC
Age-related macular degeneration (AMD) is of significant public health concern due to the impact of vision loss on quality of life together with an increasingly aged population. Substantial advances in retinal imaging has furthered our understanding of the condition through the use of optical coherence tomography (OCT) images, yet most epidemiological studies rely solely on colour fundus photographs for assessment.
WHAT THIS STUDY ADDS
The Norther Ireland Cohort for the Longitudinal Aging Study included multimodal retinal imaging (colour fundus photography, OCT and ultrawide field retinal imaging enabling an unprecedented assessment of AMD including individual retinal features of the condition. The results show disparities between assessments using different imaging modalities highlighting the importance of using multimodal imaging for future studies of prevalence and incidence.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study supports the incorporation of OCT based features into future grading schemes or severity staging systems and the need for longitudinal data on progression of OCT based features to better understand their significance in progression to late-stage disease.
Introduction
Epidemiological studies of age-related macular degeneration (AMD) have traditionally relied on colour fundus photography (CFP) to identify the characteristics used to classify each eye into disease severity stages. Landmark studies such as the Beaver Dam Eye study,1 the Rotterdam Eye Study2 and the Blue Mountain’s Eye Study3 have identified the key phenotypic features and revealed the natural history of progression and associations with risk factors especially age, smoking and genetic risk.4–7 Although studies showed the importance of drusen size and hyperpigmentation in influencing the risk of progression, the role of other colour-defined features such as reticular pseudodrusen8 (RPD; a characteristic form of extracellular drusenoid deposits) and small drusen remains controversial. This is mainly because colour grading can overestimate presence of small drusen9 and underestimate presence of RPD.10 Optical Coherence tomography (OCT permits the distinction of nodular (classic) drusen from RPD through characterisation of layer location.
The establishment of a new population-based longitudinal study of ageing in Northern Ireland (The Northern Ireland Cohort for the Longitudinal Study of Aging (NICOLA)) offered an opportunity to assess the retina using multimodal retinal imaging (CFP, OCT, ultrawide field imaging (UWFII)) approach, define previously unexplored phenotypes from an epidemiological perspective and study risk factor associations with an extended range of markers of systemic health.
Methods
NICOLA Study overview
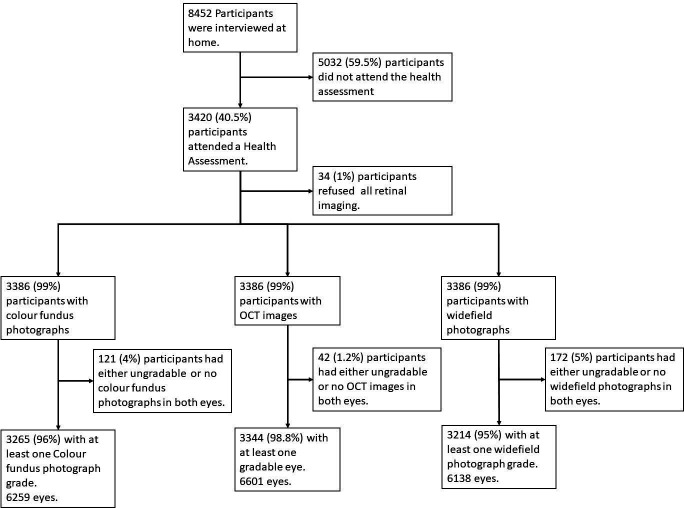
The NICOLA is a multidisciplinary prospective population-based cohort study. Wave 1 of the study commenced in December 2013 and ended in April 2018. In total, 8452 persons completed the home-based computer-assisted personal interview. Of these, 3420 (40.5%) attended for the health assessment, which consisted of anthropometric, cardiac, respiratory, cognitive and ophthalmic tests (figure 1).
Figure 1.
Flow chart showing participant pathway in wave 1 of the NICOLA Study. NICOLA, Northern Ireland Cohort for the Longitudinal Study of Aging.
Details on image acquisition, image grading, physical data collection, self-report, blood-based biomarkers, genetic analysis, outcome categorisation and covariate selection can be found in the online supplemental methods.
bjo-2021-320469supp001.pdf (242.9KB, pdf)
Statistical analysis
All statistical analysis was conducted using R (V.3.6.0).11
Prevalence analysis
Prevalence of AMD was estimated, weighting records by the population distribution of age and sex as recorded in the 2011 Census for Northern Ireland. For the eye level analysis, the frequency, prevalence and 95% CIs for each AMD feature was calculated. To facilitate comparison with prior studies,12 estimates are provided by age category and with a separate estimate for participants aged 55 years and above.
Imputation and risk factor association
Missing risk factor values were imputed using multivariate imputation by chained equations (MICE) using the R package mice (V.3.8.0),13 which yielded a set of five datasets in which the missing values had been imputed probabilistically. The regression models outlined further were fitted using each of the imputed datasets, and estimates were pooled across imputations. A single estimate was generated for each parameter of interest that encompassed the additional uncertainty introduced by imputation. Further details of the imputation procedure are given in online supplemental methods.
Associations between AMD features and risk factors were explored using logistic regression. A series of regressions were fitted for each AMD feature with the feature as the response variable. First, a set of univariate models was fitted, the sole predictor in each being one of the candidate risk factors. A second set was adjusted with the addition of age (linear and quadratic terms) and sex. Risk factors receiving some statistical support in the age-adjusted andsex-adjusted models (p<0.1) were included in a multivariable model. Finally, a saturated model including all risk factors was fitted. Where an AMD feature was particularly rare, inclusion of all the selected variables occasionally resulted in unstable estimates or a failure of multivariable or saturated models to converge. In these instances, problematic combinations of predictors were identified, and models were refitted after removing these risk factors. For the analysis of person-level AMD risk factors (dichotomised AMD stage), an additional model was fitted excluding the Genetic Risk Score (GRS) to explore the extent to which associations with other risk factors differed in the absence of the GRS.
Results
Cohort characteristics
Of the 3420 participants who attended the health assessment, retinal imaging was performed in 3386 (99%) (figure 1). Images were available for colour 6259 eyes (92%), OCT 6601 (96%) and UWFI 6138 (89%). Images were ungradable in approximately 121 (4%) (colour), 42 (1%) (OCT) and 172 (5%) (UWFI) eyes. The number of individuals for whom both eyes were gradable was: colour 2994 (88%), OCT 3257 (95%) and UWFI 2934 (85%). More than 85% of participants had gradable images from both eye on all three imaging modalities. Online supplemental table 1 shows proportions of participants with ungradable images by imaging modality. The mean (SD) age of participants with gradable colour images was 63.5 (8.9) years (online supplemental table 1), and over 99.9% were Caucasian.
bjo-2021-320469supp002.pdf (311.4KB, pdf)
Prevalence by Beckman stage
The prevalence of stage 0 and stage 1 (representing no features of AMD or small hard drusen only) declined from 62.7% and 23.7% to 54.3 % and 10.9%, respectively (table 1). For stage 2, the prevalence rose steadily from 9.6% to 17.3% in those in age band 75–84 but was 7.1% in the oldest age band. For participants in stage 3, the prevalence increased from 4.0% to 16.9%. Stage 4 representing the most advanced cases of AMD was rare in the two younger age bands, was infrequent in the age groups between 65 and 84 and occurred at 10.9% in the oldest age group. Prevalence rates were similar across sexes (online supplemental table 2).
Table 1.
Prevalence by stage of Beckman clinical classification for AMD % (95% CI) by age
| Unweighted | Weighted | ||||||||||
| Age (years) | Number at risk | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| All ≥55 | 2690 | 61.1 (59.2 to 63.0) | 16.6 (15.2 to 18.1) | 13.8 (12.5 to 15.1) | 7.5 (6.6 to 8.6) | 1.0 (0.6 to 1.4) | 60.4 (58.3 to 62.6) | 15.9 (14.5 to 17.5) | 13.7 (12.3 to 15.2) | 8.3 (7.1 to 9.8) | 1.6 (0.9 to 2.6) |
| <55 | 575 | 63.1 (59.0 to 67.1) | 23.7 (20.2 to 27.3) | 9.4 (7.1 to 12.1) | 3.8 (2.4 to 5.7) | 0.0 | 62.7 (58.5 to 66.7) | 23.7 (20.2 to 27.5) | 9.6 (7.3 to 12.4) | 4.0 (2.5 to 6.0) | 0.0 |
| 55–64 | 1257 | 64.6 (61.9 to 67.2) | 18.5 (16.3 to 20.7) | 12.2 (10.4 to 14.1) | 4.7 (3.6 to 6.0) | 0.1 (0.0 to 0.4) | 64.5 (61.8 to 67.2) | 18.6 (16.4 to 20.8) | 12.1 (10.3 to 14.0) | 4.8 (3.7 to 6.1) | 0.1 (0.0 to 0.4) |
| 65–74 | 1061 | 59.3 (56.3 to 62.3) | 16.0 (13.9 to 18.4) | 14.9 (12.8 to 17.2) | 8.8 (7.1 to 10.6) | 1.0 (0.5 to 1.8) | 59.0 (55.9 to 62.0) | 16.1 (13.9 to 18.4) | 15.2 (13.1 to 17.5) | 8.7 (7.1 to 10.6) | 1.0 (0.5 to 1.8) |
| 75–84 | 325 | 54.8 (49.2 to 60.3) | 12.3 (8.9 to 16.4) | 16.9 (13.0 to 21.5) | 13.2 (9.7 to 17.4) | 2.8 (1.3 to 5.2) | 55.6 (49.9 to 61.2) | 11.3 (8.1 to 15.2) | 17.3 (13.2 to 22.0) | 13.0 (9.5 to 17.2) | 2.8 (1.2 to 5.3) |
| ≥85 | 47 | 53.2 (38.1 to 67.9) | 10.6 (3.5 to 23.1) | 8.5 (2.4 to 20.4) | 17.0 (7.6 to 30.8) | 10.6 (3.5 to 23.1) | 54.3 (36.7 to 71.1) | 10.9 (2.9 to 26.1) | 7.1 (1.4 to 19.9) | 16.9 (6.4 to 33.3) | 10.9 (2.9 to 26.1) |
Table 1 shows the prevalence of AMD severity stages based on the Beckman clinical classification using the worse eye (person level). Unweighted and weighted estimates are shown. Data were weighted by age and gender using the 2011 Census for Northern Ireland.
AMD, age-related macular degeneration.
Prevalence by graded features
The prevalence of features of AMD based on colour or OCT grading is shown in table 2.
Table 2.
Prevalence of AMD features by age category (eye-level analysis, unweighted)
| Colour/en face grading | Multimodal grading (Infra-red and OCT) | ||||||||||||
| Age (years) |
Number at risk | Hyperpigmentation | Drusen (any size) |
Drusen ≥63 µm | Drusen ≥125 µm | Reticular Drusen | GA | Number at risk | Drusen (any size) |
SDD | cRORA | CNV | Vitelliform |
| All ≥55 | 5128 | 2.0 (1.5, 2.4) | 28.9 (27.4 to 30.4) | 14.5 (13.4 to 15.7) | 3.9 (3.3 to 4.5) | 3.2 (2.7 to 3.9) | 0.3 (0.2 to 0.6) | 5450 | 27.5 (26.1 to 29.0) | 25.7 (24.3 to 27.1) | 4.3 (3.7 to 5.0) | 0.3 (0.2 to 0.5) | 0.3 (0.2, 0.6) |
| <55 | 1131 | 1.0 (0.5, 1.7) | 26.0 (23.0 to 29.2) | 7.9 (6.1 to 9.9) | 1.3 (0.7 to 2.4) | 1.3 (0.6 to 2.5) | 0.0 | 1151 | 16.3 (13.9 to 19.0) | 13.0 (10.9 to 15.4) | 2.5 (1.5 to 3.9) | 0.0 | 0.0 |
| 55–64 | 2447 | 1.0 (0.6, 1.6) | 24.7 (22.7 to 26.8) | 10.2 (8.8 to 11.6) | 1.9 (1.4 to 2.6) | 1.1 (0.7 to 1.7) | 0.0 | 2534 | 20.4 (18.5 to 22.3) | 18.3 (16.6 to 20.1) | 2.9 (2.2 to 3.7) | 0.0 (0.0 to 0.2) | 0.0 (0.0, 0.2) |
| 65–74 | 2003 | 2.4 (1.7, 3.3) | 30.6 (28.2 to 33.1) | 16.4 (14.5 to 18.5) | 4.7 (3.6 to 6.0) | 3.8 (2.8 to 5.1) | 0.5 (0.2 to 1.1) | 2144 | 29.5 (27.1 to 31.9) | 27.3 (25.1 to 29.6) | 4.7 (3.7 to 5.8) | 0.2 (0.1 to 0.7) | 0.6 (0.2, 1.1) |
| 75–84 | 595 | 3.2 (1.8, 5.2) | 38.8 (33.9 to 43.9) | 24.7 (20.5 to 29.4) | 8.2 (5.7 to 11.4) | 8.6 (5.9 to 11.9) | 1.2 (0.4 to 2.6) | 675 | 44.3 (39.5 to 49.2) | 44.1 (39.5 to 48.9) | 6.8 (4.7 to 9.5) | 0.8 (0.3 to 1.9) | 0.6 (0.1, 2.1) |
| ≥85 | 83 | 9.6 (3.4, 20.5) | 39.8 (26.8 to 53.9) | 22.9 (12.1 to 37.1) | 9.6 (3.4 to 20.5) | 12.0 (4.0 to 26.0) | 0.0 | 97 | 54.6 (40.9 to 67.9) | 53.6 (40.1 to 66.8) | 16.5 (8.2 to 28.2) | 6.0 (2.0 to 13.5) | 2.1 (0.0, 11.1) |
Table 2 shows the prevalence of AMD features on en face and multimodal grading (non-weighted) (eye level). The all category is restricted to those over 55 years to enable comparison with other studies who restrict definition of AMD to this age category.
AMD, age-related macular degeneration; CNV, choroidal neovascular membrane; cRORA, complete retinal pigment epithelium and outer retinal atrophy; GA, geographic atrophy; OCT, optical coherence tomography; SDD, subretinal drusenoid deposit.
With colour detection, the prevalence of focal hyperpigmentation increased from 1.0% to 9.6% with age. Small drusen <63 µm rose from 7.9% in the youngest to 22.9% in those older than 85 years. The prevalence of drusen >125 µm rose steadily from 1.3% to 9.6% doubling by each age category, but the oldest two age bands were similar. RPD frequencies were similar for the two youngest age bands but showed steady rises in the three oldest age categories. On OCT images, subretinal drusenoid deposit (SDD) were observed frequently on OCT in all age bands compared with RPD on colour. These differentials were striking 18.0% on OCT versus 1.1% on colour in the youngest age category and 53.6% versus 12.0% in those >85 years. The prevalence of geographic atrophy (GA) was low on colour and only seen in the two oldest age bands at 0.5% and 1.2%. This contrasted with the higher proportions of cRORA on OCT, which rose from 2.5% from those under 55 year to 16.5% in those aged 85 years and above. The prevalence of all the graded features was not significantly different between men and women (online supplemental table 3).
Prevalence of AMD features on UWFI colour imaging
The prevalence of hard and soft drusen and hyperpigmentation in UWFI colour images by age groups are shown in table 3.
Table 3.
Prevalence of hard drusen, soft drusen and hyperpigmentation on ultrawide field retinal images (weighted)
| Age (years) | Number at risk | Centre | Mid | Far | Full |
| Hard drusen | |||||
| All ≥55 | 5023 | 62.8 (61.0 to 64.7) | 86.8 (85.4 to 88.1) | 75.6 (74.0 to 77.2) | 94.6 (93.7 to 95.4) |
| <55 | 1115 | 58.6 (55.4 to 61.7) | 85.2 (82.7 to 87.4) | 76.9 (74.1 to 79.6) | 94.4 (92.6 to 95.8) |
| 55–64 | 2392 | 57.0 (54.8 to 59.2) | 86.4 (84.8 to 87.9) | 77.2 (75.2 to 79.0) | 94.9 (93.8 to 95.8) |
| 65–74 | 1971 | 64.6 (62.2 to 67.0) | 87.8 (86.2 to 89.3) | 77.0 (74.8 to 79.0) | 94.7 (93.6 to 95.7) |
| 75–84 | 585 | 69.8 (65.2 to 74.0) | 88.2 (84.9 to 91.0) | 72.6 (68.2 to 76.6) | 94.9 (92.8 to 96.6) |
| ≥85 | 75 | 70.8 (52.3 to 85.2) | 80.4 (64.3 to 91.4) | 68.2 (52.7 to 81.2) | 91.2 (80.3 to 97.2) |
| Soft Drusen | |||||
| All≥55 | 5023 | 5.6 (4.6 to 6.9) | 3.2 (2.6 to 3.9) | 3.7 (3.1 to 4.5) | 8.6 (7.4 to 10.0) |
| <55 | 1115 | 0.8 (0.3 to 1.7) | 0.6 (0.2 to 1.3) | 2.2 (1.2 to 3.5) | 2.8 (1.7 to 4.2) |
| 55–64 | 2392 | 2.3 (1.7 to 3.1) | 2.1 (1.5 to 2.8) | 3.4 (2.5 to 4.4) | 5.5 (4.4 to 6.7) |
| 65–74 | 1971 | 6.5 (5.2 to 8.0) | 4.3 (3.2 to 5.5) | 4.7 (3.6 to 6.0) | 10.0 (8.4 to 11.8) |
| 75–84 | 585 | 10.0 (7.1 to 13.5) | 4.5 (2.6 to 7.1) | 4.2 (2.4 to 7.0) | 12.8 (9.6 to 16.7) |
| ≥85 | 75 | 10.2 (1.9 to 27.9) | 1.3 (0.1 to 4.9) | 0.0 | 10.2 (1.9 to 27.9) |
| Hyperpigmentation | |||||
| All≥55 | 5023 | 2.0 (1.4 to 2.9) | 8.5 (7.1 to 10.2) | 12.5 (10.9 to 14.2) | 14.5 (12.8 to 16.4) |
| <55 | 1115 | 0.8 (0.3 to 1.7) | 2.1 (1.2 to 3.3) | 3.2 (2.1 to 4.6) | 4.6 (3.2 to 6.2) |
| 55–64 | 2392 | 0.9 (0.5 to 1.5) | 3.9 (3.0 to 4.9) | 6.1 (5.0 to 7.4) | 7.6 (6.3 to 9.0) |
| 65–74 | 1971 | 1.9 (1.3 to 2.7) | 8.9 (7.4 to 10.5) | 13.5 (11.7 to 15.5) | 15.5 (13.5 to 17.6) |
| 75–84 | 585 | 3.1 (1.6 to 5.6) | 11.0 (8.0 to 14.6) | 19.0 (15.2 to 23.4) | 20.9 (16.8 to 25.4) |
| ≥85 | 75 | 6.8 (1.5 to 18.4) | 27.9 (13.4 to 46.8) | 27.8 (13.3 to 46.9) | 34.7 (17.9 to 54.7) |
Table 3 shows the prevalence of hard, soft drusen and hyperpigmentation on ultrawide field retinal images (weighted) (eye level). Unweighted and weighted estimates are shown. Data were weighted by age and gender using the 2011 Census for Northern Ireland. The all category is restricted to those over 55 years to enable comparison with other studies who restrict definition of AMD to this age category.
Small hard drusen were highly prevalent in the central (62.8%), mid (86.8%) and far periphery (75.8%) of the fundus with the highest prevalences seen in the midregion, but no significant differences were seen with age and sex (sex data not shown). The prevalence of soft drusen rose monotonically with age in the central fundus, with similar rises in the mid and far periphery. Hyperpigmentation increased with age in all three regions, but the prevalence by age group and rise with increasing age group was most marked in the far periphery. Table 4 shows the relationship between Beckman stage and the UWFI grades. The UWFI-based prevalence of soft drusen in central, mid and far regions rose with increasing Beckman stage.
Table 4.
Comparison of Beckman stages graded on colour with presence of hard drusen, soft drusen or hyperpigmentation on UWF
| Beckman classification | Number at risk | Centre % (95% CI) | Mid % (95% CI) | Far % (95% CI) | |
| Hard drusen | 0 | 4131 | 58.4 (56.8 to 60.0) | 86.0 (84.9 to 87.2) | 75.8 (74.3 to 77.2) |
| 1 | 836 | 67.5 (64.0 to 70.8) | 88.2 (85.7 to 90.3) | 78.1 (75.1 to 80.9) | |
| 2 | 545 | 68.6 (64.4 to 72.6) | 90.3 (87.2 to 92.9) | 85.0 (81.5 to 88.0) | |
| 3 | 277 | 69.7 (63.9 to 75.1) | 88.1 (83.4 to 91.8) | 79.1 (73.0 to 84.3) | |
| 4 | 31 | 54.8 (32.8 to 75.5) | 71.0 (50.3 to 86.8) | 67.7 (48.0 to 83.7) | |
| Ungradable | 318 | 57.2 (51.5 to 62.8) | 83.3 (78.7 to 87.3) | 63.2 (57.1 to 69.0) | |
| Soft drusen | 0 | 4131 | 1.5 (1.1 to 1.9) | 1.9 (1.5 to 2.5) | 2.9 (2.3 to 3.5) |
| 1 | 836 | 2.9 (1.8 to 4.4) | 2.8 (1.8 to 4.1) | 4.7 (3.2 to 6.5) | |
| 2 | 545 | 11.6 (8.7 to 15.0) | 5.3 (3.5 to 7.8) | 7.0 (4.8 to 9.8) | |
| 3 | 277 | 28.5 (22.3 to 35.4) | 6.5 (3.7 to 10.4) | 4.3 (1.9 to 8.3) | |
| 4 | 31 | 38.7 (18.8 to 61.9) | 25.8 (9.3 to 49.7) | 25.8 (9.3 to 49.7) | |
| Ungradable | 318 | 6.3 (3.7 to 9.9) | 2.5 (1.0 to 5.3) | 1.9 (0.7 to 4.1) | |
| Hyperpigmentation | 0 | 4131 | 1.0 (0.7 to 1.4) | 5.5 (4.7 to 6.4) | 9.0 (8.0 to 10.1) |
| 1 | 836 | 0.6 (0.2 to 1.4) | 5.9 (4.3 to 7.8) | 8.0 (6.1 to 10.3) | |
| 2 | 545 | 2.0 (0.9 to 3.8) | 9.0 (6.4 to 12.2) | 12.3 (9.3 to 15.8) | |
| 3 | 277 | 9.0 (5.7 to 13.5) | 9.0 (5.6 to 13.5) | 13.7 (9.6 to 18.8) | |
| 4 | 31 | 16.1 (5.3 to 34.1) | 16.1 (5.0 to 35.0) | 19.4 (6.9 to 38.9) | |
| Ungradable | 318 | 0.9 (0.2 to 2.7) | 5.7 (3.3 to 9.0) | 9.4 (6.3 to 13.4) |
UWF, ultrawide field.
The prevalence of hard drusen in all UWFI regions was similar across all Beckman stages. The prevalence of hyperpigmentation increased with increasing Beckman stage.
Genetic risk score (GRS)
The GRS ranged from −3.0 to 5.1 (SD 1.1) and was normally distributed. In controls (Beckman 0,1), the mean GRS was 0.4 (SD 1.1). For cases (Beckman 2,3,4), the GRS was higher 0.7 (SD 1.2) (online supplemental table 4). Mean GRS (SD) for AMD features detected on colour were hyperpigmentation: 0.4 (1.2), drusen (any size): 0.6 (1.2), drusen ≥63 µm: 0.7 (1.2), drusen >125 µm: 1.0 (1.4), reticular drusen: 1.0 (1.2) and geographic atrophy: 1.6 (0.8). The mean GRS for AMD features detected on OCT were classic drusen 0.8 (1.2), SDD 0.7 (1.2), SDD only 0.5 (1.1), cRORA 0.8 (1.3), CNV 1.4 (1.1) and vitelliform lesion 0.1 (1.1).
Risk factor associations
Comparison between stage 0 or 1 versus stages 2, 3 and 4 (online supplemental table 4) showed that only the GRS (OR 1.25, 95% CI 1.15 to 1.35, p<0.001) and serumHigh Density Lipoprotein (HDL) remained highly statistically significant in the fully adjusted model. Self-reported chronic lung disease (OR 1.71, 95% CI 1.11 to 2.64, p=0.014), Parkinson’s disease (OR 5.10, 95% CI 1.39 to 18.74, p=0.014) and the category of elevated hypertension (OR 1.44, 95% CI 1.06 to 1.96, p=0.020) just reached significance in the fully adjusted model. The association with high-sensitivity C-Reactive Protein (hsCRP) was higher in the model with GRS removed (OR 1.09, 95% CI 1.00 to 1.19, p=0.045) than when it was included (OR 1.08, 95% CI 0.99 to 1.18, p=0.065).
Risk factor associations with individual features detected by colour grading are shown in online supplemental table 5. The GRS was consistently associated with classical drusen and RPD. The OR for classical drusen <63 µm was 1.24 (1.13 to 1.37) and increased to 1.52 (1.27 to 1.81) for drusen >125 µm. The OR for RPD was 1.47 (1.23 to 1.75). There was no significant association for GRS and hyperpigmentation. The presence of a thickened choroid was significantly associated with drusen of any size.
Online supplemental table 6 shows the risk factor associations with individual grading features based on OCT. A consistent and highly significant association was seen between the GRS and classical drusen with an OR of 1.33 (95% CI 1.24 to 1.43, p<0.001). Eyes with SDD with classical drusen also had a statistically significant association OR 1.35 (95% CI 1.26 to 1.44, p<0.001). For eyes with SDD without classical drusen, the association with the GRS lost significance 1.15 (95% CI 1.02 to 1.28, p=0.017), but the direction of risk remained unchanged. The OR for cRORA was statistically significant p=0.003 at 1.26 (95% CI 1.08 to 1.46). Classical drusen were significantly associated with thick choroid OR 1.84 (95% CI 1.42 to 2.37, p<0.001) and RPD with thin choroid with an OR of 1.69 (95% CI 1.27 to 2.23, p<0.001).
Discussion
NICOLA Study ascertained the prevalence of features of early AMD through traditional colour imaging and extended this to include UWFI and SD-OCT, permitting us to distinguish between participants based on individual characteristics. We showed clear and steady almost monotonic age-related rises in the prevalence of Beckman stages 2 and 3 representing early and intermediate AMD, respectively (table 1). These findings are in accord with prior epidemiological studies.2 3
In the eye- level analysis, differences by age group in the frequencies of drusen were seen on comparing features detected by colour versus OCT. Notably, on colour, the prevalence of drusen (any size) was higher than that seen on OCT in the younger age groups, and although it rose with age, the increase was shallow. On OCT, the prevalence of any drusen, which was around 16% in the youngest age band, rose steadily to 54% in the oldest age band. These colour versus OCT discrepancies mainly occurred in the detection of drusen <63 µM. Our data suggest that small drusen were more likely to be graded as present in younger age groups and likely represented an over calling of this feature by the graders.9 14 By contrast, small drusen when present were more likely to be missed on colour grading in the older age groups in whom there is a higher prevalence of lens opacities with resultant degradation of image quality interfering with the detection process.
We observed good correspondence for prevalence of drusen >125 µM on comparing detection by colour or by OCT. We demonstrated poor agreement for the prevalence RPD comparing detecting by en face technologies versus its OCT correlate of SDD, a finding that is in keeping with previous studies.10 15 16 We expected that the OCT correlate of pseudo drusen, SDDs would be detected at higher frequency than that reported by en face imaging, but the magnitude of the difference was surprising. Even though the prevalence on en face imaging of RPD was marginally lower in NICOLA Study at 3.2% than the Rotterdam study (4.9%) or that reported from another UK cohort (5.06%),17 it was still much higher than that of a large community-based cohort study in Australia18 (0.41%). Studies that have either used OCT alone or in conjunction with other imaging modalities have generally reported high prevalence rates of SDD. Alienor found the prevalence of SDD to be 13.4% using multimodal imaging19 in contrast to the Alstar study who reported a prevalence of 32% in their clinic based enrolment cohort20 using multimodal imaging. In NICOLA Study, we found the prevalence to be 25.7% in those aged 55 years and older. We contend that NICOLA Study offers better representation of the true population prevalence of SDD in older adults as its community-based sampling strategy is less likely to be biased in the direction of persons with other ocular morbidities that are common in clinic-based samples.
As with other epidemiological studies, the prevalence of large areas of atrophy visible on colour images representing GA was low, precluding generation of robust estimates of prevalence and risk factor associations for this late stage of AMD. Nonetheless, we were able to characterise in detail the presence of cRORA, an OCT based definition of focal atrophy in the outer retina,21 using SD-OCT, and indeed NICOLA is the first epidemiological study to record its prevalence. The proportion of eyes exhibiting cRORA even in the younger age groups was around 2.5%, and this rose steadily with age allowing us to provide robust estimates by age band for focal outer retinal atrophy. This information will be particularly useful for sample size calculations when developing protocols for GA interventional trials. In this context, we recognise that while it is presently unknown if cRORA is a robust precursor of geographic atrophy, longitudinal case series22 and data from clinical trials23 that have enrolled participants with early AMD who have progressed to GA strongly support this view.
Peripheral retinal changes have been reported in several clinical AMD cohorts24 25 and one other population-based study.26 The prevalence in the periphery of small hard drusen, soft drusen and pigmentary irregularities in the UWFI images in the NICOLA population were in accord with the high rates of abnormalities reported in these prior studies. It was notable that increasing Beckman severity stages was mirrored by increases in soft drusen and hyperpigmentation in the central, mid and far periphery. By contrast, the prevalence of hard drusen was similar in the central mid and far periphery across all Beckman severity stages suggesting that these are a ubiquitous finding. Histological studies however dispute these findings and show that the pathology visible in the periphery is not the same as that in the macula.27 Widefield OCT images should be prioritised in future studies as these may help resolve these disparities in clinical cohorts.
Various risk factors for intermediate and late AMD have been identified in longitudinal epidemiological studies28 and clinical cohorts.29 30 The fully adjusted multivariate regression model revealed that age and the GRS were the only highly statistically significant associations with the Beckman severity stage person-level classification. Associations between AMD and chronic lung disease is relatively novel though has recently been reported from a populatio-based retrospective study in Taiwan31 so deserves further exploration. Some factors such as physical activity that were significant in univariate are likely to be highly collinear with age, hence the drop from age-adjusted models. The ORs and risk estimates for the GRS and Beckman stages of intermediate and late AMD are in accord those of the eye-risk consortium, which recently reported a similar mean GRS from their large, pooled analysis of cross-sectional data from the European Eye Epidemiology Consortium.32
In the AMD feature-level analysis, the GRS was strongly associated with classical drusen, on separating eyes with SDD from eyes with both SDD and classical drusen, the association with the GRS lost significance despite previous studies showing a significant association between SDD and the two major AMD risk loci independent of drusen presence (ARMS2 positively associated and CFH Y402H negatively associated).33 We also observed that GRS was not significantly associated with hyperpigmentation when this feature present in the absence of drusen. This is not surprising since focal hyperpigmentation on its own can represent pathology such as past inflammation.
All types of drusen were associated with a thicker choroid, which is in keeping with many previous studies,34–36 whereas those with SDD alone had a significantly thinner choroid. A detailed study of the relationship between choroidal thickness, choroidal vascularity index (CVI) and SDD presence by Keenan et al 35 reported a biphasic alteration in choroidal dimensions across the disease spectrum with those with large drusen showing increased choroidal thickness and increased CVI, whereas the same parameters in those with advanced AMD in the fellow eye were no different to controls. Those with just SDD had significantly thinner choroid and reduced CVI.35 Keenan et al propose CVI as a potential biomarker of ageing given its significant and negative correlation with age, and our data on SDD alone suggest that it too may be more reflective of ubiquitous ageing rather than AMD per se. It is also interesting to note SDD alone also had a lower GRS than the other drusen related features though still significantly associated in keeping with recent genetic studies showing significant associations with the major AMD susceptibility loci.37
Limitations
Our study suffers from several limitations. First, the response rate for those who attended the health assessment was moderate, but we implemented appropriate weighting strategies to mitigate the effects of such bias. Nonetheless, when weighted and unweighted prevalence estimates were compared (table 1), the differences were minor suggesting that the cohort demographic structure closely matched that of the general population. Second, we investigated a large number of potential risk factors a process that can increase the risk of false positive results. We therefore took a highly conservative approach in the creation of the multivariable models and interpreted the findings in terms of effect size and biological plausibility rather than explicit p value cut-offs. Third, we did not grade for intraretinal hyper-reflective foci an OCT feature, which is now considered a biomarker of deteriorating retinal pigment epithelium (RPE) health and a predictor for progression to late AMD.38
Conclusions
This study provides further insight into the prevalence and risk factors of AMD and AMD features using multiple imaging modalities. Interestingly, the correlation between the Beckman classification and our findings from UWF imaging provide evidence that on a pragmatic level that the former continues to have validity. It highlights the benefits of using a multimodal approach in future epidemiological studies but also the challenges in interpretating findings that can be compared with previous colour only studies. New severity stage systems that incorporate AMD-based OCT features are urgently needed.
Acknowledgments
We are grateful to all the participants of the Northern Ireland Cohort for the Longitudinal Study of Aging (NICOLA) Study, and the whole NICOLA team, which includes nursing staff, research scientists, clerical staff, computer and laboratory technicians, managers and receptionists. The Atlantic Philanthropies, the Economic and Social Research Council, the UKCRC Centre of Excellence for Public Health Northern Ireland, the Centre for Ageing Research and Development in Ireland, the Office of the First Minister and Deputy First Minister, the Health and Social Care Research and Development Division of the Public Health Agency, the Wellcome Trust/Wolfson Foundation and Queen’s University Belfast provide core financial support for NICOLA. The authors alone are responsible for the interpretation of the data and any views or opinions presented are solely those of the authors and do not necessarily represent those of the NICOLA Study team. The ophthalmic component of the NICOLA study was funded by grants from the College of Optometrists. Diabetes UK, Macular Society, Guidedogs for the Blind, Thomas Pocklington Trust and the Belfast Association for the Blind, Bayer, Novartis and Optos plc.
Footnotes
Twitter: @ruth_hogg, @MarkATully
Contributors: Conceived and designed the study: REH, UC, TP, ISY and FK.Analysed the data: DMW, LS and AJM. Acquisition of data: BH, KAM, LS and TP. Administrative, technical or material support: AJM, LS, JW, MAT, SC, BM, ISY and FK. Wrote the paper: RH. Critically revised the manuscript: DMW, NBQ, KAM, BH, LS, AJM, JW, MAT, SC, BM, ISY, FK, TP and UC. RH and DMW had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. UC is guarantor. Statistical analyses were performed by DMW.
Funding: Atlantic Philanthropies, Bayer, Belfast Association for the Blind, Centre for Aging Research and Development in Ireland, College of optometrists, Diabetes UK, Economic and Social Research Council, Macular Society, Medical Research Council, Novartis, Office of the First and Deputy First Minister, Optos Plc, Queen’s University Belfast Research and Development, Research and Development Division of the Public health Agency, Thomas Pocklington Trust and United Kingdom Clinical Research Collaboration. Grant numbers not available.
Disclaimer: The sponsors and funding organisations had no role in the design or conduct of this research.
Competing interests: Ruth Hogg: Optos PLC and Novartis; UC: Bayer. These all relate to non-restrictive grants awarded to QUB to support the retinal grading of this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Details on data access policy available at https://www.qub.ac.uk/sites/NICOLA/InformationforResearchers/.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by School of Medicine, Dentistry and Biomedical Sciences Ethics Committee, Queen’s University Belfast (Ref: 12/23). Participants gave informed consent to participate in the study before taking part.
References
- 1. Klein R, Klein BE, Moss SE. Diabetes, hyperglycemia, and age-related maculopathy. The Beaver dam eye study. Ophthalmology 1992;99:1527–34. 10.1016/s0161-6420(92)31770-1 [DOI] [PubMed] [Google Scholar]
- 2. Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology 1995;102:205–10. 10.1016/S0161-6420(95)31034-2 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell P, Smith W, Attebo K, et al. Prevalence of age-related maculopathy in Australia. the blue Mountains eye study. Ophthalmology 1995;102:1450–60. 10.1016/s0161-6420(95)30846-9 [DOI] [PubMed] [Google Scholar]
- 4. Buitendijk GHS, Rochtchina E, Myers C, et al. Prediction of age-related macular degeneration in the general population: the three continent AMD Consortium. Ophthalmology 2013;120:2644–55. 10.1016/j.ophtha.2013.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joachim N, Colijn JM, Kifley A, et al. Five-Year progression of unilateral age-related macular degeneration to bilateral involvement: the three continent AMD Consortium report. Br J Ophthalmol 2017;101:1185–92. 10.1136/bjophthalmol-2016-309729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joachim N, Kifley A, Colijn JM, et al. Joint contribution of genetic susceptibility and modifiable factors to the progression of age-related macular degeneration over 10 years: the three continent AMD Consortium report. Ophthalmol Retina 2018;2:684–93. 10.1016/j.oret.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 7. Saunier V, Merle BMJ, Delyfer M-N, et al. Incidence of and risk factors associated with age-related macular degeneration: four-year follow-up from the ALIENOR study. JAMA Ophthalmol 2018;136:473–81. 10.1001/jamaophthalmol.2018.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mimoun G, Soubrane G, Coscas G. [Macular drusen]. J Fr Ophtalmol 1990;13:511–30. [PubMed] [Google Scholar]
- 9. Graham KW, Chakravarthy U, Hogg RE, et al. Identifying features of early and late age-related macular degeneration: a comparison of multicolor versus traditional color fundus photography. Retina 2018;38:1751–8. 10.1097/IAE.0000000000001777 [DOI] [PubMed] [Google Scholar]
- 10. Hogg RE, Silva R, Staurenghi G, et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology 2014;121:1748–55. 10.1016/j.ophtha.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 11. Team RC . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 12. Colijn JM, Buitendijk GHS, Prokofyeva E, et al. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology 2017;124:1753–63. 10.1016/j.ophtha.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buuren Svan, Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 14. Farinha C, Cachulo ML, Coimbra R, et al. Age-Related Macular Degeneration Staging by Color Fundus Photography vs. Multimodal Imaging-Epidemiological Implications (The Coimbra Eye Study-Report 6). J Clin Med 2020;9. doi: 10.3390/jcm9051329. [Epub ahead of print: 02 05 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Z, Ayton LN, Luu CD, et al. Reticular Pseudodrusen in intermediate age-related macular degeneration: prevalence, detection, clinical, environmental, and genetic associations. Invest Ophthalmol Vis Sci 2016;57:1310–6. 10.1167/iovs.15-18682 [DOI] [PubMed] [Google Scholar]
- 16. De Bats F, Mathis T, Mauget-Faÿsse M, et al. Prevalence of reticular PSEUDODRUSEN in age-related macular degeneration using multimodal imaging. Retina 2016;36:46–52. 10.1097/IAE.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 17. Wilde C, Poostchi A, Mehta RL, et al. Prevalence of reticular pseudodrusen in an elderly UK Caucasian population-The Bridlington eye assessment project (BEAP): a cross-sectional study (2002-2006). Eye 2018;32:1130–7. 10.1038/s41433-018-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finger RP, Chong E, McGuinness MB, et al. Reticular Pseudodrusen and their association with age-related macular degeneration: the Melbourne Collaborative cohort study. Ophthalmology 2016;123:599–608. 10.1016/j.ophtha.2015.10.029 [DOI] [PubMed] [Google Scholar]
- 19. Chan H, Cougnard-Grégoire A, Delyfer M-N, et al. Multimodal imaging of reticular Pseudodrusen in a population-based setting: the Alienor study. Invest Ophthalmol Vis Sci 2016;57:3058–65. 10.1167/iovs.16-19487 [DOI] [PubMed] [Google Scholar]
- 20. Zarubina AV, Neely DC, Clark ME, et al. Prevalence of Subretinal Drusenoid Deposits in Older Persons with and without Age-Related Macular Degeneration, by Multimodal Imaging. Ophthalmology 2016;123:1090–100. 10.1016/j.ophtha.2015.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology 2018;125:537–48. 10.1016/j.ophtha.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaffe GJ, Chakravarthy U, Freund KB, et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration: classification of atrophy meeting report 5. Ophthalmol Retina 2021;5:855-867. 10.1016/j.oret.2020.12.009 [DOI] [PubMed] [Google Scholar]
- 23. Guymer RH, Wu Z, Hodgson LAB, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology 2019;126:829–38. 10.1016/j.ophtha.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 24., Domalpally A, Clemons TE, et al. , Writing Committee for the OPTOS PEripheral RetinA (OPERA) study (Ancillary Study of Age-Related Eye Disease Study 2) . Peripheral Retinal Changes Associated with Age-Related Macular Degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology 2017;124:479–87. 10.1016/j.ophtha.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 25. Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology 2013;120:1271–7. 10.1016/j.ophtha.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lengyel I, Csutak A, Florea D, et al. A population-based ultra-widefield digital image grading study for age-related macular Degeneration-Like lesions at the peripheral retina. Ophthalmology 2015;122:1340–7. 10.1016/j.ophtha.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 27. Rudolf M, Clark ME, Chimento MF, et al. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci 2008;49:1200–9. 10.1167/iovs.07-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joachim N, Mitchell P, Kifley A, et al. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology 2013;120:2042–50. 10.1016/j.ophtha.2013.03.029 [DOI] [PubMed] [Google Scholar]
- 29. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150–61. 10.1016/j.ophtha.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Z, Luu CD, Hodgson LAB, et al. Prospective longitudinal evaluation of nascent geographic atrophy in age-related macular degeneration. Ophthalmol Retina 2020;4:568–75. 10.1016/j.oret.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 31. Bair P-J, Hsia N-Y, Lin C-L, et al. Population-Based retrospective cohort study on risk of age-related macular degeneration in people with chronic obstructive pulmonary disease. Sci Rep 2021;11:15079. 10.1038/s41598-021-94657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colijn JM, Meester-Smoor M, Verzijden T, et al. Genetic risk, lifestyle, and age-related macular degeneration in Europe: the EYE-RISK Consortium. Ophthalmology 2021;128:1039-1049. 10.1016/j.ophtha.2020.11.024 [DOI] [PubMed] [Google Scholar]
- 33. Lin LY, Zhou Q, Hagstrom S, et al. Association of single-nucleotide polymorphisms in age-related macular degeneration with Pseudodrusen: secondary analysis of data from the comparison of AMD treatments trials. JAMA Ophthalmol 2018;136:682–8. 10.1001/jamaophthalmol.2018.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J, Kim M, Lee CS, et al. Drusen subtypes and choroidal characteristics in Asian eyes with typical neovascular age-related macular degeneration. Retina 2020;40:490–8. 10.1097/IAE.0000000000002419 [DOI] [PubMed] [Google Scholar]
- 35. Keenan TD, Klein B, Agron E. Choroidal thickness and vascularity vary with disease severity and subretinal drusenoid deposit presence in Nonadvanced age-related macular degeneration. Retina 2019. (published Online First: 2019/01/22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Velaga SB, Nittala MG, Vupparaboina KK, et al. Choroidal vascularity index and choroidal thickness in eyes with reticular Pseudodrusen. Retina 2020;40:612–7. 10.1097/IAE.0000000000002667 [DOI] [PubMed] [Google Scholar]
- 37. Domalpally A, Agrón E, Pak JW, et al. Prevalence, risk, and genetic association of reticular Pseudodrusen in age-related macular degeneration: age-related eye disease study 2 report 21. Ophthalmology 2019;126:1659–66. 10.1016/j.ophtha.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nassisi M, Lei J, Abdelfattah NS, et al. Oct risk factors for development of late age-related macular degeneration in the fellow eyes of patients enrolled in the harbor study. Ophthalmology 2019;126:1667–74. 10.1016/j.ophtha.2019.05.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjo-2021-320469supp001.pdf (242.9KB, pdf)
bjo-2021-320469supp002.pdf (311.4KB, pdf)
Data Availability Statement
Data are available on reasonable request. Details on data access policy available at https://www.qub.ac.uk/sites/NICOLA/InformationforResearchers/.